Abstract
Background
Herbal medicines are being used for treating viral diseases including viral myocarditis, and many controlled trials have been done to investigate their efficacy.
Objectives
To assess the effects of herbal medicines on clinical and indirect outcomes in patients with viral myocarditis.
Search strategy
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library Issue 3, 2009, MEDLINE (January 1966 - July 2009), EMBASE (January 1998 - July 2009), Chinese Biomedical Database (1979 - 2009), China National Knowledge Infrastructure (1979 - 2009), Chinese VIP Information (1989 - 2009), Chinese Academic Conference Papers Database and Chinese Dissertation Database (1980 - 2009), AMED (1985 - 2009), LILACS accessed in July 2009 and the trials register of the Cochrane Complementary Medicine Field. We handsearched Chinese journals and conference proceedings. No language restrictions were applied.
Selection criteria
Randomised controlled trials of herbal medicines (with a minimum of seven days treatment duration) compared with placebo, no intervention, or conventional interventions were included. Trials of herbal medicine plus conventional drug versus drug alone were also included. Only trials that reported adequate description of allocation sequence generation were included.
Data collection and analysis
Two review authors independently extracted data and evaluated trial quality. Adverse effects information was collected from the trials.
Main results
Fourteen randomised trials involving 1463 people were included. All trials were conducted and published in China. Quality of the trials was assessed to be low. No trial had diagnosis of viral myocarditis confirmed histologically, and only a few trials attempted to establish viral aetiology. Nine different herbal medicines were tested in the included trials. The trials reported electrocardiogram results, level of myocardial enzymes, cardiac function, symptoms, and adverse effects.
Astragalus membranaceus (either as an injection or granules) showed significant positive effects in symptom improvement, normalisation of electrocardiogram results, CPK levels, and cardiac function. Shengmai injection also showed significant effects in symptom improvement. Shengmai decoction triggered significant improvement in quality of life measured by SF-36. No serious adverse effects were reported.
Authors’ conclusions
Some herbal medicines may lead to improvement of symptoms, ventricular premature beat, electrocardiogram, level of myocardial enzymes, and cardiac function in viral myocarditis. However, interpretation of these findings should be taken with care due to the low methodological quality, small sample size, and limited number of trials on individual herbs. Further robust trials are needed to explore the use of herbal medicines in viral myocarditis.
Medical Subject Headings (MeSH): Astragalus membranaceus; Drug Combinations; Drugs, Chinese Herbal [adverse effects; *therapeutic use]; Myocarditis [*drug therapy; virology]; Phytotherapy [adverse effects; *methods]; Randomized Controlled Trials as Topic; Virus Diseases [*drug therapy]
MeSH check words: Humans
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON [Explanation]
| Herbal medicines versus supportive therapy for viral myocarditis | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Patient or population: patients with viral myocarditis | ||||||
| Settings: inpatients and outpatients | ||||||
| Intervention: Herbal medicines versus supportive therapy | ||||||
|
| ||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) |
No of Participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
|
|
||||||
| Control | Herbalmedicines versus supportive therapy |
|||||
|
Number of patients with-
out symptom improve ment- Shenmai injection versus support therapy |
Study population |
RR 0.32 (0.14 to 0.75) |
127 (1 study) |
 very low1,2,3 |
||
|
|
||||||
| 290 per 1000 |
93 per 1000 (41 to 218) |
|||||
|
|
||||||
| Medium risk population | ||||||
|
|
||||||
| 290 per 1000 |
93 per 1000 (41 to 218) |
|||||
|
| ||||||
|
Number of patients with-
out symptom improve ment- Xinshu Capsule versus supportive ther- apy |
Study population |
RR 0.14 (0.04 to 0.45) |
120 (1 study) |
 low1,4 |
||
|
|
||||||
| 350 per 1000 |
49 per 1000 (14 to 157) |
|||||
|
|
||||||
| Medium risk population | ||||||
|
|
||||||
| 350 per 1000 |
49 per 1000 (14 to 157) |
|||||
|
| ||||||
|
Number of patients with
premature beat - Xinshu Capsule versus support- ive therapy |
Study population |
OR 0.21 (0.06 to 0.79) |
120 (1 study) |
 very low1,2,4 |
||
|
|
||||||
| 950 per 1000 |
800 per 1000 (533 to 938) |
|||||
|
|
||||||
| Medium risk population | ||||||
|
|
||||||
| 950 per 1000 |
800 per 1000 (533 to 938) |
|||||
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio;
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
No information about allocation concealment, lack of blinding.
The confidence interval is wide.
Outcomes were selectively reported.
All the included patients were suffered from Frequent Ventricular Extrasystoles.
BACK GROUND
Viral myocarditis is the result of viral infection that leads to myocardial necrosis (Suddaby 1996; Feldman 2000; Kearney 2001; Cooper 2009). Many pathogenic mechanisms may contribute to myocardial cell loss including cytokine production contributing to myocardium inflammation; viral persistence, which may produce an autoimmune response to cardiac myosin; and viral invasion of vascular endothelium causing vascular spasm with reperfusion injury (Feldman 2000; Rose 2009). Viral myocarditis is one of the causes of dilated cardiomyopathy (Dec 1994;Kawai 1999;Cooper 2009). The severe outcomes of viral myocarditis include arrhythmias, cardiogenic shock, development of dilated cardiomyopathy and death (necrosis) of heart tissue, although the majority of cases are subclinical and self-limited.
Myocarditis is an insidious disease that is usually asymptomatic in its early stages, and it appears to be far more common in children than in adults (Feldman 2000). The true prevalence of viral myocarditis in the general population is unknown due to the invasive technique (myocardial biopsy) required for diagnosis (Haas 2001; Cooper 2009).Myocarditis is amajor cause of sudden, unexpected death (accounting for approximately 20% of cases) in adults less than 40 years of age (Drory 1991; Feldman 2000; Baughman 2006).Routine postmortemexaminations have identifiedmyocardial inflammation in one to nine percent of sudden, unexpected adult deaths taking into consideration three early studies in western countries (Feldman 2000). Viral infection is thought to be the most common cause of myocarditis. Viral myocarditis can be caused by more than 27 viruses such as coxsackie virus, parvovirus B19, enterovirus, adenovirus, rubella virus, polio virus, human immunodeficiency virus 1 (HIV-1), cytomegalovirus, hepatitis A and C viruses.
The clinical features of myocarditis are varied. The spectrum includes asymptomatic participants who may have chest pain, fever, palpitation and electrocardiographic abnormalities; signs and symptoms of clinical heart failure and ventricular dilation, of fulminant heart failure and severe left ventricular dysfunction, with or without cardiac dilations (Dec 1985; Feldman 2000). Although the endomyocardial biopsy remains the gold standard for the diagnosis of viral myocarditis, comprehensive criteria are developed for the diagnosis through evaluation of cardiac function, symptoms and signs, history of flu-like syndrome, laboratory findings, identification of the viruses, as well as elimination of other causes of global cardiac dysfunction, (see ‘Types of participants’) (Dec 1992; Feldman 2000; Andreoletti 2009; Schultz 2009).
Supportive care is the first line of therapy for left ventricular dysfunction in patients with viral myocarditis. Cardiac function support is provided by pharmacological agents such as digitalis and diuretics, extracorporeal membrane oxygenation, and implantation of a ventricular-assisting device (Topkara 2006). Current trials of treatment in chronic heart failure secondary to dilated cardiomyopathy support the use of angiotensin converting enzyme inhibitors angiotensin-receptor blockers, beta adrenoceptor blockers, and diuretics (Kearney 2001,Cooper 2009).Other treatments for viralmyocarditis are immunosuppressive agents (Parrillo 2001; Wojnicz 2001), immunoadsorption (Staudt 2001), and interferon (Miric 1995).
Complementary therapies are being used increasingly (Eisenberg 1998; Vickers 2000; Koithan 2009; O’Regan 2009). The number of randomised trials of complementary treatments has increased significantly in the recent years (Tang 1999). The Cochrane Database of Systematic Reviews has over 100 systematic reviews of complementary medicine interventions (as of Issue 1, 2010). Many people turn to this therapywhen conventionalmedicine fails them or they believe strongly in the effectiveness of complementary medicine (John 2005). Herbal medicine forms the main part of traditional Chinese medicine, which is a 3000-year-old holistic systemofmedicine combiningmedicinal herbs, acupuncture, food therapy, massage, and therapeutic exercise for both treatment and prevention of disease (Fulder 1996). Herbalmedicines are defined in this review as products derived from plants or parts of plants (e.g., leaves, stems, buds, flowers, roots, or tubers) (raw or refined) used for treatment of diseases. Synonyms of herbal medicines include herbal remedies, herbalmedications, herbal products, herbal preparations, medicinal herbs, and phyto-pharmaceuticals.
Our primary searches identified more than 400 studies tested Chinese herbalmedicines for viralmyocarditis in theChinese biomedical database (December 2001). There are four kinds of herbal therapies, i.e. single herb, Chinese proprietary medicines, mixture of different herbs, and any one of the three types plus western medicines. The most commonly tested single herbs include Astragalus membranaceus, Salviae miltiorrhizae, ginseng, and Sophorae flavescentis; and the Chinese proprietary medicines such as Shenmai and Shuanghuanglian in the clinical trials.Chinese proprietary medicines are usually based on well-established and longstanding recipes and formulated as tablets or capsules for commerce, convenience, or palatability (Yang 2006).Mixture of herbs is prescribed by Chinese herbalists according to their differentiation of symptoms through the Chinese diagnostic patterns (i.e. inspection, listening, smelling, inquiry, and palpation) (MOH 2007). However, active ingredients of these herbal medicines are largely unknown and they are combined with different herbs to regulate body functions (Jiang 1999). Several trials have shown that Astragalusmembranaceus and Shenmaimight have potential for treating viral myocarditis or alleviating symptoms and signs and decreasing cardiac enzymes and few trials reported adverse effects (Yang 1990; Li 1992; Ren 1992; Huang 1995; Liu 1996; Yang 1997; Yin 1997; Li 1998; Chen 1999). The possible modes of action include enhancing natural killer cell activity, inducing production of alpha- and gamma-interferon, improving cardiac microcirculation, and anti-free radical and lipid peroxidation (Yang 1990; Li 1992; Huang 1995; Zhao 1996). On the other hand, there are an increasing number of reports in the medical literature about liver toxicity, renal damage and even cancer from some Chinese herbal products (Ishizaki 1996;Melchart 1999; Gottieb 2000) thus, this area needs further research and systematic evaluation.
OBJECTIVES
The objective of this review was to assess the effect, both harms and benefits of treating viral myocarditis with herbal medicines.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) with adequate method of allocation sequence generation were included irrespective of blinding, publication status, and language. Adequate methods of sequence generation included computer generated random numbers, tables of random numbers or drawing lots. Randomised crossover trials would also be included if the data were available. Quasi-randomised trials, non-randomised studies and selfdescribed randomised control studies without elaboration of sequence generation method used would be excluded.
Types of participants
Male or female patients, of any age or ethnic origin, who had viral myocarditis (including acute and/or chronic viral myocarditis). Viral myocarditis was diagnosed on the basis of: a history of an antecedent flu-like syndrome accompanied by symptoms such as fever, arthralgias, and malaise; following with signs and symptoms of clinical heart failure and ventricular dilation; along with laboratory findings of leukocytosis, an elevated sedimentation rate, eosinophilia, or an elevation in the cardiac fraction of creatine phosphokinase (CPK-MB); the electrocardiogram showing ventricular arrhythmias or heart block; and excluding other causes of global cardiac dysfunction, e.g. acute myocardial infarction or pericarditis (Vignola 1984; Dec 1992; Feldman 2000; Baughman 2006; Schultz 2009). The gold standard by the finding of the endomyocardial biopsy is not imperative for diagnosis. Acute viral myocarditis was considered in patients who presented with recent (less than two weeks) onset of cardiac failure or arrhythmia. Trials in which patients presenting with recent onset of cardiac failure or arrhythmia and laboratory tests corresponding with myocarditis, but without electrocardiogram confirmation, would be included.
Types of interventions
We defined herbal medicines in this review as products derived from plants or parts of plants (e.g. leaves, stems, buds, flowers, roots, or tubers; raw or refined) used for treatment of diseases. Synonyms of herbal medicines were herbal remedies, herbal medications, herbal products, herbal preparations, medicinal herbs, and phyto-pharmaceuticals.
The intervention of herbal medicines included single herb (including extract from single herb), Chinese proprietary medicine, or compound of several herbs irrespective of preparation (e.g. decoction, oral liquid, tablet, capsule, pill, powder, granule, injection, or plaster (external use of dressings impregnated with herbal extracts)), route of administration (e.g. oral, topical, intramuscular or intravenous injection), dosage, and regimen of herbs.
We also included trials of herbal medicines plus conventional intervention versus conventional intervention alone. The control intervention included placebo, non-specific treatment such as vitamins or nutritional supplement, supportive therapy such as diuretics, beta-blocker, or antiviral therapy. Any co-intervention out of experimental and control interventions was allowed as long as all arms of the randomised allocation received the same co-intervention.
We included trials if the treatment was given for a minimum of seven days although definitive information about duration of treatment was lacking in the literature. Short (seven days) and long term (more than three weeks) duration would be explored in subgroup analysis.
The following comparisons were tabulated where data available:
herbal medicines versus placebo;
herbal medicines versus non-specific treatment;
herbal medicines versus supportive intervention;
herbal medicines versus antiviral therapy;
herbal medicines plus conventional intervention versus conventional intervention alone.
Types of outcome measures
Primary outcomes
Mortality (all-cause and myocarditis related);
Incidence of complications (heart failure and arrhythmias).
Secondary outcomes
Cardiac function;
Biochemical response, defined as decrease or normalisation of serum enzymes levels;
Number and type of adverse events;
Quality of life (assessed by validated scale);
Health economics (such as cost of interventions, length of hospital stay).
Two types of adverse events would be analysed: serious and minor adverse events. Serious adverse events were defined as those that led to death, were life-threatening, required or prolonged hospitalisation, resulted in persistent or significant disability/incapacity, or were events that could jeopardise the patient or required another intervention to prevent or treat the relevant adverse events (ICH-GCP 1997). All other adverse events were considered to be minor. For herbal medicines that were included in this review, we would use non-randomised or toxicological studies to assess potential adverse effects.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Issue 3, 2009), MEDLINE (January 1966 - July 2009), EMBASE (January 1998 - July 2009) Chinese Biomedical Database (1979 - 2009), China National Knowledge Infrastructure (1979 - 2009), Chinese VIP Information (1989 - 2009), Chinese Academic Conference Papers Database and Chinese Dissertation Database (1980 - 2009), AMED (1985 - 2009), LILACS (www.bireme.br/bvs/I/ibd.htm) accessed on July 2009 and the trials register of the Cochrane Complementary Medicine Field. We handsearched Chinese journals and conference proceedings.
No language restrictions were applied.
Searching other resources
The following journals published in Chinese were initially handsearched for the published review: Journal of Clinical Cardiology (1985 to 2003), Chinese Journal of Hypertension (1993 to 2003), Chinese Journal of Cardiac Arrhythmia (1997 to 2003), Chinese Circulation Journal (1986 to 2003), Journal of Traditional Chinese Medicine (1980 to 2003), Chinese Journal of Integrated Traditional and Western Medicine (1982 to 2003).
No handsearching was performed for this update because all the post-2003 publications in above journals were embodied in electronic databases.
We checked the reference lists of identified randomised clinical trials and review articles in order to find randomised trials not identified by the electronic searches or handsearches. We searched ongoing trials through the National Research Register https://portal.nihr.ac.uk/Pages/NRRArchive.aspx and Current Controlled Trials www.controlled-trials.com, and grey literature through the database SIGLE.
Data collection and analysis
Selection of trials for inclusion
Two reviewers (ZLL and ZJL) independently selected the trials using a pre-specified selection criteria form. Any disagreement was resolved by discussion.
Assessment of risk of bias
For this review update, we applied a stricter inclusion criteria and only included trials with clear description of the method used in generating allocation sequences. Risk of bias of included studies were assessed by two authors (ZLL, ZJL) independently. Any disagreements were resolved by consensus, or with consultation with a third author (JPL). The Cochrane Collaboration risk of bias tool was used (Higgins 2008):
was the allocation sequence adequately generated?
was the allocation adequately concealed?
was knowledge of the allocated intervention adequately prevented during the study?
were incomplete outcome data adequately addressed?
were reports of the study free of suggestion of selective outcome reporting?
was the study apparently free of other problems that could put it at a high risk of bias?
A judgement of ‘Yes’ indicates low risk of bias, ‘No’ indicates high risk of bias and ‘Unclear’ indicates unclear or uncertain risk of bias.
Furthermore, we aimed to investigate if intention-to-treat analysis and pre-sample estimation were applied in the included studies.
Data extraction
Two reviewers (ZLL andZJL) extracted data independently,which were validated by a third author (JPL) using a self-developed data extraction form. Papers not in Chinese, English, Japanese, and German were translated with the help of the Cochrane Heart Group. The following characteristics and data were extracted from each included trial: primary author, funding source, quality assessment, mean age, proportion of males, and ethnicity of patients, number of randomised patients, reason and number dropped out or lost during follow-up, patient inclusion and exclusion criteria, acute or chronic viral myocarditis, the way diagnosis was made, type of herb or herbs, method of administration, dosage and duration of intervention, details of the comparison regime, outcome measures, and number and type of adverse events.
Data on the number of participants with each outcome, by allocated treatment group, irrespective of compliance or follow-up, were sought to allow an intention-to-treat analysis. If the above data were not available in the trial reports, we contacted the principal investigator.
Data synthesis
Every type of herbal medicines was compared with a control (e.g. placebo) individually regardless of route of administration, dose, or preparation. Meta-analyses were performed for trials that compared the same herb against same control. Dichotomous data were expressed as relative risk (RR) and continuous outcomes as weighted mean difference (WMD), both with 95% confidence intervals (CI). Intention-to-treat analyses were performed where possible. For dichotomous outcomes, patients with incomplete or missing data were included in a sensitivity analysis by counting them as treatment failures, in order to explore the possible effect of loss to follow-up. Heterogeneity was assessed using the I2 test . Thresholds for the interpretation of I2 can bemisleading, since the importance of inconsistency depends on several factors. A rough guide to interpretation is as follows:
0% to 40%: low level of heterogeneity;
30% to 60%: moderate heterogeneity;
50% to 90%: substantial heterogeneity;
75% to 100%: high level of heterogeneity.
If I2 > 50% , the random effects model was used.
The following comparisons were tabulated if data were available:
herbal medicines versus placebo;
herbal medicines versus non-specific treatment;
herbal medicines versus supportive intervention;
herbal medicines versus antiviral therapy.
Trials of herbal medicines plus conventional intervention versus conventional intervention alone were presented as a separate comparison.
Data from non-randomised studies for assessment of safety were tabulated and analysed in Table 1.
Table 1.
Herbal medicines and adverse effects in the included trials
| Herbs | Formulation | Compositions | Adverse events | Study ID |
|---|---|---|---|---|
| Xinshu Capsule | capsule | herb mixtures, Cortex Eucommiae 30g, Rhi- zoma Polygonati 30g, Semen Cuscutae 30g, Radix Astra- gali 30g, Rhizoma Pinelliae 15g, Sanguis Draconis 10g. |
not reported. | Chen PY 2005 |
| Qingxin Kangyan Yin decoction |
decoction | herb mixtures, Radix Glycyrrhizae Preparata 20g, Radix Astragali 15g010045010051Ramulus Cinnamomi 8g, Radix Ophiopogonis 10g, Radix Rehmanniae 10g, Se- men Platycladi 10g, Colla Corii Asini 10g, Rhizoma Blechni 5g, Radix Trichosan- this 10g, semen nelumbinis 10g. |
not reported. | Li L 2006 |
| Shengyang Yixin decoc- tion |
decoction | herb mixtures,Radix Gin- seng 12g, semen nelumbi- nis 12g, Rhizoma Pinel- liae 12g, Schisandra chinen- sis 12g, Radix Gly-cyrrhizae 12g, Cistanche tubulosa 15g, Fructus Trichosanthis 15g, Rhizoma Coptidis 15g, Rhi- zoma Polygoni Cuspidati 15g, Fructus Forsythiae 15g, Fruc- tus Lycii 15g, Semen Platy- cladi 15g, Folium Isatidis 18g, Radix Astragali 20g, Radix Salviae Miltiorrhizae 20g. |
notreported. | Li M 2006 |
| Radix Astragali | injection | Extracts from Astragalus membranaceus |
not observed (Tan YB 2003) or not reported (Zheng R 2003;Zhou Y 2006;Yang YX 2008;Zhang ZZ 2006). |
Zheng R 2003;Zhou Y 2006;Tan YB 2003;Yang YX 2008;Zhang ZZ 2006 |
| Shortscape fleabane | injection | herb extract | not reported. | Wang Y 2005 |
| Radix astragali | pill | herb extracts | not reported. | Wu JW 2009 |
| Qi Lu decoction | decoction | herb mixtures | not reported. | Yao BJ 2005 |
| Compound Qiangqi pill | pill | herb mixtures,Radix Astra- gali, Rhizoma seu Radix No- topterygii, Radix Paeoniae Alba, Radix Puerariae, Rhi- zomaCimicifugae, Radix Bu- pleuri, Radix Saposhnikoviae, Radix Glycyrrhizae |
more leucocyte count in 15 patients and reduced to nor- mal at the end of the inter vention. |
Zheng RF 2005 |
| Shengmai | oral liquid | herb extracts | more leucocyte count in 15 patients and reduced to nor- mal at the end of the inter vention. |
Zheng RF 2005 |
| Qing Xin HuoMing de- coction |
decoction | herbmixtures, Radix Ophio- pogonis 30-60g,Radix Paeo- niae Rubra, Radix Paeoniae Alba 15g,, Cortex Moutan 15g, Radix Astragali 30-60g, Cornu Bubali 15g, Scrophu- laria 12g, Radix Sophorae flavescentis 18g, Radix Glycyrrhizae Preparata 10g, Fructus Forsythiae,15g, Lonicera japonica Tilwnb 30g, Asparagus Tuber 10g, Dwarf Lilyturf Tuber 10 g, Salvia miltiorrhiza Bunge 30g, health and long teeth 30g, Concha Margaritifera Usta 10g, Fructus Trichosan this 15g, Amber Succinum 2g |
not reported. | Zhou YW 2008 |
| Shenmai | injection | Herbal extracts | not reported. | Peng Q 2005 |
If a sufficient number of randomised trials were identified, we would have performed subgroup analyses according to clinical course (acute or chronic viral myocarditis), electrocardiogram diagnosis (yes or no), formulation of herbs (extract, single herb, or mixture of herbs), and treatment duration (short and long term). Furthermore, if we had identified a sufficient number of randomised trials, we planned to perform sensitivity analyses to explore the influence of trial quality on effect estimates. The quality components of methodology included adequacy of concealment of allocation, double blinding, the use of intention-to-treat (yes or no). Potential biases (Vickers 1998) were investigated using the funnel plot or other corrective analytical methods according to Egger 1997.
