Abstract
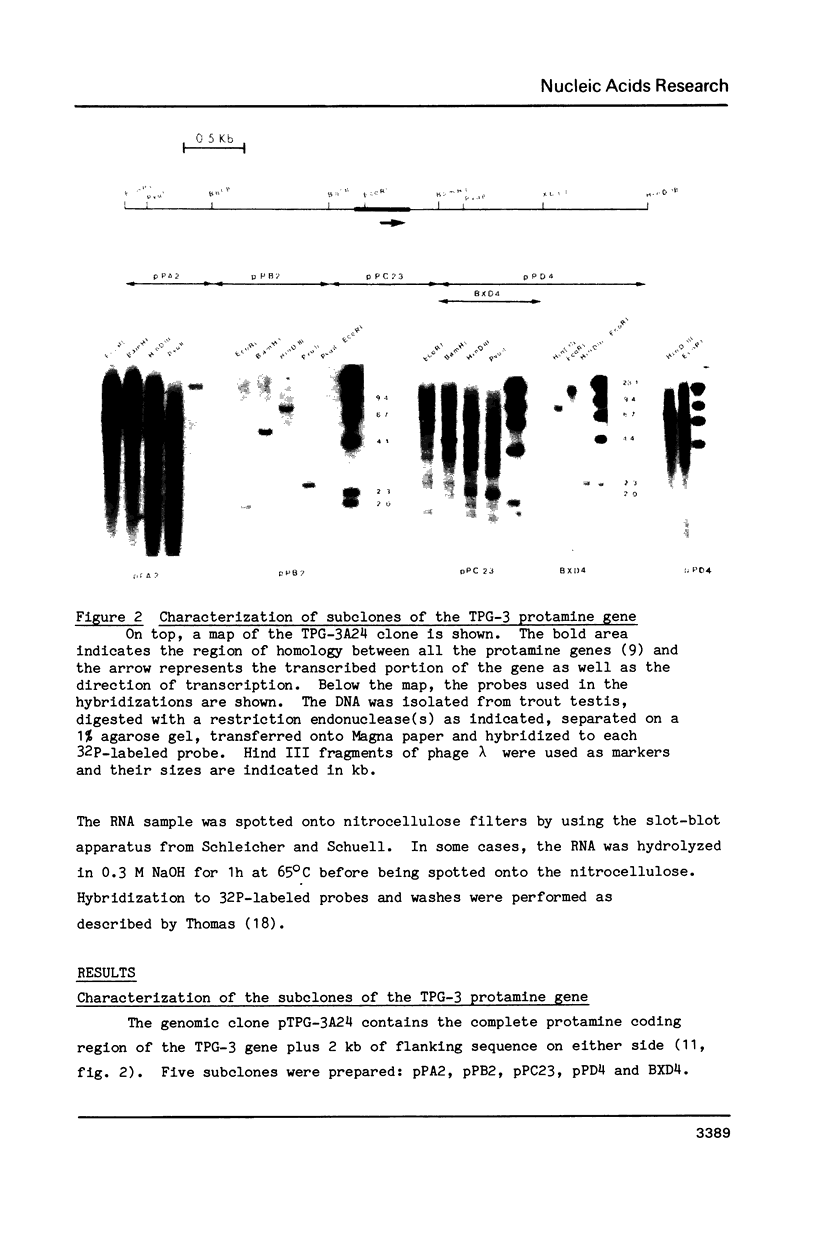
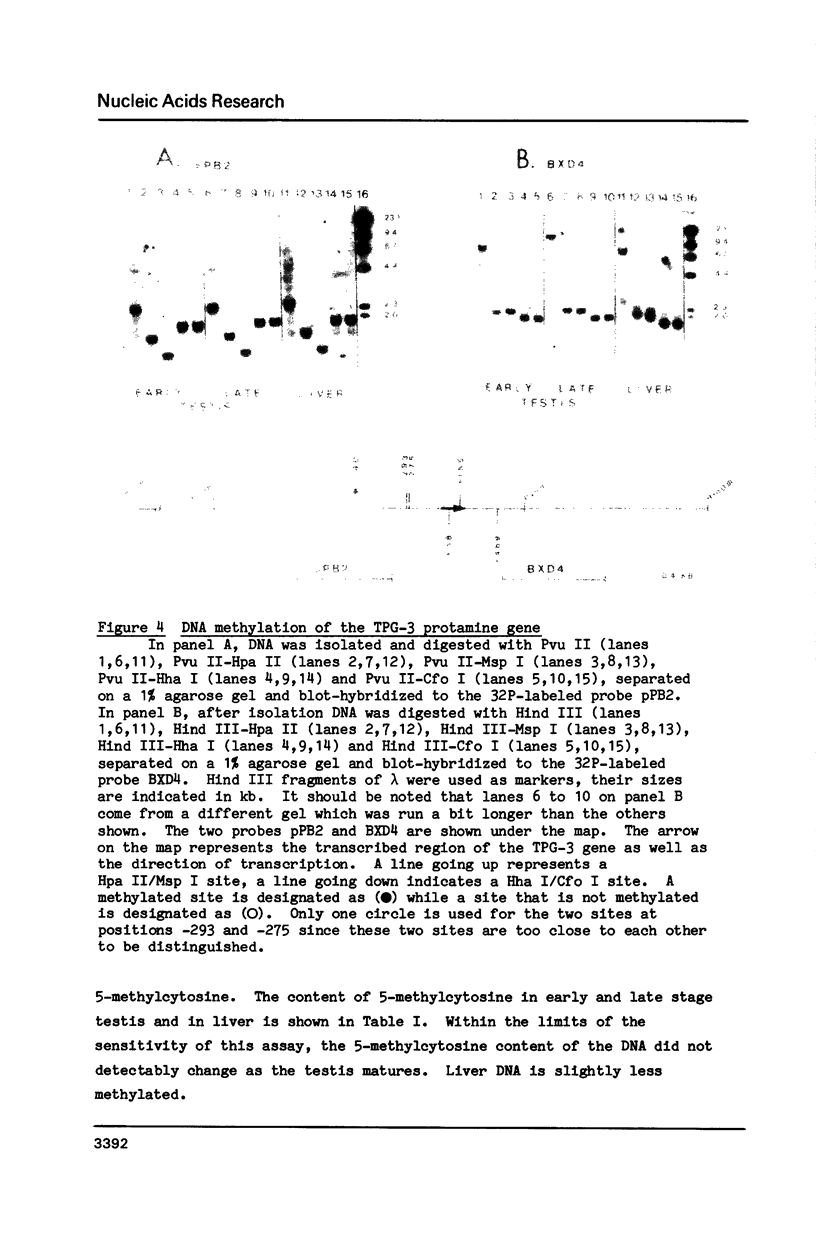
The chromatin structure of a germ-line specific gene, the TPG-3 gene, one of the rainbow trout protamine genes was analyzed in various tissues. The protamine genes are expressed in early stage testis but not in late stage testis, liver or erythrocyte. Five potential CpG methylation sites in the coding and flanking regions of the TPG-3 protamine gene were monitored in early and late stage testis, nucleoprotamine, liver and erythrocyte. In all cases the patterns of methylation were identical with only one CpG site at position -740 being methylated. Thus, the methylation pattern of this protamine gene remained the same independently of the expression of the gene. Two Msp I sites at positions -293 and/or -275 and +155 were accessible to the enzyme in the TPG-3 chromatin of early stage testis. Since the Msp I site at position -293 and/or -275 was also present in the TPG-3 chromatin of liver, only the site at position +155 within the transcribed region correlated with the expression of the protamine gene.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiken J. M., McKenzie D., Zhao H. Z., States J. C., Dixon G. H. Sequence homologies in the protamine gene family of rainbow trout. Nucleic Acids Res. 1983 Jul 25;11(14):4907–4922. doi: 10.1093/nar/11.14.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Blanco J., States J. C., Dixon G. H. General method for isolation of DNA sequences that interact with specific nuclear proteins in chromosomes: binding of the high mobility group protein HMG-T to a subset of the protamine gene family. Biochemistry. 1985 Dec 31;24(27):8021–8028. doi: 10.1021/bi00348a028. [DOI] [PubMed] [Google Scholar]
- Bülow S., Link G. A general and sensitive method for staining DNA and RNA blots. Nucleic Acids Res. 1986 May 12;14(9):3973–3973. doi: 10.1093/nar/14.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie J. R., Saunders C. A. Chemical composition of nucleosomes among domains of calf thymus chromatin differing in micrococcal nuclease accessibility and solubility properties. J Biol Chem. 1981 Dec 10;256(23):12574–12580. [PubMed] [Google Scholar]
