Summary
Background
National melanoma incidence trends with details of anatomical site have not been previously described for England.
Objectives
To describe site-specific trends in cutaneous melanoma for England as a whole during the last three decades.
Methods
Anonymized data, 1979–2006, were obtained from national cancer registrations of all patients in England up to age 89 years with incident primary invasive cutaneous melanomas (n = 124 055). Sex-specific age-standardized incidence rates and average annual percentage change in rates were calculated for each broad anatomical site.
Results
Overall incidence rates of cutaneous melanoma in England, 1979–2006, were 81 and 100 per million, in males and females, respectively. Site-specific rates were consistently highest on the lower limbs in females followed by the trunk in males. Greatest annual increases occurred on the trunk in both sexes over 45 years (males 9·9%, females 6·8%), then upper limbs (males 8·7%, females 6·8%). Incidence trends in males relative to females varied little across sites apart from a more rapid rise in head/neck melanomas in males than in females after the 1980s.
Conclusions
Invasive melanoma rates continue to rise in England, particularly on the trunk and arms, and in males on the head/neck. The steeper increases in melanoma rates among males are consistent with their greater sun exposure and poorer compliance with sun protection measures than females.
Incidence of cutaneous melanoma has been rising worldwide over the past few decades in countries with predominantly white populations.1 The overall incidence of melanoma in the U.K. has almost doubled in the past two decades to 10·2 and 11·8 per 100 000 in males and females, respectively.2 As elsewhere, background incidence markedly increases between early adulthood and seniority in the U.K., by 10-fold in males and 3·5-fold in females.2 There is, however, evidence to suggest that the rising incidence of melanoma is at least partly explained by diagnostic drift, whereby lesions previously reported as benign are now reported as malignant, resulting in the rise in incidence being predominantly early, preinvasive disease.3–5
Little recent information is available about the anatomical sites of occurrence of melanoma in the U.K. Based on a study in Scotland, 1979–2003, and another in Southeast England, 1960–98, trunk melanomas predominated in males (2·5–4·0 per 100 000), while lower limb tumours predominated in females (3·0–5·0 per 100 000).6,7 A detailed comparison of anatomical sites of melanoma in Western Scotland and Queensland, Australia, which accounted for relative surface areas of different body sites, showed that rates on the lower limbs of Scottish women under age 60 years were higher than on any other site (17·1 per 100 000 at ages 40–59 years).8 Beyond the U.K., analyses of site-specific trends have shown steep increases in incidence on the head/neck in both sexes, as well as on the trunks of males and lower limbs of females.7–12 Previous incidence studies grouping anatomical sites by the frequency with which they are exposed to the sun (i.e. occasionally exposed, regularly exposed, etc.) have shown significant increases in incident melanoma on occasionally exposed sites in both sexes.13,14
Detailed national data on melanoma incidence trends by anatomical site have not been documented for England. These data are needed in order to understand the changing patterns and causes of melanoma development and to inform targeted primary prevention strategies. The aim of this study therefore was to describe site-specific trends in cutaneous melanoma for England as a whole during the past three decades. To obviate the effect of diagnostic shift in the reporting of thin noninvasive melanomas, in situ melanomas were excluded a priori from these analyses.
Methods
Anonymized, individual-level, national cancer registry data were obtained from the Northern and Yorkshire Cancer Registry and Information Service for all people in England up to 89 years of age with a newly diagnosed, primary, invasive, cutaneous melanoma over the 28-year period, 1979–2006. Cancer registration in England is of a consistently high standard and the majority of cancers, including melanomas, are captured (98%). Histology reporting is the main source (at least 95%) for registration of new melanomas (South West Public Health Observatory, personal communication). Diagnostic criteria per se did not materially alter for invasive melanoma during the study period. Data were coded using the International Classification of Diseases (ICD) 9th revision from 1979 to 1994,15 while from 1995 onward the ICD 10th revision was used.16 For site-specific analyses, melanomas were categorized as occurring on the head and neck (ICD-09 and ICD-10 codes: 1720–1724, C43·0–C43·4), trunk (codes 1725, C43·5), upper limb (codes 1726, C43·6), lower limb (codes 1727, C43·7) or unspecified site (codes 1728–1729, and C·43·8–C·43·9). Annual mid-year national population estimates by sex, single year of age and calendar year were supplied by the Population Estimates Unit, Office of National Statistics (ONS).
