Abstract
Purpose
Autosomal dominant spastic paraplegia, type 4 (SPG4), a debilitating disorder of progressive spasticity and weakness of the lower limbs, results from heterozygous mutations in the SPAST gene. The full spectrum of SPAST mutations causing SPG4 and their mechanisms of formation remain to be determined.
Methods
We used multiplex ligation-dependent probe amplification, locus-specific array comparative genomic hybridization, and breakpoint DNA sequencing to identify and describe genomic rearrangements in three patients with a clinical presentation of hereditary spastic paraplegia.
Results
We describe three SPG4 patients with intragenic rearrangements in SPAST; all specifically delete the final exon, exon 17. Breakpoint sequence analyses provide evidence for Alu-specific microhomology-mediated deletion as the mechanism of exon loss; one complex rearrangement apparently occurred by multiple Alu-facilitated template switches.
Conclusion
We hypothesize that the high concentration of Alu family members in the introns and flanking sequence of SPAST may predispose to intragenic rearrangements. Thus, Alu-specific microhomology-mediated intragenic rearrangements in SPAST may be a common cause of SPG4. Furthermore, we propose that genomic deletions encompassing the final exon of SPAST may affect expression of SLC30A6, the most proximal downstream locus and a gene that has been implicated in the pathogenesis of Alzheimer disease, potentially explaining recent reports of dementia in selected SPG4 patients.
Keywords: SPG4, spastin, intragenic rearrangements, exon mutations, FoSTeS
INTRODUCTION
Hereditary spastic paraplegias (HSPs) are a group of nervous system disorders characterized by progressive spasticity and weakness of the lower extremities.1 HSP subtypes are described either as pure or uncomplicated, with spasticity as the sole feature, or as complicated, in which other neurologic findings, including dementia, deafness, ataxia, extrapyramidal disturbances, retinopathy, optic neuropathy, and icthyosis, can occur either individually or in selective combinations. The HSPs vary not only in clinical manifestations, but also in modes of inheritance (autosomal dominant (AD), autosomal recessive, and X-linked) and genetic causes; to date, over 40 spastic paraplegia gene (SPG) loci have been identified, defining an equal number of similarly-named HSP subtypes.1,2
Autosomal dominant spastic paraplegia-4 (SPG4; MIM 182601), characterized by relatively pure spastic paraplegia, is the most common type of AD-HSP and the most common form of HSP overall.3 SPG4 may present as either an inherited (AD) or sporadic condition that is caused by heterozygous mutations in the SPAST gene (MIM 604277; 2p22.3). SPAST point mutations, including missense, nonsense, and splice site mutations, were first identified in AD-HSP patients by Hazan et al.4 Since then, small insertions/deletions (indels) and gross deletions,5 a gross duplication,6 and a small complex rearrangement5 in SPAST have all been identified in AD-HSP patients, consistent with haploinsufficiency at this locus as the cause of SPG4.
SPAST codes for spastin, a member of the AAA (ATPases associated with diverse cellular activities) family of ATPases4 with roles in microtubule dynamics7,8 and membrane trafficking.9 Spastin appears to interact with atlastin-1 (encoded by SPG3A) and receptor expression enhancing protein 1 (encoded by REEP1); haploinsufficiency of these proteins leads to SPG3A and SPG31, respectively, two other types of AD-HSP.10 Though convincing genotype-phenotype correlations for SPAST mutations in SPG4 have been alternately reported11–13 and discounted,14,15 point mutations and indels tend to cluster in the sequences coding for the AAA domain, MIT (microtubule interacting and trafficking) domain, MTBD (microtubule-binding domain), and a yet-undefined region (residues 228–269) of spastin.16 Several sequence variants within SPAST (reviewed in 17) and one in heat shock 60kDa protein 1 (chaperonin) (HSPD1; the gene associated with SPG13)18 have been proposed to be modifiers of the SPG4 phenotype.
Intragenic CNVs in SPAST, most commonly identified by multiplex ligation-dependent probe amplification (MLPA), have been found to cause disease in a substantial proportion of mutation-negative (i.e. those lacking single nucleotide variants, or SNV, identifiable by gene sequencing) patients with HSP.12 Intragenic copy-number changes have been implicated in the pathogenesis of disparate disorders,19 most often reasoned to result from gene disruption. Mechanisms of genomic copy-number change (reviewed by Zhang, et al.20) include: 1) non-allelic homologous recombination (NAHR), characterized by recurrent rearrangements mediated by flanking low-copy repeats (LCRs). NAHR may also occur at highly homologous non-LCR interspersed repetitive sequences, for example LINEs or Alu elements (i.e. Alu-Alu recombination; 21,22); 2) non-homologous end joining (NHEJ), which generates non-recurrent rearrangements with variable breakpoint architecture; 3) fork stalling and template switching (FoSTeS) or microhomology-mediated break-induced replication (MMBIR), characterized by breakpoint microhomology and the possibility of complex rearrangements,23,24 and 4) transposition of mobile elements throughout the genome.20 FoSTeS and MMBIR, proposed, replication-based processes, have been implicated in the generation of CNV of a wide size range spanning from the genomic- (megabases) to the exonic-scale (~100bp to kb in size).25 Deletion CNVs have been recently described with breakpoints, the architecture of which suggests FoSTeS or MMBIR, that localize to Alu elements.26–30 Alu-specific microhomology-mediated deletion is the mechanism proposed to have generated these CNVs, wherein moderate sequence similarity between breakpoint Alus (in contrast to the nearly complete sequence identity of breakpoint Alus required for minimal efficient processing segments (MEPS) in homologous Alu-Alu recombination) is sufficient to provide a substrate for the microhomology-mediated processes of FoSTeS/MMBIR.
Which of the above mechanisms play(s) a role in generating intragenic CNV in SPAST is unknown, because deletion or duplication breakpoints have only been sequenced for two patients.6,31 We report three deletions of the final exon of SPAST, one of which is complex, all with breakpoints displaying microhomology and localized to Alu elements with moderate sequence similarity and in direct orientation, but displaying ≤ 89% sequence identity. These findings suggest Alu-specific microhomology-mediated generation of intragenic CNV in SPAST as a recurrent, and potentially common, cause of hereditary spastic paraplegia.
MATERIALS AND METHODS
Anonymized genomic DNA samples from three patients (A37, A38, A39), each with a suspected clinical diagnosis of hereditary spastic paraplegia, were obtained from Athena Diagnostics. Each sample was analyzed by MLPA using the MRC-Holland (Amsterdam, the Netherlands) SALSA MLPA kit P165. This kit contains one probe pair complementary to each of exons 1–16 of SPAST, and two probe pairs complementary to exon 17.
To determine the size, genomic context, and extent of each deletion, samples were analyzed using an 8 × 15 K Agilent (Santa Clara, CA, USA) oligonucleotide microarray, custom-designed using eArray (https://earray.chem.agilent.com/earray/). This array interrogated the entire SPAST gene as well as ~950,000 kilobases (kb) upstream and downstream with 11,940 probes, for an average probe spacing of ~200 base pairs (bp). The remaining probes were control probes, spread throughout the genome. Array comparative genomic hybridization (aCGH) and subsequent bioinformatic analyses were performed as in Gonzaga-Jauregui, et al.32
For each of the three patients, estimated deletion breakpoints derived from aCGH were used to design primers to PCR-amplify the breakpoint regions: (Primer A37F: 5’-CATAAATACCAAACAGAAGAAAATTACA-3’; A37R: 5’-GGGAAAATTTCACGCATCAT-3’; A39F: 5’-TCCAAAGGCAATTTAAAAGATCA-3’; A39R: 5’-GGGAAAATTTCACGCATCAT-3’; A38F: 5’-TGTCTGCCAGTGAGGTATAGTATTTT-3’; A38R: 5’-CATCCCAAATGCTTAAGACCA-3’). A 23µl PCR mix consisting of 170 µM each dNTP, 0.43 µM each primer, 75 ng DNA, 1.5 U HotStarTaq DNA polymerase (Qiagen, Valencia, CA, USA), and 1× PCR buffer was subjected to the following “touchdown” PCR reaction in a thermal cycler: 95°C × 15 minutes (min); 10 cycles of 94°C × 30 seconds (s), 61°C × 15 s (this annealing temperature dropped by 0.5°C in each cycle following the first), and 72°C × 90 s; 32 cycles of 94°C × 30 s, 56°C × 15 s, 72°C × 90 s; and 72°C × 10 min.