RESULTS
Description of studies
See:Characteristics of included studies;Characteristics of excluded studies.
Results of the search
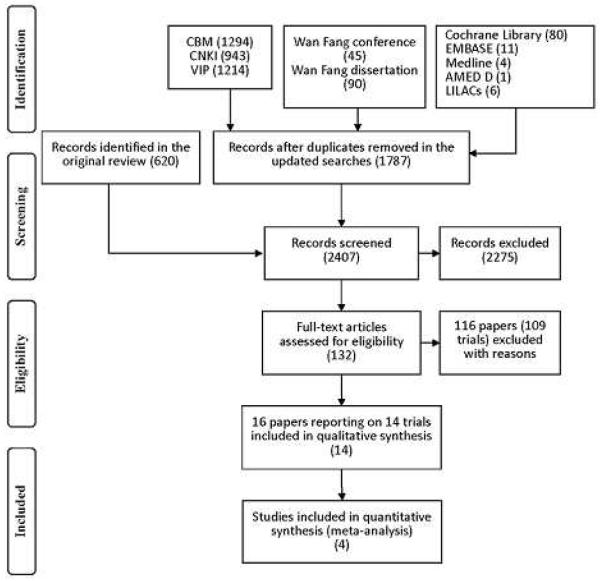
Our searches identified 2407 (620 before year 2003, and 1787 from year 2003 to year 2009) references: 2391 from the electronic searches and 16 fromthe handsearches up to July 2009. After reading titles and abstracts, we excluded 2275 of these articles because they were duplicates, non-clinical studies, or had study objectives different from this review. A total of 132 references published in Chinese or English were retrieved for further assessment.Of these, 116 references (of 109 trials) were excluded because they did not meet our inclusion criteria. A total of 14 new trials (of 16 references) were included in this update (Figure 1). The reasons for exclusion were listed under Characteristics of excluded studies. 40 trials (of 43 references) that were included in the previous version of this review were excluded in this update because of inadequate description of generation of allocation sequence.
Figure 1.
QUOROM flow chart
Included studies
FourteenRCTswere included in this reviewupdate. Further details are given in theCharacteristics of included studies.All the included trials were conducted and published in China.
Participants
A total of 1463 participants with viral myocarditis were included in the 14 trials. The proportion of male participants was 35.2% (515/1463). Eight trials included inpatients (Tan YB 2003; Zheng R 2003; Wang Y 2005; Yao BJ 2005; Li L 2006; Li M 2006; Yang YX 2008;Wu JW2009), five trials included both inpatients and outpatients (Peng Q 2005; Zheng RF 2005; Chen PY 2006; Zhang ZZ 2006; Zhou Y 2006) and one trial did not specify the study setting (Zhou YW 2008). Average size of the trials was 105 participants (ranging from 40 to 218 participants per trial).
Diagnosis
Four trials enrolled patients with acute viral myocarditis; four enrolled with a mixture of acute and chronic viral myocarditis; two enrolled patients with chronic viral myocarditis, and the remaining four trials enrolled patients with undefined phase of viral myocarditis. The diagnostic criteria were based on the national conference consensus in China (NCSSMC 1995; Li 1996;Guo 1999; Wu 2000), which included antecedent history, clinical manifestations, abnormal electrocardiogram and laboratory tests (biochemical parameters and/or aetiology), and excluded other diseases with similar presentations. No trials attempted to establish a viral aetiology for the myocarditis.
Interventions
There were large variations in the formulations, dosage, administration, duration of treatment, and control interventions in the included trials among the herbal medicines tested (Table 1). In total, nine different herbal medicines were tested. Astragalus membranaceus was tested in six trials (Tan YB 2003; Zheng R 2003; Zhang ZZ 2006; Zhou Y 2006; Yang YX 2008; Wu JW 2009). Xinshu Capsule was tested in one trial (Chen PY 2006). Four herbal decoctions including Qingxin Kangyan Yin decoction, Shengyang Yixin decoction,Qi Lu decoction andQingxinHuoming decoction were tested in four trials respectively (Yao BJ 2005; Li L 2006; Li M 2006; Zhou YW 2008). Two formulations (oral liquids and injection) of Shengmai were tested respectively in two trials (Peng Q 2005; Zheng RF 2005), shortscape fleabane injection (herb extract) was tested in one trial (Wang Y 2005) and compound Qiangqi pill (herbmixtures) was tested in one trial (Zheng RF 2005). The formulations of herbal medicines were different, ranging from capsule, pill oral liquid, decoction to injection. The compositions of the herbal medicines varied (Table 1). The duration of treatment varied from 14 days to 30 days (mostly from 14 to 21 days). No trial reported quality standard of the herbal preparations. The supportive therapy included intravenous use of glucose, ATP, co-enzyme A or Q10, inosine, vitamin C, B, E, insulin, cytochromeC, KCl, FDP, etc. The co-interventions included antiarrhythmic drugs, corticosteroids, and antiviral therapies such as ribavirin or interferon.
Outcomes
No trial reported outcomes of the incidence of complications or on health economic costs. One trial reported outcomes of quality of life (Zheng RF 2005). The outcomes that were reported included mortality, conversion to persistent myocarditis, clinical symptoms and signs, electrocardiogram, cardiac function,myocardial enzymes, and adverse effects. Only two trials reported outcomeof adverse effects (Tan YB 2003; Zheng RF 2005). Tan YB 2003 observed no adverse events whereas adverse events of high leucocyte count were seen in 15/132 (11%) patients in Zheng RF 2005. Outcomes reported in 12 trials were measured at the end of treatment. Two trials reported a follow-up period of six months.
Risk of bias in included studies
None of the trials reported sample size calculation or stated that they performed an intention-to-treat analysis to evaluate their data. No multi-centre, large scale RCTs were identified in our searches. Three trials (Zheng R 2003; Zheng RF 2005; Yang YX 2008) reported drop-outs, with a 20% drop-out rate observed in Yang YX 2008. Regrettably, no information was available in how the dropout rate was treated with. Eleven trialsmade baseline comparisons. Only two trials had clear inclusion and exclusion criteria (Zheng RF 2005; Chen PY 2006). Outcome assessment was made after treatment but there was no stated indication for further interventions.
The trials provided limited information on allocation concealment and blinding but there was a lack of description of generation of the allocation sequence. Trialists were contacted for further information but regrettably no information has been provided to date. Sensitivity analyses were performed to explore the effect of potential biases. Assessment of risk of bias is described for each included study in the Characteristics of included studies tables as well as in Figure 2 and Figure 3.
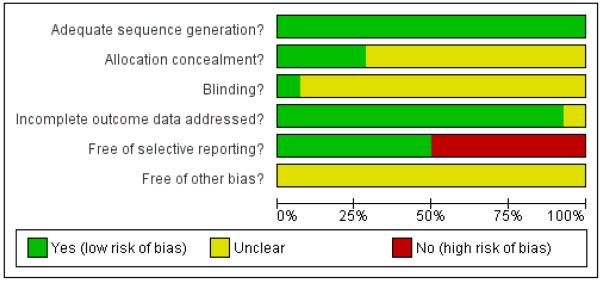
Figure 2.
Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
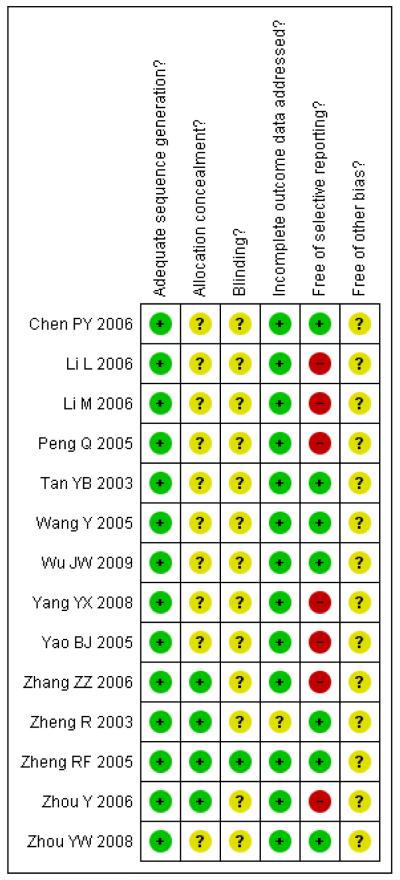
Figure 3.
Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Allocation
All 14 trials had adequate sequence generation but only four (Zheng R 2003; Zheng RF 2005; Zhang ZZ 2006; Zhou Y 2006) provided information on how the allocation sequence was concealed. In each case sealed envelopes were used.
Blinding
Among the 14 included trials, only one trial (Zheng RF 2005) was double-blinded. The remaining 13 trials did not provide information about blinding.
Incomplete outcome data
One trial (Zheng R 2003) did not provide sufficient information for making a judgement on incomplete outcome data assessment. The remaining 13 trials reported the same number of participants randomised and analysed, or addressed the incomplete outcome data or the reasons for missing outcome data.
Selective reporting
Seven of the 14 trials (Li L 2006; Li M 2006; Peng Q 2005; Yang YX 2008; Yao BJ 2005; Zhang ZZ 2006; Zhou Y 2006) were judged to be at high risk of bias in selective reporting due to the lack of information in pre-specified primary outcomes. Other potential sources of bias All included trials contained insufficient information for us to make a judgement on other potential sources of bias and thus were all judged to be unclear for the item “free of other bias?”.
Effects of interventions
See: Summary of findings for the main comparison Main findings of Herbal medicines versus supportive therapy for viral myocarditis; Summary of findings 2 Main findings of Herbal medicines plus supportive therapy versus supportive therapy for viralmyocarditis; Summary of findings 3Main findings ofHerbal medicines plus supportive therapy versus supportive therapy for viralmyocarditis; Summary of findings 4Main findings ofHerbal medicines plus supportive therapy versus supportive therapy for viral myocarditis
Astragalus membranaceus
The injections of single herb Astragalus membranaceus were tested in five trials (Tan YB 2003; Zheng R 2003; Zhang ZZ 2006; Zhou Y 2006; Yang YX 2008), and one trial tested Astragalusmembranaceus granule (Wu JW 2009). The above five trials reported outcomes for symptoms, electrocardiogram, cardiac function, and cardiac enzymes. However, since they reported different outcome measures, it was not possible to combine the data except for the outcomes of symptoms and myocardial enzymes.
Compared with supportive therapy, Astragalus membranaceus injection didn’t show significant effect on symptom improvement, reducing the number of patients died of cardiac failure and patients converting to persisting myocarditis (Yang YX 2008).
Compared with supportive therapy, a combination of Astragalus membranaceus injection and supportive therapy showed significant effect on some outcomes. As there were considerable heterogeneity among these studies (Tan YB 2003; Zheng R 2003; Zhang ZZ 2006; Zhou Y 2006), the random effects model was used to do a meta-analysis of a combination of Astragalus membranaceus injection and supportive therapy on symptom improvement and no significant effect was found (RR 0.38, 95% CI 0.14 to 1.07; Analysis 2.1). One trial showed significant effect of Astragalus membranaceus injection plus supportive therapy on a number of patients with abnormal electrocardiogram (RR 0.25, 95% CI 0.08 to 0.80; Analysis 2.2) (Tan YB 2003). A meta-analysis of two trials showed significant effect of Astragalus membranaceus injection on creatine phosphate kinase (CPK) levels (MD 21.54, 95% CI -33.80 to -9.28; Analysis 2.8) and lactate dehydrogenase (LDH) levels (MD30.33, 95%CI -46.78 to -13.88; Analysis 2.9) (random effects model was used due to significant heterogeneity) (Zhang ZZ 2006; Zhou Y 2006).One trial showed significant difference between Astragalus membranaceus injection plus supportive therapy and supportive therapy regarding left ventricular ejection time (LVET) levels (MD4.67, 95%CI 1.94 to 7.40; Analysis 2.11) (Zheng R 2003).Therewas no significant difference between the combination of Astragalus membranaceus injection and supportive therapy alone in ST-T change (Zheng R 2003), reducing the number of patients with premature beat and abnormal myocardial enzyme levels (Tan YB 2003), reducing the number of patients with abnormal LVEF and converting to persisting myocarditis (Zheng R 2003).
Referring to the intervention of Astragalus membranaceus granule, one trial showed significant difference between Astragalus membranaceus granule plus supportive therapy and supportive therapy regarding LVETlevels(MD12.00, 95&CI 5.06 to 18.94;Analysis 2.11) and creatine kinase MB (CK-MB) levels (MD 16.30, 95& CI -19.66 to -12.94; Analysis 2.12) (Wu JW 2009). A combination of Astragalus membranaceus granule and supportive therapy did not show significant effect than supportive therapy alone on symptom improvement, reducing the number of patients with abnormal electrocardiogram and patients with abnormal myocardial enzyme levels (Wu JW 2009).
Shengmai
Two trials tested Shengmai: one used Shengmai injection in 57 patients with viral myocarditis (Peng Q 2005) and the other Shengmai decoction in 80 patients (Zheng RF 2005). Compared to supportive therapy, Shengmai injection showed significant effects on symptom improvement (RR 0.32, 95% CI 0.14 to 0.75; Analysis 1.1) (Peng Q 2005). There was no significant difference between Shengmai injection and supportive therapy regarding abnormal ST-T changes (RR 1.18, 95%CI 0.58 to 2.40; Analysis 1.2) (Peng Q 2005). Shengmai decoction plus supportive therapy showed significant effect on improving quality of life measured by SF-36 (WMD 40.20, 95% CI 18.13 to 62.27; Analysis 2.13) compared to supportive therapy (Zheng RF 2005).There was no significant difference between Shengmai decoction plus supportive therapy and supportive therapy regarding symptom improvement, abnormal electrocardiogram, number of patients with premature beat (Zheng RF 2005).
Other herbal medicines that were tested once in trials
Shortscape Fleabane injection (Erigeron breviscapus)
One trial compared herbal extract from Erigeron breviscapus plus supportive therapy against supportive therapy alone in 83 patients with acute viral myocarditis (Wang Y 2005). The combination of herbal and supportive therapy appeared better than supportive therapy alone in reducing CK-MB levels (VMD -5.81, 95% CI -11.34 to -0.28; Analysis 2.12), LDH levels (MD 29.28, 95% CI -57.82 to -0.74, Analysis 2.9), symptom scores (VMD -1.41, 95% CI -2.23 to -0.59; Analysis 2.10), CPK levels (MD -41.37, 95% CI -67.00 to -16.46; Analysis 2.8). There was no significant difference between Erigeron breviscapus plus supportive therapy and supportive therapy alone regarding number of patients with premature beat.
Qingxinkangyan decoction
One trial compared Qingxinkangyan decoction versus supportive therapy in 111 participants with viralmyocarditis (Li L 2006).No other differences were observed in this study.
Xinshu capsule
One trial tested Xinshu Capsule for treatment of 120 patients with viral myocarditis (Chen PY 2006). The trial showed significant difference between the herbal medicine and supportive therapy regarding symptomimprovement (RR 0.14, 95%CI 0.04 to 0.45; Analysis 1.1) and number of patients with premature beat (RR 0.21, 95% CI 0.06 to 0.79; Analysis 1.5).
Compound Qiangqi pill
One trial compared Compound Qiangqi pill plus supportive therapywith supportive therapy alone in 80 participantswith viralmyocarditis (Zheng RF 2005). The combination of herbal medicine and supportive therapy showed significant effect on improving quality of life measured by SF-36 (WMD 88.35, 95% CI 68.01 to 108.69; Analysis 2.13) compared to supportive therapy (Zheng RF 2005). No other differences were observed in this study.
Qi Lu decoction
One trial compared herbal decoction plus supportive therapy against supportive therapy alone in 168 participants with viral myocarditis (Yao BJ 2005). The trial showed significant difference between the combined therapy and supportive therapy regarding symptom improvement (RR 0.16, 95% CI 0.04 to 0.69; Analysis 2.1). The combined therapy was not significant different from supportive therapy in number of patients with premature beats.
Shengyangyixin decoction
One trial compared Shengyangyixin Decoction plus supportive therapywith supportive therapy alone in 76 patientswith viralmyocarditis (Li M 2006). The combination of herbal medicine and supportive therapy had a significant effect on reducing the number of participants with abnormal electrocardiogram (RR 0.56, 95% CI 0.35 to 0.90; Analysis 2.2) compared with supportive therapy. The trial also showed significant difference between the combined therapy and supportive therapy regarding LDH levels (MD -186.63, 95% CI -215.02 to -158.24; Analysis 2.9) and CK-MB levels (MD -3.30, 95% CI -3.74 to -2.86; Analysis 2.12). However, the combination did not differ significantly from supportive therapy regarding symptom improvement in this study.
Qingxinhuoming decoction
One trial compared Qingxin Huoming Decoction plus supportive therapy against supportive therapy alone in 78 participants with viral myocarditis (Zhou YW 2008). The combination of herbal medicine and supportive therapy had a significant effect on reducing the number of participants with abnormal electrocardiogram (RR 0.32, 95% CI 0.13 to 0.79; Analysis 2.2) compared with supportive therapy.There was no significant difference betwee combined Qingxinhuoming Decoction and supportive therapy in symptom improvement.
ADDITIONAL SUMMARY OF FINDINGS [Explanation]
| Herbal medicines plus supportive therapy versus supportive therapy for viral myocarditis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: patients with viral myocarditis | ||||||
| Settings: inpatients and/or outpatients | ||||||
| Intervention: Herbal medicines plus supportive therapy versus supportive therapy | ||||||
|
| ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) |
No of Participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
|
|
||||||
| Assumed risk | Corresponding risk | |||||
|
|
||||||
| Control | Herbal medicines plus supportive therapy ver- sus supportive therapy |
|||||
|
Number of patients with-
out symptom improve- ment - Astragaloside injection plus support therapy versus support therapy |
Study population |
RR 0.53 (0.34 to 0.85) |
300 (4 studies) |
 low1,2 |
||
|
|
||||||
| 271 per 1000 |
144 per 1000 (92 to 230) |
|||||
|
|
||||||
| Medium risk population | ||||||
|
|
||||||
| 258 per 1000 |
137 per 1000 (88 to 219) |
|||||
|
| ||||||
|
Number of patients with-
out symptom improve- ment - Qi Lu Decoc- tion plus support therapy versus support therapy |
Study population |
RR 0.16 (0.04 to 0.69) |
168 (1 study) |
 very low1,2,3,4 |
||
|
|
||||||
| 146 per 1000 |
23 per 1000 (6 to 101) |
|||||
|
|
||||||
| Medium risk population | ||||||
|
|
||||||
| 146 per 1000 |
23 per 1000 (6 to 101) |
|||||
|
| ||||||
|
Abnormal electrocardio-
gram - Qingxinhuoming Decoction plus support therapy versus support therapy |
Study population |
RR 0.32 (0.13 to 0.79) |
78 (1 study) |
 low1,4 |
||
|
|
||||||
| 395 per 1000 |
126 per 1000 (51 to 312) |
|||||
|
|
||||||
| Medium risk population | ||||||
|
|
||||||
| 395 per 1000 |
126 per 1000 (51 to 312) |
|||||
|
| ||||||
|
Abnormal electrocardio-
gram - Shengyangyixin Decoction plus support therapy versus support therapy |
Study population |
RR 0.56 (0.35 to 0.9) |
76 (1 study) |
 low1,2,3 |
||
|
|
||||||
| 658 per 1000 |
368 per 1000 (230 to 592) |
|||||
|
|
||||||
| Medium risk population | ||||||
|
|
||||||
| 658 per 1000 |
368 per 1000 (230 to 592) |
|||||
|
| ||||||
|
Abnormal electrocardio-
gram - Astragaloside in- jection plus supportive therapy versus support- ive therapy |
Study population |
RR 0.25 (0.08 to 0.8) |
60 (1 study) |
 low1,3,4 |
||
|
|
||||||
| 400 per 1000 |
100 per 1000 (32 to 320) |
|||||
|
|
||||||
| Medium risk population | ||||||
|
|
||||||
| 400 per 1000 |
100 per 1000 (32 to 320) |
|||||
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio;
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
No information about blinding.
Outcomes were selectively reported.
No information about allocation concealment.
The confidence interval is wide.
| Herbal medicines plus supportive therapy versus supportive therapy for viral myocarditis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: patients with viral myocarditis | ||||||
| Settings: inpatients and/or outpatients | ||||||
| Intervention: Herbal medicines plus supportive therapy versus supportive therapy | ||||||
|
| ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) |
No of Participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
|
|
||||||
| Assumed risk | Corresponding risk | |||||
|
|
||||||
| Control | Herbal medicines plus supportive therapy vers- us supportive therapy |
|||||
|
Myocardial enzyme CPK
levels (U/L) - Astragalo- side injection plus sup- port therapy versus sup- port therapy U/L |
The mean Myocardial en- zyme CPK levels (U/L) - Astragaloside injection plus support therapy ver- sus support therapy in the intervention groups was 21.54 lower (33.8 to 9.28 lower) |
120 (2 studies) |
 very low1,2,3,4 |
|||
|
| ||||||
|
Myocardial enzyme CPK
levels (U/L) - Shortscape fleabane injection plus support therapy versus support therapy |
The mean Myocardial en- zyme CPK levels (U/L) - Shortscape fleabane in- jection plus support ther- apy versus support ther- apy in the intervention groups was 41.73 lower (67 to 16.46 lower) |
83 (1 study) |
 low1,3,5 |
|||
|
| ||||||
|
Myocardial enzyme LDH
lev- els (U/L) - Shengyangy- ixin Decoction plus sup- port therapy versus sup- port therapy |
The mean Myocardial en- zyme LDH levels (U/L) - Shengyangyixin Deco- ction plus support therapy versus support therapy in the intervention groups was 186.63 lower (215.02 to 158.24 lower) |
76 (1 study) |
 low1,4,5 |
|||
|
| ||||||
|
Myocardial enzyme LDH
levels (U/L) - Astragalo- side injection plus sup- port therapy versus sup- port therapy |
The mean Myocardial en- zyme LDH levels (U/L) - Astragaloside injection plus support therapy ver- sus support therapy in the intervention groups was 30.33 lower (46.78 to 13.88 lower) |
120 (2 studies) |
 very low1,2,3,4 |
|||
|
| ||||||
|
Myocardial enzyme LDH
levels (U/L) - Shortscape fleabane injection plus support therapy versus support therapy |
The mean Myocardial en- zyme LDH levels (U/L) - Shortscape fleabane in- jection plus support ther- apy versus support ther- apy in the intervention groups was 29.28 lower (57.82 to 0.74 lower) |
83 (1 study) |
 very low1,3,4,5 |
|||
|
| ||||||
|
Symptomscores - Short-
scape fleabane injec- tion plus support therapy versus support therapy |
The mean Symptom scores - Shortscape flea- bane injection plus sup- port therapy versus sup- port therapy in the inter- vention groups was 1.41 lower (2.23 to 0.59 lower) |
83 (1 study) |
 low1,4,5 |
|||
|
Cardiac function LVET
(%) - Astragaloside in- jection plus support therapy versus support therapy |
The mean Cardiac func tion LVET (%) - Astraga loside injection plus sup port therapy versus sup port therapy in the inter vention groups was 4.67 higher (1.94 to 7.4 higher) |
98 (1 study) |
 low1,3,4 |
|||
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval;
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
No information about blinding.
