Abstract
Renal transplantation has long been recognised as the gold standard treatment for children with end-stage renal failure. There has been an improvement over the years in patient and renal allograft survival because of improved immunosuppression, surgical techniques and living kidney donation. Despite reduced acute allograft rejection rates, non-viral infections continue to be a serious complication for paediatric renal transplant recipients (RTR). The risk of infections in RTR is determined by the pre-transplantation immunisation status, post-transplant exposure to potential pathogens and the amount of immunosuppression. The greatest risk of life-threatening and Cytomegalovirus infections is during the first 6 months post-transplant owing to a high immunosuppressive burden. The potential sources of bacterial infections are donor derived, transplant medium fluid, peritoneal and haemodialysis catheter and transplant ureteric stent. Urinary tract infections are frequent in patients with lower urinary tract dysfunction and can result in renal allograft damage. This review outlines the incidence, timing, risk factors, prevention and treatment of non-viral infections in paediatric RTR by critically reviewing current immunosuppressive regimens, their risk–benefit ratio in order to optimise renal allograft survival with reduced rates of rejection and infectious complications.
Keywords: Paediatric renal transplantation, Non-viral infection, Immunosuppression minimisation, Risk of infection, Graft survival
Introduction
Renal transplantation is the gold standard therapy for children with end-stage renal failure (ESRF). Renal transplantation (RT) frequently restores the potential for normal growth and development with improved morbidity and mortality rates and reduced cardiovascular complications compared with dialysis [1, 2]. Short-term renal allograft survival rates have improved with the advent of newer immunosuppressive medications, but carry the risk of increased infectious complications [3].
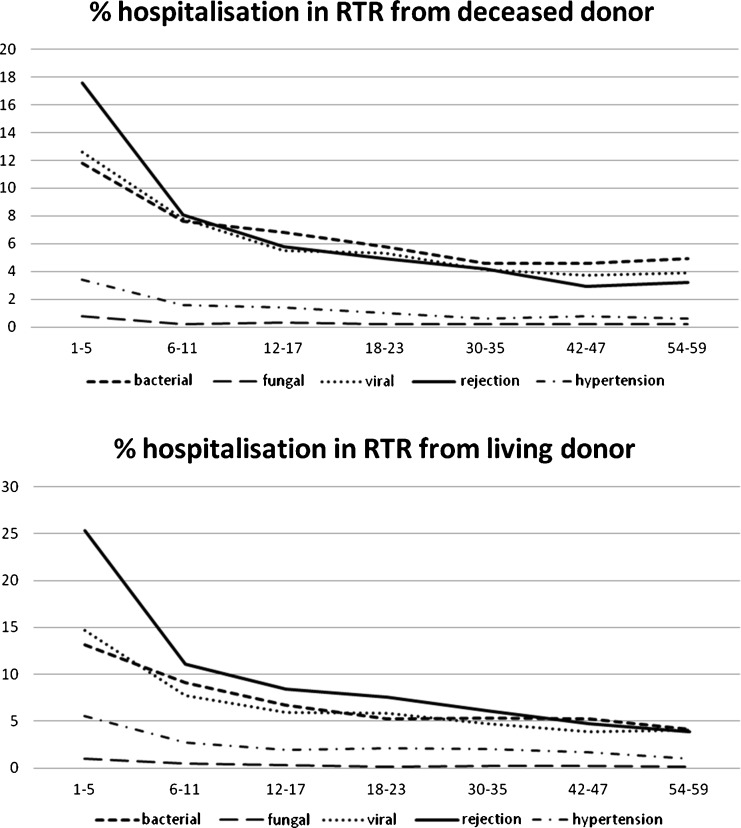
Infection is the most common cause of death after transplantation in 24–56% of cases, with cardiovascular disease accounting for 30–36% and malignancy 11–20% respectively [4–9]. In a recent analysis of the North American Pediatric Renal Trials and Collaborative Study (NAPRTCS), post-transplant infections exceed acute rejection as the cause of hospitalisation in the first 2 years following RT (Fig. 1) [10]. In 2000, the risk of hospitalisation for post-transplant infection was twice that for acute rejection. The optimal approach for patient and renal allograft survival is a balance between the prophylaxis and treatment of rejection and the risk of infections using minimal immunosuppression.
Fig. 1.
Post-transplant reasons for hospitalisation for living and deceased donor source renal transplant recipients (RTR) in the first 59 months of follow-up. (Data derived from North American Pediatric Renal Trials and Collaborative Study (NAPRTCS) Annual Report 2008.)
Complications of immunosuppression account for the most significant morbidity and mortality following organ transplantation. Infection, both sepsis-related and due to opportunistic organisms, have increased with the use of more potent immunosuppressive agents. Opportunistic infections are the most severe of these complications and are most frequent in the early post-transplant period when RTR are highly immunosuppressed [11].
There are a broad range of potential sources of infection (latent viruses, hospital and community acquired) in a host that has an impaired inflammatory response by immunosuppression, which attenuates the signs and symptoms of invasive infections. This has to be balanced with the toxicity of antimicrobial medications and their interaction with immunosuppressive therapy [12].
Increasingly, potent immunosuppressive agents have dramatically reduced the incidence of biopsy-proven acute cellular rejection of transplanted organs while increasing patients’ susceptibility to opportunistic infections and malignancy. There is also an increased number of infections due to organisms with antimicrobial resistance [13].
Paediatric RT carries a higher risk of infectious complications compared with adult counterparts, due to reduced exposure in a developing immune system. Children with chronic kidney disease (CKD) and ESRF requiring dialysis may have inadequate nutrition and growth, leaving them compromised at the time of transplantation. Their small size makes surgical procedures more complex and predisposes them to gastrointestinal, pulmonary and vascular complications that can increase the risk of infection [11].
Risk of infection in renal transplant recipients (RTR)
The risk of infections in RTR is determined by the interaction between the exposure to potential pathogens the individual encounters and the individual’s net state of immunosuppression [14–16]. This refers to all factors that contribute to the patient’s risk of infection, including the dose, duration and type of immunosuppressive therapies, the presence of metabolic factors like malnutrition and uraemia, the presence of immunomodulating viral infections (including Cytomegalovirus [CMV] and Epstein–Barr virus [EBV]; Table 1) [17]. Even minimal environmental exposure to organisms of low virulence can cause an invasive infection that is most likely early post-transplantation when the immunosuppressive burden is greatest [14]. CMV infection may cause both invasive disease and a variety of secondary immune phenomena in RT, increasing the risk of opportunistic infections [15, 18, 19].
Table 1.
Factors affecting the net state of immunosuppression in renal transplant recipients (RTR). Reproduced with permission from [17]
| Factors affecting the net state of immunosuppression in RTR |
|---|
| Immunosuppressive therapy: dose, duration and temporal sequence |
| Haematological features: neutropaenia, lymphopaenia |
| Underlying immunodeficiency: autoimmune disease, functional immune deficits |
| Integrity of the mucocutaneous barrier: catheters, epithelial surfaces, devitalised tissue, fluid collections |
| Metabolic conditions: uraemia, malnutrition, diabetes mellitus, liver disease |
| Infection with immunomodulating viruses: Cytomegalovirus, Epstein–Barr virus, hepatitis B and C viruses, human immunodeficiency virus |
Timing for infection after transplantation
Early post transplant infections
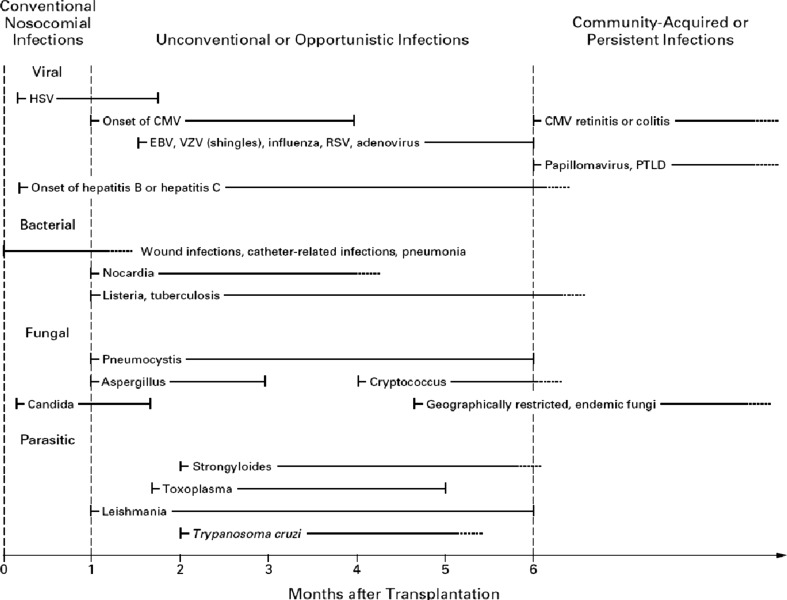
Opportunistic infections (viral, fungal, nocardial and protozoal) are almost non-existent in the first month after transplantation (Fig. 2). The major causes of infection are bacterial wound, pulmonary, urinary tract and intravenous line and catheter-related infections seen in patients after any operative procedure. The effects of these infections are obviously more complex in RT [16].
