Abstract
The present study evaluates the antioxidant activity of two Aronia melanocarpa cultivars—Viking and Aron—and of Aronia prunifolia hybrid in relationship with their phytochemical composition regarding the contents of total phenolics, flavonoids, procyanidins, and monomeric anthocyanins. The antioxidant capacity of the mentioned extracts of chokeberries was evaluated through five complementary assays: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), H2O2 scavenging potential, oxygen radical absorbance capacity, ferric reducing antioxidant power, and cupric ion reducing antioxidant capacity. A. prunifolia hybrid was found to have the highest antioxidant activity and to be the richest in polyphenols, procyanidins, and anthocyanins compared with the A. melanocarpa cultivars. A good correlation was observed between antioxidant activity and total procyanidin and anthocyanin content. Cyanidin glycosides inhibited HeLa human cervical tumor cell proliferation and increased generation of reactive oxygen species after 48 h of treatment, suggesting that they could be responsible for the antiproliferative activity. These results may be significant for industry concerning food quality and disease prevention.
Key Words: antioxidant assay, antitumor, chokeberry, reactive oxygen species
Introduction
The Aronia genus (Rosaceae family, subfamily Malodieae) includes Aronia melanocarpa, Aronia arbutifolia, and the Aronia prunifolia hybrid of native North American shrubs, introduced in Europe in the 20th century.1 Aronia berries, known as chokeberries, have been widely studied for their potential as a natural product for food and medicinal use, because of their high amounts of phenolic constituents.2,3 The chokeberries contain procyanidins,3 flavan 3-ols and flavonol glycosides,4 phenolic acids,5 and anthocyanins.
The antioxidant activity of these compounds, evaluated until now through different assays classified according to the mechanism of the reaction involved, is due to the presence of ortho-3′,4′-dihydroxy moiety in the B ring and meta-5,7-dihydroxy arrangements in the A ring.6,7 These berries, because of their high content in anthocyanins, are being studied also for their ability to protect against oral,8 colon,9,10 breast,8 and prostate11,12 cancer.
The objectives of this study were (1) to determine the contents of total flavonoids, procyanidins, anthocyanins, and polyphenols of chokeberry extracts obtained from three cultivars and to characterize their anthocyanin fraction by high-performance liquid chromatography (HPLC) analysis, (2) to measure and compare the antioxidant activity using three different single electron transfer (SET)-based assays [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), ferric reducing antioxidant power (FRAP), and cupric ion reducing antioxidant capacity (CUPRAC)], one hydrogen atom transfer (HAT)-based method (oxygen radical absorbance capacity [ORAC]), and the H2O2 scavenging potential (HPS) assay, and (3) to evaluate the cytotoxic action and the pro-oxidative effect of the anthocyanin fraction on the HeLa tumor cervical cell line.
Materials and Methods
Reagents
The standard compounds and reagents were obtained from Sigma-Aldrich (Darmstadt, Germany), Polyphenols Laboratories AS (Sandnes, Norway), or local producers. The human cervical tumor HeLa cell line was obtained from The “Prof. Dr. Ion Chiricuţă” Oncology Institute, Cluj-Napoca, Romania.
Extraction and HPLC analysis
The fruits of the two species, A. prunifolia and A. melanocarpa (cultivars Viking [AmV] and Aron [AmA]), were collected in the middle of August at a plantation near Cluj-Napoca and preserved at −20°C, immediately after harvest. Anthocyanin and non-anthocyanin compounds were extracted by homogenization of chokeberries (5 g) in methanol containing HCl (0.3%) using an Ultraturax (model Miccra D-9 KT; Digitronic GmbH, Bergheim, Germany). Extracts were concentrated at 35°C under reduced pressure (Rotavapor® model R-124; Buchi, Flawil, Switzerland). The A. prunifolia anthocyanin fraction was obtained following the procedure described previously.13 The acidified methanol fraction of anthocyanins was dissolved in deionized water and quantified using HPLC analysis.
HPLC analysis was performed on a Shimadzu (Kyoto, Japan) system equipped with a binary pump delivery system (model LC-20 AT Prominence), a degasser (model DGU-20 A3 Prominence), model SPD-M20 A UV–VISdiode array detector), and a Luna Phenomenex (Torrance, CA, USA) C-18 column (film thickness, 5 μm; 25 cm×4.6 mm). The mobile phase consisted formic acid (4.5%) in double-distilled water (solvent A) and acetonitrile (solvent B). The gradient elution system was as follows: 10% B, 0–9 min; 12% B, 9–17 min; 25% B, 17–30 min; 90% B, 30–50 min; and 10% B, 50–55 min. The flow rate was 0.8 mL/min, and the analyses were performed at 35°C. The chromatogram was monitored at 520 nm. Identification and peak assignments of anthocyanins are based on their retention times and ultraviolet–visible spectra comparing with standards and published data. Anthocyanin quantification was performed using cyanidin 3-O-galactoside.
