Abstract
This study applied multiscale entropy analysis to investigate the correlation between the complexity of intracranial pressure waveform and outcome after traumatic brain injury. Intracranial pressure and arterial blood pressure waveforms were low-pass filtered to remove the respiratory and pulse components and then processed using a multiscale entropy algorithm to produce a complexity index. We identified significant differences across groups classified by the Glasgow Outcome Scale in intracranial pressure, pressure-reactivity index and complexity index of intracranial pressure (P < 0.0001; P = 0.001; P < 0.0001, respectively). Outcome was dichotomized as survival/death and also as favourable/unfavourable. The complexity index of intracranial pressure achieved the strongest statistical significance (F = 28.7; P < 0.0001 and F = 17.21; P < 0.0001, respectively) and was identified as a significant independent predictor of mortality and favourable outcome in a multivariable logistic regression model (P < 0.0001). The results of this study suggest that complexity of intracranial pressure assessed by multiscale entropy was significantly associated with outcome in patients with brain injury.
Keywords: complexity, intracranial pressure, multiscale entropy, traumatic brain injury, outcome
Introduction
A complex system is defined as one that is composed of many parts that interact in a non-linear fashion and give rise to emergent behaviour that cannot be understood through the analysis of its constituents. In the realm of physiology, we can define a complex biological system as one that is very sensitive to initial conditions and reacts adaptively to minute changes in its environment. Therefore, the complexity of such a system can be directly correlated to its ability to react to change and hence logically, when this ability is lost, we can postulate that the complexity of the system is also adversely affected.
Loss of complexity, or decomplexification, occurs during ageing or disease and may also be associated with poor outcome (Goldstein et al., 1998; Norris et al., 2008; Riordan et al., 2009). It has been shown, for example, that decomplexification of heart rate variability is statistically associated with the worse outcome of critically ill children and adults (Goldstein et al., 1998; Norris et al., 2008; Riordan et al., 2009). Recently, several studies have demonstrated that reduced complexity of intracranial pressure waveform occurs during intracranial hypertension in paediatric patients after head injury and during infusion study in patients with hydrocephalus (Hornero et al., 2006, 2007; Santamarta et al., 2010). However, the relationship between the decomplexification of intracranial pressure waveform and the outcome of these patients has not been determined.
Traditionally, linear methods have been adopted for analysis of physiological signals. However, despite their relative success, these methods suffer from inaccuracies due to the non-linear and non-stationary nature of biological systems. Therefore, new methods have been introduced to explore their non-linear dynamics. One of the widely explored methods, attractive due to its simplicity, is the analysis of entropy. It is a concept used to describe system randomness or unpredictability. Pincus (1991) proposed ‘approximate entropy’ as a measure of irregularity of time series of various origins. It quantifies the predictability of subsequent amplitude values of data series based on the knowledge of the previous ones; the less the predictability the larger the approximate entropy. However, these calculations classically require very long data sets and a bias may exist leading to overestimation of the time series regularity. Sample entropy addresses this problem to an extent (Richman and Moorman, 2000). We must keep in mind, however, that a higher entropy value only reflects an increase in the degree of randomness and not necessarily an increase in the complexity of the time series (white noise series, for example, have high entropy but in fact low complexity). In order to provide a more meaningful measure of complexity, a multiscale entropy analysis that calculates entropy over multiple time scales was proposed by Costa et al. (2002). Multiscale entropy has been successfully utilized to analyse several biosignals and to distinguish healthy status from pathological conditions. When applied to the EEG, multiscale entropy helps to characterize patients with a variety of neuropathological conditions including Alzheimer’s disease, schizophrenia, autism and medial temporal lobe epilepsy (Mizuno et al., 2010; Protzner et al., 2010; Takahashi et al., 2010; Catarino et al., 2011). Recently, multiscale entropy has also been used to analyse arterial blood pressure to detect dynamics of the autonomic nervous system’s reaction to postural change (Turianikova et al., 2011) and for abnormalities in patients with type I diabetes mellitus (Trunkvalterova et al., 2008).
As widely demonstrated in the literature, the intracranial pressure signal carries substantially more information in its waveforms than can be summarized by the mean intracranial pressure value (Czosnyka et al., 2007). Pressure reactivity index, a running correlation coefficient between slow fluctuations in arterial blood pressure and intracranial pressure, has been accepted by many clinical practitioners as reflecting cerebrovascular reactivity, a surrogate for cerebral blood flow autoregulation. The reason that such information can be extracted from intracranial pressure is ascribed to the complex interplay of two dynamic systems: CSF and cerebral blood circulation embedded in an elastic brain tissue encompassed by a rigid skull (Czosnyka et al., 1997a; Ursino and Lodi, 1997). These interacting systems dynamically regulate the homeostasis of intracranial pressure (Rosner and Becker, 1984). Therefore, alterations in intracranial pressure waveform characteristics may reflect the dysfunction of the regulatory systems and may be associated with a bad outcome (Balestreri et al., 2006; Hiler et al., 2006). Similarly, an increase in pressure reactivity index signifies impaired cerebrovascular pressure reactivity and has been shown to be associated with poor outcome in patients after traumatic brain injury (Czosnyka et al., 1997b; Hiler et al., 2006).
To date, however, no study has applied multiscale entropy to the analysis of intracranial pressure. Considering the non-linear character of mechanisms contributing to observed dynamics of the intracranial pressure signal (Marmarou et al., 1978; Hu et al., 2006) and the reported reduced entropy of intracranial pressure caused by intracranial hypertension (Hornero et al., 2006, 2007; Santamarta et al., 2010), we decided to apply multiscale entropy to study the complexity of intracranial pressure and its relationship to outcome in a large cohort of patients with traumatic brain injury.
