Abstract
Regulation of phosphate homeostasis is critical for many biological processes, and both hypophosphatemia and hyperphosphatemia can have adverse clinical consequences. Only a very small percentage (1%) of total body phosphate is present in the extracellular fluid, which is measured by routine laboratory assays and does not reflect total body phosphate stores. Phosphate is absorbed from the gastrointestinal tract via the transcellular route [sodium phosphate cotransporter 2b (NaPi2b)] and across the paracellular pathway. Approximately 85% of the filtered phosphate is reabsorbed from the kidney, predominantly in the proximal tubule, by NaPi2a and NaPi2c, which are present on the brush border membrane. Renal phosphate transport is tightly regulated. Dietary phosphate intake, parathyroid hormone (PTH), 1,25 (OH)2 vitamin D3, and fibroblast growth factor 23 (FGF23) are the principal regulators of phosphate reabsorption from the kidney. Recent advances in genetic techniques and animal models have identified many genetic disorders of phosphate homeostasis. Mutations in NaPi2a and NaPi2c; and hormonal dysregulation of PTH, FGF23, and Klotho, are primarily responsible for most genetic disorders of phosphate transport. The main focus of this educational review article is to discuss the genetic and clinical features of phosphate regulation disorders and provide understanding and treatment options.
Keywords: Hypophosphatemia, Hyperphosphatemia, FGF23, Klotho, Sodium phosphate contransporters
Normal phosphate homeostasis
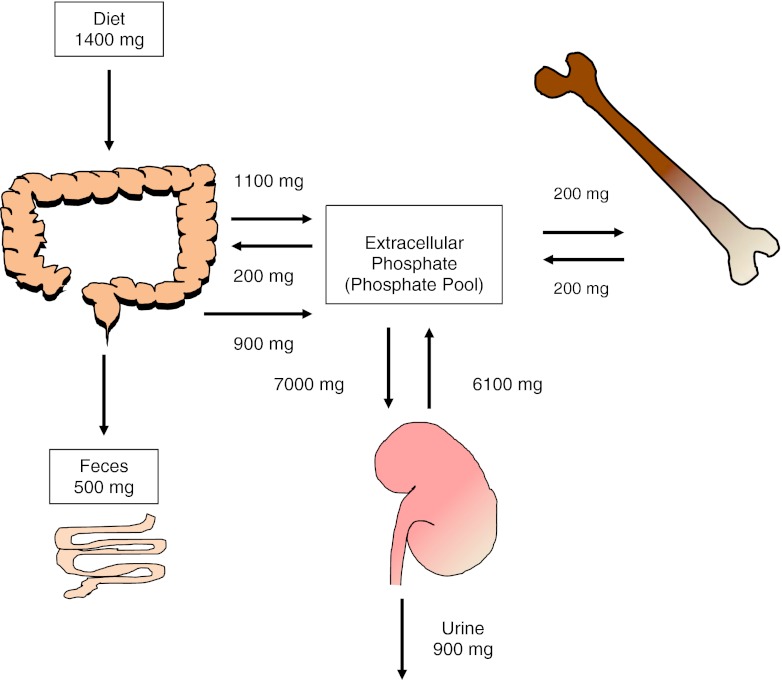
Maintenance of serum phosphate in the physiological range is critical for many biological processes. Phosphate is an essential component of bones, nucleic acids, and cell membranes, and it plays a crucial role in cellular energy metabolism, intracellular signaling by phosphorylation of proteins, and release of oxygen from hemoglobin as phosphate is a component of 2,3-diphosphoglycerate. Phosphate is an important urinary and blood acid base buffer. The adult body contains about 700 g of phosphate, of which 85% is present in the bones and teeth. The remaining 15% is distributed in the soft tissues (14%) and extracellular fluid (1%). Of the 1% that is present in the extracellular fluid, 10–20% is protein bound and 5% is complexed with cations such as calcium, magnesium, and sodium. The remaining 85% exists as HPO-24 and H2PO-14, and at a physiological pH of 7.4, the ratio of HPO-24 to H2PO-14 is 4:1. It is the extracellular (1% of total body phosphate) phosphate that is measured by routine laboratory analysis and thus it does not always reflect the total body phosphate stores [1]. The average adult phosphate intake is about 1–1.5 g, of which one-third is excreted in the stool and the remaining two-thirds is excreted in the urine and thus there is phosphate balance. Gastrointestinal secretions contain 200 mg of phosphate, which is excreted in the stool. Due to continuous bone remodeling, 200 mg of phosphate is exchanged between the bone and the extracellular phosphate. This is depicted in Fig. 1 [1–3].
Fig. 1.
Flux of phosphate between different compartments to maintain normal extracellular serum phosphate levels. In an adult in phosphate balance, the amount of phosphate absorbed from the intestinal tract is excreted in the urine, thus maintaining phosphate balance
A large body of evidence suggests that phosphate homeostasis in general is dependent upon the family of sodium-dependent phosphate transporters: solute carrier family SLC34. There are three types of SLC34 transporters: NaPi2a (SLC34A1, Npt2a, NaPi-IIa), NaPi2b (SLC34A2, Npt2b, NaPi-IIb), and NaPi2c (SLC34A3, Npt2c, NaPi-IIc) [4]. Ingested phosphate is absorbed from the gastrointestinal (GI) tract via the transcellular route and across the paracellular pathway. Transcellular phosphate absorption is an active sodium-dependent process mediated by sodium phosphate cotransporter 2b (NaPi2b). NaPi2b is regulated primarily by 1,25(OH)2 vitamin D3 (also referred to as 1,25 (OH)2D), metabolic acidosis, and dietary phosphate [5–7]. The paracellular pathway is a diffusion-driven non-saturable process in which phosphate transport increases with increasing phosphate in the diet [2]. Thus, with increasing dietary phosphate intake, phosphate absorption increases linearly. The transporter(s) responsible for phosphate exit from the basolateral membrane remains unknown.
Once phosphate enters the systemic circulation, free and complexed phosphate is freely filtered at the glomerulus. Under basal conditions, 80–90% of the filtered load of phosphate is reabsorbed by the kidney. Phosphate is primarily reabsorbed by the proximal tubule via brush border membrane sodium-dependent phosphate transporters, NaPi2a and NaPi2c [8]. Studies employing mouse models of genetic alterations in phosphate transport have demonstrated that NaPi2a is the key transporter in the proximal tubule, responsible for the reabsorption of 70% of filtered phosphate [9]. Human studies suggest that NaPi2c is the predominant transporter in humans and will be discussed later in this article [10, 11]. NaPi2a and NaPi2c have different characteristics and they are regulated differently. NaPi2a is an electrogenic transporter that transports 3Na along with 1 HPO-24 while NaPi2c transports 2Na along with 1 HPO-24 and thus is electroneutral. NaPi2c is highly expressed during weaning in mouse models relating it to growth and is located only on the S1 segment of the proximal tubule, whereas NaPi2a is located along the entire proximal tubule and highly expressed in the adult mouse [12]. Similar to the basolateral intestinal transport of phosphate, basolateral transport of phosphate from the renal tubule also remains to be identified. The principal regulators of NaPi2a and NaPi2c are dietary phosphate, parathyroid hormone (PTH), and fibroblast growth factor 23 (FGF23), although the adaptation of NaPi2a and NaPi2c to these regulators can vary with regards to time course and mechanism [12]. Another family of SLC transporters (SLC20) has recently been identified to play a role in phosphate homeostasis. These proteins were originally identified as “viral receptors” but recently have been identified as NaPi-3 cotransporters: PiT-1 and PiT-2 [13, 14]. An important discovery was made when PiT-2 was shown to be present on the brush border of the proximal tubule and was regulated by dietary phosphate [15]. Other studies have shown that PiT-2 can also be regulated by metabolic acidosis, dietary potassium intake, and FGF23 [16]. Thus far, it appears that PiT-2 plays a modest role in phosphate homeostasis under basal physiological conditions [17].
