Abstract
Trained people exhibit low plasma concentrations of triacylglcyerols in both fasting and postprandial states. Exercise practice is commonly believed to improve postprandial lipemia. In addition, elevated postprandial lipemia is an indicator of poor lipid clearance, and it has been associated with atherosclerosis, insulin resistance, and obesity. Spirulina maxima is an edible microorganism with a high nutritional value. When it is consumed, beneficial properties to health have been demonstrated, such as hypolipemic and antihypertensive properties in human beings. This work evaluates the effects of orally administrated S. maxima on postprandial lipemia in a young Mexican sporting population after 15 days of consumption, as a possible alternative treatment to improve their lipid clearance. Forty-one runners (10–26 years old; 21 men and 20 women) volunteered to participate in the study. All of them were physically active for at least 1 year before the study and were not undergoing training during the study. The subjects consumed 5 g of Spirulina during 15 days. Before and after the treatment with Spirulina, they consumed (12 h fasting) a standardized meal with high fat content (53.2% total calories). Postprandial lipemia was measured at 1.5, 3, and 4.5 h after the fatty meal. Fasting plasma triacylglycerol (TAG) concentrations were lower after Spirulina treatment than before treatment. In addition, the postprandial area under the curve of TAG concentrations was lower after the treatment with Spirulina. Sixty-two percent of the youngest runners (10–16 years) studied exhibited the best response to the treatment. Orally administered S. maxima decreased postprandial lipemia in sporting teenagers. The youngest people were the most responsive to the beneficial effects of Spirulina on postprandial lipemia.
Key Words: area under curve, exercise, nutrition, triacylglycerols
Introduction
In recent years, several studies have been performed in an attempt to support the hypothesis that accumulated physical activity has health benefits. The findings of these studies suggested that persistent physical activity is effective in lowering postprandial triacylglycerol (TAG) concentrations.1–3 Other authors have proposed that fasting plasma TAG concentration is the best predictor of postprandial lipemia.4,5
Exercise response and lipid metabolism are influenced by age and sex. These could be explained by hormonal changes, especially growth hormone and the sexual hormones, testosterone and estrogens. These hormones have an important effect on the muscular and adipose tissues and lipid transport.6–10 Other hormones that are directly involved in lipid changes observed during exercise are insulin, as well as the adrenaline/noradrenaline (A/NA) ratio, because during exercise, insulin concentration diminishes while catecholamine concentration increases, causing activation of lipoprotein lipase (LPL) and hormone-sensitive lipase.11
On the other hand, postprandial lipemia is considered to be associated with the presence or development of coronary artery disease.4 In fact, elevated postprandial hypertriacylglycerolemia indicates poor lipid clearance and is associated with atherosclerosis, insulin resistance, an elevation in cholesterol associated with low-density lipoprotein (C-LDL) concentration, a decrease in cholesterol associated with high-density lipoprotein (C-HDL) concentration, and obesity.4,12
Many studies13,14 have shown that acute and chronic exercise mitigates the elevation of plasma TAG concentration after a fatty meal. Most of these studies have employed test meals containing exceedingly high amounts of fat (at least 10 g/kg body mass or more than 60% of total energy). Other studies recently examined the effect of exercise on lipemia after a meal of high-fat content, which is closer to that of the typical Western diet (35% total energy) and found a significant reduction in postprandial lipemia.15 It has been proposed that delayed and sustained activation of LPL in the muscular capillaries is due to exercise, and this accelerates the clearance of circulating TAG in the postprandial state.16,17
Despite the beneficial effects of exercise, its practice in our country is limited. This agrees with a study conducted by Dumith et al.,18 who reported that the overall worldwide prevalence of physical inactivity was of 21.4%, being higher in women than in men. This study showed that Mauritania is the most physically inactive country with a mean prevalence of 62%. On the other hand, we face the problem of a fat-rich and protein-poor diet; both these factors affect exercise performance. A study conducted by Ortiz-Hernández and Gómez-Tello19 showed that in 7218 Mexican adolescents, two-thirds did not consume fruit and vegetables, one-third consumed sweet soft drinks, and one-fifth consumed salty snacks. In a study by Rodriguez-Oliveros et al.,20 it was shown that there are many barriers in childhood that have to be overcome to practice physical activity, which included the influence of young family members to play video games, parental time constraints, street safety, low access to sports facilities, unhealthy food preparation practices, and the consumption of junk food or bad food. However, it is possible to counteract the influence of bad food on health by using some natural products, such as Spirulina, which has been studied as food supplements for subjects in training.