Sex-matched control DNA (NA10851 and NA15510) was derived from cell lines obtained from Coriell Cell Repositories (Camden, NJ, USA). ExoSAP-IT (USB, Cleveland, OH, USA) was used to clean PCR products, according to the manufacturer’s instructions. DNA sequencing was performed by the Sanger di-deoxynucleotide method (Child Health Research Center Molecular Core Laboratory, Baylor College of Medicine, Houston, TX, USA; and SeqWright, Houston, TX, USA). The March 2006 assembly of the reference genome (NCBI36/hg18) was used to analyze both sequencing and array data. SPAST exon numbering is based on RefSeq transcript NM_014946.3.
RESULTS
MLPA revealed a heterozygous genomic deletion including exon 17, the final exon of the SPAST gene, in each of the three patients (Fig. 1 and Figs. S1–S3, Supplemental Digital Content, which show a pre-normalized plot of MLPA data). This allowed confirmation of the clinically suspected spastic paraplegia and enabled a molecular diagnosis of SPG4. To determine the boundaries of each deletion, patient DNA was analyzed on a genomic microarray, custom-designed to interrogate the SPAST locus at high resolution. Array CGH confirmed each deletion and provided an approximation of genomic breakpoints (Fig. 2a–b). In each case, PCR further confirmed the genomic deletion (Fig. 2b–c) and provided substrate for subsequent breakpoint sequencing.
Figure 1. MLPA reveals deletions in the final exon of SPAST.
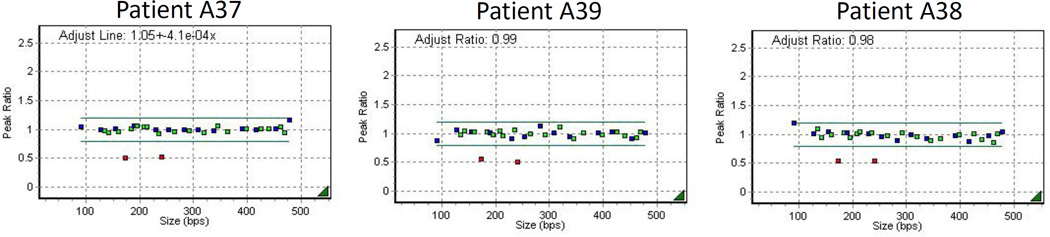
Dot plots depicting normalized MLPA values demonstrate that the genomic regions corresponding to two probe pairs – both localized to exon 17 of SPAST – are present in only a single copy in the three patients. This indicates a heterozygous genomic loss in exon 17 (the final exon) of SPAST. Green dots indicate probe pairs directed against exons of SPAST, blue dots indicate control probe pairs, and red dots indicate probe pairs in regions of copy number change.
Figure 2. Targeted aCGH and PCR assays confirm and define the range of deletions of SPAST exon 17.
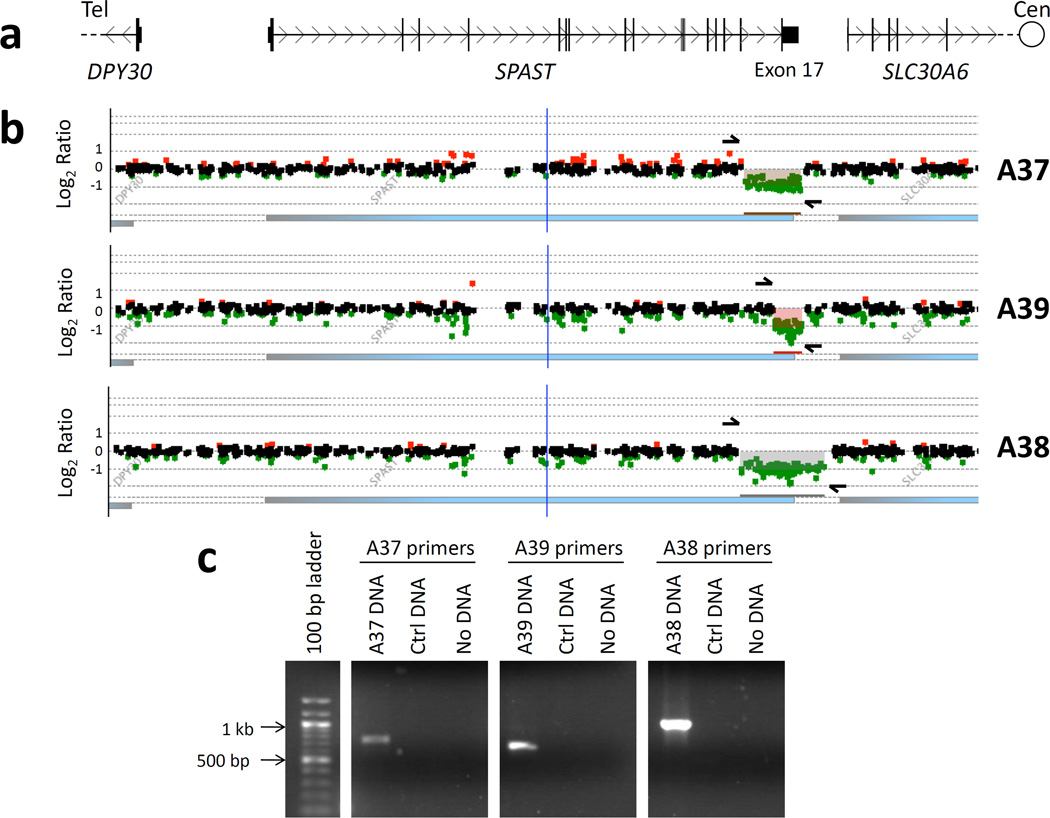
a. Local genomic map of the SPAST locus. b. Local view of aCGH results, aligned with the genomic map in [a]. Regions of copy-number loss are indicated by shading and underlined in similar color. In each patient, the deletion spans exon 17, the final exon of SPAST. Arrows indicate the locations of PCR primers used to further define each deletion. As primer pairs span the predicted breakpoints, a deletion is expected to substantially reduce the amplicon size. This enables a PCR product to be amplified from the deleted allele in the three patients, but not from control DNA. c. Results of PCR, followed by agarose gel electrophoresis, which for each patient further confirms a deletion. Cen, centromeric; tel, telomeric.
DNA sequencing revealed simple (i.e. non-complex) deletions of 10,767 bp and 5,501 bp in patients A37 and A39, respectively, which solely encompassed exon 17 of SPAST (Fig. 3). Interestingly, both sets of deletion breakpoints exhibited microhomology of 8 and 26 bp, respectively (Figs. 3c and 3e), and localized to Alu elements in direct orientation (Fig. 3a–b). Though the centromeric breakpoints of these two cases localize to the same AluY, their exact rearrangement join-point locations differ (Figs. 3c–f).
Figure 3. Deletion breakpoints in patients A37 and A39 localize to Alu elements and display microhomology.