Fig. 2.
Usual timing of infections after renal transplantation. Zero indicates the time of transplantation. Solid lines indicate the most common period for the onset of infection; dotted lines and arrows indicate periods of continued risk at reduced levels. HSV: herpes simplex virus; CMV: Cytomegalovirus; EBV: Epstein–Barr virus; VZV: Varicella zoster virus; RSV: respiratory syncytial virus; PTLD: post-transplantation lymphoproliferative disease. Reproduced with permission from [15]
The infection in the first month after transplantation can be transmitted from the donor (which may include the cause of death of deceased donors) or can be untreated infection in the recipient. Donor-derived infections can be active but not recognised at the time of donation. This group of infections may include bacteria and fungi (staphylococci, Streptococcus pneumoniae, Candida species, Salmonella, Escherichia coli). Cultures from the donor, transport medium and recipient at the time of transplantation are necessary to guide antimicrobial therapy.
Patients waiting for transplantation may become colonised with nosocomial, antimicrobial-resistant organisms, including methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, antimicrobial resistant Gram-negative bacteria, fluconazole resistant Candida species, Aspergillus species and Clostridium difficile. The incidence is increased in those who have had procedures previously (e.g. bladder augmentation, gastrostomy and other catheter exit site colonisations and infections). After transplantation these organisms may infect wounds, catheters, haematomas and ascitic fluid or potentially cause pneumonia.
Infection 1–6 months after transplantation
The first 6 months post-transplantation is the critical period for RTR in terms of the greatest risk of life-threatening infections owing to high-dose immunosuppressive medications, the risk of CMV infections (which itself is immunosuppressing) and therefore increased risk of opportunistic infections. The combination of sustained immunosuppression and immunomodulating viruses increases the likelihood of opportunistic infections (including Pneumocystis jiroveci, Aspergillus species, Listeria monocytogenes) in the absence of an excessive epidemiological hazard.
Human CMV is generally considered to be the most frequently occurring opportunistic pathogen and the most important infectious agent in RT. Ganciclovir, a synthetic nucleoside analogue, is the medication currently most efficacious in both prophylaxis and treatment of CMV disease after RT and can provoke neutropaenia (depending on the duration of the therapy), thereby predisposing RTR to a higher risk of opportunistic infections.
Infection more than 6 months after transplantation
Patient and renal allograft survival rates have improved during the first year after transplantation [6–9]. RTR with good renal allograft function are maintained on reduced immunosuppressive therapy from 6 months after transplantation with lower infectious risks, although some patients continue to have increased susceptibility (e.g. hostile bladders) [15, 20]. In this period, excluding patients with chronic viral infections, there is an increased risk of opportunistic infections in patients who have had an increased immunosuppressive burden due to acute renal allograft rejection [15].
Common non-viral infections
Bacteraemia
It is well known that bacteraemic infections are a major cause of death among organ transplant recipients. Patients receiving deceased donor renal transplants are susceptible to various bacterial infections including bacteraemia and septicaemia and the results of antibiotic treatment are dependent on both the nature of the infecting organism and the presence of serious underlying complications [21]. The great majority of patients with bacteraemia have severe underlying disease or have undergone surgical procedures, and high incidences of bacteraemia have been described following urinary tract surgery or instrumentation, in severe skin infections and burns, and in patients with impaired body defence mechanisms (Table 2).
Table 2.
Risk factors for bacteraemia
| Risk factors for bacteraemia |
|---|
| Aetiology of ESRF (autoimmune diseases with complement deficiencies (e.g. systemic lupus erythematosus and other glomerulonephritis with complement defects), glomerulonephritis with previous use of immunosuppressants) |
| Induction therapy with lymphocyte depletion |
| Early renal allograft rejection and use of pulsed-dose corticosteroids |
| Recurrence of primary disease and use of plasmapheresis, cyclophosphamide and rituximab |
| Active or latent infection in the donor or recipient |
| Technical complications: prolonged intubation, wound infection and poor healing, bleeding, anastomotic leak |
| Previous vascular line for haemodialysis, peritoneal dialysis (PD) catheter, urinary catheters |
| Previous major surgical procedures |
| Cardiac–respiratory tract diseases or abnormalities |
ESRF, end stage renal failure
Fungal infections
There are two general categories of fungal infection that can occur in RTR: disseminated primary infection with one of the geographically restricted systemic mycoses (histoplasmosis, coccidioidomycosis, blastomycosis) and reactivation infection with fungal species that rarely cause invasive infection in the normal host (Aspergillus spp., Candida spp., Cryptococcus). Infection through intravenous cannulae with candida species is one of the most common in RTR [22]. Treatment of invasive fungal infections in RTR must be prolonged and given intravenously even if it is complicated by the fact that the administration of most antifungals (such as amphotericin and fluconazole) can affect the pharmacokinetics of calcineurin inhibitors. It is important to monitor renal function and calcineurin inhibitor levels during treatment.
Parasitic infections
Some of the parasites that can cause opportunistic infections in RTR are geographically restricted like malaria, Strongyloides, Leishmania, schistosomiasis, Trypanosoma cruzi; others, like toxoplasmosis and Cryptosporidium, are more diffuse. Strongyloides is a disease caused by an intestinal nematode and has a prevalence of 0.4–4% in the USA and is transmitted by faecal–oral spread and possibly with the renal allograft [23]. The clinical presentation of strongyloidiasis in RTR is variable and often atypical, with the majority of cases presenting during the initial 4 months after transplantation with gastrointestinal and pulmonary symptoms. Leishmaniasis is a rare protozoal disease in the USA, but is endemic in Latin America, the Middle East and North Africa. Cutaneous leishmaniasis is almost the only one encountered, although visceral leishmaniasis occurs very rarely in recipients of solid organ transplants (SOT), but is associated with an elevated mortality rate despite treatment [24]. The use of donor and recipient screening for Toxoplasma in the era of universal co-trimoxazole prophylaxis is controversial [25]. A Canadian study reviewed Toxoplasma serology in 1,006 adult SOT recipients. The seroprevalence was higher among the recipients (17.8%) than the donors (13.4%). Only 4 patients seroconverted, and the authors reported no clinical disease, so routine screening should not be offered, especially when prophylaxis is given. This may differ in paediatric RTR where mismatches (positive donor, negative recipient) may be more common, especially in regions where seroprevalence is high. There is a higher risk of severe, disseminated disease in children.
Potential sources of non-viral infections
Donor-derived infections
The transmission of infections from organ donors can be due to latent infection in transplanted tissues (CMV, tuberculosis), or to active donor infections like viraemia or bacteraemia (which may have been the cause of death in deceased donors). Pre-transplantation screening is essential in preventing serious post-transplant infection, either excluding a donor or defining the need for specific antimicrobial therapy after transplantation. Organs from donors with specified known infections may be considered for specific recipients, based on the urgency of the need for transplantation and the availability of effective antimicrobial therapies.
Transplant medium-derived infections
The perfusion fluid used to perfuse and preserve the kidneys prior to transplantation represents a potential medium in which organisms can grow. Contamination rates of 7–24% have been reported and both Gram-positive and Gram-negative organisms have been isolated [26–29]. The majority of organisms isolated are coagulase-negative staphylococci [26, 30], but also Staphylococcus aureus and Pseudomonas aeruginosa, which can be associated with sepsis (local and general), anastomotic failure and renal allograft loss [31, 32]. The frequency with which contamination occurs justifies routine culturing of the perfusion fluid to ensure that potentially highly virulent organisms are not missed and treatment is instituted appropriately. The fact that culture results are invariably not available in time for transplantation further emphasises the importance of peri-operative antibiotic prophylaxis. In view of the variation in protocols and the possibility that virulent organisms requiring extended or modified antibiotic regimens may be necessary, alerting the recipient centre to the results of procurement perfusion fluid samples would be useful to ensure that potentially serious infective complications are prevented [33].