Measurement of total phenolics, flavonoid, procyanidins, and monomeric anthocyanins
The total phenolic content, expressed as gallic acid equivalents (GAE), was determined by a modified Folin–Ciocalteu method.14 The absorbance was read at 750 nm by a microplate reader (BioTek Instruments, Winooski, VT, USA).
Total monomeric anthocyanin content, expressed as cyanidin 3-galactoside equivalents, was determined and calculated using the differential pH method.15
Total proanthocyanidin content, expressed as epicatechin equivalents, was evaluated by a modified vanillin-HCl assay method described by Sun et al.16 The absorbance was measured at 500 nm using a spectrophotometer (V-630 series, JASCO, Tokyo, Japan), subtracting the anthocyanin absorbance of the extracts.
The existence of other flavonoids in chokeberry cultivars was evaluated by the aluminum chloride colorimetric method as described previously.17,18 The flavonoid content was expressed as quercetin equivalents.
Antioxidant activity of chokeberry extracts
The scavenging ability of chokeberry extracts against the radical anion ABTS+ was determined according to the procedure described by Arnao et al.,19 adapted to 96-well microplates. The FRAP assay was performed according to the method of Benzie and Strain.20 The CUPRAC of chokeberries was determined according to a method reported before by Apak et al.21 The antioxidant capacity was measured by the ORAC assay,22 performed as described previously.23 The HPS assay was carried out following the procedure of Ruch et al.24 The results of antioxidant assays were expressed as micromoles of Trolox per gram fresh weight (FW).
Cell culture and survival
The human tumor cervical HeLa cell line was maintained in Dulbecco's modified Eagle's medium containing 1 g/L glucose supplemented with 10% fetal bovine serum, 1 mM glutamine, 1% gentamicin, and 1% nonessential amino acids at 37°C in an atmosphere of 5% CO2 and 95% relative humidity. For the cell survival test HeLa cells were plated (5000 cells per well) in 96-well plates for 24 h. The culture medium was then replaced with complete medium containing 0–250 μg/mL anthocyanins. The number of viable cells was determined with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell proliferation reagent. Three phosphate-buffered saline washing steps were followed by 1 h of incubation with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution (0.5 mg/mL) in Dulbecco's modified Eagle's medium without phenol red. The formazan particles were solubilized with dimethyl sulfoxide. The absorbance was read at 550 nm and expressed relative at that 630 nm (for background) with the HT Synergy microplate plate reader. The results were expressed as survival percentage with respect to an untreated control.
Intracellular reactive species assay
The determination of intracellular reactive oxygen species (ROS) uses the fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate, which is cell membrane permeable and is hydrolyzed by cellular esterases to 2′,7′-dichlorofluorescein, which reacts with intracellular ROS to form fluorescent 2′,7′-dichlorofluorescein, which is measured by a plate reader. Cells cultured in 96-well black-bottom plates were incubated for 1 h with 20 μM 2′,7′-dichlorodihydrofluorescein diacetate in Hanks' balanced salt solution. Fluorescence was monitored at 37°C at excitation wavelengths of 485 nm and emission wavelengths of 528 nm.25
Statistical analysis
Statistical analyses were performed by an analysis of variance and Duncan's multiple range tests using Microsoft Excel (version 2003; Redmond, WA, USA). The data were expressed as mean±SD values. Differences at P≤.05 were considered to be significant. Correlations among data obtained were done using Pearson's correlation coefficient (r). Each determination was carried out in triplicate.
Results and Discussion
Total phenolic, flavonoid, procyanidin, and anthocyanin contents of chokeberries
The Folin–Ciocalteu method estimates the total content of all phenolics present in the chokeberries, including flavonoids, procyanidins, anthocyanins, and non-flavonoid phenolic compounds. The total phenolic values for chokeberry cultivars ranged from 1586.5 to 2059.5 mg of GAE/100 g FW (Table 1), which are in agreement with values reported previously: 2556 mg of GAE/100 g FW for wild chokeberry26 and 2010 mg of GAE/100 g FW3 and 1063.7 mg of GAE/100 g FW27 for A. melanocarpa. A. prunifolia had the highest total phenolic content of all chokeberry samples investigated.
Table 1.
Contents of Total Phenolics, Anthocyanins, Procyanidins, and Other Flavonoids of Chokeberry Cultivars
| Sample | Total phenols(mg GAE/100 g FW) | Total anthocyanins (mg cyanidin 3-galactoside/100 g FW) | Total procyanidins (mg epicatechin/100 g FW) | Other flavonoids (mg quercetin equivalents/100 g FW) |
|---|---|---|---|---|
| A. prunifolia | 2059.5±145.8a | 366.16±1.4a | 855.13±63.4a | 47.67±3.7c |
| A. melanocarpa cultivar | ||||
| Viking | 1713±110.2b | 277.13±25.1b | 627.57±55.3b | 64.04±5.4a |
| Aron | 1586.5±123.7c | 176.18±19.4c | 354.94±49.2c | 60.0±4.6b |
Data are means±SD.
