Abstract
Cytokine-induced cell death is recognized as a major cause of progressive β-cell loss. Tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and interferon γ (IFN-γ) in combination trigger a series of events that lead to β-cell death. In the past few decades, the use of myricetin as an anti-inflammatory and cytoprotective agent has gained much attention. The present study focused on the protective roles of myricetin against cytokine-induced cell death in insulin-secreting RIN-m5f β cells. The results showed that myricetin (especially at concentrations of 10 μM and 20 μM) increased cell viability and decreased cell apoptosis induced by the cytokine mixture of TNF-α (10 ng/mL), IL-1β (5 ng/mL), and IFN-γ (1000 IU/mL) for 3 days. Moreover, the cytokines increased the total and p65 subunit levels of nuclear factor κB, decreased inhibitor κB α levels, stimulated the accumulation of nitric oxide, increased cytochrome c release from mitochondria, and induced reactive oxygen species generation; myricetin (especially at the concentration of 20 μM) abolished all of these parameters. These results suggest that myricetin might have therapeutic value for preventing β-cell death.
Key Words: cytokines, mitochondrial death pathway, myricetin, nuclear factor κB, RIN-m5f β cells
Introduction
The failure of insulin-secreting β cells is a common feature of diabetes leading to relative or absolute insulin deficiency. The inflammatory mediators have been implicated as having a crucial role in prolonged suppression of β-cell function, β-cell apoptosis, and progressive β-cell loss.1 Currently, the mechanisms of β-cell death induced by cytokines are still being unraveled. Evidence is growing that tumor necrosis factor α (TNF-α), interleukin (IL)-1β, and interferon γ (IFN-γ) are candidate cytokines that participate in promoting β-cell death with some combined effects.2–4
Several studies during the last decade indicated that cytokine-mediated β-cell death was related to the activation of nuclear factor κB (NF-κB), which induced a pro-apoptotic expression pattern.5 Among the genes whose expression is regulated by NF-κB following exposure to cytokines, the role of inducible nitric oxide (NO) synthase (iNOS) and the subsequent generation of an excess of NO in cytokine-mediated β-cell death have been clearly established.6 Alternatively, the mitochondrial death pathway represents a new focus on the failure of β cells induced by apoptotic stimuli (e.g., cytokines). It is important that reactive oxygen species (ROS), especially mitochondrial ROS, also play a role in the initiation of apoptotic signaling.7
However, it remains unclear how to safely and effectively prevent or reverse β-cell death induced by cytokines. Clinically, a novel treatment with fewer side effects is desirable for the control of β-cell death. Myricetin (3,5,7,3′,4′,5′-hexahydroxyflavone cannabiscetin) is a naturally occurring flavonoid that occurs in fruits, vegetables, tea, berries, red wine, and medical plants.8 There has been an increasing attention to its widespread health benefits, such as antioxidative effects (free radical scavengers and potent metal chelators), antiviral and antimicrobial properties, and anticarcinogenic actions.9–11 Moreover, in vitro and animal studies showed that myricetin exerted hypoglycemic effects by ameliorating glycogen metabolism and increasing insulin sensitivity.12–14 In the United States, researchers used a composition, including myricetin, as an application for treating diabetes and metabolic disorders.15,16 In addition, myricetin was shown to possess anti-inflammatory activity. In vitro studies demonstrated that myricetin not only inhibited the production of cytokines (e.g., IL-1β and IL-6),17,18 but also affected signal pathways induced by cytokines to inhibit apoptosis and cell death.19 However, little information is available describing its effect on β-cell death induced by the synergistic action of TNF-α, IL-1β, and IFN-γ. The protective role of myricetin in β cells and the relevant mechanisms need further elucidation.
In the present study, we used the insulin-secreting β-cell line RIN-m5f to investigate the cytoprotective effects of myricetin in vitro. To test cell viability, the methyl thiazolyl tetrazolium (MTT) assay and nuclear staining with 4,6-diamino-2-phenyl indole (DAPI) were performed. To learn the mechanisms by which myricetin protected β cells against cytokine-induced cell death, the expression of NF-κB p65 and inhibitor κB (I-κB) α and the NO levels were determined. Furthermore, the mitochondrial death pathway, especially release of cytochrome c from mitochondria, and the ROS production were studied.
Materials And Methods
Materials
The insulin-secreting β-cell line RIN-m5f was generously provided by Dr. Chunyan Zhou (Department of Biochemistry and Molecular Biology, School of Basic Medical Science, Peking University). Myricetin (catalog number 70050) (≥95.0% pure myricetin by high-performance liquid chromatography), MTT, and DAPI (≥98.0%, cell cultured tested) were purchased from Sigma-Aldrich (St. Louis, MO, USA). High-glucose Dulbecco's modified Eagle's medium, fetal bovine serum, and trypsin were from GIBCO™ (Auckland, New Zealand). TNF-α, IL-1β, and IFN-γ were purchased from Peprotech (Rocky Hill, NJ, USA). Nuclear and cytoplasmic extraction reagents, BCA protein assay kit, phenylmethylsulfonyl fluoride, leupeptin, aprotinin, and pepstatin were from Pierce (Rockford, IL, USA). The fluorescent probes 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), NO assay kit, and Super ECL Plus detection reagent were purchased from Applygen Technologies Inc. (Beijing, China). The antibodies to I-κBα, NF-κB p65, cytochrome c, glyceraldehyde 3-phosphate dehydrogenase, lamin B, and immunoglobulin G horseradish peroxidase–linked secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture
The insulin-secreting RIN-m5f β cells were grown in high-glucose Dulbecco's modified Eagle's medium containing 10% fetal bovine serum at 37°C and a 5% CO2 atmosphere in 96-, 24-, or 6-well or 100-mm-diameter plates. Because of the long incubation time, cells were preincubated in serum-free medium for 4–6 h prior to each experiment once grown to 50% confluence. To induce β-cell death, cells were challenged for 3 days with the same dosages of cytokines (10 ng/mL TNF-α, 5 ng/mL IL-1β, and 1000 IU/mL IFN-γ [herein referred to as TII]) used in the recent study from this laboratory.20 In this set of experiments, cells were pretreated with different doses of myricetin for 3 h prior to TII stimulation and continuously treated with TII and different doses of myricetin. All of the determinations were performed 3 days later. Medium with TII and/or myricetin was replaced with fresh medium every day.