Age was categorized as < 25, 25–44, 45–64 and 65–89 years and time periods were defined by 5-year intervals giving five complete quinquennia for study: 1982–1986, 1987–1991, 1992–1996, 1997–2001 and 2002–2006. Sex-specific incidence rates were calculated for each site and expressed per million person-years at risk. All incidence rates were age-standardized to the European standard population.17 Person-years at risk were calculated for each subgroup from the population data provided. Average annual percentage change in incidence in each subgroup was estimated using the slope of the linear trend line fitted to the logarithm of incidence rates, by year of diagnosis. Thus, the average percentage change per year in incidence rates was estimated by 100*(eb–1), where b = slope of the linear trend line.18 Significance was set at P < 0·05.
Results
Between 1979 and 2006 there were a total of 124 055 cases of primary invasive cutaneous melanoma diagnosed in England in persons up to age 89 years, three-quarters (n = 94 470) being diagnosed from 1990 to 2006. The overall age-standardized incidence rates 1982–2006 across all sites for males and females were 81 and 100 per million person-years at risk, respectively. Since 1979 the greatest proportion of all incident invasive melanoma in both sexes combined occurred on the lower limb (33%), followed by the trunk (25%), the upper limb (18%), and the head/neck (16%) with anatomical site unspecified in 8%. Highest site-specific incidence rates consistently occurred on female lower limbs followed by the male trunk.
Overall age-standardized invasive melanoma rates increased between consecutive 5-year intervals (Table 1) and across the whole study period at all specified anatomical sites in both sexes (Figs 1 and 2). Melanoma incidence on unspecified sites rose early in the study period but then plateaued so was not considered further. Comparing rates in 1982–86 to those in 2002–06, the most marked rises in incidence occurred on the trunk where rates rose almost fourfold from 15 to 56 per million in males, and over threefold in females from 9 to 29 per million (Table 1).
Table 1.
Age-standardized site-specific incidence rates (per 106 person-years at risk) of cutaneous melanoma in males and females in England, by 5-year period (1982–2006)
| Head/neck |
Trunk |
Upper limb |
Lower limb |
All sitesa |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
|||||||||||
| Period | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate |
| 1982–1986 | 858 | 7 | 1031 | 7 | 1524 | 15 | 1049 | 9 | 731 | 7 | 1466 | 12 | 911 | 8 | 4353 | 38 | 4537 | 40 | 8469 | 66 |
| 1987–1991 | 1334 | 11 | 1422 | 9 | 2448 | 23 | 1457 | 12 | 1079 | 10 | 1876 | 16 | 1290 | 11 | 5350 | 45 | 7005 | 60 | 11 122 | 84 |
| 1992–1996 | 1809 | 14 | 1715 | 10 | 3334 | 30 | 1869 | 16 | 1460 | 13 | 2528 | 20 | 1515 | 13 | 6035 | 49 | 9110 | 75 | 13 335 | 97 |
| 1997–2001 | 2409 | 18 | 2004 | 12 | 4605 | 39 | 2502 | 20 | 2038 | 15 | 3117 | 24 | 1798 | 15 | 6716 | 51 | 11 979 | 94 | 15 419 | 109 |
| 2002–2006 | 3439 | 25 | 2602 | 16 | 6931 | 56 | 3742 | 29 | 2997 | 26 | 4503 | 33 | 2469 | 19 | 8310 | 60 | 16 928 | 125 | 20 236 | 138 |
| 1982–2006 | 9849 | 15 | 8774 | 11 | 18 842 | 34 | 10 619 | 18 | 8305 | 14 | 13 490 | 21 | 7983 | 14 | 30 764 | 49 | 49 559 | 81 | 68 581 | 100 |
All sites includes 4580 male and 4934 female cases with unspecified site (age-standardized rate of 7 per million for both sexes).