Means with different letters in the same column have a statistically significant difference at P≤.05.
GAE, gallic acid; FW, fresh weight.
Using a rapid and easy screening method for total monomeric anthocyanin quantification of chokeberry, we estimated for the first time the anthocyanin content of the AmA, AmV, and A. prunifolia cultivars, ranging from 176 to 366 mg/100 g FW (Table 1). The anthocyanin content previously reported, determined with the differential pH method, for A. melanocarpa fruits was 428 mg/100 g FW,26 460.5 mg/100 g FW,28 and 434.1 mg/100 g FW.27
The presence of other flavonoids was evaluated using the aluminum chloride colorimetric method. In chokeberry cultivars the flavonoid content ranged from 47.67 mg/100 g FW (A. prunifolia) to 64.04 mg/100 g FW (AmV) (Table 1). Our results are in agreement with data obtained by HPLC/mass spectrometry analysis, which revealed for A. melanocarpa a flavonoid content of 71 mg/100 g FW, as quercetin glycosides.5
The remaining difference value between total phenols and anthocyanins relative to flavonoid content corresponds to polymeric procyanidins and to other insoluble phenols. Acoording to the vanillin-HCl assay A. prunifolia had a higher procyanidin content (855.1 mg/100 g) compared with the AmA and AmV cultivars. Literature data report that proanthocyanidins of chokeberries are represented only by polymeric procyanidins. The previously reported procyanidin content for A. melanocarpa berries was about 633 mg/100 g FW.3
To our knowledge, we report for the first time data regarding the A. prunifolia hybrid, which showed higher statistically significant polyphenols, flavonoid, procyanidin, and anthocyanin contents than either AmA or AmV. The small quantitative differences in content of polyphenolic compounds compared with literature data might be due to different physiological and developmental stage or environmental factors, such as light intensity and nutrient availability29 (and references therein).
Antioxidant activity
Because different antioxidant assays give very different results, various methods based on different mechanisms must be used in parallel to evaluate the antioxidant capacity of compounds or extracts. Taking into account the pros and cons of each assay and their reaction mechanism (HAT or SET), we chose five antioxidant methods to be used in this study. SET-based assays (ABTS, FRAP, and CUPRAC) measures the capacity of the antioxidants to reduce an oxidant. ORAC, an HAT-based assay, measures the capability of antioxidants to quench peroxyl radicals by hydrogen donation.30 The ABTS, FRAP, and ORAC assays are the most common methods for measuring in vitro antioxidant capacity. Besides these common methods, we used the HPS and CUPRAC assays, untested on chokeberry extracts, in order to provide comprehensive information regarding their antioxidant activity.
The ABTS assay uses K2S2O8 as an oxidant and measures the antioxidant's ability to scavenge the radical ABTS+∙ compared with Trolox, a vitamin E analog. The chokeberry radical scavenging activity (Table 2) ranged from 95 to 171 μmol of Trolox/g FW, with an elevated value for the AmV extract, but not statistically significant compared with that obtained for A. prunifolia. Our values for scavenging activity toward the ABTS+∙ radical are similar to that reported in a recent study: 37.44 mg of Trolox/g of sample.31 Considering that the water content of berries is around 85% of the fresh mass,32 our data are also in the same range of concentration with scavenging effect expressed as dry weight: 439.4 μM Trolox/100 g dry weight.33
Table 2.
Antioxidant Capacity of Selected Chokeberry Cultivars Measured by Different Complementary Assays
| Chokeberry cultivar | ABTS(μmol Trolox equivalents/g FW) | FRAP (μmol Fe2+/g FW) | CUPRAC (μmol Trolox equivalents/g FW) | ORAC (μmol Trolox equivalents/g FW) | HPS (μmol Trolox equivalents/g FW) | WA* | Rank |
|---|---|---|---|---|---|---|---|
| A. prunifolia | 167.6±6.2a | 300.2±10.6a | 232.3±14.2a | 42.3±2.0a | 65.0±2.6a | 1.16 | 1 |
| A. melanocarpa cultivar | |||||||
| Viking | 171.7±6.8a | 206.2±9.4b | 158.8±8.3c | 41.7±1.2a | 61.9±1.9a | 0.99 | 2 |
| Aron | 95.9±7.3b | 185±8.6b | 177.8±9.6b | 35.3±1.7b | 53.3±1.5b | 0.83 | 3 |
| Average capacity | 145.1 | 230.5 | 189.6 | 39.8 | 60.1 | ||
Data are means±SD.
Means with different letters in the same column have a statistically significant difference at P≤.05.
For weighted average (WA) calculation, see Results and Discussion.
ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid; CUPRAC, cupric ion reducing antioxidant capacity; FRAP, ferric reducing antioxidant potential; HPS, H2O2 scavenging potential; ORAC, oxygen radical absorbance capacity.