Cell viability assay
The MTT assay was performed to determine the nontoxic dose of myricetin and to monitor the cell viability. Cells were cultured in 96-well plates at a density of 2×105/mL. After the designated treatment, 100 μL of MTT solution (1 mg/mL) was added. After 4 h, blue formazan crystals were resolved with 100 μL of dimethyl sulfoxide. Absorbance was measured at 595 nm. Cell viability (percentage of control) was calculated relative to the untreated control.
DAPI staining
Chromatin condensation and fragmentation are typical markers of apoptosis. Morphologic evidence of apoptosis was estimated by nuclear staining with DAPI. After treatment, RIN-m5f β cells, seeded on coverslips in 24-well plates, were fixed with 4% paraformaldehyde for 30 min at room temperature, washed with phosphate-buffered solution three times, and stained with DAPI (0.1 μg/mL) for 5 min in the dark. Then cells were observed under a fluorescence microscope (Olympus, Tokyo, Japan). DAPI-stained cells with intensely fluorescing and/or with condensed nuclei were considered apoptotic.
Protein extract
For whole-cell extracts, RIN-m5f β cells were rinsed twice with ice-cold phosphate-buffered saline and lysed in lysis buffer containing 20 mM Tris-HCl, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1 mM Na3VO4, 11 mM β-mercaptoethanol, 0.1% Triton X-100, 2.5 mM Na4P2O7, 250 mM phenylmethylsulfonyl fluoride, 1 μg/mL leupeptin, 1 μg/mL aprotinin, and 1 μg/mL pepstatin for 30 min on ice. Lysates were centrifuged at 12,000 g for 5 min at 4°C, and the supernatants were collected.
For differential nuclear and cytoplasmic extraction, a protocol following the instructions for nuclear and cytoplasmic extraction reagents (Pierce) was used. Cells were washed with ice-cold phosphate-buffered saline and centrifuged at 500 g for 5 min. The pellets were vortex-mixed with ice-cold CER I and incubated on ice for 10 min. Then cells were vortex-mixed with ice-cold CER II and incubated on ice for 1 min. After that, cells were centrifuged at 16,000 g for 5 min, and the supernatants (cytoplasmic extract) were immediately collected. The insoluble fraction, which contains nuclei, was resuspended with ice-cold NER. After being placed on ice and vortex-mixed for 15 s every 10 min (for a total of 40 min), cells were centrifuged at 16,000 g for 10 min, and the supernatants (nuclear extract) were immediately collected.
To observe the release of cytochrome c from mitochondria, mitochondria was collected. In brief, RIN-m5f β cells were harvested and suspended with 5 volumes of the solution (20 mM HEPES-KOH, 1.5 mM MgCl2, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 250 mM sucrose, and 0.1 mM phenylmethylsulfonyl fluoride). Cells were homogenized and centrifuged at 750 g for 10 min at 4°C. Supernatants were then centrifuged at 10,000 g for 15 min, and the pellets contained cellular mitochondria. Pellets were then lysed in lysis buffer (10 mM Tris-HCl, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1 μg/mL leupeptin, 1 μg/mL aprotinin, 1 μg/mL pepstatin, 250 mM phenylmethylsulfonyl fluoride, 2 mM Na3VO4, and 1% sodium dodecyl sulfate). The lysed solution was used for the identification of mitochondrial cytochrome c. The supernatants were centrifuged at 100,000 g for 15 min, and the supernatants obtained were used for identification of cytosolic cytochrome c.
Protein content was then determined using a BCA protein assay kit (Pierce), and proteins were frozen at −80°C until further analysis.
Western blot analysis
An aliquot of 40 μg of protein from each sample was used for electrophoresis through either 10% or 12% sodium dodecyl sulfate–polyacrylamide gels and transferred to 0.45-μm (pore size) polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). The membranes were blocked for 1 h with 5% nonfat dried milk in Tris-buffered saline at ambient temperature. After washing in Tris-buffered saline containing 0.05% Tween 20, the membranes were incubated with primary antibodies in blocking buffer overnight at 4°C. For detection, the membranes were incubated with horseradish peroxidase–conjugated anti-rabbit immunoglobulin G or anti-mouse immunoglobulin G secondary antibodies for 1 h at 37°C, and the blots were visualized autoradiographically with ECL reagent. Relative protein quantification was done with Quantity One® software (Bio-Rad Laboratories, Hercules, CA, USA).
NO determination
The level of NO was determined by assaying the concentration of nitrite in the whole cell extracts and cell culture medium. Fifty microliters of whole-cell extracts or cell culture medium was incubated with 100 μL of Griess reagent (Applygen Technologies Inc.) for 10 min at room temperature in the dark. Sodium nitrite was used to generate a standard curve. The optical density value of the samples at 540 nm was measured. Results were indicated as the NO to protein ratio, expressed as micromoles of NO per gram of protein.
Measurement of intracellular ROS
To analyze the intracellular generation of ROS, cells were detached by trypsinization. The cellular fluorescence intensity was measured after a 30-min incubation with the oxidation-sensitive probe DCFH-DA (10 μmol/L), by using a FACScan™ flow cytometer (BD Biosciences, San Jose, CA, USA). For each analysis, 10,000 events were recorded.
Statistical analysis
The data are given as mean±SE values of three or more independent experiments. Comparisons between multiple groups were made by one-way analysis of variance followed by the least significant difference test. P<.05 was considered statistically significant.