Fig 1.
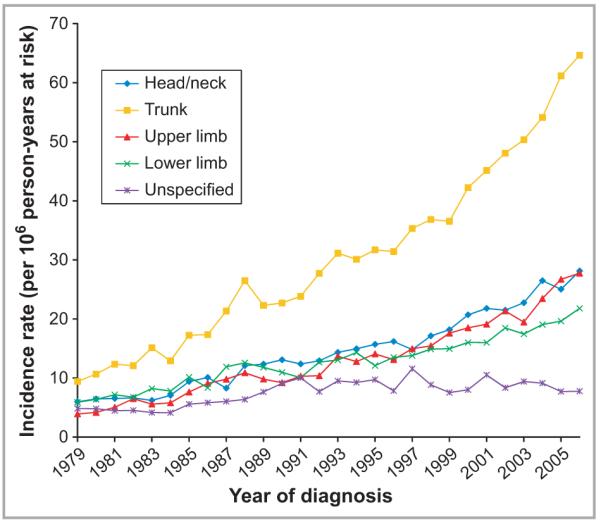
Incidence of cutaneous melanoma in males (per million person-years at risk) by anatomical site, in England, 1979–2006.
Fig 2.
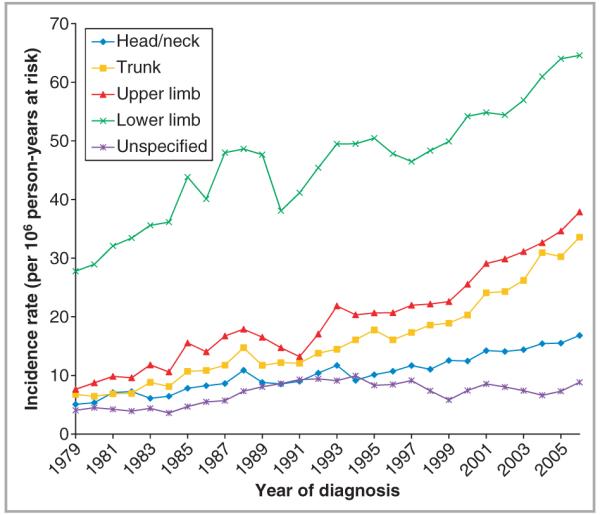
Incidence of cutaneous melanoma in females (per million person-years at risk) by anatomical site, in England, 1979–2006.
In every age group there were significant increases in melanoma rates over time for each anatomical site (P-values < 0·01) (Table 2). Greatest annual increases occurred on the trunk (9·9%) and upper limbs (8·7%) in males aged > 64 years. In females, the greatest increases were on the upper limb for the oldest age group (6·8%) and on the trunk in 45–64-year-olds (6·8%). Lowest annual increases in melanoma occurred on the lower limb in females < 65 years. Across all sites and age groups, the annual increase in incidence was 5·8% in males and 3·8% in females.
Table 2.