Materials and methods
Patient eligibility
We retrospectively examined digital recordings from 325 prospective patients after severe head injury admitted to the Neurosciences Critical Care Unit, Addenbrooke’s Hospital, Cambridge, between 2002 and 2010. Patients with available recordings of intracranial pressure, arterial blood pressure, Glasgow Coma Scale and Glasgow Outcome Scale were eligible for analysis. All patients were sedated, mechanically ventilated and managed according to cerebral perfusion pressure oriented protocol during their stay in the Neurosciences Critical Care Unit (Menon, 1999). Therapeutic options including head elevation, sedation, hyperosmolar therapy, moderate hyperventilation, inotropics, CSF drainage (insertion of a catheter through a ventriculostomy into the anterior horn of one of the ventricles) and even decompressive craniectomy were adopted to keep cerebral perfusion pressure above 60–70 mm Hg and intracranial pressure below 20 mm Hg. Patients after craniectomy were not excluded from this study since even in those patients, despite lower mean intracranial pressure values, the waveforms’ make-up contributing to widely understood intracranial pressure dynamics still reflects their clinical course and correlates with outcome. It has been shown, for example, that after craniectomy, fast increases of intracranial pressure to moderately elevated values (15–20 mm Hg) are associated with worse outcome (Li et al., 2010). In general, physiological phenomena affecting intracranial pressure waveforms after craniectomy remain qualitatively unchanged. Therefore, excluding these patients from the analysis seemed unjustifiable, at least at this exploratory stage. Data collection and analysis were approved by the Neurocritical Care Users’ Committee and the Research Ethics Committee. The same database has been used for other studies from this research group (Sorrentino et al., 2011; Aries et al., 2012). However, assessment of the complexity of intracranial pressure as presented here has never been investigated in this data set.
Data acquisition and analysis
Arterial blood pressure was monitored predominantly from a radial artery using a standard pressure monitoring kit (Baxter Healthcare Corp.). Intracranial pressure was monitored using an intraparenchymal probe (Codman & Shurtleff, Inc.) in all patients, including those with an external drainage device. All signals were continuously sampled at a frequency from 30 to 200 Hz using ICM+ software (http://www.neurosurg.cam.ac.uk/icmplus). Time trends of mean intracranial pressure and other derived parameters were constructed with a rate of one sample every 10 s, thus suppressing pulse and respiratory waves and focusing entirely on the slow fluctuations of intracranial pressure. For calculation of pressure reactivity index, a moving Pearson correlation coefficient was obtained from changes of arterial blood pressure and intracranial pressure in a window of 30 consecutive 10-s time averages. Only segments of intracranial pressure, when cerebral perfusion pressure was ≥30 mm Hg, were included in order to avoid analysing data from dead brain.
Patient demographics
Seven patients in a low awareness state (vegetative or minimally conscious) were excluded from analysis because of the small number and greater heterogeneity compared with other outcome groups. A total of 290 patients were enrolled for analysis and the baseline characteristics divided into different Glasgow Outcome Scale groups are shown in Table 1. The average age was 38 years [standard deviation (SD) 16.5 years] with 78% male and 22% female. Moderate disability was observed in the youngest group (32.8 ± 13.9 years; 61.2% male) while the oldest (45.1 ± 17.2 years; 89% male) group tended more towards fatal outcome. The median admission Glasgow Coma Scale was 6 [interquartile range (IQR) 3–9], and unsurprisingly the group with a fatal outcome had the lowest median Glasgow Coma Scale (5; IQR 3–8) compared with the other groups. The proportion of patients with a Glasgow Coma Scale of ≤8 was 73.5% versus 78.9% in survival versus death outcome group and 68% versus 80.5% in favourable versus unfavourable outcome group. The median data length for analysis was 4 days (range 0.2–19.6 days) and there was no significant difference between groups in this respect.
Table 1.
Demographic data of 290 subjects
| Good outcome | Moderate disability | Severe disability | Dead | P-value | |
|---|---|---|---|---|---|
| n | 49 | 77 | 93 | 71 | |
| Age (years) | 34.2 ± 17.7 | 32.8 ± 13.9 | 39.9 ± 15.3 | 45.1 ± 17.2 | 0.00001 |
| Male (%) | 61.2 | 84.4 | 75.3 | 85.9 | 0.005 |
| GCS | 7 (4–10) | 8 (5–11) | 6 (3–8) | 5 (3–8) | 0.015 |
| Data length (days) | 4.3 ± 3.8 | 5.6 ± 4.4 | 5.0 ± 4.1 | 5.1 ± 4.1 | 0.348 |
Numerical data are expressed as mean ± SD and compared with one-way ANOVA. Categorical data are expressed as number (percentage) or median (IQR) and compared with chi-square test. GCS = Glasgow Coma Scale.
Multiscale entropy analysis
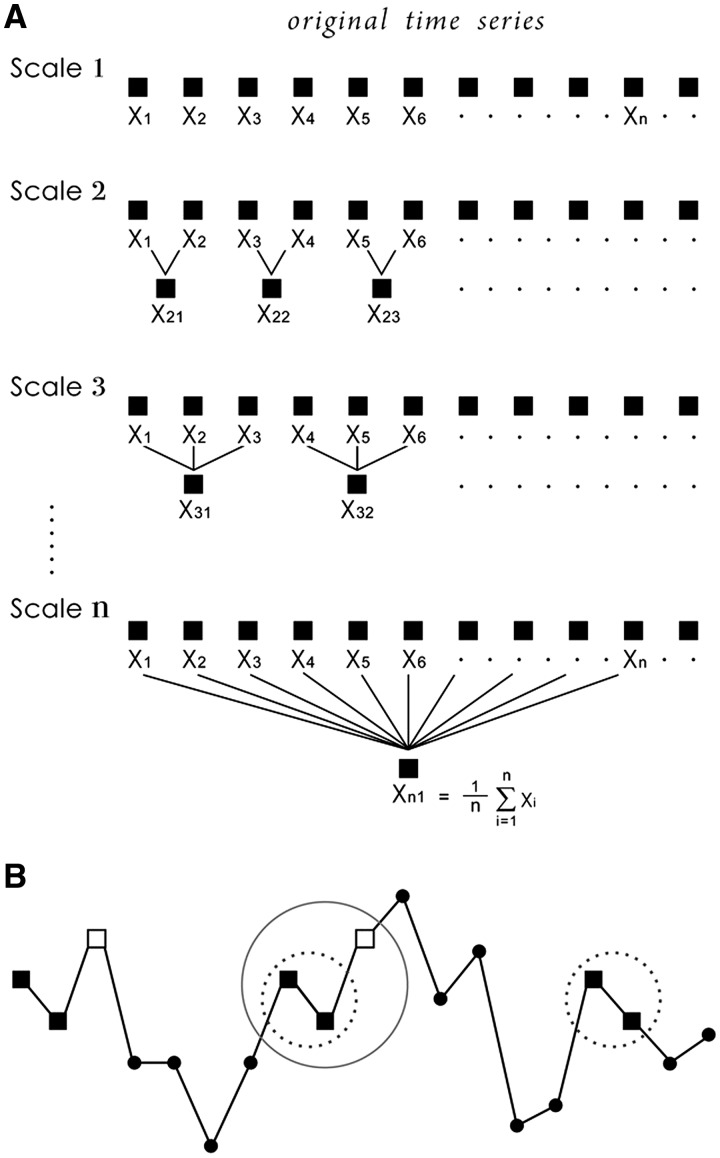
Multiscale entropy was calculated as described by Costa et al. (2002, 2005). Briefly, the multiscale entropy analysis constructs a set of coarse-grained time series constructed by replacing a progressively increasing number of data points in non-overlapping windows by their mean values. This procedure starts with the original times series, described as the Scale 1 series. The Scale 2 time series is made up of averages of consecutive pairs of data points, and so it is represented by half as many data points. Scale 3 uses averages of three data points and its length decreases 3-fold, and so on (Fig. 1A). In this study, we coarse-grained the original time series up to scale factor 20.
