Abstract
Patients rarely experience visual hallucinations while being observed by clinicians. Therefore, instruments to detect visual hallucinations directly from patients are needed. Pareidolias, which are complex visual illusions involving ambiguous forms that are perceived as meaningful objects, are analogous to visual hallucinations and have the potential to be a surrogate indicator of visual hallucinations. In this study, we explored the clinical utility of a newly developed instrument for evoking pareidolic illusions, the Pareidolia test, in patients with dementia with Lewy bodies—one of the most common causes of visual hallucinations in the elderly. Thirty-four patients with dementia with Lewy bodies, 34 patients with Alzheimer’s disease and 26 healthy controls were given the Pareidolia test. Patients with dementia with Lewy bodies produced a much greater number of pareidolic illusions compared with those with Alzheimer’s disease or controls. A receiver operating characteristic analysis demonstrated that the number of pareidolias differentiated dementia with Lewy bodies from Alzheimer’s disease with a sensitivity of 100% and a specificity of 88%. Full-length figures and faces of people and animals accounted for >80% of the contents of pareidolias. Pareidolias were observed in patients with dementia with Lewy bodies who had visual hallucinations as well as those who did not have visual hallucinations, suggesting that pareidolias do not reflect visual hallucinations themselves but may reflect susceptibility to visual hallucinations. A sub-analysis of patients with dementia with Lewy bodies who were or were not treated with donepzil demonstrated that the numbers of pareidolias were correlated with visuoperceptual abilities in the former and with indices of hallucinations and delusional misidentifications in the latter. Arousal and attentional deficits mediated by abnormal cholinergic mechanisms and visuoperceptual dysfunctions are likely to contribute to the development of visual hallucinations and pareidolias in dementia with Lewy bodies.
Keywords: acetylcholine, dementia with Lewy bodies, visual hallucinations, visual illusions
Introduction
‘A perception without an object’ is the classic definition of a hallucination and has been in use since the end of the 19th century (Ey, 1973). According to this definition, a visual hallucination is experienced when one sees something where nothing actually exists. However, it is inevitable that when patients are awake and have their eyes open, they see things. It is difficult to determine whether false perceptions arise independently of real visual scenes or as a result of their distortion.
Dementia with Lewy bodies is arguably the most common cause of visual hallucinations in the elderly. Although visual hallucinations are one of the core clinical features differentiating dementia with Lewy bodies from other dementias, clinicians rarely witness patients as they experience them (McKeith et al., 1996, 2005). Although questionnaires or interviews with patients or caregivers are usually used to assess visual hallucinations, visual hallucinations are commonly under-reported by patients and are often not discovered by caregivers and health professionals (Mosimann et al., 2008). Tests that detect visual hallucinations directly from the patients would thus be a helpful clinical tool.
Patients with dementia with Lewy bodies frequently experience a variety of visual illusions as well as visual hallucinations (Iseki et al., 2002; Nagahama et al., 2007). Pareidolias, which are illusions of meaningful objects such as faces and animals, are thought to arise from ambiguous forms embedded in visual scenes and have a striking phenomenological resemblance to visual hallucinations. For instance, patients with dementia with Lewy bodies may incorrectly see a person in a curtain or perceive blobs on the wall as faces. In this study, we attempted to evoke and measure pareidolic illusions using a simple experimental paradigm, the Pareidolia test, and we explored the clinical utility of this test as a surrogate indicator of visual hallucinations in dementia with Lewy bodies.
Materials and methods
Participants
We recruited 34 patients with probable dementia with Lewy bodies and 34 with probable Alzheimer’s disease from the dementia clinics at the Tohoku University Hospital, the Akita Prefectural Centre of Rehabilitation and Psychiatric Medicine and the Niigata Rehabilitation Hospital. Additionally, 26 healthy controls were recruited though an advertisement. The three groups were comparable in age, sex, educational level and visual acuity. The severity of cognitive impairment, which was assessed by the Mini-Mental State Examination (MMSE) (Folstein et al., 1975), was matched between the dementia with Lewy bodies and Alzheimer’s disease groups (Table 1). All patients underwent an examination by experienced behavioural neurologists as well as MRI and routine laboratory investigations. Probable dementia with Lewy bodies and probable Alzheimer’s disease were diagnosed according to the international workshop criteria of dementia with Lewy bodies (McKeith et al., 2005) and the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al., 1984), respectively. The exclusion criteria were as follows: (i) a history of other neurological, psychiatric or severe ocular diseases; (ii) a best corrected visual acuity of <20/70; and (iii) language deficits hindering task executions. At the time of examination, 12 patients with dementia with Lewy bodies were treated with donepezil, one was treated with levodopa, four were treated with quetiapine and two were treated with fluvoxamine. In the Alzheimer’s disease group, eight patients were treated with donepezil and one was treated with fluvoxamine. The demographic and clinical characteristics of the participants are summarized in Table 1 and Supplementary Table 1.
Table 1.
Demographic and clinical profiles of the participants
| Variables | DLB (n = 34) | AD (n = 34) | HC (n = 26) | P-value |
|---|---|---|---|---|
| Age, years | 81.0 (3.9) | 80 (3.6) | 79.2 (4.9) | 0.238 |
| Sex (female/male)a | 19/15 | 10/24 | 8/18 | 0.385 |
| Education, years | 9.7 (2.5) | 9.3 (1.8) | 10.2 (1.8) | 0.265 |
| Visual acuityb | 20/25 (20/30–20/25) | 20/25 (20/30–20/25) | 20/25 (20/30–20/25) | 0.357 |
| Neuropsychology | ||||
| MMSE [30] | 18.9 (4.0) | 19.2 (3.1) | 29.0 (1.0) | <0.001c,d |
| Digit span [24] | 9.0 (2.3) | 8.8 (2.2) | 10.6 (1.7) | <0.001c,d |
| Object naming [60] | 54.9 (4.3) | 55.3 (2.3) | 58.7 (1.5) | <0.001c,d |
| Shape detection [20] | 18.1 (1.5) | 19.3 (1.0) | 19.7 (0.5) | <0.001c,e |
| Position discrimination [20] | 18.2 (1.7) | 19.1 (1.1) | 19.9 (0.4) | <0.001c,d,e |
| Object decision [32] | 24.3 (2.8) | 27.7 (2.3) | 29.8 (1.5) | <0.001c,d,e |
| Face recognition [30] | 23.9 (3.0) | 26.8 (2.1) | 28.6 (1.3) | <0.001c,d,e |
| NPIf | ||||
| Persecutory delusions | 1.3 (2.8) | 0.9 (2.5) | 0.379 | |
| Delusional misidentifications | 2.9 (4.1) | 0.1 (0.5) | <0.001 | |
| Hallucinations | 3.0 (3.8) | 0.1 (0.3) | <0.001 | |
| Agitation/aggression | 1.4 (2.6) | 0.9 (1.5) | 0.231 | |
| Dysphoria | 1.1 (2.3) | 0.6 (1.6) | 0.117 | |
| Anxiety | 2.4 (3.1) | 0.9 (1.7) | 0.088 | |
| Euphoria | 0.3 (0.8) | 0.0 (0.0) | 0.021 | |
| Apathy | 5.0 (3.5) | 2.9 (3.3) | 0.007 | |
| Disinhibition | 0.7 (2.1) | 1.2 (2.9) | 0.731 | |
| Irritability/lability | 0.7 (2.1) | 1.2 (2.9) | 0.819 | |
| Aberrant motor behaviour | 1.4 (2.6) | 1.3 (2.8) | 0.476 | |
| Fluctuations in cognition | 3.9 (3.3) | 0.1 (0.2) | <0.001 | |
| Prevalence [/34]a | ||||
| Delusional misidentifications | 22 | 2 | <0.001 | |
| Visual hallucinations | 25 | 3 | <0.001 | |
| Fluctuations in cognition | 31 | 2 | <0.001 |
The values in the second to fourth columns indicate the mean (standard deviation). The visual acuity is indicated as the median (IQR). The full scores for the neuropsychological tests are indicated in square brackets. The prevalence of behavioural symptoms are indicated as number of patients.
