Abstract
Background
Measurement of the serum thyroglobulin (Tg) level with TSH stimulation (sTg) is the cornerstone of monitoring for the recurrence or persistence of differentiated thyroid cancer (DTC) in patients who have undergone surgery and remnant ablation. However, there have been several reports that an undetectable sTg could not predict the absence of future recurrence. The aim of this study was to evaluate the long-term outcome of DTC patients who achieved biochemical remission (BR, defined as sTg<1 ng/mL) after initial treatment, and to determine the role of repeated sTg measurement in detecting a clinical recurrence.
Methods
This is a retrospective observational cohort study in a tertiary referral hospital. There were 1010 DTC patients who achieved BR at 12 months after the initial treatment (surgery and ablation), and they were eligible for analysis. Among them, 787 patients had values of repeated sTg.
Results
Thirteen out of 1010 (1.3%) patients had clinical recurrences during a median 84 months of follow-up. All of the clinical recurrences were limited to the cervical lymph nodes without clinical evidence of distant metastasis. Among 787 patients with available repeated sTg, 10 had clinical recurrences (5 out of 750 patients with repeated sTg<1 ng/mL and 5 out of 37 patients with repeated sTg≥1 ng/mL). Patients with repeated sTg ≥1 ng/mL had a much greater chance of disease recurrence (log-rank statistics=43.7, df=1, p<0.001).
Conclusions
About 1% of DTC patients who had sTg<1 ng/mL 12 months after initial treatment had a clinical recurrence. All of clinical recurrences were loco-regional recurrences. Although repeated sTg measurement can be helpful to predict recurrence, we could not recommend it for surveillance in patients with BR due to its very low yield.
Introduction
Serum thyroglobulin (Tg) is a very useful tumor marker for differentiated thyroid cancer (DTC), after all noncancerous thyroid tissue has been eliminated by the initial surgery and radioiodine remnant ablation. Serum Tg measurement during TSH stimulation (sTg) is recommended to monitor for the recurrence or persistence of DTC. Especially, undetectable or very low sTg obtained at 12 months after treatment (defined as biochemical remission, BR) can predict complete and persistent remission of disease and a very low recurrence rate (0.6%–1.0%) (1–4).
There have been debates on the issue of follow-up modalities in DTC patients. The early detection of persistent or recurrent disease is important because a 40-year disease recurrence rate has been reported up to 35% (5), despite of the low risk of disease-related mortality. In the American Thyroid Association (ATA) guidelines, neck ultrasonography (US) was recommended within the first year after treatment and then periodically (6). Neck US is useful for detecting recurrence, because most of clinical recurrences are limited to the neck area (7,8). For another surveillance modality, recent reports suggested that the second sTg during TSH stimulation was informative in patients who have had a positive first sTg, but not in those who had a negative first sTg (9–12). There was a study that a negative second sTg with a negative neck US finding provided a negative predictive value of 100% and sensitivity of 100% (13). As yet, the utility of repeated sTg measurement is uncertain in patients who achieved BR.
This study was used to evaluate the long-term outcome of DTC patients who achieved BR after initial treatment, and to determine the role of repeated sTg measurement in early detection of recurrence or persistent disease.
Methods
Definitions
BR was defined as sTg <1 ng/mL and negative thyroglobulin autoantibody (TgAb). sTg1 and TgAb1 were serum Tg and TgAb values measured at 12 months after ablation during thyroid hormone withdrawal. sTg2 and TgAb2 were serum Tg and TgAb values measured 1–3 years after sTg1 measurement under the same condition. Clinical recurrence/persistence was defined as the detection of pathologically proven malignant tissue or of distant metastatic lesions such as lung, bone, and brain metastasis. No clinical evidence of disease (NCED) was defined, as no evidence of disease based on physical examination, neck US, and any other imaging modalities (usually neck/chest computed tomography [CT] and F-18-fluorodeoxyglucose positron emission tomography [FDG-PET]) performed as a part of the clinical evaluation, performed at the end of follow-up, regardless of the level of Tg.
Patients
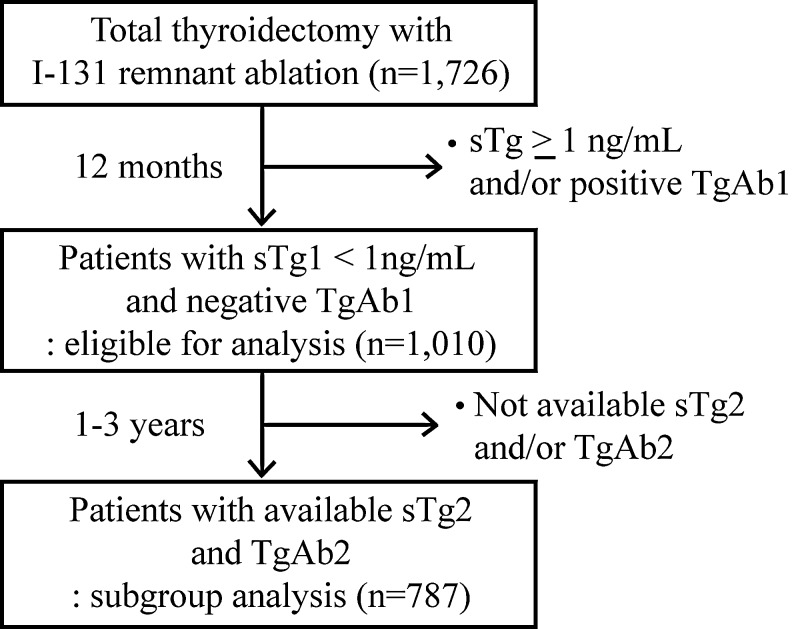
This was a retrospective observational cohort study from 1995 to 2004 in the Asan Medical Center (a tertiary referral hospital in Seoul, Korea). During that period, there were 1726 consecutive patients with DTC who underwent total thyroidectomy with or without neck dissection followed by immediate 131I remnant ablation. Patients with clinical evidence of distant metastasis or those without available sTg1 and TgAb1 were excluded. Among them, 1010 patients achieved BR at 12 months after ablation and were eligible for analysis. Of the 1010 patients, 787 had values of sTg2 and TgAb2, and were included for the subgroup analysis. A flow diagram for description of eligible patients is shown in Figure 1. The local ethics committee approved the retrospective review protocol.