Figure 1.
The illustration of (A) the coarse graining procedure and (B) sample entropy. For length m = 2, two sequences (dotted circle) match the first two data points and one sequence (circle) matches the first three data points (length m + 1). This matching process is repeated for the next two data points and then all sequences to determine the total number of matches of length m and m + 1. Sample entropy is calculated as the negative natural logarithm of the ratio between the number of length m + 1 matches and the number of length m matches.
For each time series, a sample entropy analysis was performed as described in detail elsewhere (Richman and Moorman, 2000). In brief, sample entropy estimates the likelihood that two similar sequences of m consecutive data points (m = 2 in this study) will remain similar when the sequence length increases to m + 1 data points (Fig. 1B). It is computed as the negative natural logarithm of the ratio of number of m + 1 length patterns to the number of corresponding m length patterns. Therefore, higher sample entropy values signify higher irregularity of the time series. A multiscale entropy curve was drawn by plotting the sample entropy of each coarse-grained time series as a function of time scale. A single value named complexity index was obtained as the area under the multiscale entropy curve (Manor et al., 2010). The calculations were performed on a complete data set for each patient. In addition, episodes of plateau waves and refractory intracranial hypertension were analysed separately, using a complete intracranial pressure signal for multiscale entropy analysis. In order to deal with the problem of different sampling frequencies of recorded data, intracranial pressure signal was resampled to 100 Hz using band-limited interpolation.
Statistical analysis
The calculated complexity index and the patient-averaged values of derived parameters, including pressure reactivity index, obtained from the whole recording periods were used for statistical analysis. Outcome was assessed 6 months after head injury using the Glasgow Outcome Scale (Jennett and Bond, 1975). Outcome groups were further dichotomized into survival and death, as well as favourable and unfavourable outcome (good recovery and moderate disability versus severe disability, persistent vegetative state and fatal outcome). Statistical analysis was performed using SPSS 13.0 (IBM). Interval data were expressed as mean ± SD, or median (with IQR), and compared with one-way ANOVA or Kruskal–Wallis non-parametric test where appropriate. Categorical data were coded and compared with chi-square test. One-way ANOVA was used to compare the interval variables between the dichotomized outcome groups. A multiple logistic regression model was used to identify the independent predictors with the dichotomized outcome. Variables that failed to pass the tests for normal distribution were normalized before introduced into the logistic regression model. P < 0.05 was considered to represent a significant difference. To enhance robustness of the logistical regression model analysis, a 10-fold cross-validation approach was used.
Results
Observation of the complexity during increases in intracranial pressure
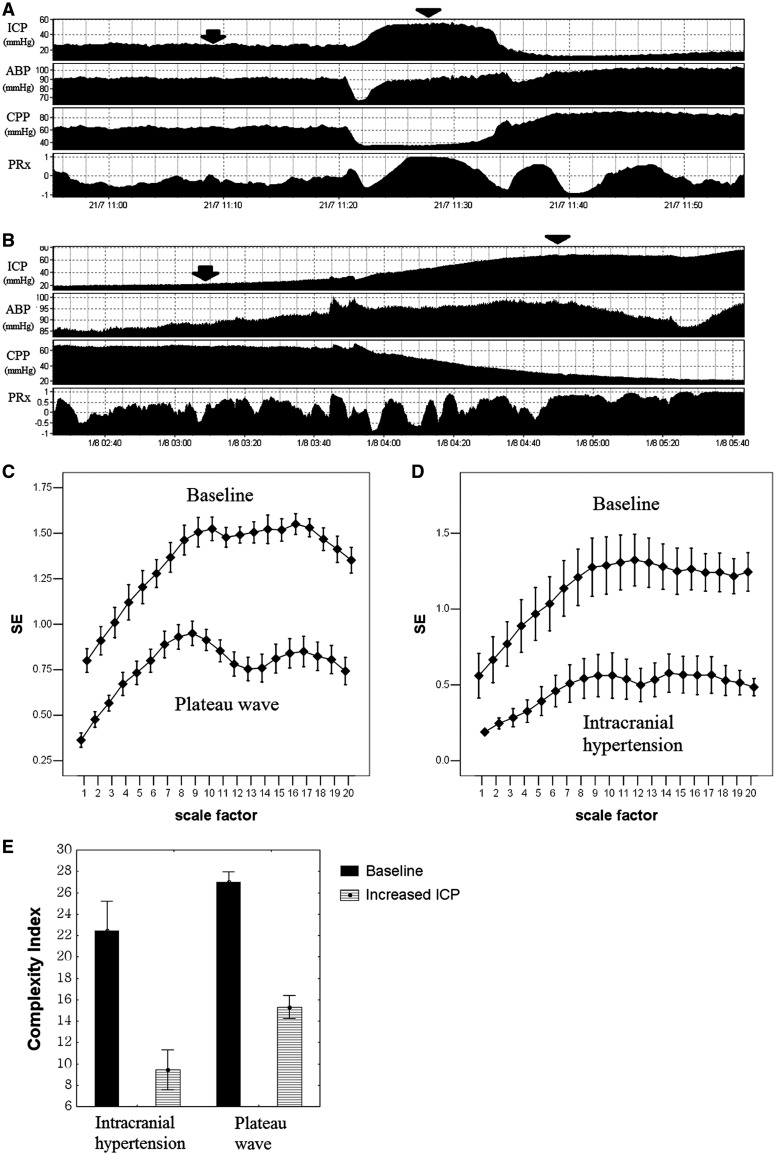
Figure 2 illustrates time course of intracranial pressure, arterial blood pressure, cerebral perfusion pressure and pressure reactivity index during an episode of a plateau wave of intracranial pressure and during an uncontrolled development of intracranial hypertension. In both cases, an abrupt increase in pressure reactivity index to values close to one associated with high level of intracranial pressure signified maximal cerebral vasodilatation and exhaustion of cerebrovascular reactivity. This was reflected in a decrease in entropy of intracranial pressure signal across all measured scales resulting in a decrease in overall complexity index during those periods. Ten episodes of plateau waves and seven cases of refractory intracranial hypertension were analysed (Fig. 2C–E).