Studies yield widely differing estimates of effect.
The confidence interval is wide.
Outcomes were selectively reported.
No information about allocation concealment.
Herbal medicines plus supportive therapy versus supportive therapy for viral myocarditis
Patient or population: patients with viral myocarditis
Settings: inpatients and/or outpatients
Intervention: Herbal medicines plus supportive therapy versus supportive therapy
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) |
No of Participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Control | Herbal medicines plus supportive therapy ver- sus supportive therapy |
|||||
|
Cardiac function LVET (%) - Astragalus mem- branaceus plus support therapy versus support therapy |
The mean Cardiac func- tion LVET (%) - As- tragalus membranaceus plus support therapy ve- rsus support therapy in the intervention groups was 12 higher (5.06 to 18.94 higher) |
50 (1 study) |
 low1,2,3 |
|||
|
Myocardial enzyme CK- MB levels (U/L) - As- tragalus membranaceus plus support therapy versus support therapy |
The mean Myocardial en- zyme CK-MB levels (U/ L) - Astragalus mem- branaceus plus support therapy versus support therapy in the intervention groups was 16.3 lower (19.66 to 12.94 lower) |
50 (1 study) |
 low1,2,3 |
|||
|
Myocardial enzyme CK- MB levels (U/L) - Shengyangyixin Decoc- tion plus support therapy versus support therapy |
The mean Myocardial en- zyme CK-MB levels (U/ L) - Shengyangyixin De- coction plus support ther- apy versus support ther- apy in the intervention groups was 3.3 lower (3.74 to 2.86 lower) |
76 (1 study) |
 low1,2,3 |
|||
|
Myocardial enzyme CK- MB levels (U/L) - Short- scape fleabane injec- tion plus support therapy versus support therapy |
The mean Myocardial en- zyme CK-MB levels (U/L) - Shortscape fleabane in- jection plus support ther- apy versus support ther- apy in the intervention groups was 5.81 lower (11.34 to 0.28 lower) |
83 (1 study) |
 low1,2,4 |
|||
|
scores of quality of life measured by SF-36 - Compound Qiangqi pill plus supportive therapy versus supportive the- rapy |
The mean scores of qual- ity of life measured by SF- 36 - Compound Qiangqi pill plus supportive ther- apy versus supportive therapy in the intervention groups was 88.35 higher (68.01 to 108.69 higher) |
80 (1 study) |
 moderate4 |
|||
|
scores of quality of life measured by SF- 36 - Shengmai decoction plus supportive therapy versus supportive ther- apy |
The mean scores of qual- ity of life measured by SF- 36 - Shengmai decoction plus supportive therapy versus supportive therapy in the intervention groups was 40.2 higher (18.13 to 62.27 higher) |
80 (1 study) |
 high |
|||
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval;
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
No information about blinding.
No information about allocation concealment.
Outcomes were selectively reported.
The confidence interval is wide.
DISCUSSION
The present systematic review suggests that some herbalmedicines may appear to have positive effects in patients with suspected viral myocarditis.
Astragalus membranaceus plus supportive therapy was effective on symptomimprovement, improvedmyocardial enzymes, abnormal electrocardiogram and cardiac function. Shengmai injection was effective on symptom improvement. Shortscape Fleabane , Xinshu Capsule, Compound Qiangqi pill, Qi Lu Decoction, Shengyangyixin Decoction, and Qingxinhuoming Decoction appeared to be effective in improving symptoms as well as cardiac function, electrocardiogram, and/or myocardial enzymes. However, at present there is no strong evidence to recommend any of these herbal medicines for the treatment of viral myocarditis due to the weak methodological quality of the trials, lack of confirmed diagnosis, the variations of the population, the regimens and duration of the herbalmedicines tested, and the outcomes reported. More specifically, the positive findings should be interpreted conservatively due to the following facts:
Risk of bias
All the randomised trials included in this review had risk of bias in terms of design, reporting, and methodology. They provided only limited descriptions of study design, randomisation, and baseline data. Some of the trials reported skewed distribution of data,which cannot be explained by the randomisation principle. Methodologically poorly designed trials show larger differences between experimental and control groups than those conducted rigorously (Schulz 1995; Moher 1998; Kjaergard 2001). The insufficient number of trials prohibited us from performing meaningful sensitivity analysis to illuminate how robust the results of the review are to the exclusion of the trials with inadequate methodology. The included trials were heterogeneous in the populations (adults, children, or mixture with acute or undefined viral myocarditis), interventions (few herbal medicines tested more than twice), and the reported outcomes. No multi-centre, large scale RCTs were identified.
Publication bias
Although we conducted comprehensive searches, we only identified and included trials which were conducted and published in Chinese. Most of the trials are small, with positive findings. We tried to avoid language bias and location bias, but we could not exclude potential publication bias. Vickers and colleagues (Vickers 1998) found that some countries including China publish unusually high proportions of positive results within the complementary medicine field. Publication bias may be a possible explanation. We have undertaken extensive searches for unpublished material, few trials of the identified qualified for inclusion, but at the same time we cannot disregard the fact that trials with negative findings remain unpublished.
Diagnostic criteria
No trial used endomyocardial biopsy (the gold standard) for diagnosis of viral myocarditis. Most of the trials made their diagnosis based on the national conference consensus on diagnosis of viral myocarditis, which basically conforms with the international recommended criteria. No trials reported aetiological confirmation. Therefore, the participants in the included trials are considered as ‘suspected’ viral myocarditis. Due to the fact of lack in information about diagnosis of acute and chronic types with subgroup outcomes reported as well as electrocardiogram diagnosis, we could not perform pre-specified subgroup analyses on diagnosis.
Interventions
There are wide variations among tested herbal medicines and control interventions. No trial used placebo control. The herbal medicines were compared with supportive therapy or added to supportive therapy compared with supportive therapy alone. Even for a same herbal intervention, it is still different in the treatment regimens including the dosage, co-interventions, and duration. Therefore, it is difficult to undertake subgroup analyses to explore factors that may affect these effects. There is still a lack of information about quality standard for the development of the herbal preparations or for themanufacture of the herbal products. Future trials should provide information about standardisation including compositions, quality control, detailed regimen, and fixed duration of treatment.
Surrogate outcomes
The primary goal of treatment for viral myocarditis is to prevent death or progression to complications. Only one trial reported death in patients with viral myocarditis (Yang YX 2008). Other outcomes from the included trials are mainly symptom improvement, myocardial enzyme, electrocardiogram, cardiac function, i.e. surrogate outcomes. Only one trial reported outcome of quality of life. There is a lack of data from most trials on clinically relevant outcomes such as mortality, incidence of complications, and quality of life. There were 109 randomised trials on herbal medicines in viral myocarditis excluded from this review. The main reasons are inadequate reporting of the outcomes and methods of sequence generation. Most of the excluded studies reported a global improvement of outcomes combined of symptoms and signs, electrocardiogram, and/or myocardial enzymes. Data from individual outcome is not available.
Nevertheless, herbal medicines are widely used for treating viral myocarditis in China. We have identified more than 100 randomised trials on this topic until now. However, over half of them are not eligible for the review due to inadequate design, conducting, and reporting of the trials. Chinese researchers must be aware of the need to design and use appropriate statistical power in future randomised controlled trials of herbal medicines and to measure clinical outcomes rather than physiological (surrogate) outcomes.
Adverse outcomes
There is inadequate reporting on adverse events in the included trials. Only two trial reported results about adverse effects (Tan YB 2003; Zheng RF 2005). A conclusion about the safety of herbal medicines cannot be made. In China, there is a general perception that it is safe to use herbal medicines for various conditions and some studies support this perception (Haines 2008; May 2009; Ramesh 2009;Wang 2009;). The low level of reporting on adverse events may reflect this. However, there are some reports of liver toxicity and other adverse events associated with using Chinese herbal medicines (Ishizaki 1996; Melchart 1999; Gottieb 2000; Pinn 2001; Liu 2006). For this reason, safety of herbal medicines needs to be monitored and reported in clinical trials.
AUTHORS’ CONCLUSIONS
Implications for practice
Based on this systematic review, the effectiveness and safety of herbal medicines in suspected viral myocarditis is uncertain. The evidence is inconclusive due to poorly designed and low quality trials and uncertain diagnosis of viral myocarditis. Further randomised trials with robust methodology are warranted in future.
Implications for research
Future research needs to emphasise not only good clinical trial methods, but also more rigorous description of the pharmacology of the interventions and histological diagnosis of the myocarditis. Trials on Chinese herbal medicines for viral myocarditis should be designed in order to meaningfully record clinical outcomes.
From the results of the present review, it would be interesting to evaluate preparations of Astragalus membranaceus and Shengmai in comparing with supportive therapy in patients of established viral myocarditis. Information about species, geographical origin of herbs, season for collecting, quality of the preparations should be provided. Standardised monitoring and reporting should be used for assessment of adverse events.
Future research should also pay attention to the quality of the trials. To improve the quality of future trials, we suggest that all researchers should receive necessary training on clinical trials methodology before designing a trial and registered the trial in authorised registered organizations. And the following methodological issues should be particularly addressed: (i) methods used to generate random sequence; (ii) methods used to allocation concealment; (iii) double blindingwith the use of adequate placebo. In the stage of data analysis, clear descriptions of withdrawal/dropout during the trial and use of intention-to-treat analysis were preferred. We suggest that the authors report trials according to the CONSORT 2010 Statement (Schulz 2010).
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Trial design: Parallel design. Study duration: March 2004 to March 2005. Intention-to-treat analyses: no. Baseline comparability: no description. Statistics: adequate (chi2 and t test). |
|
| Participants | Ethnic: Chinese 120 patients (60 in herb group, M/F 36/24, mean age 35.26 years, ranging from 16 to 46 years, duration of disease from 1 week to 3 months; 60 in control group, M/F 35/ 25, mean age 35.12, ranging from 16 to 47 years, duration of disease from 1 week to 3 months) Setting: outpatients and inpatients Diagnostic criteria: viral myocarditis, by the national criteria in 1995 Exclusion criteria: specified |
|
| Interventions | Experimental: Xinshu Capsule (herb mixtures), 10g, three times a day, orally Control: Conventional treatment: Co-enzyme Q10 capsule, 20mg, three times a day, orally Duration of treatment: 30 days |
|
| Outcomes | Symptoms and signs, electrocardiogram, myocardial enzyme The outcomes were measured at the end of treatment |
|
| Notes | No information on the periods of follow-up. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number table |
| Allocation concealment? | Unclear | No information about concealment |
| Blinding? All outcomes |
Unclear | No information about blinding |
| Incomplete outcome data addressed? All outcomes |
Yes | Same number of participants randomised and analysed |
| Free of selective reporting? | Yes | Data collection clearly described and re- ported in results |
| Free of other bias? | Unclear | Insufficient information to make judge- ments |
| Methods | Trial design: Parallel design. Study duration: Jannuary 2003 to October 2005. Intention-to-treat analyses: no. Baseline comparability: basic characters (P > 0.05). Statistics: adequate (chi2 test). |
|
| Participants | Ethnic: Chinese; 111 patients (57 in herb group, M/F 35/22, mean age 31.4 ± 8.1 years, mean disease duration 1.43 ± 9.6 days, 50 in cardiac function II group, 7 in cardiac function III- IV group; 54 in control group, M/F 34/20, mean age 30.3 ± 6.5 years, mean disease duration 15.2 ± 10.1 days, 48 in cardiac function II group, 6 in cardiac function III-IV group). Setting: inpatients. Diagnostic criteria: viral myocarditis, by the national criteria in 1999. Exclusion criteria: not specified. |
|
| Interventions | Experimental: Qingxinkangyan yin decoction (herb mixtures), 10ml/time, three times a day, for 30 days, plus conventional treatment Control: Conventional treatment: Oral Liquid Ribavirin, 0.1g/time, 4 times a day, conventional nursing. Co-invention: anti-arrhythmia when necessary. Duration of treatment: 30 days |
|
| Outcomes | Symptoms and signs, electrocardiogram, and chest-x ray, cardiac function,myocardial enzyme, troponin (I or T), LDH, serum CVB-IgM, CK, CK-MB, glutamic oxaloacetic transaminase enzyme. The outcomes were measured at the end of treatment and follow-up. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number table |
| Allocation concealment? | Unclear | No information about concealment |
| Blinding? All outcomes |
Unclear | No information about blinding |
| Incomplete outcome data addressed? All outcomes |
Yes | Same number of participants randomised and analysed |
| Free of selective reporting? | No | No information on electrocardiogram, chest-x ray, LDH, CK tropin, CK-MB |
| Free of other bias? | Unclear | Insufficient information to make judge- ments |
| Methods | Trial design: Parallel design. Study duration: March 2002 to Febuary 2006. Intention-to-treat analyses: no. Baseline comparability: age, gender, duration of disease, electrocardiogram, patient his- tory (P > 0.05). Statistics: adequate (chi2 test and t test). |
|
| Participants | Ethnic: Chinese; 76 patients (38 in herb group, M/F 23/15, mean age 31.7 ± 7.7 years, mean disease duration 61.6days, ranging from 16 to 92 days; 38 in control group, M/F 24/14, mean age 32.4 ± 7.8 years, mean disease duration 60.9 days, ranging from 17 to 91 days). Setting: inpatients. Diagnostic criteria: viral myocarditis, by the national criteria in 1999. Exclusion criteria: not specified. |
|
| Interventions | Experimental: Sheng Yang Yi Xin decoction (herb mixtures), 1 dose, 3 times/day, for 3 weeks, plus conventional treatment Control: Conventional treatment: Vitamin C 5g, 10%250ml glucose, 100U co-enzyme A, 40mg ATP, 400mg Inoine, intravenously, daily. GIK (10% 500ml glucose, 12U insulin, 10% 1g K3N), , intravenously, daily. Co-enzyme Q10 capsule 40mg, Moroxydine 100mg, oral, 3 times/day. Duration of treatment: 21 days |
|
| Outcomes | Symptoms and signs, electrocardiogram, myocardial enzyme. The outcomes were measured at the end of treatment. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number table |
| Allocation concealment? | Unclear | No information about concealment |
| Blinding? All outcomes |
Unclear | No information about blinding |
| Incomplete outcome data addressed? All outcomes |
Yes | Same number of participants randomised and analysed |
| Free of selective reporting? | No | Some outcomes were reported but not pre- specified |
| Free of other bias? | Unclear | Insufficient information to make judge- ments |
| Methods | Trial design: Parallel design Study duration: December 1999 to June 2004 Intention-to-treat analyses: no Baseline comparability: gender, duration of diagnosis and severity of disease, indexes of symptoms, time of occurrence (P > 0.05) Statistics: adequate (Ridit analysis, Chi2 , U and t test) |
|
| Participanst | Ethnic: Chinese; 127 patients (65 in herb group, 62 in control group). Setting: inpatients and outpatients. Diagnostic criteria: no description. Exclusion criteria: not specified. |
|
| Interventions | Experimental: Sheng Mai injection(herb extracts), 20 - 40ml, 5% 250 glucose or 0.09% 250 physiolo- gical salt solution, intravenously, daily, 20 - 45 days Control: Conventional treatment: Energy Mixture, 50U co-enzyme A, 200mg ATP, 4U insulin, 10% 250 glucose, intravenously, daily. 1 month. Duration of treatment: 30 days |
|
| Outcomes | Symptoms and signs, electrocardiogram, myocardial enzyme. The outcomes were measured after 1 month. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number table |
| Allocation concealment? | Unclear | No information about concealment |
| Blinding? All outcomes |
Unclear | No information about blinding |
| Incomplete outcome data addressed? All outcomes |
Yes | Same number of participants randomised and analysed |
| Free of selective reporting? | No | The pre-specified outcome of myocardial enzyme was not reported |
| Free of other bias? | Unclear | Insufficient information to make judge- ment |
| Methods | Trial design: Parallel design Study duration: June 1999 to June 2002 Intention-to-treat analyses: no Baseline comparability: age, gender, disease condition, duration of diagnosis, symptoms, signs, results of electrocardiogram and myocardial enzymes (P > 0.05) Statistics: adequate (Ridit analysis and chi2 test) |
|
| Participants | Ethnic: Chinese; 60 patients (30 in herb group, 30 in control group) Setting: inpatients. Diagnostic criteria: viral myocarditis, by the national criteria in 1994. Exclusion criteria: not specified. |
|
| Interventions | Experimental: Radix Astragali injection (herb extract), 0.5ml/kg (no more than 20ml daily) in 100 - 250ml of 10% glucose, intravenously, daily, for 21 days, plus supportive treatment. Control: Supportive treatment: rest, anti-inflammatory, regulate immunity treatment, and symptomatic treatments, high dose glucose-insulin-potassium solutions, intravenously, daily, for 21 days. Duration of treatment: 21 days |
|
| Outcomes | Symptoms, signs, myocardial enzyme and electrocardiogram,monitoring blood-routine, urine-routine, stool-routine, hepatic and renal function. The outcomes were measured at the end of treatment. |
|
| Notes | There were no basic characteristics of the patients in intervention group and control group. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Drawing lots |
| Allocation concealment? | Unclear | No information about concealment |
| Blinding? All outcomes |
Unclear | No information about blinding |
| Incomplete outcome data addressed? All outcomes |
Yes | Same number of participants randomised and analysed |
| Free of selective reporting? | Yes | Data collection clearly described and re- ported in results |
| Free of other bias? | Unclear | Insufficient information for making judge- ment |
| Methods | Trial design: Parallel design Study duration: August 1999 to December 2003 Intention-to-treat analyses: no Baseline comparability: age, gender, duration of diagnosis and severity of disease (P > 0.05) Statistics: adequate (Ridit analysis and t test used for ‘overall improvement‘) |
|
| Participants | Ethnic: Chinese; 83 patients (43 in herb group, M/F 19/24, mean age 29.94 years (from 18 - 43), mean disease duration 30.56 days, ranging from 3 to 52 days); 40 in control group, M/F 18/ 22, mean age 27.35 years (from 17 - 41), mean disease duration 32.32 days, ranging from 5 to 59 days). Setting: inpatients. Diagnostic criteria: viral myocarditis, by the national criteria in 1999. Exclusion criteria: specified. |
|
| Interventions | Experimental: Shortscape fleabane injection (herb extract), 30ml in 250 ml of 5% glucose, intravenously, daily, for 15 days, plus supportive treatment. Control: Supportive treatment: 500 ml of 10% glucose plus 8 U insulin and Vitamin C 2g, co- enzyme A 100u, intravenously, daily, for 15 days. Co-intervention: supportive therapy arrhythmia. Duration of treatment: 15 days |
|
| Outcomes | Traditional Chinese symptom indexes, myocardial enzyme and electrocardiogram. The outcomes were measured at the end of treatment. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number table |
| Allocation concealment? | Unclear | No information about concealment |
| Blinding? All outcomes |
Unclear | No information about blinding |
| Incomplete outcome data addressed? All outcomes |
Yes | Same number of participants randomised and analysed |
| Free of selective reporting? | Yes | Data collection clearly described and re- ported in results |
| Free of other bias? | Unclear | Insufficient information for making judge- ment |
| Methods | Trial design: Parallel design. Study duration: January 2005 to Febuary 2007. Intention-to-treat analyses: no. Baseline comparability: age, sex (P > 0.05). Statistics: adequate( u or Chi2 test). |
|
| Participants | Ethnic: Chinese 50 patients (25 in herb group, M/F 12/13, age no more than 12 years; 25 in control group, M/F 14/11, age no more than 12 years) Setting: inpatients Diagnostic criteria: viral myocarditis, by the national criteria 1999 Exclusion criteria: not specified. |
|
| Interventions | Experimental: Radix astragali (herb extracts), infantile, 2g/time, children, 4g/time, twice a day, for 14 days, plus conventional treatment. Control: Conventional treatment: Vitamin C, 1,6-FDP, co-enzyme A 100U, ATP, for 14 days. Duration of treatment: 14 days |
|
| Outcomes | Symptoms, chest-x ray, electrocardiogram,myocardial enzyme (CK-mB), cardiac tro- ponin I (cTnI), immunoglobulin, T-cells, adverse effects, CoX virus IgM. The outcomes were measured at the end of treatment. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number table |
| Allocation concealment? | Unclear | No information about concealment |
| Blinding? All outcomes |
Unclear | No information about blinding |
| Incomplete outcome data addressed? All outcomes |
Yes | No missing outcome data |
| Free of selective reporting? | Yes | Data collection clearly described and re- ported in results |
| Free of other bias? | Unclear | Insufficent information to make judge- ment |
| Methods | Trial design: Parallel design Study duration: June 2004 to March 2006 Intention-to-treat analyses: no Baseline comparability: no statistical testing Statistics: adequate (Chi2 test) |
|
| Participants | Ethnic: Chinese 218 patients ( age from 14 to 50) enrolled, 164 analysed Setting: inpatients Diagnostic criteria: viralmyocarditis, by the national criteria in 1995 and 1999, duration of disease less than 3 months Exclusion criteria: not specified. |
|
| Interventions | Experimental: Radix astragali injection (herb extract), 40g in 250 ml of 5% glucose, intra- venously, daily, for 14 days. Control: Supportive treatment: 500 ml of 5% glucose plus 6U insulin and 10% kcl 10ml, intravenously, daily, for 14 days. Co-intervention: after the first 14 days, Vitamin C 0.2g, co-enzyme Q10, three times per day, until the end of 6 months’ follow-up. Duration of treatment: 14 days |
|
| Outcomes | Symptoms, electrocardiogram, and chest-x ray, cardiac function,myocardial enzyme, troponin (I or T), serum CVB-IgM or CVB-Ab, blood EVs-RNA, hepatic function, antibody and antigen of hepatitis A, B AND C. The outcomes were measured at the end of follow-up. |
|
| Notes | There were no basic characters of the patients in intervention group and control group. Only 139 of the 164 patients were analysed on symptom improvements, which was not specified by the investigators. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | random number table |