Results
Myricetin increased cell viability down-regulated by cytokines
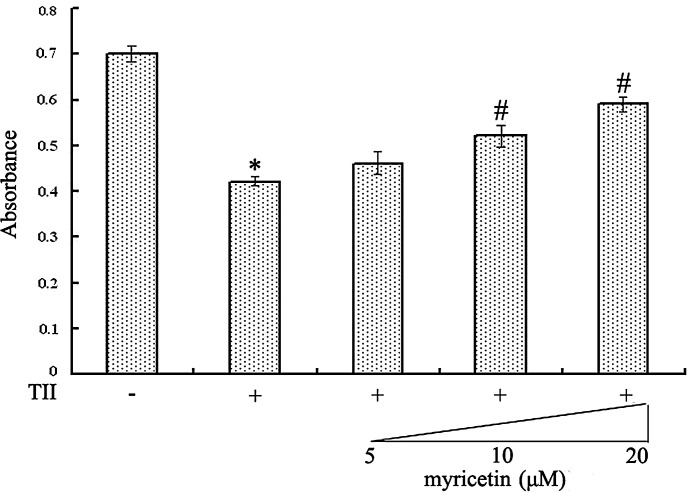
In the initial experiments, the nontoxic dose of myricetin was examined by the MTT assay. Myricetin did not influence the viability of RIN-m5f β cells in the dose range from 5 to 20 μM (data not shown). The effect of TII on cell viability and the cytoprotective properties of myricetin were then determined. As indicated in Figure 1, in contrast with the control group, exposure to the cytokine mixture for 3 days significantly decreased viability of RIN-m5f β cells (P<.05). However, myricetin pretreatment at concentrations (5, 10, and 20 μM) rescued cells viability to 65.89%, 74.24%, and 84.27% of the control level, respectively. These results suggest that myricetin pretreatment may partially preserve cell viability in TII-treated RIN-m5f β cells in a concentration-dependent manner but will not restore viability back to control levels.
FIG. 1.
Comparison of methyl thiazolyl tetrazolium assay in RIN-m5f β cells. Data are mean±SE values expressed as the absorbance (at 595 nm) from six experiments. P<.05, statistically significant difference from *control group or #10 ng/mL tumor necrosis factor-α, 5 ng/mL interleukin-1β, and 1000 IU/mL interferon-γ (TII)-alone group.
Myricetin decreased cell apoptosis induced by cytokines
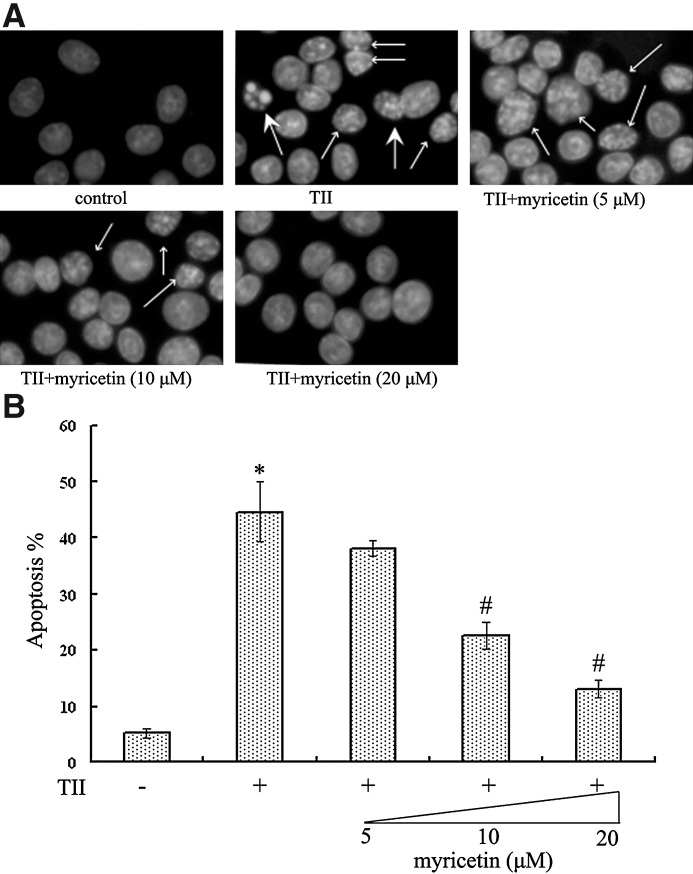
RIN-m5f β cells undergoing apoptosis were shown in Figure 2. Healthy cells stained with DAPI exhibited homogeneous and diffused staining with regular contours. Treatment with TII for 3 days resulted in increased apoptosis (P<.05) characterized by intense fluorescence in the nucleus and even apoptotic bodies. Although 5 μM myricetin had no notable effect on cell apoptosis, 10 μM and 20 μM myricetin significantly decreased cell apoptosis (both P<.05), and the morphology of cell nuclei tended to be normal after co-treatment with 20 μM myricetin.
FIG. 2.
4,6-Diamino-2-phenyl indole staining of RIN-m5f β cell nucleus. (A) The large arrows showed the late phage of apoptosis with apoptotic bodies, and the small arrows showed the early phase of apoptosis with intense fluorescence in the nucleus. Original magnification, ×400. (B) Apoptotic and total cells were counted in three random fields of three different slides. Data are mean±SE values. The number of apoptotic cells was expressed as a percentage to total cells. P<.05, statistically significant difference from *control group or #TII-alone group.