Figure 2.
Time-related changes of intracranial pressure (ICP), arterial blood pressure (ABP), cerebral perfusion pressure (CPP) and pressure reactivity index (PRx) during (A) plateau wave and (B) refractory intracranial hypertension. Multiscale entropy (MSE) during baseline (arrow) and increased intracranial pressure (arrowhead) in (C) 10 episodes of plateau wave and (D) seven episodes of refractory intracranial hypertension. (E) Complexity index of intracranial pressure. Results are the mean ± SEM. SE = sample entropy.
Correlation of the complexity of intracranial pressure with outcome
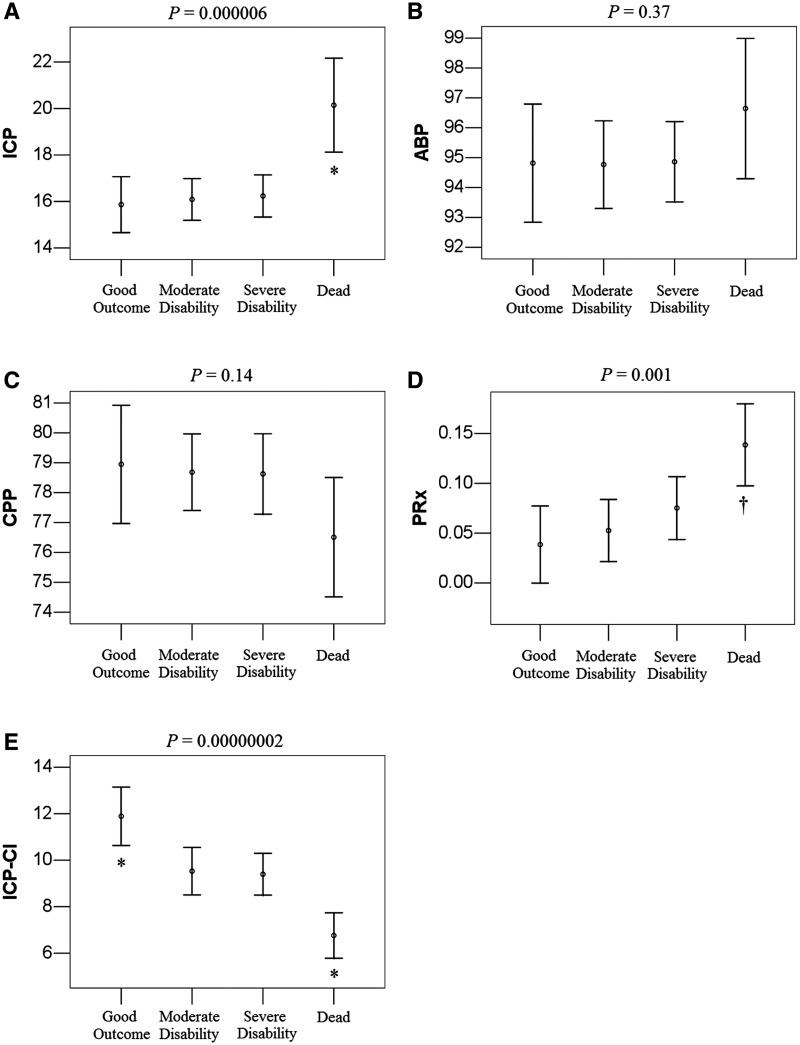
Figure 3 summarizes the studied parameters across different outcome groups. In a cohort analysis, intracranial pressure and pressure reactivity index were significantly higher in the fatal group (P < 0.0001 and P < 0.001, respectively) and there was no significant difference in arterial blood pressure and cerebral perfusion pressure between groups. When multiscale entropy was used to assess the complexity of intracranial pressure, the complexity index of intracranial pressure was the highest in the good recovery group (P < 0.0001). Table 2 shows the results of different variables compared by dichotomized outcome group using one-way ANOVA. A higher F-value indicates better differentiation of a variable across different outcome groups. The F-value was the highest for complexity index of intracranial pressure irrespective of the division of the outcome groups into death/survival or unfavourable/favourable (F = 28.7; P < 0.0001 and F = 17.21; P < 0.0001, respectively).
Figure 3.
Calculated parameters in different Glasgow Outcome Scale (GOS) groups. (A) Intracranial pressure (ICP), (B) arterial blood pressure (ABP), (C) cerebral perfusion pressure (CPP), (D) pressure reactivity index (PRx) and (E) complexity index of intracranial pressure (ICP-CI). Results represent the mean and 95% confidence intervals. Symbols express the difference between groups in one-way ANOVA followed by Bonferroni post hoc analysis with a confidence level of P < 0.05. *Group differed from three other groups. †Dead differed from good outcome and moderate disability.
Table 2.