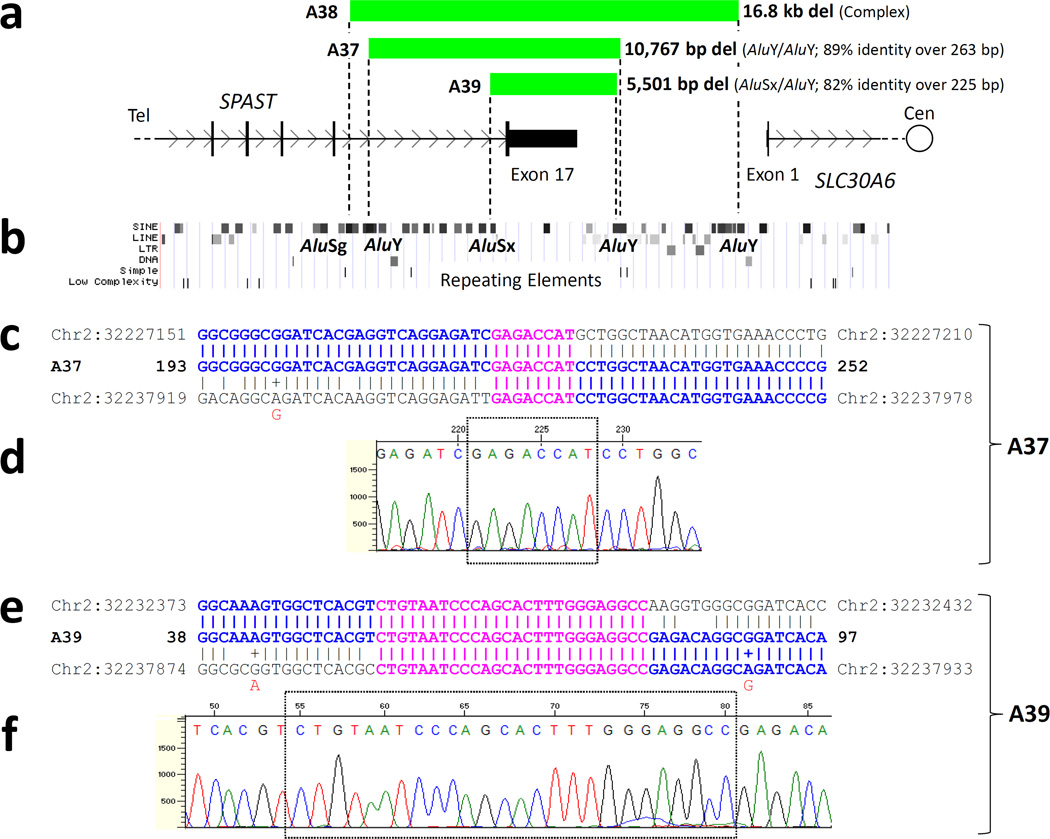
a. Green bars represent the regions deleted in patients A37, A38, and A39, superimposed on a genomic map of the 3’ end of SPAST. In each case, only exon 17 is deleted. b. A map of repeating elements, obtained from the UCSC genome browser (http://genome.ucsc.edu/) is aligned with [a]. All deletion breakpoints (indicated by dotted lines in [a]) fall within Alu elements; each pair of Alus flanking a deletion are in direct orientation with each other. (c–f). Sequencing of the breakpoint regions in patients A37 and A39 reveals microhomology. c and e. The DNA sequences of the deletion breakpoint regions of mutant patient chromosomes (“A37” and “A39”) are displayed between the telomeric (top) and centromeric (bottom) reference sequences. Perfect sequence identity with one of the reference sequences is indicated with bold blue text. Microhomology (sequence identity among all three sequences) is indicated with bold pink text. A “+” and red lettering indicate a known SNP, as listed by the UCSC genome browser, which matches the sequenced patient DNA. d and f. DNA sequencing traces. The regions of microhomology are boxed. Though the centromeric breakpoints of patients A37 and A39 fall within the same AluY element, their exact locations differ. Del, deletion; cen, centromeric; tel, telomeric.
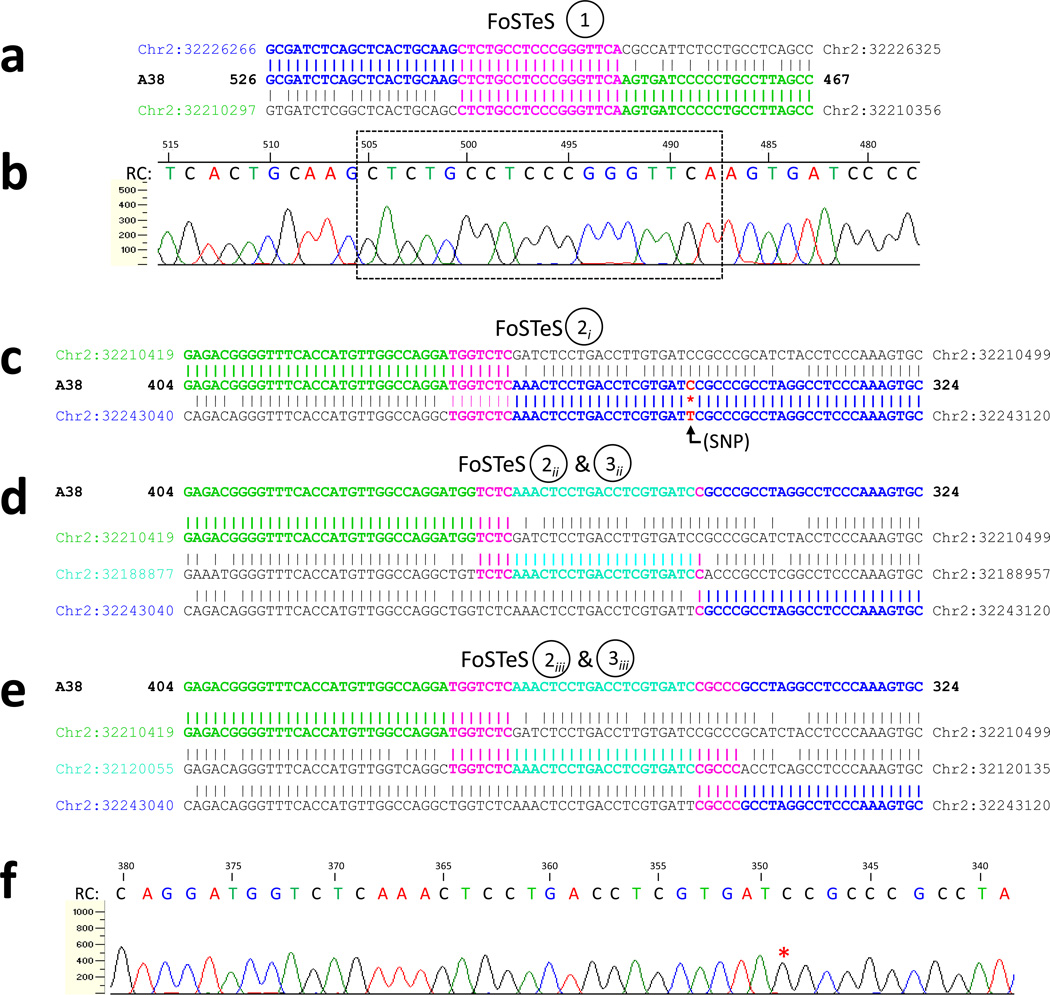
Patient A38 was found by DNA sequencing to have a complex rearrangement involving a 16.8 kb genomic loss encompassing exon 17 of SPAST (Fig. 3a). The boundaries of the deletion map to Alu elements of differing families (AluY and AluSg) in direct orientation. Interestingly, in place of the deleted genomic region, >100 bp of inserted sequence was found (Figs. 4a and 5). This insertion consists of either one or two discrete regions that map to intronic/intergenic Alu elements within or near SPAST. This uncertainty exists owing to several possible inferred series of events that could result in the recombinant, mutant sequence product derived from patient DNA (Figs. 4b–f and 5).
