Abstract
In progressive kidney diseases, fibrosis represents the common pathway to end-stage kidney failure. Transforming growth factor-β1 (TGF-β1) is a pleiotropic cytokine that has been established as a central mediator of kidney fibrosis. Emerging evidence demonstrates a complex scheme of signaling networks that enable multifunctionality of TGF-β1 actions. Specific targeting of TGF-β signaling pathway is seemingly critical and attractive molecular therapeutic strategy. TGF-β1 signals through the interaction of type I (TβRI) and type II (TβRII) receptors to activate distinct intracellular pathways involving the Smad and the non-Smad. The Smad signaling axis is known as the canonical pathway induced by TGF-β1. Importantly, recent investigations show that TGF-β1 also induces various non-Smad signaling pathways. In this review, we focus on current insights into the mechanism and function of Smad-independent signaling pathway via TGF-β-activated kinase 1 (TAK1) and its role in mediating the profibrotic effects of TGF-β1.
Keywords: Transforming growth factor-β1, intracellular signaling, TGF-β-activated kinase 1, fibrosis, chronic kidney disease
Introduction
Most chronic kidney diseases, regardless of the nature of the initial injury, progress to end-stage renal disease (ESRD) with irreversible loss of tissue and function. Chronic kidney disease (CKD) has become a major public health concern worldwide as the incidence continues to rise and portends high rates of morbidity and mortality.1 Thus, improved and more effective therapies are critical. The hallmark of progressive CKD is the development of kidney fibrosis that is thought to be the final common mechanism leading to ESRD.2–4 The pathogenesis of kidney fibrosis is characterized by relentless production and progressive accumulation of extracellular matrix (ECM) proteins, such as collagen and fibronectin, within the kidney which strongly correlates with deterioration of kidney function.4,5 Transforming growth factor-beta 1 (TGF-β1) has been firmly established as a central mediator of kidney fibrosis associated with progressive kidney diseases.6,7 Fibrosis represents the final common pathway of tissue injury response that ultimately leads to end-stage kidney failure and, therefore, blocking TGF-β signaling pathways is an attractive approach to anti-fibrotic therapy.
TGF-β1 is the prototype member of TGF-β superfamily of multifunctional cytokines that acts as a key regulator of diverse cellular functions such as cellular differentiation, proliferation, apoptosis, and wound healing, and is a potent inducer of ECM synthesis.8,9 In response to tissue injury, chronically dysregulated expression and actions of TGF-β1 lead to the pathogenesis of kidney fibrosis seen in chronic progressive kidney diseases.6,10,11 However, more acutely, TGF-β1 promotes wound repair and tissue regeneration, as well as anti-inflammatory effects, and thereby exerts cytoprotective effects to mitigate tissue injury.11–16 Thus, TGF-β1 plays a dual, seemingly paradoxical, role in tissue injury response. This paradigm suggests that in developing therapeutic interventions, it may be unwise to devise strategies to simply indiscriminately inhibit TGF-β1 actions. Hence, a better understanding of the cellular and molecular mechanisms of TGF-β1 actions will provide the basis that will guide in the development of therapeutic strategies that specifically target its signaling pathway responsible for the deleterious effects of TGF-β1
TGF-β signaling receptors
TGF-β1 actions are mediated through heteromeric interactions of serine/threonine kinases, TGF-β type I (TβRI) and type II (TβRII) receptors, to activate intracellular signaling pathways.17 Recent investigations have helped to unravel a complex scheme of signaling network downstream of the TGF-β receptors that transduce signals from the cell surface to the nucleus and enable multi-functionality of TGF-β1 in controlling diverse biological functions in a cell-specific and context-specific manner. Initiation of the TGF-β signaling cascade requires ligand binding to TβRII, and in turn, TβRI and TβRII form hetero-tetrameric complexes, by which TβRI is phosphorylated in the cytoplasmic GS domain and activated, which is followed by activation of a number of intracellular signaling mediators of TGF-β1.
Intracellular signaling mediators: The Smad and the non-Smad
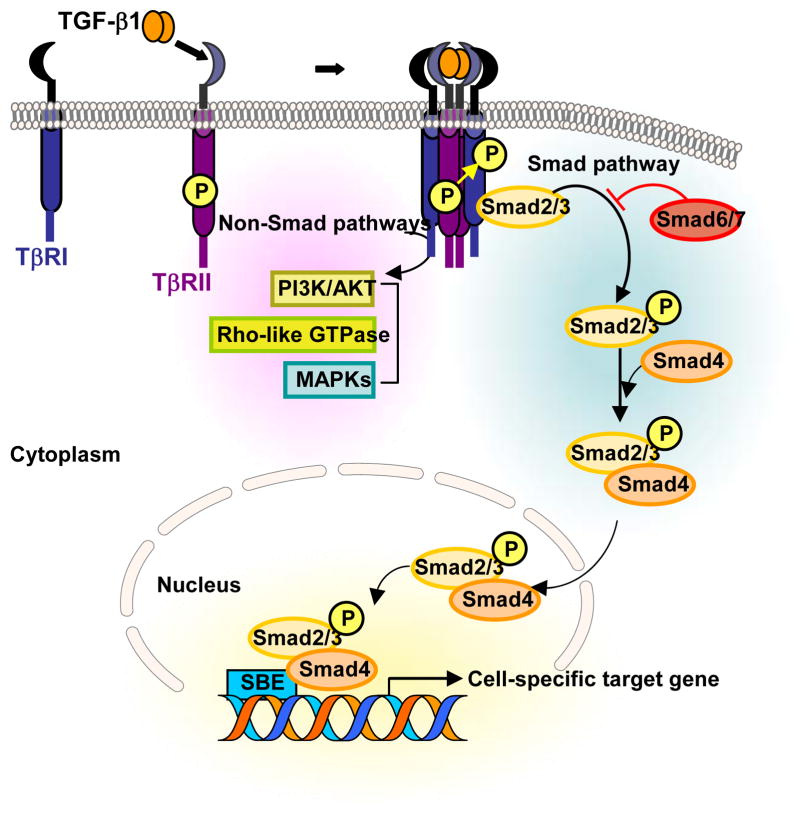
The Smads comprise a family of structurally related proteins that represent the human analogues of the Drosophila protein MAD (Mothers Against Decapentaplegic) and the Caenorhabditis elegans protein SMA (Small body size).17,18 Receptor-regulated Smads (R-Smads) are recruited and activated by the activated TβRI (Fig. 1). The phosphorylation in the GS domain19 and L45 loop20 of TβRI are thought to be crucial for its interaction with R-Smads. Subsequent serine/threonine phosphorylation of the R-Smads, Smad2 and Smad3, leads them to be rapidly dissociated from TβRI and interact to form complexes with common-mediator, Smad4, followed by nuclear translocation where they recognize regulatory Smad binding elements to transcriptionally activate or repress target genes.21 Both Smad 6 and Smad 7 compete with the R-Smads for binding to the activated receptors and thus function as inhibitory Smads.22
Fig. 1. Overview of TGF-β Signaling.