Differences in intracranial pressure, pressure reactivity index and complexity index related to outcome using ANOVA
| Survival versus death |
Favourable versus unfavourable |
|||
|---|---|---|---|---|
| F | P | F | P | |
| ICP | 28.31 | 0.0000002 | 7.89 | 0.005 |
| PRx | 14.72 | 0.0002 | 9.44 | 0.002 |
| ICP-CI | 28.7 | 0.0000002 | 17.21 | 0.00004 |
ICP = intracranial pressure; ICP-CI = complexity index of ICP; PRx = pressure reactivity index.
Our results showed that the complexity index of intracranial pressure in patients with good outcome (11.9 ± 4.4) was significantly larger than those with moderate disability and severe disability (9.5 ± 4.5, P = 0.02; 9.4 ± 4.4, P = 0.008; one-way ANOVA followed by Bonferroni post hoc analysis). This suggests that patients with a higher complexity of intracranial pressure tend to have a good outcome due to their better dynamics in response to varying stimuli. The patients included in this study were admitted between 2002 and 2010 and managed according to the tiered head injury protocol (Menon, 1999). We did not see any significant difference in association between the complexity index and outcome in patients admitted during the early phases of the study versus those admitted in the later years.
The complexity of intracranial pressure is an independent predictor of outcome
Multivariable logistic regression identified age, Glasgow Coma Scale, intracranial pressure and pressure reactivity index to be significantly associated with mortality (P < 0.0001; P = 0.01; P < 0.0001; P = 0.006, respectively) when complexity index of intracranial pressure was excluded from analysis (Table 3). However, when complexity index of intracranial pressure was included in the model, complexity index of intracranial pressure was identified as a significant predictor (P < 0.0001), while pressure reactivity index became statistically insignificant (P = 0.23) and others remained significant independent predictors (Table 3). Similarly, in the multivariable logistic regression to identify the independent predictors of the favourable outcome (Table 4), the inclusion of complexity index of intracranial pressure (P < 0.0001) converted pressure reactivity index into an insignificant predictor (P = 0.31).
Table 3.
Multivariable logistic regression for factors associated with mortality in patients after traumatic brain injury
| Without ICP-CI |
With ICP-CI |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age | 1.04 | 1.02–1.07 | 0.00002 | 1.06 | 1.03–1.08 | 0.000002 |
| GCS > 8 | 0.37 | 0.17–0.80 | 0.01 | 0.37 | 0.17–0.83 | 0.02 |
| ICP | 1.18 | 1.10–1.25 | 0.0000006 | 1.18 | 1.10–1.27 | 0.000004 |
| PRxa | 17.58 | 2.28–135.82 | 0.006 | 4.07 | 0.41–44.04 | 0.23 |
| ICP-CIb | NA | NA | NA | 0.16 | 0.08–0.32 | 0.0000003 |
GCS = Glasgow Coma Scale; ICP = intracranial pressure; ICP-CI = complexity index of ICP; NA = not applicable; OR = odds ratio; PRx = pressure reactivity index.
a Fisher transformation was used to normalize the distribution of PRx.
b Logarithmic transformation was used to normalize the distribution of ICP-CI.
Table 4.
Multivariable logistic regression for factors associated with favourable outcome in patients after traumatic brain injury
| Without ICP-CI |
With ICP-CI |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age | 0.96 | 0.94–0.98 | 0.000002 | 0.95 | 0.94–0.97 | 0.0000006 |
| GCS > 8 | 3.44 | 1.82–6.50 | 0.0002 | 3.85 | 2.00–7.39 | 0.00005 |
| ICP | 0.9 | 0.86–0.95 | 0.0003 | 0.91 | 0.86–0.93 | 0.001 |
| PRxa | 0.14 | 0.02–0.86 | 0.03 | 0.37 | 0.05–2.58 | 0.31 |
| ICP-CIb | NA | NA | NA | 3.02 | 1.74–5.27 | 0.00009 |
GCS = Glasgow Coma Scale; ICP = intracranial pressure; ICP-CI = complexity index of ICP; NA = not applicable; OR = odds ratio; PRx = pressure reactivity index.
a Fisher transformation was used to normalize the distribution of PRx.
b Logarithmic transformation was used to normalize the distribution of ICP-CI.
Ten-fold cross-validation process confirmed the significance of intracranial pressure and its complexity for prediction of outcome. When intracranial pressure was excluded and complexity index of intracranial pressure was included in the model of predicting mortality, the accuracy, sensitivity and specificity were 0.80 ± 0.06, 0.33 ± 0.18 and 0.95 ± 0.07, respectively. When intracranial pressure was included and the complexity index of intracranial pressure was excluded from the model, the averaged accuracy, sensitivity and specificity were 0.79 ± 0.09, 0.24 ± 0.20 and 0.96 ± 0.05, respectively. When both intracranial pressure and complexity index of intracranial pressure were included in the model, the averaged accuracy, sensitivity and specificity were 0.82 ± 0.07, 0.50 ± 0.19 and 0.94 ± 0.06, respectively. Analysis based on favourable/unfavourable outcome dichotomy gave similar results, thus confirming the significance of our model predictors.
Correlation of the complexity of intracranial pressure with intracranial pressure, cerebral perfusion pressure and pressure reactivity index
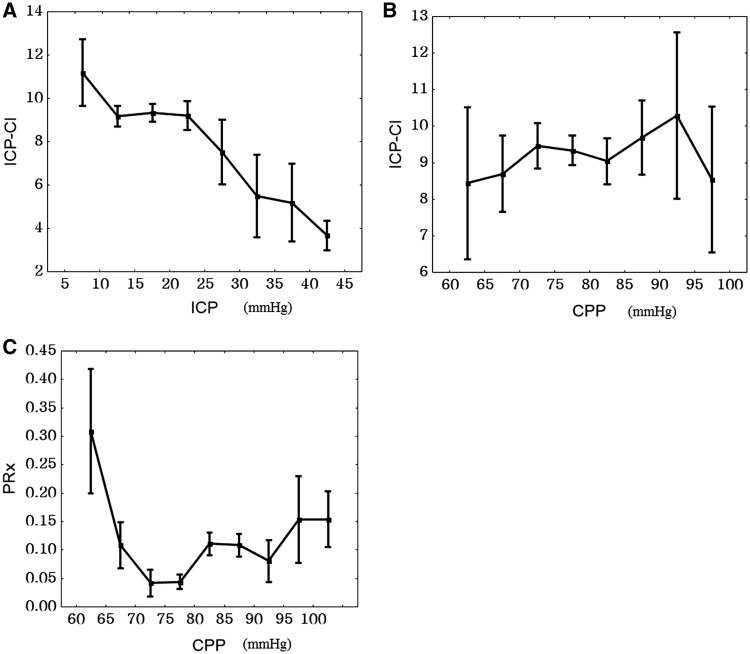
When data from all patients were pooled, complexity index of intracranial pressure showed an inverse relationship with intracranial pressure (Fig. 4A). The relationship with cerebral perfusion pressure was less apparent and showed a convex shape (Fig. 4B) as opposed to the concave relationship between pressure reactivity index and cerebral perfusion pressure (Fig. 4C). Overall, the complexity index of intracranial pressure correlated weakly with intracranial pressure and pressure reactivity index (R = −0.13; P = 0.02 and R = −0.25; P < 0.0001, respectively) indicating lower intracranial pressure complexity during intracranial hypertension and disturbed vascular control.