Myricetin inhibited NF-κB activation stimulated by cytokines
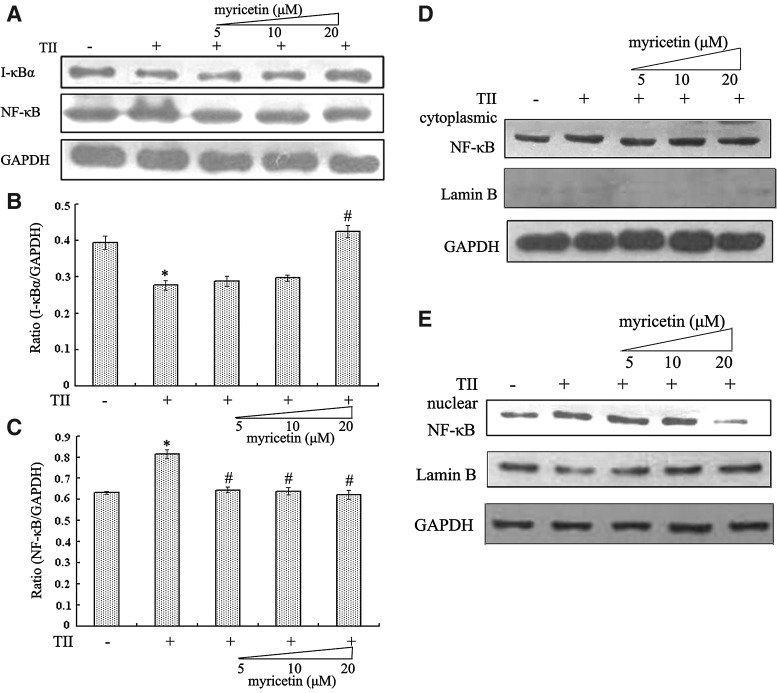
Figure 3B and 3C showed that, in response to TII, NF-κB p65 subunit, comprising a powerful transcriptional activation domain, was significantly increased (P<.05). I-κBα, one of three major I-κB isoforms preventing the translocation of NF-κB to the nucleus, was decreased (P<.05). However, the pretreatment with myricetin for 3 h prior to TII stimulation clearly down-regulated the NF-κB p65 level, and myricetin (especially at the concentration of 20 μM) treatment clearly caused an increase in I-κBα levels (P<.05). Furthermore, the nuclear translocation of NF-κB p65 was also observed (Fig. 3D and E). The results showed that, in contrast to the control group, TII enhanced the level of NF-κB p65 in cell nucleus, whereas myricetin (20 μM) treatment obviously reduced NF-κB p65 nuclear translocation. In addition, the level of NF-κB p65 in cytoplasm was slightly increased in the presence of TII, but no obvious change was seen after myricetin treatment. Collectively, these results indicate that myricetin's protective effect on TII-treated RIN-m5f β cells may be at least in part due to its effect on NF-κB activation.
FIG. 3.
Myricetin modulated nuclear factor κB (NF-κB) activity in TII-treated RIN-m5f β cells. (A) An aliquot of protein from whole-cell extracts was subjected to western blot analysis using inhibitor κB (I-κB) α and NF-κB p65 antibodies. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein levels were used as a control. Relative protein quantification was done with Quantity One software. (B, C) A ratio between specific protein and GAPDH was calculated as mean±SE of three experiments. P<.05, statistically significant difference from *control group or #TII-alone group. (D, E) Nuclear and cytoplasmic proteins were assayed for NF-κB p65 proteins by western blot analysis. GAPDH and Lamin B are cytoplasmic and nuclear markers, respectively. Three independent experiments were done, and all gave similar results.
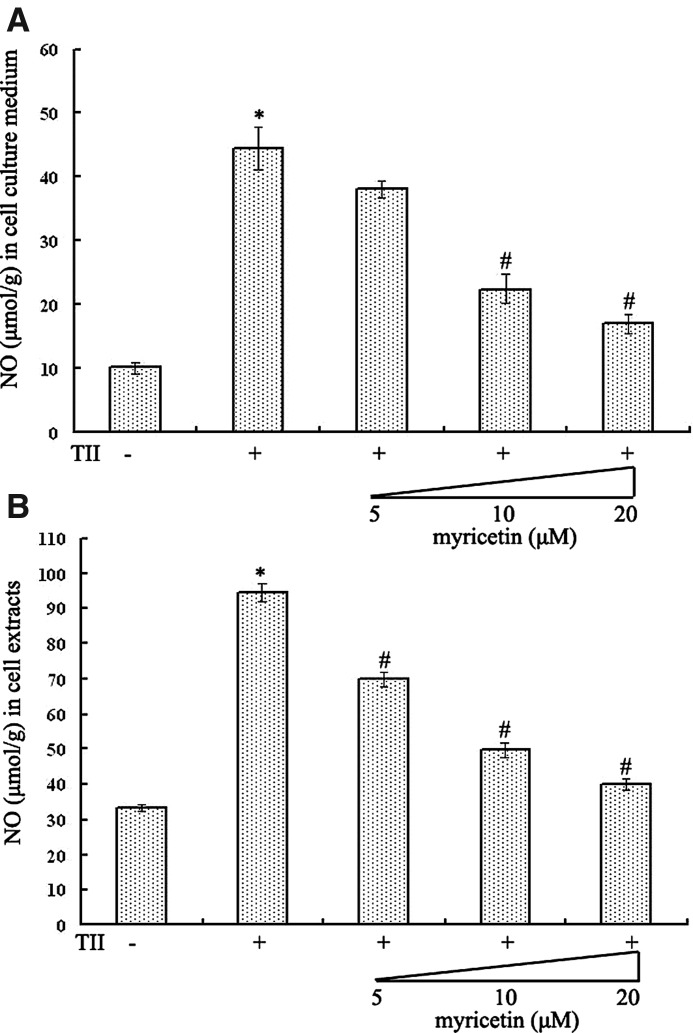
Myricetin reduced the accumulation of NO in cytokine-treated RIN-m5f β cells
NO has been considered an effector molecule leading to β-cell death in autoimmune diabetes. As can be seen in Figure 4, NO generation was significantly increased in both cell culture medium and RIN-m5f β cells incubated with TII compared with the control group (both P<.05). However, the stimulated NO production in cell culture medium was suppressed by the addition of myricetin in a dose-dependent way (Fig. 4A). Moreover, in contrast to the TII-stimulated group, the level of NO in cell extracts was decreased by 26.18%, 47.39%, and 57.65% with 5 μM, 10 μM, and 20 μM myricetin treatment (Fig. 4B).