Initiation of the TGF-β signaling cascade occurs upon ligand binding to TβRII and subsequent TβRI-TβRII hetero-tetrameric complex formation, and TβRI is activated through phosphorylated in the cytoplasmic GS domain. This is followed by activation of intracellular signaling mediators of TGF-β1. The canonical Smad pathway involves activation of Smad2/3 through recruitment and phosphorylation by activated TβRI, and requires kinase activity of TβRI. Smad2/3 is then released from the receptor complex to interact with Smad4 to transmit TGF-β1 signals. TGF-β1 also induces various non-Smad signaling pathways, including the PI3K/AKT, Rho-like GTPases, and the MAPKs.
During the past decade, important advances in our understanding of TGF-β1-induced signaling have been made and much of the early investigations were focused on studies of Smad signaling which is widely accepted as a canonical pathway induced by TGF-β1.23 The role of Smads in the context of kidney health and disease is a topic of several previous reviews.24,25 However, it has become quite evident that the Smad signaling pathway does not explain all of the diverse actions of TGF-β1. In addition to the Smad, a growing body of evidence demonstrates that TGF-β1 also activates various Smad-independent signaling pathways, with or without direct crosstalk with the Smad.26,27
A number of noncanonical TGF-β signaling pathways has been identified, including the Rho-like GTPases,28,29 phosphatidylinositol-3-kinase (PI3K)/AKT,30–33 and the mitogen-activated protein kinases (MAPKs), namely extracellular signal-regulated kinase (Erk) 1/2,34,35 c-Jun N-terminal kinase (JNK),36–38 and p38 MAPK.39–42 Studies implicate the p38 MAPK signaling pathway in the development of fibrosis in animal models of glomerular and tubulointerstitial injury43,44 and in human kidney disease.45 We and others have demonstrated that TGF-β-activated kinase 1 (TAK1) is a major upstream signaling molecule in TGF-β1-induced type I collagen and fibronectin expression through activation of the MAPK kinase (MKK)3-p38 and MKK4-JNK signaling cascades, respectively (Fig. 2).46–48 Below, we review our current understanding of the molecular mechanisms of Smad-independent signaling pathway via TAK1 and its role in mediating the profibrotic effects of TGF-β1.
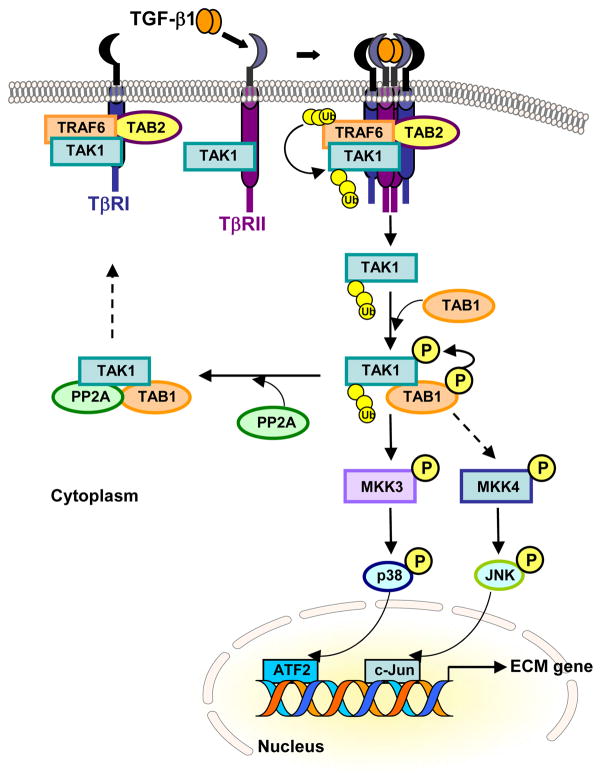
Fig. 2. Schema of TAK1 Signaling pathway.
Under unstimulated condition, TAK1 associates with TβRI through complex formation with TAB2 and TRAF6. Upon TGF-β1 stimulation and formation of TβRI-TβRII hetero-tetrameric complexes, autopolyubiqitination of TRAF6 leads to polyubiquitination (Ub) of TAK1, triggering release of TAK1 from the receptor complexes. TAK1 interacts with TAB1, which in turn induces autophosphorylation of TAK1. The activated TAK1 transmits TGF-β1 signal to downstream signaling pathways such as the MKK3-p38 or MKK4-JNK cascade, or is rapidly deactivated by phosphatase PP2A. TGF-β1-induced TAK1 activation occurs independent of TβRI kinase activity.
TGF-β-activated kinase 1 (TAK1)
TAK1, originally identified as a member of the MAPK kinase kinase (MAP3K) family and known as MAP3K7, is a serine/threonine kinase that is rapidly activated by TGF-β1.49,50 To date, TAK1 is the only MAP3K family member that has been directly implicated in TGF-β1 signaling. TAK1 can also be activated by various stimuli including environmental stress,51 proinflammatory cytokines such as tumor necrosis factor (TNF)-α52 and interleukin (IL)-1,53 and lipopolysaccharides (LPS).54 For TAK1 activation, phosphorylation at Thr-187 and Ser-192 in the activation loop of TAK1 is essential.55,56 Activated TAK1 can transduce signals to several downstream signaling cascades, including the MKK4/7-JNK, MKK3/6-p38 MAPK, and Nuclear Factor-kappa B (NF-kB)-inducing kinase (NIK)-IkB kinase (IKK).52–54 Recent report has shown that TAK1 is activated by agonists of AMP-activated kinase (AMPK) and ischemia, which in turn activates the LKB1/AMPK pathway, a key energy-sensor pathway.57 TAK1 is also involved in non-canonical Wnt signaling that functions as a negative feedback mechanism of canonical Wnt signaling.58 Furthermore, studies indicate that TAK1 can regulate TGF-β-induced activation of Smad signaling by inducing Smad7 expression59 and also interfering with R-Smad transactivation by direct interaction with the MH2 domain of Smad proteins.60 In addition to the role of TAK1 in the regulation of Smad function, there is cross-talk between the Smad and downstream targets of TAK1 such as p38 MAPK and ATF2 in regulation of certain TGF-β1 target gene expression.39,61,62 Collectively, these observations suggest that TAK1 might be the point of convergence in various signaling pathways activated by a variety of stimuli and play a pivotal role in regulating cellular responses.