a χ2 test.
b Kruskal–Wallis test.
c Scheffé test (P < 0.05): DLB < AD.
d Scheffé test (P < 0.05): DLB < HC.
e Scheffé test (P < 0.05): AD < HC.
f Mann–Whitney U-test.
The remaining variables were tested using a one-way analysis of variance (ANOVA) and post hoc Scheffé tests.
DLB = dementia with Lewy bodies; AD = Alzheimer’s disease; HC = healthy controls.
All procedures in this study were approved by the ethical committee of the Tohoku University Graduate School of Medicine. All participants provided written informed consent after receiving a detailed explanation of the study.
Neuropsychology
The Digit Span subtest from the Wechsler Memory Scale-Revised (Sugishita, 2001) and the Object Naming subtest of the Western Aphasia Battery (Sugishita, 1986) were used to assess attention/working memory and language, respectively. Visuoperceptual and visuospatial functions were assessed using the Shape Detection Screening and Position Discrimination subtests of the Visual Object and Space Perception battery (Warrington and James, 1991), the Object Decision subtest (Easy B) of the Birmingham Object Recognition Battery (Riddoch and Humphreys, 1992) and the Face Recognition subtests (face-to-face matching of unknown faces, same/different judgement of unknown faces in different views, gender and age judgements of unknown faces) of the Visual Perception Test for Agnosia (Japan Society for Higher Brain Dysfunction, 1997).
Behavioural assessment
The Neuropsychiatric Inventory (NPI) (Cummings et al., 1994) was administered to the caregivers of the patients. We made some modifications to the original NPI as follows. First, the ‘delusion’ domain was separated into two different categories: persecutory delusions and delusional misidentifications. The questions regarding the former included ‘believing that others are planning to hurt him/her’ and ‘believing that others are stealing from him/her’; the questions regarding the latter included ‘believing that unwelcome guests are living in the house’, ‘believing that television or magazine figures are actually present in the home’ and ‘believing that patient’s family is an imposter’. Second, we employed an additional domain for fluctuations in cognition, which included questions such as ‘does the patient sometimes show a lack of attention or a slow reaction to others’ call than usual?’ and ‘does the patient sometimes show a poor understanding of things that he can usually understand?’ (Mori et al., 2006). The frequency (range 1–4), the severity (1–3) and the domain total scores (the product of the frequency score multiplied by the severity score) were recorded for each behaviour.
The Pareidolia test
Stimuli and task administration
Twenty-five coloured scenery pictures (25 × 19 cm2) containing animals, plants and artefacts were used. The pictures were selected on the basis of preliminary experiments in patients with dementia with Lewy bodies, Alzheimer’s disease and healthy controls. Each picture was filtered by Gaussian blurring (0.17 mm full-width at half-maximum) (Fig. 3B). Immediately before the administration of the test, a detailed explanation and three training trials were given. Subjects were instructed to point to and describe in as much detail as possible the objects shown in each picture. When the subjects provided few responses, they were prompted to describe as many things as possible. When subjects responded with such comments as, ‘It looks like X’, we determined whether the object (X) was actually in the picture or whether the subject saw something that looked like X. Each picture was presented for 60 s. No feedback was given to subjects, regardless of correct or incorrect responses. Subjects’ responses were recorded by video camera for later analysis.
Figure 3.
(A) Contents of illusory responses on the Pareidolia test. Numbers denote a percentage of the total illusory responses in each patient group. (B) Examples of illusory responses. Patients with dementia with Lewy bodies often misidentified objects or patterns in the picture as real faces (yellow triangle) or as figures of people and animals (white triangle).
Scoring
Subjects’ responses were classified into three types: (i) correct responses; (ii) illusory responses, in which subjects falsely identified objects that were not in the pictures; and (iii) other responses, in which subjects provided no response or said ‘I don’t know’. On the basis of the preliminary experiments in healthy subjects, correct answers were defined in advance. We calculated the sum of correct responses and illusory responses in the 25 images for each subject. In some cases, subjects provided two or more responses to a single picture. Accordingly, the total numbers of correct responses and of illusory responses were more than 25 in some cases.
Patients with dementia with Lewy bodies frequently see hallucinatory images in places where people do not usually direct their attention, such as the corners of rooms, trees in the background or the roofs of neighbouring houses. To assess where subjects saw pareidolias, we categorized the objects in the pictures into the ‘gist’ and the ‘detail/background’ (Adolphs et al., 2001). The ‘gist’ is the general information or major photographic subject that is important for the meaning of the image, and the ‘detail/background’ is the information that is of little importance for the meaning of the image.
To conduct a phenomenological analysis of pareidolias, the content of the illusory responses was classified into four categories: people, animals (e.g. animals, birds and fishes), objects (man-made objects, vegetables and flowers) and others (flash, stream of water, flame).
Test–retest and inter-rater reliabilities
Ten patients with dementia with Lewy bodies and 10 patients with Alzheimer’s disease underwent the test twice with a 2-week interval. The scoring was performed by two independent raters who were not informed about the diagnosis or other conditions of the subjects.
Statistical analyses
A Kruskal–Wallis test and a post hoc Mann–Whitney U-test with a Bonferroni correction (P < 0.05/3) were used for between-group comparisons of the Pareidolia test. To evaluate the ability of the test to differentiate dementia with Lewy bodies from Alzheimer’s disease, a receiver operating characteristic analysis was performed on the neuropsychological tests, the NPI domain total scores and the Pareidolia test. Relationships between performances on the Pareidolia test and other neuropsychological and behavioural variables were assessed using Pearson’s correlation coefficient or Spearman’s rank correlation coefficient.
Results
Neuropsychology and behaviours
The results are summarized in Table 1. There were no significant differences between the dementia with Lewy bodies and Alzheimer’s disease groups in the MMSE, Digit Span or the Object Naming tests. On all visuoperceptual and visuospatial tests, the performance of the dementia with Lewy bodies group was worse than the performance of the Alzheimer’s disease group. The dementia with Lewy bodies group had significantly higher scores for delusional misidentifications, hallucinations, euphoria, apathy and fluctuations in cognition than did the Alzheimer’s disease group on the NPI. All of the patients with dementia with Lewy bodies with a positive NPI hallucination score had visual hallucinations and none had hallucinations in other modalities.