FIG. 1.
Description of the study cohort. sTg1 and TgAb1, serum Tg and TgAb measured 12 months after ablation during thyroid hormone withdrawal; sTg2 and TgAb2, serum Tg and TgAb measured 1–3 years after sTg1 during thyroid hormone withdrawal; sTg, serum Tg measurement during TSH stimulation; Tg, thyroglobulin; TgAb, thyroglobulin autoantibody.
Institutional initial treatment and follow-up strategy
Patients who underwent total thyroidectomy received ablative doses of 131I (1.11–5.55 GBq) according to their tumor stage, 5–6 weeks after the initial surgery. Suppressive treatment with the thyroid hormone was initiated just after the remnant ablation. Thyroid hormone levels were titrated every 3–6 months with measurement of serum-free T4 and TSH levels, and all patients had physical examinations, including neck palpation, by experts at each visits. The diagnostic whole-body scan with 148 MBq 131I and serum sTg1 and TgAb1 were usually obtained at 12 months after the remnant ablation. Neck US was checked in about 30% of enrolled patients simultaneously with sTg1 measurement. Stimulated Tg was defined as a Tg value measured when TSH was >30 mU/L. Among patients who achieved BR, sTg2 and TgAb2 were checked 1–3 years thereafter. Neck US was also performed at the time of sTg2 measurement and followed up annually or biannually after that, regardless of sTg2 values. When there was clinical suspicion of recurrence, FDG-PET and neck/chest CT were performed. Fine-needle aspiration (FNA) cytology was done in patients with abnormal neck US finding. Recently, we also measured Tg concentrations in FNA washout fluid in some patients.
Tg, TgAb, and TSH measurement
All Tg, TgAb, and TSH measurements were performed by the same laboratory using the same kits until 2007. Serum Tg levels were measured by an immunoradiometric assay (ELSA-hTG kit; Schering-CIS Bio-International, Gif-sur-Yvette, France) with a functional sensitivity of 1 ng/mL. We were unable to standardize the serum Tg against the CRM-457 protocol, but instead developed our own Tg-reference interval according to the laboratory medicine practice guidelines suggested by the National Academy of Clinical Biochemistry (available at www.nacb.org/Impg/main.stm). Our generated Tg-reference interval was ∼1.0–27.4 μg/L (mean 5.2 μg/L). Serum TgAb levels were determined by a radioligand assay (HENNING test anti-Tg kit; BRAHMS Diagnostica, Berlin, Germany), and TgAb values >100 U/mL were considered positive. The functional sensitivity (20% interassay variation coefficient) was ∼31 U/mL, whereas the analytical sensitivity from the optimal curve was 20 U/mL. Intraassay and interassay coefficients of variation were 3.1% and 4.5%, respectively. Serum TSH was measured by a radioimmunometric assay (SPAC-S TSH kit; Daiichi, Tokyo, Japan), with a normal range of 0.5–5.0 mU/L, an intraassay coefficient of variation of 2.1%, and an interassay coefficient of variation of 2.5%.
After 2008, there were changes in assay kits for measurement of Tg, TgAb, and TSH. Tg levels were measured with the Tg-pluS RIA kit (BRAHMS AG, Henningsdorf, Germany) having a functional sensitivity (20% interassay variation coefficient) of 0.2 ng/mL. Serum TgAb levels were measured with the anti-Tg RIA kit (BRAHMS AG) with a functional sensitivity (20% interassay variation coefficient) of 20 U/mL. Serum TSH was measured by the TSH-CTK-3 kit (DiaSorin S.p.A., Saluggia, Italy) with a functional sensitivity (20% interassay variation coefficient) of 0.07 mU/mL.
Comparability between the ELSA-hTG kit (Schering-CIS Bio-International) and the Tg-pluS RIA kit (BRAHMS AG) was verified through a regression analysis of values of samples measured by both kits (regression coefficient 0.97, data not shown).
Statistical analysis
Categorical variables are presented as numbers and percentages. Continuous variables are expressed as medians with range. We used R version 2.12 and R libraries survival (R Foundation for Statistical Computing, Vienna, Austria; www.R-project.org) to analyze the data. For the univariate analysis, survival curves were plotted by the Kaplan–Meier methods with the log-rank statistics. For the multivariate survival analysis, a Cox-proportional hazards model was developed by forward, stepwise regression for all independent variables. The relative importance of prognostic factors is presented as a hazard ratio (HR) with a 95% confidence interval (CI) calculated using a binominal distribution. p<0.05 was considered statistically significant.