FIG. 4.
Myricetin decreased the accumulation of nitric oxide (NO) in TII-treated RIN-m5f β cells. Accumulation of NO in (A) cell culture medium and (B) cells was determined. Results were represented as NO to protein ratio expressed as micromoles of NO per gram of protein of six separate experiments. P<.05, statistically significant difference from *control group or #TII-alone group.
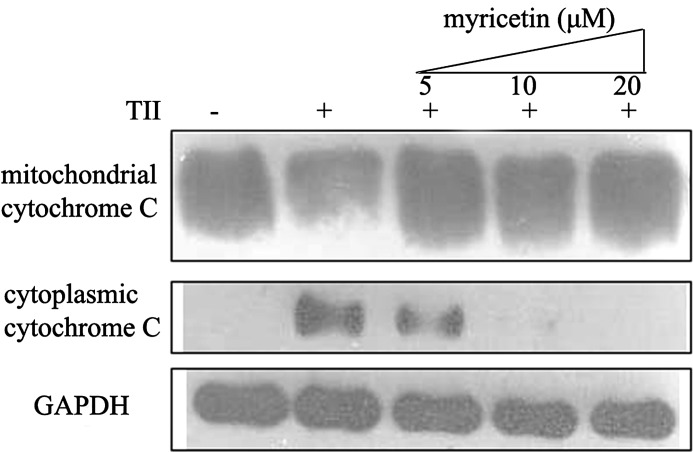
Myricetin inhibited cytochrome c release from mitochondria in cytokine-treated RIN-m5f β cells
Mitochondrial dysfunction has also been proposed as a common feature of defective β-cell function and survival. As expected, the treatment of cells with TII clearly increased cytochrome c release from mitochondria into the cytosol. However, the myricetin treatment markedly decreased the release of cytochrome c from mitochondria in a dose-dependent manner (Fig. 5). Myricetin therefore might produce its cytoprotective effect by suppressing the mitochondrial death pathway.
FIG. 5.
Myricetin inhibited cytochrome c release from mitochondria in TII-treated RIN-m5f β cells. Mitochondrial and cytoplasmic proteins were assayed for cytochrome c proteins by western blot analysis. GAPDH protein levels were used as a control. The result is one representative example of three separate experiments.
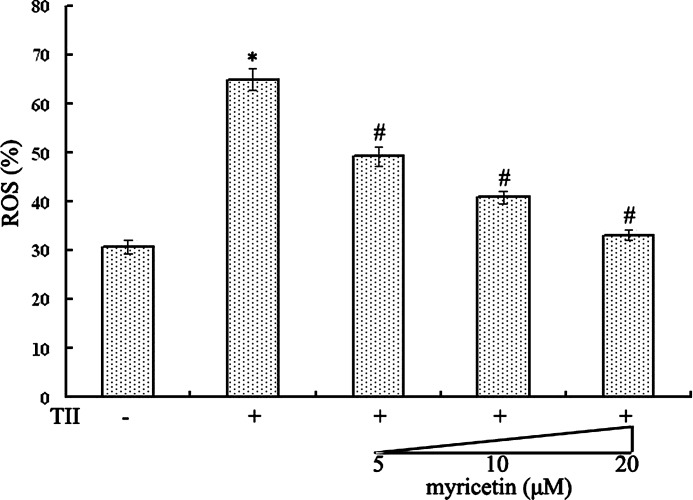
Myricetin decreased ROS levels induced by cytokines
Understanding that ROS are important factors in the pathogenesis of diabetes raises a concern regarding the generation of ROS in cytokine-treated RIN-m5f β cells. The intracellular ROS were analyzed by using the oxidation-sensitive probe DCFH-DA (Fig. 6). Flow cytometric analysis of RIN-m5f β cells exposed to TII revealed a dramatic increase in ROS generation (64.84%). In marked contrast, the level of ROS was reduced to 49.25%, 40.90%, and 33.14% with 5 μM, 10 μM, and 20 μM myricetin treatment (all P<.05), suggesting that myricetin's cytoprotective effect is partly attributable to its regulation of ROS.
FIG. 6.
Myricetin suppressed reactive oxygen species (ROS) levels in TII-treated RIN-m5f β cells. The cellular fluorescence intensity was measured after a 30-min incubation with an oxidation-sensitive dye (2′,7′-dichlorodihydrofluorescein diacetate) and analyzed with a FACScan flow cytometer to determine the percentage of cells displaying an increase in ROS levels. Data are mean±SE values from six experiments. P<.05, statistically significant difference from *control group or #TII-alone group.
Discussion
The determination of β-cell death in vivo has proven difficult because of the slow kinetics of inflammatory process and the rapid clearance of dead cells. So it might be corroborated by in vitro studies. In the present study, the in vitro model of cytokine-induced pancreatic β-cell death was established. In the study of Delaney et al.,2 prolonged (6–9 days) exposure of human pancreatic islets to a combination of cytokines (TNF-α [1000 U/mL]+IL-1β [50 U/mL]+IFN-γ [1000 U/mL]) induced DNA strand breaks and cell death by apoptosis. In the recent study from this laboratory, exposure to combination of cytokines (TNF-α [10 ng/mL]+IL-1β [5 ng/mL]+IFN-γ [1000 U/mL]) for 24 h decreased viability and cell function in RIN-m5f β cells.20 In this study, we observed that a 3-day exposure of the same dosages of cytokines severely impaired RIN-m5f β cells and induced cell apoptosis. The cytotoxic effects of cytokines on β cells might depend on their concentrations, duration of exposure, and cell types.