Figure 4. A complex rearrangement in patient A38 deletes the final exon of SPAST and probably occurs by multiple Alu-specific FoSTeS events.
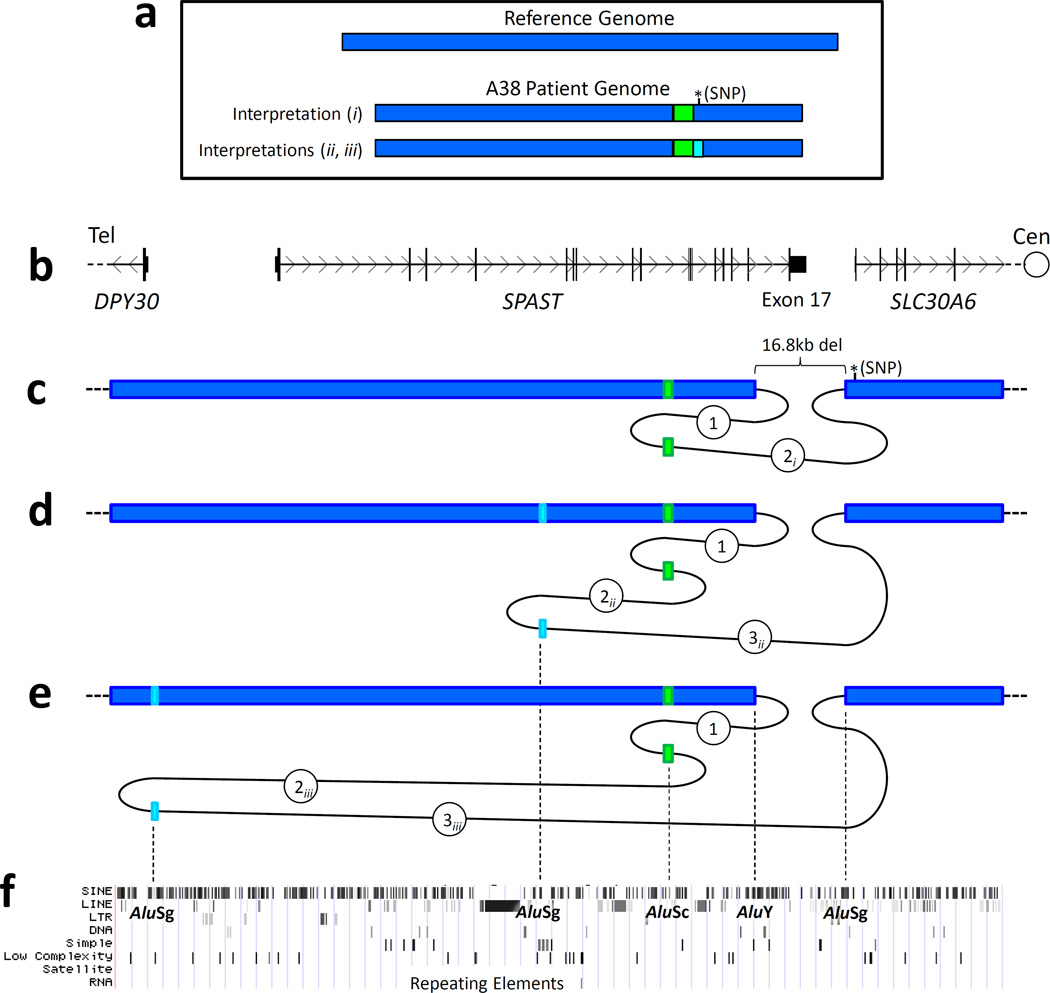
a. With respect to the reference genome, patient A38 has a heterozygous loss of 16.8 kb and in its place either one or two shorter, inserted sequences. This uncertainty exists owing to several possible inferred series of events that could result in the recombinant, mutant sequence product derived from patient DNA. b. Local genomic map of the SPAST locus, aligned with [b–f]. (c–e). Schematic diagrams of three potential series of FoSTeS events: c. FoSTeS × 2, deleting 16.8 kb (including exon 17) and inserting (creating a non-tandem duplication) between 113 and 136 bp. This mechanistic explanation requires a novel SNP to exist centromeric to breakpoint 2 (see Fig. 5c and text); d. One possible FoSTeS × 3 event, deleting 16.8 kb, inserting/duplicating 113–136 bp, and inserting/duplicating 21–24 bp; and e. Another (of multiple) possible FoSTeS × 3 events, deleting 16.8 kb, inserting/duplicating 113–136 bp, and inserting/duplicating 21–31 bp. f. A map of repeating elements, obtained from the UCSC genome browser (http://genome.ucsc.edu/). All five potential deletion breakpoints (indicated by dotted lines) fall within Alu elements in direct orientation. Del, deletion, cen, centromeric; tel, telomeric.
Figure 5. Breakpoint sequencing in patient A38 reveals a complex rearrangement and suggests multiple Alu-specific FoSTeS events.
Sequencing of the breakpoint region reveals that this is a complex rearrangement with multiple instances of microhomology. This suggests one of several multiple-FoSTeS schemes as having generated this rearrangement. The DNA sequence of the breakpoint regions of the mutant patient chromosome (“A38”) is displayed either in between or on top of relevant reference sequences. Perfect sequence identity with one of the reference sequences is indicated by bold colored text, corresponding with the coloring in Fig. 4c–e. Microhomology (homology among three sequences) is indicated by bold pink text. As sequencing was performed in reverse orientation to the reference sequence, sequence traces have been flipped horizontally and a reverse complement (RC) sequence accompanies them. Circled numbers and subscripts signify a correspondence to similarly-labeled FoSTeS events depicted in Fig. 4c–e. As displayed in Fig. 4, all breakpoints localize to directly oriented Alu elements. a. This breakpoint is shared by all possible rearrangement schemes. b. DNA sequencing trace, corresponding to [a], in which the 18 bp region of microhomology is boxed. (c–e). Three of several possibilities for the remaining FoSTeS event(s): c. The second of two breakpoints in this scheme, displaying 7 bp of microhomology and necessitating that the base at Chr2:32,243,095 (in red text with an accompanying asterisk) be a novel SNP on this allele in the patient (see text); d. The second and third breakpoints of one possible FoSTeS × 3 event, featuring four and one bp of microhomology, respectively; e. The second and third breakpoints of another possible FoSTeS × 3 event, featuring seven and five bp of microhomology, respectively. The light blue regions in this figure and Fig. 4 were found by searching for the sequence CAAACTCCTGACCTCGTGATCC, the minimum sequence needed to complete this rearrangement with no requirement for a novel SNP. Though multiple copies of this short sequence exist on chromosome 2, those displayed in [d–e] and in Fig. 4c–d are the copies located most near to the final exon of SPAST (all others are >1 Mb away). f. DNA sequencing trace, corresponding to (c–e) in which the base at Chr2:32,342,095 is marked with a red asterisk.
For each proposed mechanism, breakpoints are localized to Alu elements in direct orientation (Fig. 4c–f) and exhibit microhomology (Fig. 5), and all combinations of potential sequential events in the rearrangement process result in the experimentally identified breakpoint displayed in Figure 5a–b (also illustrated in Fig. 4c–e). Moreover, all inferred avenues for deriving the recombined chromosome involve a replication-based mechanism of rearrangement, for example FoSTeS or MMBIR (see Discussion).