Figure 4.
Empirical regression plots for all patients. (A) Complexity index of intracranial pressure (ICP-CI) versus mean intracranial pressure (ICP). (B) Complexity index of intracranial pressure versus cerebral perfusion pressure (CPP). (C) Pressure-reactivity index (PRx) versus cerebral perfusion pressure. Results are mean ± SEM.
Discussion
The results of the cohort analysis showed that a significant relationship exists between the complexity of mean intracranial pressure fluctuations and outcome after severe head injury. The complexity of mean intracranial pressure waveform was the highest in patients with good recovery and lowest in patients with fatal outcomes. This finding supports the theory that critical illness can lead to the decomplexification of dynamics of normal physiology (Buchman, 2004). Using multiscale entropy analysis, we illustrated that the complexity index of the intracranial pressure signal was reduced during episodes of plateau waves and intracranial hypertension and this is consistent with the findings of previous studies (Hornero et al., 2005, 2006, 2007). However, entropy/complexity analysis of the complete intracranial pressure signal mainly reflects ‘irregularity’ of the pulse morphology. It is not well suited to probe slower process, originating in or modulated by the cerebrovascular regulation system.
The frequency domain analysis (Lundberg, 1960) divides intracranial pressure waveforms into three components: the first and fastest component comes from cardiac pulse wave, the second component is associated with respiration and the lowest frequency component forms the so-called slow waves. The 10-s long moving average filter used in this study effectively removed the effects of cardiac and respiratory subsystems. As a result, the complexity index was generated exclusively from the analysis of variations within slow waves. These have a frequency range between 0.05 and 0.008 Hz and are less well understood, although a number of underlying mechanisms have been suggested (Lundberg, 1960; Auer and Sayama, 1983). The slow waves are often classified as ‘A’ waves (plateau waves), ‘B’ waves and ‘M’ waves (Mayer waves). This classification is based on a visual description of different morphological and temporal patterns of the slow waves. These slow waves are thought to be vasogenic and largely driven by cerebrovascular haemodynamic control systems. Their presence may or may not be associated with pathological processes. Plateau waves, and often B waves, for example, are associated with depleted brain compliance. However, the occurrence of plateau waves is not directly linked to poorer outcome in patients with head injury (Castellani et al., 2009) and B waves have also been observed in healthy subjects (Lemaire et al., 1994). On the other hand, reduced activity of slow waves has been shown to be associated with poor prognosis after traumatic brain injury (Balestreri et al., 2005). Our results showed decreased complexity of intracranial pressure is associated with poorer outcome, thus it is not the occurrence of slow waves that predicts worse outcome but their increased regularity (which is not the same as increased intensity).
In concordance with previous research (Eide et al., 2007), the pressure reactivity index was identified as an independent predictor of outcome. However, when the complexity index of intracranial pressure was also included in the regression model, pressure reactivity index became statistically insignificant. This suggests that loss of autoregulation is partially responsible for decomplexification of intracranial pressure fluctuations. This is further reflected in the weak but significant direct correlation between the complexity index of intracranial pressure and pressure reactivity index. Higher predictive power achieved by the complexity index may suggest that it reflects development of other patho-physiological processes in addition to those responsible for failure of cerebrovascular reactivity.
Other studies have previously looked into the regularity/chaotic nature of the intracranial pressure signal. A study by Burr et al. (2008) in a group of 147 patients with traumatic brain injury analysed intracranial pressure slow wave time series complexity by looking at their fractal nature. Using detrended fluctuation analysis of intracranial pressure time series, they were able to demonstrate, similar to this study, its significant predictive power for outcome. However, the fractal nature of a time series can only be studied when examining its fluctuations over several levels of magnitude of time scale. In practice, this means that in order to obtain meaningful results, data spans of up to several days should be used for analysis. Furthermore, fractal methods such as detrended fluctuation analysis require the underlying process to possess a fractal nature and, more precisely, a mono-fractal characteristic. If this assumption is not valid, the results could be uninterpretable. The crux of the matter lies in the fact that the nature of intracranial pressure slow waves is not known and therefore the above cannot be assumed. In contrast, the multiscale entropy method should give reliable results from a span of several hours and does not make any assumptions about the nature of the time series analysed. Thus, the multiscale entropy-based complexity index appears to be a practical and readily interpretable measure of irregularity of slow waves with a potential for clinical use. Further cross-validation studies, however, are required for complexity index of intracranial pressure. However, one potential drawback of using the complexity index of intracranial pressure in a clinical environment is the fact that the complexity index of intracranial pressure does not have a direct physiological interpretation.
Limitations of the study
Some limitations should be addressed in this study. First, in this retrospective study we cannot investigate the influence of treatments (including CSF drainage) or medications on the complexity of intracranial pressure although therapeutic management was standardized (Menon, 1999). Therefore, further prospective studies should be conducted to examine the individual impact of different treatments on the complexity of intracranial pressure.