Peritoneal and haemodialysis catheters
As a foreign body, peritoneal dialysis (PD) and haemodialysis catheters represent a potential source of infection, particularly for immunosuppressed transplant patients. Early bacterial infections remain a significant problem during the first few post-operative months. Any catheter left in situ for a prolonged period can increase the risk of infection. Despite this increased risk, early primary non-function and delayed graft function remain a significant problem in deceased donor RTR, which can necessitate the use of a PD or haemodialysis catheter for dialysis. Published recommendations regarding the time of PD catheter removal have varied from as early as the time of transplantation to 4 months later [34, 35]. Two retrospective paediatric studies in 1994 and 2001 reported a higher use of PD catheter during the first month post-transplant (38–50% of deceased donor RTR) for acute rejection or primary allograft non-function. There have also been reported risks of infection by 3 months post-transplantation [35].
We advocate the removal of the peritoneal or haemodialysis catheter at the time of transplantation in living donor (LRDT) transplants if there are no surgical complications and at the same time as the removal of the transplant ureteric stent (TUS) in deceased donor RTR (which is up to 6 weeks post-transplantation), although it can be removed earlier if there is good renal allograft function to reduce the risk of infectious complications.
Transplant ureteric stent
Major urological complications (e.g. urinary leak, obstruction) after RT contribute to patient morbidity and compromised renal allograft function. The majority arise from the vesico-ureteric anastomosis and present early after transplantation. TUS have been successfully used to treat such complications. A recent Cochrane review of seven studies (1,154 patients) reports that the incidence of major urological complications is significantly reduced by the use of prophylactic stenting and that urinary tract infections (UTI) are more common in patients with TUS. The addition of antibiotic prophylaxis (with co-trimoxazole) does not alter the incidence of UTI [36]. However, there can be more complications with TUS rather than UTI, like migration, macroscopic haematuria and pain. These complications may be related to how long the TUS remains in situ. In our experience we reviewed retrospectively 180 RTR from 2002 to 2008 with cystoscopic TUS removal performed in 151 patients at 8–90 (median 42) days with TUS complications in 20% (16 UTI, 9 macroscopic haematuria, 4 migration, 1 blocked stent) [37]. These resulted in early TUS removal in 64% of patients, with ureteric complications found in 5% of patients (two leaks and five stenoses). Six patients had TUS sutured with the catheter, although no patients in this group developed stent or ureteric complications. Suturing the TUS to the catheter facilitates early removal and may reduce the incidence of stent complications [37].
Urinary tract infection
Urinary tract infections (UTI) are the most common form of bacterial infection in RTR and an important clinical problem encountered in 15–33% of paediatric RTR [38–41]. Meticulous surveillance, diagnosis and treatment of UTI are necessary to minimise acute morbidity and compromise of long-term renal allograft function from transplant pyelonephritis and scarring. It is important to distinguish asymptomatic bacteriuria, lower UTI (cystitis) and febrile UTI (transplant pyelonephritis) as the latter can cause organ damage and can promote the development of chronic allograft dysfunction with biopsy-confirmed chronic changes with interstitial fibrosis and tubular atrophy (IF/TA). Many children with ESRF who receive renal transplants have lower urinary tract abnormalities, bladder pathology with abnormal urodynamic assessment and a significant post-micturition residual requiring urinary catheterisation. It is common for these patients to have asymptomatic bacteriuria and develop UTI. Febrile UTI may cause significant morbidity and is usually associated with acute renal allograft dysfunction. The acute deterioration of renal function during UTI is a well-known feature [37, 40], which, together with the significant inflammatory parenchymal response, emphasises the potential risk of tissue damage in the transplanted kidney in acute and long-term follow-up, despite normalisation of renal function after UTI. In addition, acute rejection episodes may be triggered by febrile UTI [42].
Although Escherichia coli remains the most frequently isolated microorganism [43–45], it is isolated less frequently than in the general paediatric population. This may be due to underlying immunosuppression, colonisation, antibiotic prophylaxis and mainly the higher risk of bladder malformations in this population. Risk factors for developing febrile UTI include anatomical, functional and demographic factors, as well as baseline immunosuppression and foreign bodies, such as catheters and stents. Underlying urological abnormalities have to be regarded as risk factors for UTI even before transplantation, such as hydronephrosis, vesico-ureteric reflux (VUR), and/or neurogenic bladder, often in combination. Bladder augmentation has been found to be the most significant predisposing factor for bacteriuria in RTR, which may result in scarring of the transplant [44].
99-Tc-Dimercaptosuccinic acid (DMSA) scintigraphy is regarded as the gold standard in the diagnosis of acute pyelonephritis as well as in the documentation of residual scarring in repeat scans performed 6 months after UTI. Long-term data suggest that febrile UTI might lead to focal defects on DMSA scanning [46, 47], but other events, such as vascular complications and renal biopsy, may also do so, and need to be considered in the interpretation of the images [48]. We perform a baseline DMSA scan within the first 3 weeks post-transplantation in all patients at risk of developing UTI (such as those with hostile bladders and at risk of pyelonephritis in the future).
Transplantation in a patient with lower urinary tract dysfunction (LUTD) is associated with a high incidence of urological and infectious complications. However, despite this, several studies have found no differences in patient survival or transplant outcome between RTR with and without LUTD [49–52]. Surgery of the urinary tract before listing for transplantation is often necessary and should decrease the risk of infections. There is a higher incidence of UTI in RTR with neurogenic bladder, with associated morbidity and poorer renal allograft function [53]. There are no controlled data available demonstrating that bladder augmentation procedures prevent or decrease the rate of UTI after transplantation. However, it seems mandatory that evaluation and treatment of neurogenic bladders are very important, should be performed before renal transplantation and require specific follow-up after transplantation. Because of the high urological complication rates, careful surveillance of lower urinary tract function by urodynamic evaluation is essential before transplantation. A review of 25 articles on transplantation in patients with urinary tract dysfunction has suggested that bladder reconstruction should be performed before transplantation when clinically indicated [51], although one study suggests that there is no adverse outcome with regard to long-term renal allograft survival or function from transplantation into the unaugmented valve bladder [54]. There is still considerable controversy over the best way to manage RTR with LUTD, with no established guidelines defining criteria for reconstructive surgery, the optimal surgical procedure; or when, in relation to renal transplantation, surgery should be carried out. Treatment options for LUTD are divided into conservative measures, including bladder training with or without pharmacological agents or clean intermittent catheterisation or both, and reconstructive surgery procedures (drainage, augmentation, urinary diversion). Our current approach is for accurate urodynamic assessment before transplantation in paediatric patients with ESRF and LUTD. If the cystometric bladder capacity is lower than that expected and/or in presence of high changes in detrusor pressure with or without VUR, we consider a trial of bladder cycling and repeat urodynamics. We perform bladder surgery prior to renal transplant if the urodynamics continue to show signs of reduced bladder capacity [55].
Vesico-ureteric reflux (VUR) is present in many patients as an underlying diagnosis, and UTI could affect the native and the transplanted kidney (or both). Some authors recommend native nephrectomies with VUR to decrease the risk of native-kidney UTI after transplantation, although no controlled studies are available [56]. Pre-transplant anti-reflux surgery does not reduce the risk of febrile UTI after transplantation. In a recent study, Basiri et al. compared 36 patients without VUR, 12 children with VUR who underwent reimplantation and 17 children who did not. The frequency of febrile UTI after surgical correction of VUR before transplantation remained higher than in RTR without VUR [57]. VUR does not need to be corrected before transplantation, unless it is causing symptoms or infection [58–60]. Parenteral antibiotics are usually indicated for RTR with transplant pyelonephritis, although controlled data are not available [61].