Average annual percentage change (AAPC) and 95% confidence limits (CL) of cutaneous melanoma incidence according to anatomical site, by sex and age group (1979–2006)
| Head/neck |
Trunk |
Upper limb |
Lower limb |
All sitesa |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AAPC (%) | 95% CL | AAPC (%) | 95% CL | AAPC (%) | 95% CL | AAPC (%) | 95% CL | AAPC (%) | 95% CL | |
| Males | ||||||||||
| < 25 years | 6·3 | 5·4, 7·2 | 4·2 | 3·5, 5·0 | 2·9 | 1·9, 3·8 | 3·2 | 2·4, 4·0 | 3·8 | 3·6, 4·0 |
| 25–44 years | 4·0 | 3·6, 4·4 | 4·9 | 4·6, 5·2 | 5·5 | 5·0, 6·0 | 4·1 | 3·7, 4·5 | 4·4 | 4·1, 4·7 |
| 45–64 years | 5·3 | 4·9, 5·7 | 6·7 | 6·4, 7·0 | 6·6 | 6·3, 7·0 | 4·5 | 4·2, 4·9 | 5·7 | 5·5, 6·0 |
| 65–89 years | 6·8 | 6·6, 7·0 | 9·9 | 9·6, 10·2 | 8·7 | 8·4, 9·1 | 4·4 | 4·0, 4·8 | 7·2 | 7·1, 7·3 |
| 0–89 years | 5·9 | 5·7, 6·1 | 6·8 | 6·5, 7·0 | 6·7 | 6·5, 7·0 | 4·3 | 4·0, 4·5 | 5·8 | 5·6, 6·0 |
| Females | ||||||||||
| < 25 years | 5·1 | 3·9, 6·3 | 4·2 | 3·5, 4·8 | 5·2 | 4·4, 5·7 | 3·1 | 2·5, 3·6 | 3·9 | 3·5, 4·3 |
| 25–44 years | 3·1 | 2·5, 3·6 | 5·8 | 5·4, 6·1 | 4·3 | 3·9, 4·6 | 1·3 | 1·0, 1·6 | 2·9 | 2·7, 3·2 |
| 45–64 years | 4·1 | 3·8, 4·4 | 6·8 | 6·5, 7·0 | 5·3 | 5·0, 5·7 | 2·5 | 2·3, 2·7 | 3·8 | 3·6, 4·0 |
| 65–89 years | 4·0 | 3·8, 4·3 | 5·1 | 4·7, 5·4 | 6·8 | 6·5, 7·0 | 4·6 | 4·3, 4·9 | 4·7 | 4·6, 4·9 |
| 0–89 years | 3·9 | 3·6, 4·1 | 5·9 | 5·7, 6·1 | 5·3 | 5·0, 5·6 | 2·5 | 2·3, 2·8 | 3·8 | 3·6, 4·0 |
All sites includes cases with unspecified site.
The magnitude of incidence rates in males relative to females (M : F) did not vary greatly over time across sites apart from divergence for head/neck melanoma (Fig. 3). Specifically, rate ratios for upper (~0·6) and lower (~0·3) limbs were consistent over the study period, with incidence remaining higher in females than in males at these sites. Despite M : F rate ratio fluctuations (1·4–2·1), incidence of trunk melanoma was always higher in males. In contrast, head/neck melanoma showed similar rates among men and women in the 1980s (0·9) but from the early 1990s rates in men began to rise noticeably faster than the rise in women (M : F ratio ~1·6).
Fig 3.
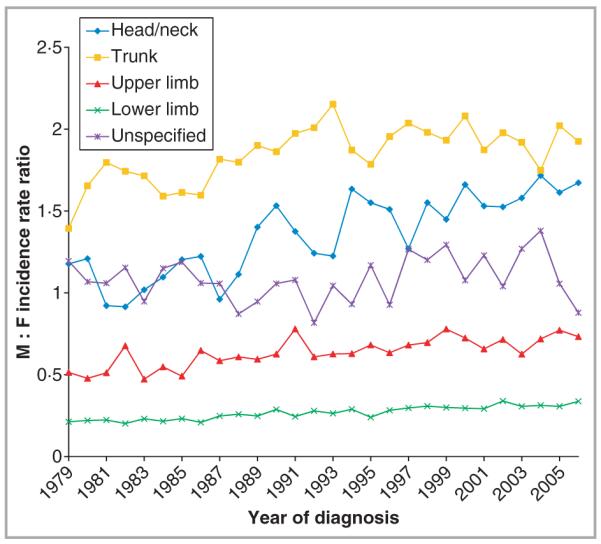
Cutaneous melanoma male to female (M : F) incidence rate ratios by anatomical site, in England, 1979–2006.