In the past few decades, the use of myricetin as a cytoprotective agent has gained much attention. In vitro studies indicated that preincubation with various concentrations (0–200 μM) of myricetin before H2O2 exposure significantly protected Caco-2 and HepG2 cells against H2O2-induced cell damage.21 Moreover, myricetin (5 and 30 μM) decreased the apoptotic rate of cardiomyocytes.22 However, it was also proposed that myricetin (25–200 μM) induced apoptotic cell death in pancreatic cancer cells without influencing the viability of normal ductal cells.23,24 The effect of myricetin on cell death appears to be cell type-dependent and dosage-dependent. In this study, we present, for the first time, a mode of action for myricetin protection against TII-induced cytotoxicity in RIN-m5f β cells.
It is well known that NF-κB plays an important role in cytokine-induced β-cell destruction. In resting cells, NF-κB, combined with I-κB isoforms (e.g., I-κBα), is present in the cytosol as an inactive form. Signals from cytokines might induce the activation of proteolytic degradation of I-κB and/or the phosphorylation of I-κB, thereby allowing released NF-κB to enter the nucleus, where it binds to DNA and regulates gene transcription. Both in vitro and in vivo studies demonstrated that inhibition of NF-κB translocation or activation could prevent β-cell dysfunction and death induced by cytokines (e.g., TNF-α, IL-1β, and IFN-γ).25,26 In this study, we observed that the combined actions of cytokines increased the total and nuclear levels of NF-κB p65 and decreased the level of I-κBα. It was interesting that myricetin inhibited NF-κB nucleus translocation and increased the level of I-κBα, which may be one of its mechanisms of cytoprotective action. However, it is unclear here whether production of I-κBα protein is increased or degradation of the protein is inhibited after myricetin treatment. Additional studies will be needed to address this issue.
As mentioned above, iNOS and NO are implicated in β-cell death. Previous studies established that TNF-α and IL-1β mediated activation of the NF-κB pathway to stimulate the expression of iNOS.27 Moreover, IL-1β, alone or in synergy with IFN-γ, was proposed to induce NF-κB p65 translocation into the cell nucleus and increase the expression of iNOS mRNA and NO production.28 In the present study, we found that TII increased NO generation. On the other hand, an in vivo study indicated that myricetin could attenuate the lipopolysaccharide-induced outburst of iNOS gene expression and decrease NO production in intact rat liver.29 Our results also provided evidence that myricetin could reduce cytokine-induced NO formation. Thus, we conclude that myricetin might reduce NO production through the inhibition of NF-κB-dependent iNOS expression in this study. Such mechanisms against cytokine-induced cytotoxicity in β cells have also been shown with other flavonoids, such as curcumin, resveratrol, and sulfuretin.30–32
A growing body of evidence has demonstrated that intracellular signal responses controlled by mitochondria plays a central role in another relevant cell death pathway. As an electron carrier in the mitochondrial respiratory chain, cytochrome c is thought to mediate mitochondrial death pathway. We found that cytokines promoted the release of cytochrome c from mitochondria, which was coincident with β-cell apoptosis. As our results showed, Grunnet et al.33 reported that exposure to a cytokine cocktail (IL-1β, IFN-γ, and/or TNF-α) increased cytochrome c release from mitochondria in human and rat islets and INS-1 β cells. Moreover, we also observed that myricetin blocked mitochondria from releasing cytochrome c in the presence of TII. Taken together, these results suggest that, apart from the inhibition of NF-κB activation, myricetin might prevent TII-induced cytotoxicity in RIN-m5f β cells through a suppression of the mitochondrial death pathway. However, the modification of certain genes in the overlapping NF-κB controlled gene network and also induced mitochondrial death pathway with apoptotic features, such as cytochrome c release from mitochondria and mitochondrial permeability transition.7 Therefore, it is still possible that myricetin does not directly affect the mitochondrial death pathway; it might act by preventing the damaging effects of NF-κB on mitochondrial function. It remains to be determined which mechanisms are accountable for the present observations.
Additionally, the release of cytochrome c from the mitochondrial respiratory chain can also lead to the accumulation of ROS in cells. Recently, the generation of ROS was observed in MIN6N8 β cells stimulated by TNF-α and IFN-γ.34 Myricetin, with a large number of active hydroxyl groups, can effectively remove a variety of ROS and has an effective antioxidative activity. Investigators reported that myricetin significantly decreased chemical-induced increases in oxidative stress parameters of cells (e.g., erythrocytes from type 2 diabetic patients, osteoblastic MC3T3-E1 cells, and MES23.5 cells).35–37 Our results were in agreement with these reports. The synergetic effect of TII induced the production of ROS; however, the increase was reversible by myricetin treatment. Thus, removing the accumulation of ROS may be the underlying mechanism for its protective effect. It is notable that β cells are considered especially susceptible to attacks by ROS because of the very low expression of antioxidant enzymes. It was reported that myricetin caused an increase in antioxidant enzymes like glutathione, catalase, and glutathione peroxidase.38,39 Therefore, it is still possible that antioxidant effect of myricetin in the present study might be exerted through mechanism of activation of endogenous antioxidant defenses, such as glutathione production.
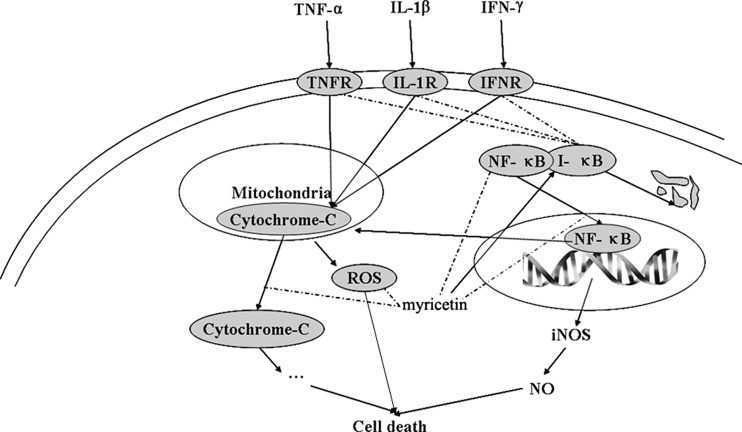
Collectively, the findings presented here suggest that the effect of myricetin on TII-induced cytotoxicity in the RIN-m5f β cell line may be mediated via inhibition of signaling by NF-κB and mitochondrial death pathways, in addition to its antioxidative activity (Fig. 7). It is important that using another cell line or primary pancreatic islet cells will generalize the effect of myricetin as a protective agent against cytokine-mediated cell death. Furthermore, the in vitro experiments does not consider the bioavailability and Phase II metabolism, and the effect of myricetin is less clear in vivo. Thus, further in vivo research into the effect of myricetin on pancreatic β-cell death induced by cytokines will contribute to our understanding of the identification of new molecular targets and therapeutic agents for protection against β-cell damage diseases like diabetes.