Results
Baseline clinicopathological characteristics (Table 1)
Table 1.
Baseline Clinicopathological Characteristics of Patients
| Initial characteristics | No. of patients (%) |
|---|---|
| Sex | |
| Male/female | 107 (10.6%)/903 (89.4%) |
| Pathology | |
| PTC | 888 (87.9%) |
| PTC c follicular variant | 60 (5.9%) |
| PTC c oncocytic variant | 1 (0.1%) |
| FTC | 37 (3.7%) |
| WDCa-NOS | 14 (1.4%) |
| Hurthle cell thyroid cancer | 10 (1.0%) |
| Tumor size (cm) | |
| ≤4 | 953 (94.4%) |
| >4 | 57 (5.6%) |
| Extrathyroidal extension | |
| Absent | 489 (48.4%) |
| Present | |
| Microscopic invasion | 518 (51.3%) |
| Macroscopic invasion | 3 (0.3%) |
| Lymph node dissection | |
| Not done | 151 (15.0%) |
| Central neck dissection | 775 (76.7%) |
| Central+lateral neck dissection | 84 (8.3%) |
| Lymph node metastasis | |
| pN0/Nx | 550 (54.5%) |
| pN1a (central neck node) | 386 (38.2%) |
| pN1b (lateral neck node) | 74 (7.3%) |
| AJCC TNM stage (2002) | |
| Stage I | 573 (56.7%) |
| Stage II | 35 (3.5%) |
| Stage III | 375 (37.1%) |
| Stage IV | 27 (2.7%) |
WDCa-NOS (well-differentiated carcinoma, not otherwise specified): tumors with features intermediate between PTC and FTC. PTC, papillary thyroid cancer; FTC, follicular thyroid cancer; AJCC TNM, American Joint Committee on Cancer tumor–node–metastasis.
There were 1010 patients (107 male and 903 female) who achieved BR at 12 months after ablation and were included in the study (Fig. 1). The median age of patients was 46.3 years (range 13.0–76.4 years), and the median size of tumor was 1.5 cm (range 0.1–8.5 cm). The frequencies of the following characteristics were calculated: tumor size >4 cm (5.6%); extrathyroidal extension (51.6%); central neck lymph node metastasis (N1a) (38.2%); lateral neck lymph node metastasis (N1b) (7.3%). According to the TNM staging (tumor, lymph node, metastasis—a classification system of the International Union Against Cancer and the American Joint Committee on Cancer, revised in 2002), 573 patients had stage I, 35 had stage II, 375 had stage III, and 27 had stage IV.
Long-term clinical outcome in patients who achieved BR after initial treatment (Table 2)
Table 2.
Clinical Characteristics and Course of 13 Patients Who Had Clinical Evidence of a Tumor Recurrence Confirmed by Cytologic/Histopathologic Information
| No. | Age/Sex | Primary tumor size (cm) | Lymph node metastsis | sTg1 (ng/mL) | TgAb1 | sTg1 WBS | sTg2 (ng/mL) | TgAb2 | Tg at recurrence (ng/mL) | TgAb at recurrence | NCED duration (months) | Site of recurrence | FNA washout Tg (ng/mL) | Detection method | Second treatment | Final outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 33/F | 2 | N1b | <1 | Neg | Neg | <1 | Neg | <1 | Neg | 31.9 | Neck | NA | Neck US | Surgery+RAI | Recurrence at cervical node |
| 2 | 49/F | 1.2 | N1b | <1 | Neg | Neg | <1 | Neg | <1 | Neg | 59.9 | Neck | NA | Neck US | Surgery+RAI | US (−) |
| 3 | 52/F | 1 | N0 | <1 | Neg | Neg | <1 | Neg | <1 | Neg | 71.3 | Neck | 133 | Neck US | Surgery+RAI | US (−) |
| 4 | 45/F | 1.3 | N1b | <1 | Neg | Neg | <1 | Neg | <1 | Neg | 31.7 | Neck | NA | Neck US | Surgery | Planning for disease evaluation |
| 5 | 28/F | 1.7 | N1a | <1 | Neg | Neg | <1 | Neg | <1 | Neg | 55.8 | Neck | 20.1 | Neck US | Surgery | US (−) |
| 6 | 63/F | 4 | N1a | <1 | Neg | Neg | 1.5 | Neg | <1 | Neg | 84.2 | Neck | NA | Neck US | Surgery | Planning for disease evaluation |
| 7 | 30/F | 0.2 | N1b | <1 | Neg | Neg | 1.9 | Neg | <1 | Neg | 113.3 | Neck | 29.9 | Neck US | RFA | Planning for disease evaluation |
| 8 | 70/F | 4 | N0 | <1 | Neg | Neg | 2.4 | Neg | 1.2 | Neg | 80.5 | Neck | 188 | Neck US | Surgery | Recurrence at superior mediastinal node |
| 9 | 58/M | 6 | N1a | <1 | Neg | Neg | 9 | Neg | 9 | Neg | 60 | Neck | NA | Neck US | Surgery | Planning for disease evaluation |
| 10 | 57/F | 4.5 | N1a | <1 | Neg | Neg | 12.8 | Neg | 12.8 | Neg | 45.3 | Neck | NA | Neck US | Surgery | US (−) |
| 11 | 65/M | 4 | N1a | <1 | Neg | Neg | NA | NA | <1 | Neg | 87.1 | Neck | 324 | Neck US | Surgery | US (−) |
| 12 | 48/F | 2.8 | N1a | <1 | Neg | Neg | NA | NA | <1 | Neg | 18.7 | Neck | NA | Neck US | Surgery | US (−) |
| 13 | 43/F | 1 | N1a | <1 | Neg | Neg | NA | NA | <1 | Neg | 27.1 | Neck | NA | Neck US | Surgery+RAI | US (−) |
sTg1 and TgAb1, serum thyroglobulin (Tg) and thyroglobulin autoantibody (TgAb) measured 1 year after remnant ablation during thyroid hormone withdrawal; sTg2 and TgAb2, Tg and TgAb measured 1–3 years after sTg1 during thyroid hormone withdrawal; WBS, diagnostic whole-body scan; negative TgAb, TgAb<60 U/mL; NCED, no clinical evidence of disease; FNA, fine-needle aspiration; NA, not available; US, ultrasonography; RFA, radiofrequency ablation; RAI, radioactive iodine ablation; Neg, negative.
