Abstract
Mutations in the ACTA2 gene lead to diffuse and diverse vascular diseases; the Arg179His mutation is associated with an early onset severe phenotype due to global smooth muscle dysfunction. Cerebrovascular disease associated with ACTA2 mutations has been likened to moyamoya disease, but appears to have distinctive features. This study involved the analysis of neuroimaging of 13 patients with heterozygous missense mutations in ACTA2 disrupting Arg179. All patients had persistent ductus arteriosus and congenital mydriasis, and variable presentation of pulmonary hypertension, bladder and gastrointestinal problems associated with this mutation. Distinctive cerebrovascular features were dilatation of proximal internal carotid artery, occlusive disease of terminal internal carotid artery, an abnormally straight course of intracranial arteries, and absent basal ‘moyamoya’ collaterals. Patterns of brain injury supported both large and small vessel disease. Key differences from moyamoya disease were more widespread arteriopathy, the combination of arterial ectasia and stenosis and, importantly, absence of the typical basal ‘moyamoya’ collaterals. Evaluation of previously published cases suggests some of these features are also seen in the ACTA2 mutations disrupting Arg258. The observation that transition from dilated to normal/stenotic arterial calibre coincides with where the internal carotid artery changes from an elastic to muscular artery supports the hypothesis that abnormal smooth muscle cell proliferation caused by ACTA2 mutations is modulated by arterial wall components. Patients with persistent ductus arteriosus or congenital mydriasis with a label of ‘moyamoya’ should be re-evaluated to ensure the distinctive neuroimaging features of an ACTA2 mutation have not been overlooked. This diagnosis has prognostic and genetic implications, and mandates surveillance of other organ systems, in particular the aorta, to prevent life-threatening aortic dissection.
Keywords: ACTA2, moyamoya, stroke, child
Introduction
There is increasing appreciation of the adverse health and economic impacts of arterial ischaemic stroke in children (Perkins et al., 2009). Most cases are associated with abnormalities of the cerebral circulation, termed arteriopathies, currently distinguished on the basis of radiological features (Ganesan et al., 2003; Amlie-Lefond et al., 2009). The morphology and evolution of cerebral arteriopathy is currently the most robust predictor of arterial ischaemic stroke recurrence risk (Ganesan et al., 2006; Fullerton et al., 2007), and thus its characterization, and distinguishing between different radiological phenotypes, is clinically important. In particular, the specific ‘moyamoya’ pattern of arteriopathy, characterized by bilateral occlusive disease of the terminal internal carotid or proximal middle cerebral arteries and basal collaterals (Fukui, 1997), is especially ominous as it is associated with a high rate of stroke recurrence (Ganesan et al., 2006).
Heterozygous missense mutations in ACTA2 cause diffuse and diverse vascular diseases, including thoracic aortic aneurysms, aortic dissections, early onset coronary artery disease, stroke and moyamoya disease (Guo et al., 2007, 2009). However, ACTA2 mutations are not a major genetic cause of moyamoya disease in Japanese or European patients (Shimojima and Yamamoto 2009; Roder et al., 2011). Recently, a novel de novo mutation in ACTA2 (p.Arg179His) was reported in association with a unique syndrome characterized by aortic and cerebrovascular disease, persistent ductus arteriosus, congenital fixed dilated pupils and dysfunction of organs dependent on smooth muscle function, including the bladder and gut (Milewicz et al., 2010a). Cerebrovascular features include dolichoectasia of the internal carotid arteries and more distal occlusive arterial disease, described as ‘moyamoya’. However, having made the diagnosis on the basis of distinctive neuroimaging features in a child subsequently shown to have the Arg179His mutation, we were interested to investigate whether cerebrovascular disease in all these patients had distinctive features. Here we describe this neuroimaging phenotype in detail, highlight the differences compared with moyamoya disease, and emphasize clinical features that should lead to consideration of this diagnosis. Finally, the angiographic features shed light on the pathological mechanisms underlying the cerebrovascular disease associated with ACTA2 mutations, with future treatment implications.