FIG. 7.
Schematic diagram for myricetin-stimulated signal pathways in TII-treated RIN-m5f β cells. Arrows and dashed lines indicate activation and repression of the following targets, respectively. IFN-γ, interferon-γ; IFNR, interferon-γ receptor; IL-1β, interleukin-1β; IL-1R, interleukin-1β receptor; iNOS, inducible NO synthase; TNF-α, tumor necrosis factor-α; TNFR, tumor necrosis factor-α receptor.
Acknowledgments
The authors would like to acknowledge Dr. Chunyan Zhou (Department of Biochemistry and Molecular Biology, School of Basic Medical Science, Peking University) for providing the insulin-secreting β-cell line RIN-m5f for this study. The present study was supported by the Foundation (grant number 2006BAD27B01) from the Ministry of Science and Technology of the People's Republic of China.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Eizirik DL. Mandrup-Poulsen T. A choice of death: the signal-transduction of immune-mediated β-cell apoptosis. Diabetologia. 2001;44:2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 2.Delaney CA. Pavlovic D. Hoorens A. Pipeleers DG. Eizirik DL. Cytokines induce deoxyribonucleic acid strand breaks and apoptosis in human pancreatic islet cells. Endocrinology. 1997;138:2610–2614. doi: 10.1210/endo.138.6.5204. [DOI] [PubMed] [Google Scholar]
- 3.Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003;66:1433–1440. doi: 10.1016/s0006-2952(03)00494-5. [DOI] [PubMed] [Google Scholar]
- 4.Guest CB. Park MJ. Johnson DR. Freund GG. The implication of proinflammatory cytokines in type 2 diabetes. Front Biosci. 2008;13:5187–5194. doi: 10.2741/3074. [DOI] [PubMed] [Google Scholar]
- 5.Eizirik DL. Moore F. Flamez D. Ortis F. Use of a systems biology approach to understand pancreatic beta-cell death in Type 1 diabetes. Biochem Soc Trans. 2008;36:321–327. doi: 10.1042/BST0360321. [DOI] [PubMed] [Google Scholar]
- 6.Corbett JA. Wang JL. Sweetland MA. Lancaster JR., Jr McDaniel ML. Interleukin 1 beta induces the formation of nitric oxide by beta-cells purified from rodent islets of Langerhans. Evidence for the beta-cell as a source and site of action of nitric oxide. J Clin Invest. 1992;90:2384–2391. doi: 10.1172/JCI116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabadkai G. Duchen MR. Mitochondria mediated cell death in diabetes. Apoptosis. 2009;14:1405–1423. doi: 10.1007/s10495-009-0363-5. [DOI] [PubMed] [Google Scholar]
- 8.Harnly JM. Doherty RF. Beecher GR, et al. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem. 2006;54:9966–9977. doi: 10.1021/jf061478a. [DOI] [PubMed] [Google Scholar]
- 9.Mira L. Fernandez MT. Santos M. Rocha R. Florencio MH. Jennings KR. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res. 2002;36:1199–1120. doi: 10.1080/1071576021000016463. [DOI] [PubMed] [Google Scholar]
- 10.Cushnie TP. Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang NJ. Jung SK. Lee KW. Lee HJ. Myricetin is a potent chemopreventive phytochemical in skin carcinogenesis. Ann N Y Acad Sci. 2011;1229:124–132. doi: 10.1111/j.1749-6632.2011.06122.x. [DOI] [PubMed] [Google Scholar]
- 12.Ong KC. Khoo HE. Effects of myricetin on glycemia and glycogen metabolism in diabetic rats. Life Sci. 2000;67:1695–1705. doi: 10.1016/s0024-3205(00)00758-x. [DOI] [PubMed] [Google Scholar]
- 13.Liu IM. Tzeng TF. Liou SS. Lan TW. Myricetin, a naturally occurring flavonol, ameliorates insulin resistance induced by a high-fructose diet in rats. Life Sci. 2007;81:1479–1488. doi: 10.1016/j.lfs.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 14.Liu IM. Tzeng TF. Liou SS. Lan TW. Improvement of insulin sensitivity in obese Zucker rats by myricetin extracted from Abelmoschus moschatus. Planta Med. 2007;73:1054–1060. doi: 10.1055/s-2007-981577. [DOI] [PubMed] [Google Scholar]
- 15.Ahrens MJ. Thompson DL. Composition, method for treating diabetes, metabolic disorders. Sep 25, 2008. U.S. Patent Application 2008/0234364 A1.
- 16.Mazed MA. Linda Y. Mazed S. Nutritional supplement for the prevention of cardiovascular disease, Alzheimer's disease, diabetes, regulation, reduction of blood sugar, insulin resistance. Oct 8, 2009. U.S. Patent Application 2009/0252796 A1.