Molecular mechanism of TAK1 activation
Role of TAK1-binding proteins: (TAB1, 2, 3)
TAK1 is unique among the MAP3K family members in that its activation requires complexing with specific binding partner known as TAK1-binding protein-1, -2, -3 (TAB1, 2, 3).63–65 The requirement of these TAK1-binding proteins appears to be dependent on the stimuli. Studies in Tab1-deficient mouse embryonic fibroblasts reveal that osmotic stress-induced activation of TAK1 is TAB1-dependent, but not in the case of certain cytokines such as TNF-α and IL-1-induced activation of TAK1,66 whereas TAB1 is essential for TAK1 activity and necessary for TGF-β signal transduction.67 We have shown that TAB1 is indispensable for TGF-β1-induced TAK1 activation in glomerular mesangial cells.68 Studies in vivo also demonstrate that the TAK1–TAB1 complex is crucial for normal embryonic development and morphogenesis, as genetic inactivation of TAB1 resulted in embryonic lethality and defects in development of major organs including the heart and lung.67 Moreover, evidence supports cell-type specificity in the involvement of the specific TAK1-binding proteins, such that, in HeLa cells, TNF-α-induced TAK1 activation involved signaling complex with TAB1 as well as TAB2.56 TAB3 is a TAB2-related protein which has been shown to play a role in TNF-α and IL-1 signaling pathways.64,65
Role of ubiquitin ligase TNF receptor-associated factor 6 (TRAF6)
The molecular events that link the activated TGF-β receptor complex and the intracellular signaling mediators have been well characterized from the standpoint of Smad signaling, whereas events that link the non-Smad signaling molecules are less clearly understood. We know that there are notable differences in the mechanism of Smad2/3 and TAK1 activation. TGF-β1-induced TAK1 activation occurs independent of TβRI kinase activity,68,69 whereas activation of Smad2/3 involves recruitment and phosphorylation by TβRI and requires kinase activity of TβRI.19,20 Activated Smad2/3 is then released from the receptor complex to interact with Smad4 to transmit TGF-β1 signals. We have reported that in glomerular mesangial cells, in the absence of ligand stimulation, TAK1 stably associates with TβRI.68 TGF-β1 stimulation causes rapid dissociation from the receptor complex and in turn activates TAK1. Thus, TGF-β1-induced formation of TβRI-TβRII complex triggers dissociation of TAK1 from TβRI, and subsequently TAK1 is activated through TAB1-mediated autophosphorylation, independent of receptor kinase activity of TβRI.
Although TAB1 is required for TGF-β1-induced TAK1 activation, TAB1 does not interact with TGF-β receptors. On the other hand, TAB2 and another adaptor protein TRAF6 are necessary for the interaction of TAK1 with TβRI and TGF-β1-induced TAK1 activation in glomerular mesangial cells.68 TRAF6 is a member of a family of RING (really interesting new gene) domain ubiquitin ligases that catalyzes synthesis of polyubiquitin chains linked through Lys-63 of ubiquitin. The highly conserved ubiquitin-binding zinc finger domains in TAB2 preferentially bind Lys-63-linked polyubiquitin chains on TRAF6 and facilitates TGF-β1-induced TAK1 activation.69–71 TβRI has been found to harbour a consensus binding site for TRAF6 and recent evidence reveals that TRAF6 physically interacts with TβRI and promotes Lys-63-dependent polyubiquitination of TAK1 Lys-34 and subsequent TAK1 activation.69–72 Thus, TβRI kinase activity is required for activation of the canonical Smad signaling pathway, whereas ubiquitin ligase activity of TRAF6 regulates the activation of TAK1 in a receptor kinase-independent manner. TGF-β1 specifically activates TAK1 through the interaction of TβRI with TRAF6, whereas Smad activation is not dependent on TRAF6.
Molecular mechanism of TAK1 inactivation
Dysregulation and persistent TGF-β1 actions are thought to lead to pathological states. Once TAK1 is activated, efficient down-regulation of TAK1 activity is important to prevent excessive TGF-β1 responses. Cyclic phosphorylation and dephosphorylation of kinases represent a fundamental mechanism responsible for tight regulation of intracellular signaling cascades. TAK1 requires phosphorylation at Thr-187 and Ser-192 within the activation loop of TAK1 for its activation55,56 and these sites, in turn, serve as substrates for protein phosphatases to inactivate TAK1. Several members of type 2A Ser/Thr protein phosphatase family have been identified as negative regulators of TAK1. Protein phosphatase 2C (PP2C) is capable of binding and dephosphorylating TAK1 in 293 cells under non-stimulated condition.73,74 More recently, a new Ser/Thr protein phosphatase family member PP6 has been shown to interact with and negatively regulate IL-1-induced TAK1 in 293 cells75 and TNF-induced TAK1 in fibroblasts.76 We reported that another type 2A protein phosphatase family member PP2A functions as a negative regulator of TAK1 activation in response to TGF-β1 in glomerular mesangial cells.77 PP2A associates with TAK1 and TAB1, and Thr-187 in the activation loop of TAK1 is a major dephosphorylation target of PP2A. Our findings in mesangial cells reveal that TAK1 is activated and deactivated very rapidly. This rapid activation and inactivation of TAK1 by TGF-β1 stimulation have been also observed in cardiac myocytes.78 These findings suggest that TAK1 activation is tightly regulated and may be controlled through rapid phosphorylation and dephosphorylation, and dysregulation of protein phosphatase-dependent dephosphorylation may cause of prolonged activation of TAK1.
TGF-β/TAK1 signaling and profibrotic response
TGF-β1 is considered the most potent profibrogenic cytokine, and there is increasing evidence implicating a critical role of TAK1 signaling in ECM production and pathogenesis of kidney fibrosis. In vitro studies show that TAK1 mediates TGF-β-induced expression of types I and IV collagens and fibronectin in cultured mesangial cells.46 We have previously reported that TGF-β1-induced activation of the downstream MKK3-p38 MAPK cascade leads to type I collagen expression40,41 and that TAK1 is a major upstream signaling molecule mediating TGF-β1-induced MKK3 activation and collagen induction in mesangial cells.47 In fibroblasts, TGF-β-induced fibronectin expression is mediated by TAK1 through MKK4-JNK signaling cascade48 and TAK1-deficient fibroblasts exhibited reduced profibrotic response to TGF-β1 stimulation.79 These studies help establish TAK1 as a major regulator of TGF-β signaling and pathogenic mechanisms in renal cellular injury and profibrotic response (Fig. 3).
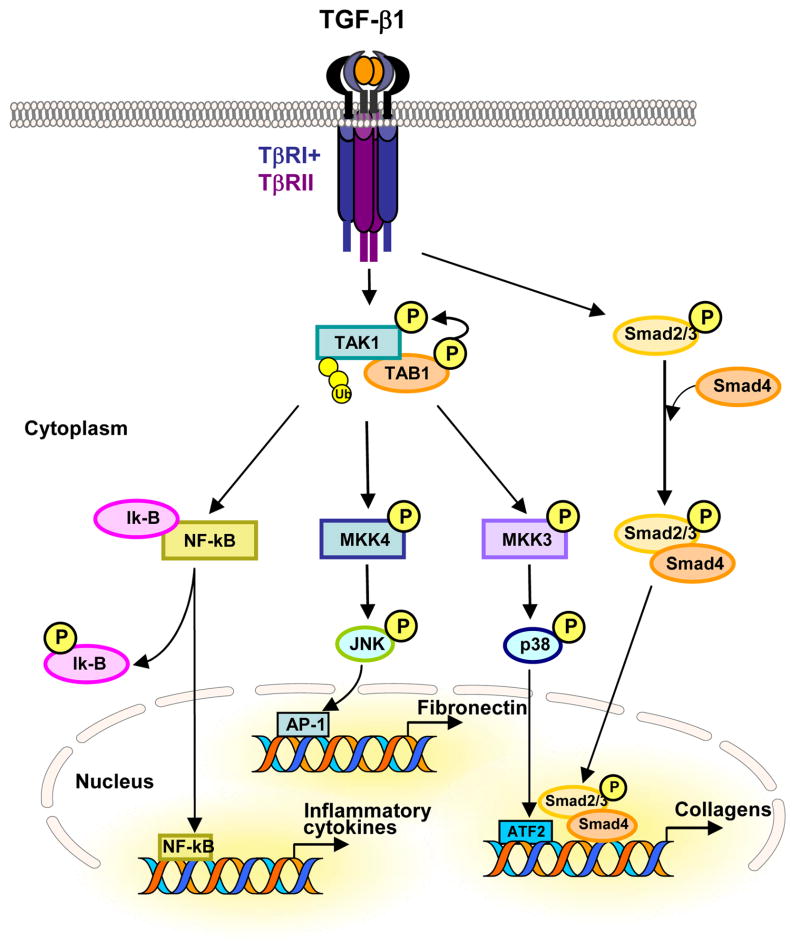
Fig. 3. TGF-β-induced TAK1 signaling and profibrotic response.