| Allocation concealment? | Unclear | No information about concealment |
| Blinding? All outcomes |
Unclear | No information about concealment |
| Incomplete outcome data addressed? All outcomes |
Yes | Number of participants with loss to follow up reported, 54/218. |
| Free of selective reporting? | No | Results of chest-x ray, myocardial enzyme and troponin (I or T) were not reported. |
| Free of other bias? | Unclear | Insufficient information for making judge- ment |
| Methods | Trial design: Parallel design. Study duration: January 2002 to December 2003. Intention-to-treat analyses: no. Baseline comparability: age, gender and duration of diagnosis (P > 0.05). Statistics: inadequate (No description in the methods). |
|
| Participants | Ethnic: Chinese 168 patients (86 in herb group, M/F 49/37; 82 in control group, M/F 47/35), duration of disease no more than 90 days Setting: inpatients Diagnostic criteria: viral myocarditis by the national criteria in 1995 Exclusion criteria: duration of disease more than 90 da |
|
| Interventions | Experimental: Qi Lu decoction (herb mixtures), daily, for 15 days, plus conventional treatment Control: Conventional treatment: rest, anti-inflammatory regulate immunity treatment, drugs including Vitamin C 0.2g, co-enzyme Q10 20mg, 3 times/day, Metoprolol when necessary. Duration of treatment: 15 days |
|
| Outcomes | Symptoms,signs and electrocardiogram. The outcomes were measured at the end of treatment. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number table |
| Allocation concealment? | Unclear | No information about concealment |
| Blinding? All outcomes |
Unclear | No information about blinding |
| Incomplete outcome data addressed? All outcomes |
Yes | Same number of participants randomised and analysed |
| Free of selective reporting? | No | Information on electrocardiogramwere not reported |
| Free of other bias? | Unclear | Insufficient information to make judge- ment. |
| Methods | Trial design: Parallel design Study duration: January to December 2004 Intention-to-treat analyses: no Baseline comparability: age, gender, duration of diagnosis and severity of disease (P > 0.05) Statistics: adequate (Chi2 and t test) |
|
| Participants | Ethnic: Chinese; 40 patients (20 in herb group, M/F 13/7, age from 1 year 8 months to 11 years; 20 in control group, M/F 14/6, age from 2 years 5 months to 12 years). Setting: inpatients and outpatients. Diagnostic criteria: viral myocarditis by the national criteria in 1999. Exclusion criteria: not stated. |
|
| Interventions | Experimental: Radix Astragali injection (herb extract), 5-10ml in 100-150 ml of 5% glucose, intravenously, daily, for 14 days, plus supportive treatment. Control: Supportive treatment: rest, anti-inflammatory regulate immunity treatment, drugs including Vitamin C, Energy Mixture, Vitamin E, co-enzyme A. Duration of treatment: 14 days |
|
| Outcomes | Symptoms, myocardial enzyme and electrocardiogram. The outcomes were measured at the end of treatment. |
|
| Notes | Detailed information on supportive treatment were not specified. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | random number table |
| Allocation concealment? | Yes | Sealed envelops |
| Blinding? All outcomes |
Unclear | No information about Blinding |
| Incomplete outcome data addressed? All outcomes |
Yes | Same number of participants randomised and analysed |
| Free of selective reporting? | No | Information on electrocardiogramwere not reported |
| Free of other bias? | Unclear | Insufficient information for making judge- ment |
| Methods | Trial design: Parallel design. Study duration: Jannuary 1996 to October 1999. Intention-to-treat analyses: no. Baseline comparability: age, gender, antiarrhythmic drugs(P > 0.05). Statistics: adequate (Chi2 test and t test used for ‘overall improvement‘). |
|
| Participants | Ethnic: Chinese; 120 patients, duration of diagnosis less than 3months,M/F 48/72, age from14-68 years (70 in herb group; 50 in control group). Setting: inpatients. Diagnostic criteria: viral myocarditis, by the national criteria in 1995. Exclusion criteria: not stated. |
|
| Interventions | Experimental: Radix Astragali injection (herb extract), 40g in 500 ml of 5% glucose, intravenously, daily, for 2 weeks. Control: Supportive treatment: 500ml of 5%glucose plus 6 Uinsulin and 10ml KCl, for 2 weeks. Co-intervention: supportive therapy for arrhythmia. Duration of treatment: 14 days |
|
| Outcomes | Symptoms, electrocardiogram, and cardiac function. The outcomes were measured at the end of treatment. |
|
| Notes | There was a slightly skew distribution (1.4:1) of patients in the groups, which was not specified by the investigators. |
|
| Risk of bias | ||
| Item | Authors‘ judgement | Description |
| Adequate sequence generation? | Yes | Allocation sequence were generated using a calculator random number generator and patients were randomly allocated to treatment group(the odd numbers) and control group(the even numbers) |
| Allocation concealment? | Yes | Sealed envelops |
| Blinding? All outcomes |
Unclear | No information about blinding |
| Incomplete outcome data addressed? All outcomes |
Unclear | Insufficient information for making judge- ment |
| Free of selective reporting? | Yes | Data collection clearly described and re- ported in results |
| Free of other bias? | Unclear | Insufficient information for making judge- ment |
| Methods | Trial design: Parallel design; Study duration: November 2003 to February 2005; Intention-to-treat analyses: no; Baseline comparability: type of patient, vocation, age, gender, duration of diagnosis, type of condition, severity of disease (P > 0.05); Statistics: adequate (Chi2 test,H test,LSD). |
|
| Participants | Ethnic: Chinese;132 patients ( 81 outpatients, 51 inpatients) enrolled, 120 analysed. Setting: inpatients and outpatient; Diagnostic criteria: viral myocarditis, by the national criteria in 1999. Exclusion criteria: specified. |
|
| Interventions | Experimental: Compound Qiangqi pill ,1.5g, three times per day plus placebo of Shengmai Yin oral liquid ,10ml, three times per day, plus conventional treatment, for 1 month. Control A: Shengmai Yin oral liquid ,10ml, three times per day, plus placebo of compound Qiangqi pill ,1.5g, three times per day, plus conventional treatment, for 1 month. Control B: conventional treatment, plus placebo of Compound Qiangqi pill ,1.5g, three times per day plus placebo of Shengmai Yin oral liquid ,10ml, three times per day, for 1 month. conventional treatment: co-enzyme Q10,20mg,oral,there times per day.500 ml of 5% GS plus vitaminC6g plus ATP 40mg plus co-enzyme A 100u plus potassiummagnesium aspartate 20 ml intravenously, daily, and symptomatic treatments. Duration of treatment: 30 days |
|
| Outcomes | Symptoms, sign,electrocardiogram, urine routine, blood routine, stool routine, liver function, and renal function. The outcomes were measured at the start and end of treatment. |
|
| Notes | There were no basic characters of the patients in intervention group and control group. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Using SAS to generate random number. |
| Allocation concealment? | Yes | Sealed envelops. |
| Blinding? All outcomes |
Yes | Three blinding. |
| Incomplete outcome data addressed? All outcomes |
Yes | Number of participants with loss to follow up reported, 120/132. |
| Free of selective reporting? | Yes | Data collection clearly described and re- ported in results. |
| Free of other bias? | Unclear | Insufficient information for making judge- ment. |
| Methods | Trial design: Parallel design Study duration: January 2005 to May 2006 Intention-to-treat analyses: no Baseline comparability: age, gender, duration of diagnosis and severity of disease (P>0.05) Statistics: adequate (Chi2 and t test) |
|
| Participants | Ethnic: Chinese; 80 patients (40 in herb group, M/F 32/8, age from 2 year 8 months to 14 years; 40 in control group, M/F 30/10, age from 2 years 5 months to 13 years). Setting: inpatients and outpatients. Diagnostic criteria: viral myocarditis by the national criteria in 1999. Exclusion criteria: not stated. |
|
| Interventions | Experimental: Radix Astragali injection (herb extract), 5-10ml in 100-150 ml of 5% glucose, intravenously, daily, for 14 days, plus supportive treatment. Control: Supportive treatment: rest, anti-inflammatory regulate immunity treatment, drugs including Vitamin C, Energy Mixture, Vitamin E, co-enzyme Q10. Duration of treatment: 14 days |
|
| Outcomes | Symptoms, myocardial enzyme and electrocardiogram. The outcomes were measured at the end of treatment. |
|
| Notes | Detailed information on supportive treatment were not specified. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number table |
| Allocation concealment? | Yes | Sealed envelopes |
| Blinding? All outcomes |
Unclear | No information about blinding |
| Incomplete outcome data addressed? All outcomes |
Yes | Same number of participants randomised and analysed |
| Free of selective reporting? | No | Information on electrocardiogramwere not reported |
| Free of other bias? | Unclear | Insufficient information for making judge- ment |
| Methods | Trial design: Parallel design Study duration: No description Intention-to-treat analyses: no Baseline comparability: No description Statistics: adequate (Chi2 and rank-sum test) |
|
| Participants | Ethnic: Chinese 78 patients, M/F 32/46, mean age 23 years, ranging from 6 to 40 years (40 in herb group, 38 in control group) Setting: No description Diagnostic criteria: viral myocarditis by the national criteria in 1999 Exclusion criteria: not stated |
|
| Interventions | Experimental: Qing Xin HuoMing decoction (herbmixtures), one dose, three times a day, for 4 weeks, plus conventional treatment Control: Conventional treatment: rest, drugs including Vitamin C5-10g, EnergyMixture, 500ml glucose, 10U co-enzyme A, 10mg ATP, 600mg Inoine, intravenously, daily. Co-enzyme Q10 capsule, 3 times a day anti-inflammatory when necessary. Duration of treatment: 28 days |
|
| Outcomes | Symptoms,signs and electrocardiogram. The outcomes were measured at the end of treatment. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number table |
| Allocation concealment? | Unclear | No information about concealment |
| Blinding? All outcomes |
Unclear | No information about blinding |
| Incomplete outcome data addressed? All outcomes |
Yes | Same number of participants randomised and analysed |
| Free of selective reporting? | Yes | Data collection clearly described and reported in results |
| Free of other bias? | Unclear | Insufficient information to make judge- ment |
| Study | Reason for exclusion |
|---|---|
| An XF 1997 | Randomised clinical trial comparing herbal extract (Salviae miltiorrhizae injection) versus propafenone plus isoptin and amiodarone in 100 patients of viralmyocarditis with arrhythmia. Co-interventions included ribavirin, interferon, and supportive treatment. A global outcome measure including symptoms, electrocardiogram, X-ray, and laboratory tests was reported, but data for each separate parameter was not available. |
| Cao GM 1996 | Randomised clinical trial comparing Xinyikang (Kang Er Xin) oral liquid versus supportive therapy in 218 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Chen BY 1993 | Randomised clinical trial comparing three herbal mixtures (self-prescribed formulations based on differentiation of symptoms) versus supportive treatment in 349 children with viral myocarditis. The experimental intervention included three different preparations, but the outcome measures were not reported adequately. It is impossible to extract data for each individual herbal preparation. |
| Chen BY 1994 | Randomised clinical trial comparing Tongmai oral liquid (herbal formulation) versus supportive therapy in 65 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Chen H 1999 | Randomised clinical trial comparing Astragalus injection (herb extract) versus supportive therapy in 202 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Chen LJ 1997 | Randomised clinical trial comparing herbal mixture (self-prescribed Yixinyin decoction) versus ribavirin plus supportive treatment in 78 patients of viral myocarditis. The outcome measures of symptoms and signs as well as laboratory tests were inadequately reported and raw data could not be abstracted. |
| Chen SX 1992 | Randomised clinical trial comparing Kushen preparation (mixture of 7 herbs) versus interferon therapy in 33 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Feng D 1996 | Randomised clinical trial comparing herbal medicine (Xinluning) with propafenone in chronic heart diseases patients with frequent ventricular premature beat including viral myocarditis . The outcomes from viral myocarditis were not reported separately. |
| Geng J 1996 | Randomised clinical trial comparing a self-prescribed herbal formulation (composed of eight herbs) versus ATP, co-enzyme A, inosine, vitamin C, KCl, co-vitamin B, and anti-viral drug in 44 patients of acute viral myocarditis. A global outcome measure including symptoms, signs, and electrocardiogram was reported, but data for each separate parameter was not available due to inadequate reporting. |
| Gong LH 2001 | Randomised clinical trial comparing herbal extract (Astragalusmembranaceus injection) versus supportive therapy including ATP, co-enzyme A, vitamin C, and FDP in 66 patients of viral myocarditis. A global outcome measure including symptoms, signs, and electrocardiogram was reported, but data for each separate parameter was not available. |
| Gu W 1996 | Randomised clinical trial comparing Astragalus injection (herb extract) versus supportive therapy in 48 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Guo FQ 2008 | Randomised clinical trial comparing three herbal medicines versus supportive treatment in 30 children with viral myocarditis. The experimental intervention included three different preparations in two stages, but the outcome measures were not reported adequately. It is impossible to extract data for each individual herbal preparation. |
| Guo WX 2000 | Multicentre, blinded, randomised controlled trial comparing herbal extract (Xinyikang oral liquid) versus another herbal oral liquid (Qidong Yixin) in 460 patients of viral myocarditis. The control intervention did not meet the inclusion criteria of this review. |
| Han DS 1997 | Randomised clinical trial comparing herbal mixture (self-prescribed Yixin decoction) plus supportive treatment versus supportive treatment in 80 patients of viral myocarditis. The outcomes were inadequately reported and raw data could not be abstracted. |
| Han Y 2000 | Randomised clinical trial comparing Astragalus injection (herb extract) versus supportive therapy in 60 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| He AY 1999 | Randomised clinical trial comparing Shuanghuanglian powder injection (herb extract) versus supportive therapy in 120 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| He P 1995 | Randomised clinical trial comparing Astragalus (single herb) versus Propafenone therapy in 110 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methodso f allocation sequence generation were not reported. |
| Hu SY 1995 | Randomised clinical trial comparing Tongmaiye (herbal extract) versus supportive therapy in 64 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Hu SY 1999 | Randomised clinical trial comparing three herbal medicines (Qingxinye, Shuanghuanglian, and Astragalus injection) versus supportive therapy plus antiviral and antibiotics treatment in 136 children with viral myocarditis. The three herbal medicines were not equally given to the patients in experimental group, which may contribute to the confounding to the intervention. |
| Huang W 1999 | Randomised clinical trial comparing herbal decoction (BuyangHuanwuTang) versus supportive therapy including ATP, co-enzyme A, vitamin C and B6 in 90 patients of viral myocarditis. A global outcome measure including symptoms, signs, electrocardiogram, and X-ray chest was reported, but data for each separate parameter was not available due to inadequate reporting. |
| Huang ZQ 1995 | Randomised clinical trial comparing herbal extract Astragalus membranaceus plus supportive treatment versus supportive treatment alone in 54 patients with viral myocarditis. The primary objective of the trial was to explore the effect of the herb on T-lymphocyte subsets of peripheral blood. Other outcomes were not reported. |
| Huang ZQ 1996 | Randomised clinical trial comparing herbal extract Yixinling plus supportive treatment versus supportive treatment alone in 52 patients with viral myocarditis. The primary objective of the trial was to explore the effect of the herb on NK cell activity and T-lymphocyte subsets of peripheral blood. |
| Ji XL 1995 | Randomised clinical trial comparing herbal extract Xinjiyin versus supportive treatment in 108 patients with viral myocarditis. There was a significant skew distribution of patient characteristics in two groups. A global improvement as primary outcome was reported, but data for individual outcomes were not available. |
| Jia WH 1998 | Randomised clinical trial comparing Huangqi Shengmaisan (herbalmixture) decoction versus supportive therapy in 66 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Jiang Y 2000 | Randomised clinical trial comparing herbal mixture (self-prescribed and modified based on different syndromes)v ersus ribavirin plus supportive treatment in 98 patients of viral myocarditis. The outcomes were inadequately reported and raw data could not be abstracted. |
| Jin W 2002 | Randomised clinical trial comparing Shenmai injection (herb extract) versus supportive therapy in 60 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that them ethods of allocation sequence generation were not reported. |
| Kuo C 1986 | A case report of Chinese herbs for treating acute viral myocarditis. |
| Li DP 2005 | Randomised clinical trial comparing herbal extract (Qing Xin Huo Xue decoction) plus conventional treatment versus conventional treatment in 48 patients of viral myocarditis.A global outcome measure including symptoms, electrocardiogram, myocardial enzymes and laboratory tests was reported, but data for each separate parameter was not available. |
| Li JL 1999 | Randomised clinical trial comparing herbal extract (Shengmaisan combined with Guipi Tang) versus supportive therapy (KCl injection, insulin, ATP, and co-enzyme A) in 95 patients of viral myocarditis. A global outcome measure including symptoms, electrocardiogram, heart rate, heartX-ray, ultrasound assay, andmyocardial enzyme profile was reported, but data for each separate parameter were not available. |
| Li Y 2000 | Randomised clinical trial comparing herbal mixture (Erhuang Wendan decoction) versus supportive treatment in patients of acute viral myocarditis. The study was published four times with different number of patients. All the publications reported global outcome and raw data for individual parameters were not available. |
| Li YR 1996 | Randomised clinical trial comparing Shengmai injection (herb extract) versus Danshen injection (composita Salviae miltiorrhizae) in 50 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Li YW 1997 | Randomised clinical trial comparing Chinese herbal medicine (Yangxinshi) versus supportive treatment in 62 patients of viralmyocarditis.The outcomemeasures including symptoms and electrocardiogramwere inadequately reported and raw data were not available. |
| Li ZY 1998 | Randomised clinical trial comparing Astragalus injection (herb extract) versus supportive therapy in 54 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Lin GZ 1998 | Randomised clinical trial comparing Shuanghuanglian powder injection (herb extract) versus supportive therapy in 62 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| LIU AD 2005 | Randomised clinical trial comparing different herbal medicines versus conventional treatment in 127 patients with viral myocarditis. The herbal treatment was composed of 8 different herbal mixtures and prescribed based on different stages, different types of Chinese differential diagnosis. However, the outcome was not reported separately. |
| Liu GJ 1996 | Randomised clinical trial comparing herbal extract (Shenmai injection) versus supportive treatment in 45 patients of acute viral myocarditis. The outcomes were inadequately reported and raw data were not available. |
| Liu HQ 2000 | Randomised clinical trial comparing herbal mixture (Shenqiyin decoction) versus supportive treatment in 129 patients of viral myocarditis. The outcome measures included symptoms and signs, electrocardiogram, and Xray image, but were reported globally and raw data were not available. |
| Liu J 1995 | Randomised clinical trial comparing Huangqi Shengmaisan (mixture of more than 4 herbs) versus supportive therapy in 66 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Liu MD 1999 | Randomised clinical trial comparing herbal extract (Astragalusmembranaceus injection) versus supportive therapy (glucose, insulin, co-enzyme Q10, and vitamin C) in 87 patients of viral myocarditis. A global outcome measure including symptoms, signs, electrocardiogram, and cardiac enzyme profile was reported, but data for each separate parameter was not available. |
| Liu SS 1997 | Randomised clinical trial comparing Astragalus (single herb) versus supportive therapy in 63 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Liu YJ 1997 | Randomised clinical trial comparing extract of Astragalus membranaceus versus supportive treatment in 50 patients with viral myocarditis. After the randomisation, 12 patients who did not respond well to the supportive treatment was changed to receive herbal treatment. There was a confounding due to this change of intervention during trial. |
| Lu Y 1997 | Randomised clinical trial comparingDengzhanhua injection (breviscapine) versus supportive therapy in 64 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Luo L 1998 | Randomised clinical trial comparing herbal mixture (self-prescribed Wushen Jiawei Shengmai Powder) versus ribavirin plus supportive treatment in 61 patients of viral myocarditis. The outcomes were inadequately reported and raw data were not available. |
| Ma CH 1995 | Randomised clinical trial comparing herbal mixture (self-prescribed formula) plus ribavirin and supportive treatment versus supportive treatment alone in 40 patients of viral myocarditis. The outcome measures included symptoms and signs, and electrocardiogram, but were reported globally and raw data were not available. |
| Ma GL 1998 | Randomised clinical trial comparing Astragalus compound (mixture of 15 herbs) decoction versus supportive therapy in 100 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Ma HB 1997 | Randomised clinical trial comparing herbal mixture (basic formula modified according to different types of Chinese medical diagnosis) versus supportive treatment in 60 children with viral myocarditis. The outcome measures included symptoms and signs, electrocardiogram, X-ray, and cardiac spectrum of enzymes, but were reported globally and raw data were not available. |
| Ma YL 1984 | Randomised clinical trial comparing different herbal medicines versus supportive treatment in 40 patients of children with viral myocarditis. The herbal treatment was composed of 8 different herbal mixtures and prescribed based on different stages, different types of Chinese differential diagnosis, and different complications. However, the outcome was not reported separately. |
| Qin FH 2001 | Randomised clinical trial comparing herbal formulation (Xiao Chaihu Tang) versus supportive therapy (ATP, co-enzyme A, vitamin C, and FDP) in 62 patients of viral myocarditis. A global outcome measure including symptoms, signs, and electrocardiogram was reported, but data for each separate parameter were not available. |
| Qin HS 2006 | Randomised clinical trial comparing herbal extract (radix astragali) versus another herbal tablet (Composita Salviae miltiorrhizae) in 62 patients with acute viral myocarditis. The control intervention did not meet the inclusion criteria of this review. |