- Dillon N. O., Spencer V. M., Butterworth P. H. Transcription of a cloned rainbow trout protamine gene is accurately initiated following transfection into HeLa cells but the majority of the transcripts fail to polyadenylate at the correct site. Nucleic Acids Res. 1985 Dec 20;13(24):8715–8727. doi: 10.1093/nar/13.24.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drance M. G., Hollenberg M. J., Smith M., Wylie V. Histological changes in trout testis produced by injections of salmon pituitary gonadotropin. Can J Zool. 1976 Aug;54(8):1285–1293. doi: 10.1139/z76-146. [DOI] [PubMed] [Google Scholar]
- Fey E. G., Krochmalnic G., Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol. 1986 May;102(5):1654–1665. doi: 10.1083/jcb.102.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam S., Aline R., Jr, Wylie V., Ingles C. J., Smith M. RNA synthesis and RNA polymerase activities in germ cells of developing rainbow trout testis. Biochim Biophys Acta. 1979 Dec 17;565(2):275–292. doi: 10.1016/0005-2787(79)90205-3. [DOI] [PubMed] [Google Scholar]
- Gregory S. P., Dillon N. O., Butterworth P. H. The localisation of the 5'-termini of in vivo transcripts of a cloned rainbow trout protamine gene. Nucleic Acids Res. 1982 Dec 11;10(23):7581–7592. doi: 10.1093/nar/10.23.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Conkin K. F. Chromatin structure and de novo methylation of sperm DNA: implications for activation of the paternal genome. Science. 1985 May 31;228(4703):1061–1068. doi: 10.1126/science.2986289. [DOI] [PubMed] [Google Scholar]
- Huang L. H., Farnet C. M., Ehrlich K. C., Ehrlich M. Digestion of highly modified bacteriophage DNA by restriction endonucleases. Nucleic Acids Res. 1982 Mar 11;10(5):1579–1591. doi: 10.1093/nar/10.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatrou K., Dixon G. H. Protamine messenger RNA: its life history during spermatogenesis in rainbow trout. Fed Proc. 1978 Sep;37(11):2526–2533. [PubMed] [Google Scholar]
- Iatrou K., Spira A. W., Dixon G. H. Protamine messenger RNA: evidence for early synthesis and accumulation during spermatogenesis in rainbow trout. Dev Biol. 1978 May;64(1):82–98. doi: 10.1016/0012-1606(78)90062-3. [DOI] [PubMed] [Google Scholar]