FRAP assay values are summarized in Table 2. A. prunifolia extract demonstrated the highest FRAP (300.2±10.6 μmol of Fe2+/g FW), compared with the other two cultivars, AmA and AmV. The reduction of Fe3+ to Fe2+ by polyphenolic-containing extracts, obtained from hybrids of rowanberry with Malus, Pyrus, Aronia, or Mespilus, showed values ranging from 61 to 105 μmol of Fe2+/g FW.34
Compared with FRAP, the CUPRAC assay is carried out at pH 7.0, close to physiological pH, and the reaction assay involves faster kinetics.35 The ability of chokeberry extracts to reduce cupric ion (Cu2+) is shown in Table 2. The A. prunifolia cultivar had the highest reducing potential, more than 230 μmol of Trolox equivalents/g FW, a value statistically different from those obtained for the AmA and AmV cultivars: respectively, 158.8 and 177.8 μmol of Trolox equivalents/g FW. CUPRAC values were recently reported to range between 47.1 and 127.9 μmol Trolox equivalents/g FW for some raspberry and blackberry cultivars.36 To our knowledge, there are no prior literature data about cupric reducing antioxidant capacity of chokeberry extracts.
The ORAC assay measures the scavenging capacity of antioxidants against peroxyl radical. The ORAC values for chokeberry extracts ranged from 35.3 to 42.3 μmol of Trolox/g FW (Table 2), being smaller than those reported previously: 138.2 μmol of Trolox/g FW26 and 161 μmol of Trolox equivalents/g of FW.3 These differences could be due to the use of a different fluorescent reagent. Wu et al.3 used β-phycoerythrin, instead of the fluorescein we used. The disadvantages of using β-phycoerythrin, such as the inconsistent reactivity toward peroxyl radicals, the interaction with polyphenols due to the nonspecific protein binding, and the instability after exposure to excitation light, were the reasons why its replacement by fluorescein was recommended.37
H2O2 is a strong oxidizing agent, produced in vivo as a by-product of oxygen metabolism. As a main source of hydroxyl radicals it can be toxic for many cell types.38 AmA, AmV, and A. prunifolia exhibited 53.3, 61.9, and 65.0 μmol/g FW H2O2 scavenging activity, respectively (Table 2). The A. prunifolia cultivar was the most effective scavenger toward H2O2, and the AmV cultivar exhibited the lowest inhibition of H2O2. A black rice anthocyanin-rich extract (100 μg/mL) showed higher H2O2 scavenging activity than butylated hydroxyanisole, butylated hydroxytoluene, and α-tocopherol.39 As far as we know no study has revealed the H2O2 scavenging ability of chokeberry extracts.
We established a sample ranking calculating the weighted average (in μmol/g) (Table 2) according to a recent study.40 The ranking order for the antioxidant assay obtained after the weighted average calculation was A. prunifolia > AmV > AmA.
The Pearson's correlation coefficient (r) between chokeberry polyphenolic content and antioxidant activity showed a weak correlation between antioxidant activity and total flavonoid content (r=−0.93 [FRAP], r=−0.23 [ABTS], r=−0.35 [ORAC] and r=−1.0 [CUPRAC], and r=−0.52 [HPS]), a slight correlation between antioxidant activity and total phenolics (r=0.99 [FRAP], r=0.67 [ABTS], r=0.76 [ORAC] and r=0.87 [CUPRAC], and r=0.86 [HPS]), and a high correlation between antioxidant activity versus total anthocyanin and procyanidin contents (for total anthocyanins, r=0.91 [FRAP], r=0.86 [ABTS], r=0.91 [ORAC] and r=0.69 [CUPRAC], and r=0.97 [HPS]; for procyanidins, r=0.91 [FRAP], r=0.87 [ABTS], r=0.92 [ORAC] and r=0.68 (CUPRAC), and r=0.98 [HPS]). The positive correlations between antioxidant assays and the polyphenolic content suggest that the antioxidant capacity of chokeberries would derive more from anthocyanins and procyanidins than from the other phenolic compounds contribution.