Spirulina maxima is a filamentous unicellular cyanobacterium belonging to the Oscillatoraceae family that usually grows in the alkaline waters of Africa, Asia, and North and South America.21 Spirulina has been used as a food additive due to its high protein content, as well as its essential nutrients such as carotenoids, vitamins, and minerals.22 In addition, previous studies have demonstrated that Spirulina (rich in iron and vitamins) prevents anemia, inhibits herpes simplex infection, decreases HIV replication velocity, increases antibody production, and has hypoglycemic, hypolipemic, and antihypertensive properties in experimental models and humans,23,24 as well as hepatoprotective properties through decreasing liver lipid profile and lipoperoxidation.25 In fact Kalafati et al.26 recently studied the effect of 6 g per day of Spirulina on nine moderately trained men. They measured exercise performance and some antioxidant enzymes after placebo and Spirulina supplementation, and found that the Spirulina supplement induced a significant increase in exercise performance, fat oxidation, and reduced glutathione concentration, and attenuated the increase of exercise-induced lipid peroxidation.
Furthermore, Lu et al.,27 demonstrated that Spirulina platensis prevented skeletal muscle damage in eight untrained subjects progressively submitted to treadmill exercise according to Bruce protocol, suggesting that this could explain the postponement of exhaustion time among athletes fed on S. platensis, similar to those of Nagpur, India.
The purpose of this study was to assess the effects of orally administered S. maxima on postprandial lipemia in a young Mexican sporting population after 15 days of treatment.
Materials and Methods
Forty-one young volunteer runners participated in the study; 10–26 years old (15.7±4.5 years old), 21 men and 20 women. All of them were undergoing systematic training at a nonprofessional level by a qualified trainer, and they had also had recreational sport practice for at least 1 year before the study. At the onset and during the study, they were prohibited from participating in a race or any kind of exercise. At this point, they were encouraged to make no modification in their diet. The athletes were informed of all the clinical and biochemical procedures and signed the informed consent form. The protocol was previously approved by the Facultad de Medicina's ethics committee. The S. maxima used in this study was a donation from Alimentos Esenciales para la Humanidad (AEH, Mexico City, Mexico).
The basal blood plasma levels of TAG, C-HDL, total cholesterol (TC), and glucose were taken after 12 h fasting and 36 h without exercise. Blood for plasma analyses was drawn into 100-unit sodium heparin freeze-dried sterile glass tubes (BD Vacutainer) and then centrifuged at 4000 g for 5 min at 4°C. Glucose, TC, C-HDL, and TAG concentrations were analyzed by using standard enzymatic procedures (Jas Diagnostics, Inc.) with a spectrophotometer (Genesys 10 UV; Thermo Electron Corporation. The postprandial lipemia was evaluated each 90 min until 4½ h was reached, using Accutrend Tryglicerides strips (Roche Diagnostic) and Accutrend GCT (Roche Diagnostics). The results were reported in mg/dL. Blood lactate concentrations were assessed using Accutrend Lactate strips (Roche Diagnostic), before and after exercise in subjects consuming or not Spirulina (or before and after exercise and also with and without Spirulina).
The study was conducted during 2 weeks, and the tests were performed between 09:00 a.m. and 14:00 p.m. The first postprandial lipemia test was conducted at 9:00 am after 36 h of minimal physical activity and 12 h of fasting. Then, they consumed one standardized Mexican collation (1000 kcal and 63% fat) (Table 1). The participants initially consumed S. maxima powder (5 g in 250 mL water) daily for breakfast during 15 days (the next 2 weeks and a day). Then, the postprandial lipemia test was performed again (Table 1).
Table 1.