| Ren GH 1996 | Randomised clinical trial comparing Astragalus injection (herb extract) versus supportive therapy in 61 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Ren W 1991 | Randomised clinical trial comparing Astragalus injection (herb extract) versus supportive therapy in 66 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Rong YS 2001 | Randomised clinical trial comparing herbal medicine (decoction) plus supportive therapy versus supportive therapy (ATP, co-enzyme A, vitamin C and B6, and glucose) in 110 children with viral myocarditis. A global outcome measure including symptoms, signs, electrocardiogram and serum enzymes was reported, but data for each separate parameter were not available. |
| Song JM 1999 | Randomised clinical trial comparing Yangyin Qingxin (herbal extract) oral liquid versus supportive therapy in 119 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Su CT 1999 | Quasi-randomised clinical trial comparing herbal mixture (modified based on Shengmaisan) plus supportive treatment versus herbal extract composita Salviae miltiorrhizae injection plus supportive treatment in 36 patients of viral myocarditis. The outcome measures including symptoms and signs, electrocardiogram as well as serum enzymes were inadequately reported and raw data were not available. |
| Sun DX 2000 | Randomised clinical trial comparing Shengmai injection (herb extract) versus supportive therapy in 56 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Sun J 1998 | Randomised clinical trial comparing herbalmedicine ‘Xinankang’ versus supportive treatment in 60 patients with viral myocarditis. The outcome was reported selectively, and data from other patients were not available. |
| Sun KJ 1998 | Randomised clinical trial comparing herbal extract (Shengmai injection) versus supportive treatment in 22 patients of viral myocarditis with arrythmia at the convalescent stage. The outcome measure was reduction of arrhythmia, but only rates were reported and raw data were not available. |
| Sun WM 1999 | Randomised clinical trial comparing herbalmixture (self-prescribedWushen Jiawei Shengmaisan) plus supportive treatment versus supportive treatment alone in 102 patients of viral myocarditis. The outcomes of symptoms, cardiac function, and electrocardiogram were inadequately reported and raw data were not available. |
| Sun Y 1997 | Randomised clinical trial comparing Acanthopanacis senticosi plus Salviae miltiorrhizae versus supportive therapy in 320 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Tan JC 1995 | Randomised clinical trial comparing herbal extract (Shengmai injection) versus supportive treatment in 50 patients of acute viral myocarditis. The outcomes were inadequately reported and raw data were not available. |
| Tang SY 2000 | Randomised clinical trial comparing Qingxinkang (self-prescribed herbal extract) decoction versus supportive therapy in 67 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Tu XH 1996 | Randomised clinical trial comparing herbal extract ‘Qidong Yixin’ (oral liquid) versus another herbal extract ‘Shengmaiyin’ (oral liquid) in 500 patients of viral myocarditis. The control intervention did not meet the inclusion criteria of this review. |
| Wang JM 2003 | Randomised clinical trial comparing radix astragali(herbal extracts) injection and Meglumine Cyclic Adenylate injection (non-herbal extracts) in 68 inpatients of viral myocarditis. The intervention group did not meet the inclusion criteria of this review. |
| Wang K 2000 | Randomised clinical trial comparing herbal mixture (Huangzhihua oral liquid) plus supportive treatment versus supportive treatment alone in 66 children with viral myocarditis. The outcome measures included symptoms and signs, electrocardiogram, and/or cardiac spectrum of enzymes, but raw data for individual outcomes were not available. |
| Wang WR 2001 | Randomised clinical trial comparing herbalmixture (self-prescribed ShenyingWendan decoction) plus supportive treatment versus supportive treatment in 106 patients of viral myocarditis. The outcome measures including symptoms and signs as well as electrocardiogram, but raw data for individual outcomes were not available. |
| Wang XF 1997 | Randomised clinical trial comparing Chaihu Qingxin Yin (self-developed herbal extract) decoction versus supportive therapy in 84 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Wang XJ 1995 | Randomised clinical trial comparing single herb Astragalus versus supportive treatment in 116 patients with viral myocarditis. The outcomes were inadequately reported and raw data were not available. |
| Wang ZH 1998 | Randomised clinical trial comparing herbal extract (Astragalus membranaceus injection) versus supportive treatment in 48 patients of viral myocarditis. The objective of the trial was to explore the effect of the herbal extract on tumour necrotic factor (TNF) and interleukin-1 (IL-1) in viral myocarditis. |
| Wang ZH 2001 | Randomised clinical trial comparing Astragalus injection (herb extract) versus supportive therapy in 116 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Wang ZL 2000 | Randomised clinical trial comparing herbal extract (Chuanhuning injection) plus supportive treatment versus supportive treatment in 40 patients of viral myocarditis. The outcomes were inadequately reported and raw data were not available. |
| Wang ZM 2000 | Randomised clinical trial comparing herbal medicines (Astragalus membranaceus injection and Yangxintang) versus supportive therapy (ATP, co-enzyme Q10, insulin, and KCl) in 95 patients of viral myocarditis. A global outcome measure including symptoms, electrocardiogram, heart rate, X-ray chest,myocardial enzyme profile was reported, but data for each separate parameter were not available. |
| Wang ZT 2004 | Randomised clinical trial comparing herbal extract (Yixin decoction) plus conventional treatment versus conventional treatment in 54 patients of viral myocarditis.A global outcome measure including symptoms, electrocardiogram, and premature beat was reported, but data for each separate parameter was not available. |
| Wei QH 2004 | Randomised clinical trial comparing herbal extract Fufang Sishen Decoction treatment versus conventional treatment in 102 patients with viral myocarditis. The primary objective of the trial was to explore the effect of the herb on arrhythmia. |
| Wei YL 1998 | Randomised clinical trial comparing herbal extract (Weierxin tablet) versus another herbal tablet (Composita Salviae miltiorrhizae) in 420 children with viral myocarditis. The control intervention did not meet the inclusion criteria of this review. |
| Wu CS 1988 | Randomised clinical trial comparing Shengmai injection (herb extract) versus supportive therapy in 100 patientso f viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Wu XN 2002 | Randomised clinical trial comparing herbal mixture (self-prescribed decoction) plus supportive treatment versus supportive treatment in 44 patients of acute viral myocarditis. The outcomes were inadequately reported and raw data were not available. |
| Xia DC 2000 | Randomised clinical trial comparing herbal mixture (self-prescribed Qinggong decoction) versus supportive treatment in 60 patients of acute viral myocarditis. The outcomes were inadequately reported and raw data were not available. |
| Xing YH 1998 | Randomised clinical trial comparing Royal jelly (non-herbal medicine) versus supportive treatment in 50 patients of viral myocarditis. The intervention did not fall into our category. |
| Xu MM 2000 | Randomised clinical trial comparing Chinese herbs (mixture of herbs) decoction versus supportive therapy in 102 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Xu T 1996 | Randomised clinical trial comparing Composita Salviae miltiorrhizae injection (extract of herbs) versus supportive therapy in 86 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Yang CJ 2005 | Randomised clinical trial comparing herbal mixtures (Buqishengmai decoction) plus conventional treatment versus conventional treatment in 35 patients of viral myocarditis. A global outcome measure including symptoms, electrocardiogram, and laboratory tests was reported, but data for each separate parameter was not available. |
| Yang FQ 1998 | Randomised clinical trial comparing herbal mixture (self-prescribed Qingxin Jiedu formula) plus herbal extract (Shengmai injection) versus ribavirin and corticosteroid plus supportive treatment in 61 young children with viral myocarditis. The outcomes were inadequately reported and raw data were not available. |
| Yang GF 2002 | Randomised clinical trial comparing herbal mixture Yiqi Yangxin decoction (self-prescribed formula) plus supportive treatment versus supportive treatment in 87 patients of viralmyocarditis. The outcomes were inadequately reported and raw data were not available. |
| Yang HB 1997 | Randomised clinical trial comparing herbal extract (Shengmai injection) versus supportive treatment in 100 patients of viral myocarditis. The outcomes were inadequately reported and raw data were not available. |
| Yang SJ 1997 | Randomised clinical trial comparing herbal extract (Shenmai injection) versus supportive treatment in 120 patients of acute viral myocarditis. The outcome measures included symptoms and signs, and laboratory tests, but were reported globally and raw data were not available. |
| Yang YZ 1990 | Non-randomised controlled study testing Astragalus membranaceus in treating patients with coxsackie B viral myocarditis with depressed natural killer activity compared with conventional therapy. |
| Yao ZP 1995 | Randomised clinical trial comparing herbal extract (Qingkailing) versus supportive treatment plus antibiotics and Poly I:C in 31 patients with acute viral myocarditis. The outcome measures included symptoms and signs, electrocardiogram, and laboratory tests, but were reported globally and raw data were not available. |
| Yin YS 1997 | Randomised clinical trial comparing Shengmai injection (herb extract) versus supportive therapy in 162 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that them ethods of allocation sequence generation were not reported. |
| Yu ZK 1996 | Randomised clinical trial comparing Yiqi Yangyin Jiedu Huayu (mixture of herbs) decoction versus supportive therapy in 61 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Zeng CF 1997 | Randomised clinical trial comparing Astragalus membranaceus (herb powder) versus supportive therapy in 45 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Zhang DF 2005 | Randomised clinical trial comparing FDP, astragalus injection(herbal extracts) versus supportive therapy in 60 patients of viral myocarditis. The intervention did not meet the inclusion criteria of this review. |
| Zhang PY 1997 | Randomised clinical trial comparing herbal medicines (Qingkailing injection plus Shengmai injection) plus supportive therapy and ribavirin versus supportive therapy and ribavirin in 160 patients of viralmyocarditis (acute phase). A global outcome measure including symptoms, signs, X-ray chest and electrocardiogram was reported, but data for each separate parameter were not available. |
| Zhang SY 2000 | Randomised clinical trial comparing Salviae miltiorrhizae injection (extract of herbs) versus supportive therapy in 68 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Zhang XL 1999 | Randomised clinical trial comparing Qidong Yixin (extract of herbs) oral liquid versus supportive therapy in 60 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Zhang XM 2000 | Randomised clinical trial comparing combined herbal medicines (Astragalus injection and composita Salviae miltiorrhizae injection) with supportive treatment versus supportive treatment in 68 patients of acute viral myocarditis. The outcome measures including symptoms, electrocardiogram, myocardiac enzymes, cardiac X ray,u ltrasound cardiac image, and cardiac function as well as enzyme test were inadequately reported and raw data were not available. |
| Zhang ZX 2000 | Randomised clinical trial comparing Yiqi Yangxin Tang (mixture of herbs) decoction versus supportive therapy in 81 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Zhao CS 2005 | Randomised clinical trial comparing Qing Xin Tang decoction and Yan Po Nings injections(herbal extracts) injection plus conventional treatment versus conventional treatmentwhich include radix astragali injection (herbal extracts) in 72 inpatients of viral myocarditis. The control group did not meet the inclusion criteria of this review. |
| Zhao MH 1996 | Randomised clinical trial comparing Shengmaisan (mixture of herbs) granule versus placebo in 62 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Zhao QC 1996 | Randomised clinical trial comparing Ginseng (single herb) versus supportive therapy in 62 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Zhao XS 2008 | Randomised clinical trial comparing herbal extract (Yixin decoction) plus conventional treatment versus conventional treatment in 54 patients of viral myocarditis.A global outcome measure including symptoms, electrocardiogram, and laboratory tests was reported, but data for each separate parameter was not available. |
| Zhao YT 1994 | Randomised clinical trial comparing herbal extract ‘Yangxin Fumai Tang’ (self-prescribed decoction) versus ribavirin plus supportive treatment in 75 patients of viral myocarditis. The outcomes were inadequately reported and raw data were not available. |
| Zhao YZ 1998 | Randomised clinical trial comparing Yixintang (mixture of herbs) decoction versus supportive therapy in 80 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Zhou D 2004 | Randomised clinical trial comparing herbal extract (ShengMai San HeWuWei Zi decoction) plus conventional treatment versus conventional treatment in 54 patients of viral myocarditis.A global outcome measure including symptoms, electrocardiogram, and laboratory tests was reported, but data for each separate parameter was not available. |
| Zhou FR 2001 | Randomised clinical trial comparing Xinjikang (extracts of herbs) capsule versus supportive therapy in 76 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Zhou L 2000 | Randomised clinical trial comparing herbal medicine (self-prescribed Qingjie Huangmai Yin) versus supportive therapy (co-enzyme Q10, vitamin C, inosine, glucose, insulin, KCl), plus Poly I:C, and ribavirin in 120 patients of acute viral myocarditis. A global outcome measure including symptoms, signs, X-ray chest, electrocardiogram and laboratory tests was reported, but data for each separate parameter were not available. |
| Zhou MY 1996 | Randomised clinical trial comparing herbalmixture (modified based on Shengmai powder and Licorice decoction) plus supportive treatment versus supportive treatment alone in 60 patients of viral myocarditis. The outcomes of symptoms, electrocardiogram, and cardiac enzymes were inadequately reported and raw data were not available. |
| Zhou ZY 2000 | Randomised clinical trial comparing herbal preparations (Shuanghuanglian injection plus Astragalus membranaceus injection) plus supportive treatment versus supportive treatment alone in 163 patients with viral myocarditis. A global outcome including symptoms, signs, and electrocardiogram were reported and separate outcome data were not available. |
| Zhu Q 1997 | Randomised clinical trial comparing Gualou Xiebai Wenxin (extracts of herbs) oral liquid versus supportive therapy in 68 patients of viral myocarditis was included in the original review. It was excluded in the updating review due to that the methods of allocation sequence generation were not reported. |
| Zhu YD 1997 | Randomised clinical trial comparing herbal mixture (self-prescribed decoction) versus another herbal extract (Shenqi tablet) in 85 patients of viral myocarditis. The control intervention did not meet the inclusion criteria of this review. |
DATA AND ANALYSES
Comparison 1.
Herbal medicines versus supportive therapy
| Outcome or subgroup title | No. of studies |
No. of participants |
Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients without symptom improvement |
4 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Qingxinkangyan Yin Decoction versus support therapy |
1 | 111 | Risk Ratio (M-H, Fixed, 95% CI) | 0.34 [0.12, 1.02] |
| 1.2 Xinshu Capsule versus supportive therapy |
1 | 120 | Risk Ratio (M-H, Fixed, 95% CI) | 0.14 [0.04, 0.45] |
| 1.3 Astragaloside injection versus support therap |
1 | 164 | Risk Ratio (M-H, Fixed, 95% CI) | 0.77 [0.37, 1.59] |
| 1.4 Shenmai injection versus support therapy |
1 | 127 | Risk Ratio (M-H, Fixed, 95% CI) | 0.32 [0.14, 0.75] |
| 2 Abnormal ST-T changes | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Shenmai injection versus support therapy |
1 | 57 | Risk Ratio (M-H, Fixed, 95% CI) | 1.18 [0.58, 2.40] |
| 3 Number of patients died of cardiac failure |
1 | 164 | Risk Ratio (M-H, Fixed, 95% CI) | 1.22 [0.34, 4.38] |
| 3.1 Astragaloside injection versus support therapy |
1 | 164 | Risk Ratio (M-H, Fixed, 95% CI) | 1.22 [0.34, 4.38] |
| 4 Number of patients converting to persisting myocarditis |
1 | 164 | Risk Ratio (M-H, Fixed, 95% CI) | 0.65 [0.11, 3.79] |
| 4.1 Astragaloside injection versus support therapy |
1 | 164 | Risk Ratio (M-H, Fixed, 95% CI) | 0.65 [0.11, 3.79] |
| 5 Number of patients with premature beat |
1 | 120 | Odds Ratio (M-H, Fixed, 95% CI) | 0.21 [0.06, 0.79] |
| 5.1 Xinshu Capsule versus supportive therapy |
1 | 120 | Odds Ratio (M-H, Fixed, 95% CI) | 0.21 [0.06, 0.79] |
Comparison 2.
Herbal medicines plus supportive therapy versus supportive therapy
| Outcome or subgroup title | No. of studies |
No. of participants |
Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients without symptom improvement |
9 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Astragaloside injection plus support therapy versus support therapy |
4 | 300 | Risk Ratio (M-H, Random, 95% CI) | 0.38 [0.14, 1.07] |
| 1.2 Compound Qiangqi pill plus supportive therapy versus supportive therapy |
1 | 80 | Risk Ratio (M-H, Random, 95% CI) | 0.4 [0.14, 1.17] |
| 1.3 Shengmai decoction plus supportive therapy versus supportive therapy |
1 | 80 | Risk Ratio (M-H, Random, 95% CI) | 0.8 [0.35, 1.82] |
| 1.4 Qi Lu Decoction plus support therapy versus support therapy |
1 | 168 | Risk Ratio (M-H, Random, 95% CI) | 0.16 [0.04, 0.69] |
| 1.5 Shengyangyixin Decoction plus support therapy versus support therapy |
1 | 76 | Risk Ratio (M-H, Random, 95% CI) | 0.25 [0.06, 1.10] |
| 1.6 Astragalus membranaceus plus support therapy versus support therapy |
1 | 50 | Risk Ratio (M-H, Random, 95% CI) | 0.2 [0.03, 1.59] |
| 1.7 Qingxinhuoming Decoction plus support therapy versus support therapy |
1 | 78 | Risk Ratio (M-H, Random, 95% CI) | 0.36 [0.10, 1.24] |
| 2 Abnormal electrocardiogram | 5 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Astragalus membranaceus plus support therapy versus support therapy |
1 | 48 | Risk Ratio (M-H, Fixed, 95% CI) | 0.64 [0.29, 1.41] |
| 2.2 Qingxinhuoming Decoction plus support therapy versus support therapy |
1 | 78 | Risk Ratio (M-H, Fixed, 95% CI) | 0.32 [0.13, 0.79] |
| 2.3 Compound Qiangqi pill plus supportive therapy versus supportive therapy |
1 | 32 | Risk Ratio (M-H, Fixed, 95% CI) | 0.81 [0.53, 1.23] |
| 2.4 Shengmai decoction plus supportive therapy versus supportive therapy |
1 | 31 | Risk Ratio (M-H, Fixed, 95% CI) | 0.95 [0.67, 1.36] |
| 2.5 Shengyangyixin Decoction plus support therapy versus support therapy |
1 | 76 | Risk Ratio (M-H, Fixed, 95% CI) | 0.56 [0.35, 0.90] |
| 2.6 Astragaloside injection plus supportive therapy versus supportive therapy |
1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 0.25 [0.08, 0.80] |
| 3 Abnormal ST-T changes | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Astragaloside injection plus support therapy versus support therapy |
1 | 96 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.63, 1.09] |
| 4 Number of patients with premature beat |
4 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Qi Lu Decoction plus support therapy versus support therapy |
1 | 111 | Risk Ratio (M-H, Fixed, 95% CI) | 0.71 [0.50, 1.00] |
| 4.2 Shortscape fleabane injection plus support therapy versus support therapy |
1 | 83 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.67, 1.20] |
| 4.3 Astragaloside injection plus support therapy versus support therapy |
1 | 93 | Risk Ratio (M-H, Fixed, 95% CI) | 1.09 [0.69, 1.71] |
| 4.4 Compound Qiangqi pill plus supportive therapy versus supportive therapy |
1 | 14 | Risk Ratio (M-H, Fixed, 95% CI) | 0.8 [0.36, 1.77] |
| 4.5 Shengmai decoction plus supportive therapy versus supportive therapy |
1 | 14 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.52, 1.94] |
| 5 Number of patients with abnormal myocardial enzyme levels |
2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Astragalus membranaceus plus support therapy versus support therapy |
1 | 47 | Risk Ratio (M-H, Fixed, 95% CI) | 0.39 [0.12, 1.30] |
| 5.2 Astragaloside injection plus supportive therapy versus supportive therapy |
1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 0.25 [0.06, 1.08] |
| 6 Abnormal LVEF | 1 | 98 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.66, 1.23] |
| 6.1 Astragaloside injection plus support therapy versus support therapy |
1 | 98 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.66, 1.23] |
| 7 Number of patients converting to persisting myocarditis |
1 | 120 | Odds Ratio (M-H, Fixed, 95% CI) | 1.21 [0.27, 5.29] |
| 7.1 Astragaloside injection plus support therapy versus support therapy |
1 | 120 | Odds Ratio (M-H, Fixed, 95% CI) | 1.21 [0.27, 5.29] |
| 8 Myocardial enzyme CPK levels (U/L) |
3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Astragaloside injection plus support therapy versus support therapy |
2 | 120 | Mean Difference (IV, Random, 95% CI) | −21.54 [−33.80, − 9.28] |
| 8.2 Shortscape fleabane injection plus support therapy versus support therapy |
1 | 83 | Mean Difference (IV, Random, 95% CI) | −41.73 [−65.00, − 16.46] |
| 9 Myocardial enzyme LDH levels (U/L) |
4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Shengyangyixin Decoction plus support therapy versus support therapy |
1 | 76 | Mean Difference (IV, Random, 95% CI) | −186.63 [−215.02, − 158.24] |
| 9.2 Astragaloside injection plus support therapy versus support therapy |
2 | 120 | Mean Difference (IV, Random, 95% CI) | −30.33 [−46.78, − 13.88] |
| 9.3 Shortscape fleabane injection plus support therapy versus support therapy |
1 | 83 | Mean Difference (IV, Random, 95% CI) | −29.28 [−57.82, − 0.74] |
| 10 Symptom scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Shortscape fleabane injection plus support therapy versus support therapy |
1 | 83 | Mean Difference (IV, Fixed, 95% CI) | −1.41 [−2.23, -0.59] |
| 11 Cardiac function LVET (%) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Astragaloside injection plus support therapy versus support therapy |
1 | 98 | Mean Difference (IV, Fixed, 95% CI) | 4.67 [1.94, 7.40] |
| 11.2 Astragalus membranaceus plus support therapy versus support therapy |
1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 12.0 [5.06, 18.94] |
| 12 Myocardial enzyme CK-MB levels (U/L) |
3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Astragalus membranaceus plus support therapy versus support therapy |
1 | 50 | Mean Difference (IV, Fixed, 95% CI) | −16.30 [−19.66, − 12.94] |
| 12.2 Shengyangyixin Decoction plus support therapy versus support therapy |
1 | 76 | Mean Difference (IV, Fixed, 95% CI) | −3.30 [−3.74, -2.86] |
| 12.3 Shortscape fleabane injection plus support therapy versus support therapy |
1 | 83 | Mean Difference (IV, Fixed, 95% CI) | −5.81 [−11.34, -0.28] |
| 13 Scores of quality of life measured by SF-36 |
1 | 160 | Mean Difference (IV, Fixed, 95% CI) | 66.24 [51.28, 81.20] |
| 13.1 Compound Qiangqi pill plus supportive therapy versus supportive therapy |
1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 88.35 [68.01, 108.69] |
| 13.2 Shengmai decoction plus supportive therapy versus supportive therapy |
1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 40.20 [18.13, 62.27] |
Analysis 1.1.
Comparison 1 Herbal medicines versus supportive therapy, Outcome 1 Number of patients without symptom improvement.