The Pareidolia test
Reliability
The inter-class correlation coefficients were 0.98 for illusory responses, 0.97 for correct responses and 0.89 for other responses, indicating a good inter-rater reliability. The intra-class correlation coefficients were 0.98 for illusory responses, 0.98 for correct responses and 0.95 for other responses, indicating good test–retest reliability.
Group comparisons
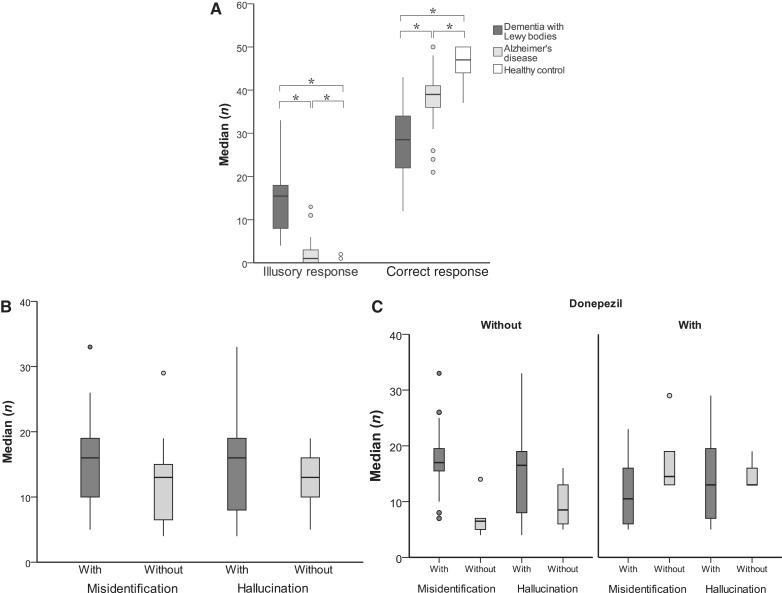
The median [interquartile range (IQR)] numbers of illusory responses were 15.5 (11.0) for dementia with Lewy bodies, 1.0 (3.0) for Alzheimer’s disease and 0 (0) for healthy controls (Fig. 1A). A Kruskal–Wallis test demonstrated a significant group difference (χ2 = 46.1, P < 0.001). The number of illusory responses in the dementia with Lewy bodies group was significantly larger than that in the Alzheimer’s disease (U = 28.5, P < 0.001) and the healthy control groups (U < 0.001, P < 0.001). The patients with Alzheimer’s disease gave more illusory responses than the healthy control subjects (U = 252.0, P < 0.001). The numbers of correct responses was 28.5 (13.0) for the dementia with Lewy bodies group, 39.0 (5.0) for the Alzheimer’s disease group and 47.0 (6.0) for the healthy control group (Fig. 1A). There was a significant difference among the three groups (χ2 = 23.8, P < 0.001). Post hoc tests showed significant differences in all of the pairwise comparisons (P < 0.001).
Figure 1.
(A) Numbers of illusory responses and correct responses on the Pareidolia test. Significance is denoted by an asterisk (P < 0.05/3). (B) Numbers of illusory responses in patients with dementia with Lewy bodies with and without delusional misidentifications and hallucinations. (C) Numbers of illusory responses in patients with dementia with Lewy bodies with and without delusional misidentifications and hallucinations, stratified by medication status.
When we classified the patients with dementia with Lewy bodies on the basis of the use or non-use of donepezil therapy, the median (IQR) numbers of illusory responses were 13.0 (9.0) in the patients with dementia with Lewy bodies with donepezil and 16.0 (11.0) in those without donepezil. There was no significant difference in illusory responses between the patients with and without donepezil (U = 123.0, P = 0.75; Supplementary Table 1). The numbers of correct responses was 21.0 (12.5) in the patients with dementia with Lewy bodies with donepezil and 32.0 (9.75) in those without donepezil. There was a significant difference in the number of correct responses between the two groups (U = 65.0, P = 0.002; Supplementary Table 1).
Next, we examined the impact of the presence of delusional misidentifications and hallucinations on the number of illusory responses (Fig. 1B). The median (IQR) numbers of illusory responses was 16.0 (10.0) in patients with dementia with Lewy bodies with delusional misidentifications (n = 22) and 13.0 (9.0) in patients without (n = 12). This difference in illusory responses did not reach the level of statistical significance (U = 81.0, P = 0.065). The numbers of illusory responses in patients with (n = 25) and without hallucinations (n = 9) were 16.0 (12.0) and 13.0 (8.0), respectively. There was no significant difference between these two groups (U = 83.5, P = 0.256).
We further stratified the patients with dementia with Lewy bodies according to both their medication status and the presence of delusional misidentifications and hallucinations (Fig. 1C). In the patients without donepezil, the median (IQR) numbers of illusory response in patients with (n = 16) and without (n = 6) delusional misidentifications were 17.0 (5.0) and 6.5 (4.0), respectively; and the numbers of illusory response in patients with (n = 18) and without hallucinations (n = 4) were 16.5 (12.0) and 8.5 (9.0), respectively. In the patients with donepezil, the numbers of illusory response in the patients with (n = 6) and without delusional misidentifications (n = 6) were 10.5 (12.0) and 14.5 (9.0), respectively; and the numbers of illusory response in patients with (n = 7) and without hallucinations (n = 5) were 13.0 (17.0) and 13.0 (5.0), respectively. In the absence of donepezil, although we were unable to perform formal statistical comparisons because of the small sample size, the number of illusory responses appeared to be larger in the patients with delusional misidentifications than those without; however, the difference was less clear between those with hallucinations and those without. In the patients treated with donepezil, there were no significant differences in the number of illusory responses between the patients with delusional misidentifications and hallucinations and those without.
The relationships among the number of correct responses, the presence of delusional misidentifications and hallucinations and medication status are shown in Supplementary Fig. 1.