Prevention of infection
Vaccinations
Pre-transplantation immunisation is one of the best means of preventing serious infections. RTR have an advantage compared with recipients of other solid organs as they have time before transplantation to be fully immunised. RTR should be protected against tuberculosis (TB), pneumococcus, meningococcal group C, diphtheria, tetanus, pertussis, inactivated poliomyelitis, Haemophilus influenzae B, measles, mumps, rubella, hepatitis B, varicella and influenza. All these vaccines are safe and efficacious in patients with CKD and ESRF on dialysis, although vaccine responses may be reduced because of proteinuria (from nephrotic syndrome) or in inherently immunocompromised children (such as systemic lupus erythematosus) or those on immunosuppressive medications for their underlying condition [62–64]. The incidence of TB in RTR varies in different groups of patients from around the world with rates of 0.5–1% in North America, 1–4% in Europe and the Middle East, and 10–13% in India [65]. In the USA, the minimal annual incidence of TB in RTR has been reported to be 37 times higher than in the general population. Uraemia, with its immunosuppressive effects, and high-dose corticosteroids and other immunosuppressive medications may promote reactivation of latent TB. There can be a good response to treatment of TB in RTR, but there are interactions between antituberculous medications and immunosuppressive agents. It is advocated to vaccinate all patients younger than 6 years of age against TB and to vaccinate older patients if the tuberculin skin test is negative. The tuberculin skin test is required before vaccination in children who were born or lived for more than 1 month in countries where the TB incidence is high. Varicella is a common disease in childhood and it is very contagious, although usually it is a very mild infection, but in the immunocompromised host could be very serious with complications like hepatitis, pneumonia, encephalitis and even death. All patients who are seronegative must be vaccinated before transplantation. The live-attenuated vaccine is safe and gives persisting immunity to chickenpox, even if cases of disease are described in immunocompromised patients that had received the vaccine [66].
Peri-operative antibiotic prophylaxis
In our centre, we advocate antibiotic therapy with ciprofloxacin as part of the operative and postoperative management of RTR with intravenous ciprofloxacin 5 mg/kg over 30–60 min, to be given with pre-medication and continued on a once daily basis until a negative culture has been obtained from the transplant medium fluid. Other broad-spectrum antibiotics can be used as perioperative prophylaxis. Cephalosporins such as ceftriaxone can be administered intravenously on a once-daily basis.
Antimicrobial prophylaxis has significantly altered the severity and incidence of infections post-transplantation. By far the most effective prophylactic therapy is co-trimoxazole (trimethoprim and sulfamethoxazole) for the first 6 months after transplantation. The incidence of Pneumocystis jiroveci pneumonia (PJP) among RTR not receiving co-trimoxazole prophylaxis has been reported to be 5–10% [22]. Their routine use has eliminated Pneumocystis jiroveci and UTI. The major side-effect of co-trimoxazole are rashes and haematological effects including leucopaenia and thrombocytopaenia.
A recent report described 33 RTR who developed PJP without prophylaxis in Japan [67]. PJP prophylaxis has been abandoned in Japan because of the nephrotoxicity of co-trimoxazole. The European Renal Transplant Guidelines recommends PJP prophylaxis for at least 4 months post-transplantation [68], whereas another report recommended the use of PJP prophylaxis for 6–12 months. However, in this decade, large outbreaks of PJP after an index case have been reported in RTR in France (2004), Germany (2005 and 2008) and the Netherlands (2007) [69–72]. We recommend PJP prophylaxis for 6 months post-transplantation (upper limit of incubation period) in all RTR.
Antibiotic prophylaxis with co-trimoxazole is also useful in view of the high prevalence of UTI and transplant vesico-ureteric reflux. Most paediatric renal transplant centres prescribe prophylactic antibiotics with co-trimoxazole for the first 3–6 months post-transplantation, which also covers against PJP [33, 73]. Several studies have shown how co-trimoxazole is effective in preventing UTI and Gram-negative sepsis of urinary tract origin [74, 75]. However, not all studies confirmed a beneficial effect and even demonstrated a high bacterial resistance rate [44]. If prophylactic antibiotics are used, it seems that they should be administered for prolonged periods, as UTI may occur late, especially in girls. The rate of UTI in adolescent girls has been reported to be twice as high as in male RTR for anatomical reasons such as a shorter urethra and predisposing immunological factors [61].
Role of immunosuppression
Immunosuppression has an important impact on defence mechanisms and risk of infections after renal transplantation. There have been considerable changes in the use of immunosuppression over the years. A recent report of the NAPRTCS shows that the use of cyclosporin has decreased from 82.3% in 1996 to 20.7% in 2003. In contrast, use of tacrolimus has increased from 5.5% to 67.1% over the same period. There has also been a move away from azathioprine (AZA), from 56.4% to 1.9%, towards mycophenolate mofetil (MMF), the use of which has now reached approximately 57.4%. Antibody induction has also moved away from antithymocyte globulin (ATG) and OKT3 monoclonal antibody to anti-IL2 receptor blockers. The use of steroid-sparing regimens is also becoming common [76]. Several studies have analysed the possible association between immunosuppression regimens (induction and maintenance therapy) and viral, bacterial or fungal infections.
Induction therapy
Almost 70% of RTR receive induction therapy with either rabbit ATG or non-lymphocyte-depleting monoclonal antibodies that target interleukin-2 receptor (basiliximab). Their impact on infections has been analysed in several studies. While the use of induction agents has been associated with a lower incidence of acute rejection [77, 78], their role in renal allograft survival is more controversial and there is a higher incidence of infections in patients receiving antibody induction therapy with both monoclonal and polyclonal antibodies [79, 80].
In a recent analysis of the NAPRTCS database, 3,106 children undergoing a transplant between 1996 and 2002 with 2-year follow-up were analysed [79]. This NAPRTCS report showed that in comparison to those receiving no induction therapy, patients receiving monoclonal antibody were more likely to have infections (42% vs 34%), both bacterial (24% vs 21%) and viral (29% vs 21%). The hospitalisation rates for infections were similarly higher in patients receiving polyclonal antibodies in comparison to those with no induction therapy (45% vs 34%), both bacterial (28% vs 21.2%) and viral (30% vs 21%). At 2 years post-transplant there was no difference in the risk of having one acute rejection or in the percentage of graft failure comparing patients who had received polyclonal or monoclonal antibodies and patients who did not receive induction therapy [79].
Many studies reported a lower risk of infections and of CMV disease with basiliximab compared with ATG [81–83]. One recent prospective, randomised, international study comparing the use of ATG and basiliximab in 278 patients reported a higher incidence of non-viral infections, particularly UTI, in the ATG-treated group (86% vs 75%, p = 0.03) ,but a lower incidence of CMV disease (8% vs 18%, p = 0.02) [84].
It may be prudent to select patients for antibody induction therapy for those RTR undergoing second or subsequent renal transplant, patients with cytotoxic antibodies and those in whom there is a need to minimise other immunosuppressive agents such as utilising steroid minimisation protocols. The use of ATG is reserved for patients with corticosteroid-resistant acute rejection.
Maintenance therapy
A large prospective study with intention-to-treat analysis has evaluated 1,398 adult transplant recipients of the RESISTRA Transplant Network. With the exception of sirolimus there was no association between the immunosuppression regimens and the development of specific infection. The probability of developing CMV infection in patients not receiving sirolimus was significantly higher than in patients who did receive sirolimus. Sirolimus was associated with a higher risk of surgical site infection [85].
Sirolimus inhibits growth factor production in response to tissue injury and it has been associated with a higher number of bacterial infections [86–88] and pneumonia [89–91]. Different studies have confirmed a decreased rate of CMV infection in patients receiving sirolimus [92–94] or everolimus [95, 96].
Mycophenolate mofetil has been associated with a higher risk of CMV infection in some studies [97, 98] and a decrease in Pneumocystis jiroveci infections in others [99]. Different studies confirmed that in maintenance therapy, no differences in infection rates have been observed among patients receiving cyclosporin or tacrolimus [100–102]. A recent study reviewed the incidence of infections in RTR who have received intravenous rituximab. They were at a significantly lower risk of viral infections, a higher risk of fungal infections and there was a high percentage of deaths related to infections, mostly in patients who had also received ATG [103].
Summary
Renal transplantation is the gold standard treatment for children with ESRF. There has been a significant improvement in patient and renal allograft survival in the last few decades with newer potent immunosuppressive agents and the use of antimicrobial prophylaxis. However, non-viral infections continue to be a serious complication for RTR. Most of the opportunistic infections occur in the first 6 months post-transplantation and can be related to catheters or lines left in situ post-transplantation. UTI are the most frequent infections that can provoke renal allograft damage and occur more frequently in patients with LUTD. Therefore, it is mandatory to perform an accurate urodynamic assessment pre-transplantation and have multi-disciplinary discussion of the best surgical approach. The use of potent immunosuppressive agents with monoclonal or polyclonal antibodies increases the risk of infection. Our current opinion is that the optimal approach to preserving renal allograft function is to minimise the immunosuppressive burden, thereby achieving a balance between the risk of rejection and infectious complications.