Genetic disorders of phosphate regulation
This section briefly describes the conditions in which there are alterations in phosphate homeostasis due to either genetic defects in phosphate transporters or due to genetic abnormalities in the hormonal regulation of phosphate homeostasis.
Primary phosphate transporter disorders
The primary phosphate transporters on the apical membrane of the proximal tubule are NaPi2a and NaPi2c. In rodents, NaPi2a is the predominant transporter, as deletion of the NaPi2a gene results in phosphaturia, hypophosphatemia, elevated 1,25 (OH)2 vitamin D3 levels, hypercalcemia, hypercalciuria, and urolithiasis [9]. Abnormalities in the trabecular bone with expansion of the epiphyseal plate and decreased secondary ossification at weaning is seen in NaPi2a-null mice [9, 18]. The bone abnormalities in NaPi2a-null mice normalize with age, and this is likely due to increased 1,25 (OH)2 vitamin D3 levels [18]. In addition, a missense mutation of NaPi2a in mice has demonstrated an autosomal recessive inheritance pattern with hypophosphatemia, phosphaturia, hypercalcemia, and hypercalciuria [19]. In humans, the essential role of NaPi2a in phosphate reabsorption remains controversial. Prie et al. reported two patients with urolithiasis and persistent hypophosphatemia who had heterozygous mutations of NaPi2a, suggesting a dominant negative effect or autosomal dominant inheritance [20]. Subsequently, Virkki et al. could not confirm the dominant negative effect of the same mutations when messenger RNA (mRNA) was expressed in Xenopuslaevis oocytes [21]. Another group reported that polymorphisms of the NaPi2a gene were not clinically associated with significant hypophosphatemia in a group of patients with urolithiasis [22]. Recently, Magen et al. reported two siblings with Fanconi’s syndrome who had homozygous mutations of NaPi2a that resulted in phosphaturia and hypophosphatemia. These siblings were initially reported to have hereditary hypophosphatemic rickets with hypercalciuria (HHRH) along with proximal tubulopathy without metabolic acidosis. Two decades later, the siblings developed renal insufficiency, had lower 1,25 (OH)2 vitamin D3 levels, normal urinary calcium excretion, likely due to low 25 vitamin D levels. Furthermore, expression of the mutant NaPi2a in X. laevis oocytes showed a functional defect of NaPi2a, with accumulation of the mutant protein in the cytoplasm. It remains to be proven if accumulation of intracellular mutant protein directly results in the disruption of other proximal tubular transporters [23].
On the contrary, the essential role of NaPi2c in humans has been very well described. HHRH is an autosomal recessive disorder due to either missense mutations or large deletions in the NaPi2c gene [10, 11]. HHRH was first described by Tieder et al. in six patients from a Bedouin tribe [24]. These patients had short stature, bone pain, muscle weakness, deformities of their lower extremities, and rickets. Patients with HHRH had severe phosphaturia, hypophosphatemia, hypercalciuria, increased 1,25 (OH)2 vitamin D3 levels, and suppressed PTH levels [10, 11]. Elevated serum 1,25 (OH)2 vitamin D3 levels in HHRH is due to the stimulation of 1α- hydroxylase by hypophosphatemia. Elevated 1,25 (OH)2 vitamin D3 levels result in increased absorption of calcium and phosphate from the GI tract, which results in hypercalcemia. Hypercalcemia leads to PTH suppression, contributing to hypercalciuria. Due to increased urinary calcium and phosphate excretion, patients with HHRH have nephrolithiasis. Patients with HHRH are treated with phosphate supplements, which improves bone mineralization and decreases 1,25 (OH)2 vitamin D3 levels. Administration of activated vitamin D supplements will worsen hypercalcemia and hypercalciuria, thus increasing the risk of nephrolithiasis. The main contrasting feature between HHRH and other inherited hypophosphatemic disorders, such as X- linked hypophosphatemic rickets (XLH), autosomal recessive hypophosphatemic rickets (ARHR), and autosomal dominant hypophosphatemic rickets (ADHR), is the inappropriately low level of 1,25 (OH)2 vitamin D3 in XLH, ARHR, and ADHR are hypophosphatemic disorders due to excess of FGF23, which is a phosphaturic hormone that also suppresses 1,25 (OH)2 vitamin D3 synthesis. These conditions will be discussed in detail later. In contrast to humans with NaPi2c mutation (HHRH), mice with deletion of NaPi2c do not develop hypophosphatemia but have hypercalcemia, hypercalciuria, and elevated 1,25 (OH)2 vitamin D3 levels. Thus, in mice, NaPi2c plays an important role in calcium and 1,25 (OH)2vitamin D3 homeostasis [12].
Sodium hydrogen exchanger regulatory factor 1 (NHERF1) is a scaffolding protein that has two PDZ domains and is important in the regulation of protein trafficking, especially G-protein-coupled receptors, transporters, channels, and other structural elements of the cytoskeleton [25]. NHERF1 interacts with NaPi2a and NaPi2c and is important for the trafficking of NaPi2a to the cell surface and its transcription [25, 26]. NHERF1-/- mice have hypophosphatemia, phosphaturia, decreased expression of NaPi2a on the brush border membrane of the proximal tubule, hypercalciuria, and hyperuricosuria [26]. Karim et al. described seven patients with NHERF1 mutations with hyperphosphaturia; the majority of them had nephrolithiasis and bone demineralization [27]. This identified another new cause for hypophosphatemia in both humans and in mice.