The proposed alternative mechanisms are each based upon the presumed reference haploid human genome sequence and differ as follows: 1) Figures 4c and 5c illustrate the possibility that a single non-tandem duplication of between 113 and 136 base pairs (bp) accounts for the inserted genomic material in the patient (a range of sizes must be proposed, as microhomology limits the resolution with which a breakpoint position may be known). This proposed sequence of events necessitates that a single-nucleotide polymorphism (SNP) exists at Chr2:32,243,095 (C>T). Though this SNP is not present either in dbSNP (build 130) or as one of the additional personal genome variants listed in the UCSC genome browser (http://genome.ucsc.edu), this nucleotide position occurs in non-coding sequence and lacks conservation even among primates, so a benign SNP at this location is quite plausible in the personal genome of this individual patient with SPG4. As this was an anonymized sample, parental DNA is not available for SNP genotyping; 2) It is also possible that an additional (third) FoSTeS/MMBIR event occurred, producing a second, smaller duplication (Figs. 4d–e and 5d–e). This latter inference may more parsimoniously explain the observed data, as it eliminates the need for the aforementioned novel SNP to exist at Chr2:32,243,095. The locations depicted in figures 4d–e and 5d–e for this second, smaller duplicated sequence are not the only possibilities, as a sufficient length of identical sequence exists in multiple other copies throughout the genome; they are pictured on account of being the only such sequence within one megabase (Mb) of the SPAST locus.
Given the overwhelming proclivity of our patients’ deletion breakpoints to exist in Alu elements, we computationally examined the architecture of the SPAST locus to assess whether a local enhancement of Alu concentration may exist. We tabulated each Alu family member from 10 kb upstream of SPAST to 8,225 bp downstream, where the solute carrier family 30 (zinc transporter), member 6 gene (SLC30A6), the most proximal downstream gene, begins. One hundred sixty-three Alu family members exist in this 112,252 bp region, totaling 40,639 bp, or 36%, of the genomic sequence. This significantly exceeds the ~11% genome-wide average (p < 0.0001; Pearson’s chi-square test; see calculation, Supplemental Digital Content)33. Furthermore, the sequences flanking exon 17, the exon deleted in our three patients, are particularly enriched for Alu family members (intron 16: 4,045/7,115 bp (57%); 3’ flanking intergenic region: 2,951/8,225 bp (36%)).
DISCUSSION
We describe three SPG4 patients with intragenic rearrangements in SPAST, each deleting its final exon (exon 17). This finding confirms the importance of exonic CNV in SPAST as a cause of SPG4. Table 1 lists known intragenic rearrangements of >30 bp in SPAST, and demonstrates that pathogenic exonic deletions can exist throughout the gene. The use of a custom genomic microarray and DNA sequencing allowed us to determine the exact breakpoint sequences of intragenic CNVs in SPAST. These techniques also have enabled us to identify a complex exonic rearrangement in this gene in one patient (A38) and have provided insight into the mechanism of origin of this and the other deletion CNVs we report.
Table 1.
Published intragenic CNVs in SPAST of size >30 bp.
| No. | Exon(s) Involved |
Clinical Diagnosis (No. different families if >1) |
Method of Detection |
Coordinatesa | Reference |
|---|---|---|---|---|---|
| Deletions | |||||
| 1 | 1 | AD-HSP | MLPA | - | 15 |
| 2 | 1 | AD-HSPb (3 families) |
MLPA | - | 12 |
| 3 | 1 | HSP | MLPA | - | 14 |
| 4 | 1 | AD-HSP (1 or 2 families) |
MLPA | - | 17 |
| 5 | 1 (partial) |
AD-HSP | Southern blot, seq |
Chr2:32,142,293 −32,144,598 |
31 |
| 6 | 1–3 | AD-HSP | MLPA | - | 15 |
| 7 | 1–3 | HSP | MLPA | - | 16 |
| 8 | 1–4 | AD-HSPb | MLPA | - | 12 |
| 9 | 1–7 | AD-HSP | MLPA | - | 15 |
| 10 | 1–17 | AD-HSPb (4 families) |
MLPA | - | 12 |
| 11 | 2–5 | AD-HSP | MLPA | - | 15 |
| 12 | 2–9 | Familial HSP | MLPA | - | 16 |
| 13 | 2–16 | AD-HSP | MLPA | - | 49 |
| 14 | 2–16 | Familial HSP | MLPA | - | 16 |
| 15 | 2–17 | HSP | MLPA | - | 14 |
| 16 | 3–17 | Spastic paraplegia | MLPA | - | 50 |
| 17 | 4 | HSP | MLPA | - | 50 |
| 18 | 4–17 | AD-HSP | MLPA | - | 49 |
| 19 | 4–17 | AD-HSPb | MLPA | - | 12 |
| 20 | 5 | HSP | MLPA | - | 14 |
| 21 | 5–6 | AD-HSPb | MLPA | - | 12 |
| 22 | 5–7 | AD-HSP | MLPA | - | 15 |
| 23 | 5–7 | AD-HSPb | MLPA | - | 12 |
| 24 | 5–15 | AD-HSP (2 families) |
MLPA | - | 15 |
| 25 | 6 | AD-HSPb | MLPA | - | 12 |
| 26 | 6–17 | AD-HSP | MLPA | - | 15 |
| 27 | 8 | Familial HSP | MLPA | - | 16 |
| 28 | 8–9 | AD-HSP | MLPA | - | 17 |
| 29 | 8–12 | AD-HSPb | MLPA | - | 12 |
| 30 | 8–17 | AD-HSP | MLPA | - | 49 |
| 31 | 8–17 | AD-HSPb (2 families) |
MLPA | - | 12 |
| 32 | 9 | AD-HSPb | MLPA | - | 12 |
| 33 | 9–12 | AD-HSPb | MLPA | - | 12 |
| 34 | 9–17 | Familial HSP | MLPA | - | 16 |
| 35 | 10 | AD-HSP | MLPA | - | 15 |
| 36 | 10–12 | AD-HSP | MLPA | - | 15 |
| 37 | 10–12 | AD-HSP | MLPA | - | 49 |
| 38 | 10–12 | HSP | MLPA | - | 14 |
| 39 | 10–12 | AD-HSP | MLPA | - | 51 |
| 40 | 10–16 | AD-HSPb | MLPA | - | 12 |
| 41 | 10–16 | Familial HSP | MLPA | - | 52 |
| 42 | 13 | AD-HSPb (2 families) |
MLPA | - | 12 |
| 43 | 13–16 | HSP | PCR and electrophoresis of cDNA |
- | 5 |
| 44 | 14–17 | Spastic paraplegia | MLPA | - | 50 |
| 45 | 16 | AD-HSP | MLPA | - | 49 |
| 46 | 16 | AD-HSPb (2 families) |
MLPA | - | 12 |
| 47 | 16–17 | AD-HSP | MLPA | - | 15 |
| 48 | 16–17 | AD-HSPb | MLPA | - | 12 |
| 49 | 17 | AD-HSP | MLPA | - | 15 |
| 50 | 17 | AD-HSP (2 families) |
MLPA | - | 49 |
| 51 | 17 | AD-HSPb | MLPA | - | 12 |
| 52 | 17 | HSP | MLPA | - | 16 |
| 53 | 17 | Sporadic spastic paraplegia |
MLPA | - | 51 |
| 54 | 17 | HSPc |
MLPA, LS- aCGH, seq |
See text and figures |
Present Investigation (Patient A37) |
| 55 | 17 | HSPc |
MLPA, LS- aCGH, seq |
See text and figures |
Present Investigation (Patient A39) |
| Duplication | |||||
| 56 | 10–12 | AD-HSP | MLPA, seq | Chr2:32,212,239 −32,216,284 |
6 |
| Complex | |||||
| 57 | 17 | HSPc |
MLPA, LS- aCGH, seq |
See text and figures |
Present Investigation (Patient A38) |
March 2006 assembly of the reference genome (NCBI36/hg18).