Questions (answers are provided following the reference list)
- What proportion of the mortality rate is due to infections after renal transplantation ?
- 5–15%
- 15–25%
- 25–55%
- 55–75%
- 75–80%
- What is the most frequent infection in the first month after renal transplantation ?
- Fungal infections
- Parasitic infections
- TB
- UTI
- Viral infections
- Which is the current statement regarding prophylaxis with co-trimoxazole after renal transplantation:
- Must be given for 1 year post-transplant
- Not currently advocated
- Significant side-effects
- Useful in preventing PJP
- Useful in preventing PJP and UTI
- What is the optimal time for the removal of PD catheters in children who receive a successful living, related renal transplant?
- At the time of the transplant
- In the first month post-transplantation
- Within 2 months post-transplantation
- Within 3 months post-transplantation
- Within 6 months post-transplantation
- Which immunosuppressive treatment is most associated with a higher risk of non-viral infections?
- ATG
- Everolimus
- MMF
- Sirolimus
- Tacrolimus
Footnotes
Answers
1. c
2. d
3. e
4. a
5. a
References
- 1.Fine RN. Renal transplantation for children—the only realistic choice. Kidney Int Suppl. 1985;17:15–17. [PubMed] [Google Scholar]
- 2.Ingelfinger JR, Grupe WE, Harmon WE, Femback SK, Levey RH. Growth acceleration following renal transplantation in children less than 7 years of age. Pediatrics. 1981;68:255–259. [PubMed] [Google Scholar]
- 3.Dharnidharka VR, Caillard S, Agodoa LY, Abbott KC. Infection frequency and profile in different age groups of kidney transplant recipients. Transplantation. 2006;27:1662–1667. doi: 10.1097/01.tp.0000226068.66819.37. [DOI] [PubMed] [Google Scholar]
- 4.NAPRTCS 2005 annual report, available at https://web.emmes.com/study/ped/resources/annlrept2005.pdf
- 5.Groothoff JW, Gruppen MP, Offringa M, Hutten J, Lilien MR, Kar NJ, Wolff ED, Davin JC, Heymans HS. Mortality and causes of death of endstage renal disease in children: a Dutch cohort study. Kidney Int. 2002;61:621–629. doi: 10.1046/j.1523-1755.2002.00156.x. [DOI] [PubMed] [Google Scholar]
- 6.Damme-Lombaerts R, Herman J, Coosemans W, Pirenne J. Pediatric renal transplantation: a single Belgian center experience over 20 years. Pediatr Transplant. 2001;5:447–451. doi: 10.1034/j.1399-3046.2001.t01-1-00008.x. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RJ, Armstrong S, Belger MA, Fuggle SV, Martin S, Middleton D, Ray TC, Rigden SP, Verrier-Jones K, Morris PJ. Paediatric Task Force of United Kingdom, Bristol, UK: the outcome of pediatric cadaveric renal transplantation in the UK and Eire. Pediatr Transplant. 2002;6:367–377. doi: 10.1034/j.1399-3046.2002.02027.x. [DOI] [PubMed] [Google Scholar]
- 8.Harzallah K, Floret D, Martin X, Cochat P. Mortality in pediatric renal transplants: 15 years’ experience. Arch Pediatr. 2004;11:916–920. doi: 10.1016/j.arcped.2004.03.129. [DOI] [PubMed] [Google Scholar]
- 9.Rees L, Shroff R, Hutchinson C, Fernando ON, Trompeter RS. Long-term outcome of paediatric renal transplantation: follow-up of 300 children from 1973 to 2000. Nephron Clin Pract. 2007;105:c68–c76. doi: 10.1159/000097601. [DOI] [PubMed] [Google Scholar]
- 10.Dharnidharka VR, Stablein DM, Harmon WE. Post-transplant infections now exceed acute rejection as cause for hospitalization: a report of the NAPRTCS. Am J Transplant. 2004;4:384–389. doi: 10.1111/j.1600-6143.2004.00350.x. [DOI] [PubMed] [Google Scholar]
- 11.Harmon WE. Opportunistic infections in children following renal transplantation. Pediatr Nephrol. 1991;5:118–125. doi: 10.1007/BF00852868. [DOI] [PubMed] [Google Scholar]
- 12.Rubin RH. Infectious disease complications of renal transplantation. Kidney Int. 1993;44:221–236. doi: 10.1038/ki.1993.234. [DOI] [PubMed] [Google Scholar]
- 13.Bilal J, Nicholls K, Becker GJ, Walker RG. Impact of acute rejection therapy on infections and malignancies in renal transplant recipients. Transplantation. 1999;68:1597–1603. doi: 10.1097/00007890-199911270-00027. [DOI] [PubMed] [Google Scholar]
- 14.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 15.Fishman JA, Rubin RH. Infection in organ transplant recipients. N Engl J Med. 1998;24:1743–1752. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 16.Rubin RH, Wolfson JS, Cosimi AB, Tolkoff-Rubin NE. Infections in renal transplant recipients. Am J Med. 1981;70:405–411. doi: 10.1016/0002-9343(81)90780-4. [DOI] [PubMed] [Google Scholar]
- 17.Fishman JA. Infection in renal transplant recipients. Semin Nephrol. 2007;27:445–461. doi: 10.1016/j.semnephrol.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Rubin RH, Cosimi AB, Tolkoff-Rubin NE, Russell PS, Hirsch MS. Infectious disease syndromes attributable to cytomegalovirus and their significance among renal transplant recipients. Transplantation. 1977;24:458–464. doi: 10.1097/00007890-197712000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Rubin RH, Sester M, Gärtner BC. The indirect effects of cytomegalovirus infection on the outcome of organ transplantation. JAMA. 1989;261:3607–3609. doi: 10.1001/jama.1989.03420240121038. [DOI] [PubMed] [Google Scholar]
- 20.Fonseca-Aten M, Michaels MG. Infections in pediatric solid organ transplant recipients. Semin Pediatr Surg. 2006;15:153–161. doi: 10.1053/j.sempedsurg.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leigh JDA. Bacteraemia in patients receiving human cadaveric renal transplants. Pathol Clin. 1971;24:295–299. doi: 10.1136/jcp.24.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tolkoff-Rubin NE, Rubin RH. Opportunistic fungal and bacterial infection in the renal transplant recipient. J Am Soc Nephrol. 1992;2:S264–S269. doi: 10.1681/ASN.V212s264. [DOI] [PubMed] [Google Scholar]
- 23.DeVault GA, Jr, King JW, Rohr MS, Landreneau MD, Brown ST, McDonald JC. Opportunistic infections with Strongyloides stercoralis in renal transplantation. Rev Infect Dis. 1990;12:653–671. doi: 10.1093/clinids/12.4.653. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Guerrero ML, Aguado JM, Buzon L, Barros C, Montalbán C, Martín T, Bouza E. Visceral leishmaniasis in immunocompromised hosts. Am J Med. 1987;83:1098–1102. doi: 10.1016/0002-9343(87)90948-X. [DOI] [PubMed] [Google Scholar]
- 25.Gourishankar S, Doucette K, Fenton J, Purych D, Kowlewska-Grochowska K, Preiksaitis J. The use of donor and recipient screening for toxoplasma in the era of universal trimethoprim sulphametoxazole prophylaxis. J Transplant. 2008;85:980–985. doi: 10.1097/TP.0b013e318169bebd. [DOI] [PubMed] [Google Scholar]
- 26.Anderson CB, Haid SD, Hruska KA, Etheredge EA. Significance of microbial contamination of stored cadaver kidneys. Arch Surg. 1978;113:269–271. doi: 10.1001/archsurg.1978.01370150041006. [DOI] [PubMed] [Google Scholar]
- 27.Benoit G, Tiguert R, Bensadoun H, Hammoudi Y, Hiesse C, Jacques L, Fries D, Jardin A. Incidence of transport medium contamination in cadaver kidney procurement. Transplant Proc. 1988;20:895. [PubMed] [Google Scholar]