Locations of illusory responses
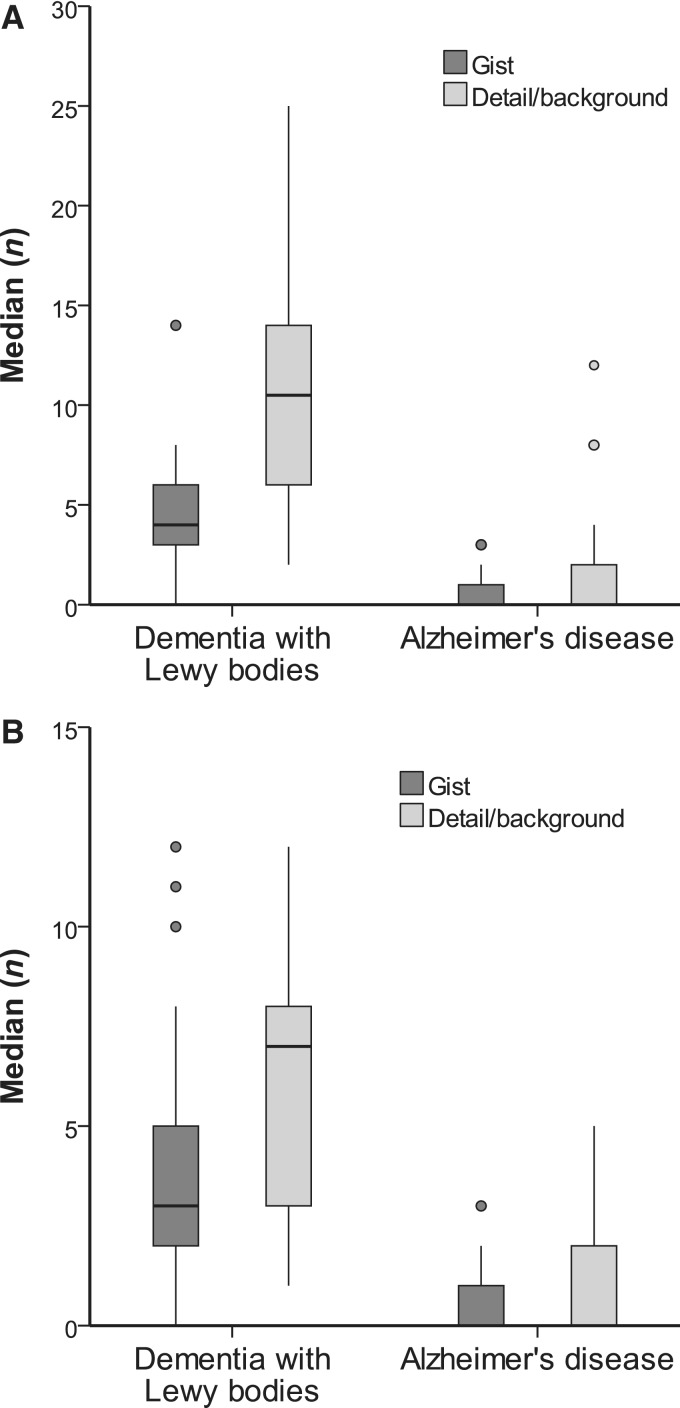
In the dementia with Lewy bodies group, the median (IQR) numbers of illusory responses were 4.0 (3.0) for the gist and 10.5 (8.0) for the detail/background (Fig. 2A). In the Alzheimer’s disease group, the median (IQR) numbers of illusory responses were 0 (1.0) for the gist and 0 (2.0) for the detail/background.
Figure 2.
(A) Locations of illusory responses. (B) Locations of first illusory responses.
As the items in the detail/background outnumbers that in the gist in the Pareidolia test, the number of illusory responses in the detail/background is inevitably larger than that in the gist even if one attends equally to the gist and the detail/background. In order to clarify whether the patients saw pareidolic illusions in the detail/background than in the gist, we performed the following analyses: (i) a comparison of weighted numbers of illusory responses between the location categories (gist and detail/background), which were calculated as: number of illusory responses in each location category was divided by the total number of items in each location category; and (ii) a comparison of the numbers of first illusory responses that were made in the gist and those that were made in the detail/background. In the dementia with Lewy bodies group, the median (IQR) of the weighted numbers of illusory response were 0.18 (0.15) for the gist and 0.52 (0.42) for the detail/background. In the Alzheimer’s disease group, the medians of the weighted numbers of illusory responses were 0 (0.05) for the gist and 0 (0.09) for the detail/background. First illusory responses were made for the gist in 3.0 (3.0) pictures and for the detail/background in 7.0 (5.0) pictures in the dementia with Lewy bodies group. In Alzheimer’s disease group, first illusory responses were made for the gist in 0 (1.0) pictures and for the detail/background in 0 (2.0) pictures (Fig. 2B).
In sum, the patients with dementia with Lewy bodies had a tendency to see pareidolic illusions more in the detail/background than in the gist.
Content of illusory responses and visual hallucinations
The content of illusory responses on the Pareidolia test are shown in Fig. 3A. Illusions of people and animals accounted for >80% of illusory responses. Faces and full-length figures of people and animals dominated whereas other body parts and man-made artefacts were infrequent. These characteristics were common to the dementia with Lewy bodies group and to a subset of patients with Alzheimer’s disease who gave illusory responses. In general, illusory images were not familiar to subjects. In the dementia with Lewy bodies group, familiar people, such as parents and spouses, and familiar animals, such as pets, accounted for only 2.1 and 2.6% of the total illusory responses, respectively. In the patients with Alzheimer’s disease, the illusory responses of familiar people and those of familiar animals accounted for 0 and 2.6% of all illusory responses, respectively. Illusory responses often appeared in bizarre and paradoxical contexts. For instance, patients often saw faces in flowers and found a man in a tie after they correctly identified the flowers and the tie (Fig. 3B). Even though they accepted the examiner’s suggestions that this was impossible, the patients failed to correct their illusory descriptions.
We determined the contents of visual hallucinations in each of 25 patients with dementia with Lewy bodies with visual hallucinations using medical records. Visual hallucinations of people, animals, plants and man-made objects were found in 21 (84%), 12 (48%), 2 (8%) and 2 patients (8%), respectively. Overall, the dominant contents of illusory responses in the Pareidolia test were consistent with those of visual hallucinations, and in both of these, people and animals accounted for >80% of contents. When comparing the contents of visual hallucinations and those of illusory responses on a patient-by-patient basis, they did not always agree with each other. The contents of illusory responses depended on the contents of the pictorial stimuli; for instance, patients with dementia with Lewy bodies frequently saw human faces but rarely found animals in petals of flowers and they tended to find animals but hardly saw people around the feet of a leopard (Fig. 3B).
Receiver operating characteristic analysis
In the receiver operating characteristic analysis, the area under the curve (AUC) was calculated for the neuropsychological tests and the NPI domain total scores in which significant differences were found between the dementia with Lewy bodies and Alzheimer’s disease groups and for illusory responses in the Pareidolia tests. The AUC (95% confidence interval) were 0.98 (0.94–1.0) for the illusory responses of the Pareidolia test, 0.95 (0.89–1.0) for the NPI fluctuations in cognition score, 0.86 (0.77–0.94) for the object decision test, 0.84 (0.75–0.94) for correct responses, 0.84 (0.74–0.94) for the NPI hallucinations score and 0.80 (0.69–0.91) for the NPI delusional misidentifications score. The optimal screening cut-off point for the illusory responses was ≥4 (sensitivity = 1.00, specificity = 0.88). With this criterion, four patients with Alzheimer’s disease were incorrectly classified as having dementia with Lewy bodies.
To evaluate the impact of dementia severity on the determination of cut-off point for illusory responses, we separately performed receiver operating characteristic analyses in the higher MMSE (≥20; 18 patients with dementia with Lewy bodies and 16 patients with Alzheimer’s disease) and the lower MMSE (≤19; 16 patients with dementia with Lewy bodies and 18 patients with Alzheimer’s disease) groups. In both groups, the number of illusory responses had the greatest AUC compared with any other variables (0.96 in the higher MMSE group and 0.98 in the lower MMSE group). The optimal cut-off points for the illusory responses were similar between the higher and the lower MMSE groups: ≥4 (sensitivity = 1.00, specificity = 0.88) in the former and ≥5 (sensitivity = 1.00, specificity = 0.94) in the latter.