Hormonal dysregulation resulting in alteration of phosphate homeostasis
Until about two decades ago, the known principal regulators of proximal tubular phosphate reabsorption were PTH, 1,25 (OH)2vitamin D3, and dietary phosphate intake. PTH decreases phosphate reabsorption from the kidney and increases the synthesis of 1,25 (OH)2vitamin D3. In 1994, Cai et al. described for the first time a new phosphate-regulating substance from a patient with tumor-induced osteomalacia (TIO) that was different from PTH. This newly identified substance inhibited phosphate transport in the cultured opossum kidney epithelial cells (OK cells) [28]. The term phosphatonin was coined in an editorial that accompanied the paper [29]. This phosphate-regulating substance was identified as FGF23 in 2000 [30]. Importantly, tumor removal normalized serum levels of FGF23 and phosphate. This important discovery aided in the understanding of the pathophysiology of many inherited hypophosphatemic conditions. FGF23 is primarily synthesized in the bone by osteocytes, but to a lesser extent by osteoblasts [31, 32]. FGF23 causes hypophosphatemia by decreasing the expression of NaPi2a and NaPi2c on the brush border of the proximal tubule and by decreasing the serum levels of 1,25 (OH)2vitamin D3, which decreases phosphate absorption from the intestine [33, 34].
Animal models have been used to further explore the role of FGF23 in phosphate and 1,25 (OH)2vitamin D3 homeostasis. Transgenic mice that overexpress FGF23 demonstrated lower serum phosphate levels and lower serum 1,25 (OH)2vitamin D3 levels than wild-type mice [34, 35]. On the contrary, deletion of FGF23 in the mouse models showed hyperphosphatemia, increased 1,25 (OH)2vitamin D3 levels, ectopic calcifications, and shortened life spans compared with their wild-type counterparts [34, 36]. Administration of neutralizing antibodies confirmed the findings from FGF23 null mice [34].
FGF23 is an important physiologic hormone that regulates phosphate homeostasis. A high phosphate diet results in an increase in FGF23 levels, and low phosphate diet results in a decrease in FGF23 levels, which has been demonstrated in both rodents and humans [34, 37–39]. In addition, high serum 1,25 (OH)2vitamin D3 levels also stimulate FGF23 production, resulting in a negative feedback mechanism, as FGF23 decreases 1,25 (OH)2vitamin D3 synthesis [40].
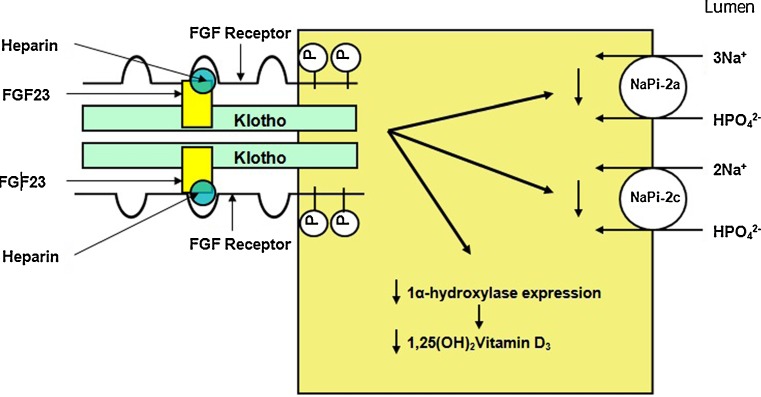
Which receptors mediate the various actions of FGF23 is a topic of intense research. Fibroblast growth factors (FGFs) in general mediate their actions by interacting with FGF receptors (FGFRs). We have shown that FGFR1 is the primary receptor that regulates phosphate reabsorption from the proximal tubule by regulating NaPi2a and NaPi2c [33]. We and others have shown that FGFR3 and FGFR4 are the receptors that regulate 1,25 (OH)2vitamin D3 homeostasis [41, 42]. Thus, different receptors regulate different functions of FGF23 in the proximal tubule, as the proximal tubule is the principal site of phosphate reabsorption and 1,25 (OH)2vitamin D3 synthesis. Whereas FGFRs are present in many tissues, the direct actions of FGF23 are limited to only a few organs; namely, kidneys and parathyroid glands. This organ specificity is likely provided by Klotho, an essential cofactor for the interaction of FGF23 with its receptors. Klotho was first identified in a mouse model of premature aging, and Klotho knockout mice have hyperphosphatemia, hypercalcemia, elevated 1,25 (OH)2vitamin D3 levels, and soft tissue calcifications [43]. Klotho knockout mice and FGF23 knockout mice share a similar phenotype, including shortened life spans, hyperphosphatemia, hypercalcemia, elevated 1,25 (OH)2vitamin D3 levels, and soft tissue calcifications. This provided the first clue that both Klotho and FGF23 work in a common pathway that regulates phosphate homeostasis [44]. Other hormones, such as growth hormone, glucocorticoids, thyroid hormone, and insulin-like growth factor, play a relatively minor role in the regulation of phosphate homeostasis [45]. The proposed interaction of FGF23, Klotho, heparin, and FGF receptors is shown in Fig. 2.
Fig. 2.
Interaction of fibroblast growth factor 23 (FGF23) with FGF receptors on the cell surface, with Klotho and heparin stabilizing the complex. The well-known functions of FGF23 in the proximal tubule are also shown (Adapted from [34] with permission)
Conditions of FGF23 excess
Autosomal dominant hypophosphatemic rickets (ADHR)
ADHR is a rare disorder that was first described by Bianchine et al. in 1971 [46]. ADHR is a result of activating mutations of the FGF23 gene [47]. The mutant FGF23 protein has a missense mutation in either Arg176 or Arg179. The cleavage site, R176XXR179, is normally identified by subtilisin-like proprotein convertases that cleave the FGF23 protein. The mutant FGF23 protein is resistant to these proteases, resulting in an increase in serum FGF23 levels [34, 47]. Elevated FGF23 levels result in phosphaturia, hypophosphatemia, and inappropriately low levels of 1,25 (OH)2vitamin D3. Econs et al. studied a large kindred with ADHR and showed that there can be incomplete penetrance with variable presentation. Patients who manifested the disease in their childhood developed short stature, rickets, bone pain, lower extremity deformities, and dental abscess. Two of the children had spontaneous resolution of symptoms during adulthood. On the other hand, patients with ADHR who manifested the disease in adulthood had symptoms similar to patients with TIO. Adults had bone pain, weakness, osteomalacia, and fractures/pseudofractures, but did not have short stature or lower-extremity deformities. Interestingly, the majority of patients who developed the disease in adulthood were women, and pregnancy triggered the onset of symptoms [48]. FGF23 levels correlated with disease severity [49]. Treatment of ADHR is similar to XLH, which includes phosphate replacement and 1,25 (OH)2vitamin D3 supplementation.