TGF-β1-induced TAK1 activation triggers downstream signaling pathways such as the MKK4-JNK, or the MKK3-p38 cascade, and promotes degradation of Ik-B, which in turn lead to the activation of transcription factors ATF-2, AP-1 and NF-kB, respectively, to regulate the expression of extracellular matrix proteins, including collagens and fibronectin, and inflammatory cytokines. In addition, TGF-β1-induced Smad activation is also indispensible for TGF-β1-induced type I collagen expression through the crosstalk with TAK1-MKK3-p38 signaling axis.
Several recent investigations have provided in vivo evidence that help to corroborate the critical role of TAK1 signaling pathway in tissue injury response and fibrosis. Studies using pharmacologic inhibitors and gene-deficient mice in experimental models of glomerular and tubulointerstitial injury, have shown that activation of MKK3-p38 MAPK44,45,80–82 and JNK83–86 pathways promotes renal inflammation and fibrosis. Blockade of MKK3-p38 MAPK or JNK pathways in unilateral ureteral obstruction (UUO) model of renal fibrosis resulted in substantial amelioration and protection against renal inflammation and fibrosis.44,86,87 Examination of human biopsy tissues have also implicated p38 MAPK45,88,89 and JNK84,85 signaling in the development of inflammation and fibrosis in human kidney disease. Both the p38 MAPK and JNK pathways are downstream targets of TAK1 activation. Ma et al.90 using conditional tak1 gene deletion in mice and the UUO model of renal fibrosis, reported that TAK1 deletion suppressed interstitial myofibroblast accumulation, collagen deposition, and expression of profibrotic molecules.90 Taken altogether, these studies establish strong evidence for a pathologic role for TAK1 signaling pathway in the development of inflammatory response and ECM elaboration and the pathogenesis of renal fibrosis.
The profibrotic function of TAK1 signaling is also demonstrated in the heart. Increased expression and activation of TAK1 lead to enhanced p38 MAPK phosphorylation and promote interstitial fibrosis in the myocardium of Tak1 transgenic mice.78 Interestingly, hepatocyte-specific deletion of Tak1 gene in mice resulted in spontaneous hepatocyte death, inflammation, fibrosis, and carcinogenesis, indicating that TAK1 signaling is an essential component for cellular homeostasis in the liver.91 The seemingly opposite effects suggest that TAK1 signaling is capable of exerting dual functions, like TGF-β1, in tissue/cell type and context dependent manner.
TAK1 signaling in apoptosis and autophagy
TGF-β1 is known to regulate cell survival and cell death, and TAK1 in a similar fashion possesses pro- and anti-apoptotic functions. The TAK1-null phenotype is lethal early in embryonic development, and knockdown of TAK1 expression or inhibition of TAK1 activation augments cell apoptosis induced by TGF-β1 in various cell types in vitro and in vivo, including the kidney, indicating that TAK1 is required for prevention of apoptosis and plays a role as a cell survival factor.90,92 Conditional tak1 gene deletion in mice resulted in a two-fold increase in the apoptotic response in the obstructed kidney by UUO.90 In contrast, abrogation of TAK1 activation inhibits TGF-β-induced apoptosis in embryonic fibroblasts, prostate cancer cells, and AML12 liver cells, indicating that TAK1 also acts as a mediator of apoptosis.69,70
The role of TGF-β1 as an inducer of autophagy is just beginning to be appreciated, and TAK1 signaling pathway in the regulation of autophagy has been hereto understudied. Autophagy, also known as macro-autophagy (literally, self-eating), is a fundamental cellular homeostatic process by which cells degrade and recycle proteins and remove damaged organelles.93 In HuH7 human hepatocellular carcinoma cells, MDA-MB-231 breast cancer cells, and primary mouse mesangial cells, TGF-β1 induces accumulation of autophagosomes and conversion of microtubule-associated protein 1 light chain 3 (LC3) to the lapidated form, LC3-II.33,94 LC3-II serves as a valuable molecular biomarker for the detection and assessment of autophagic activity.95 TGF-β1 also increases the mRNA expression levels of several autophagy-related genes (ATG), such as Beclin 1, ATG5, ATG7, DAPK (death-associated protein kinase) and LC3 through the non-Smad signaling pathways as well as the Smad pathway.33,94,96 Recent studies have demonstrated that TAK1-MKK3-p38 signaling axis is critical for the regulation of LC3 expression,33 whereas NF-kB and JNK are positive regulators of Beclin 1 expression.97 Moreover, JNK phosphorylation of Bcl-2 results in Beclin 1 activation by promoting the dissociation of Beclin 1 from Bcl-2.98 Thus, a number of distinct mechanisms are implicated in TAK1-mediated signaling pathways that activate autophagy (Fig. 4).
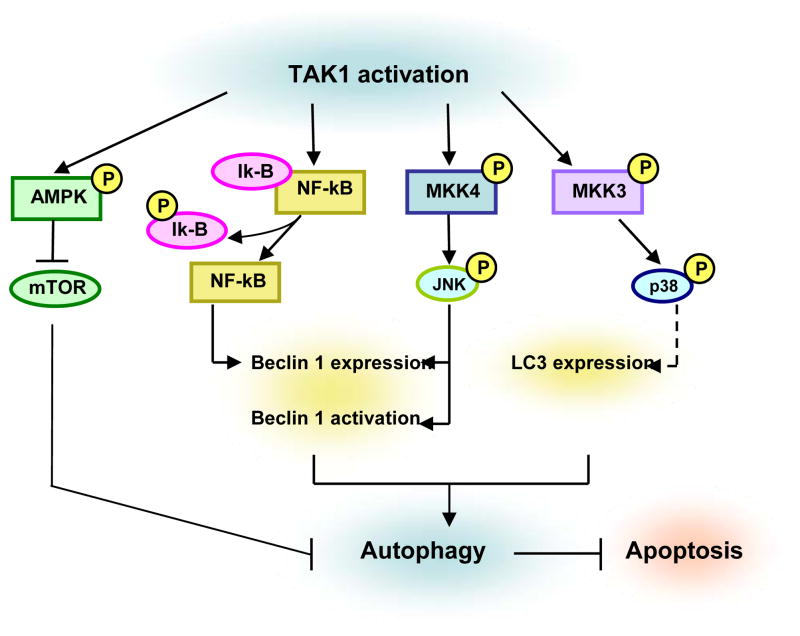
Fig. 4. Signaling pathways implicated in TAK1-mediated autophagy induction.