Discussion
In this study based on national cancer registry data, we have shown that incidence rates of invasive cutaneous melanoma have been increasing on all body sites, in both males and females, across the whole of England since 1979, to 2006. As elsewhere in the U.K., highest incidence rates were observed on the lower limb in females, but during the last three decades, increases in invasive melanoma in England have been most pronounced on the trunk in both sexes.
Several studies of anatomical incidence of invasive cutaneous melanoma have been conducted around the world, over varying time periods, and our results show that the actual site distribution of cutaneous melanomas appears similar to other countries with predominantly white-skinned populations, with trunk melanomas predominating in males and lower limb melanomas in females.6–13 Ours is the first study to characterize site-specific incidence trends of cutaneous melanoma in the English population, however, and to have analysed changes in site-specific rates in males relative to females. Most sites have maintained steady differences in melanoma incidence between men and women, but this was not true of the head/neck where rates in men and women were similar in the 1980s but diverged after the early 1990s, with rates in men rising faster, not only at older ages > 65 years, but also in the youngest men < 25 years.
Given that solar ultraviolet radiation (UVR) is the primary environmental cause of melanoma,19,20 a widespread increase in sun exposure is the likely explanation for rising melanoma trends in England and worldwide. Increases in recreational sun exposure have been facilitated by a growing industry of budget airlines in England and declining airfares21,22 making travel abroad to sunny destinations both accessible and affordable to people of all ages. Such vacations abroad have been linked directly to increasing melanoma incidence.21,22 At the same time, changes in clothing fashions have also resulted in increases in sun exposure on previously littleexposed body sites like the trunk and limbs,7,11,13,23,24 and such exposure is specifically associated with melanoma at these sites.20,25
Meanwhile, the sunbed industry has provided a means of artificial UVR exposure in temperate England. Sunbed use, now recognized as a cause of melanoma,26 is particularly prevalent among teens and young adults,27 and thus may be the cause of some of the increase in melanoma in younger adults in England. Several studies have explored the relationship between sunbed use and melanoma and have noted significantly increased risk among sunbed users, particularly at earlier ages of first use.28–31 Further studies specifically among those highly exposed to UVR and among adolescents and young adults have noted significant positive dose–response relationships between sunbed usage and melanoma and have described site-specific increases in risk particularly for trunk melanomas.30,31 It is hoped that regulations recently implemented in the U.K. banning sunbed use among those under 18 years of age32 may mitigate these trends.
There is some controversy about whether or not the increases in melanoma incidence on trunk and limbs might be artefact, entirely explained by the shift from diagnosis of a proportion of benign naevi to stage I melanomas.5 While this diagnostic drift may indeed partly explain some recent general increases on trunk and limbs, it is more difficult to explain the continuing incidence rises on the head and neck (in males much more than females) as this anatomical region has been relatively unaffected by the diagnostic shift5 and is not sex-specific. With high cumulative UVR exposure leading to the development of melanoma on the head and neck7,11,13,33 the steeper increase in males than in females on this site is consistent with ongoing higher levels of exposure of the face to UVR in males than in females at all ages, including youth. This is consistent with the general observation that women tend to be less photodamaged than men and have sustained fewer sunburns.34–36 They are also more informed about, and compliant with, health promotion measures than men.34,35 Women tend to be more conscious of their appearance and to perceive a suntan as a greater threat to attractiveness and health than their male counterparts.35 Similarly, it is notable that melanoma mortality rates have stabilized in women although are still rising in men in England37 suggesting that improved diagnosis has yet to benefit men with more advanced disease to the same degree as women.38