The antioxidant capacity evaluated with the SET methods ABTS, FRAP, and CUPRAC showed elevated values for correlation coefficient, whereas for the HAT assay22 lower values were obtained, so it is possible that the antioxidant mechanism of the chokeberry extracts could be based on SET, a property due to the ability of the aromatic ring of anthocyanins to support an unpaired electron.41 To the total antiradical activity of chokeberries anthocyanins have the highest contribution (53–56%), but proanthocyanidins and insoluble phenols contribute 35%, followed by phenolic acids and flavonols (9%).26,42 The antioxidant activity was found to be positively correlated with anthocyanin content also in blackberries, red raspberries, black raspberries, and strawberries.43,44
Inhibition of HeLa tumor cell survival
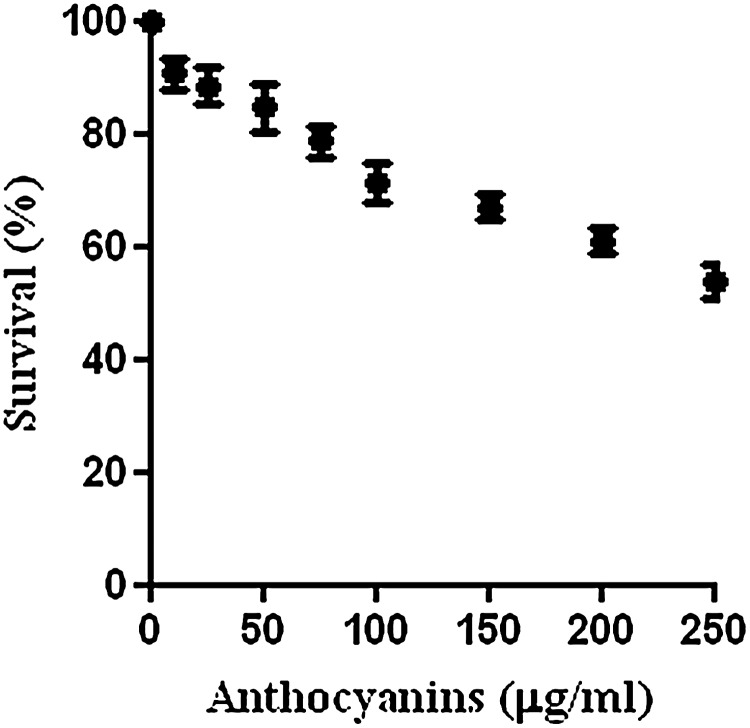
In order to prove the antitumor effect of chokeberry anthocyanins, we administered the purified anthocyanin fraction, containing only cyanidin glycosides (67.1% cyanidin 3-galactoside, 2.6% cyanidin 3-glucoside, 24.3% cyanidin 3-arabinoside, and 5.8% cyanidin 3-xyloside), to HeLa tumor cells. The influence of cyanidin glycosides on survival and metabolism was evaluated by measuring the mitochondrial succinate dehydrogenase activity in HeLa cells incubated for 48 h in the absence or in the presence of the extract. The anthocyanin fraction inhibited the survival of HeLa cells by 40% at a concentration of 200 μg/mL after 48 h (Fig. 1). Suppression of survival by 50% of the HT29 cell line by 25 μg of cyanidin 3-glucoside/mL after a 48-h exposure to anthocyanins from chokeberry, with no effect on the growth of normal non-transformed colon epithelial cells (NCM460), was observed.10 The effects of bilberry extract anthocyanins on cervical cancer HeLa cells showed that the inhibitory rate increased after 48 h of treatment.45
FIG. 1.
Cytotoxic effects of chokeberry anthocyanins at different concentrations (μg/mL) on the human cervical cancer HeLa cell line, expressed as the decrease of survival percentage. Cells were exposed to extract treatment for 48 h, and cell survival was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Data are mean±SD values (n=3). P<.05.
Intracellular ROS
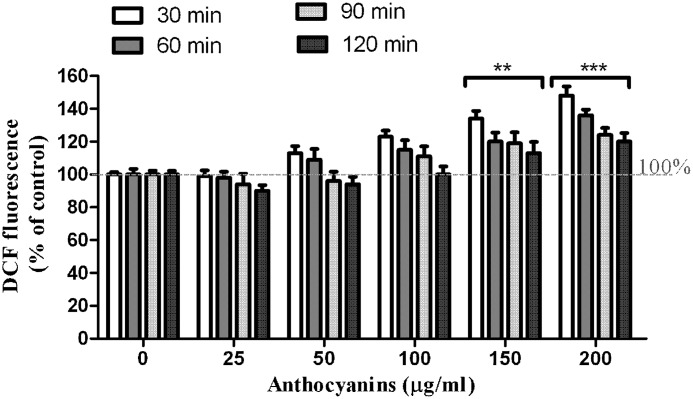
In HeLa tumor cell culture, in contrast to their antioxidant potential in vitro, we observed that cyanidin glycosides induced the accumulation of peroxides. To determine the ROS generation in cyanidin glycoside–treated HeLa cells we used 2′,7′-dichlorodihydrofluorescein, a dye specific for detection of H2O2. Significant increases in dose– and time–response effects of intracellular ROS in chokeberry anthocyanin–treated HeLa cells were observed at 30 min (Fig. 2). An initial reduction of ROS for doses of 25 μg/mL and 50 μg/mL anthocyanins after 4 h was noted. For the 100, 150, and 200 μg/mL concentrations tested, ROS levels were increased above the control value after 30 min, but the accumulated level of ROS was gradually reduced with time. It is possible that the increased oxidative stress, being toxic, was responsible for the cell death. Similar observations were reported in a recent study of cyanidin 3-rutinoside, an anthocyanin extracted and purified from black raspberry, administered to HL-60 leukemic cells, which determined the increase of ROS in human leukemia cells, but the ROS accumulation decreased with time.46 A dose-dependent increase of intracellular ROS level was observed when the cells were treated with 25–125 μM delphinidin 3-sambubioside obtained from the dried flower of Hibiscus sabdariffa.47 Also, oxidative stress-induced ROS production was observed in HepG2 cells treated with an anthocyanin-rich extract from Thai black sticky rice.48
FIG. 2.