Composition of Diet for Postprandial Lipemia
| Meala | Amount (g) | Protein (g) | Lipids (g) | Carbohydrates (g) | Energy (kcal) |
|---|---|---|---|---|---|
| Pork rinds | 20 | 11 | 9 | 0 | 125 |
| Muffin | 125 | 7.4 | 32.8 | 61.2 | 572 |
| Yoghurt | 250 | 5.76 | 6.5 | 31.7 | 211.3 |
| Small pork sausage | 58 | 5.4 | 14.8 | 0.7 | 160 |
| Total | 453 | 29.5 | 63.1 | 93.6 | 1068.3 |
Each one of these products represents a normal typical Mexican collation.
This time was selected, because previous studies conducted on rats had shown that TAG is decreased after 1 week of feeding with Spirulina, and as it is more difficult to control program adherence in young populations. Furthermore, it has been demonstrated that detraining effects could occur within 2 weeks or more after stopping the training.9
Participants were divided (on the basis of the principal changes during growth) into three age groups: 10–12 years (n=9 women and n=3 men), 13–16 years (n=6 women and n=8 men), and 17–26 years of age (n=5 women and n=10 men).
Statistical analysis
Statistical analyses were performed using GraphPad Prism software, version 4. We compared the means between groups using an unpaired t test. In order to evaluate changes in lipemia, we used area under curve (AUC) of the TAG concentrations versus the time calculated starting from the value just before the consumption of the meal and using the trapezoidal rule. Results were represented as a percentage of ΔAUC of each group of participants with and without treatment. We also used the Student t-test to evaluate the differences in TC and TAG concentrations between participants with and without Spirulina treatment.
Results
The study initially included 41 volunteers, but only 29 participants concluded the tests. No differences in basal characteristics between men and women were observed, according to age and sex (data not shown), indicating a homogenous group. The fat meal used for postprandial lipemia consisted of a high amount of lipids (34%) and adequate carbohydrate (50%) and protein (16%) content. We observed that the most important support of energy in this diet was derived from lipids (53.2%), then carbohydrates (35.1%), and, finally, proteins (11.04%). These kinds of products are usually consumed in the urban Mexican population (Table 1).
The effects of Spirulina on TAG, TC, and C-HDL fasting concentrations are depicted in Table 2. Differences in neither TC nor C-HDL were observed when comparing concentrations before and after treatment; but lower values of fasting TAG concentrations were found after Spirulina treatment. Figure 1 represents the percentage of change in plasma TAG both before and after Spirulina treatment in all participants at 1.5, 3, and 4.5 h after the fat meal. This figure shows that 18 out of the 29 final participants had reduced lipemia at the three times (prevalence 62%) and that the most important reduction was observed at 4.5 h with 42%.
Table 2.
Spirulina Effects on Fasting Plasma Lipids
| |
Total subjects |
|
|---|---|---|
| Before treatment | After treatment | |
| TAG (mg/dL) | 71.47±5.8 | 57.06±24* |
| TC (mg/dL) | 154.4±28.8 | 148.7±25.4 |
| HDL-C (mg/dL) | 56.04±10.8 | 51.4±9.4 |
TAG, triacylglycerols; TC, total cholesterol; HDL-C, cholesterol associated to high density lipoproteins.
P=.04, n=29.
FIG. 1.
Effects of Spirulina on postprandial lipemia. Results are expressed as a percentage of change in plasma triacylglycerol, before and after Spirulina treatment, at three times selected for postprandial analyses (1.5, 3, and 4.5 h, n=29).