- 28.Mora M, Wilms H, Kirste G. Significance of bacterial contamination of cadaver donor renal allografts before transplantation. Transplant Proc. 1991;23:2648. [PubMed] [Google Scholar]
- 29.Bijnen AB, Weimar W, Dik P, Oberop H, Jeekel J. The hazard of transplanting contaminated kidney. Transplant Proc. 1984;16:27. [Google Scholar]
- 30.McCoy GC, Loening S, Braun WE, Magnusson MO, Banowsky LH, McHenry MC. The fate of cadaver renal allografts contaminated before transplantation. Transplantation. 1975;20:467. doi: 10.1097/00007890-197512000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Wilson CH, Gregory RT, Wheeler JR, Hurwitz RL, Vansant JH, Thomas FT. Complications of end-to-side renal artery anastomosis in renal transplant infection. Vasc Surg. 1979;13:207. doi: 10.1177/153857447901300310. [DOI] [Google Scholar]
- 32.Doig RL, Boyd PJR, Eykyn S. Staphylococcus aureus transmitted in transplanted kidneys. Lancet. 1975;9:243. doi: 10.1016/S0140-6736(75)90960-5. [DOI] [PubMed] [Google Scholar]
- 33.Wakelin SJ, Casey J, Robertson A, Friend P, Bryon C, Hester Yorke J, Rigden SP, Xavier EFS, Pareja-Cebrian L, Forsythe JLR, Morris PJ. The incidence and importance of bacterial contaminants of cadaveric renal perfusion fluid. Transpl Int. 2005;17:680–686. doi: 10.1007/s00147-004-0792-6. [DOI] [PubMed] [Google Scholar]
- 34.Arbeiter K, Pichler A, Muerwald G, Mueller T, Bidmon B, Balzar E, Ruffingshofer D, Greenbaum L, Aufricht C. Timing of peritoneal dialysis catheter removal after pediatric renal transplantation. Perit Dial Int. 2001;21:467–470. [PubMed] [Google Scholar]
- 35.Palmer JA, Kaiser BA, Polinsky MS, Dunn SP, Braas C, Waltz R, Baluarte HJ. Peritoneal dialysis catheter infections in children after renal transplantation: choosing the time of removal. Pediatr Nephrol. 1994;8:715–718. doi: 10.1007/BF00869099. [DOI] [PubMed] [Google Scholar]
- 36.Wilson CH, Bhatti AB, Rix DA, Manas DM. Routine intraoperative ureteric stenting for kidney transplant recipients. Cochrane Database Syst Rev. 2005;19:4 CD004925. doi: 10.1002/14651858.CD004925.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Patel P, Olsburgh J, Marks SD. Timing of ureteric stent removal in pediatric renal transplant recipients (RTR) Pediatr Nephrol. 2010;25:1779–2004. doi: 10.1007/s00467-010-1577-z. [DOI] [Google Scholar]
- 38.Mueller T, Resinger C, Ruffingshofer D, Arbeiter K, Balzar E, Aufricht C. Urinary tract infections beyond the early posttransplant period in pediatric renal graft recipients. Wien Klin Wochenschr. 2003;115:385–388. doi: 10.1007/BF03040357. [DOI] [PubMed] [Google Scholar]
- 39.Ranchin B, Chapuis F, Dawhara M, Canterino I, Hadj-Aissa A, Said MH, Parchoux B, Dubourg L, Pouillaude JM, Floret D, Martin X, Cochat P. Vesicoureteral reflux after kidney transplantation in children. Nephrol Dial Transplant. 2000;15:1852–1858. doi: 10.1093/ndt/15.11.1852. [DOI] [PubMed] [Google Scholar]
- 40.Dunn SP, Vinocur CD, Hanevold C, Wagner CW, Weintraub WH. Pyelonephritis following pediatric renal transplant: increased incidence with vesicoureteral reflux. J Pediatr Surg. 1987;22:1095–1099. doi: 10.1016/S0022-3468(87)80716-9. [DOI] [PubMed] [Google Scholar]
- 41.Neuhaus TJ, Schwobel M, Schlumpf R, Offner G, Leumann E, Willi U. Pyelonephritis and vesicoureteral reflux after renal transplantation in young children. J Urol. 1997;157:1400–1403. doi: 10.1016/S0022-5347(01)64999-1. [DOI] [PubMed] [Google Scholar]
- 42.Audard V, Amor M, Desvaux D, Pastural M, Baron C, Philippe R, Pardon A, Dahmane D, Lang P, Grimbert P. Acute graft pyelonephritis: a potential cause of acute rejection in renal transplant. Transplantation. 2005;80:1128–1130. doi: 10.1097/01.TP.0000174343.05590.9F. [DOI] [PubMed] [Google Scholar]
- 43.Chuang P, Parikh CR, Langone A. Urinary tract infections after renal transplantation: a retrospective review at two US transplant centers. Clin Transplant. 2005;19:230–235. doi: 10.1111/j.1399-0012.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 44.Sharifian M, Rees L, Trompeter RS. High incidence of bacteriuria following renal transplantation in children. Nephrol Dial Transplant. 1998;13:432–435. doi: 10.1093/oxfordjournals.ndt.a027842. [DOI] [PubMed] [Google Scholar]
- 45.Ergin F, Arslan H, Yapar G, Karakayali H, Haberal M. Urinary tract infections in renal transplant recipients. Transplant Proc. 2003;35:2685–2686. doi: 10.1016/j.transproceed.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 46.Cairns HS, Spencer S, Hilson AJ, Rudge CJ, Neild GH. 99mTc-DMSA imaging with tomography in renal transplant recipients with abnormal lower urinary tracts. Nephrol Dial Transplant. 1994;9:1157–1161. doi: 10.1093/ndt/9.8.1157. [DOI] [PubMed] [Google Scholar]
- 47.Coulthard MG, Keir MJ. Reflux nephropathy in kidney transplants, demonstrated by dimercaptosuccinic acid scanning. Transplantation. 2006;82:205–210. doi: 10.1097/01.tp.0000226165.06196.84. [DOI] [PubMed] [Google Scholar]
- 48.Hutchinson C, Beckett M, Kiratli P, Gordon I, Trompeter RS, Rees L. The significance of a defect on DMSA scan in children with renal transplants. Pediatr Transplant. 2003;7:441–445. doi: 10.1046/j.1399-3046.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 49.Ali-El-Dein B, Abol-Enein H, El-Husseini A, Osman Y, Shehab El-Din AB, Ghoneim MA. Renal transplantation in children with abnormal lower urinary tract. Transplant Proc. 2004;36:2968–2973. doi: 10.1016/j.transproceed.2004.11.095. [DOI] [PubMed] [Google Scholar]
- 50.Otukesh H, Sharifian M, Simfroosh N, Basiri A, Hoseini R, Sedigh N, Golnari P, Rezai M, Fereshtenejad M. Outcome of renal transplantation in children with low urinary tract abnormality. Transplant Proc. 2005;37:3071–3074. doi: 10.1016/j.transproceed.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 51.Mendizabal S, Estornell F, Zamora I, Sabater A, Ibarra FG, Simon J. Renal transplantation in children with severe bladder dysfunction. J Urol. 2005;173:226–229. doi: 10.1097/01.ju.0000148369.34519.86. [DOI] [PubMed] [Google Scholar]
- 52.Franc-Guimond J, Gonzalez R. Renal transplantation in children with reconstructed bladders. Transplantation. 2004;77:1116–1120. doi: 10.1097/01.TP.0000116898.59741.97. [DOI] [PubMed] [Google Scholar]
- 53.Neild GH, Dakmish A, Wood S, Nauth-Misir R, Woodhouse CR. Renal transplantation in adults with abnormal bladders. Transplantation. 2004;77:1123–1127. doi: 10.1097/01.TP.0000116712.56265.78. [DOI] [PubMed] [Google Scholar]
- 54.Ross JH, Kay R, Novick AC, Hayes JM, Hodge EE, Streem SB. Long-term results of renal transplantation into the valve bladder. J Urol. 1994;151:1500–1504. doi: 10.1016/s0022-5347(17)35286-2. [DOI] [PubMed] [Google Scholar]
- 55.Riley P, Marks SD, Desai DY, Mushtaq I, Koffman G, Mamode N. Dysfunction challenges facing renal transplantation in pediatric patients with lower urinary tract. Transplantation. 2010;89(11):1299–1307. doi: 10.1097/TP.0b013e3181de5b8c. [DOI] [PubMed] [Google Scholar]