Relationships between performances on the Pareidolia test and neuropsychological and behavioural variables in dementia with Lewy bodies
The results are shown in Table 2. There were no significant correlations between performances on the Pareidolia test and age, education or visual acuity. There were no significant correlations between illusory responses and neuropsychological performance in the entire dementia with Lewy bodies group and in the patients without donepezil. In the patients with dementia with Lewy bodies with donepezil, illusory responses were significantly correlated with the face recognition tests.
Table 2.
Correlations between illusory responses on the Pareidolia test and neuropsychological and behavioural variables
| Variables | Entire DLB (n = 34) |
Without donepezil (n = 22) |
With donepezil (n = 12) |
|||
|---|---|---|---|---|---|---|
| r, rs | P-value | r, rs | P-value | r, rs | P-value | |
| Age | 0.17 | 0.335 | 0.247 | 0.268 | 0.079 | 0.808 |
| Education | 0.061 | 0.551 | 0.168 | 0.454 | −0.225 | 0.48 |
| Visual acuity | −0.045 | 0.801 | −0.107 | 0.636 | 0.099 | 0.761 |
| Neuropsychology | ||||||
| MMSE | −0.065 | 0.713 | 0.059 | 0.795 | −0.252 | 0.429 |
| Digit span | 0.077 | 0.664 | 0.174 | 0.439 | −0.064 | 0.844 |
| Object naming | 0.014 | 0.937 | 0.117 | 0.604 | −0.447 | 0.145 |
| Shape detection | −0.148 | 0.404 | 0.035 | 0.625 | −0.233 | 0.465 |
| Position discrimination | <0.001 | 0.999 | 0.211 | 0.346 | −0.529 | 0.077 |
| Object decision | −0.214 | 0.224 | −0.258 | 0.247 | −0.097 | 0.764 |
| Face recognition | −0.156 | 0.379 | 0.061 | 0.786 | −0.601 | 0.039* |
| NPI | ||||||
| Delusional misidentifications | ||||||
| Domain total | 0.42 | 0.013* | 0.704 | <0.001* | −0.256 | 0.422 |
| Frequency | 0.337 | 0.051 | 0.706 | <0.001* | −0.163 | 0.034* |
| Severity | 0.304 | 0.008* | 0.729 | <0.001* | −0.63 | 0.028* |
| Hallucinations | ||||||
| Domain total | 0.311 | 0.074 | 0.474 | 0.026* | −0.06 | 0.854 |
| Frequency | 0.397 | 0.02* | 0.471 | 0.027* | 0.094 | 0.072 |
| Severity | 0.218 | 0.217 | 0.44 | 0.041* | −0.231 | 0.471 |
| Fluctuations in cognition | ||||||
| Domain total | 0.253 | 0.149 | 0.198 | 0.377 | 0.349 | 0.266 |
| Frequency | 0.219 | 0.33 | 0.13 | 0.308 | 0.389 | 0.211 |
| Severity | 0.214 | 0.057 | 0.565 | 0.164 | 0.253 | 0.428 |
Spearman’s correlation coefficients (rs) were used for visual acuity and behavioural symptoms; otherwise, Pearson’s correlation coefficients (r) were used.
*P < 0.05.
DLB = dementia with Lewy bodies.
In the entire dementia with Lewy bodies group, the number of illusory responses was significantly correlated with the domain total score of the NPI delusional misidentifications domain and the frequency score of the hallucination domain. In the patients with dementia with Lewy bodies without donepezil, the number of illusory responses was significantly correlated with the domain total, severity and frequency scores of the NPI delusional misidentifications and hallucination domains.
Discussion
Phenomenological similarities between visual hallucinations and pareidolias in dementia with Lewy bodies
Although it is conceptually easy to distinguish between hallucinations and illusions, the phenomenological distinction between the two conditions is ambiguous and difficult to determine. There have been longstanding criticisms that hallucinations should be defined not by the presence or absence of objects but as ‘perceptions without objects which are to be perceived’ (Ey, 1973; Oyebode, 2008). The following findings in this study support the view that visual hallucinations and illusions are continuous conditions. First, faces and whole bodies of people and animals constituted a large portion of the content of pareidolic illusions, whereas man-made objects were considerably less frequent. The pareidolic illusions often appeared in bizarre and paradoxical contexts, such as a man in a tie or human faces in flowers. These characteristics are similar to those of visual hallucinations in dementia with Lewy bodies. Patients with dementia with Lewy bodies experience visual hallucinations of meaningful objects (complex visual hallucinations) far more frequently than dots, flashes or amorphous forms (simple visual hallucinations) (Collerton et al., 2005; Mosimann et al., 2006). Their contents are dominated by people and animals (Mosimann et al., 2006; Nagahama et al., 2007) and they often develop into delusions with bizarre contents (McKeith et al., 1996). Second, the patients saw illusory images more frequently in the details/backgrounds than in the gist of scenes. This finding reminds us that patients with dementia with Lewy bodies often see human figures in the corners of rooms or small insects on floors and walls (Mosimann et al., 2006). Finally, the lack or impairment of insight is a common feature of pareidolias and visual hallucinations in dementia with Lewy bodies. The patients maintain the existence of illusory images, even though the examiner prompts them to verify their judgement. Similarly, previous studies have documented that most patients with dementia with Lewy bodies are convinced of the reality (or unconvinced of the unreality) of hallucinations (Ballard et al., 1997).
Clinical utility of the Pareidolia test
Due to the spontaneous nature of visual hallucinations, clinicians rarely observe patients seeing visual hallucinations. Therefore, the detection and the measurement of visual hallucinations have relied on self and family reports. However, reliable information is difficult to obtain from patients themselves when they do not have insight into their visual hallucinations or from their family when patients live alone. In such cases, techniques to evoke pareidolias directly from patients would be helpful as they are an analogous phenomenon to visual hallucinations. In addition, a quantitative measure of pareidolias may be useful as a surrogate indicator of therapeutic responses in dementia with Lewy bodies.
A receiver operating characteristic analysis demonstrated that the AUC of the number of illusory responses was larger than those of any other neuropsychological and neuropsychiatric variables. With the optimal cut-off score, the Pareidolia test successfully differentiated dementia with Lewy bodies from Alzheimer’s disease with a sensitivity and specificity of 100 and 88.2%, respectively, and four of the 34 patients with Alzheimer’s disease were incorrectly classified as having dementia with Lewy bodies. Surprisingly, a longitudinal follow-up of three of the four patients with Alzheimer’s disease with pareidolias demonstrated that two had fulfilled the clinical diagnostic criteria for probable dementia with Lewy bodies during the following 2 years (Supplementary Table 2). In contrast, none of the eight patients with Alzheimer’s disease with no pareidolias have met the criteria of dementia with Lewy bodies in the following years. Given that pareidolias were observed in the patients with dementia with Lewy bodies who had visual hallucinations as well as those who did not have visual hallucinations, pareidolias do not reflect visual hallucinations themselves. We suggest that the Pareidolia test may be able to identify patients with subclinical hallucinations or a predisposition to visual hallucinations.