X-linked hypophosphatemic rickets (XLH)
XLH has an incidence of 1:20,000 and is the most common inherited form of rickets. XLH presents within the first 2 years of life. Patients have hypophosphatemia, phosphaturia, inappropriately low levels of 1,25 (OH)2vitamin D3, short stature, bowing of the lower extremities, rickets/osteomalacia, enthesopathy, and dental abscess [50]. Winters et al. first described the X-linked dominant inheritance of this disorder in a family from North Carolina, USA, of English–Scottish descent. They noted that the degree of hypophosphatemia was similar in males and females, but females had a less severe bone phenotype. There was no male to male transmission, and all the daughters of the affected males demonstrated the XLH phenotype, indicating that this condition was X-linked dominant [50, 51]. Approximately 20 years after the inheritance was described, a patient with XLH who was previously thought to have vitamin-D-resistant rickets underwent a renal transplant due to chronic glomerulonephritis/calcium deposition in the kidney. Unfortunately, after the transplant, he continued to have significant renal phosphate wasting despite the fact that he had undergone semitotal parathyroidectomy [52]. This indicated that the defect causing phosphaturia was not in the kidney but, instead, was a systemic circulating factor. A few years later, parabiosis experiments performed between Hyp (mouse model of XLH) mice and wild-type mice and cross-transplantation of kidneys between Hyp and wild-type mice confirmed that, in fact, the defect causing phosphaturia was indeed a systemic circulating factor [53–55]. Inactivating mutations of the PHEX (phosphate regulating gene with homologies to endopeptidases on the X-chromosome) gene were then identified as the genetic defect in patients with XLH [56]. PHEX encodes for an endopeptidase and is highly expressed in bone [57]. As FGF23 was already identified as the causative factor in ADHR, FGF23 levels were measured in the patients with XLH and Hyp mice. Hyp mice have elevated levels of FGF23, as do the majority of the patients with XLH [34, 58–60]. In addition, breeding of fgf23-null mice with Hyp mice resulted in a phenotype similar to fgf23-null mice, indicating that FGF23 is required for the hypophosphatemia in Hyp mice, and FGF23 is downstream of PHEX [36]. Administration of neutralizing antibodies to FGF23 corrected the hypophosphatemia and the low levels of 1,25 (OH)2vitamin D3 in Hyp mice [61]. These studies confirmed the role of FGF23 in the pathogenesis of XLH. FGF23 was once thought to be a substrate of PHEX but was later not confirmed by other studies [34]. It is yet not understood how mutations in the PHEX gene result in elevated FGF23 levels.
Treatment of XLH includes phosphate supplements and 1,25 (OH)2vitamin D3 (calcitriol). Calcitriol increases phosphate absorption from the intestine. Nephrocalcinosis is a well-described iatrogenic complication of phosphate therapy in patients with XLH. Moreover, high doses of calcitriol further increase FGF23 levels in these patients [62, 63]. Due to high phosphate supplements and high FGF23 levels, there is a mild decrease in serum calcium in these patients, which results in secondary hyperparathyroidism. Cinacalcet (calcimimetic agent), is a potential therapy for these patients, as it can decrease phosphate excretion, mitigating the need for orally administered pharmacologic doses of phosphate [64].
Autosomal-recessive hypophosphatemic rickets (ARHR)
ARHR is a rare form of rickets caused by inactivating mutations in the gene encoding for dentin matrix protein 1 (DMP1) [34, 58]. DMP1 belongs to the short integrin-binding ligand interacting N-linked glycoprotein (SIBLING) family of proteins and is an important regulator of the development of bone, cartilage, and teeth. Patients with ARHR present in childhood with symptoms similar to ADHR and XLH, with phosphaturia, hypophosphatemia, inappropriately low levels of 1,25 (OH)2vitamin D3, elevated alkaline phosphatase levels, rickets, dental abscess, and osteosclerotic bone lesions [65]. FGF23 levels are elevated or inappropriately normal for the low serum phosphate levels. Recently, Levi-Litan et al. identified an inactivating mutation in the ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) gene that caused ARHR in a Bedouin family. ENPP1 is a cell-surface enzyme responsible for generating inorganic pyrophosphates that inhibit calcification [66]. ENPP1 mutation was initially identified in patients with infantile arterial calcifications [67]. Interestingly, patients who have ARHR due to ENPP1 mutation do not typically have arterial calcifications, and it is speculated to be due to hypophosphatemia.
Fibrous dysplasia
Fibrous dysplasia (FD) is a disorder in which the normal bone is replaced by fibrous tissue that contains immature osteogenic cells. FD can occur in monostotic or polyostotic forms and can occur in isolation or in conjunction with McCune Albright syndrome. FD is a characteristic feature of McCune Albright syndrome. Patients with McCune Albright syndrome are characterized by polyostotic bone lesions, precocious puberty, thyrotoxicosis, Cushing syndrome, and café-au-lait spots. Some patients with McCune Albright syndrome develop renal phosphate wasting, which is due to increased secretion of FGF23 from the bone lesions. The severity of hypophosphatemia is dependent upon the number of fibrous dysplasia lesions. Patients also have low 1,25 (OH)2vitamin D3, elevated alkaline phosphatase, and rickets/osteomalacia [68]. Patients with McCune Albright syndrome have activating mutations of GNAS1, the gene that encodes for the alpha subunit of stimulatory G protein (Gs α) [69]. G proteins function to couple specific receptors to intracellular signaling molecules. The mechanism behind how mutations in GNAS1 result in elevated levels of FGF23 remains unknown. As McCune Albright syndrome is not inherited, it is possible that GNAS1 mutation is lethal when present in the germline. Bisphosphonates have been used as treatment, with some success [70].
Osteoglophonic dysplasia (OGD)
OGD is a rare autosomal dominant disorder due to an activating mutation of FGFR1. Patients with OGD have facial hypoplasia with prominent supraorbital ridge, craniosynostosis, depressed nasal bridge, and rhizomelic dwarfism. Some patients with OGD have been noted to have elevated FGF23 levels and the reason is unclear. Elevated FGF23 levels result in phosphaturia and inappropriately normal 1,25 (OH)2vitamin D3 levels. Activation of FGFR1 in and of itself results in decreased expression of NaPi2a and NaPi2c, and increased FGF23 levels have an additional effect, resulting in phosphaturia [71].
Conditions of FGF23 deficiency
Conditions causing FGF23 deficiency are characterized by hyperphosphatemia, hypercalcemia, increased serum levels of 1,25 (OH)2vitamin D3, and ectopic and vascular calcifications [72]. This phenotype is similar to that seen in fgf23 and Klotho-null mice. Tumoral calcinosis is an autosomal recessive disorder that results in ectopic deposition of calcium and phosphate. Patients with tumoral calcinosis have painful joints due to deposition of calcium and phosphate in the joints and soft tissues. Mutations in both GALNT3 and FGF23 can result in this phenotype. GALNT3 encodes for a protein called UDP-N-acetyl-alpha-D-galactosamine: polypeptide N-acetylgalactosaminyl transferase 3, which is responsible for O-linked glycosylation of threonine 178 just adjacent to cleavage site, R176XXR179 that stabilizes FGF23. The mutated FGF23 protein with defective O-glycosylation is rapidly degraded by proteases. There are two assays available to measure serum FGF23 levels; intact assay that measures intact FGF23 protein, and the C-terminal assay that measures both the C-terminal fragment and the intact FGF23 protein. In patients with GALNT3 mutation, as expected, intact FGF23 levels are low due to its rapid degradation, but the C-terminal assay demonstrates elevated levels [34, 58]. Inactivating mutations in the conserved region of FGF23 also result in decreased levels of FGF23. The mutated protein is either not secreted in its intact from or is easily prone to proteolytic cleavage. Similar to patients with GALNT3 mutation, patients with inactivating FGF23 mutations also have low intact FGF23 levels but elevated FGF23 levels when measured by C-terminal assay [73].