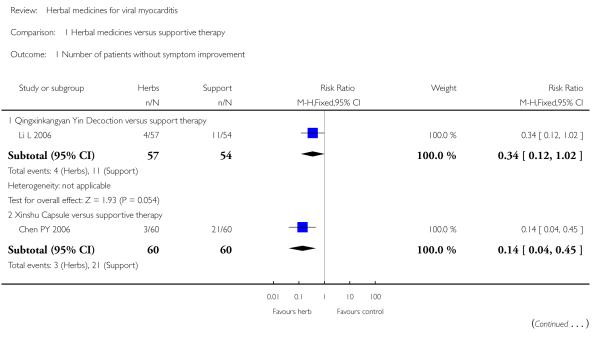
|
Analysis 1.2.
Comparison 1 Herbal medicines versus supportive therapy, Outcome 2 Abnormal ST-T changes.
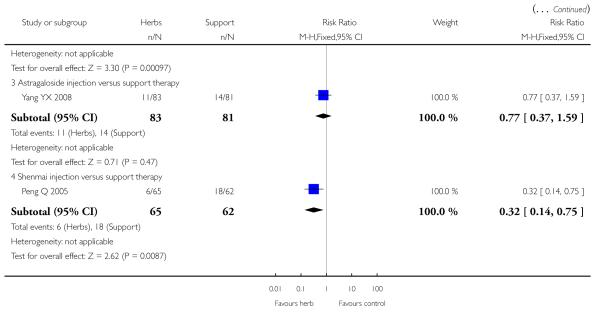
|
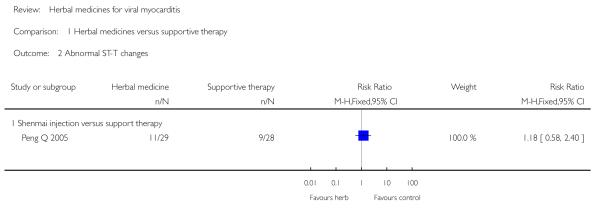
|
Analysis 1.3.
Comparison 1 Herbal medicines versus supportive therapy, Outcome 3 Number of patients died of cardiac failure.
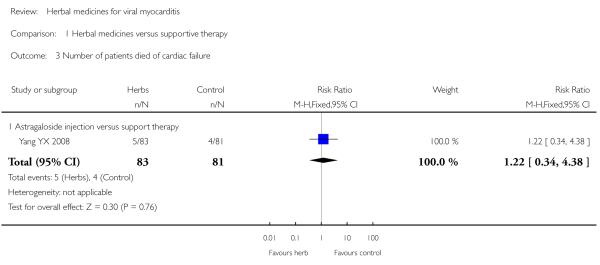
|
Analysis 1.4.
Comparison 1 Herbal medicines versus supportive therapy, Outcome 4 Number of patients converting to persisting myocarditis.
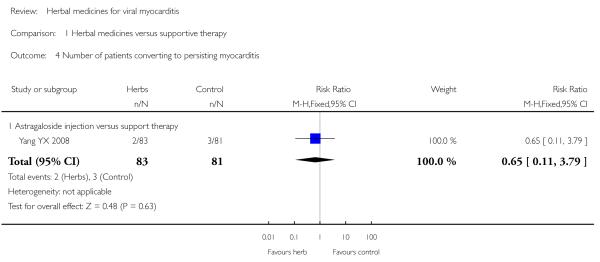
|
Analysis 1.5.
Comparison 1 Herbal medicines versus supportive therapy, Outcome 5 Number of patients with premature beat.
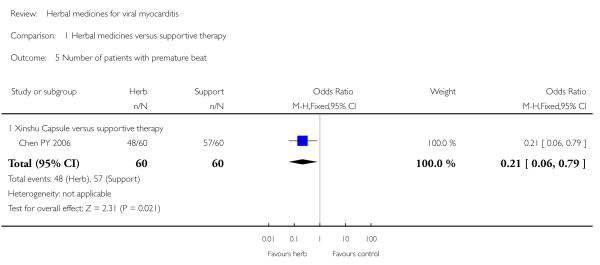
|
Analysis 2.1.
Comparison 2 Herbal medicines plus supportive therapy versus supportive therapy, Outcome 1 Number of patients without symptom improvement.
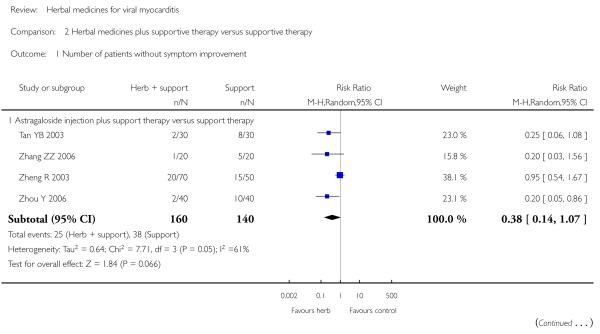
|
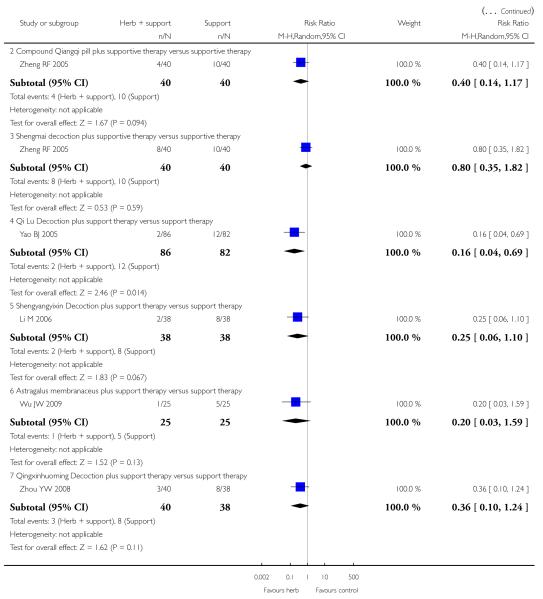
|
Analysis 2.2.
Comparison 2 Herbal medicines plus supportive therapy versus supportive therapy, Outcome 2 Abnormal electrocardiogram..
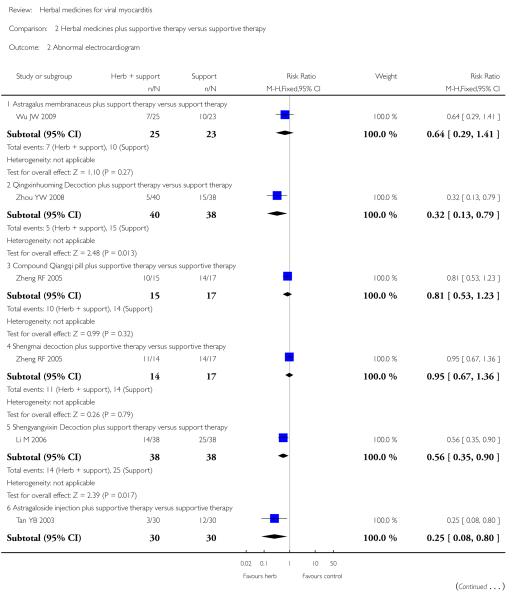
|
|
Analysis 2.3.
Comparison 2 Herbal medicines plus supportive therapy versus supportive therapy, Outcome 3 Abnormal ST-T changes
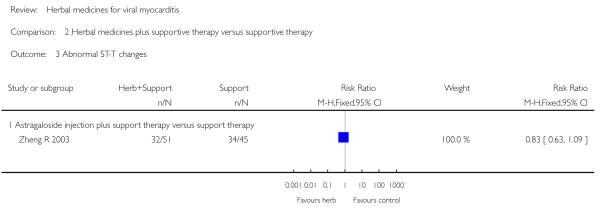
|
Analysis 2.4.
Comparison 2 Herbal medicines plus supportive therapy versus supportive therapy, Outcome 4 Number of patients with premature beat.
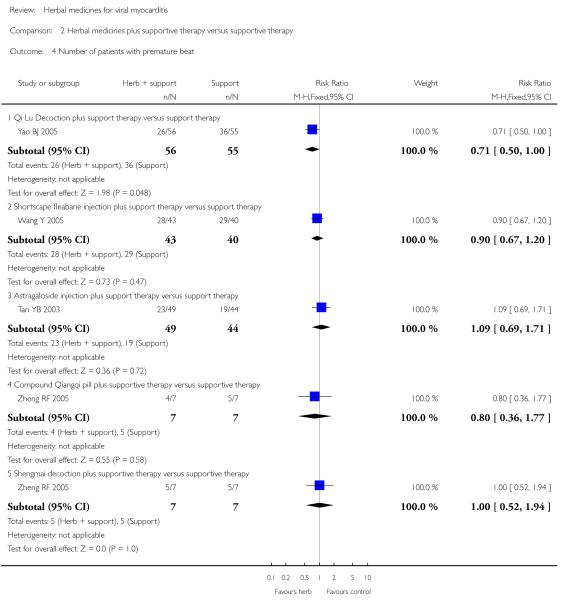
|
Analysis 2.5.
Comparison 2 Herbal medicines plus supportive therapy versus supportive therapy, Outcome 5 Number of patients with abnormal myocardial enzyme levels.
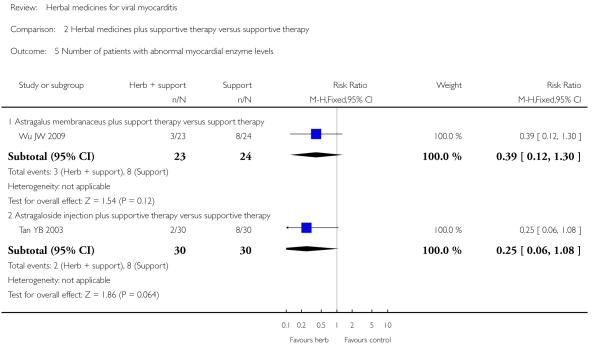
|
Analysis 2.6.
Comparison 2 Herbal medicines plus supportive therapy versus supportive therapy, Outcome 6 Abnormal LVEF.
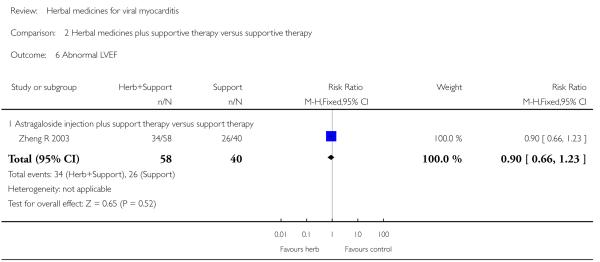
|
Analysis 2.7.
Comparison 2 Herbal medicines plus supportive therapy versus supportive therapy, Outcome 7 Number of patients converting to persisting myocarditis.
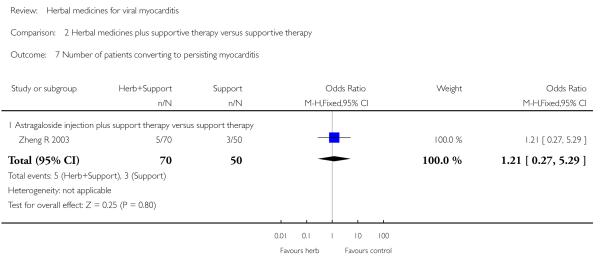
|
Analysis 2.8.
Comparison 2 Herbal medicines plus supportive therapy versus supportive therapy, Outcome 8 Myocardial enzyme CPK levels (U/L).
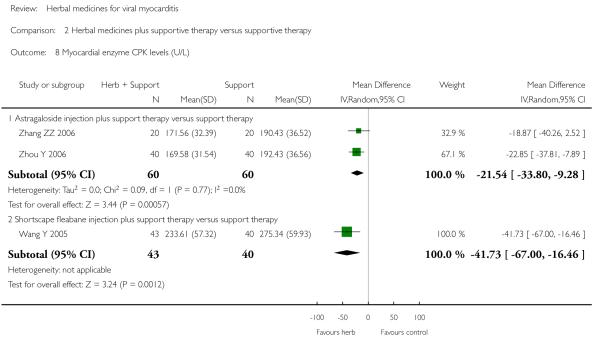
|
Analysis 2.9.
Comparison 2 Herbal medicines plus supportive therapy versus supportive therapy, Outcome 9 Myocardial enzyme LDH levels (U/L).
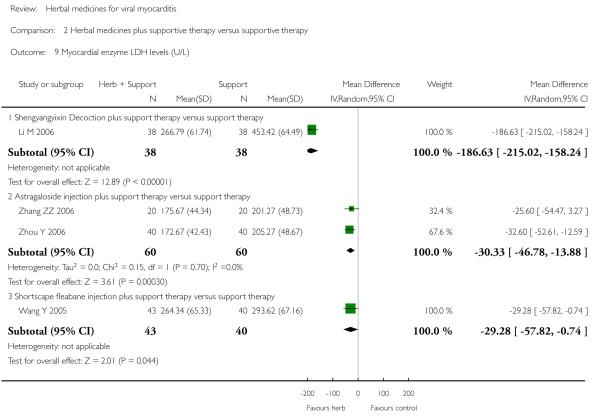
|
Analysis 2.10.
Comparison 2 Herbal medicines plus supportive therapy versus supportive therapy, Outcome 10 Symptom scores.
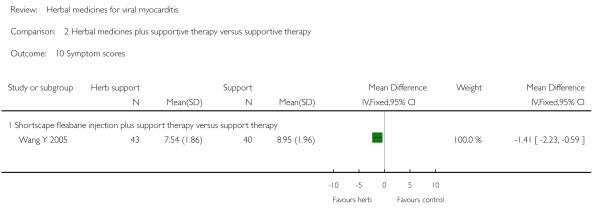
|
Analysis 2.11.
Comparison 2 Herbal medicines plus supportive therapy versus supportive therapy, Outcome 11 Cardiac function LVET (%).
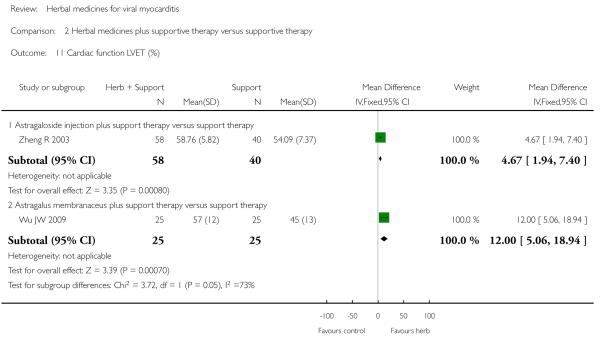
|
Analysis 2.12.
Comparison 2 Herbal medicines plus supportive therapy versus supportive therapy, Outcome 12 Myocardial enzyme CK-MB levels (U/L).
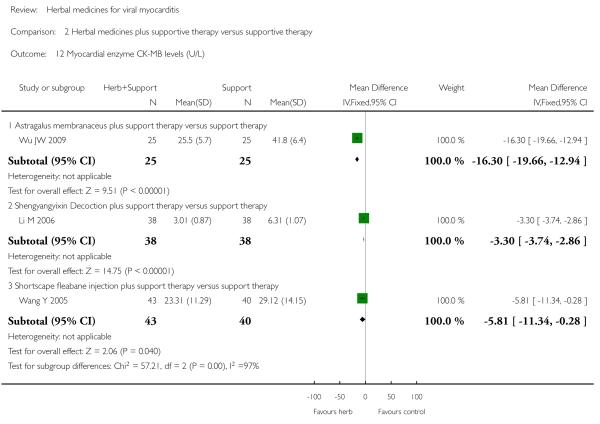
|
Analysis 2.13.
Comparison 2 Herbal medicines plus supportive therapy versus supportive therapy, Outcome 13 Scores of quality of life measured by SF-36.
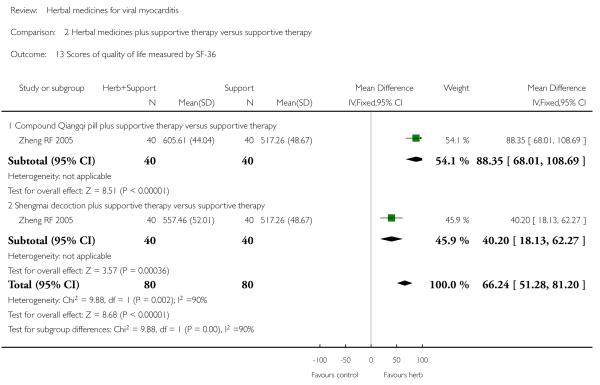
|
PLAIN LANGUAGE SUMMARY.
Herbal medicines for viral myocarditis
Viral myocarditis is a disease where the muscles in the walls of heart become infected with a virus. This systematic review evaluates the effect of various herbal formulations (including single herbs, ingredients, and mixtures of different herbs) for treating acute and chronic viral myocarditis patients. Fourteen identified clinical trials were performed and published in China. The review of trials found that some of the herbal medicines may have a positive effect on improving cardiac function, lowering blood enzymes and relieving symptoms in viral myocarditis patients. Data on adverse events were only available from one trial. However, the methodological quality of the clinical trials evaluating these herbal medicines was generally poor.
ACKNOWLEDGEMENTS
We thank Margaret Burke of the Cochrane Heart Group for her help in the development of search strategy. We are grateful to Theresa Moore for her previous constructive suggestions in the development of the protocol. We also wish to acknowledge Xinmiao Du for her help in trials search, study selection, quality assessment of trials and data extraction with the initial review and previous review update.
SOURCES OF SUPPORT
Internal sources
• Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, China.
• National Research Centre in Complementary and Alternative Medicine (NAFKAM), Norway.
External sources
• Grant number 2006CB504602 from the National Basic Research Program (973 Program), China.
• Grant number 2009DFA31460 from the Internaitonal Cooperation Project of the Ministry of Science and Technology, China.
• Grant Number R24 AT001293 from the National Center for Complementary and Alternative Medicine (NCCAM) of the US National Institutes of Health, USA.
Appendix 1. Search strategies 2009
CENTRAL on The Cochrane Library
#1 MeSH descriptor myocarditis this term only
#2 MYOCARDITIS in All Text
#3 (MYOCARD* in All Text near/6 INFLAMM* in All Text)
#4 (HEART in All Text near/6 INFLAMM* in All Text)
#5 (#1 or #2 or #3 or #4)
#6 MeSH descriptor Medicine, Traditional explode all trees
#7 MeSH descriptor Plant Extracts explode all trees
#8 MeSH descriptor Plants, Medicinal explode all trees
#9 ( (HERB in All Text or HERBAL in All Text) or HERBS in All Text)
#10 ALTERNATIVE next MEDICIN* in All Text
#11 COMPLEMENTARY next MEDICINE* in All Text
#12 (TRADITIONAL in All Text near/6 MEDICINE* in All Text)
#13 (PLANT* in All Text near/6 MEDICIN* in All Text)
#14 (CHINESE in All Text near/6 MEDICIN* in All Text)
#15 PHYTODRUG* in All Text
#16 PHYTOPHARMACEUTIC* in All Text
#17 AYURVEDIC in All Text
#18 (ORIENTAL in All Text near/6 MEDICINE* in All Text)
#19 MeSH descriptor phytotherapy this term only
#20 (#6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19)
#21 (#5 and #20)
MEDLINE (on Ovid)
1 exp Myocarditis/
2 myocarditis.tw.
3 or/1-2
4 exp Medicine, Traditional/
5 Alternative Medicine/
6 exp Plant Extracts/
7 exp Plants, Medicinal/
8 Drugs, Non-Prescription/
9 exp Phytotherapy/
10 (herb or herbs or herbal).tw.
11 alternative medicine$.tw.
12 complementary medicine$.tw.
13 traditional medicine$.tw.
14 (plant or plants).tw.
15 ((chinese or oriental) adj3 medicine$).tw.
16 ayurvedic.tw.
17 (phytodrug$ or phyto-drug$ or phytopharmaceutical$).tw.
18 or/4-17
19 3 and 18
20 limit 19 to yr=“2003 - 2009”
21 exp animals/ not humans/
23 20 not 21
EMBASE (on Ovid)
1 exp Myocarditis/
2 myocarditis.tw.
3 or/1-2
4 exp traditional medicine/
5 Alternative Medicine/
6 exp Plant Extracts/
7 exp Medicinal Plant/
8 Phytotherapy/
9 (herb or herbs or herbal).tw.
10 alternative medicine$.tw.
11 complementary medicine$.tw.
12 traditional medicine$.tw.
13 (plant or plants).tw.
14 ((chinese or oriental) adj3 medicine$).tw.
15 ayurvedic.tw.
16 (phytodrug$ or phyto-drug$ or phytopharmaceutical$).tw.
17 or/4-16
18 3 and 17
19 limit 18 to yr=“2003 - 2009”
20 (exp animals/ or nonhuman/) not human/
21 19 not 20
AMED (Allied and Complementary Medicine; on Ovid)
1 myocarditis.tw.
2 limit 1 to yr=“2003 - 2009”
LILACs (on BIREME)
(myocarditis or Miocarditis or miocardite) and not (animals and not humans) [Words] and (random$ or trial$ or RCT or placebo$ or comparative or prospective or groups) [Words] and 2003 or 2004 or 2005 or 2006 or 2007 or 2008 or 2009 [Country, year publication]
Appendix 2. Search strategy 2003
MEDLINE
1 exp Myocarditis/
2 myocarditis.tw.
3 or/1-2
4 exp Medicine, Traditional/
5 Alternative Medicine/
6 exp Plant Extracts/
7 exp Plants, Medicinal/
8 Drugs, Non-Prescription/
9 Herbs/
10 (herb or herbs or herbal).tw.
11 alternative medicine$.tw.
12 complementary medicine$.tw.
13 traditional medicine$.tw.
14 (plant or plants).tw.
15 ((Chinese or oriental) adj3 medicine$).tw.
16 (phytodrug$ or phyto-drug$ or phytopharmaceutical$).tw.
17 or/4-16
18 3 and 17
19 a RCT filter (Dickersin 1994)
20 18 and 19.
[/ indicates MeSH term, exp = exploded, tw = textword, $ = truncation]
WHAT’S NEW Last assessed as up-to-date: 28 January 2010.
| Date | Event | Description |
|---|---|---|
| 13 May 2010 | New citation required but conclusions have not changed | Change of authors. |
| 13 May 2010 | New search has been performed | The inclusion criteria were revised to include only trials that included an adequate description of the generation of allocation sequence. This resulted in 40 trials (of 43 references) that were included in the previous review being excluded in this update. Searches were updated and 14 new trials were identified. The conclusions were not changed. |
HISTORY Protocol first published: Issue 3, 2002 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 8 September 2008 | Amended | Converted to new review format. |
| 29 March 2004 | New citation required and conclusions have changed | Substantive amendment |
Footnotes
CONTRIBUTIONS OF AUTHORS Jianping Liu: defined the review question, developed the protocol and search strategy, selected studies, assessed quality, extracted data, analysed data, developed the final review.