One of 121 families studied by Depienne et al.12 displayed disease in two siblings, rather than strict AD inheritance. It is unclear whether this family is represented by any exonic CNV reported in the table.
Inheritance is not known for these anonymized samples.
AD, autosomal dominant; HSP, hereditary spastic paraplegia; MLPA, multiplex ligation-dependent probe amplification; LS-aCGH, locus-specific aCGH; seq, DNA sequencing.
In all three patients, it is the final exon of SPAST that is deleted, raising the possibility that expression of the most proximal downstream gene, SLC30A6, may be altered. The downstream (centromeric) breakpoint of each patient’s CNV lies within 6.5 to 1.3 kb of the start of SLC30A6, potentially a proximity that could either disrupt promoter elements or convey a position effect.34,35 A final-exon deletion in SPAST could also allow a fusion transcript to be made that joins some portions of SPAST and the directly-oriented SLC30A6.36,37 Conceptual transcription and translation suggest reading frame preservation for a theoretical fusion transcript resulting from splicing of the penultimate exon of SPAST (exon 16) to SLC30A6 exon 2 (Fig. 6). Nervous system mRNA is not available from the patients presented in this study, precluding further experimental investigation of the above possibilities.
Figure 6. DNA and protein sequences demonstrating that a (heterozygous) deletion of the final exon (exon 17) of SPAST may result in a fusion protein.
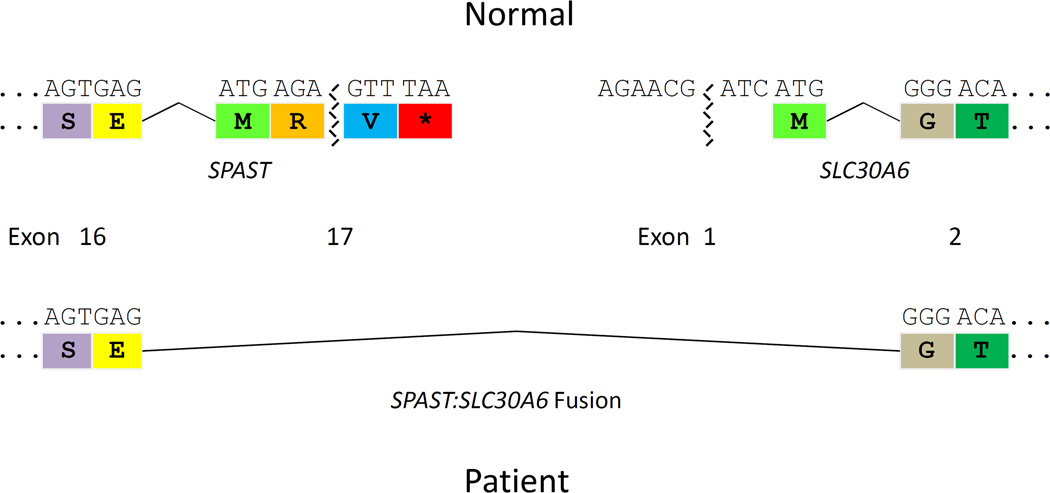
Removal of the final exon of SPAST may allow in-frame splicing from the penultimate exon (exon 16) of SPAST to exon 2 of SLC30A6 and a fusion protein product to be made.
SLC30A6 encodes a zinc transport protein38 that may play a role in the pathogenesis of Alzheimer disease39–42. Intriguingly, a subset of patients with SPG4 has been reported to have dementia and/or cognitive impairment (reviewed in ref. 43). Murphy et al.43 recently studied a family of SPG4 patients with deletions of SPAST exon 17 and described a co-morbid, age-dependent cognitive decline and dementia in patients carrying the deletion, though any potential effect on SLC30A6 was not assessed. If altered expression of SLC30A6 (or function of the zinc transporter it encodes, in the case of a fusion protein) is potentially associated with cognitive phenotypes in such individuals, then perhaps either SNVs or CNVs in or near this gene that do not affect SPAST may either cause or predispose to isolated cognitive decline or dementia.
In all three patients, breakpoints exhibit microhomology and localize to Alu elements. This breakpoint architecture provides evidence for Alu-specific microhomology-mediated deletion as the mechanism of exon loss. Although Alu-mediated NAHR cannot be excluded as a possible CNV-generating mechanism in patients A37 and A39, the observed breakpoint Alus are either of different families or, in the case of the AluYs at the breakpoint junction of patient A37’s deletion, share only 89% identity over 263 bp based upon the reference haploid human genome. The experimentally suggested minimum requirement for favorable homologous recombination (i.e. the minimal efficient processing segment or MEPS) for mammalian genomes is thought to be ~200–300 bp.29,44 Thus, Holliday structures potentially formed by Alu members from unrelated families or with <95% identity may be broken down by the mismatch repair pathway. The CNV in patient A38 is complex and more parsimoniously explained by template switching than multiple NAHR events.
The concentration of Alu family members in the introns and flanking sequence of SPAST is more than three times greater than the genomic average. In intron 16, it is more than five times greater. A similar, though less pronounced, phenomenon is seen in the CDKL5 gene, in which Erez et al.26 have reported Alu-mediated intragenic deletions with breakpoints localizing to regions of Alu concentration greater than that of the gene as a whole – itself enriched for Alus above the baseline genomic level (data not shown). We hypothesize that this genomic architecture may predispose to Alu-specific microhomology-mediated intragenic rearrangements within SPAST, in particular involving exons near the highest Alu density (e.g. exon 17). Thus, we predict that this mechanism is not only a possible, but frequent cause of pathogenic CNV in this gene, leading to SPG4. Beetz et al.15 noted that both the Alu density and the frequency of intragenic CNV in the SPG3A locus (in a small sample of AD-HSP patients) were lower than those of SPAST, and hypothesized a causal relationship including potentially Alu-mediated NAHR. We suggest that Alu-specific microhomology-mediated intragenic rearrangement may explain their observation.
Exonic deletions predominate among known, pathogenic, intragenic CNV in SPAST (Table 1); indeed, only a single exonic duplication has been reported in an SPG4 patient.6 Duplication (or higher order copy-number gain) of one or several exons could be benign whereas deletion of the same exon(s) would be pathogenic. Nonetheless, the number of reported exonic duplications appears to be lower than expected (see calculation, Supplemental Digital Content). This incongruity, first noted by Beetz et al.,15 may derive from an unforeseen quality of the SPAST locus and/or an unexpected feature of the mutational mechanism(s) creating these intragenic CNV that favors deletions, though more likely from a bias of ascertainment; i.e. perhaps MPLA, the predominant CNV detection method in previous studies (Table 1), fails to detect copy-number gains with the same accuracy as losses. Using locus-specific aCGH (as in the present study) or genome-wide aCGH with exonic coverage19 may improve detection of duplication and higher-order gain CNVs at the SPAST locus, though detecting copy-number gains using aCGH and the clinical interpretation of such CNV gains each possesses its own challenges.45,46
It is also of note that no intronic CNVs in SPAST, nor CNVs in flanking intergenic regions, including regulatory regions, have been reported in SPG4 patients. CNV in non-coding regions such as these have been shown to cause disease in an increasing number of conditions.35,47,48 As MLPA, the diagnostic methodology by which the majority of intragenic CNVs in SPAST has been discovered (Table 1), is usually performed in a strictly exon-targeted manner, it is an insufficient method to detect such CNV, should they exist in SPG4 patients. In contrast, aCGH with sufficient coverage of non-coding regions of and near SPAST may be a more appropriate methodology if all SPG4-causing mutations are to be detected.