Disorders of klotho regulation
Homozygous missense mutations of Klotho have also been identified as a cause of tumoral calcinosis. Ichikawa et al. described a 13-year-old who initially presented with tumoral calcinosis with increased levels of phosphate, calcium, 1,25 (OH)2vitamin D3, and PTH. This patient also had elevated intact and C-terminal FGF23 levels, thus prompting further investigation. A missense mutation in the highly conserved region of the Klotho gene was identified. As Klotho is an essential cofactor in the interaction of FGF23 with its receptors, absence of active Klotho decreases the activity of FGF23 and thus results in a phenotype analogous to tumoral calcinosis. The increase in serum FGF23 levels were likely due to elevated serum phosphate and 1,25 (OH)2vitamin D3 levels [74]. On the contrary, a translocation causing elevated levels of Klotho resulted in hypophosphatemic rickets in a 13-month-old infant [75].
Inherited disorders of parathyroid hormone (PTH) and its receptor
PTH is an important regulator of phosphate homeostasis. PTH results in decreased phosphate reabsorption from the proximal tubule while stimulating the synthesis of 1,25 (OH)2vitamin D3. PTH receptors are present on both the basolateral and the apical side of the proximal tubule, and the intracellular signaling is dependent on which receptor is activated. Activation of the basolateral receptor will preferentially activate the protein kinase A pathway, whereas activation of the apical receptor in the presence of NHERF1 will predominantly activate the protein kinase C pathway [76]. Familial causes of hyperparathyroidism include multiple endocrine neoplasias (MEN). Patients with hyperparathyroidism will present with hypophosphatemia, hypercalcemia, and hypercalciuria. An activating mutation of the PTH receptor (PTH1R) has been described in Jansen’s metaphyseal chondrodysplasia (JMC), a rare cause of short-limbed dwarfism inherited in an autosomal dominant fashion. PTH1R is abundantly present in the kidneys and bones, and the constitutively active mutated PTH1R receptor results in phosphaturia, hypophosphatemia, hypercalcemia, and low PTH levels due to a negative feedback mechanism [77]. Inactivating mutations of PTH1R have been described in Blomstrand’s disease, which is a rare autosomal recessive disorder with early mortality and advanced bone age [78]. On the contrary, inherited causes of hypoparathyroidism include mutations of glial cells missing B (GCMB), regulator of parathyroid gland development and Di George syndrome, deletion of 22q11.2 resulting in hypoplasia of the thymus, parathyroid gland, cardiac defects, and craniofacial abnormalities [79]. Hypoparathyroidism results in hypocalcemia, hyperphosphatemia, and decreased fractional excretion of phosphate.
Nongenetic etiologies and clinical manifestations of hypophosphatemia and hyperphosphatemia
Hyperphosphatemia results either from decreased renal excretion, increased intake, or redistribution of phosphate. Decreased renal excretion of phosphate occurs in the face of acute kidney injury (AKI), chronic kidney disease (CKD), hypoparathyroidism, and nonfamilial tumoral calcinosis. Increased intake of phosphate is primarily seen in patients who have received phosphate-containing enemas or oral phosphate as laxatives, and even patients who have normal renal function can develop hyperphosphatemia after an excessive phosphate load. Whereas hyperphosphatemia is transient, rarely, acute phosphate ingestion can result in AKI, known as phosphate nephropathy, which has a poor prognosis for renal recovery [80]. Cellular redistribution of phosphate leading to hyperphosphatemia can be seen in patients with tumor lysis syndrome, rhabdomyolysis, or respiratory acidosis. These conditions can result in a temporary increase in serum phosphate that might require renal replacement therapy. Once the underlying condition has resolved, serum phosphate levels return to normal. The symptoms of acute hyperphosphatemia are primarily due to secondary hypocalcemia resulting from calcium precipitation [81]. Chronic effects of hyperphosphatemia, as seen in CKD and end-stage renal disease (ESRD), result in ectopic deposition of calcium in the vasculature, skin, eyes, joints, and other organs. Many factors, including uremia, calcium phosphate product, and serum phosphate levels influence vascular calcification and conversion of vascular smooth muscle cells into osteoblast-like cells [82]. Vascular calcifications in patients with ESRD increase morbidity and mortality. Chronic hyperphosphatemia as seen in CKD and ESRD results in secondary hyperparathyroidism and renal osteodystrophy.
Hypophosphatemia results either from increased renal losses of phosphate, impaired or decreased absorption of phosphate from the GI tract, decreased dietary intake, or cellular redistribution. Increased renal losses can be due to hyperparathyroidism or vitamin D deficiency. Decreased GI absorption is seen with ingestion of phosphate-binding agents or chronic diarrhea. Severe malnutrition can also result in hypophosphatemia. Cellular redistribution of phosphate is seen in acute respiratory alkalosis, acute leukemia, or refeeding syndrome. Hypophosphatemia seen in refeeding syndrome is due to rapid uptake of phosphate in to the cells along with glucose, potassium, and magnesium. Symptoms and signs of hypophosphatemia usually occur when the serum phosphorus is <1 mg/dl (normal range 3–4.5 mg/dl or 1–1.5 mmol/L) and include hemolysis, rhabdomyolysis, leucocyte dysfunction, decreased delivery of oxygen to the tissues, and respiratory failure. In addition, chronic phosphate depletion results in rickets and osteomalacia [2].
Summary
This review describes the inherited conditions in which phosphate handling is altered. FGF23 emerges as a key regulator of phosphate homeostasis, and its alteration results in many inherited disorders. Klotho is an important player in the interaction of FGF23 with its receptors and thus alterations in Klotho significantly affect phosphate homeostasis. Mutations of PHEX, DMP1, ENPP1, and GNAS1 result in altered FGF23 levels, but the precise underlying mechanism is not understood. Table 1 lists some of the inherited disorders of phosphate homeostasis, their genetic mutations, and their pathophysiology.
Table 1.