The strengths of these findings are their national population base and their coverage of recent trends over almost three decades. While melanoma registration and data capture are currently of high quality,39 there were some deficiencies in melanoma registration in England at the outset of the study period. Improved registration late in the study period, including the recording of multiple primary melanomas on different sites, could therefore have contributed to the observed increase in incidence rates overall, although this should not have affected anatomical sites differentially. Thus the relative site-specific distributions reported here should be accurate. Lack of information about precise anatomical subsites of melanomas prevented further insights into exact sites of UVR exposure which might be amenable to sun-protection measures (e.g. face vs. ear or leg vs. thigh). Laterality details were not available to us, although a previous report has shown a left-sided excess of cutaneous melanoma in England along with several other mainly white populations around the world.40 Lack of adjustment for body surface area in the calculation of anatomical site-specific incidence rates may also be seen as a potentially limiting factor, but as we were assessing only trends in melanoma incidence over time, body surface area would not have influenced the results and indeed our analyses are quite comparable to those of other studies which have adjusted for body surface area.8,11
In conclusion, the incidence of invasive cutaneous melanoma on all body sites continued to rise in England among both sexes over the last three decades, with the greatest increases on the trunk, upper limbs and male head and neck. Melanoma prevention messages emphasizing the dangers of high UVR exposure, both outdoors in summer or on holiday abroad, and indoors from sunbeds, should continue to be a focus of public health initiatives in England. Men in particular should be encouraged to protect themselves from harmful sun exposure. Continued monitoring of trends in the incidence of cutaneous melanoma is required to track future changes in incidence rates and to assess the effectiveness of current public health messages.
What’s already known about this topic?
Incidence of cutaneous melanoma is still rising world-wide, particularly in countries with predominantly white populations.
Diagnostic drift may account for some of the increases observed in rates.
Rates increase with age and are generally highest on the trunk in males and on the lower limb in females.
National-level site-specific melanoma incidence trends have not previously been described in England.
What does this study add?
To our knowledge this is the first study to describe trends in invasive melanoma incidence trends by anatomical site, for England as a whole.
Invasive melanoma rates continue to rise in England across all body sites, but particularly on the trunk and arms, and on the head/neck of males.
The steeper increases in male rates are consistent with their greater ultraviolet radiation exposure and poorer compliance with health promotion messages compared with females.
Acknowledgments
We thank staff at the South West Public Health Observatory for information about quality of melanoma registration. Data used in this study were contributed by the eight regional cancer registries in England. Census output is Crown copyrighted and is reproduced with the permission of the Controller of Her Majesty’s Stationary Office and the Queen’s Printer for Scotland.
Funding sources S.C.W. and A.C.G. are funded by a Fellowship from the Medical Research Council (no. 89912). R.D.A. and J.M.B. are funded by Cancer Research U.K.
Footnotes
Conflicts of interest None declared.