Second, the multiscale entropy technique requires substantially more samples to provide a reliable estimate value than single scale sample entropy. For analysis over 20 scales, it has been estimated that at least 2000 data samples are required (Angelini et al., 2007). As the analysed intracranial pressure time series were ultimately sampled at periods of 10 s, this means that nearly 6 h of data are required to provide a meaningful value of the complexity index; making clinical application of the technique more difficult. In addition, multiscale entropy, as its building block is sample entropy, assumes stationarity. If this assumption is invalid, which is likely to be the case for prolonged time periods, the sample entropy estimation becomes less reliable (its variance increases). However, by taking into account more temporal scales, multiscale entropy attenuates the effects of this problem. Perhaps an improved multiscale entropy methodology using adaptive approaches, similar to the one applied by Hu et al. (2008), would help to reduce these effects even further.
Conclusion
We have demonstrated that reduced complexity of mean intracranial pressure is associated with poor outcome in patients after traumatic brain injury. A prospective study is necessary to verify the role of complexity in the management of patients with traumatic brain injury.
Funding
This work was supported by the National Institute of Health Research, Biomedical Research Centre (Neuroscience Theme), the Medical Research Council (grants G0600986 and G9439390), NIHR Senior Investigator Awards (J.D.P.). This research was also supported by the Center for Dynamical Biomarkers and Translational Medicine which is sponsored by National Science Council (grant number: NSC 100-2911-I-008-001).
Conflict of interest
The software for brain monitoring ICM+ is licensed by the University of Cambridge (Cambridge Enterprise Ltd.). P.S. and M.C. have a financial interest in a part of the licensing fee.
Acknowledgements
The authors would like to thank the visiting scholars and PhD students of Brain Physics Laboratory and Neurocritical Care staff, who contributed to prospective data collection during 2002–10.
References
- Angelini L, Maestri R, Marinazzo D, Nitti L, Pellicoro M, Pinna GD, et al. Multiscale analysis of short term heart beat interval, arterial blood pressure, and instantaneous lung volume time series. Artif Intell Med. 2007;41:237–50. doi: 10.1016/j.artmed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Aries MJH, Czosnyka M, Budohoski K, Steiner L, Lavinio A, Kolias A, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012 doi: 10.1097/CCM.0b013e3182514eb6. Advance Access published on May 2, 2012, doi: 10.1097/CCM.0b013e3182514eb6. [DOI] [PubMed] [Google Scholar]
- Auer LM, Sayama I. Intracranial pressure oscillations (B-waves) caused by oscillations in cerebrovascular volume. Acta Neurochir (Wien) 1983;68:93–100. doi: 10.1007/BF01406205. [DOI] [PubMed] [Google Scholar]
- Balestreri M, Czosnyka M, Hutchinson P, Steiner LA, Hiler M, Smielewski P, et al. Impact of intracranial pressure and cerebral perfusion pressure on severe disability and mortality after head injury. Neurocrit Care. 2006;4:8–13. doi: 10.1385/NCC:4:1:008. [DOI] [PubMed] [Google Scholar]
- Balestreri M, Czosnyka M, Steiner LA, Hiler M, Schmidt EA, Matta B, et al. Association between outcome, cerebral pressure reactivity and slow ICP waves following head injury. Acta Neurochir Suppl. 2005;95:25–8. doi: 10.1007/3-211-32318-x_6. [DOI] [PubMed] [Google Scholar]
- Buchman TG. Nonlinear dynamics, complex systems, and the pathobiology of critical illness. Curr Opin Crit Care. 2004;10:378–82. doi: 10.1097/01.ccx.0000139369.65817.b6. [DOI] [PubMed] [Google Scholar]
- Burr RL, Kirkness CJ, Mitchell PH. Detrended fluctuation analysis of intracranial pressure predicts outcome following traumatic brain injury. IEEE Trans Biomed Eng. 2008;55:2509–18. doi: 10.1109/TBME.2008.2001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani G, Zweifel C, Kim DJ, Carrera E, Radolovich DK, Smielewski P, et al. Plateau waves in head injured patients requiring neurocritical care. Neurocrit Care. 2009;11:143–50. doi: 10.1007/s12028-009-9235-7. [DOI] [PubMed] [Google Scholar]
- Catarino A, Churches O, Baron-Cohen S, Andrade A, Ring H. Atypical EEG complexity in autism spectrum conditions: a multiscale entropy analysis. Clin Neurophysiol. 2011;122:2375–83. doi: 10.1016/j.clinph.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett. 2002;89:068102. doi: 10.1103/PhysRevLett.89.068102. [DOI] [PubMed] [Google Scholar]
- Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71(2 Pt 1):021906. doi: 10.1103/PhysRevE.71.021906. [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Piechnik S, Richards HK, Kirkpatrick P, Smielewski P, Pickard JD. Contribution of mathematical modelling to the interpretation of bedside tests of cerebrovascular autoregulation. J Neurol Neurosurg Psychiatry. 1997a;63:721–31. doi: 10.1136/jnnp.63.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997b;41:11–7. doi: 10.1097/00006123-199707000-00005. discussion 17–9. [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Smielewski P, Timofeev I, Lavinio A, Guazzo E, Hutchinson P, et al. Intracranial pressure: more than a number. Neurosurg Focus. 2007;22:E10. doi: 10.3171/foc.2007.22.5.11. [DOI] [PubMed] [Google Scholar]
- Eide PK, Czosnyka M, Sorteberg W, Pickard JD, Smielewski P. Association between intracranial, arterial pulse pressure amplitudes and cerebral autoregulation in head injury patients. Neurol Res. 2007;29:578–82. doi: 10.1179/016164107X172167. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Fiser DH, Kelly MM, Mickelsen D, Ruttimann U, Pollack MM. Decomplexification in critical illness and injury: relationship between heart rate variability, severity of illness, and outcome. Crit Care Med. 1998;26:352–7. doi: 10.1097/00003246-199802000-00040. [DOI] [PubMed] [Google Scholar]