Materials and methods
This study comprises analysis of the neuroimaging features of 13 unrelated patients with heterozygous mutations in ACTA2 disrupting Arg179. Patients with either an Arg179His mutation (n = 12) or Arg179Leu mutation (n = 1) were identified via D.M.M.’s laboratory (n = 7) or referrals to V.G.’s paediatric cerebrovascular service (n = 6). Anonymized clinical and radiological data were analysed with appropriate human subjects research approval. Seven patients (Patients 1–6 and Patient 11, Table 1) have been included in previous publications focusing on clinical, rather than neuroimaging, features (Milewicz et al., 2010a; Richer et al., 2012). For the purposes of this study the primary radiological material was reviewed and analysed afresh. DNA sequencing of the ACTA2 gene was performed using previously described sequencing methods in a research or clinical diagnostic laboratory (Milewicz et al., 2010a).
Table 1.
Demographics, neurological presentation and neuroimaging findings
| Age/gender | Ethnicity | Neurological presentation | Brain imaging | Transition from dilated to stenosed or normal arterial calibre; arterial course |
|---|---|---|---|---|
| (1) 11 years/female (Patient A in Milewicz et al., 2010a) | White | Recurrent left hemiparesis; headaches | Focal infarction, white matter signal change, generalized volume loss | Cavernous internal carotid artery; straight arterial course |
| (2) 14 years/male (Patient B in Milewicz et al., 2010a) | White | Left hemiparesis. Headaches with tinnitus | White matter signal change | Laceral segment internal carotid artery; straight arterial course |
| (3) 17 years/male (Patient C in Milewicz et al., 2010a) | White | Global developmental delay | White matter signal change, dysplatic corpus callosum | Laceral segment internal carotid artery; straight arterial course |
| (4) 11 years/female (Patient D in Milewicz et al., 2010a) | White | Episodic confusion and vertigo | White matter signal change, prominent perivascular spaces | Cavernous internal carotid artery; straight arterial course |
| (5) 26 years/female (Patient E in Milewicz et al., 2010a) | White | Right hemisphere arterial ischaemic stroke | Focal arterial infarct, white matter signal change | Cavernous internal carotid artery; straight arterial course |
| (6) 27 years/female (2nd addendum patient in Milewicz et al., 2010a)a | White | Transient unilateral vision loss and weakness | Unavailable | Cavernous internal carotid artery; normal arterial course |
| (7) 3 years/female | White | Acute right hemiparesis; recurrent transient ischaemic attacks | Focal arterial infarct, white matter signal change, calcification in region of previous infarct | Laceral segment internal carotid artery; straight arterial course |
| (8) 6 years/femalea | Mixed (White and Black African) | Acute right hemiparesis; recurrent transient ischaemic attacks | Focal arterial infarct, white matter signal change | Cavernous internal carotid artery; straight arterial course |
| (9) 5 years/maleb | White | Headaches | White matter signal change | Cavernous internal carotid artery; straight arterial course |
| (10) 2 years/female | Pakistani | Acute right hemiparesis | Focal infarction, white matter signal change, generalized volume loss | Laceral segment internal carotid artery; straight arterial course |
| (11) 2 years/male (included in Richer et al., 2012)a | Pakistani | Nil (brain imaging undertaken after genetic diagnosis; presented with bladder, persistent ductus arteriosus and pupillary features) | White matter signal change | Cavernous internal carotid artery; straight arterial course |
| (12) 6 years/male | White | Nil (brain imaged after genetic diagnosis; presented with persistent ductus arteriosus and pupillary features) | White matter signal change | Terminal internal carotid artery; normal arterial course |
| (13) 3 months/female | White | No acute neurological events; gross motor delay | Normal | Cavernous internal carotid artery; straight arterial course |
a Both parents of Patients 8 and 11 and the deceased father of Patient 6 did not undergo genetic testing but had no clinical features of ACTA2 mutation. All other parents were negative for ACTA2 mutations.
b Patient 9 had an R179L mutation.