Genetic disorders of phosphate regulation
| Hypophosphatemic Disorders | ||
|---|---|---|
| Disease | Genetic mutation | Pathogenesis of the disease |
| Autosomal recessive Fanconi syndrome, hypophosphatemic rickets | NaPi2a (SLC34A1, Npt2a, NaPi-IIa) | Loss of function of NaPi2a at the brush border of the proximal tubule |
| Hereditary hypophosphatemic rickets with hypercalciuria | NaPi2c (SLC34A3, Npt2c, NaPi-IIc) | Loss of function of NaPi2c at the brush border of the proximal tubule |
| Hypophosphatemia, nephrocalcinosis and osteopenia | NHERF1 | Loss of function of the anchoring protein resulting in decreased expression of brush border NaPi2a |
| Autosomal dominant hypophosphatemic rickets | FGF23 | FGF23 protein that is resistant to degradation |
| Autosomal recessive hypophosphatemic rickets | DMP1 | Increased expression of FGF23 protein from the bone |
| ENPP1 | ||
| X-linked hypophosphatemic rickets | PHEX | Increased expression of FGF23 protein from the bone |
| Fibrous dysplasia/McCune-Albright syndrome | GNAS-1 | Increased expression of FGF23 protein rom the bone lesions |
| Hypophosphatemic rickets | Klotho | Overexpression of Klotho results in hypophosphatemia |
| Hyperphosphatemic disorders | ||
| Disease | Genetic mutation | Pathogenesis of the disease |
| Tumoral calcinosis | FGF23 | Decreased production or increased degradation of FGF23 or resistance to FGF23 due the absence of Klotho |
| GALNT3 | ||
| Klotho | ||
Acknowledgments
This work was supported by Children’s Medical Center Research Foundation Grant (JG) and NIH grant K08DK089295-01 (JG), NIH grant DK41612 and DK078596 (MB), T32 DK07257 (Peter Igarashi and MB), O’Brien Center P30DK079328 (Peter Igarashi, PI).
Questions
Question 1.
A 5-year-old boy presents with fracture of his right femur with minimal trauma. X-ray shows diffuse osteopenia. Workup revealed a serum phosphorus of 1.8 mg/dl (4–5.4 mg/dl), elevated fractional excretion of phosphate (40%), 1,25 vitamin D levels of 129 pg/ml (15–75 pg/ml), 24-h urinary calcium of 7 mg/kg/day, and serum calcium 9.8 mg/dl (8.5–11 mg/dl). He has nephrocalcinosis on renal sonogram. The most likely diagnosis in this patient is:
X- Linked hypophosphatemic rickets
Autosomal recessive hypophosphatemic rickets
Tumoral calcinosis
Hereditary hypophosphatemic rickets with hypercalciuria
Autosomal dominant hypophosphatemic rickets
Question 2.
True or False
Fibroblast growth factor 23:
Is a phosphaturic hormone
Is regulated by 1,25 vitamin D
Increases the expression of 1 hydroxylase and thereby increases serum 1,25 vitamin D levels
Is primarily secreted by the bone
Levels are detected in healthy normal individuals
Question 3.
Patients with XLH, ADHR, and ARHR share common features, except:
Elevated FGF23 levels
Decreased fractional excretion of phosphate
Inappropriately low levels of 1,25 vitamin D
Normal serum calcium levels
Normal 25 vitamin D levels
Question 4. Match the following:
| I. X- Linked hypophosphatemic rickets | a. Mutation of DMP1 |
| II. Autosomal dominant hypophosphatemic rickets | b. Mutation of PHEX |
| III. Autosomal recessive hypophosphatemic rickets | c. NaPi-2c mutation |
| IV. Hereditary hypophosphatemic rickets with hypercalciuria | d. Activating mutation of FGF23 |
| V. Tumoral calcinosis | e. Inactivating mutation of FGF23 |
Footnotes
Answers:
1. d
2. a. True
b. True
c. False
d. True
e. True
3. b
4. I - b
II - d
III - a
IV - c
V - e
References
- 1.Amanzadeh J, Reilly RF. Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nat Clin Pract Nephrol. 2006;2:136–148. doi: 10.1038/ncpneph0124. [DOI] [PubMed] [Google Scholar]
- 2.Naderi AS, Reilly RF. Hereditary disorders of renal phosphate wasting. Nat Rev Nephrol. 2010;6:657–665. doi: 10.1038/nrneph.2010.121. [DOI] [PubMed] [Google Scholar]
- 3.Shaikh A, Berndt T, Kumar R. Regulation of phosphate homeostasis by the phosphatonins and other novel mediators. Pediatr Nephrol. 2008;23:1203–1210. doi: 10.1007/s00467-008-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murer H, Forster I, Biber J. The sodium phosphate cotransporter family SLC34. Pflugers Arch. 2004;447:763–767. doi: 10.1007/s00424-003-1072-5. [DOI] [PubMed] [Google Scholar]
- 5.Danisi G, Bonjour JP, Straub RW. Regulation of Na-dependent phosphate influx across the mucosal border of duodenum by 1,25-dihydroxycholecalciferol. Pflugers Arch. 1980;388:227–232. doi: 10.1007/BF00658486. [DOI] [PubMed] [Google Scholar]
- 6.Hattenhauer O, Traebert M, Murer H, Biber J. Regulation of small intestinal Na-P(i) type IIb cotransporter by dietary phosphate intake. Am J Physiol. 1999;277:G756–G762. doi: 10.1152/ajpgi.1999.277.4.G756. [DOI] [PubMed] [Google Scholar]
- 7.Hilfiker H, Hattenhauer O, Traebert M, Forster I, Murer H, Biber J. Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc Natl Acad Sci U S A. 1998;95:14564–14569. doi: 10.1073/pnas.95.24.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virkki LV, Biber J, Murer H, Forster IC. Phosphate transporters: a tale of two solute carrier families. Am J Physiol Renal Physiol. 2007;293:F643–F654. doi: 10.1152/ajprenal.00228.2007. [DOI] [PubMed] [Google Scholar]
- 9.Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U S A. 1998;95:5372–5377. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, bu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet. 2006;78:179–192. doi: 10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenz-Depiereux B, et-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet. 2006;78:193–201. doi: 10.1086/499410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segawa H, Aranami F, Kaneko I, Tomoe Y, Miyamoto K. The roles of Na/Pi-II transporters in phosphate metabolism. Bone. 2009;45(Suppl 1):S2–S7. doi: 10.1016/j.bone.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Kavanaugh MP, Miller DG, Zhang W, Law W, Kozak SL, Kabat D, Miller AD. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci U S A. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller DG, Miller AD. A family of retroviruses that utilize related phosphate transporters for cell entry. J Virol. 1994;68:8270–8276. doi: 10.1128/jvi.68.12.8270-8276.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villa-Bellosta R, Ravera S, Sorribas V, Stange G, Levi M, Murer H, Biber J, Forster IC. The Na + -Pi cotransporter PiT-2 (SLC20A2) is expressed in the apical membrane of rat renal proximal tubules and regulated by dietary Pi. Am J Physiol Renal Physiol. 2009;296:F691–F699. doi: 10.1152/ajprenal.90623.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breusegem SY, Takahashi H, Giral-Arnal H, Wang X, Jiang T, Verlander JW, Wilson P, Miyazaki-Anzai S, Sutherland E, Caldas Y, Blaine JT, Segawa H, Miyamoto K, Barry NP, Levi M. Differential regulation of the renal sodium-phosphate cotransporters NaPi-IIa, NaPi-IIc, and PiT-2 in dietary potassium deficiency. Am J Physiol Renal Physiol. 2009;297:F350–F361. doi: 10.1152/ajprenal.90765.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villa-Bellosta R, Sorribas V. Compensatory regulation of the sodium/phosphate cotransporters NaPi-IIc (SCL34A3) and Pit-2 (SLC20A2) during Pi deprivation and acidosis. Pflugers Arch. 2010;459:499–508. doi: 10.1007/s00424-009-0746-z. [DOI] [PubMed] [Google Scholar]