TAK1 activation of MKK3-p38 induces the expression of LC3, whereas JNK activation induces the expression and activation of Beclin 1. NF-kB directly interacts with Beclin 1 promoter region and enhances Beclin 1 transcription. TAK1 activation also leads to the activation of AMPK and inhibits mTOR activity resulting in induction of autophagy. Accumulating evidence indicates that activation of autophagy inhibits apoptosis and can function as a cytoprotective response.
Autophagy can lead to cell death in response to stress, but it can also act as a protective mechanism for cell survival. It is plausible that the functions of TGF-β1 as both an apoptosis promoter and apoptosis suppressor may relate to its regulation of autophagy. Indeed, it has recently reported that tumor necrosis factor-related apoptosis inducing ligand (TRAIL), which triggers apoptosis preferentially in cancer cells, spares normal untransformed cells from apoptosis by inducing cytoprotective autophagy via TAK1-dependent AMPK activation.99 AMPK inhibits mammalian target of rapamycin (mTOR), a potent inhibitor of autophagy. We have reported that TGF-β1 induces autophagy through TAK1 and Akt activation, and protects glomerular mesangial cells from undergoing apoptosis during serum deprivation.33 Although the precise mechanism by which TGF-β1-induced autophagy prevents cell death via apoptosis is not well understood, recent evidence indicates that active caspase-8, a death receptor effector, can be degraded through autophagy.100
Implications in anti-TGF therapy
Although there has been a plethora of evidence in preclinical studies that TGF-β blockade diminishes fibrosis in experimental models, limited advances have been made to date in treatment of human disease. A phase I/II randomized, placebo-controlled trial of TGF-β inhibitor therapy using a human anti-TGF-β antibody (Cat-192) in 45 patients with scleroderma was not associated with any clinical benefits in these patients.101 Recently, the results of the first phase I clinical study using neutralizing anti-TGF-β antibodies for the treatment of kidney disease were reported.102 Fresolimumab is a human monoclonal antibody that neutralizes all three isoforms of TGF-β. The phase I open-label study designed to assess the safety, tolerability, and pharmacokinetics of single-dose infusion of fresolimumab was conducted in patients with treatment-resistant primary focal segmental glomerulosclerosis (FSGS).101 The results of this phase I clinical trial indicate that fresolimumab is relatively safe and well tolerated in patient. Larger randomized clinical trial to assess efficacy of this agent is anticipated.
Other anti-fibrotic therapy that is garnering interest is pirfenidone, which may be partially mediated by the inhibition of TGF-β TGF-β promoter activity and TGF-β protein secretion, and inhibiting TGF-β–induced Smad2 phosphorylation.103 In early clinical studies, beneficial effects of pirfenidone were noted in a multicenter, double-blind, placebo-controlled, randomized phase III clinical trial of patients with idiopathic pulmonary fibrosis,104 and pirfenidone has shown promise as a therapy to slow renal function decline in patients with FSGS.105
Better anti-fibrotic targets are still needed for an effective therapy in human fibrotic diseases. Excessive TGF-β1 activity leads to fibrotic conditions. A potential candidate is targeting the TAK1 signaling pathway to block TGF-β-induced fibrotic responses. Blockade of TAK1 is a plausible and attractive strategy that targets major proinflammatory, proapoptotic, and profibrotic pathways, such as the p38 MAPK and JNK, in the development of progressive kidney disease. In this endeavor, many formidable challenges are anticipated for future investigations. We are keenly cognizant that we still have a great need to gain increased insights into complex TGF-β signal transduction pathways, and that we have much to discover in this exciting field.
Acknowledgments
This work was supported in part by NIH R01#DK57661 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the M. James Scherbenske Grant from the American Society of Nephrology (ASN) to MEC; and the Carl W. Gottschalk Research Scholar Grant to SIK.
Footnotes
Financial disclosure and conflict of interest statements: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–59. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 2.Iwano M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens. 2004;13:279–84. doi: 10.1097/00041552-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–9. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;6:643–56. doi: 10.1038/nrneph.2010.120. [DOI] [PubMed] [Google Scholar]
- 5.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol. 2002;283:F861–75. doi: 10.1152/ajprenal.00362.2001. [DOI] [PubMed] [Google Scholar]
- 6.Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994;331:1286–92. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 7.Cheng J, Grande JP. Transforming growth factor-beta signal transduction and progressive renal disease. Exp Biol Med. 2002;227:943–56. doi: 10.1177/153537020222701102. [DOI] [PubMed] [Google Scholar]
- 8.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–71. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 9.Massagué J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–91. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 10.Lee LK, Meyer TW, Pollock AS, Lovett DH. Endothelial cell injury initiates glomerular sclerosis in the rat remnant kidney. J Clin Invest. 1995;96:953–964. doi: 10.1172/JCI118143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blobe GC, Schiemann WP, Lodish HF. Role of Transforming Growth Factor β in Human Disease. N Engl J Med. 2000;342:1350–8. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 12.Majesky MW, Volkhard L, Twardzik DR, Schwartz SM, Reidy MA. Production of transforming growth factor β1 during repair of arterial injury. J Clin Invest. 1991;88:904–10. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 14.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–9. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, et al. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–4. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Huang XR, Li AG, Liu F, Truong LD, Wang XJ, et al. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: role of Smad7. J Am Soc Nephrol. 2005;16:1371–83. doi: 10.1681/ASN.2004121070. [DOI] [PubMed] [Google Scholar]