Zhaolan Liu: searched for trials, selected studies, assessed quality of trials, extracted data, analysed data, and updated the review.
Zhijun Liu: searched for trials, selected studies, assessed quality of trials, extracted data.
Min Yang: selected studies and extracted data.
Joey Kwong: interpretated data and their analyses; provided methodological perspective and general advice on this review update.
DECLARATIONS OF INTEREST None known.
DIFFERENCES BETWEEN PROTOCOL AND REVIEW During the updating of the published review, we applied a more strict inclusion criteria regarding the study design. We restricted all randomised trials to adequate description of the generation of allocation sequence. Therefore, some RCTs included in original review were excluded due to unclear method for generation of the allocation sequence. This resulted in 40 trials (43 references) that were included in the previous version of the review being excluded from this update. Searches were updated and 14 new trials were identified.
REFERENCES
References to studies included in this review
- Chen PY, Zhou F, Ceng Y, Li G, Luo LY. Clinical observation on the effect of Xinshu Capsule (XC) in treating frequent ventricular extrasystoles due to viral myocarditis and the influence on ET, MDA AND CRP [in Chinese] Shanxi Journal of Traditional Chinese Medicine. 2006;22(3):13–5. [Google Scholar]
- Chen PY, Zhou F, Chen WL, Ceng Y, Luo LY. Clinical observation on the effect of Xinshu Capsule (XC) in treating frequent ventricular extrasystoles due to viral myocarditis and the influence on NO, NOS AND SOD [in Chinese] Chinese Journal of Current Traditional and Western Medicine. 2005;3(10):893–5. [Google Scholar]
- Li L, Zhang SL, Zhong XL, Xu LL, Duan AY. Observation on therapeutic effect of Qingxinkangyan Yin Decoction in treating patients with CVB myocarditis and nursing strategy. Journal of Nursing (China) 2006;13(3):59–60. [Google Scholar]
- Li M. 38 cases of viral myocarditis treated by Sheng Yang Yi Xin decoction [in Chinese] Shaanxi Journal of Traditional Chinese Medicine. 2006;27(7):791–3. [Google Scholar]
- Ma XB. Observation of Shengmai injection in treating 127 patients with viral myocarditis [in Chinese] Chinese Community Doctors. 2008;23(10):132. [Google Scholar]
- Peng Q, Liu JQ. Observation of Shengmai injection in treating 127 patients with viral myocarditis [in Chinese] Maternal and Child Health Care of China. 2005;20(23):3096. [Google Scholar]
- Tan YB. Clinical observation on the effect of Radix Astragali injection in the treatment of infantile viral myocarditis [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care. 2003;10(1):60–1. [Google Scholar]
- Wang Y, Chen YR, Ye L, Wu YZ, Chen BW. Clinical observation of shortscape fleabane injection in treating patients with viral myocarditis [in Chinese] Journal of Emergency in Traditional Chinese Medicine. 2005;14(9):813–4. [Google Scholar]
- Wu JW, Tao N, Jiang RF, Feng HD. Clinical observation on infantile viral myocarditis treated with radix astragali [in Chinese] Journal of Pediatric Pharmacy. 2009;15(1):23–5. [Google Scholar]
- Yang YX. Clinical research on treatment of acute viral myocarditis by radix astragali [in Chinese] World Health Digest Medical Periodical. 2008;5(9):35. [Google Scholar]
- Yao BJ. Qi Lu decoction in the treatment of viral myocarditis with arrhythmia. Journal of Henan University of Chinese Medicine. 2005;20(3):39–40. [Google Scholar]
- Zhang ZZ, Yu XH, Zhang P, Yang YH. Observation on 20 cases of viral myocarditis treated with radix astragali injection [in Chinese] Journal of Nanhua University (medical Edition) 2006;34(1):143–4. [Google Scholar]
- Zheng Rong. 120 cases of viral myocarditis treated with integrated Chinese and western medicine [in Chinese] The Journal of Medical Theory and Practice. 2003;16(8):907–8. [Google Scholar]
- Zheng Ruifeng. The double-blinding study on the effects of Compound recipe Qiang Qi pill in treating patients with VMC. Full paper database of Chinese excellent dissertations by doctors and masters. 2005:1–72. [Google Scholar]
- Zhou Y, Yu XH, Yang YH. Observation of astragaloside on treating viral myocarditis in 40 cases [in Chinese] Chinese Journal of the Practical with Modern Medicine. 2006;19(8):880–1. [Google Scholar]
- Zhou YW, Zhang ZJ. Observation of Huo Ming decoction in treatment of viral myocarditis. Chinese Journal of Misdiagnosis. 2008;8(23):5584–5. References to studies excluded from this review
- An XF. 50 cases of viral myocarditis complicated with arrhythmia treated with Radix Salviae miltiorrhizae [in Chinese] Tianjin Medical Journal. 1997;25(8):502–3. [Google Scholar]
- Cao GM, Zhang SF, Hu YH, Lu JZ, Wang JC, Li LS, et al. Clinical observation on acute viral myocarditis treated with Xinyikang oral liquid [in Chinese] Chinese Journal of Traditional Chinese Medical Science and Technology. 1996;3(6):35–7. [Google Scholar]
- Chen BY, Zhang XL, Ying XZ, Dong YQ, Liu H, Qiao WP, et al. Clinical research on treatment of children viral myocarditis by the principle of nourishing Qi and Yin and promoting blood circulation by removing blood stasis [in Chinese] Chinese Journal of Traditional Chinese Medicine and Pharmacy. 1993;8(5):20–2. [Google Scholar]
- Chen BY, Yin XZ, Hu SY, Liu H, Qiao WP, He AY. Controlled observation on 65 infantile acute viral myocarditis treated with traditional and western medicine [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1994;14(4):216–9. [PubMed] [Google Scholar]
- Chen H. 104 cases of acute viral myocarditis treated with Huangqi injection [in Chinese] Chinese Journal of Information on Traditional Chinese Medicine. 1999;6(4):49. [Google Scholar]
- Chen LJ. Observation on 48 cases of viral myocarditis by treatment with Chinese herbs Yixinyin [in Chinese] Journal of Practical Traditional Chinese Medicine. 1997;12(1):8–9. [Google Scholar]
- Chen SX, Chang PL, Bao SH, Zheng XJ, Mei SW, Zhang LQ. A study of integrated traditional Chinese and western medicines for treatment of severe viral myocarditis [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1992;12(7):398–401. [PubMed] [Google Scholar]
- Feng DX, Chen KJ. Observation on the effect of Xinluning in the treatment of frequent ventricular premature beat [in Chinese] Integrated Traditional Chinese and Western Medicicine in Practical Clinical Emergency. 1996;3(10):444–5. [Google Scholar]
- Geng J. Yiqi Yangyin Huoxue recipe for treatment of 44 cases of acute viral myocarditis [in Chinese] Chinese Journal of School Doctor. 1996;10(6):453–4. [Google Scholar]
- Gong LH, Wu JW. Radix Astragali injection for treatment of 36 cases of viral myocarditis [in Chinese] Study Journal of Traditional Chinese Medicine. 2001;19(2):167. [Google Scholar]
- Gu W, Yang YZ, He MX. A study on combination therapy of western and traditional Chinese medicine of acute viral myocarditis [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1996;16(12):713–6. [PubMed] [Google Scholar]
- Guo FQ, Yang YH, Deng H. 30 cases of infantile viral myocarditis treated by radix astragali, Caculus Bovis and Coenzyme Q10[in Chinese] Journal of Nanhua University (Medical Edition) 2008;36(2):208–9. [Google Scholar]
- Guo WX, Liu WM, Lin HJ, Wang CP, Zhang H. Clinical observation on oral liquid of Xinyikang used in the treatment ofviral myocarditis [in Chinese] Chinese Journal of Information on Traditional Chinese Medicine. 2000;7(7):38–41. [Google Scholar]
- Han DS, Li CL, Lou AG. 42 cases of viral myocarditis treated with Yixin decoction [in Chinese] Journal of Traditional Chinese Medicine and Chinese Materia Medica of Jilin. 1997;17(5):11. [Google Scholar]
- Han Y, Zhang XJ, Li JY. 30 cases of infantile viral myocarditis treated by integrated Chinese and western drugs [in Chinese] Journal of Practical Traditional Chinese Medicine. 2000;16(4):21. [Google Scholar]
- He AY, Hu SY, Chen BY. Shuanghuanglian injection and Tongmaiye for treatment of children with viral myocarditis [in Chinese] Liaoning Journal of Traditional Chinese Medicine. 1999;26(10):450–1. [Google Scholar]
- He P, Yang SZ. Clinical observation on the effect of Radix Astragali in the treatment of viral myocarditis complicated with ventricular premature beat and in the regulation of immunologic function [in Chinese] Journal of Traditional Chinese Medicine and Chinese Materia Medica of Jilin. 1995;15(2):7–8. [Google Scholar]
- Hu SY, He AY, Liu H, Hu SP, Chen Y, Chen BY. Effect of Tongmaiye on left cardiac function in children with acute viral myocarditis [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1995;15(7):432–3. [Google Scholar]
- Hu SY, He AY, Liu H, Qiao WP, Xiang Y, Liu YZ, et al. Clinical study on infantile coxsackie viral myocarditis with heart invaded by toxic pathogen treated with Qingxin solution [in Chinese] Journal of Traditional Chinese Medicine. 1999;40(5):297–9. [Google Scholar]
- Huang W. Clinical observation on viral myocarditis treated with decoction Invigoration Yang for recuperation [in Chinese] Journal of Practical Traditional Chinese Medicine. 1999;15(8):6–7. [Google Scholar]
- Huang ZQ, Qin NP, Ye W, Guo P, Wang HR. Effect of Astragalus membranaceus on T-lymphocyte subsets in patients with viral myocarditis [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1995;15(6):328–30. [PubMed] [Google Scholar]
- Huang ZQ, Qin NP, Zhou Y. Effect of herbal extract Yixinling on NK cell activity and T-lymphocyte subsets in patients with viral myocarditis [in Chinese] Traditional Chinese Drug Research & Clinical Pharmacology. 1996;7(3):7–9. [Google Scholar]
- Ji XL, Guo H. Clinical observation on 54 cases of viral myocarditis treated by Xinjiyin [in Chinese] Tianjin Journal of Traditional Chinese Medicine. 1995;12(1):19–20. [Google Scholar]
- Jia WH. 43 cases of viral myocarditis treated by the principle of nourishing Qi and Yin. Chinese Journal of Integrated Traditional and Western Medicine. 1998;18(5):308. [Google Scholar]
- Jia WH. Clinical study of patients with viral myocarditis treated with supplemented Huangqi Shengmai Powder [in Chinese] Chinese Journal of Experimental Traditional Medical Formulae. 1998;4(2):35–7. [Google Scholar]
- Jiang Y, Hu QY, Hu XY. Clinical study on viral myocarditis treated by differential diagnosis of syndromes [in Chinese] Journal of Traditional Chinese Medicine and Chinese Materia Medica of Jilin. 2000;20(5):12. [Google Scholar]
- Jin W, Chen XR, Rong ZM. Treatment of viral myocarditis with vitamin C and Shenmai injection [in Chinese] Modern Journal of Integrated Chinese Traditional and Western Medicine. 2002;11(4):287–8. [Google Scholar]
- Kuo C. Successful treatment of complete left bundle branch block complicating acute viral myocarditis employing Chinese herbs. American Journal of Chinese Medicine. 1986;14(3-4):124–30. doi: 10.1142/S0192415X8600020X. [DOI] [PubMed] [Google Scholar]
- Li DP, Li Y, Liu XF. 48 cases of viral myocarditis treated with integrated Chinese and western medicine[in Chinese] Modern Medicine and Health. 2005;21(19):2664–5. [Google Scholar]
- Li JL, Zhao J. Clinical study of Chinese medicine for treatment of viral myocarditis [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1999;19(4):246–7. [Google Scholar]
- Li Y. Report of 265 cases of acute viral myocarditis treated with Erhuang Wendan decoction [in Chinese] Jiangxi Journal of Traditional Chinese Medicine. 1999;30(5):61. [Google Scholar]
- Li Y. Treatment of 268 cases of acute viral myocarditis by ingredient-modified “Erhuang Wendan Decoction” [in Chinese] Shanghai Journal of Traditional Chinese Medicine. 2000;34(7):22–3. [Google Scholar]
- Li Y. Treatment of viral myocarditis with ingredient-modified “HuangLian WenDan Decoction” [in Chinese] Shanghai Journal of Traditional Chinese Medicine. 1995;29(7):43. [Google Scholar]
- Li Y, Shen LM. Chinese traditional medicine fractionally treating acute viral myocarditis [in Chinese] Chinese Traditional Patent Medicine. 2002;24(6):436–7. [Google Scholar]
- Li YR, Liu XP, Bai CL, Li RS, Jia XL, Yang YL, et al. Effect of Shenmai Injection on cardiac function and cellular immune function in children viral myocarditis [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1996;16(8):477–9. [Google Scholar]
- Li YW, Tan XJ, Zhang WF. Chinese medicine Yangxinshi for treatment of 32 cases of viral myocarditis [in Chinese] Shandong Journal of Traditional Chinese Medicine. 1997;16(10):445–6. [Google Scholar]
- Li ZY, Liu BG, Liu YM. Observation on viral myocarditis treated with Huangqi injection [in Chinese] Chinese Journal of Information on Traditional Chinese Medicine. 1998;5(12):51. [Google Scholar]
- Lin GZ, Liu DM, Zhu L, Qiu DZ. Clinical study on Shuanghuanglian powder in treating children viral myocarditis [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1998;18(10):601–2. [PubMed] [Google Scholar]
- Liu AD. Clinical observation on viral myocarditis treated with new Chinese medicinal regimens [in Chinese] Chinese Community Doctors. 2005;7(23):58. [Google Scholar]
- Liu GJ, Liu QP. Clinical observation on 45 cases of acute viral myocarditis treated with Shenmai injection [in Chinese] Research of Traditional Chinese Medicine. 1996;12(6):18. [Google Scholar]
- Liu HQ, Li JX. Study on therapeutic effect of Shenqiyin for 66 cases of viral myocarditis [in Chinese] Jiangxi Journal of Traditional Chinese Meidicine. 2000;31(3):40. [Google Scholar]
- Liu J, Cai HB, Yang XW. Clinical observation of Huangqi Shengmaisan for treatment of 36 cases of viral myocarditis [in Chinese] Guang Ming Journal Traditional Chinese Medicine. 1995;10(1):17–8. [Google Scholar]
- Liu MD, Zhang YX. Integrated Chinese and western medicine for treatment of 45 cases of viral myocarditis [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1999;19(2):123. [Google Scholar]
- Liu SS. Study of adjunct treatment of Astragali membranaceus for viral myocarditis [in Chinese] Clinical Focus. 1997;12(14):657–8. [Google Scholar]
- Liu YJ, Huang P. Clinical observation on viral myocarditis treated with Astragalus membranaceus injection [in Chinese] Acta Chinese Medicine and Pharmacology. 1997;25(1):18. [Google Scholar]
- Lu Y, Lang YQ, Zhou WL, Wang JH. Application of Dengzhanhua injection in treatment of acute viral myocarditis [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1997;17(12):753. [Google Scholar]
- Luo L. 38 cases of viral myocarditis treated with Wushen Jiwei Shengmai Powder [in Chinese] Hubei Journal of Traditional Chinese Medicine. 1998;20(3):16. [Google Scholar]
- Ma CH, Wu X, Cheng SS. Integrated traditional Chinese and western medicines for treatment of viral myocarditis [in Chinese] Jiangsu Journal of Traditional Chinese Medicine. 1995;16(6):19. [Google Scholar]
- Ma GL, Wang CY, Diao WX. Clinical study on viral myocarditis treated with integrated Chinese and western medicine [in Chinese] Acta Chinese Medicine and Pharmacology. 1998;26(1):9–10. [Google Scholar]
- Ma HB, Su BL, Zhang RF. Clinical study on 30 cases of children viral myocarditis treated by Chinese differentiated therapy [in Chinese] Shanxi Traditional Chinese Medicine. 1997;13(3):9–10. [Google Scholar]
- Ma YL, Xiong YQ. Clinical observation on 40 cases of infantile viral myocarditis treated by differential diagnosis of syndromes [in Chinese] Journal of Traditional Chinese Medicine. 1984;25(6):25–7. [Google Scholar]
- Qin FH. Ingredient-modified “Minor Bupleurum Decoction” for myocarditis in 31 cases. Shanghai Journal of Traditional Chinese Medicine. 2001;35(4):22–3. [Google Scholar]
- Qin HS. Observation on the effect of radix astragali combined salviae miltiorrhizae in the treatment of acute viral myocarditis [in Chinese] Modern Journal of Integrated Traditional Chinese and Western Medicine. 2006;15(8):1033–4. [Google Scholar]
- Ren GH. Therapeutic study on Astragalus injection for children with viral myocarditis [in Chinese] Central Plains Medical Journal. 1996;23(4):17. [Google Scholar]
- Ren W, Zhu HW, Zhang DY. Clinical observation on effect of Radix Astragali treating 66 patients with viral myocarditis complicated with cardiac dysfunction [in Chinese] Chinese Journal of Critical Care Medicine. 1991;11(3):38–40. [Google Scholar]
- Ren W, Zhu HW, Zhang DY. Observation on the effect of Radix Astragali in the treatment of viral myocarditis complicated with cardiac insufficiency [in Chinese] Chinese Journal of Internal Medicine. 1992;31(10):644–5. [Google Scholar]
- Rong YS, Jiao SL. Treatment of 66 cases of viral myocarditis using integrated Chinese and western medicine [in Chinese] Journal of Hebei Traditional Chinese Medicine and Pharmacology. 2001;16(1):28–9. [Google Scholar]
- Song JM, Xu CQ, Zhang DR, Xu GC, Wang YC. Clinical study on oral liquid of Yangyin Qingxin used in the treatment of acute viral myocarditis [in Chinese] Guang Ming Journal of Traditional Chinese Medicine. 1999;14(1):41–5. [Google Scholar]
- Su CT, Fan DM, Yu MX. Therapeutic study on Shengmai San modified for treatment of viral myocarditis [in Chinese] Acta Chinese Medicine and Pharmacology. 1999;27(5):14. [Google Scholar]
- Sun DX, Yu J. Clinical study on treatment of acute viral myocarditis with Shenmai injection [in Chinese] Jiangxi Journal of Traditional Chinese Medicine. 2000;31(5):19–20. [Google Scholar]
- Sun J, Song GW, Sun F, Liu ZQ, Zhang SQ, Yu QF. Clinical observation on viral myocarditis treated with Xinankang [in Chinese] Journal of Traditional Chinese Medicine and Chinese Materia Medica of Jilin. 1998;18(6):11. [Google Scholar]
- Sun KJ, Wang LP, Me HY, Mei CJ. Clinical observation on 12 cases of viral myocarditis complicated with arrhythmia in the convalescent period treated with Shengmai injection [in Chinese] Acta Chinese Medicine and Pharmacology. 1998;26(1):19. [Google Scholar]
- Sun WM, Liu XY, Ma LX. Clinical observation on treatment of viral myocarditis by combined method of Chinese and western medicine [in Chinese] Journal of Traditional Chinese Medicine and Chinese Materia Medica of Jilin. 1999;19(6):39. [Google Scholar]
- Sun Y, Sun SF, Sun H. Clinical observation on viral myocarditis treated with Radix Acanthopanacis senticosi and Radix Salviae miltiorrhizae [in Chinese] Guizhou Medical Journal. 1997;21(3):178–9. [Google Scholar]
- Tan JC, Xie HF, Zhang CY, Qi LJ. 30 cases of acute viral myocarditis treated with Shengmai injection [in Chinese] Hebei Journal of Traditional Chinese Medicine. 1995;17(4):47–8. [Google Scholar]
- Tang SY. Observation on the effect of Qingxinkang in the treatment of viral myocarditis [in Chinese] Journal of Traditional Chinese Medicine and Chinese Materia Medica of Jilin. 2000;20(3):18. [Google Scholar]
- Tu XH, Xu FQ, Miao Y, Wang XF, Xu MY, Huang YS, et al. Clinical trial of Qidong Yixin oral liquid for treatment of viral myocarditis [in Chinese] Traditional Chinese Drug Research & Clinical Pharmacology. 1996;7(4):6–9. [Google Scholar]
- Wang JM, Tian ZX, Yang SW, Meng QJ, Li WC. Clinical observation of Meglumine Cyclic Adenylate combined radix astragali injections in treating 36 patients with viral myocarditis [in Chinese] Chinese Journal of Integrative Medicine on Cardio/ Cerebrovascular Disease. 2003;1(2):93. [Google Scholar]
- Wang K, Gao LZ, Yang L, Lin T. Study on 36 children with viral myocarditis treated with Huangzhihua oral liquid [in Chinese] Journal of Beijing University of Traditional Chinese Medicine. 2000;23(1):75. [Google Scholar]
- Wang WR, Zhu RH. Study on integrated traditional Chinese and western medicines for treatment of 53 cases of viral myocarditis [in Chinese] Journal of Practical Traditional Chinese Medicine. 2001;17(3):24. [Google Scholar]
- Wang XF, Yan WC, Guo ZW, Zhang J, Bai XH. The effect of Chaihu Qingxin Yin on left cardiac function and T-cell subgroup in peripheral blood in children with viral myocarditis [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1997;17(2):73–5. [PubMed] [Google Scholar]
- Wang XJ. Evaluation of the effect of Astragali in treating 58 patients with viral myocarditis complicated with cardiac dysfunction [in Chinese] The Practical Journal of Integrating Chinese with Modern Medicine. 1995;8(5):309–10. [Google Scholar]
- Wang ZH, Li DM, Zhou CH. Effect of Astragalus membranaceus injection on TNF and IL-1 in patients with viral myocarditis [in Chinese] Journal of Changchun College of Traditional Chinese Medicine. 1998;14(1):12. [Google Scholar]
- Wang ZH, Liao YH. Combined treatment of viral myocarditis with traditional Chinese medicine and western medicine [in Chinese] Journal of Clinical Cardiology (China) 2001;17(8):353. [Google Scholar]
- Wang ZL, Sun BQ. 24 cases of viral myocarditis treated with combination of Chinese and western drugs [in Chinese] Journal of Practical Traditional Chinese Medicine. 2000;16(1):32–3. [Google Scholar]
- Wang ZM. Chinese herbal medicine for viral myocarditis [in Chinese] Hubei Journal of Traditional Chinese Medicine. 2000;22(6):26–7. [Google Scholar]