- 56.Casale P, Grady RW, Mitchell ME, Healey P. Recurrent urinary tract infection in the post-transplant reflux nephropathy patient: is reflux in the native ureter the culprit? Pediatr Transplant. 2005;9:324–327. doi: 10.1111/j.1399-3046.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 57.Basiri A, Otookesh H, Simforoosh N, Hosseini R, Hosseini-Moghaddam SM, Sharifian M. Does pre-transplantation antireflux surgery eliminate post-renal transplantation pyelonephritis in children? J Urol. 2006;175:1490–1492. doi: 10.1016/S0022-5347(05)00670-1. [DOI] [PubMed] [Google Scholar]
- 58.Luke PP, Herz DB, Bellinger MF, Chakrabarti P, Vivas CA, Scantlebury VP, Hakala TR, Jevnikar AM, Jain A, Shapiro R, Jordan ML. Long-term results of pediatric renal transplantation into a dysfunctional lower urinary tract. Transplantation. 2003;76:1578–1582. doi: 10.1097/01.TP.0000090866.00241.0C. [DOI] [PubMed] [Google Scholar]
- 59.Lopez Pereira P, Jaureguizar E, Martinez Urrutia MJ, Meseguer C, Navarro M. Does treatment of bladder dysfunction prior to renal transplant improve outcome in patients with posterior urethral valves? Pediatr Transplant. 2000;4:118–122. doi: 10.1034/j.1399-3046.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- 60.Ozcan O, Tekgul S, Duzova A, Aki F, Yuksel S, Bakkaloglu A, Erkan I, Bakkaloglu M. How does the presence of urologic problems change the outcome of kidney transplantation in the pediatric age group. Transplant Proc. 2006;38:552–553. doi: 10.1016/j.transproceed.2005.12.101. [DOI] [PubMed] [Google Scholar]
- 61.John U, Kemper JM. Urinary tract infection after renal transplantation. Pediatr Nephrol. 2009;24:1129–1136. doi: 10.1007/s00467-007-0690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flynn JT, Frisch K, Kershaw DB, Sedman AB, Bunchman TE. Response to early measles-mumps-rubella vaccination in infants with chronic renal failure and/or receiving peritoneal dialysis. Adv Perit Dial. 1999;15:269–272. [PubMed] [Google Scholar]
- 63.Kruger S, Mueller-Steinhardt M, Kirchner H, Kreft B. A 5-year follow-up on antibody response after diphtheria and tetanus vaccination in hemodialysis patients. Am J Kidney Dis. 2001;38:1264–1270. doi: 10.1053/ajkd.2001.29223. [DOI] [PubMed] [Google Scholar]
- 64.Webb NJ. Immunization against varicella in end stage and pre-end stage renal failure. Arch Dis Child. 2000;82:141–143. doi: 10.1136/adc.82.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jha V, Kohli HS, Sud K, Gupta KL, Minz M, Joshi K, Sakhuja V. Laryngeal tuberculosis in renal transplant recipients. Transplantation. 1999;68:153–155. doi: 10.1097/00007890-199907150-00028. [DOI] [PubMed] [Google Scholar]
- 66.Prelog M, Zimmerhackl LB. Varicella vaccination in pediatric kidney and liver transplantation. Pediatr Transplant. 2010;14:41–47. doi: 10.1111/j.1399-3046.2009.01204.x. [DOI] [PubMed] [Google Scholar]
- 67.Yazaki H, Goto N, Uchida K, Kobayashi T, Gatanaga H, Oka S. Outbreak of Pneumocystis jiroveci pneumonia in renal transplant recipients: P. jiroveci is contagious to the susceptible host. Transplantation. 2009;88:380–385. doi: 10.1097/TP.0b013e3181aed389. [DOI] [PubMed] [Google Scholar]
- 68.EBPG Expert Group on Renal Transplantation European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV. 7.1 Late infections. Pneumocystis carinii pneumonia. Nephrol Dial Transplant. 2002;17(Suppl 4):36. [PubMed] [Google Scholar]
- 69.Rabodonirina M, Vanhems P, Couray-Targe S, Gillibert RP, Ganne C, Nizard N, Colin C, Fabry J, Touraine JL, Melle G, Nahimana A, Francioli P, Hauser PM. Molecular evidence of interhuman transmission of Pneumocystis pneumonia among renal transplant recipients hospitalised with HIV-infected patients. Emerg Infect Dis. 2004;10:1766. doi: 10.3201/eid1010.040453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hocker B, Wendt C, Nahimana A, Tönshoff B, Hauser PM. Molecular evidence of Pneumocystis transmission in pediatric transplant unit. Emerg Infect Dis. 2005;11:330. doi: 10.3201/eid1102.040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boer MG, Bruijnesteijn van Coppenraet LE, Gaasbeek A, Berger SP, Gelinck LB, Houwelingen HC, Broek P, Kuijper EJ, Kroon FP, Vandenbroucke JP. An outbreak of Pneumocystis jiroveci pneumonia with 1 predominant genotype among renal transplant recipients: interhuman transmission or a common environmental source? Clin Infect Dis. 2007;44:1143. doi: 10.1086/513198. [DOI] [PubMed] [Google Scholar]
- 72.Schmoldt S, Schuhegger R, Wendler T, Huber I, Söllner H, Hogardt M, Arbogast H, Heesemann J, Bader L, Sing A. Molecular evidence of nosocomial Pneumocystis jirovecii transmission among 16 patients after kidney transplantation. J Clin Microbiol. 2008;46:966. doi: 10.1128/JCM.02016-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fontana I, Ginevri F, Arcuri V, Basile G, Nocera A, Beatini M, Bonato L, Barocci S, Bertocchi M, Manolitsi O, Valente R, Draghi P, Gusmano R, Valente U. Vesico-ureteral reflux in pediatric kidney transplants: clinical relevance to graft and patient outcome. Pediatr Transplant. 1999;3:206–209. doi: 10.1034/j.1399-3046.1999.00017.x. [DOI] [PubMed] [Google Scholar]
- 74.Tolkoff-Rubin NE, Cosimi AB, Russell PS, Rubin RH. A controlled study of trimethoprim-sulfamethoxazole prophylaxis of urinary tract infection in renal transplant recipients. Rev Infect Dis. 1982;4:614–618. doi: 10.1093/clinids/4.2.614. [DOI] [PubMed] [Google Scholar]
- 75.Fox BC, Sollinger HW, Belzer FO, Maki DG. A prospective, randomized, double-blind study of trimethoprim-sulfamethoxazole for prophylaxis of infection in renal transplantation: clinical efficacy, absorption of trimethoprim-sulfamethoxazole, effects on the microflora, and the cost-benefit of prophylaxis. Am J Med. 1990;89:255–274. doi: 10.1016/0002-9343(90)90337-D. [DOI] [PubMed] [Google Scholar]
- 76.Harmon WE, McDonald RA, Reyes JD, Bridges ND, Sweet SC, Sommers CM, Guidinger MK. Pediatric transplantation, 1994–2003. Am J Transplant. 2005;5:887–903. doi: 10.1111/j.1600-6135.2005.00834.x. [DOI] [PubMed] [Google Scholar]
- 77.Baron PW, Ojogho ON, Yorgin P, Sahney S, Cutler D, Ben-Youssef R, Baqai W, Weissman J, Franco E, Zuppan C, Concepcion W. Comparison of outcomes with low dose anti-thymocyte globulin, basiliximab or no induction therapy in pediatric kidney transplant recipients: a retrospective study. Pediatr Transplant. 2008;12:32–39. doi: 10.1111/j.1399-3046.2007.00764.x. [DOI] [PubMed] [Google Scholar]
- 78.Flaman F, Zieroth S, RaoV RH, Delgado DH. Basiliximab versus rabbit anti-thymocyte globulin for induction therapy in patients after heart transplantation. J Heart LungTransplant. 2006;25:1358–1362. doi: 10.1016/j.healun.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 79.Puliyandaa DP, Stableinb DM, Dharnidharkac VR. Younger age and antibody induction increase the risk for infection in pediatric renal transplantation: a NAPRTCS Report. Am J Transplant. 2007;7:662–666. doi: 10.1111/j.1600-6143.2006.01675.x. [DOI] [PubMed] [Google Scholar]
- 80.Ettenger RB. Antibody therapy as an induction regimen in pediatric renal transplantation. Transplant Proc. 1999;31:2677–2678. doi: 10.1016/S0041-1345(99)00527-8. [DOI] [PubMed] [Google Scholar]