References
- 1.Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol. 2009;27:3–9. doi: 10.1016/j.clindermatol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Giblin AV, Thomas JM. Incidence, mortality and survival in cutaneous melanoma. J Plast Reconstr Aesthet Surg. 2007;60:32–40. doi: 10.1016/j.bjps.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Shuster S. Malignant melanoma: how error amplification by screening creates spurious disease. Br J Dermatol. 2009;161:977–9. doi: 10.1111/j.1365-2133.2009.09399.x. [DOI] [PubMed] [Google Scholar]
- 4.Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331:481–4. doi: 10.1136/bmj.38516.649537.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levell NJ, Beattie CC, Shuster S, et al. Melanoma epidemic: a midsummer night’s dream? Br J Dermatol. 2009;161:630–4. doi: 10.1111/j.1365-2133.2009.09299.x. [DOI] [PubMed] [Google Scholar]
- 6.MacKie RM, Bray C, Vestey J, et al. Melanoma incidence and mortality in Scotland 1979–2003. Br J Cancer. 2007;96:1772–7. doi: 10.1038/sj.bjc.6603801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newnham A, Moller H. Trends in the incidence of cutaneous malignant melanomas in the south east of England, 1960–1998. J Public Health Med. 2002;24:268–75. doi: 10.1093/pubmed/24.4.268. [DOI] [PubMed] [Google Scholar]
- 8.Whiteman DC, Bray CA, Siskind V, et al. A comparison of the anatomic distribution of cutaneous melanoma in two populations with different levels of sunlight: the west of Scotland and Queensland, Australia 1982–2001. Cancer Causes Control. 2007;18:485–91. doi: 10.1007/s10552-007-0123-1. [DOI] [PubMed] [Google Scholar]
- 9.Bulliard J-L, Cox B, Semenciw R. Trends by anatomic site in the incidence of cutaneous malignant melanoma in Canada, 1969–93. Cancer Causes Control. 1999;10:407–16. doi: 10.1023/a:1008964621225. [DOI] [PubMed] [Google Scholar]
- 10.Marrett LD, Nguyen HL, Armstrong BK. Trends in the incidence of cutaneous malignant melanoma in New South Wales, 1983–1996. Int J Cancer. 2001;92:457–62. doi: 10.1002/ijc.1203. [DOI] [PubMed] [Google Scholar]
- 11.Green A, MacLennan R, Youl P, et al. Site distribution of cutaneous melanoma in Queensland. Int J Cancer. 1993;53:232–6. doi: 10.1002/ijc.2910530210. [DOI] [PubMed] [Google Scholar]
- 12.Downing A, Newton-Bishop JA, Forman D. Recent trends in cutaneous malignant melanoma in the Yorkshire region of England; incidence, mortality and survival in relation to stage of disease, 1993–2003. Br J Cancer. 2006;95:91–5. doi: 10.1038/sj.bjc.6603216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elwood JM, Gallagher RP. Body site distribution of cutaneous malignant melanoma in relationship to patterns of sun exposure. Int J Cancer. 1998;78:276–80. doi: 10.1002/(SICI)1097-0215(19981029)78:3<276::AID-IJC2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.Mowbray M, Stockton DL, Doherty VR. Changes in the site distribution of malignant melanoma in South East Scotland (1979–2002) Br J Cancer. 2007;96:832–5. doi: 10.1038/sj.bjc.6603612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . International Statistical Classification of Disease, Injuries and Causes of Death. 9th Revision World Health Organization; Geneva: 1977. [Google Scholar]
- 16.World Health Organization . International Statistical Classification of Disease, Injuries and Causes of Death. 10th Revision World Health Organization; Geneva: 1992. [Google Scholar]
- 17.Quinn M, Wood HC, Rowan N, editors. Cancer Atlas of the United Kingdom and Ireland 1991–2002: Studies on Medical and Population Subjects. Vol. 68. Palgrave MacMillan; London: 2005. [Google Scholar]
- 18.de Vries E, Bray FI, Coebergh JWW, et al. Changing epidemiology of malignant cutaneous melanoma in Europe 1953–1997: rising trends in incidence and mortality but recent stabilizations in Western Europe and decreases in Scandinavia. Int J Cancer. 2003;107:119–26. doi: 10.1002/ijc.11360. [DOI] [PubMed] [Google Scholar]
- 19.IARC . Solar and Ultraviolet Radiation. IARC; Lyon, France: 1992. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 55). [PMC free article] [PubMed] [Google Scholar]