The contribution of visuoperceptual impairment to visual hallucinations and pareidolias
Charles Bonnet syndrome in patients with ocular diseases and visual hallucinations in the hemianopic field in patients with damage to the central visual pathway have led to the idea that visuoperceptual impairment plays a critical role in the emergence of visual hallucinations (Kölmel, 1988; Ffytche, 2005, 2009). In accord with this idea, visuoperceptual dysfunction is reportedly more pronounced in patients who have visual hallucinations with dementia with Lewy bodies and its related disorder, Parkinson’s disease, than in those without visual hallucinations (Mori et al., 2000; Mosimann et al., 2004). The contribution of defective visuoperceptual processing is also supported by neuroimaging studies showing dysfunction of the occipitotemporal visual association cortices in patients with dementia with Lewy bodies and patients with Parkinson’s disease with visual hallucinations (Oishi et al., 2005; Perneczky et al., 2008; Meppelink et al., 2009).
In this study, there was a negative correlation between the number of illusory responses in the Pareidolia test and scores on the face recognition tests in patients with dementia with Lewy bodies with donepezil. This finding suggests that visuoperceptual mechanisms are involved in pareidolias. However, the Pareidolia test may be no more than a test of visuoperceptual function itself rather than a measure of pathological processes associated with visual hallucinations. To address this possibility, we performed a supplementary experiment on two patients with visual agnosia arising from cerebral infarction (Supplementary Fig. 2). Although the performance of the two agnosics on the visuoperceptual tests was similar to that of the patients with dementia with Lewy bodies, one agnosic provided no illusory responses and the other provided only a small number of illusory responses that was below the cut-off score. These findings indicate that visuoperceptual impairment alone is insufficient to cause the emergence of pareidolias.
The roles of arousal and attention: relevance to acetylcholine neurotransmission
The deafferentation hypothesis, in which visual hallucinations are understood as release phenomena of the visual association cortices that arise because of defective visual input, does not explain why most patients with visual impairment do not develop visual hallucinations (Ffytche, 2009). In fact, there should be additional non-visual mechanisms in the pathogenesis of visual hallucinations. Evidence suggests that the alteration of arousal and abnormalities of the modulatory neurotransmitter systems, particularly cholinergic systems, contribute to the emergence of visual hallucinations. Visual hallucinations usually occur during sleep-wake transitions (hypnagogic or hypnopompic hallucinations) in narcolepsy and in healthy people (Manni, 2005). Recent studies indicate that the arousal deficits in narcolepsy are associated with the activation failure of the basal forebrain cholinergic systems resulting from orexinergic deficiencies (Arrigoni et al., 2010). Furthermore, damage to the upper brainstem tegmentum occasionally results in visual hallucinations (referred to as peduncular hallucinosis) (Manford and Andermann, 1998; Nishio et al., 2007). Cholinergic, dopaminergic, serotoninergic and noradrenergic systems are densely packed in this brain region and play critical roles in generating sleep-wake cycles. Finally, sleep abnormalities and electroencephalographic slowing, a hallmark of the low-arousal state, are prevalent in dementia with Lewy bodies and Parkinson’s disease (Diederich et al., 2005; McKeith et al., 2005; Bonanni et al., 2008). The amelioration of visual hallucinations and fluctuating arousal/attention by cholinesterase inhibitors has been repeatedly documented in dementia with Lewy bodies and Parkinson’s disease (McKeith et al., 2005; Diederich et al., 2009).
In their proposition of the perception and attention deficit model, Collerton et al. (2005) proposed that visual hallucinations arise from the combination of visuoperceptual deficits and top–down attentional impairment. According to this model, in normal scene perception, an external visual input activates a number of potentially seen visual object representations, which they called ‘proto-objects’. Then, top–down attentional processes allow only one proto-object to enter conscious visual awareness to accomplish visual object recognition. In the case of visual hallucinations, top–down attentional processes are defective, and therefore, patients aberrantly recognize incorrect proto-objects as real objects. Accordingly, the current observation suggests that abnormal top–down attentional orientation is involved in the pathomechanisms of pareidolias in dementia with Lewy bodies; the patients with dementia with Lewy bodies saw pareidolias more in the detail/background, which is usually of little semantic importance and less noticeable, than in the gist of scenes, which carries semantically important information and has attentional priority.
The perception and attention deficit model proposes that attentional impairment in visual hallucinations is associated with cholinergic insufficiency (Collerton et al., 2005). This view is constructed on the grounds of the following clinical and neuroscientific findings. First, it has long been known that anticholinergic agents produce a hypoarousal state (also referred to as a confusional state) associated with severe attentional deficits and visual hallucinations (Collerton et al., 2005). There is also abundant evidence suggesting that cholinomimetic drugs improve attentional deficits and visual hallucinations (McKeith et al., 2000; Mori et al., 2006). Second, acetylcholine facilitates local cortical processing of external input and at the same time suppresses internal information processing, consequently enhancing the neuronal signal-to-noise ratio for external information (Perry et al., 1999; Hasselmo and McGaughy, 2004). Under low acetylcholine states, information processing is susceptible to internal ‘noise’ and thereby attentional control processes are disrupted. In the current study, the NPI hallucination score and the number of illusory responses were smaller in the patients with dementia with Lewy bodies with donepezil than in those without (Supplementary Table 1). Moreover, the number of illusory responses was not correlated with the NPI hallucination score but with performance on the visual perception tests in the patients treated with donepezil (Table 2). These results suggest that the role of visuoperceptual deficits is larger in pareidolias than in visual hallucinations, whereas the role of attentional deficits is larger in visual hallucinations than in pareidolias. Accordingly, pareidolias may tend to persist after cholinomimetic drugs alleviate attentional deficits and improve visual hallucinations. When patients are treated with cholinomimetic drugs, pareidolias may primarily reflect residual visuoperceptual impairment.
Lack of insight into visual hallucinations and pareidolias
Insight into visual hallucinations is lacking or impaired in most patients with dementia with Lewy bodies (Ballard et al., 1997). These patients often develop secondary delusions of the real existence of persons and animals that appear in visual hallucinations (McKeith et al., 2005). Similarly, the current study demonstrated that insight into pareidolias is lacking in dementia with Lewy bodies. The contexts in which pareidolias occur were bizarre and paradoxical, e.g. a man appearing in a tie and human faces appearing in flowers. These findings suggest that abnormal insight is a key feature of both visual hallucinations and pareidolias in patients with dementia with Lewy bodies. Thus, how do these patients falsely accept the bizarre and paradoxical contents of visual hallucinations and pareidolias as reality? A similar question is found in research on psychosis in schizophrenia: how do patients acquire delusional beliefs and false convictions of reality of hallucinatory perceptions? Kapur (2003) proposed that the initial step in the development of psychosis is heightened awareness or aberrant assignment of motivational salience to unimportant perceptual experiences. In a similar way, we could consider a model in which the aberrant assignment of salience to illusory perceptual information (or incorrect proto-objects in the terminology of the perception and attention deficit model) leads to abnormal insight into visual hallucinations and pareidolias in dementia with Lewy bodies. We suggest that abnormal insight may be a common underlying pathology of visual hallucinations, pareidolias and delusions in dementia with Lewy bodies. In fact, in the current study, the number of illusory responses was significantly correlated with both hallucination and delusional misidentifications scores on the NPI.