- 17.Massagué J. How cells read TGF-β signals. Nature Rev. 2000;1:169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 18.Massagué J, Wotton D. Transcriptional control by the TGF-β/Smad signaling system. EMBO J. 2000;19:1745–54. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu G, Chen YG, Ozdamar B, Gyuricza CA, Chong PA, Wrana JL, Massagué J, Shi Y. Structural basis of Smad2 recognition by the Smad anchor for receptor activation. Science. 2000;287:92–7. doi: 10.1126/science.287.5450.92. [DOI] [PubMed] [Google Scholar]
- 20.Chen YG, Hata A, Lo RS, Wotton D, Shi Y, Pavletich N, Massagué J. Determinants of specificity in TGF-beta signal transduction. Genes Dev. 1998;12:2144–52. doi: 10.1101/gad.12.14.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 23.Massagué J, Blain SW, Lo RS. TGFβ signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 24.Bottinger EP, Bitzer M. TGF-β Signaling in Renal Disease. J Am Soc Nephrol. 2002;13:2600–10. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 25.Schnaper HW, Hayashida T, Poncelet AC. It’s a Smad World: Regulation of TGF-β Signaling in the Kidney. J Am Soc Nephrol. 2001;13:1126–8. doi: 10.1681/ASN.V1341126. [DOI] [PubMed] [Google Scholar]
- 26.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 27.Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–84. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 28.Edlund S, Landström M, Heldin CH, Aspenström P. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13:902–14. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–10. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- 31.Yi JY, Shin I, Arteaga CL. Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase. J Biol Chem. 2005;280:10870–6. doi: 10.1074/jbc.M413223200. [DOI] [PubMed] [Google Scholar]
- 32.Wilkes MC, Mitchell H, Penheiter SG, Doré JJ, Suzuki K, Edens M, et al. Transforming growth factor-beta activation of phosphatidylinositol 3-kinase is independent of Smad2 and Smad3 and regulates fibroblast responses via p21-activated kinase-2. Cancer Res. 2005;65:10431–40. doi: 10.1158/0008-5472.CAN-05-1522. [DOI] [PubMed] [Google Scholar]
- 33.Ding Y, Kim JK, Kim SI, Na HJ, Jun SY, Lee SJ, Choi ME. TGF-beta1 protects against mesangial cell apoptosis via induction of autophagy. J Biol Chem. 2010;285:37909–19. doi: 10.1074/jbc.M109.093724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartsough MT, Mulder KM. Transforming growth factor β activation of p44mapk in proliferating cultures of epithelial cells. J Biol Chem. 1995;270:7117–24. doi: 10.1074/jbc.270.13.7117. [DOI] [PubMed] [Google Scholar]
- 35.Mucsi I, Skorecki KL, Goldberg HJ. Extracellular signal-regulated kinase and the small GTP-binding protein, Rac, contribute to the effects of transforming growth factor-beta1 on gene expression. J Biol Chem. 1996;271:16567–72. doi: 10.1074/jbc.271.28.16567. [DOI] [PubMed] [Google Scholar]
- 36.Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor beta-mediated signaling. J Biol Chem. 1997;272:1429–32. doi: 10.1074/jbc.272.3.1429. [DOI] [PubMed] [Google Scholar]
- 37.Hocevar BA, Brown TL, Howe PH. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–56. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yue J, Sun B, Liu G, Mulder KM. Requirement of TGF-beta receptor-dependent activation of c-Jun N-terminal kinases (JNKs)/stress-activated protein kinases (Sapks) for TGF-beta up-regulation of the urokinase-type plasminogen activator receptor. J Cell Physiol. 2004;199:284–92. doi: 10.1002/jcp.10469. [DOI] [PubMed] [Google Scholar]
- 39.Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, Matsumoto K, Nishida E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274:27161–7. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- 40.Chin BY, Mohsenin A, Li SX, Choi AM, Choi ME. Stimulation of pro-alpha(1)(I) collagen by TGF-beta(1) in mesangial cells: role of the p38 MAPK pathway. Am J Physiol Renal Physiol. 2001;280:F495–504. doi: 10.1152/ajprenal.2001.280.3.F495. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Ma R, Flavell RA, Choi ME. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for activation of p38alpha and p38delta MAPK isoforms by TGF-beta 1 in murine mesangial cells. J Biol Chem. 2002;277:47257–62. doi: 10.1074/jbc.M208573200. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Barbero A, Obreo J, Yuste L, Montero JC, Rodriguez-Pena A, Pandiella A, et al. Transforming growth factor-beta1 induces collagen synthesis and accumulation via p38 mitogen-activated protein kinase (MAPK) pathway in cultured L(6)E(9) myoblasts. FEBS Lett. 2002;513:282–8. doi: 10.1016/s0014-5793(02)02337-2. [DOI] [PubMed] [Google Scholar]
- 43.Lim AK, Nikolic-Paterson DJ, Ma FY, Ozols E, Thomas MC, Flavell RA, et al. Role of MKK3-p38 MAPK signaling in the development of type 2 diabetes and renal injury in obese db/db mice. Diabetologia. 2009;52:347–58. doi: 10.1007/s00125-008-1215-5. [DOI] [PubMed] [Google Scholar]
- 44.Stambe C, Atkins RC, Tesch GH, Masaki T, Schreiner GF, Nikolic-Paterson DJ. The role of p38alpha mitogen-activated protein kinase activation in renal fibrosis. J Am Soc Nephrol. 2004;15:370–9. doi: 10.1097/01.asn.0000109669.23650.56. [DOI] [PubMed] [Google Scholar]
- 45.Adhikary L, Chow F, Nikolic-Paterson DJ, Stambe C, Dowling J, Atkins RC, et al. Abnormal p38 mitogen-activated protein kinase signaling in human and experimental diabetic nephropathy. Diabetologia. 2004;47:1210–22. doi: 10.1007/s00125-004-1437-0. [DOI] [PubMed] [Google Scholar]
- 46.Ono K, Ohtomo T, Ninomiya-Tsuji J, Tsuchiya M. A dominant negative TAK1 inhibits cellular fibrotic responses induced by TGF-β. Biochem Biophys Res Commun. 2003;307:332–7. doi: 10.1016/s0006-291x(03)01207-5. [DOI] [PubMed] [Google Scholar]
- 47.Kim SI, Kwak JH, Zachariah M, He Y, Wang L, Choi ME. TGF-beta-activated kinase 1 and TAK1-binding protein 1 cooperate to mediate TGF-beta1-induced MKK3-p38 MAPK activation and stimulation of type I collagen. Am J Physiol Renal Physiol. 2007;292:F1471–8. doi: 10.1152/ajprenal.00485.2006. [DOI] [PubMed] [Google Scholar]
- 48.Hocevar BA, Prunier C, Howe PH. Disabled-2 (Dab2) Mediates Transforming Growth Factor β (TGFβ)-stimulated Fibronectin Synthesis through TGFβ-activated Kinase 1 and Activation of the JNK Pathway. J Biol Chem. 2005;280:25920–7. doi: 10.1074/jbc.M501150200. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–11. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 50.Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E, Matsumoto K. TAB1: an activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science. 1996;272:1179–82. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- 51.Shirakabe K, Yamaguchi K, Shibuya H, Irie K, Matsuda S, Moriguchi T, Gotoh Y, Matsumoto K, Nishida E. TAK1 mediates the ceramide signaling to stress-activated protein kinase/c-Jun N-terminal kinase. J Biol Chem. 1997;272:8141–4. doi: 10.1074/jbc.272.13.8141. [DOI] [PubMed] [Google Scholar]