This is the first report of a complex rearrangement within SPAST that spans an exon. Given recent data suggesting the importance of replication-based mechanisms in generating copy number change, for example MMBIR/FoSTeS/Alu-specific microhomology-mediated copy-number change25,26 and, specifically, the complex rearrangements they can generate,29 one might expect that some number of the CNVs in Table 1 may in fact represent complex rearrangements. Such complexity, if it exists, has likely gone undetected, as MLPA enables detection of only the presence or absence of assayed exons.
In summary, local genome architecture, including distribution and frequency of repetitive elements such as Alu, may facilitate genomic rearrangements. Such rearrangements can have consequences not only for the involved gene, but also for other genes in proximity to the breakpoints. We hypothesize that the high concentration of Alu family members in the introns and flanking sequence of SPAST may predispose to intragenic rearrangements in this gene, such as those described in the present study. Thus, Alu-specific microhomology-mediated intragenic rearrangements in SPAST may be a common cause of SPG4. Furthermore, we speculate that genomic deletions encompassing the final exon of SPAST may affect expression of SLC30A6, the most proximal downstream locus and a gene that has been implicated in the pathogenesis of Alzheimer disease, potentially explaining recent reports of dementia in SPG4 patients with this particular exon deletion.
Supplementary Material
Acknowledgements
P.M.B. is supported by the Baylor College of Medicine Medical Scientist Training Program (T32GM007330-34). This work was supported in part by a National Institute of Neurological Disorders and Stroke (NINDS) grant (R01NS058529) from the United States National Institutes of Health (J.R.L.). J.R.L. is a paid consultant for Athena Diagnostics and Ion Torrent Systems and is a co-inventor on multiple United States and European patents related to molecular diagnostics for inherited neuropathies. The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from the chromosomal microarray analysis offered in the Medical Genetics Laboratory.
Footnotes
List of Supplemental Digital Content:
Supplemental Digital Content.pdf
References
- 1.Salinas S, Proukakis C, Crosby A, Warner TT. Hereditary spastic paraplegia: clinical features and pathogenetic mechanisms. Lancet Neurol. 2008;7:1127–1138. doi: 10.1016/S1474-4422(08)70258-8. [DOI] [PubMed] [Google Scholar]
- 2.HUGO Gene Nomenclature Committee. [Accessed Oct. 15, 2010];HGNC Home Page. 2010 http://www.genenames.org.
- 3.McDermott CJ, Burness CE, Kirby J, et al. Clinical features of hereditary spastic paraplegia due to spastin mutation. Neurology. 2006;67:45–51. doi: 10.1212/01.wnl.0000223315.62404.00. [DOI] [PubMed] [Google Scholar]
- 4.Hazan J, Fonknechten N, Mavel D, et al. Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nat Genet. 1999;23:296–303. doi: 10.1038/15472. [DOI] [PubMed] [Google Scholar]
- 5.Sauter S, Miterski B, Klimpe S, et al. Mutation analysis of the spastin gene (SPG4) in patients in Germany with autosomal dominant hereditary spastic paraplegia. Hum Mutat. 2002;20:127–132. doi: 10.1002/humu.10105. [DOI] [PubMed] [Google Scholar]
- 6.Mitne-Neto M, Kok F, Beetz C, et al. A multi-exonic SPG4 duplication underlies sex-dependent penetrance of hereditary spastic paraplegia in a large Brazilian pedigree. Eur J Hum Genet. 2007;15:1276–1279. doi: 10.1038/sj.ejhg.5201924. [DOI] [PubMed] [Google Scholar]
- 7.Salinas S, Carazo-Salas RE, Proukakis C, et al. Human spastin has multiple microtubule-related functions. J Neurochem. 2005;95:1411–1420. doi: 10.1111/j.1471-4159.2005.03472.x. [DOI] [PubMed] [Google Scholar]
- 8.Roll-Mecak A, Vale RD. Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature. 2008;451:363–367. doi: 10.1038/nature06482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connell JW, Lindon C, Luzio JP, Reid E. Spastin couples microtubule severing to membrane traffic in completion of cytokinesis and secretion. Traffic. 2009;10:42–56. doi: 10.1111/j.1600-0854.2008.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SH, Zhu P-P, Parker RL, Blackstone C. Hereditary spastic paraplegia proteins REEP1, spastin, and atlastin-1 coordinate microtubule interactions with the tubular ER network. J Clin Invest. 2010;120:1097–1110. doi: 10.1172/JCI40979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svenson IK, Ashley-Koch AE, Gaskell PC, et al. Identification and expression analysis of spastin gene mutations in hereditary spastic paraplegia. Am J Hum Genet. 2001;68:1077–1085. doi: 10.1086/320111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Depienne C, Fedirko E, Forlani S, et al. Exon deletions of SPG4 are a frequent cause of hereditary spastic paraplegia. J Med Genet. 2007;44:281–284. doi: 10.1136/jmg.2006.046425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bönsch D, Schwindt A, Navratil P, et al. Motor system abnormalities in hereditary spastic paraparesis type 4 (SPG4) depend on the type of mutation in the spastin gene. J Neurol Neurosurg Psychiatry. 2003;74:1109–1112. doi: 10.1136/jnnp.74.8.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erichsen AK, Inderhaug E, Mattingsdal M, Eiklid K, Tallaksen CME. Seven novel mutations and four exon deletions in a collection of Norwegian patients with SPG4 hereditary spastic paraplegia. Eur J Neurol. 2007;14:809–814. doi: 10.1111/j.1468-1331.2007.01861.x. [DOI] [PubMed] [Google Scholar]
- 15.Beetz C, Nygren AOH, Schickel J, et al. High frequency of partial SPAST deletions in autosomal dominant hereditary spastic paraplegia. Neurology. 2006;67:1926–1930. doi: 10.1212/01.wnl.0000244413.49258.f5. [DOI] [PubMed] [Google Scholar]
- 16.Shoukier M, Neesen J, Sauter SM, et al. Expansion of mutation spectrum, determination of mutation cluster regions and predictive structural classification of SPAST mutations in hereditary spastic paraplegia. Eur J Hum Genet. 2009;17:187–194. doi: 10.1038/ejhg.2008.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svenstrup K, Bross P, Koefoed P, et al. Sequence variants in SPAST, SPG3A and HSPD1 in hereditary spastic paraplegia. J Neurol Sci. 2009;284:90–95. doi: 10.1016/j.jns.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Hewamadduma CAA, Kirby J, Kershaw C, et al. HSP60 is a rare cause of hereditary spastic paraparesis, but may act as a genetic modifier. Neurology. 2008;70:1717–1718. doi: 10.1212/01.wnl.0000311395.31081.70. [DOI] [PubMed] [Google Scholar]
- 19.Boone PM, Bacino CA, Shaw CA, et al. Detection of clinically relevant exonic copy-number changes by array CGH. Hum Mutat. 2010 doi: 10.1002/humu.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang F, Gu W, Hurles ME, Lupski JR. Copy number variation in human health, disease, and evolution. Annu Rev Genomics Hum Genet. 2009;10:451–481. doi: 10.1146/annurev.genom.9.081307.164217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deininger PL, Batzer MA. Alu repeats and human disease. Mol Genet Metab. 1999;67:183–193. doi: 10.1006/mgme.1999.2864. [DOI] [PubMed] [Google Scholar]