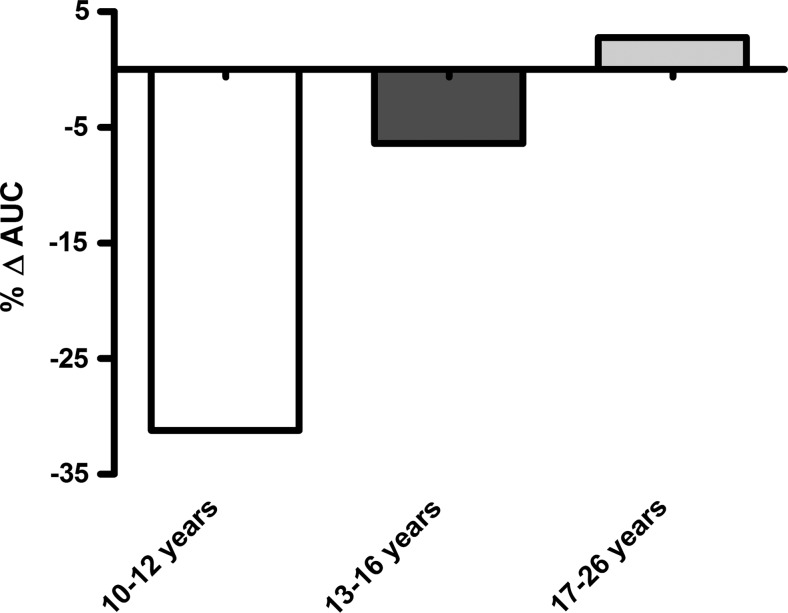
Then, we analyzed the effects of Spirulina according to age, comparing the percentage of changes observed in AUC. We noted that the main decrease occurred in the youngest group with 30%, and similarly in the group of participants between 13 and 16 years of age with 7%. However, this decrease is not evident in the oldest group, Figure 2.
FIG. 2.
Effects of Spirulina on lipemia according to age group. Results are expressed as a percentage of change in area under curve (AUC) values of postprandial lipemia in the three age groups (10–12 years, n=12; 13–16 years, n=14; 17–26 years, n=15).
Before exercise, fasting lactate concentration was 2.25±1.19 without Spirulina versus 1.70±0.58 with Spirulina. After exercise, fasting lactate concentration was 15.53±4.15 without Spirulina versus 12.78±6.25 with Spirulina. Despite the trend to lower lactate concentration with Spirulina, the differences were not significant.
Discussion
A few studies have shown that exercise performed 1 day, or even 1 h, before the fat meal effectively diminishes postprandial lipemia. These studies demonstrate this effect in the form of changes in AUC.3,12,28–30 However, Zhang et al.,12 observed this effect when exercise was performed before but not after the meal. These studies have used high-fat test meals. In our study, participants were asked to suspend exercise practice 36 h both before treatment and during the study, in order to view the changes induced by treatment with Spirulina in physically active subjects.
Other studies have determined that Spirulina increases exercise performance, possibly through an increase in β-oxidation pathway rate and increases in reduced glutathione levels.26 Furthermore, it is already known that exercise increases LPL activity,6 and in our study, we propose that changes seen in lipemia could be associated with an increase in LPL activity, previously increased by the effect of exercise. On the other hand, we have seen that S. maxima is a good food source with a high nutritional value31 and has hypolipidemic and anti-inflammatory activities, factors which have a positive impact on exercise performance.
In this study, we have demonstrated that Spirulina has an important effect as a hypotriacylglycerolemic product. Furthermore, we observed no changes in C-HDL levels and a tendency to diminish TC. However, the studies conducted by Slentz et al.32 and Orio et al.,33 demonstrate that detraining for >1 week decreases C-HDL and increases postprandial lipemia, C-LDL, and TAG-VLDL concentrations. Spirulina seems to attenuate these negative effects.
In addition, in a previous study conducted in our laboratory, we demonstrated that non-trained adult subjects show an effective response to the antihyperlipemic effect of Spirulina. We cannot possibly see changes in TC and C-HDL in these participants because of their age. We should consider that 22 of the 36 subjects (61%) were adolescents with hormonal changes which can affect cholesterolemia.24
The action mechanisms of Spirulina on lipid metabolism are not yet well understood. Nagaoka et al.34 found that a concentrate of S. platensis inhibited jejunal cholesterol absorption and ileal bile acid reabsorption, proposing that C-phycocyanin is the molecule responsible for this effect. Han et al. isolated an active component designed as glycolipid H-b2, and they found that this glycolipid inhibits pancreatic lipase activity in a dose-dependent manner, lowering rat plasma TAG levels.35 These effects could explain the hypotriacylglycerolemic effects seen among patients treated with S. maxima, but no study has been conducted in humans so far.