Intracellular reactive oxygen species level in human cervical cancer HeLa cells treated with chokeberry anthocyanins, as determined by the fluorescence test with 2′,7′-dichlorofluorescein (DCF). The cells treated with chokeberry extract showed increased fluorescence compared with untreated cells. Data are mean±SD values (n=3), **P<0.01 (very significant) from corresponding control, ***P<0.001 (extremely significant) from corresponding control.
According to literature data the in vitro antioxidant potential of anthocyanins is related to their highly electron-deficient form of flavylium cation.44 Crucial for cell–target interaction seems to be the ortho-3′,4′-dihydroxy groups on the B ring.49–51 The meta-5,7-dihydroxy arrangements in the A ring and the 3-hydroxyl group (C ring) increase the antioxidant potential of anthocyanins.6,7 Glycosylation of anthocyanins also modulates their potency, influenced by the orientation, number, and distribution of hydroxyl groups in sugars.26
Our data seem therefore to support the hypothesis that cyanidin glycosides may be responsible for the ROS accumulation of tumor cells. A possible mechanism for pro-oxidant activity of anthocyanins in tumor cells could be related to their reduced capacity to scavenge ROS, causing its accumulation and finally triggering the mitochondrial apoptotic pathway,52 through both intrinsic (mitochondrial) and extrinsic pathways.53,54 This proposed mechanism can interfere with the glutathione antioxidant system.55 A precise mechanism of death induced by ROS generation after anthocyanin administration in tumor cells still remains unknown and must be further investigated.
Conclusions
The present study provides for the first time information regarding the phytochemical content of A. prunifolia berries, in relation to their antioxidant potentials, being richer in anthocyanins and procyanidins compared with A. melanocarpa cultivars. Two antioxidant methods—the cupric reducing antioxidant capacity and the H2O2 scavenging ability—were tested for the first time on chokeberry extracts. In vitro, cell culture studies showed that the cyanidin glycosides of A. prunifolia inhibited gradually tumor cell proliferation after 48 h of treatment, proving to be cytotoxic for HeLa cells, and the intracellular ROS generation was increased in a dose-dependent manner. The anthocyanins in chokeberries could have a health benefit, acting as antitumor agents, so these fruits can be recommended for daily consumption.
Acknowledgments
Work by D.R. was supported by the European Social Fund Postdoctoral Program grant POSDRU/89/1.5/S/60746. We are also grateful for the financial assistance provided by National Project number PN-II-RU-TE-109-2010.
Author Disclosure Statement
No competing financial interests exist. All the authors report no conflict of interest.
References
- 1.Kokotkiewicz A. Jaremicz Z. Luczkiewicz M. Aronia plants: a review of traditional use, biological activities, and perspectives for modern medicine. J Med Food. 2010;13:255–269. doi: 10.1089/jmf.2009.0062. [DOI] [PubMed] [Google Scholar]
- 2.Kähkönen MP. Hopia AI. Heinonen M. Berry phenolics and their antioxidant activity. J Agric Food Chem. 2001;49:4076–4082. doi: 10.1021/jf010152t. [DOI] [PubMed] [Google Scholar]
- 3.Wu X. Gu L. Prior RL. McKay S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J Agric Food Chem. 2004;52:7846–7856. doi: 10.1021/jf0486850. [DOI] [PubMed] [Google Scholar]
- 4.Määttä-Riihinen KR. Kamal-Eldin A. Mattila PH. González-Paramás AM. Törrönen R. Distribution and contents of phenolic compounds in eighteen Scandinavian berry species. J Agric Food Chem. 2004;52:4477–4486. doi: 10.1021/jf049595y. [DOI] [PubMed] [Google Scholar]
- 5.Slimestad R. Torskangerpoll K. Nateland HS. Johannessen T. Giske NH. Flavonoids from black chokeberries, Aronia melanocarpa. J Food Comp Anal. 2005;18:61–68. [Google Scholar]
- 6.Rice-Evans C. Miller N. Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- 7.Rice-Evans CA. Miller NJ. Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 8.Seeram NP. Adams LS. Zhang Y, et al. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J Agric Food Chem. 2006;54:9329–9339. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- 9.Dai J. Patel JD. Mumper RJ. Characterization of blackberry extract and its antiproliferative and anti-inflammatory properties. J Med Food. 2007;10:258–265. doi: 10.1089/jmf.2006.238. [DOI] [PubMed] [Google Scholar]