- 22.Lehrman MA, Russell DW, Goldstein JL, Brown MS. Alu-Alu recombination deletes splice acceptor sites and produces secreted low density lipoprotein receptor in a subject with familial hypercholesterolemia. J Biol Chem. 1987;262:3354–3361. [PubMed] [Google Scholar]
- 23.Lee JA, Carvalho CMB, Lupski JR. A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell. 2007;131:1235–1247. doi: 10.1016/j.cell.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 24.Hastings PJ, Ira G, Lupski JR. A microhomology-mediated break-induced replication model for the origin of human copy number variation. PLoS Genet. 2009;5:e1000327. doi: 10.1371/journal.pgen.1000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang F, Khajavi M, Connolly AM, Towne CF, Batish SD, Lupski JR. The DNA replication FoSTeS/MMBIR mechanism can generate genomic, genic and exonic complex rearrangements in humans. Nat Genet. 2009;41:849–853. doi: 10.1038/ng.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erez A, Patel AJ, Wang X, et al. Alu-specific microhomology-mediated deletions in CDKL5 in females with early-onset seizure disorder. Neurogenetics. 2009;10:363–369. doi: 10.1007/s10048-009-0195-z. [DOI] [PubMed] [Google Scholar]
- 27.Vissers LELM, Bhatt SS, Janssen IM, et al. Rare pathogenic microdeletions and tandem duplications are microhomology-mediated and stimulated by local genomic architecture. Hum Mol Genet. 2009;18:3579–3593. doi: 10.1093/hmg/ddp306. [DOI] [PubMed] [Google Scholar]
- 28.Stankiewicz P, Sen P, Bhatt SS, et al. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet. 2009;84:780–791. doi: 10.1016/j.ajhg.2009.05.005. Erratum 85:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang F, Carvalho CMB, Lupski JR. Complex human chromosomal and genomic rearrangements. Trends Genet. 2009;25:298–307. doi: 10.1016/j.tig.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bi W, Sapir T, Shchelochkov OA, et al. Increased LIS1 expression affects human and mouse brain development. Nat Genet. 2009;41:168–177. doi: 10.1038/ng.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwanaga H, Tsujino A, Shirabe S, et al. Large deletion involving the 5'-UTR in the spastin gene caused mild phenotype of autosomal dominant hereditary spastic paraplegia. Am J Med Genet A. 2005;133A:13–17. doi: 10.1002/ajmg.a.30510. [DOI] [PubMed] [Google Scholar]
- 32.Gonzaga-Jauregui C, Zhang F, Towne CF, Batish SD, Lupski JR. GJB1 /Connexin 32 whole gene deletions in patients with X-linked Charcot-Marie-Tooth disease. Neurogenetics. 2010;11:465–470. doi: 10.1007/s10048-010-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 34.McCarroll SA, Huett A, Kuballa P, et al. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn's disease. Nat Genet. 2008;40:1107–1112. doi: 10.1038/ng.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang F, Seeman P, Liu P, et al. Mechanisms for nonrecurrent genomic rearrangements associated with CMT1A or HNPP: rare CNVs as a cause for missing heritability. Am J Hum Genet. 2010;86:892–903. doi: 10.1016/j.ajhg.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsh T, McClellan JM, McCarthy SE, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 37.Lifton RP, Dluhy RG, Powers M, et al. A chimaeric 11 β-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355:262–265. doi: 10.1038/355262a0. [DOI] [PubMed] [Google Scholar]
- 38.Huang L, Kirschke CP, Gitschier J. Functional characterization of a novel mammalian zinc transporter, ZnT6. J Biol Chem. 2002;277:26389–26395. doi: 10.1074/jbc.M200462200. [DOI] [PubMed] [Google Scholar]
- 39.Lyubartseva G, Smith JL, Markesbery WR, Lovell MA. Alterations of zinc transporter proteins ZnT-1, ZnT-4 and ZnT-6 in preclinical Alzheimer's disease brain. Brain Pathol. 2010;20:343–350. doi: 10.1111/j.1750-3639.2009.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L-H, Wang X, Stoltenberg M, Danscher G, Huang L, Wang Z-Y. Abundant expression of zinc transporters in the amyloid plaques of Alzheimer's disease brain. Brain Res Bull. 2008;77:55–60. doi: 10.1016/j.brainresbull.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Lovell MA, Smith JL, Markesbery WR. Elevated zinc transporter-6 in mild cognitive impairment, Alzheimer disease, and pick disease. J Neuropathol Exp Neurol. 2006;65:489–498. doi: 10.1097/01.jnen.0000229237.98124.91. [DOI] [PubMed] [Google Scholar]
- 42.Smith JL, Xiong S, Markesbery WR, Lovell MA. Altered expression of zinc transporters-4 and-6 in mild cognitive impairment, early and late Alzheimer's disease brain. Neuroscience. 2006;140:879–888. doi: 10.1016/j.neuroscience.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 43.Murphy S, Gorman G, Beetz C, et al. Dementia in SPG4 hereditary spastic paraplegia: clinical, genetic, and neuropathologic evidence. Neurology. 2009;73:378–384. doi: 10.1212/WNL.0b013e3181b04c6c. [DOI] [PubMed] [Google Scholar]
- 44.Metzenberg AB, Wurzer G, Huismant THJ, Smithies O. Homology requirements for unequal crossing over in humans. Genetics. 1991;128:143–161. doi: 10.1093/genetics/128.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stankiewicz P, Pursley AN, Cheung SW. Challenges in clinical interpretation of microduplications detected by array CGH analysis. Am J Med Genet A. 2010;152A:1089–1100. doi: 10.1002/ajmg.a.33216. [DOI] [PubMed] [Google Scholar]
- 46.Bacino CA, Cheung S-W. Introductory comments on special section–Genomic microduplications: When adding may equal subtracting. Am J Med Genet A. 2010;152A:1063–1065. doi: 10.1002/ajmg.a.33346. [DOI] [PubMed] [Google Scholar]
- 47.Weterman MAJ, van Ruissen F, de Wissel M, et al. Copy number variation upstream of PMP22 in Charcot-Marie-Tooth disease. Eur J Hum Genet. 2010;18:421–428. doi: 10.1038/ejhg.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang LL, Worley K, Gannavarapu A, Chintagumpala MM, Levy ML, Plon SE. Intron-size constraint as a mutational mechanism in Rothmund-Thomson syndrome. Am J Hum Genet. 2002;71:165–167. doi: 10.1086/341234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beetz C, Zuchner S, Ashley-Koch A, et al. Linkage to a known gene but no mutation identified: comprehensive reanalysis of SPG4 HSP pedigrees reveals large deletions as the sole cause. Hum Mutat. 2007;28:739–740. doi: 10.1002/humu.20508. [DOI] [PubMed] [Google Scholar]
- 50.de Bot ST, van den Elzen RTM, Mensenkamp AR, et al. Hereditary spastic paraplegia due to SPAST mutations in 151 Dutch patients: new clinical aspects and 27 novel mutations. J Neurol Neurosurg Psychiatry. 2010;81:1073–1078. doi: 10.1136/jnnp.2009.201103. [DOI] [PubMed] [Google Scholar]
- 51.Magariello A, Muglia M, Patitucci A, et al. Mutation analysis of the SPG4 gene in Italian patients with pure and complicated forms of spastic paraplegia. J Neurol Sci. 2010;288:96–100. doi: 10.1016/j.jns.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 52.Battini R, Fogli A, Borghetti D, et al. Clinical and genetic findings in a series of Italian children with pure hereditary spastic paraplegia. Eur J Neurol. 2010 doi: 10.1111/j.1468-1331.2010.03102.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.