Thirteen out of 1010 (1.3%) patients had a clinical recurrence during the median follow-up of 83.7 months (range 7.5–185.0) as shown in Table 2. All of the clinical recurrences were limited to the cervical lymph nodes and were detected by routine neck US. None of them had distant metastasis at the time of recurrence. Cytologic evaluation of cervical lymph node by FNA was done, and malignancy was confirmed in all patients. Of those 13 patients, eight patients received a second surgery alone, four received a second surgery with adjuvant radioactive iodine therapy, and one patient was treated with radiofrequency ablation. After the second treatment, seven patients had no residual disease on the follow-up neck US, two relapsed in the neck or mediastinal lymph nodes, and the others are waiting for the next evaluation.
Result of follow-up of patients according to sTg2 value: subgroup analysis
We performed a subgroup analysis in 787 (out of 1010) patients who had sTg2 and TgAb2 data (Fig. 1). sTg2 and TgAb2 were not checked in 223 patients according to attending physicians' decision or to patients' condition, and 3 patients (patients no. 11–13 in Table 2) among them had a clinical recurrence. There was no significant difference in clinical characteristics (data not shown) and in the recurrence rate (10/787 [1.3%] vs. 3/223 [1.3%]) between patients with sTg2 information and those without information.
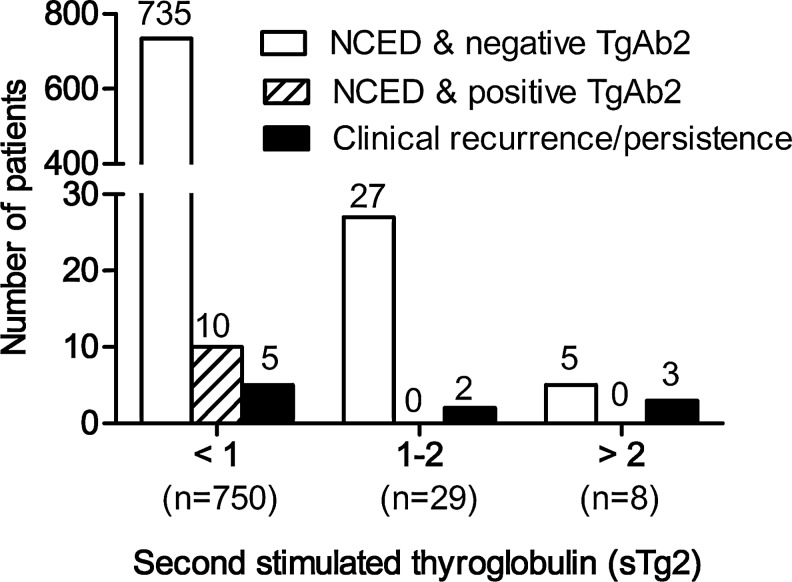
Of the 787 patients, 750 (95.3%) had a negative sTg2 value. Interestingly, five patients (patients no. 1–5 in Table 2 and Fig. 2) had persistently undetectable or very low sTg2 (and negative TgAb2) in spite of recurrent disease. TgAb2 changed to positive in 10 patients, but none showed a clinical recurrence during the follow-up.
FIG. 2.
Result of follow-up of patients with biochemical remission according to the sTg2 value. In total, 10 patients (1.3%) showed clinical evidence of disease during the follow-up. Of the 750 patients who had sTg2<1 ng/mL, 5 (5/750, 0.7%) showed a clinical recurrence. Of the 37 patients with sTg2≥1 ng/mL, clinical recurrence was detected in 5 patients (5/37, 13.5%). There were no recurred patients with positive TgAb2. sTg2 and TgAb2, Tg and TgAb measured 1–3 years after sTg1 (stimulated Tg measured 12 months after ablation) during thyroid hormone withdrawal; NCED, no clincial evidence of disease (based on physical examination, neck ultrasonography, and any other imaging performed as part of the clinical evaluation, performed at the end of follow-up, regardless of the level of Tg); clinical recurrence/persistence, reappearance of pathologically proven malignant tissue or metastatic lesion such as lung, bone, and brain metastasis.