Some patients with dementia with Lewy bodies treated with cholinesterase inhibitors report their experiences in ways such as ‘I had thought it was real before, but now I realize that that was just an illusion.’ Such an introspective description suggests a relationship between cholinergic insufficiency and abnormal insight. Other evidence for cholinergic mechanisms in abnormal insights comes from peduncular hallucinosis, a condition of recurrent visual hallucinations after damage to the upper brainstem tegmentum in which the visual pathways are spared (Manford and Andermann, 1998; Nishio et al., 2007). Hypoarousal or attentional impairment due to ascending modulatory neurotransmitter systems is a primary pathomechanism of visual hallucinations in this condition. Insight into visual hallucinations is usually lacking or impaired in most cases of peduncular hallucinosis (Feinberg and Rapcsak, 1989; Kolmel, 1991; Furuta et al., 2002; Benke, 2006; Nishio et al., 2007) (for a counter view, see Manford and Andermann, 1998). Moreover, there have been previous reports of the concomitance of visual hallucinations and delusional misidentifications in peduncular hallucinosis and those of delusions subsequent to upper brainstem lesions (Drake et al., 1990; Mitsuhata and Tsukagoshi, 1992; Benke, 2006). The roles of cholinergic insufficiency in abnormal insight into visual hallucinations and pareidolias and the pathomechanisms of delusional misidentifications in dementia with Lewy bodies should be addressed in future pharmacological intervention studies.
Limitations
Several limitations of this study should be noted. First, the high sensitivity and moderate specificity of the Pareidolia test in differentiating between dementia with Lewy bodies and Alzheimer’s disease may be associated with an inclusion bias. Since the current diagnostic criteria for dementia with Lewy bodies emphasize the existence of visual hallucinations, the differential diagnosis of dementia with Lewy bodies and Alzheimer’s disease depends heavily on whether patients have visual hallucinations. In addition, given the low sensitivity of the criteria for dementia with Lewy bodies, there could be cases in which pathological dementia with Lewy bodies is misdiagnosed as Alzheimer’s disease (McKeith et al., 2005). Second, a cross-sectional comparison between patients with and without donepezil does not provide definitive evidence for cholinergic mechanisms. Longitudinal investigations are needed to elucidate the relationship between pareidolias and acetylcholine and to examine the usefulness of the Pareidolia test in measuring the therapeutic effects of cholinergic agents. Third, although there has been a substantial interest in the dopaminergic and prefrontal mechanisms of psychosis, we did not address these issues in our study (Kapur, 2003; Coltheart et al., 2011; Feinberg, 2011). Finally, illusory responses in the Pareidolia test may not purely reflect visual illusions and may be contaminated by confabulatory responses. Doubleday et al. (2002) reported that confabulatory responses on memory tests were observed in 17% of patients with dementia with Lewy bodies. Although the memory load is fairly low in the Pareidolia test, the prompts requesting more description may evoke confabulatory responses. To diminish the possibilities of contamination of confabulation, we are currently developing a new task to evoke pareidolias in which subjects are not required to give detailed descriptions.
Conclusion and future directions
Using a simple task, we successfully evoked pareidolias, which are complex visual illusions phenomenologically analogous to visual hallucinations, in patients with dementia with Lewy bodies. Compared with patients with Alzheimer’s disease and healthy controls, patients with dementia with Lewy bodies experienced a much larger number of pareidolias. A sub-analysis of patients with or without donepezil suggested that cholinergic mechanisms are involved in visual hallucinations and in pareidolias. Our results suggest that tests that quantify pareidolias may be beneficial in the differential diagnosis of dementing disorders and may be a useful indicator of therapeutic response in dementia with Lewy bodies. In addition, the pathomechanisms of visual hallucinations in Parkinson’s disease have been considered similar to those observed in dementia with Lewy bodies (Ffytche, 2007). Visual illusions and minor hallucinations, such as presence and passage hallucinations, are reportedly common in Parkinson’s disease without visual hallucinations (Fenelon et al., 2000). We suggest that pareidolias may be a phenomenon bridging visual illusions, minor hallucinations and visual hallucinations. We are currently involved in a study on this topic.
Funding
Grant-in-Aid for Scientific Research for Young Scientists from the Ministry of Education, Culture, Sports, Science and Technology of Japan (90451591 to Y.N.) and Grant-in-Aid for Scientific Research on Innovative Areas (Face Perception and Recognition) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (30368477 to E.M.).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We thank the patients who participated in this study. We also thank Akiko Hayashi and Mayumi Shinohara for their help in data acquisition.
Glossary
Abbreviations
- MMSE
Mini-Mental State Examination
- NPI
Neuropsychiatric Inventory
References
- Adolphs R, Denburg NL, Tranel D. The amygdala’s role in long-term declarative memory for gist and detail. Behav Neurosci. 2001;115:983–92. doi: 10.1037//0735-7044.115.5.983. [DOI] [PubMed] [Google Scholar]
- Arrigoni E, Mochizuki T, Scammell TE. Activation of the basal forebrain by the orexin/hypocretin neurones. Acta Physiol. 2010;198:223–35. doi: 10.1111/j.1748-1716.2009.02036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, McKeith I, Harrison R, O’Brien J, Thompson P, Lowery K, et al. A detailed phenomenological comparison of complex visual hallucinations in dementia with Lewy bodies and Alzheimer’s disease. Int Psychogeriatr. 1997;9:381–8. doi: 10.1017/s1041610297004523. [DOI] [PubMed] [Google Scholar]
- Benke T. Peduncular hallucinosis: a syndrome of impaired reality monitoring. J Neurol. 2006;253:1561–71. doi: 10.1007/s00415-0060-0254-4. [DOI] [PubMed] [Google Scholar]
- Bonanni L, Thomas A, Tiraboschi P, Perfetti B, Varanese S, Onofrj M. EEG comparisons in early Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease with dementia patients with a 2-year follow-up. Brain. 2008;131:690–705. doi: 10.1093/brain/awm322. [DOI] [PubMed] [Google Scholar]
- Collerton D, Perry E, McKeith I. Why people see things that are not there: a novel Perception and Attention Deficit model for recurrent complex visual hallucinations. Behav Brain Sci. 2005;28:737–57. doi: 10.1017/S0140525X05000130. discussion 757–94. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Langdon R, McKay R. Delusional belief. Annu Rev Psychol. 2011;62:271–98. doi: 10.1146/annurev.psych.121208.131622. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Fenelon G, Stebbins G, Goetz CG. Hallucinations in Parkinson disease. Nat Rev Neurol. 2009;5:331–42. doi: 10.1038/nrneurol.2009.62. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Goetz CG, Stebbins GT. Repeated visual hallucinations in Parkinson’s disease as disturbed external/internal perceptions: focused review and a new integrative model. Mov Disord. 2005;20:130–40. doi: 10.1002/mds.20308. [DOI] [PubMed] [Google Scholar]