- 17.Blonska M. Czuba ZP. Krol W. Effect of flavone derivatives on interleukin-1β (IL-1β) mRNA expression and IL-1β protein synthesis in stimulated RAW 264.7 macrophages. Scand J Immunol. 2003;57:162–166. doi: 10.1046/j.1365-3083.2003.01213.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee YS. Choi EM. Myricetin inhibits IL-1beta-induced inflammatory mediators in SW982 human synovial sarcoma cells. Int Immunopharmacol. 2010;10:812–814. doi: 10.1016/j.intimp.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Kuo PL. Myricetin inhibits the induction of anti-Fas IgM-, tumor necrosis factor-α- and interleukin-1β-mediated apoptosis by Fas pathway inhibition in human osteoblastic cell line MG-63. Life Sci. 2005;77:2964–2976. doi: 10.1016/j.lfs.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z. Ding Y. Dai X. Wang J. Li Y. Epigallocatechin-3-gallate protects pro-inflammatory cytokine induced injuries in insulin-producing cells through the mitochondrial pathway. Eur J Pharmacol. 2011;670:311–316. doi: 10.1016/j.ejphar.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 21.Aherne SA. O'Brien NM. Protection by the flavonoids myricetin, quercetin, and rutin against hydrogen peroxide-induced DNA damage in Caco-2 and HepG2 cells. Nutr Cancer. 1999;34:160–166. doi: 10.1207/S15327914NC3402_6. [DOI] [PubMed] [Google Scholar]
- 22.Wang LW. Liu FZ. Lu JK. Liu YC. Yang BF. Effect of flavonols on caspase-3, Bcl-2 and Bax expression in cardiomyocytes apoptosis. Chin Pharm J. 2007;42:749–753. [Google Scholar]
- 23.Zwolak P. Borja-Cacho D. Phillips PA, et al. Myricetin induces apoptosis via caspase activation and inhibition of PI-3 kinase/Akt and Erk pathways in human pancreatic cells. Pancreas. 2007;35:439. [Google Scholar]
- 24.Phillips PA. Sangwan V. Borja-Cacho D. Dudeja V. Vickers SM. Saluja AK. Myricetin induces pancreatic cancer cell death via the induction of apoptosis and inhibition of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer Lett. 2011;308:181–188. doi: 10.1016/j.canlet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heimberg H. Heremans Y. Jobin C, et al. Inhibition of cytokine-induced NF-κB activation by adenovirus-mediated expression of a NF-κB super-repressor prevents β-cell apoptosis. Diabetes. 2001;50:2219–2224. doi: 10.2337/diabetes.50.10.2219. [DOI] [PubMed] [Google Scholar]
- 26.Eldor R. Yeffet A. Baum K, et al. Conditional and specific NF-κB blockade protects pancreatic β cells from diabetogenic agents. Proc Natl Acad Sci USA. 2006;103:5072–5077. doi: 10.1073/pnas.0508166103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kharroubi I. Ladriere L. Cardozo AK. Dogusan Z. Cnop M. Eizirik DL. Free fatty acids and cytokines induce pancreatic β-cell apoptosis by different mechanisms: role of nuclear factor-κB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 28.Darville MI. Eizirik DL. Regulation by cytokines of the inducible nitric oxide synthase promoter in insulin-producing cells. Diabetologia. 1998;41:1101–1108. doi: 10.1007/s001250051036. [DOI] [PubMed] [Google Scholar]
- 29.Rostoka E. Baumane L. Isajevs S, et al. Effects of kaempferol and myricetin on inducible nitric oxide synthase expression and nitric oxide production in rats. Basic Clin Pharmacol Toxicol. 2010;106:461–466. doi: 10.1111/j.1742-7843.2009.00526.x. [DOI] [PubMed] [Google Scholar]
- 30.Kanitkar M. Gokhale K. Galande S. Bhonde RR. Novel role of curcumin in the prevention of cytokine-induced islet death in vitro and diabetogenesis in vivo. Br J Pharmacol. 2008;155:702–713. doi: 10.1038/bjp.2008.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen F. Zhou XH. Lin Y, et al. Resveratrol prevents interleukin-1β-induced dysfunction of pancreatic β-cells. J Biomed Res. 2010;24:381–388. doi: 10.1016/S1674-8301(10)60051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song MY. Jeong GS. Kwon KB, et al. Sulfuretin protects against cytokine-induced β-cell damage and prevents streptozotocin-induced diabetes. Exp Mol Med. 2010;42:628–638. doi: 10.3858/emm.2010.42.9.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grunnet LG. Aikin R. Tonnesen MF, et al. Proinflammatory cytokines activate the intrinsic apoptotic pathway in β-cells. Diabetes. 2009;58:1807–1815. doi: 10.2337/db08-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim WH. Lee JW. Gao B. Jung MH. Synergistic activation of JNK/SAPK induced by TNF-alpha and IFN-gamma: apoptosis of pancreatic beta-cells via the p53 and ROS pathway. Cell Signal. 2005;17:1516–1532. doi: 10.1016/j.cellsig.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Pandey KB. Mishra N. Rizvi SI. Myricetin may provide protection against oxidative stress in type 2 diabetic erythrocytes. Z Naturforsch C. 2009;64:626–630. doi: 10.1515/znc-2009-9-1004. [DOI] [PubMed] [Google Scholar]
- 36.Lee KH. Choi EM. Myricetin, a naturally occurring flavonoid, prevents 2-deoxy-d-ribose induced dysfunction and oxidative damage in osteoblastic MC3T3-E1 cells. Eur J Pharmacol. 2008;591:1–6. doi: 10.1016/j.ejphar.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Zhang K. Ma Z. Wang J. Xie A. Xie J. Myricetin attenuated MPP+-induced cytotoxicity by anti-oxidation and inhibition of MKK4 and JNK activation in MES23.5 cells. Neuropharmacology. 2011;61:329–335. doi: 10.1016/j.neuropharm.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 38.Pandey KB. Mishra N. Rizvi SI. Myricetin can mitigate altered redox status in type 2 diabetic erythrocytes. http://pubcouncil.kuniv.edu.kw. [May;2010 ]. http://pubcouncil.kuniv.edu.kw
- 39.Nirmala P. Ramanathan M. Effect of myricetin on 1,2 dimethylhydrazine induced rat colon carcinogenesis. J Exp Ther Oncol. 2011;9:101–108. [PubMed] [Google Scholar]