Of the 787 patients, 37 (4.7%) converted to positive sTg (sTg2 ≥1 ng/mL) during the follow-up. The median value of the sTg2 was 1.3 ng/mL (range 1.0–12.8 ng/mL), and clinical recurrences were detected in five patients (patients no. 6–10 in Table 2. and Fig. 2). Two (patients no. 6 and 7 in Table 2) had sTg2 >1 ng/mL, but <2 ng/mL. The other three (patients no. 8–10 in Table 2) had sTg2 >2 ng/mL. The remaining 32 patients with NCED had a relatively low value of sTg2 (<5 ng/mL) and average 3.1 times of neck US were performed after sTg2 measurement during the follow-up. Some of those patients (26/32) were checked for one or more additional sTg during thyroid hormone withdrawal at 1- to 5-year intervals. The value of sTg decreased to <1 ng/mL in 23 patients and remained >1 ng/mL (similar to their sTg2 level or a little more) in 3 patients during a median 70.5 months (range 2.0–142.0) of further follow-up. Patients with positive sTg conversion were more likely to have recurrent disease by univariate analysis (log-rank statistics=43.7, df=1, p<0.001) and in a multivariate analysis (HR, 18.44; 95% CI, 5.24–64.94; p<0.001).
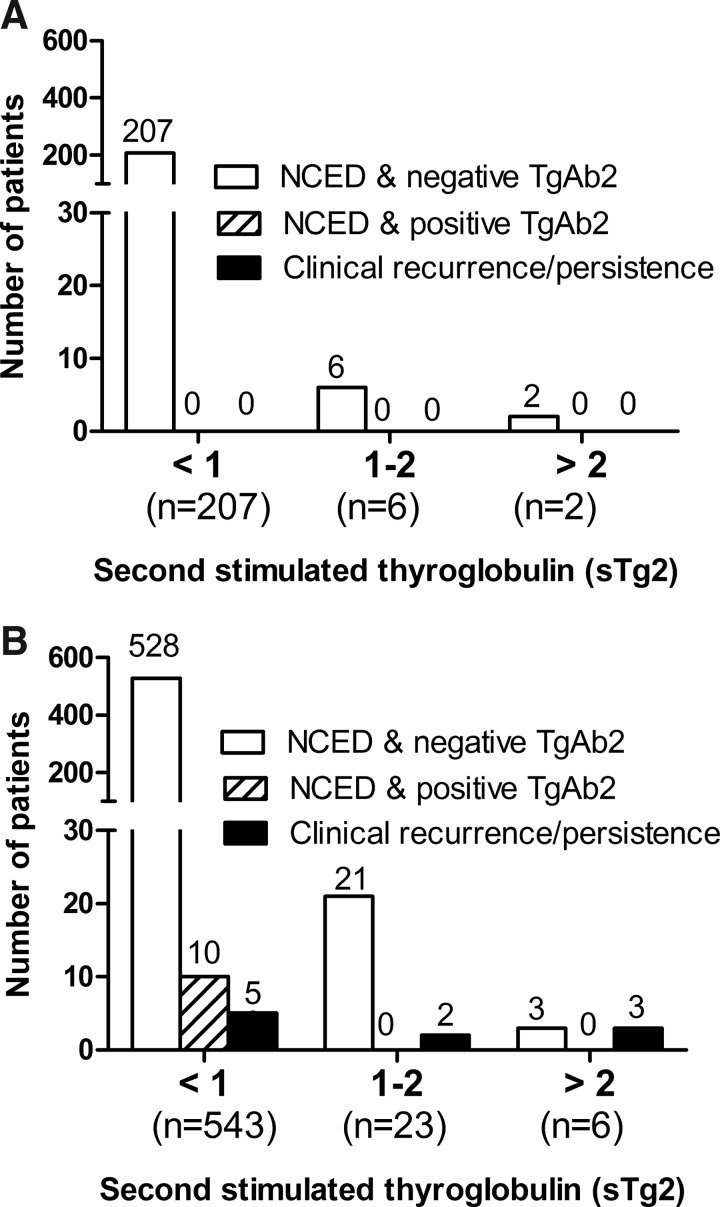
We also performed a subgroup analysis based on risk stratification of the 2009-revised ATA guidelines (low-risk group vs. intermediate- to high-risk group) (6). Of the 787 patients, 215 (27.3%) belonged to the low-risk group and 572 (72.7%) belonged to the intermediate- to high-risk group (Fig. 3). In 215 patients in the low-risk group, 8 patients had positive sTg conversion and none had a clinical recurrence or positive TgAb2 value during the follow-up. In 572 patients of the intermediate- to high-risk group, 29 patients had sTg2>1 ng/mL, and among them, clinical recurrences were detected in 5 patients. Of the 543 patients with persistently undetectable or very low sTg2 value, 10 patients had positive sTg2Ab conversion, and 5 patients had clinical recurrences. Patients with positive sTg conversion of the intermediate- to high-risk group were more likely to have recurrent disease (log-rank statistics=37.8, df=1, p<0.001).
FIG. 3.