According to our results, we have seen an important decrease in lipemia between 3.0 and 4.5 h. Sixty-two percent of these participants decreased lipemia at all the times measured.
We did not find differences in lipemia between sexes (data not shown). Instead, as shown in Figure 2, we found differences among groups of age. We have demonstrated that children between 10 and 12 years old are more sensitive to S. maxima and, to a lower degree, teenagers between 13 and 16 years old. We could not observe this effect on populations between 17 and 26 years of age, possibly due to higher desertion or because it takes more time to see any effect after cyanobacteria consumption. In our study, young population responds faster than the older non-trained population, as seen in a previous work where an open population consumed Spirulina for 6 weeks. In that study, a hypotriacylglycerolemic effect was observed.24
Finally, blood lactate concentration has been used as the physiological determiner of success in endurance sports. Blood lactate is a natural metabolite that is produced by glycolysis and is accumulated during exercise, according to the intensity and duration of physical activity, and it is also associated with a decrease in buffer capacity. Thus, maintaining a lower lactate concentration during exercise improves aerobic capacity. Trained individuals with a high-endurance capacity display lower blood lactate levels at a given cycling power output testing or running velocity than a sedentary individual. In other words, trained individuals will be able to work at a higher absolute intensity.36 Before treatment (without Spirulina), lactate concentration was higher at the end of the exercise than in those with Spirulina, which probably means that Spirulina either improves aerobic capacity or decreases the activity of lactate dehydrogenase. Unfortunately, we were unable to find significant differences.
The effects on lipids in men and women are different, but in this study, there were no differences, probably because it was a trained small population; however, this is a preliminary report that provides us significant information for further studies.
Conclusion
Spirulina diminishes postprandial lipemia after a high-fat meal in young athletes, especially between 10 and 16 years of age. We observed this effect since 1.5 h, although at 3–4.5 h, the decrease is higher. This effect was observed despite a short detraining period.
Acknowledgments
The authors acknowledge the grant from PAPIIT IN205410, UNAM. They thank Salomon Shamosh Halabe for Spirulina donation. They also thank Dr. Jose Luis Perez-Garcia for reviewing the correct usage of English in this article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Altena TS. Michaelson JL. Ball SD. Thomas TR. Single sessions of intermittent and continuous exercise and postprandial lipemia. Med Sci Sports Exerc. 2004;36:1364–1371. doi: 10.1249/01.mss.0000135793.43808.6c. [DOI] [PubMed] [Google Scholar]
- 2.Gill JM. Murphy MH. Hardman AE. Postprandial lipemia: effects of intermittent versus continuous exercise. Med Sci Sports Exerc. 1998;30:1515–1520. doi: 10.1097/00005768-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Murphy MH. Nevill AM. Hardman AE. Different patterns of brisk walking are equally effective in decreasing postprandial lipaemia. Int J Obes Relat Metab Disord. 2000;24:1303–1309. doi: 10.1038/sj.ijo.0801399. [DOI] [PubMed] [Google Scholar]
- 4.Karpe F. Hellenius ML. Hamsten A. Differences in postprandial concentrations of very-low-density lipoprotein and chylomicron remnants between normotriglyceridemic and hypertriglyceridemic men with and without coronary heart disease. Metabolism. 1999;48:301–307. doi: 10.1016/s0026-0495(99)90076-8. [DOI] [PubMed] [Google Scholar]