- 18.Miedlich SU, Zhu ED, Sabbagh Y, Demay MB. The receptor-dependent actions of 1,25-dihydroxyvitamin D are required for normal growth plate maturation in NPt2a knockout mice. Endocrinology. 2010;151:4607–4612. doi: 10.1210/en.2010-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwaki T, Sandoval-Cooper MJ, Tenenhouse HS, Castellino FJ. A missense mutation in the sodium phosphate co-transporter Slc34a1 impairs phosphate homeostasis. J Am Soc Nephrol. 2008;19:1753–1762. doi: 10.1681/ASN.2007121360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prie D, Huart V, Bakouh N, Planelles G, Dellis O, Gerard B, Hulin P, que-Blanchet F, Silve C, Grandchamp B, Friedlander G. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med. 2002;347:983–991. doi: 10.1056/NEJMoa020028. [DOI] [PubMed] [Google Scholar]
- 21.Virkki LV, Forster IC, Hernando N, Biber J, Murer H. Functional characterization of two naturally occurring mutations in the human sodium-phosphate cotransporter type IIa. J Bone Miner Res. 2003;18:2135–2141. doi: 10.1359/jbmr.2003.18.12.2135. [DOI] [PubMed] [Google Scholar]
- 22.Lapointe JY, Tessier J, Paquette Y, Wallendorff B, Coady MJ, Pichette V, Bonnardeaux A. NPT2a gene variation in calcium nephrolithiasis with renal phosphate leak. Kidney Int. 2006;69:2261–2267. doi: 10.1038/sj.ki.5000437. [DOI] [PubMed] [Google Scholar]
- 23.Magen D, Berger L, Coady MJ, Ilivitzki A, Militianu D, Tieder M, Selig S, Lapointe JY, Zelikovic I, Skorecki K. A loss-of-function mutation in NaPi-IIa and renal Fanconi’s syndrome. N Engl J Med. 2010;362:1102–1109. doi: 10.1056/NEJMoa0905647. [DOI] [PubMed] [Google Scholar]
- 24.Tieder M, Modai D, Samuel R, Arie R, Halabe A, Bab I, Gabizon D, Liberman UA. Hereditary hypophosphatemic rickets with hypercalciuria. N Engl J Med. 1985;312:611–617. doi: 10.1056/NEJM198503073121003. [DOI] [PubMed] [Google Scholar]
- 25.Hernando N, Gisler SM, Pribanic S, Deliot N, Capuano P, Wagner CA, Moe OW, Biber J, Murer H. NaPi-IIa and interacting partners. J Physiol. 2005;567:21–26. doi: 10.1113/jphysiol.2005.087049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunningham R, Biswas RS, Steplock D, Shenolikar S, Weinman EJ. Role of NHERF and scaffolding proteins in proximal tubule transport. Urol Res. 2010;4:257–262. doi: 10.1007/s00240-010-0294-1. [DOI] [PubMed] [Google Scholar]
- 27.Karim Z, Gerard B, Bakouh N, Alili R, Leroy C, Beck L, Silve C, Planelles G, Urena-Torres P, Grandchamp B, Friedlander G, Prie D. NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med. 2008;359:1128–1135. doi: 10.1056/NEJMoa0802836. [DOI] [PubMed] [Google Scholar]
- 28.Cai Q, Hodgson SF, Kao PC, Lennon VA, Klee GG, Zinsmiester AR, Kumar R. Brief report: inhibition of renal phosphate transport by a tumor product in a patient with oncogenic osteomalacia. N Engl J Med. 1994;330:1645–1649. doi: 10.1056/NEJM199406093302304. [DOI] [PubMed] [Google Scholar]
- 29.Econs MJ, Drezner MK. Tumor-induced osteomalacia–unveiling a new hormone. N Engl J Med. 1994;330:1679–1681. doi: 10.1056/NEJM199406093302310. [DOI] [PubMed] [Google Scholar]
- 30.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 32.Mirams M, Robinson BG, Mason RS, Nelson AE. Bone as a source of FGF23: regulation by phosphate? Bone. 2004;35:1192–1199. doi: 10.1016/j.bone.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009;297:F282–F291. doi: 10.1152/ajprenal.90742.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gattineni J, Baum M. Regulation of phosphate transport by fibroblast growth factor 23 (FGF23): implications for disorders of phosphate metabolism. Pediatr Nephrol. 2010;25:591–601. doi: 10.1007/s00467-009-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bai XY, Miao D, Goltzman D, Karaplis AC. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem. 2003;278:9843–9849. doi: 10.1074/jbc.M210490200. [DOI] [PubMed] [Google Scholar]
- 36.Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Juppner H, Lanske B. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 38.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 39.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–1524. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 40.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280:2543–2549. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- 41.Li H, Martin AC, David V, Quarles LD (2010) Compound Deletion of FGFR3 and FGFR4 Partially Rescues the Hyp Mouse Phenotype. Am J Physiol Endocrinol Metab 300:E508–517 [DOI] [PMC free article] [PubMed]
- 42.Gattineni J, Twombley K, Goetz R, Mohammadi M, Baum M. Regulation of serum 1,25(OH)2vitamin D3 levels by fibroblast growth factor 23 is mediated by FGF receptors 3 and 4. Am J Physiol Renal Physiol. 2011;301:F371–F377. doi: 10.1152/ajprenal.00740.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 44.Alon US. Clinical practice. Fibroblast growth factor (FGF)23: a new hormone. Eur J Pediatr. 2011;170:545–554. doi: 10.1007/s00431-010-1382-5. [DOI] [PubMed] [Google Scholar]
- 45.Biber J, Hernando N, Forster I, Murer H. Regulation of phosphate transport in proximal tubules. Pflugers Arch. 2009;458:39–52. doi: 10.1007/s00424-008-0580-8. [DOI] [PubMed] [Google Scholar]
- 46.Bianchine JW, Stambler AA, Harrison HE. Familial hypophosphatemic rickets showing autosomal dominant inheritance. Birth Defects Orig Artic Ser. 1971;7:287–295. [PubMed] [Google Scholar]
- 47.ADHR Consortium Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 48.Econs MJ, McEnery PT. Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab. 1997;82:674–681. doi: 10.1210/jc.82.2.674. [DOI] [PubMed] [Google Scholar]
- 49.Imel EA, Hui SL, Econs MJ. FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res. 2007;22:520–526. doi: 10.1359/jbmr.070107. [DOI] [PubMed] [Google Scholar]