- Wang ZT, Zeng CY, Shi XQ, Han LH. Xinjikang for Adults’ Chronic Viral Myocarditis. Journal of Henan University of Chinese Medicine. 2004;19(6):48–49. [Google Scholar]
- Wei QH, Shi YY, Shen BS, Zhang JR. Clinical study on Fufang Sishen Decoction in treating arrhythmia after virus myocarditis[in Chinese] Journal of Chinese Integrative Medicine. 2004;2(2):97–99. doi: 10.3736/jcim20040206. [DOI] [PubMed] [Google Scholar]
- Wei YL, Wu XM, Li Q. Study of Wei Er Xin for treatment of 300 cases of children with viral myocarditis [in Chinese] Chinese Journal of Information on Traditional Chinese Medicine. 1998;5(8):24–5. [Google Scholar]
- Wu CS. Clinical observation of effect of Shenmai injection in treating 100 patients with viral myocarditis [in Chinese] Zhejiang Journal of Traditional Chinese Medicine. 1988;23:369–70. [Google Scholar]
- Wu XN, Zhang XL. Therapeutic study on integrated traditional and western medicines for 24 cases of acute viral myocarditis [in Chinese] New Journal of Traditional Chinese Medicine. 2002;34(5):38. [Google Scholar]
- Xia DC. Modified Qinggong decoction treated 32 cases of acute viral myocarditis [in Chinese] Hunan Guiding Journal of Traditional Chinese Medicine and Pharmacology. 2000;6(6):25–6. [Google Scholar]
- Xing YH, Meng FL. Study on the effect of Royal jelly in the treatment of viral myocarditis. Journal of Binzhou Medical College. 1998;21(1):47. [Google Scholar]
- Xu MM. 54 cases of infantile viral myocarditis treated by integrated Chinese and western drugs [in Chinese] Journal of Practical Traditional Chinese Medicine. 2000;16(8):17–8. [Google Scholar]
- Xu T. Composita Salviae miltiorrhizae injection for treatment of children with viral myocarditis [in Chinese] Zhejiang Journal of Integrated Traditional Chinese and Western Medicine. 1996;6(2):73–4. [Google Scholar]
- Yang CJ. 35 cases of infantile viral myocarditis treated with integrated Chinese and western medicine [in Chinese] Journal of Sichuan of Traditional Chinese Medicine. 2005;23(10):90–1. [Google Scholar]
- Yang FQ, Xie WH. The clinical observation of the treatment of viral myocarditis by clearing away the heat evil and toxic materialsand by Shengmai injection [in Chinese] Nei Mongol Journal of Traditional Chinese Medicine. 1998;17(3):8–9. [Google Scholar]
- Yang GF. Study on integrated traditional and western medicines for treatment of 87 cases of viral myocarditis [in Chinese] Heilongjiang Journal of Traditional Chinese Medicine. 2002;37(3):13–4. [Google Scholar]
- Yang HB. Clinical observation on viral myocarditis treated with Shengmai injection [in Chinese] Acta Chinese Medicine and Pharmacology. 1997;25(3):11. [Google Scholar]
- Yang SJ, Yin SY, Peng JH. Shenmai injection for treatment of 60 cases of acute viral myocarditis with deficiency of both Qi and Yin [in Chinese] Liaoning Journal of Traditional Chinese Medicine. 1997;24(10):452. [Google Scholar]
- Yang YZ, Jin PY, Guo Q, Wang QD, Li ZS, Ye YC, et al. Effect of Astragalus membranaceus on natural killer cell activity and induction of alpha- and gamma-interferon in patients with coxsackie B viral myocarditis. Chinese Medical Journal. 1990;103(4):304–7. [PubMed] [Google Scholar]
- Yao ZP, Huang WQ. Study on 16 cases of acute viral myocarditis treated by herbal extract Qing Kai Ling [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1995;15(10):633–4. [Google Scholar]
- Yin YS, Lu ZF. Observation on effect of Shengmai injection in treating of viral myocarditis [in Chinese] The Practical Journal of Integrating Chinese with Modern Medicine. 1997;10(15):1477–8. [Google Scholar]
- Yu ZK, Chen ZH. Clinical observation on 61 cases of viral myocarditis treated with mainly Chinese herbal medicine [in Chinese] Sichuan Journal of Traditional Chinese Medicine. 1995;13(9):34–5. [Google Scholar]
- Yu ZK, Chen ZH, Yang XG. 61 cases of viral myocarditis treated with Chinese herbs [in Chinese] Guang Ming Journal Traditional Chinese Medicine. 1996;11(4):24–5. [Google Scholar]
- Zeng CF. Clinical observation on 25 cases of acute viral myocarditis treated with combined method of Chinese and western medicine [in Chinese] Journal of Gansu College of Traditional Chinese Medicine. 1997;14(3):28–30. [Google Scholar]
- Zhang DF, Liu XQ. Combination treatment with FDP and Astragus injection for patients with viral myocarditis [in Chinese] Chinese modern medicine. 2005;3(3):48. [Google Scholar]
- Zhang PY, Xu X, Wang J, Qu SQ, Sun ZH, Guo SW. Combination treatment with Qing Kai Ling and Shengmai injection in 100 patients with acute stage of viral myocarditis [in Chinese] Journal of Emergency Syndromes in Chinese Medicine. 1997;6(6):265–6. [Google Scholar]
- Zhang SY, Wu SH, Shao XS, Wang JH. Observation on viral myocarditis in children (34 cases) treated with Red Sage injection [in Chinese] Journal of Practical Traditional Chinese Medicine. 2000;16(2):3–4. [Google Scholar]
- Zhang XL, Yuan XD. 30 cases of children with viral myocarditis treated with oral liquid of Qidong Yixin [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1999;19(6):339. [Google Scholar]
- Zhang XM, Zhao SY, Jia YZ. Integrated traditional Chinese and western medicines for treatment of 36 cases of acute viral myocarditis [in Chinese] Journal of Practical Traditional Chinese Medicine. 2000;16(10):28–9. [Google Scholar]
- Zhang ZX. Clinical observation on 46 cases of viral myocarditis treated with Yiqi Yangyin Decoction [in Chinese] Chinese Journal of Information on Traditional Chinese Medicine. 2000;7(6):71–2. [Google Scholar]
- Zhao CS, Dong KD, Dong M, Lv J. The Qing Xin Tang and Yan Po Nings treat the curative effect observation of the AVMC. Chinese Journal of the Practical Chinese with Modern Medicine. 2005;18(17):824–5. [Google Scholar]
- Zhao CS, Dong KD, Dong M, Lv J. The Qing Xin Tang and Yan Po Nings treat the curative effect observation of the AVMC. Guangming Journal of Chinese Medicine. 2006;21(5):47–8. [Google Scholar]
- Zhao MH, Rong HZ, Lu BJ, Zhu XY, Huang GF, Yang JW. Effect of Shengmaisan on serum lipid peroxidation in acute viral myocarditis [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1996;16(3):142–5. [PubMed] [Google Scholar]
- Zhao QC, Yu JY. 32 cases of viral myocarditis treated with Ginseng [in Chinese] Clinical Nugget. 1996;11(1):41–2. [Google Scholar]
- Zhao XS, He ZJ, Huang SS. Clinical observation of 54 cases of viral myocarditis treated with integrated Chinese and western medicine [in Chinese] Nei Mongol Journal of Traditional Chinese Medicine. 2008;27(9):79–80. [Google Scholar]
- Zhao YT, Lu M, Shang BQ, Yang ZT. Study on Yangxin Fumai Tang for treatment of 40 cases of viral myocarditis [in Chinese] Heilongjiang Journal of Traditional Chinese Medicine. 1994;29(3):12. [Google Scholar]
- Zhao YZ, Wang GF, Wang LQ. Clinical observation on 60 cases of acute viral myocarditis treated with the method of integration of traditional and western medicine [in Chinese] Henan Journal of Traditional Chinese Medicine and Pharmacy. 1998;13(5):36–8. [Google Scholar]
- Zhou D. Integrated Traditional Chinese and Western Medicine in Treating 30 Cases of Viral Myocarditis. Journal of Henan University of Chinese Medicine. 2004;19(6):50. [Google Scholar]
- Zhou FR, Su Y. Observation on effect of Xinjikang capsule in the treatment of infantile viral myocarditis [in Chinese] Liaoning Journal of Traditional Chinese Medicine. 2001;28(2):101–2. [Google Scholar]
- Zhou L, Wu SS, Liu GM. Integrated Chinese and western medicine for treatment of 60 cases of acute viral myocarditis [in Chinese] Journal of Henan College of Traditional Chinese Medicine. 2000;15(4):39–40. [Google Scholar]
- Zhou MY, Wan YH. 30 cases of viral myocarditis treated with integrated Chinese and western medicine [in Chinese] Journal of Nanjing University of Traditional Chinese Medicine. 1996;12(5):53–4. [Google Scholar]
- Zhou ZY, Ni FX. Integrated Chinese and western drugs for treatment of 102 cases of viral myocarditis in acute stage [in Chinese] Liaoning Journal of Traditional Chinese Medicine. 2000;27(5):223. [Google Scholar]
- Zhu Q, Liu SJ. Exploration on treatment and relationship between Chest Bi-Syndrome and infantile viral myocarditis [in Chinese] Zhejiang Journal of Traditional Chinese Medicine. 1997;32(10):451–2. [Google Scholar]
- Zhu YD, Sun XX, Hao SR, Huang JL. Report of self-prescribed herbal mixture for treatment of 45 cases of viral myocarditis [in Chinese] Hunan Journal of Traditional Chinese Medicine. 1997;13(3):40. [Google Scholar]
Additional references
- Andreoletti L, Leveque N, Boulagnon C, Brasselet C, Fornes P. Viral causes of human myocarditis. Archives of cardiovascular diseases. 2009;102(6-7):559–68. doi: 10.1016/j.acvd.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Baughman KL. Diagnosis of myocarditis: death of Dallas criteria. Circulation. 2006;113(4):593–5. doi: 10.1161/CIRCULATIONAHA.105.589663. [DOI] [PubMed] [Google Scholar]
- Chen H. 104 cases of acute viral myocarditis treated with Astragalus membranaceus injection [in Chinese] Chinese Journal of Information on Traditional Chinese Medicine. 1999;6(4):49. [Google Scholar]
- Cooper LT., Jr Myocarditis. The New England journal of medicine. 2009;360(15):1526–38. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dec GW, Jr, Palacios IF, Fallon JT. Active myocarditis in the spectrum of acute dilated cardiomyopathies: clinical features, histologic correlates, and clinical outcome. The New England Journal of Medicine. 1985;312:885–90. doi: 10.1056/NEJM198504043121404. [DOI] [PubMed] [Google Scholar]
- Dec GW, Jr, Waldman H, Southern J, Fallon JT, Hutter AM, Jr, Palacios I. Viral myocarditis mimicking acute myocardial infarction. Journal of the American College of Cardiology. 1992;20:85–9. doi: 10.1016/0735-1097(92)90141-9. [DOI] [PubMed] [Google Scholar]
- Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. The New England Journal of Medicine. 1994;331(23):1564–75. doi: 10.1056/NEJM199412083312307. [DOI] [PubMed] [Google Scholar]
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ. 1994;309:1286–91. doi: 10.1136/bmj.309.6964.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drory Y, Turetz Y, Hiss Y, Lev B, Fisman EZ, Pines A, et al. Sudden unexpected death in persons less than 40 years of age. American Journal of Cardiology. 1991;68(13):1388–92. doi: 10.1016/0002-9149(91)90251-f. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith DG, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Rompay MV, et al. Trends in alternative medicine use in the United States, 1990-1997. Results of a follow-up national survey. JAMA. 1998;280(18):1569–75. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- Feldman AM, McNamara D. Myocarditis. The New England Journal of Medicine. 2000;343(19):1388–98. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- Fulder S. The Handbook of Alternative and Complementary Medicine. Oxford University Press; Oxford: 1996. [Google Scholar]
- Gottieb S. Chinese herb may cause cancer (news) BMJ. 2000;320:1623. [PMC free article] [PubMed] [Google Scholar]
- Guo LN. National Symposium on myocarditis and myopathy. Chinese Journal of Cardiology. 1999;27(6):408–12. [Google Scholar]
- Haas GJ. Etiology, evaluation, and management of acute myocarditis. Cardiology in Review. 2001;9(2):88–95. doi: 10.1097/00045415-200103000-00007. [DOI] [PubMed] [Google Scholar]
- Haines CJ, Lam PM, Chung TK, Cheng KF, Leung PC. A randomized, double-blind, placebo-controlled study of the effect of a Chinese herbal medicine preparation (Dang Gui Buxue Tang) on menopausal symptoms in Hong Kong Chinese women. Climacteric : the journal of the International Menopause Society. 2008;11(3):244–51. doi: 10.1080/13697130802073029. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.0. The Cochrane Collaboration; 2008. [updated February 2008] Available from www.cochrane-handbook.org. [Google Scholar]
- Huang ZQ, Qin NP, Ye W, Guo P, Wang HR. Effect of Astragalus membranaceus on T-lymphocyte subsets in patients with viral myocarditis [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1995;15(6):328–30. [PubMed] [Google Scholar]
- International Conference on Harmonisation Expert Working Group Code of Federal Regulations & International Conference on Harmonisation Guidelines; Pennsylvania: Parexel/Barnett; 1997. [Google Scholar]
- Ishizaki T, Sasaki F, Ameshima S, Shiozaki K, Takahashi H, Abe Y, et al. Pneumonitis during interferon and/or herbal drug therapy in patients with chronic active hepatitis. European Respiratory Journal. 1996;9(12):2691–6. doi: 10.1183/09031936.96.09122691. [DOI] [PubMed] [Google Scholar]
- Jiang TL. The significance of study on material foundation and mechanism of medicinal effectiveness of compound prescriptions of Chinese medicine. Chinese Journal of Integated Traditional and Western Medicine. 1999;19(4):195–6. [PubMed] [Google Scholar]
- Jackson John. Why do people use alternative medicine? Jason Braithwaite & Wendy Cousins; John Jackson: 2005. [Google Scholar]
- Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99(8):1091–100. doi: 10.1161/01.cir.99.8.1091. [DOI] [PubMed] [Google Scholar]
- Kearney MT, Cotton JM, Richardson PJ, Shah AM. Viral myocarditis and dilated cardiomyopathy: mechanisms, manifestations, and management. Postgraduate Medicine. 2001;77(903):4–10. doi: 10.1136/pmj.77.903.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine. 2001;135(11):982–9. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- Koithan M. Introducing Complementary and Alternative Therapies. The journal for nurse practitioners : JNP. 2009;5(1):18–20. doi: 10.1016/j.nurpra.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yang YZ, Ye YC, Jin PY, Wu WZ, Shan YF. An oral solution of Radix Astragali for therapy of viral myocarditis in 50 patients [in Chinese] New Drugs and Clinical Remedies. 1992;11(1):12–4. [Google Scholar]
- Li JY. The diagnostic criteria of viral myocarditis in Children. Chinese Journal of Practical Pediatrics. 1996;11(5):316. [Google Scholar]
- Li ZY, Liu BG, Liu YM. Observation on viral myocarditis treated with Astragalus membranaceus injection [in Chinese] Chinese Journal of Information on Traditional Chinese Medicine. 1998;5(12):51. [Google Scholar]
- Liu GJ, Liu QP. Clinical observation on 45 cases of acute viral myocarditis treated with Shenmai injection [in Chinese] Research of Traditional Chinese Medicine. 1996;9(6):18. [Google Scholar]
- Liu J, Zhang J, Shi Y, Grimsgaard S, Alraek T, Fonnebo V. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta-analysis of randomized controlled trials. Chinese medicine. 2006;1:4. doi: 10.1186/1749-8546-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May BH, Lit M, Xue CC, Yang AW, Zhang AL, Owens MD, et al. Herbal medicine for dementia: a systematic review. Phytotherapy research : PTR. 2009;23(4):447–59. doi: 10.1002/ptr.2656. [DOI] [PubMed] [Google Scholar]
- Melchart D, Linde K, Weidenhammer W, Hager S, Shaw D, Bayer R. Liver enzyme elevations in patients treated with traditional Chinese medicine. JAMA. 1999;282(1):28–9. doi: 10.1001/jama.282.1.28. [DOI] [PubMed] [Google Scholar]
- Miric M, Miskovic A, Vasiljevic JD, Keserovic N, Pesic M. Interferon and thymic hormones in the therapy of human myocarditis and idiopathic dilated cardiomyopathy. European Heart Journal. 1995;(Suppl 0):150–2. doi: 10.1093/eurheartj/16.suppl_o.150. [DOI] [PubMed] [Google Scholar]
- Ministry of Health of the People’s Republic of China Prescription Administrative Policy. 2007 http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohylfwjgs/s6706/200907/41707.htm.
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–13. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- National Committee of Special Symposium on Myocarditis and Cardio-myopathy National Symposium on myocarditis and myopathy. Journal of Clinical Cardiology. 1995;11(6):324–326. [Google Scholar]
- O’Regan P, Wills T. The growth of complementary therapies: and their benefits in the perioperative setting. Journal of perioperative practice. 2009;19(11):382–6. doi: 10.1177/175045890901901102. [DOI] [PubMed] [Google Scholar]
- Parrillo JE. Inflammatory cardiomyopathy (myocarditis): which patients should be treated with anti-inflammatory therapy? Circulation. 2001;104(1):4. doi: 10.1161/hc2601.092124. [DOI] [PubMed] [Google Scholar]
- Pinn G. Adverse effects associated with herbal medicine. Australian family physician. 2001;30(11):1070–5. [PubMed] [Google Scholar]
- Ramesh T, Lee K, Lee HW, Kim SJ. Subacute toxicological evaluation of Asiasari radix methanol extract. Drug and chemical toxicology. 2009;32(3):243–51. doi: 10.1080/01480540902873837. [DOI] [PubMed] [Google Scholar]
- Ren W, Zhu HW, Zhang DY. Observation on the effect of Radix Astragali in the treatment of viral myocarditis complicated with cardiac insufficiency [in Chinese] Chinese Journal of Internal Medicine. 1992;31(10):644–5. [Google Scholar]
- Rose NR. Myocarditis: infection versus autoimmunity. Journal of clinical immunology. 2009;29(6):730–7. doi: 10.1007/s10875-009-9339-z. [DOI] [PubMed] [Google Scholar]
- Schultz JC, Hilliard AA, Cooper LT, Jr, Rihal CS. Diagnosis and treatment of viral myocarditis. Mayo Clinic proceedings. Mayo Clinic. 2009;84(11):1001–9. doi: 10.1016/S0025-6196(11)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KF, Chalmers I, Hayes R, Altman D. Empirical evidence of bias. JAMA. 1995;273(5):408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ (Clinical research ed.) 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt A, Schaper F, Stangl V, Plagemann A, Bohm M, Merkel K, et al. Immunohistological changes in dilated cardiomyopathyinduced by immunoadsorption therapy and subsequent immunoglobulin substitution. Circulation. 2001;103:2681–6. doi: 10.1161/01.cir.103.22.2681. [DOI] [PubMed] [Google Scholar]
- Suddaby EC. Viral myocarditis in children. Critical Care Nurse. 1996;16(4):73–82. [PubMed] [Google Scholar]
- Tang JL, Zhan SY, Ernst E. Review of randomised controlled trials of traditional Chinese medicine. BMJ (Clinical research ed.) 1999;319(7203):160–1. doi: 10.1136/bmj.319.7203.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topkara VK, Dang NC, Barili F, Martens TP, George I, Cheema FH, et al. Ventricular assist device use for the treatment of acute viral myocarditis. The Journal of thoracic and cardiovascular surgery. 2006;131(5):1190–1. doi: 10.1016/j.jtcvs.2005.08.073. [DOI] [PubMed] [Google Scholar]
- Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Controlled Clinical Trials. 1998;19:159–66. doi: 10.1016/s0197-2456(97)00150-5. [DOI] [PubMed] [Google Scholar]
- Vickers A. Recent advances: complementary medicine. BMJ. 2000;321:683–6. doi: 10.1136/bmj.321.7262.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignola PA, Aonuma K, Swaye PS. Lymphocytic myocarditis presenting as unexplained ventricular arrhythmias: diagnosis with endomyocardial biopsy and response to immunosuppression. Journal of the American College of Cardiology. 1984;4:812–9. doi: 10.1016/s0735-1097(84)80411-8. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang ZZ, Tu XH, Zou ZD, Liu JH, Wang Y. Safety and efficacy of Qingre Buyi Decoction in the treatment of acute radiation proctitis: a prospective, randomized and controlled trial. Chinese journal of integrative medicine. 2009;15(4):272–8. doi: 10.1007/s11655-009-0272-z. [DOI] [PubMed] [Google Scholar]
- Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, Glanowska G, Wilczewski P, Niklewski T, et al. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: two-year follow-up results. Circulation. 2001;104(1):39–45. doi: 10.1161/01.cir.104.1.39. [DOI] [PubMed] [Google Scholar]
- Wu TJ. The diagnostic criteria of viral myocarditis. Chinese Journal of Pediatrics. 2000;38(2):75. [Google Scholar]
- Yang YZ, Jin PY, Guo Q, Wang QD, Li ZS, Ye YC, et al. Effect of Astragulas membranaceus on natural killer cell activity and induction of alpha- and gamma-interferon in patients with coxsackie B viral myocarditis. Chinese Medical Journal. 1990;103(4):304–7. [PubMed] [Google Scholar]
- Yang HB. Clinical observation on treatment of viral myocarditis with Injection of Restoring Pulse Beat [in Chinese] Acta Chinese Medicine and Pharmacology. 1997;25(3):11. [Google Scholar]
- Yang XZ, Cheng LH, Huang J. The brief history and development of Chinese propriety medicines. Jounal of Jiangxi University of TCM. 2006;18(5):18–9. [Google Scholar]
- Yin YS, Lu ZF. Observation on effect of Shengmai Injection in treating viral myocarditis [in Chinese] The Practical Journal of Integrated Chinese with Modern Medicine. 1997;10(15):1477–8. [Google Scholar]
- Zhao MH, Rong HZ, Lu BJ, Zhu XY, Huang GF, Yang JW. Effect of Shengmaisan on serum lipid peroxidation in acute viral myocarditis [in Chinese] Chinese Journal of Integrated Traditional and Western Medicine. 1996;16(3):142–5. [PubMed] [Google Scholar]