- 5.van Oostrom AJ. Alipour A. Sijmonsma TP, et al. Comparison of different methods to investigate postprandial lipaemia. Neth J Med. 2009;67:13–20. [PubMed] [Google Scholar]
- 6.Ramos-Jiménez A. Hernández-Torres RP. Wall-Medrano A. Munoz-Daw M. Torres-Durán PV. Juárez-Oropeza MA. Cardiovascular and metabolic effects of intensive Hatha Yoga training in middle-aged and older women from northern Mexico. Int J Yoga. 2009;2:49–54. doi: 10.4103/0973-6131.60044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu MH. Maher AC. Hamadeh MJ. Ye C. Tarnopolsky MA. Exercise, sex, menstrual cycle phase, and 17beta-estradiol influence metabolism-related genes in human skeletal muscle. Physiol Genomics. 2009;40:34–47. doi: 10.1152/physiolgenomics.00115.2009. [DOI] [PubMed] [Google Scholar]
- 8.Herd SL. Lawrence JE. Malkova D. Murphy MH. Mastana S. Hardman AE. Postprandial lipemia in young men and women of contrasting training status. J Appl Physiol. 2000;89:2049–2056. doi: 10.1152/jappl.2000.89.5.2049. [DOI] [PubMed] [Google Scholar]
- 9.Horton TJ. Pagliassotti MJ. Hobbs K. Hill JO. Fuel metabolism in men and women during and after long-duration exercise. J Appl Physiol. 1998;85:1823–1832. doi: 10.1152/jappl.1998.85.5.1823. [DOI] [PubMed] [Google Scholar]
- 10.Mittendorfer B. Horowitz JF. Klein S. Effect of gender on lipid kinetics during endurance exercise of moderate intensity in untrained subjects. Am J Physiol Endocrinol Metab. 2002;283:E58–E65. doi: 10.1152/ajpendo.00504.2001. [DOI] [PubMed] [Google Scholar]
- 11.Hernández-Torres R. Ramos-Jiménez A. Mascher D, et al. Contracción muscular y bioquímica del ejercicio. In: Díaz-Zagoya JC, editor; Juárez-Oropeza MA, editor. Bioquímica. Mc Graw Hill; India: 2007. pp. 663–667. [Google Scholar]
- 12.Zhang JQ. Thomas TR. Ball SD. Effect of exercise timing on postprandial lipemia and HDL cholesterol subfractions. J Appl Physiol. 1998;85:1516–1522. doi: 10.1152/jappl.1998.85.4.1516. [DOI] [PubMed] [Google Scholar]
- 13.Gill JM. Hardman AE. Exercise and postprandial lipid metabolism: an update on potential mechanisms and interactions with high-carbohydrate diets (review) J Nutr Biochem. 2003;14:122–132. doi: 10.1016/s0955-2863(02)00275-9. [DOI] [PubMed] [Google Scholar]
- 14.Singhal A. Trilk JL. Jenkins NT. Bigelman KA. Cureton KJ. Effect of intensity of resistance exercise on postprandial lipemia. J Appl Physiol. 2009;106:823–829. doi: 10.1152/japplphysiol.90726.2008. [DOI] [PubMed] [Google Scholar]
- 15.Kolifa M. Petridou A. Mougios V. Effect of prior exercise on lipemia after a meal of moderate fat content. Eur J Clin Nutr. 2004;58:1327–1335. doi: 10.1038/sj.ejcn.1601968. [DOI] [PubMed] [Google Scholar]
- 16.Hardman AE. The influence of exercise on postprandial triacylglycerol metabolism. Atherosclerosis. 1998;141(Suppl 1):S93–S100. doi: 10.1016/s0021-9150(98)00225-1. [DOI] [PubMed] [Google Scholar]
- 17.Hardman AE. Herd SL. Exercise and postprandial lipid metabolism. Proc Nutr Soc. 1998;57:63–72. doi: 10.1079/pns19980011. [DOI] [PubMed] [Google Scholar]
- 18.Dumith SC. Hallal PC. Reis RS. Kohl HW. Worldwide prevalence of physical inactivity and its association with human development index in 76 countries. Prev Med. 2011;53:24–28. doi: 10.1016/j.ypmed.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz-Hernández L. Gómez-Tello BL. Food consumption in Mexican adolescents. Rev Panam Salud Publica. 2008;24:127–135. doi: 10.1590/s1020-49892008000800007. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Oliveros G. Haines J. Ortega-Altamirano D, et al. Obesity determinants in Mexican preschool children: parental perceptions and practices related to feeding and physical activity. Arch Med Res. 2011;42:532–539. doi: 10.1016/j.arcmed.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Ciferri O. Spirulina, the edible microorganism. Microbiol Rev. 1983;47:551–578. doi: 10.1128/mr.47.4.551-578.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamorro G. Salazar M. Favila L. Bourges H. [Pharmacology and toxicology of Spirulina alga] Rev Invest Clin. 1996;48:389–399. [PubMed] [Google Scholar]