- Hiler M, Czosnyka M, Hutchinson P, Balestreri M, Smielewski P, Matta B, et al. Predictive value of initial computerized tomography scan, intracranial pressure, and state of autoregulation in patients with traumatic brain injury. J Neurosurg. 2006;104:731–7. doi: 10.3171/jns.2006.104.5.731. [DOI] [PubMed] [Google Scholar]
- Hornero R, Aboy M, Abasolo D. Analysis of intracranial pressure during acute intracranial hypertension using Lempel-Ziv complexity: further evidence. Med Biol Eng Comput. 2007;45:617–20. doi: 10.1007/s11517-007-0194-x. [DOI] [PubMed] [Google Scholar]
- Hornero R, Aboy M, Abasolo D, McNames J, Goldstein B. Interpretation of approximate entropy: analysis of intracranial pressure approximate entropy during acute intracranial hypertension. IEEE Trans Biomed Eng. 2005;52:1671–80. doi: 10.1109/TBME.2005.855722. [DOI] [PubMed] [Google Scholar]
- Hornero R, Aboy M, Abasolo D, McNames J, Wakeland W, Goldstein B. Complex analysis of intracranial hypertension using approximate entropy. Crit Care Med. 2006;34:87–95. doi: 10.1097/01.ccm.0000190426.44782.f0. [DOI] [PubMed] [Google Scholar]
- Hu X, Miller C, Vespa P, Bergsneider M. Adaptive computation of approximate entropy and its application in integrative analysis of irregularity of heart rate variability and intracranial pressure signals. Med Eng Phys. 2008;30:631–9. doi: 10.1016/j.medengphy.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Nenov V, Glenn TC, Steiner LA, Czosnyka M, Bergsneider M, et al. Nonlinear analysis of cerebral hemodynamic and intracranial pressure signals for characterization of autoregulation. IEEE Trans Biomed Eng. 2006;53:195–209. doi: 10.1109/TBME.2005.862546. [DOI] [PubMed] [Google Scholar]
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–4. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- Lemaire JJ, Boire JY, Chazal J, Irthum B. A computer software for frequential analysis of slow intracranial pressure waves. Comput Methods Programs Biomed. 1994;42:1–14. doi: 10.1016/0169-2607(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Li LM, Timofeev I, Czosnyka M, Hutchinson PJ. The surgical approach to the management of increased intracranial pressure after traumatic brain injury. Anesth Analg. 2010;111:736–48. doi: 10.1213/ANE.0b013e3181e75cd1. [DOI] [PubMed] [Google Scholar]
- Lundberg N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr Scand Suppl. 1960;36:1–193. [PubMed] [Google Scholar]
- Manor B, Costa MD, Hu K, Newton E, Starobinets O, Kang HG, et al. Physiological complexity and system adaptability: evidence from postural control dynamics of older adults. J Appl Physiol. 2010;109:1786–91. doi: 10.1152/japplphysiol.00390.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmarou A, Shulman K, Rosende RM. A nonlinear analysis of the cerebrospinal fluid system and intracranial pressure dynamics. J Neurosurg. 1978;48:332–44. doi: 10.3171/jns.1978.48.3.0332. [DOI] [PubMed] [Google Scholar]
- Menon DK. Cerebral protection in severe brain injury: physiological determinants of outcome and their optimisation. Br Med Bull. 1999;55:226–58. doi: 10.1258/0007142991902231. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Takahashi T, Cho RY, Kikuchi M, Murata T, Takahashi K, et al. Assessment of EEG dynamical complexity in Alzheimer’s disease using multiscale entropy. Clin Neurophysiol. 2010;121:1438–46. doi: 10.1016/j.clinph.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris PR, Anderson SM, Jenkins JM, Williams AE, Morris JA., Jr Heart rate multiscale entropy at three hours predicts hospital mortality in 3,154 trauma patients. Shock. 2008;30:17–22. doi: 10.1097/SHK.0b013e318164e4d0. [DOI] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA. 1991;88:2297–301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protzner AB, Valiante TA, Kovacevic N, McCormick C, McAndrews MP. Hippocampal signal complexity in mesial temporal lobe epilepsy: a noisy brain is a healthy brain. Arch Ital Biol. 2010;148:289–97. [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278:H2039–49. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Riordan WP, Jr., Norris PR, Jenkins JM, Morris JA., Jr Early loss of heart rate complexity predicts mortality regardless of mechanism, anatomic location, or severity of injury in 2178 trauma patients. J Surg Res. 2009;156:283–9. doi: 10.1016/j.jss.2009.03.086. [DOI] [PubMed] [Google Scholar]
- Rosner MJ, Becker DP. Origin and evolution of plateau waves. Experimental observations and a theoretical model. J Neurosurg. 1984;60:312–24. doi: 10.3171/jns.1984.60.2.0312. [DOI] [PubMed] [Google Scholar]
- Santamarta D, Hornero R, Abasolo D, Martinez-Madrigal M, Fernandez J, Garcia-Cosamalon J. Complexity analysis of the cerebrospinal fluid pulse waveform during infusion studies. Childs Nerv Syst. 2010;26:1683–9. doi: 10.1007/s00381-010-1244-5. [DOI] [PubMed] [Google Scholar]
- Sorrentino E, Budohoski KP, Kasprowicz M, Smielewski P, Matta B, Pickard JD, et al. Critical thresholds for transcranial Doppler indices of cerebral autoregulation in traumatic brain injury. Neurocrit Care. 2011;14:188–93. doi: 10.1007/s12028-010-9492-5. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Cho RY, Mizuno T, Kikuchi M, Murata T, Takahashi K, et al. Antipsychotics reverse abnormal EEG complexity in drug-naive schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51:173–82. doi: 10.1016/j.neuroimage.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trunkvalterova Z, Javorka M, Tonhajzerova I, Javorkova J, Lazarova Z, Javorka K, et al. Reduced short-term complexity of heart rate and blood pressure dynamics in patients with diabetes mellitus type 1: multiscale entropy analysis. Physiol Meas. 2008;29:817–28. doi: 10.1088/0967-3334/29/7/010. [DOI] [PubMed] [Google Scholar]
- Turianikova Z, Javorka K, Baumert M, Calkovska A, Javorka M. The effect of orthostatic stress on multiscale entropy of heart rate and blood pressure. Physiol Meas. 2011;32:1425–37. doi: 10.1088/0967-3334/32/9/006. [DOI] [PubMed] [Google Scholar]
- Ursino M, Lodi CA. A simple mathematical model of the interaction between intracranial pressure and cerebral hemodynamics. J Appl Physiol. 1997;82:1256–69. doi: 10.1152/jappl.1997.82.4.1256. [DOI] [PubMed] [Google Scholar]