- 81.Mourad G, Rostaing L, Legendre C, Garrigue V, Thervet E, Durand D. Sequential protocols using basiliximab versus antithymocyte globulins in renal-transplant patients receiving mycophenolate mofetil and steroids. Transplantation. 2004;78:584–590. doi: 10.1097/01.TP.0000129812.68794.CC. [DOI] [PubMed] [Google Scholar]
- 82.Lebranchu Y, Bridoux F, Buchler M, Meur Y, Etienne I, Toupance O, Hurault de Ligny B, Touchard G, Moulin B, Pogamp P, Reigneau O, Guignard M, Rifle G. Immunoprophylaxis with basiliximab compared with antithymocyte globulin in renal transplant patients receiving MMF containing triple therapy. Am J Transplant. 2002;2:48–56. doi: 10.1034/j.1600-6143.2002.020109.x. [DOI] [PubMed] [Google Scholar]
- 83.Clark G, Walsh G, Deshpande P, Koffman G. Improved efficacy of basiliximab over antilymphocyte globulin induction therapy in paediatric renal transplantation. Nephrol Dial Transplant. 2002;17:1304–1309. doi: 10.1093/ndt/17.7.1304. [DOI] [PubMed] [Google Scholar]
- 84.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 85.Fortun J, Martin-Davila P, Pascual J, Cervera C, Moreno A, Gavalda J, Aguado JM, Pereira P, Gurguí M, Carratala J, Fogueda M, Montejo M, Blasco F, Bou G, Torre-Cisneros J, RESITRA Transplant Network (2010) Immunosuppressive therapy and infection after kidney transplantation. Transpl Infect Dis. doi:10.1111/j.1399-3062.2010.00526.x [DOI] [PubMed]
- 86.Knight RJ, Villa M, Laskey R, Benavides C, Schoenberg L, Welsh M, Kerman RH, Podder H, Buren CT, Katz SM, Kahan BD. Risk factors for impaired wound healing in sirolimus-treated renal transplant recipients. Clin Transplant. 2007;21:460–465. doi: 10.1111/j.1399-0012.2007.00668.x. [DOI] [PubMed] [Google Scholar]
- 87.Grim SA, Slover CM, Sankary H, Oberholzer J, Benedetti E, Clark NM. Risk factors for wound healing complications in sirolimus-treated renal transplant recipients. Transplant Proc. 2006;38:3520–3523. doi: 10.1016/j.transproceed.2006.10.065. [DOI] [PubMed] [Google Scholar]
- 88.Valente JF, Hricik D, Weigel K, Seaman D, Knauss T, Siegel CT, Bodziak K, Schulak JA. Comparison of sirolimus vs. mycophenolate mofetil on surgical complications and wound healing in adult kidney transplantation. Am J Transplant. 2003;3:1128–1134. doi: 10.1034/j.1600-6143.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 89.McWilliams TJ, Levvey BJ, Russell PA, Milne DG, Snell GI. Interstitial pneumonitis associated with sirolimus: a dilemma for lung transplantation. J Heart LungTransplant. 2003;22:210–213. doi: 10.1016/S1053-2498(02)00564-8. [DOI] [PubMed] [Google Scholar]
- 90.Howard L, Gopalan D, Griffiths M, Mahadeva R. Sirolimus-induced pulmonary hypersensitivity associated with a CD4 T-cell infiltrate. Chest. 2006;129:1718–1721. doi: 10.1378/chest.129.6.1718. [DOI] [PubMed] [Google Scholar]
- 91.Pham PT, Pham PC, Danovitch GM, Ross DJ, Gritsch HA, Kendrick EA, Singer J, Shah T, Wilkinson AH. Sirolimus-associated pulmonary toxicity. Transplantation. 2004;77:1215–1220. doi: 10.1097/01.TP.0000118413.92211.B6. [DOI] [PubMed] [Google Scholar]
- 92.Marty FM, Bryar J, Browne SK, Schwarzberg T, Ho VT, Bassett IV, Koreth J, Alyea EP, Soiffer RJ, Cutler CS, Antin JH, Baden LR. Sirolimus-based graft-versus host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: a cohort analysis. Blood. 2007;110:490–500. doi: 10.1182/blood-2007-01-069294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ozaki KS, Camara NO, Nogueira E, Pereira MG, Granato C, Melaragno C, Camargo LF, Pacheco-Silva A. The use of sirolimus in ganciclovir-resistant cytomegalovirus infections in renal transplant recipients. Clin Transplant. 2007;21:675–680. doi: 10.1111/j.1399-0012.2007.00699.x. [DOI] [PubMed] [Google Scholar]
- 94.Trotter JF, Wallack A, Steinberg T. Low incidence of cytomegalovirus disease in liver transplant recipients receiving sirolimus primary immunosuppression with 3-day corticosteroid taper. Transpl Infect Dis. 2003;5:174–180. doi: 10.1111/j.1399-3062.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 95.Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, Starling RC, Sørensen K, Hummel M, Lind JM, Abeywickrama KH, Bernhardt P, RAD B253 Study Group Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847–858. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 96.Vitko S, Margreiter R, Weimar W, Dantal J, Viljoen HG, Li Y, Jappe A, Cretin N, RAD B201 Study Group Everolimus (Certican) 12-month safety and efficacy versus mycophenolate mofetil in de novo renal transplant recipients. Transplantation. 2004;78:1532–1540. doi: 10.1097/01.TP.0000141094.34903.54. [DOI] [PubMed] [Google Scholar]
- 97.Mathew TH. A blinded, long-term, randomized multicenter study of mycophenolate mofetil in cadaveric renal transplantation: results at three years. Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation. 1998;65:1450–1454. doi: 10.1097/00007890-199806150-00007. [DOI] [PubMed] [Google Scholar]
- 98.Fisher RA, Stone JJ, Wolfe LG, Rodgers CM, Anderson ML, Sterling RK, Shiffman ML, Luketic VA, Contos MJ, Mills AS, Ferreira-Gonzalez A, Posner MP. Four-year follow-up of a prospective randomized trial of mycophenolate mofetil with cyclosporine microemulsion or tacrolimus following liver transplantation. Clin Transplant. 2004;18:463–472. doi: 10.1111/j.1399-0012.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 99.Kobashigawa J, Miller L, Renlund D, Mentzer R, Alderman E, Bourge R, Costanzo M, Eisen H, Dureau G, Ratkovec R, Hummel M, Ipe D, Johnson J, Keogh A, Mamelok R, Mancini D, Smart F, Valantine H. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Mycophenolate Mofetil Investigators. Transplantation. 1998;66:507–515. doi: 10.1097/00007890-199808270-00016. [DOI] [PubMed] [Google Scholar]
- 100.Webster AC, Caring for Australians with Renal Impairment (CARI) The CARI guidelines. Calcineurin inhibitors in renal transplantation: the addition of anti-CD25 antibody induction to standard immunosuppressive therapy for kidney transplant recipients. Nephrology. 2007;12:S75–S84. doi: 10.1111/j.1440-1797.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 101.Jose M, Caring for Australians with Renal Impairment (CARI) The CARI guidelines. Calcineurin inhibitors in renal transplantation: adverse effects. Nephrology. 2007;12:S66–S74. doi: 10.1111/j.1440-1797.2007.00731.x. [DOI] [PubMed] [Google Scholar]
- 102.Gea-Banacloche JC, Opal SM, Jorgensen J, Carcillo JA, Sepkowitz KA, Cordonnier C. Sepsis associated with immunosuppressive medications: an evidence-based review. Crit Care Med. 2004;32(11 Suppl):S578–S590. doi: 10.1097/01.CCM.0000143020.27340.FF. [DOI] [PubMed] [Google Scholar]
- 103.Kamara N, Miliotoa O, Puissant-Lubranoc B, Espositoa L, Pierrea MC, Ould Mohameda Lavayssierea A, Cointaulta O, Ribesa D, Cardeaua I, Nogiera MB, Duranda D, Abbalc M, Blancherc A, Rostainga L. Incidence and predictive factors for infectious disease after rituximab therapy in kidney-transplant patients. Am J Transplant. 2010;10:89–98. doi: 10.1111/j.1600-6143.2009.02785.x. [DOI] [PubMed] [Google Scholar]