Clinical outcome of patients with biochemical remission according to recurrence risk stratification. (A) 215 patients belonged to the low-risk group. Of the 215 patients, none showed clinical recurrence. (B) 572 patients belonged to the intermediate- to high-risk group. Ten out of 572 patients showed clinical recurrence, and among them, 5 patients had sTg2≥1 ng/mL.
Discussion
Of the 1010 patients, 787 patients had information for sTg2, and these patients could be divided into two groups according to the sTg2 value: 750 patients with sTg2<1 ng/mL; 37 patients with sTg2≥1 ng/mL (Fig. 2). In the group of sTg2 <1 ng/mL, 0.7% (5/750, patients no. 1–5 in Table 2) relapsed even though they had achieved BR after curative treatment and a persistently negative sTg value. After total thyroidectomy and remnant ablation, previous studies have been reported that the sensitivity of sTg was 80%–100%, and the negative predictive value during the first year of follow-up was up to 99% (3,14,15). Conversely, some studies have suggested that the undetectable sTg level did not totally exclude the persistence of small neoplastic foci or recurrent disease (8,16–19). In the present study, 5/13 patients had recurrent disease with persistently undetectable or very low (<1 ng/mL) sTg2 and negative TgAb2. The value of the serum Tg principally reflects the tumor burden or the ability of the tumor to synthesize and secrete Tg (20). Therefore, these negative sTg2 results in our study may indicate that Tg was produced, but confined within the intra-nodal space, and Tg was not secreted into the systemic circulation. This hypothesis is supported by the fact that washout Tg measured in FNA fluid was detected and much higher than serum Tg value. Washout Tg was 133 ng/mL in patient no. 3 and 20.1 ng/mL in patient no. 5 (in Table 2), even though serum sTg was undetectable. Alternatively, the current Tg measurement that was based on monoclonal antibodies may not detect Tg in serum because the quantity of Tg produced by cervical lymph node metastasis was too small, or the Tg was immunologically inactive relating to a change in tumor properties and the nature of the Tg produced, such as might occur with dedifferentiation. Also, low titers of TgAb might have existed, which interfered with measurement of Tg, but could not be detected by current methods. Spencer et al. (21) recently reported that the use of manufacturer-recommended cutoffs to determine the presence of TgAb often led to false-negative misclassifications, which caused significant underestimation of Tg values in DTC patients.
In our study, 37 patients showed rise of sTg2 ≥1 ng/mL during the follow-up (Fig. 2), and they had a much greater chance of disease recurrence. Among them, 13.5% (5/37, patients no. 6–10 in Table 2) had clinical evidence of recurrent disease. The reason for positive sTg conversion might be that Tg production of malignant thyroid tissue decreased after treatment and increased as the relapsed or persistent tumor grew. Previously studies have shown that the sTg level measured 6–12 months after therapy was the most important predictive factor. Furthermore, age, tumor size, extrathyroidal invasion, lymph node invasion, presence of distant metastasis, presence of TgAb at 6–12 months, and ablation Tg levels have all been reported to be associated with the recurrence-free survival (22–25).
We found that sTg2 could be a possible marker for predicting recurrence in patients who achieved BR at one year after the initial therapy. However, the clinical utility of sTg2 to predict a clinical recurrence is questionable, even if there is statistical significance. The number of patients who had sTg2>1 ng/mL and recurrence was only 5 (0.5% of total 1010 patients), and patients had to use recombinant human TSH with high cost or undergo thyroid hormone withdrawal with significant morbidity to obtain sTg2. Therefore, measurement of sTg2 is not practically useful considering its high cost and inconvenience. Of the 37 patients with sTg2 ≥1 ng/mL, 32 have had NCED during the median 70.5 months (range 2.0–124.0) of follow-up. Among them, 23 had a decreased sTg value<1 ng/mL and 3 had a similar or slightly increased value.
Clinical recurrence in our study cohort was limited to loco-regional disease, and all of them were detected by routine neck US, which was demonstrated in other studies (7,8,19). Even though only negative FDG-PET and neck/chest CT results could not warrant NCED, we considered all of relapsed cases as local recurrence, because at least these imaging studies were negative. All cases were detected by neck US regardless of FDG-PET results. Thus, neck US appeared to be the most sensitive and practical modality for the detection of recurrent disease rather than the costly and cumbersome measurement of sTg2.
The present study had several limitations due to its retrospective nature. First, the time of sTg2 follow-up was not standardized, because the interval between sTg1 and sTg2 varied according to attending physicians. Second, before the year 2000, the quality of US was poor, and some patients were not checked by neck US in their early follow-up period. Third, the serum Tg assay kit was changed from the ELSA-hTG kit (functional sensitivity of 1 ng/mL) to the Tg-pluS RIA kit (functional sensitivity of 0.2 ng/mL) in 2008. In the present study, the cutoff value of sTg was 1 ng/mL because the initial Tg assay did not measure a Tg value below 1 ng/mL. We verified comparability between two kits by a regression analysis (regression coefficient 0.97). Castagna et al. reported the usefulness of ultrasensitive Tg assays recently. When the basal serum Tg was <0.1 ng/mL and neck US was unremarkable, patients might be considered free of disease and could avoid an TSH stimulation (26). Although conventional Tg assays and 1 ng/mL of cutoff value of Tg are used in current clinical guidelines, the ultrasensitive Tg assay could help risk stratification instead of repeated sTg measurement, and further studies using it are needed.