- 23.Khan Z. Bhadouria P. Bisen PS. Nutritional and therapeutic potential of Spirulina. Curr Pharm Biotechnol. 2005;6:373–379. doi: 10.2174/138920105774370607. [DOI] [PubMed] [Google Scholar]
- 24.Torres-Durán PV. Ferreira-Hermosillo A. Juárez-Oropeza MA. Antihyperlipemic and antihypertensive effects of Spirulina maxima in an open sample of Mexican population: a preliminary report. Lipids Health Dis. 2007;6:33. doi: 10.1186/1476-511X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira-Hermosillo A. Torres-Durán PV. Juárez-Oropeza MA. Hepatoprotective effects of Spirulina maxima in patients with non-alcoholic fatty liver disease: a case series. J Med Case Reports. 2010;4:103. doi: 10.1186/1752-1947-4-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalafati M. Jamurtas AZ. Nikolaidis MG, et al. Ergogenic and antioxidant effects of spirulina supplementation in humans. Med Sci Sports Exerc. 2010;42:142–151. doi: 10.1249/MSS.0b013e3181ac7a45. [DOI] [PubMed] [Google Scholar]
- 27.Lu HK. Hsieh CC. Hsu JJ. Yang YK. Chou HN. Preventive effects of Spirulina platensis on skeletal muscle damage under exercise-induced oxidative stress. Eur J Appl Physiol. 2006;98:220–226. doi: 10.1007/s00421-006-0263-0. [DOI] [PubMed] [Google Scholar]
- 28.Cohen H. Goldberg C. Effect of physical exercise on alimentary lipaemia. Br Med J. 1960;2:509–511. doi: 10.1136/bmj.2.5197.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlierf G. Dinsenbacher A. Kather H. Kohlmeier M. Haberbosch W. Mitigation of alimentary lipemia by postprandial exercise—phenomena and mechanisms. Metabolism. 1987;36:726–730. doi: 10.1016/0026-0495(87)90107-7. [DOI] [PubMed] [Google Scholar]
- 30.Hardman AE. Aldred HE. Walking during the postprandial period decreases alimentary lipaemia. J Cardiovasc Risk. 1995;2:71–78. [PubMed] [Google Scholar]
- 31.Torres-Durán PV. Paredes-Carbajal MC. Mascher D. Zamora-González J. Díaz-Zagoya JC. Juárez-Oropeza MA. Protective effect of Arthrospira maxima on fatty acid composition in fatty liver. Arch Med Res. 2006;37:479–483. doi: 10.1016/j.arcmed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Slentz CA. Houmard JA. Johnson JL, et al. Inactivity, exercise training and detraining, and plasma lipoproteins. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol. 2007;103:432–442. doi: 10.1152/japplphysiol.01314.2006. [DOI] [PubMed] [Google Scholar]
- 33.Orio F. Vuolo L. Palomba S. Lombardi G. Colao A. Metabolic and cardiovascular consequences of polycystic ovary syndrome. Minerva Ginecol. 2008;60:39–51. [PubMed] [Google Scholar]
- 34.Nagaoka S. Shimizu K. Kaneko H, et al. A novel protein C-phycocyanin plays a crucial role in the hypocholesterolemic action of Spirulina platensis concentrate in rats. J Nutr. 2005;135:2425–2430. doi: 10.1093/jn/135.10.2425. [DOI] [PubMed] [Google Scholar]
- 35.Han LK. Li DX. Xiang L, et al. [Isolation of pancreatic lipase activity-inhibitory component of Spirulina platensis and it reduce postprandial triacylglycerolemia] Yakugaku Zasshi. 2006;126:43–49. doi: 10.1248/yakushi.126.43. [DOI] [PubMed] [Google Scholar]
- 36.Hernández-Torres RP. Ramos-Jiménez A. Torres-Durán PV, et al. Effects of single sessions of low-intensity continuous and moderate-intensity intermittent exercise on blood lipids in the same endurance runners. J Sci Med Sport. 2009;12:323–331. doi: 10.1016/j.jsams.2007.12.002. [DOI] [PubMed] [Google Scholar]