Brain imaging (CT or MRI) was reviewed by D.E.S. and T.C.C. in order to describe the distribution of brain injury (categorized as: focal arterial territory infarction, basal ganglia infarction, internal carotid artery territory cerebral volume loss, periventricular white matter signal change, subcortical white matter signal change and/or deep white matter signal change). Cerebrovascular imaging (CT angiography/magnetic resonance angiography/catheter cerebral angiography) was reviewed segment by segment, as far as possible, from the aortic arch to the terminal branches of the middle, anterior and posterior cerebral arteries, respectively. Thus the segments analysed were: aortic arch, common carotid artery, external carotid artery, internal carotid artery [divided into proximal (internal carotid artery origin to level of foramen lacerum), laceral (foramen lacerum to proximal cavernous segment), cavernous (within the cavernous sinus) and terminal segments (distal cavernous sinus to terminal internal carotid artery)], anterior cerebral artery (divided into A1, distal anterior cerebral artery and terminal anterior cerebral artery branches), middle cerebral artery (divided into M1, distal middle cerebral artery and terminal middle cerebral artery branches), posterior cerebral artery (divided into P1, distal posterior cerebral artery and terminal posterior cerebral artery branches), vertebral artery (divided into vertical and horizontal segments) and basilar artery.
Vessel calibre and course were subjectively assessed by the two experienced paediatric neuroradiologists (D.E.S. and T.C.C.). Each of the arterial segments listed was evaluated as to whether vessel calibre appeared dilated, normal, stenosed or showing abnormally variable calibre. In addition, arterial course was assessed as normal, abnormally straight, or abnormally tortuous. The presence or absence of basal ‘moyamoya’ collaterals was recorded.
Histological examination of dural specimens was undertaken if these had been sent at the time of surgical revascularization. Dural samples were formalin-fixed and paraffin-embedded using standard diagnostic protocols. Paraffin sections were cut at 4 µm and stained with haematoxylin and eosin or with elastic van Gieson stain. In addition, immunohistochemistry was undertaken for smooth muscle actin (Leica, PA0943) with no antigen retrieval on a Leica BOND-MAX™ automated immuostainer (Protocol F).
Results
The patients’ details are summarized in Table 1. Of the 13 patients assessed in this study, 12 had a heterozygous missense Arg179His mutation and one, with an identical clinical phenotype, had an Arg179Leu mutation (Patient 9). All parents were negative for clinical features of ACTA2 mutations. All parents, except both parents of Patients 8, 11 and the father of Patient 6 (who was deceased) underwent genetic testing and were negative. Clinical features present in all cases were congenital fixed and dilated pupils and persistent ductus arteriosus. Eleven had respiratory involvement, five had pulmonary hypertension, ten had bladder problems and six had gut motility problems. One child (Patient 10) presented with acute hemiparesis secondary to acute arterial ischaemic stroke after acute bronchiolitis and developed fulminant, ultimately fatal, acute liver failure with markedly elevated hepatic transaminases and normal ammonia. Liver biopsy in life was unsuccessful and an autopsy was not undertaken. Patient 7 had transiently elevated hepatic transaminases, which subsequently normalized. Patient 3 and Patient 13 have not had acute neurological symptoms but have global developmental delay, which prompted brain imaging. Patient 11 had a brain scan after finding of the ACTA2 p.Arg179 mutation accounting for bladder and lung symptoms (Richer et al., 2012). Patient 12 presented with persistent ductus arteriosus and unreactive pupils; the finding of the ACTA2 p.Arg179 mutation prompted brain scanning though the child was neurologically intact. None of the patients had any other thrombotic/embolic features or markers of thrombotic or inflammatory disease.