- Liberator P. A., Lingrel J. B. Restriction endonuclease accessibility of the developmentally regulated goat gamma-, beta C-, and beta A-globin genes in chromatin. Differences in 5' regions which show unusually high sequence homology. J Biol Chem. 1984 Dec 25;259(24):15497–15501. [PubMed] [Google Scholar]
- Louie A. J., Dixon G. H. Trout testis cells. I. Characterization by deoxyribonucleic acid and protein analysis of cells separated by velocity sedimentation. J Biol Chem. 1972 Sep 10;247(17):5490–5497. [PubMed] [Google Scholar]
- McClelland M., Nelson M. The effect of site specific methylation on restriction endonuclease digestion. Nucleic Acids Res. 1985;13 (Suppl):r201–r207. doi: 10.1093/nar/13.suppl.r201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Wood W. I., Dolan M., Engel J. D., Felsenfeld G. A 200 base pair region at the 5' end of the chicken adult beta-globin gene is accessible to nuclease digestion. Cell. 1981 Nov;27(1 Pt 2):45–55. doi: 10.1016/0092-8674(81)90359-7. [DOI] [PubMed] [Google Scholar]
- Razin A., Szyf M. DNA methylation patterns. Formation and function. Biochim Biophys Acta. 1984 Sep 10;782(4):331–342. doi: 10.1016/0167-4781(84)90043-5. [DOI] [PubMed] [Google Scholar]
- Reeves R. Transcriptionally active chromatin. Biochim Biophys Acta. 1984 Sep 10;782(4):343–393. doi: 10.1016/0167-4781(84)90044-7. [DOI] [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- States J. C., Connor W., Wosnick M. A., Aiken J. M., Gedamu L., Dixon G. H. Nucleotide sequence of a protamine component CII gene of Salmo gairdnerii. Nucleic Acids Res. 1982 Aug 11;10(15):4551–4563. doi: 10.1093/nar/10.15.4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet R. W., Chao M. V., Axel R. The structure of the thymidine kinase gene promoter: nuclease hypersensitivity correlates with expression. Cell. 1982 Dec;31(2 Pt 1):347–353. doi: 10.1016/0092-8674(82)90128-3. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Gilbert W. Tissue-specific exposure of chromatin structure at the 5' terminus of the rat preproinsulin II gene. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1577–1580. doi: 10.1073/pnas.78.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]