- 10.Zhao C. Giusti MM. Malik M. Moyer MP. Magnuson BA. Effects of commercial anthocyanin-rich on colonic cancer and nontumorigenic colonic cell growth. J Agric Food Chem. 2004;52:6122–6128. doi: 10.1021/jf049517a. [DOI] [PubMed] [Google Scholar]
- 11.Hafeez BB. Siddiqui IA. Asim M, et al. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: involvement of nuclear factor-kappaB signaling. Cancer Res. 2008;68:8564–8572. doi: 10.1158/0008-5472.CAN-08-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weaver J. Briscoe T. Hou M, et al. Strawberry polyphenols are equally cytotoxic to tumourigenic and normal human breast and prostate cell lines. Int J Oncol. 2009;34:777–786. doi: 10.3892/ijo_00000203. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Saona LE. Wrolstad RE. Unit F2.1 Anthocyanins. Extraction, isolation and purification of anthocyanins. In: King S, editor; Gates M, editor; Scalettar L, editor. Current Protocols in Food Analytical Chemistry. John Wiley & Sons; New York: 2001. pp. 1–11. [Google Scholar]
- 14.Singleton VL. Orthofer R. Lamuela-Raventós RM. Lester P. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 15.Giusti MM. Wrolstad RE. Unit F1.2: Characterization, measurement of anthocyanins by UV-visible spectroscopy. In: Wrolstad RE, editor. Handbook of Analytical Food Chemistry. John Wiley & Sons, Inc.; New York: 2001. pp. 19–31. [Google Scholar]
- 16.Sun B. Ricardo-da-Silva JM. Spranger I. Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem. 1998;46:4267–4274. [Google Scholar]
- 17.Kim DO. Jeong SW. Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–326. [Google Scholar]
- 18.Zhishen J. Mengcheng T. Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]
- 19.Arnao MB. Cano A. Alcolea JF. Acosta M. Estimation of free radical-quenching activity of leaf pigment extracts. Phytochem Anal. 2001;12:138–143. doi: 10.1002/pca.571. [DOI] [PubMed] [Google Scholar]
- 20.Benzie FF. Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–23. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 21.Apak R. Güçlü K. Ozyürek M. Karademir S. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem. 2004;52:7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- 22.Rocha-Guzmán NE. Gallegos-Infante JA. González-Laredo RF, et al. Antioxidant activity and genotoxic effect on HeLa cells of phenolic compounds from infusions of Quercus resinosa leaves. Food Chem. 2009;115:1320–1325. [Google Scholar]
- 23.Huang D. Ou B. Hampsch-Woodill M. Flanagan JA. Prior RL. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J Agric Food Chem. 2002;50:4437–4444. doi: 10.1021/jf0201529. [DOI] [PubMed] [Google Scholar]
- 24.Ruch RJ. Cheng SJ. Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 25.LeBel CP. Ischiropoulos H. Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 26.Zheng W. Wang SY. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J Agric Food Chem. 2003;51:502–509. doi: 10.1021/jf020728u. [DOI] [PubMed] [Google Scholar]
- 27.Jakobek L. Šeruga M. Medvidović-Kosanović M. Novak I. Antioxidant activity and polyphenols of Aronia in comparison to other berry species. Agric Conspec Sci. 2007;72:301–306. [Google Scholar]
- 28.Benvenuti S. Pellati F. Melegari M. Bertelli D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. J Food Sci. 2004;69:164–169. [Google Scholar]
- 29.Garzón GA. Narváez CE. Riedl KM. Schwartz SJ. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chem. 2010;122:980–986. [Google Scholar]
- 30.Prior RL. Wu X. Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 31.Gramza-Michałowska A. Człapka-Matyasik M. Evaluation of the antiradical potential of fruit and vegetable snacks. Acta Sci Pol Technol Aliment. 2011;10:63–72. [PubMed] [Google Scholar]
- 32.Ogawa K. Sakakibara H. Iwata R, et al. Anthocyanin composition and antioxidant activity of the crowberry (Empetrum nigrum) and other berries. J Agric Food Chem. 2008;56:4457–4462. doi: 10.1021/jf800406v. [DOI] [PubMed] [Google Scholar]
- 33.Oszmiański J. Wojdyło A. Aronia melanocarpa phenolics and their antioxidant activity. Eur Food Res Technol. 2005;221:809–813. [Google Scholar]
- 34.Hukkanen AT. Polonen SS. Karenlampi SO. Kokko HI. Antioxidant capacity and phenolic content of sweet rowanberries. J Agric Food Chem. 2006;54:112–119. doi: 10.1021/jf051697g. [DOI] [PubMed] [Google Scholar]