Despite of these limitations, this is one of the largest studies of the clinical outcome of DTC patients who had a BR after treatment, to determine if sTg2 measurements are needed for surveillance during their follow-up. In summary, 99% of patients with DTC who had sTg1<1 ng/mL 12 months after their initial treatment showed NCED during long-term follow-up, and only 1% had a clinical recurrence. All of the clinical recurrences were limited to the cervical lymph nodes without clinical evidence of distant metastasis. Although repeated sTg measurement (sTg2) may be helpful to predict recurrence, we can not recommend it for surveillance due to its very low yield in patients with BR.
Acknowledgment
This study was supported by a grant (2011-289) from the Asan Institute for Life Sciences, Seoul, Korea.
Disclosure Statement
Young Kee Shong is a consultant for Bayer and Genzyme.
References
- 1.Cailleux AF. Baudin E. Travagli JP. Ricard M. Schlumberger M. Is diagnostic iodine-131 scanning useful after total thyroid ablation for differentiated thyroid cancer? J Clin Endocrinol Metab. 2000;85:175–178. doi: 10.1210/jcem.85.1.6310. [DOI] [PubMed] [Google Scholar]
- 2.Schlumberger M. Baudin E. Serum thyroglobulin determination in the follow-up of patients with differentiated thyroid carcinoma. Eur J Endocrinol. 1998;138:249–252. doi: 10.1530/eje.0.1380249. [DOI] [PubMed] [Google Scholar]
- 3.Pacini F. Capezzone M. Elisei R. Ceccarelli C. Taddei D. Pinchera A. Diagnostic 131-iodine whole-body scan may be avoided in thyroid cancer patients who have undetectable stimulated serum Tg levels after initial treatment. J Clin Endocrinol Metab. 2002;87:1499–1501. doi: 10.1210/jcem.87.4.8274. [DOI] [PubMed] [Google Scholar]
- 4.Tuttle RM. Tala H. Shah J. Leboeuf R. Ghossein R. Gonen M. Brokhin M. Omry G. Fagin JA. Shaha A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20:1341–1349. doi: 10.1089/thy.2010.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzaferri EL. Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86:1447–1463. doi: 10.1210/jcem.86.4.7407. [DOI] [PubMed] [Google Scholar]
- 6.Cooper DS. Doherty GM. Haugen BR. Hauger BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 7.Pacini F. Molinaro E. Castagna MG. Agate L. Elisei R. Ceccarelli C. Lippi F. Taddei D. Grasso L. Pinchera A. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:3668–3673. doi: 10.1210/jc.2002-021925. [DOI] [PubMed] [Google Scholar]
- 8.Torlontano M. Attard M. Crocetti U. Tumino S. Bruno R. Costante G. D'Azzo G. Meringolo D. Ferretti E. Sacco R. Arturi F. Filetti S. Follow-up of low risk patients with papillary thyroid cancer: role of neck ultrasonography in detecting lymph node metastases. J Clin Endocrinol Metab. 2004;89:3402–3407. doi: 10.1210/jc.2003-031521. [DOI] [PubMed] [Google Scholar]
- 9.Nascimento C. Borget I. Al Ghuzlan A. Deandreis D. Chami L. Travagli JP. Hartl D. Lumbroso J. Chougnet C. Lacroix L. Baudin E. Schlumberger M. Leboulleux S. Persistent disease and recurrence in differentiated thyroid cancer patients with undetectable postoperative stimulated thyroglobulin level. Endocr Relat Cancer. 2011;18:R29–R40. doi: 10.1677/ERC-10-0292. [DOI] [PubMed] [Google Scholar]
- 10.Castagna MG. Brilli L. Pilli T. Montanaro A. Cipri C. Fioravanti C. Sestini F. Capezzone M. Pacini F. Limited value of repeat recombinant human thyrotropin (rhTSH)-stimulated thyroglobulin testing in differentiated thyroid carcinoma patients with previous negative rhTSH-stimulated thyroglobulin and undetectable basal serum thyroglobulin levels. J Clin Endocrinol Metab. 2008;93:76–81. doi: 10.1210/jc.2007-1404. [DOI] [PubMed] [Google Scholar]
- 11.Crocetti U. Durante C. Attard M. Maniglia A. Tumino S. Bruno R. Bonfitto N. Dicembrino F. Varraso A. Meringolo D. Filetti S. Trischitta V. Torlontano M. Predictive value of recombinant human TSH stimulation and neck ultrasonography in differentiated thyroid cancer patients. Thyroid. 2008;18:1049–1053. doi: 10.1089/thy.2008.0160. [DOI] [PubMed] [Google Scholar]
- 12.Kloos RT. Mazzaferri EL. A single recombinant human thyrotropin-stimulated serum thyroglobulin measurement predicts differentiated thyroid carcinoma metastases three to five years later. J Clin Endocrinol Metab. 2005;90:5047–5057. doi: 10.1210/jc.2005-0492. [DOI] [PubMed] [Google Scholar]
- 13.Klubo-Gwiezdzinska J. Burman KD. van Nostrand D. Wartofsky L. Does an undetectable rhTSH-stimulated Tg level 12 months after initial treatment of thyroid cancer indicate remission? Clin Endocrinol (Oxf) 2011;74:111–117. doi: 10.1111/j.1365-2265.2010.03898.x. [DOI] [PubMed] [Google Scholar]