- 50.HYPOPHOSPHATEMIC RICKETS, X-LINKED DOMINANT; XLHR. OMIM . 1-13-2011. Ref Type: Electronic Citation http://www.omim.org/entry/307800
- 51.Winters RW, Graham JB, Williams TF, McFalls VW, Burnett CH. (1991) A genetic study of familial hypophosphatemia and vitamin D resistant rickets with a review of the literature. Medicine (Baltimore) 1958;70:215–217. [PubMed] [Google Scholar]
- 52.Morgan JM, Hawley WL, Chenoweth AI, Retan WJ, Diethelm AG. Renal transplantation in hypophosphatemia with vitamin D-resistant rickets. Arch Intern Med. 1974;134:549–552. doi: 10.1001/archinte.1974.00320210159025. [DOI] [PubMed] [Google Scholar]
- 53.Marie PJ, Travers R, Glorieux FH. Mineral and skeletal changes in parabiotic normal and hypophosphatemic mice. Clin Res. 1981;29:414a. [Google Scholar]
- 54.Nesbitt T, Coffman TM, Griffiths R, Drezner MK. Crosstransplantation of kidneys in normal and Hyp mice. Evidence that the Hyp mouse phenotype is unrelated to an intrinsic renal defect. J Clin Invest. 1992;89:1453–1459. doi: 10.1172/JCI115735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyer RA, Jr, Meyer MH, Gray RW. Parabiosis suggests a humoral factor is involved in X-linked hypophosphatemia in mice. J Bone Miner Res. 1989;4:493–500. doi: 10.1002/jbmr.5650040407. [DOI] [PubMed] [Google Scholar]
- 56.HYP Consortium A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 57.Du L, Desbarats M, Viel J, Glorieux FH, Cawthorn C, Ecarot B. cDNA cloning of the murine Pex gene implicated in X-linked hypophosphatemia and evidence for expression in bone. Genomics. 1996;36:22–28. doi: 10.1006/geno.1996.0421. [DOI] [PubMed] [Google Scholar]
- 58.Farrow EG, White KE. Recent advances in renal phosphate handling. Nat Rev Nephrol. 2010;6:207–217. doi: 10.1038/nrneph.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–E49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 60.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 61.Aono Y, Yamazaki Y, Yasutake J, Kawata T, Hasegawa H, Urakawa I, Fujita T, Wada M, Yamashita T, Fukumoto S, Shimada T. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res. 2009;24:1879–1888. doi: 10.1359/jbmr.090509. [DOI] [PubMed] [Google Scholar]
- 62.Alon U, Donaldson DL, Hellerstein S, Warady BA, Harris DJ. Metabolic and histologic investigation of the nature of nephrocalcinosis in children with hypophosphatemic rickets and in the Hyp mouse. J Pediatr. 1992;120:899–905. doi: 10.1016/S0022-3476(05)81957-2. [DOI] [PubMed] [Google Scholar]
- 63.Cho HY, Lee BH, Kang JH, Ha IS, Cheong HI, Choi Y. A clinical and molecular genetic study of hypophosphatemic rickets in children. Pediatr Res. 2005;58:329–333. doi: 10.1203/01.PDR.0000169983.40758.7B. [DOI] [PubMed] [Google Scholar]
- 64.Alon US, Levy-Olomucki R, Moore WV, Stubbs J, Liu S, Quarles LD. Calcimimetics as an adjuvant treatment for familial hypophosphatemic rickets. Clin J Am Soc Nephrol. 2008;3:658–664. doi: 10.2215/CJN.04981107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perry W, Stamp TC. Hereditary hypophosphataemic rickets with autosomal recessive inheritance and severe osteosclerosis. A report of two cases. J Bone Joint Surg Br. 1978;60-B:430–434. doi: 10.1302/0301-620X.60B3.681423. [DOI] [PubMed] [Google Scholar]
- 66.Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, Manor E, Buriakovsky S, Hadad Y, Goding J, Parvari R. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Genet. 2010;86:273–278. doi: 10.1016/j.ajhg.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Hohne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, Epplen JT, Knisely A, Superti-Furga A, McGill J, Filippone M, Sinaiko AR, Vallance H, Hinrichs B, Smith W, Ferre M, Terkeltaub R, Nurnberg P. Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet. 2003;34:379–381. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- 68.Imel EA, Econs MJ. Fibrous dysplasia, phosphate wasting and fibroblast growth factor 23. Pediatr Endocrinol Rev. 2007;4(Suppl 4):434–439. [PubMed] [Google Scholar]
- 69.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto T, Imanishi Y, Kinoshita E, Nakagomi Y, Shimizu N, Miyauchi A, Satomura K, Koshiyama H, Inaba M, Nishizawa Y, Juppner H, Ozono K. The role of fibroblast growth factor 23 for hypophosphatemia and abnormal regulation of vitamin D metabolism in patients with McCune-Albright syndrome. J Bone Miner Metab. 2005;23:231–237. doi: 10.1007/s00774-004-0589-9. [DOI] [PubMed] [Google Scholar]
- 71.White KE, Cabral JM, Davis SI, Fishburn T, Evans WE, Ichikawa S, Fields J, Yu X, Shaw NJ, McLellan NJ, McKeown C, Fitzpatrick D, Yu K, Ornitz DM, Econs MJ. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am J Hum Genet. 2005;76:361–367. doi: 10.1086/427956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V, Zelikovic I, Raimer S, Metzker A, Richard G, Sprecher E. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36:579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 73.et-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 74.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci U S A. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaufmann M, Muff R, Stieger B, Biber J, Murer H, Fischer JA. Apical and basolateral parathyroid hormone receptors in rat renal cortical membranes. Endocrinology. 1994;134:1173–1178. doi: 10.1210/en.134.3.1173. [DOI] [PubMed] [Google Scholar]
- 77.Schipani E, Kruse K, Juppner H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science. 1995;268:98–100. doi: 10.1126/science.7701349. [DOI] [PubMed] [Google Scholar]
- 78.Blomstrand S, Claesson I, Save-Soderbergh J. A case of lethal congenital dwarfism with accelerated skeletal maturation. Pediatr Radiol. 1985;15:141–143. doi: 10.1007/BF02388725. [DOI] [PubMed] [Google Scholar]
- 79.Scambler PJ, Carey AH, Wyse RK, Roach S, Dumanski JP, Nordenskjold M, Williamson R. Microdeletions within 22q11 associated with sporadic and familial DiGeorge syndrome. Genomics. 1991;10:201–206. doi: 10.1016/0888-7543(91)90501-5. [DOI] [PubMed] [Google Scholar]
- 80.Markowitz GS, Stokes MB, Radhakrishnan J, D’Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389–3396. doi: 10.1681/ASN.2005050496. [DOI] [PubMed] [Google Scholar]
- 81.Shiber JR, Mattu A. Serum phosphate abnormalities in the emergency department. J Emerg Med. 2002;23:395–400. doi: 10.1016/S0736-4679(02)00578-4. [DOI] [PubMed] [Google Scholar]
- 82.Neven E, D’Haese PC. Vascular calcification in chronic renal failure: what have we learned from animal studies? Circ Res. 2011;108:249–264. doi: 10.1161/CIRCRESAHA.110.225904. [DOI] [PubMed] [Google Scholar]