- 35.Apak R. Guclu K. Demirata B, et al. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 2007;12:1496–1547. doi: 10.3390/12071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sariburun E. Sahin S. Demir C. Turkben C. Uylaser V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J Food Sci. 2010;75:C328–C335. doi: 10.1111/j.1750-3841.2010.01571.x. [DOI] [PubMed] [Google Scholar]
- 37.Ou B. Hampsch-Woodill M. Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 38.Chai PC. Long LH. Halliwell B. Contribution of hydrogen peroxide to the cytotoxicity of green tea and red wines. Biochem Biophys Res Commun. 2003;304:650–654. doi: 10.1016/s0006-291x(03)00655-7. [DOI] [PubMed] [Google Scholar]
- 39.Park YS. Kim SJ. Chang HI. Isolation of anthocyanin from black rice (Heugjinjubyeo) and screening of its antioxidant activities. Korean J Microbiol Biotechnol. 2008;36:55–60. [Google Scholar]
- 40.Tabart J. Kevers C. Pincemail J. Defraigne J-O. Dommes J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2009;113:1226–1233. [Google Scholar]
- 41.van Acker SA. van den Berg DJ. Tromp MN, et al. Structural aspects of antioxidant activity of flavonoids. Free Radic Biol Med. 1996;20:331–342. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- 42.Jakobek L. Šeruga M. Krivak P. The influence of interactions among phenolic compounds on the antiradical activity of chokeberries (Aronia melanocarpa) Int J Food Sci Nutr. 2011;62:345–352. doi: 10.3109/09637486.2010.534438. [DOI] [PubMed] [Google Scholar]
- 43.Castañeda-Ovando A. Pacheco-Hernández MdL. Páez-Hernández ME. Rodríguez JA. Galán-Vidal CA. Chemical studies of anthocyanins: a review. Food Chem. 2009;113:859–871. [Google Scholar]
- 44.Dai J. Gupte A. Gates L. Mumper RJ. A comprehensive study of anthocyanin-containing extracts from selected blackberry cultivars: extraction methods, stability, anticancer properties and mechanisms. Food Chem Toxicol. 2009;47:837–847. doi: 10.1016/j.fct.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Jiang Y-X. Lei J-T. Lv S-J. Bilberry extract anthocyanins on effect of cervical cancer Hela cells. Matern Child Health J. 2010;14:46. (Abstract). [Google Scholar]
- 46.Feng R. Ni HM. Wang SY, et al. Cyanidin-3-rutinoside, a natural polyphenol antioxidant, selectively kills leukemic cells by induction of oxidative stress. J Biol Chem. 2007;282:13468–13476. doi: 10.1074/jbc.M610616200. [DOI] [PubMed] [Google Scholar]
- 47.Hou DX. Tong X. Terahara N. Luo D. Fujii M. Delphinidin 3-sambubioside, a Hibiscus anthocyanin, induces apoptosis in human leukemia cells through reactive oxygen species-mediated mitochondrial pathway. Arch Biochem Biophys. 2005;440:101–109. doi: 10.1016/j.abb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Sangkitikomol W. Tencomnao T. Rocejanasaroj A. Effects of Thai black sticky rice extract on oxidative stress and lipid metabolism gene expression in HepG2 cells. Genet Mol Res. 2010;9:2086–2095. doi: 10.4238/vol9-4gmr912. [DOI] [PubMed] [Google Scholar]
- 49.Bors W. Michel C. Chemistry of the antioxidant effect of polyphenols. Ann NY Acad Sci. 2002;957:57–69. doi: 10.1111/j.1749-6632.2002.tb02905.x. [DOI] [PubMed] [Google Scholar]
- 50.Marko D. Puppel N. Tjaden Z. Jakobs S. Pahlke G. The substitution pattern of anthocyanidins affects different cellular signaling cascades regulating cell proliferation. Mol Nutr Food Res. 2004;48:318–325. doi: 10.1002/mnfr.200400034. [DOI] [PubMed] [Google Scholar]
- 51.Shahidi F. Wanasundara PK. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- 52.Wang LS. Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269:281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang YC. Huang HP. Hsu JD. Yang SF. Wang CJ. Hibiscus anthocyanins rich extract-induced apoptotic cell death in human promyelocytic leukemia cells. Toxicol Appl Pharmacol. 2005;205:201–212. doi: 10.1016/j.taap.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 54.Reddivari L. Vanamala J. Chintharlapalli S. Safe SH. Miller JC., Jr Anthocyanin fraction from potato extracts is cytotoxic to prostate cancer cells through activation of caspase-dependent and caspase-independent pathways. Carcinogenesis. 2007;28:2227–2235. doi: 10.1093/carcin/bgm117. [DOI] [PubMed] [Google Scholar]
- 55.Cvorovic J. Tramer F. Granzotto M, et al. Oxidative stress-based cytotoxicity of delphinidin and cyanidin in colon cancer cells. Arch Biochem Biophys. 2010;501:151–157. doi: 10.1016/j.abb.2010.05.019. [DOI] [PubMed] [Google Scholar]