- 20.Chang Y, Barrett JH, Bishop DT, et al. Sun exposure and melanoma risk at different latitudes: a pooled analysis of 5700 cases and 7216 controls. Int J Epidemiol. 2009;38:814–30. doi: 10.1093/ije/dyp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bentham G, Aase AR. Incidence of malignant melanoma of the skin in Norway, 1955–1989: associations with solar ultraviolet radiation, income and holidays abroad. Int J Epidemiol. 1996;25:1132–8. doi: 10.1093/ije/25.6.1132. [DOI] [PubMed] [Google Scholar]
- 22.Agredano YZ, Chan JL, Kimball RC, et al. Accessibility to air travel correlates strongly with increasing melanoma incidence. Melanoma Res. 2006;16:77–81. doi: 10.1097/01.cmr.0000195696.50390.23. [DOI] [PubMed] [Google Scholar]
- 23.Bulliard J-L, Cox B. Cutaneous malignant melanoma in New Zealand: trends by anatomical site, 1969–1993. Int J Epidemiol. 2000;29:416–23. [PubMed] [Google Scholar]
- 24.Green A, McCredie M, Giles G, et al. Occurrence of melanomas on the upper and lower limbs in eastern Australia. Melanoma Res. 1996;6:387–94. doi: 10.1097/00008390-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24:3172–7. doi: 10.1200/JCO.2006.06.1325. [DOI] [PubMed] [Google Scholar]
- 26.El Ghissassi F, Baan R, Straif K, et al. WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens – part D: radiation. Lancet Oncol. 2009;10:751–2. doi: 10.1016/s1470-2045(09)70213-x. [DOI] [PubMed] [Google Scholar]
- 27.Webb M. Health and Economic Impact of Sunbed Use in Wales – A Rapid Review of the Evidence. National Public Health Service for Wales; Cardiff: 2009. [Google Scholar]
- 28.The International Agency for Research on Cancer Working Group on artificial ultraviolet light and skin cancer The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120:1116–22. doi: 10.1002/ijc.22453. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher RP, Spinelli JJ, Lee TK. Tanning beds, sunlamps, and risk of cutaneous malignant melanoma. Cancer Epidemiol Biomarkers Prev. 2005;14:562–6. doi: 10.1158/1055-9965.EPI-04-0564. [DOI] [PubMed] [Google Scholar]
- 30.Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2010;128:2425–35. doi: 10.1002/ijc.25576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: a case–control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557–68. doi: 10.1158/1055-9965.EPI-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sunbed (Regulation) Act. vol. c. 20. HMSO; London: 2010. pp. 1–9. [Google Scholar]
- 33.Bulliard J-L. Site-specific risk of cutaneous malignant melanoma and pattern of sun exposure in New Zealand. Int J Cancer. 2000;85:627–32. doi: 10.1002/(sici)1097-0215(20000301)85:5<627::aid-ijc5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 34.Stanton WR, Janda M, Baade PD, et al. Primary prevention of skin cancer: a review of sun protection in Australia and internationally. Health Promot Int. 2004;19:369–78. doi: 10.1093/heapro/dah310. [DOI] [PubMed] [Google Scholar]
- 35.Arthey S, Clarke VA. Suntanning and sun protection: a review of the psychological literature. Soc Sci Med. 1995;40:265–74. doi: 10.1016/0277-9536(94)e0063-x. [DOI] [PubMed] [Google Scholar]
- 36.Stott MA. Tanning and sunburn: knowledge, attitudes and behaviour of people in Great Britain. J Public Health Med. 1999;21:377–84. doi: 10.1093/pubmed/21.4.377. [DOI] [PubMed] [Google Scholar]
- 37.Karim-Kos HE, de Vries E, Soerjomataram I, et al. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44:1345–89. doi: 10.1016/j.ejca.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 38.Baade P, Meng X, Youlden D, et al. Time trends and latitudinal differences in melanoma thickness distribution in Australia, 1990–2006. Int J Cancer. 2011 doi: 10.1002/ijc.25996. [Epub ahead of print]. DOI:10.1002/ijc.25996. [DOI] [PubMed] [Google Scholar]
- 39.Goodwin RG, Holme SA, Roberts DL. Variations in registration of skin cancer in the United Kingdom. Clin Exp Dermatol. 2004;29:328–30. doi: 10.1111/j.1365-2230.2004.01523.x. [DOI] [PubMed] [Google Scholar]
- 40.Brewster DH, Horner M-JD, Rowan S, et al. Left-sided excess of invasive cutaneous melanoma in six countries. Eur J Cancer. 2007;43:2634–7. doi: 10.1016/j.ejca.2007.09.021. [DOI] [PubMed] [Google Scholar]