- 14.Mazzaferri EL. Kloos RT. Is diagnostic iodine-131 scanning with recombinant human TSH useful in the follow-up of differentiated thyroid cancer after thyroid ablation? J Clin Endocrinol Metab. 2002;87:1490–1498. doi: 10.1210/jcem.87.4.8338. [DOI] [PubMed] [Google Scholar]
- 15.Baudin E. Do Cao C. Cailleux AF. Leboulleux S. Travagli JP. Schlumberger M. Positive predictive value of serum thyroglobulin levels, measured during the first year of follow-up after thyroid hormone withdrawal, in thyroid cancer patients. J Clin Endocrinol Metab. 2003;88:1107–1111. doi: 10.1210/jc.2002-021365. [DOI] [PubMed] [Google Scholar]
- 16.Bachelot A. Cailleux AF. Klain M. Baudin E. Ricard M. Bellon N. Caillou B. Travagli JP. Schlumberger M. Relationship between tumor burden and serum thyroglobulin level in patients with papillary and follicular thyroid carcinoma. Thyroid. 2002;12:707–711. doi: 10.1089/105072502760258686. [DOI] [PubMed] [Google Scholar]
- 17.Bachelot A. Leboulleux S. Baudin E. Hartl DM. Caillou B. Travagli JP. Schlumberger M. Neck recurrence from thyroid carcinoma: serum thyroglobulin and high-dose total body scan are not reliable criteria for cure after radioiodine treatment. Clin Endocrinol (Oxf) 2005;62:376–379. doi: 10.1111/j.1365-2265.2005.02228.x. [DOI] [PubMed] [Google Scholar]
- 18.Kloos RT. Thyroid cancer recurrence in patients clinically free of disease with undetectable or very low serum thyroglobulin values. J Clin Endocrinol Metab. 2010;95:5241–5248. doi: 10.1210/jc.2010-1500. [DOI] [PubMed] [Google Scholar]
- 19.Torlontano M. Crocetti U. D'Aloiso L. Bonfitto N. Di Giorgio A. Modoni S. Valle G. Frusciante V. Bisceglia M. Filetti S. Schlumberger M. Trischitta V. Serum thyroglobulin and 131I whole body scan after recombinant human TSH stimulation in the follow-up of low-risk patients with differentiated thyroid cancer. Eur J Endocrinol. 2003;148:19–24. doi: 10.1530/eje.0.1480019. [DOI] [PubMed] [Google Scholar]
- 20.Spencer CA. LoPresti JS. Fatemi S. Nicoloff JT. Detection of residual and recurrent differentiated thyroid carcinoma by serum thyroglobulin measurement. Thyroid. 1999;9:435–441. doi: 10.1089/thy.1999.9.435. [DOI] [PubMed] [Google Scholar]
- 21.Spencer C. Petrovic I. Fatemi S. Current thyroglobulin autoantibody (TgAb) assays often fail to detect interfering TgAb that can result in the reporting of falsely low/undetectable serum Tg IMA values for patients with differentiated thyroid cancer. J Clin Endocrinol Metab. 2011;96:1283–1291. doi: 10.1210/jc.2010-2762. [DOI] [PubMed] [Google Scholar]
- 22.Tubiana M. Schlumberger M. Rougier P. Laplanche A. Benhamou E. Gardet P. Caillou B. Travagli JP. Parmentier C. Long-term results and prognostic factors in patients with differentiated thyroid carcinoma. Cancer. 1985;55:794–804. doi: 10.1002/1097-0142(19850215)55:4<794::aid-cncr2820550418>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 23.Tennvall J. Biorklund A. Moller T. Ranstam J. Akerman M. Is the EORTC prognostic index of thyroid cancer valid in differentiated thyroid carcinoma? Retrospective multivariate analysis of differentiated thyroid carcinoma with long follow-up. Cancer. 1986;57:1405–1414. doi: 10.1002/1097-0142(19860401)57:7<1405::aid-cncr2820570728>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.Kim TY. Kim WB. Kim ES. Ryu JS. Yeo JS. Kim SC. Hong SJ. Shong YK. Serum thyroglobulin levels at the time of 131I remnant ablation just after thyroidectomy are useful for early prediction of clinical recurrence in low-risk patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2005;90:1440–1445. doi: 10.1210/jc.2004-1771. [DOI] [PubMed] [Google Scholar]
- 25.Kim WG. Yoon JH. Kim WB. Kim TY. Kim EY. Kim JM. Ryu JS. Gong G. Hong SJ. Shong YK. Change of serum antithyroglobulin antibody levels is useful for prediction of clinical recurrence in thyroglobulin-negative patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2008;93:4683–4689. doi: 10.1210/jc.2008-0962. [DOI] [PubMed] [Google Scholar]
- 26.Castagna MG. Tala Jury HP. Cipri C. Belardini V. Fioravanti C. Pasqui L. Sestini F. Theodoropoulou A. Pacini F. The use of ultrasensitive thyroglobulin assays reduces but does not abolish the need for TSH stimulation in patients with differentiated thyroid carcinoma. J Endocrinol Invest. 2011;34:e219–e223. doi: 10.3275/7571. [DOI] [PubMed] [Google Scholar]