- Doubleday EK, Snowden JS, Varma AR, Neary D. Qualitative performance characteristics differentiate dementia with Lewy bodies and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;72:602–7. doi: 10.1136/jnnp.72.5.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake ME, Jr, Pakalnis A, Phillips B. Secondary mania after ventral pontine infarction. J Neuropsychiatry Clin Neurosci. 1990;2:322–5. doi: 10.1176/jnp.2.3.322. [DOI] [PubMed] [Google Scholar]
- Ey H. Paris: Masson; 1973. Traité des hallucinations. [Google Scholar]
- Feinberg TE. Neuropathologies of the self: clinical and anatomical features. Conscious Cogn. 2011;20:75–81. doi: 10.1016/j.concog.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Feinberg WM, Rapcsak SZ. ‘Peduncular hallucinosis’ following paramedian thalamic infarction. Neurology. 1989;39:1535–6. doi: 10.1212/wnl.39.11.1535. [DOI] [PubMed] [Google Scholar]
- Fenelon G, Mahieux F, Huon R, Ziegler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123(Pt 4):733–45. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- Ffytche DH. Visual hallucinations and the Charles Bonnet syndrome. Curr Psychiatry Rep. 2005;7:168–79. doi: 10.1007/s11920-005-0050-3. [DOI] [PubMed] [Google Scholar]
- Ffytche DH. Visual hallucinatory syndromes: past, present, and future. Dialogues Clin Neurosci. 2007;9:173–89. doi: 10.31887/DCNS.2007.9.2/dffytche. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffytche DH. Visual hallucinations in eye disease. Curr Opin Neurol. 2009;22:28–35. doi: 10.1097/wco.0b013e32831f1b3f. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Furuta T, Nobutou H, Nobutou F. [Peduncular hallucinosis due to a small hemorrhage around the substantia nigra: a case report] No To Shinkei. 2002;54:423–6. [PubMed] [Google Scholar]
- Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res. 2004;145:207–31. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Iseki E, Marui W, Nihashi N, Kosaka K. Psychiatric symptoms typical of patients with dementia with Lewy bodies - similarity to those of levodopa-induced psychosis. Acta Neuropsychiatr. 2002;14:237–41. doi: 10.1034/j.1601-5215.2002.140507.x. [DOI] [PubMed] [Google Scholar]
- Japan Society for Higher Brain Dysfunction. Tokyo: Shinko Igaku Shuppan; 1997. Visual perception test for agnosia. [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kolmel HW. Peduncular hallucinations. J Neurol. 1991;238:457–9. doi: 10.1007/BF00314654. [DOI] [PubMed] [Google Scholar]
- Kölmel HW. Berlin: Springer; 1988. Die homonymen Hemianopsien - Klinik und Pathophysiologie zentraler Sehstörungen. [Google Scholar]
- Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(Pt 10):1819–40. doi: 10.1093/brain/121.10.1819. [DOI] [PubMed] [Google Scholar]
- Manni R. Rapid eye movement sleep, non-rapid eye movement sleep, dreams, and hallucinations. Curr Psychiatry Rep. 2005;7:196–200. doi: 10.1007/s11920-005-0053-0. [DOI] [PubMed] [Google Scholar]
- McKeith I, Del Ser T, Spano P, Emre M, Wesnes K, Anand R, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356:2031–6. doi: 10.1016/S0140-6736(00)03399-7. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–24. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Meppelink AM, de Jong BM, Renken R, Leenders KL, Cornelissen FW, van Laar T. Impaired visual processing preceding image recognition in Parkinson’s disease patients with visual hallucinations. Brain. 2009;132:2980–93. doi: 10.1093/brain/awp223. [DOI] [PubMed] [Google Scholar]
- Mitsuhata Y, Tsukagoshi H. [Cerebellar infarction presenting erotic delusion and delusion of jealousy in the acute phase] Rinsho Shinkeigaku. 1992;32:1256–60. [PubMed] [Google Scholar]
- Mori S, Mori E, Iseki E, Kosaka K. Efficacy and safety of donepezil in patients with dementia with Lewy bodies: preliminary findings from an open-label study. Psychiatry Clin Neurosci. 2006;60:190–5. doi: 10.1111/j.1440-1819.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- Mori E, Shimomura T, Fujimori M, Hirono N, Imamura T, Hashimoto M, et al. Visuoperceptual impairment in dementia with Lewy bodies. Arch Neurol. 2000;57:489–93. doi: 10.1001/archneur.57.4.489. [DOI] [PubMed] [Google Scholar]
- Mosimann UP, Collerton D, Dudley R, Meyer TD, Graham G, Dean JL, et al. A semi-structured interview to assess visual hallucinations in older people. Int J Geriatr Psychiatry. 2008;23:712–8. doi: 10.1002/gps.1965. [DOI] [PubMed] [Google Scholar]
- Mosimann UP, Mather G, Wesnes KA, O’Brien JT, Burn DJ, McKeith IG. Visual perception in Parkinson disease dementia and dementia with Lewy bodies. Neurology. 2004;63:2091–6. doi: 10.1212/01.wnl.0000145764.70698.4e. [DOI] [PubMed] [Google Scholar]
- Mosimann UP, Rowan EN, Partington CE, Collerton D, Littlewood E, O’Brien JT, et al. Characteristics of visual hallucinations in Parkinson disease dementia and dementia with lewy bodies. Am J Geriatr Psychiatry. 2006;14:153–60. doi: 10.1097/01.JGP.0000192480.89813.80. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okina T, Suzuki N, Matsuda M, Fukao K, Murai T. Classification of psychotic symptoms in dementia with Lewy bodies. Am J Geriatr Psychiatry. 2007;15:961–7. doi: 10.1097/JGP.0b013e3180cc1fdf. [DOI] [PubMed] [Google Scholar]
- Nishio Y, Ishii K, Kazui H, Hosokai Y, Mori E. Frontal-lobe syndrome and psychosis after damage to the brainstem dopaminergic nuclei. J Neurol Sci. 2007;260:271–4. doi: 10.1016/j.jns.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Oishi N, Udaka F, Kameyama M, Sawamoto N, Hashikawa K, Fukuyama H. Regional cerebral blood flow in Parkinson disease with nonpsychotic visual hallucinations. Neurology. 2005;65:1708–15. doi: 10.1212/01.wnl.0000187116.13370.e0. [DOI] [PubMed] [Google Scholar]
- Oyebode F. Sim’s symptoms in the mind. Philadelphia: Elsevier; 2008. [Google Scholar]
- Perneczky R, Drzezga A, Boecker H, Forstl H, Kurz A, Haussermann P. Cerebral metabolic dysfunction in patients with dementia with Lewy bodies and visual hallucinations. Dement Geriatr Cogn Disord. 2008;25:531–8. doi: 10.1159/000132084. [DOI] [PubMed] [Google Scholar]
- Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22:273–80. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- Riddoch M, Humphreys G. Birmingham Object Recognition Battery. Hove (UK): Lawrence Erlbaum; 1992. [Google Scholar]
- Sugishita M. Tokyo: Igakushoin; 1986. The Western Aphasia Battery [Japanese edition] [Google Scholar]
- Sugishita M. Tokyo: Nihonbunkakagakusha; 2001. Japanese Wechsler Memory Scale-Revised. [Google Scholar]
- Warrington EK, James M. Bury St Edmunds (UK): Thames Valley Test Company; 1991. Visual object and space perception battery. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.