- 52.Sakurai H, Suzuki S, Kawasaki N, Nakano H, Okazaki T, Chino A, Doi T, Saiki I. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J Biol Chem. 2003;278:36916–23. doi: 10.1074/jbc.M301598200. [DOI] [PubMed] [Google Scholar]
- 53.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–6. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 54.Irie T, Muta T, Takeshig K. TAK1 mediates an activation signal from toll-like receptor(s) to nuclear factor-kappaB in lipopolysaccharide-stimulated macrophages. FEBS Lett. 2000;467:160–4. doi: 10.1016/s0014-5793(00)01146-7. [DOI] [PubMed] [Google Scholar]
- 55.Kishimoto K, Matsumoto K, Ninomiya-Tsuji J. TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J Biol Chem. 2000;275:7359–64. doi: 10.1074/jbc.275.10.7359. [DOI] [PubMed] [Google Scholar]
- 56.Singhirunnusorn P, Suzuki S, Kawasaki N, Saiki I, Sakurai H. Critical roles of threonine 187 phosphorylation in cellular stress-induced rapid and transient activation of transforming growth factor-beta-activated kinase 1 (TAK1) in a signaling complex containing TAK1-binding protein TAB1 and TAB2. J Biol Chem. 2005;280:7359–68. doi: 10.1074/jbc.M407537200. [DOI] [PubMed] [Google Scholar]
- 57.Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL, Taffet GE, Baldini A, Khoury DS, Schneider MD. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci U S A. 2006;103:17378–83. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smit L, Baas A, Kuipers J, Korswagen H, van de Wetering M, Clevers H. Wnt activates the Tak1/Nemo-like kinase pathway. J Biol Chem. 2004;279:17232–40. doi: 10.1074/jbc.M307801200. [DOI] [PubMed] [Google Scholar]
- 59.Dowdy SC, Mariani A, Janknecht R. HER2/Neu- and TAK1-mediated up-regulation of the transforming growth factor beta inhibitor Smad7 via the ETS protein ER81. J Biol Chem. 2003;278:44377–84. doi: 10.1074/jbc.M307202200. [DOI] [PubMed] [Google Scholar]
- 60.Hoffmann A, Preobrazhenska O, Wodarczyk C, Medler Y, Winkel A, Shahab S, et al. Transforming growth factor-beta-activated kinase-1 (TAK1), a MAP3K, interacts with Smad proteins and interferes with osteogenesis in murine mesenchymal progenitors. J Biol Chem. 2005;280:27271–83. doi: 10.1074/jbc.M503368200. [DOI] [PubMed] [Google Scholar]
- 61.Sano Y, Harada J, Tashiro S, Gotoh-Mandeville R, Maekawa T, Ishii S. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-beta signaling. J Biol Chem. 1999;274:8949–57. doi: 10.1074/jbc.274.13.8949. [DOI] [PubMed] [Google Scholar]
- 62.Abécassis L, Rogier E, Vazquez A, Atfi A, Bourgeade MF. Evidence for a role of MSK1 in transforming growth factor-beta-mediated responses through p38alpha and Smad signaling pathways. J Biol Chem. 2004;279:30474–9. doi: 10.1074/jbc.M403294200. [DOI] [PubMed] [Google Scholar]
- 63.Sakurai H, Miyoshi H, Mizukami J, Sugita T. Phosphorylation-dependent activation of TAK1 mitogen-activated protein kinase kinase kinase by TAB1. FEBS Lett. 2000;474:141–5. doi: 10.1016/s0014-5793(00)01588-x. [DOI] [PubMed] [Google Scholar]
- 64.Ishitani T, Takaesu G, Ninomiya-Tsuji J, Shibuya H, Gaynor RB, Matsumoto K. Role of the TAB2-related protein TAB3 in IL-1 and TNF signaling. EMBO J. 2003;22:6277–88. doi: 10.1093/emboj/cdg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheung PC, Nebreda AR, Cohen P. TAB3, a new binding partner of the protein kinase TAK1. Biochem J. 2004;378:27–34. doi: 10.1042/BJ20031794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inagaki M, Omori E, Kim JY, Komatsu Y, Scott G, Ray MK, et al. TAK1-binding protein 1, TAB1, mediates osmotic stress-induced TAK1 activation but is dispensable for TAK1-mediated cytokine signaling. J Biol Chem. 2008;283:33080–6. doi: 10.1074/jbc.M807574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Komatsu Y, Shibuya H, Takeda N, Ninomiya-Tsuji J, Yasui T, Miyado K, et al. Targeted disruption of the Tab1 gene causes embryonic lethality and defects in cardiovascular and lung morphogenesis. Mech Dev. 2002;119:239–49. doi: 10.1016/s0925-4773(02)00391-x. [DOI] [PubMed] [Google Scholar]
- 68.Kim SI, Kwak JH, Na HJ, Kim JK, Ding Y, Choi ME. Transforming growth factor-beta (TGF-β1) activated TAK1 via TAB1-mediated autophosphorylation, independent of TGF-beta receptor kinase activity in mesangial cells. J Biol Chem. 2009;284:22285–96. doi: 10.1074/jbc.M109.007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, et al. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10:1199–207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- 70.Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE. TRAF6 Mediates Smad-Independent Activation of JNK and p38 by TGF-β. Mol Cell. 2008;31:918–24. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, et al. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–48. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 72.Mu Y, Sundar R, Thakur N, Ekman M, Gudey SK, Yakymovych M, et al. TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer. Nat Commun. 2011;2:330. doi: 10.1038/ncomms1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanada M, Ninomiya-Tsuji J, Komaki K, Ohnishi M, Katsura K, Kanamaru R, et al. Regulation of the TAK1 signaling pathway by protein phosphatase 2C. J Biol Chem. 2001;276:5753–9. doi: 10.1074/jbc.M007773200. [DOI] [PubMed] [Google Scholar]
- 74.Li MG, Katsura K, Nomiyama H, Komaki K, Ninomiya-Tsuji J, Matsumoto K, et al. Regulation of the interleukin-1-induced signaling pathways by a novel member of the protein phosphatase 2C family (PP2Cepsilon) J Biol Chem. 2003;278:12013–21. doi: 10.1074/jbc.M211474200. [DOI] [PubMed] [Google Scholar]
- 75.Kajino T, Ren H, Iemura S, Natsume T, Stefansson B, Brautigan DL, et al. Protein phosphatase 6 down-regulates TAK1 kinase activation in the IL-1 signaling pathway. J Biol Chem. 2006;281:39891–6. doi: 10.1074/jbc.M608155200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Broglie P, Matsumoto K, Akira S, Brautigan DL, Ninomiya-Tsuji J. Transforming growth factor beta-activated kinase 1 (TAK1) kinase adaptor, TAK1-binding protein 2, plays dual roles in TAK1 signaling by recruiting both an activator and an inhibitor of TAK1 kinase in tumor necrosis factor signaling pathway. J Biol Chem. 2010;285:2333–9. doi: 10.1074/jbc.M109.090522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim SI, Kwak JH, Wang L, Choi ME. Protein phosphatase 2A is a negative regulator of transforming growth factor-beta1-induced TAK1 activation in mesangial cells. J Biol Chem. 2008;283:10753–63. doi: 10.1074/jbc.M801263200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang D, Gaussin V, Taffet GE, Belaguli NS, Yamada M, Schwartz RJ, et al. TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat Med. 2000;6:556–63. doi: 10.1038/75037. [DOI] [PubMed] [Google Scholar]
- 79.Shi-wen X, Parapuram SK, Pala D, Chen Y, Carter DE, Eastwood M, et al. Requirement of transforming growth factor β-activated kinase 1 for transforming growth factor β-induced alpha-smooth muscle actin expression and extracellular matrix contraction in fibroblasts. Arthritis Rheum. 2009;60:234–41. doi: 10.1002/art.24223. [DOI] [PubMed] [Google Scholar]
- 80.Lim AK, Nikolic-Paterson DJ, Ma FY, Ozols E, Thomas MC, Flavell RA, Davis RJ, Tesch GH. Role of MKK3-p38 MAPK signaling in the development of type 2 diabetes and renal injury in obese db/db mice. Diabetologia. 2009;52:347–58. doi: 10.1007/s00125-008-1215-5. [DOI] [PubMed] [Google Scholar]
- 81.Ma FY, Tesch GH, Flavell RA, Davis RJ, Nikolic-Paterson DJ. MKK3-p38 signaling promotes apoptosis and the early inflammatory response in the obstructed mouse kidney. Am J Physiol Renal Physiol. 2007;293:F1556–63. doi: 10.1152/ajprenal.00010.2007. [DOI] [PubMed] [Google Scholar]
- 82.Tan HB, Feng Y, Liu M, Wu YC. Protective effects of FR167653 on chronic allograft nephropathy by inhibiting p38 MAPK in rats. Transplant Proc. 2008;40:1685–9. doi: 10.1016/j.transproceed.2008.01.073. [DOI] [PubMed] [Google Scholar]
- 83.Flanc RS, Ma FY, Tesch GH, Han Y, Atkins RC, Bennett BL, et al. A pathogenic role for JNK signaling in experimental anti-GBM glomerulonephritis. Kidney Int. 2007;72:698–708. doi: 10.1038/sj.ki.5002404. [DOI] [PubMed] [Google Scholar]
- 84.de Borst MH, Prakash J, Sandovici M, Klok PA, Hamming I, Kok RJ, et al. c-Jun NH2-terminal kinase is crucially involved in renal tubulo-interstitial inflammation. J Pharmacol Exp Ther. 2009;331:896–905. doi: 10.1124/jpet.109.154179. [DOI] [PubMed] [Google Scholar]
- 85.Kanellis J, Ma FY, Kandane-Rathnayake R, Dowling JP, Polking-horne KR, Bennett BL, et al. JNK signaling in human and experimental renal ischaemia/reperfusion injury. Nephrol Dial Transplant. 2010;25:2898–908. doi: 10.1093/ndt/gfq147. [DOI] [PubMed] [Google Scholar]
- 86.Ma FY, Flanc RS, Tesch GH, Han Y, Atkins RC, Bennett BL, Friedman GC, Fan JH, Nikolic-Paterson DJ. A pathogenic role for c-Jun amino-terminal kinase signaling in renal fibrosis and tubular cell apoptosis. J Am Soc Nephrol. 2007;18:472–84. doi: 10.1681/ASN.2006060604. [DOI] [PubMed] [Google Scholar]
- 87.Ma FY, Tesch GH, Flavell RA, Davis RJ, Nikolic-Paterson DJ. MKK3-p38 signaling promotes apoptosis and the early inflammatory response in the obstructed mouse kidney. Am J Physiol Renal Physiol. 2007;293:F1556–63. doi: 10.1152/ajprenal.00010.2007. [DOI] [PubMed] [Google Scholar]
- 88.Sakai N, Wada T, Furuichi K, Iwata Y, Yoshimoto K, Kitagawa K, et al. p38 MAPK phosphorylation and NF-kappa B activation in human crescentic glomerulonephritis. Nephrol Dial Transplant. 2002;17:998–1004. doi: 10.1093/ndt/17.6.998. [DOI] [PubMed] [Google Scholar]
- 89.Stambe C, Nikolic-Paterson DJ, Hill PA, Dowling J, Atkins RC. p38 Mitogen-activated protein kinase activation and cell localization in human glomerulonephritis: correlation with renal injury. J Am Soc Nephrol. 2004;15:326–36. doi: 10.1097/01.asn.0000108520.63445.e0. [DOI] [PubMed] [Google Scholar]
- 90.Ma FY, Tesch GH, Ozols E, Xie M, Schneider MD, Nikolic-Paterson DJ. TGF-β1 activated kinase-1 (TAK1) regulates inflammation and fibrosis in the obstructed kidney. Am J Physiol Renal Physiol. 2011;300:F1410–21. doi: 10.1152/ajprenal.00018.2011. [DOI] [PubMed] [Google Scholar]
- 91.Inokuchi S, Aoyama T, Miura K, Osterreicher CH, Kodama Y, Miyai K, Akira S, Brenner DA, Seki E. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci U S A. 2010;107:844–9. doi: 10.1073/pnas.0909781107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Omori E, Matsumoto K, Zhu S, Smart RC, Ninomiya-Tsuji J. Ablation of TAK1 upregulates reactive oxygen species and selectively kills tumor cells. Cancer Res. 2010;70:8417–25. doi: 10.1158/0008-5472.CAN-10-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–77. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 94.Kiyono K, Suzuki HI, Matsuyama H, Morishita Y, Komuro A, Kano MR, et al. Autophagy is activated by TGF-beta and potentiates TGF-beta-mediated growth inhibition in human hepatocellular carcinoma cells. Cancer Res. 2009;69:8844–52. doi: 10.1158/0008-5472.CAN-08-4401. [DOI] [PubMed] [Google Scholar]
- 95.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36:2503–18. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li DD, Wang LL, Deng R, Tang J, Shen Y, Guo JF, et al. The pivotal role of c-Jun NH2-terminal kinase-mediated Beclin 1 expression during anticancer agents-induced autophagy in cancer cells. Oncogene. 2009;28:886–898. doi: 10.1038/onc.2008.441. [DOI] [PubMed] [Google Scholar]
- 98.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–88. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Herrero-Martin G, Hoyer-Hansen M, Garcia-Garcia C, Fumarola C, Farkas T, Lopez-Rivas A, et al. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28:677–85. doi: 10.1038/emboj.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hou W, Han J, Lu C, Goldstein LA, Rabinowich H. Autophagic degradation of active caspase-8: a crosstalk mechanism between autophagy and apoptosis. Autophagy. 2010;6:891–900. doi: 10.4161/auto.6.7.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Denton CP, Merkel PA, Furst DE, Khanna D, Emery P, Hsu VM, et al. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 2007;56:L323–33. doi: 10.1002/art.22289. [DOI] [PubMed] [Google Scholar]
- 102.Trachtman H, Fervenza FC, Gipson DS, Heering P, Jayne DR, Peters H, et al. A phase 1, single-dose study of fresolimumab, an anti-TGF-β antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int. 2011;79:1236–43. doi: 10.1038/ki.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.RamachandraRao SP, Zhu Y, Ravasi T, McGowan TA, Toh I, Dunn SR, et al. Pirfenidone is renoprotective in diabetic kidney disease. J Am Soc Nephrol. 2009;20:1765–75. doi: 10.1681/ASN.2008090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cho ME, Smith DC, Branton MH, Penzak SR, Kopp JB. Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2007;2:906–13. doi: 10.2215/CJN.01050207. [DOI] [PubMed] [Google Scholar]
- 105.Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–9. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]