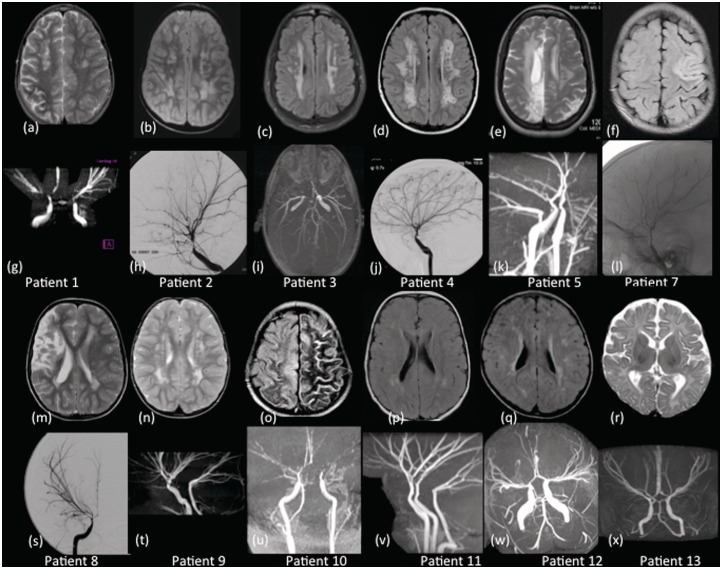
Brain MRI was available in 12 of the 13 cases (not available for Patient 6). Five patients, all of whom had clinical arterial ischaemic stroke, had evidence of cerebral ischaemic injury (volume loss in internal carotid artery territory in two patients, basal ganglia infarction in two patients, other focal arterial territory infarct in five patients; Fig. 1). Twelve patients had white matter signal change on MRI (hyperintensity on T2-weighted or FLAIR sequences). This was in periventricular white matter in 11 patients, deep white matter in nine and subcortical white matter in seven (Fig. 1). Patient 4 had markedly prominent perivascular spaces (Fig. 1D); Patient 7 had calcification in the region of an old cerebral infarct. Patient 3 had a dysplastic corpus callosum of uncertain significance (image not shown). No other brain imaging abnormalities were identified. Age was not a significant predictor of the pattern of parenchymal change, specifically of volume loss, presence of focal infarction or white matter signal change; however, it was interesting to observe that the youngest patient, presenting at 3 months of age (Patient 13), was the only one without white matter abnormalities (Fig. 1R and X).
Figure 1.
Axial T2-weighted and FLAIR images of Patients 1–13 (excluding Patient 6) (a–f, m–r) and their corresponding intracranial magnetic resonance angiograms and internal carotid artery injection of a cerebral angiography (g–l, s–x). The imaging of the brain parenchyma reveals the periventricular and deep white matter lesions (a–e, m, n, p and q) and cortical infarctions (e, f, m and o). Patient 13 has normal brain parenchyma (r). The lateral projections (h, j, l) and the (s) anterior-posterior view of the intracranial angiograms show dilatation of the internal carotid artery to the terminal portion, occlusive disease of distal intracranial circulation, an abnormally straight course of intracranial arteries and absence of ‘moyamoya’, other basal and leptomeningeal collaterals. Tortuous small vessels can be seen on the cerebral angiograms on Patients 2 and 4 (h and j). Posterior circulation involvement was manifest as basilar artery and posterior cerebral artery stenosis (i) and vertebral artery dilatation (u and v).
Cerebrovascular imaging was available for review in all patients. This was CT angiography in one patient (Patient 1, who also had magnetic resonance angiography), magnetic resonance angiography in 10 and catheter cerebral angiography in four (one of whom also had magnetic resonance angiography). The most proximal extent of the cerebrovascular imaging study was the aortic arch in three cases, common carotid artery in two cases and proximal internal carotid artery in the remaining cases.
Cervical and cerebral arterial abnormalities were generally symmetrical, both in terms of calibre change and arterial course. The proximal vasculature (aortic arch/common carotid arteries), visualized in five cases, was always dilated (Fig. 2). Transition from dilatation to stenosis (n = 7, e.g. Fig. 1H) or normal calibre (n = 6, Fig. 1T) was between the cavernous and terminal segments of the internal carotid artery in eight cases, laceral and cavernous segments of internal carotid artery in four, and the terminal internal carotid artery and proximal middle/anterior cerebral artery in one. Considering arterial course, the arteries assumed an abnormally straight course, almost as if stretched, at some point in 11 of the 13 cases (Fig. 1I, S and X). This was chiefly apparent between the terminal internal carotid artery and the distal segments of the middle, anterior and posterior cerebral arteries, i.e. the vessels forming the circle of Willis. Basal lenticulostriate ‘moyamoya’ collaterals were not observed in any cases, a key distinguishing feature between ACTA2 arteriopathy and moyamoya disease.
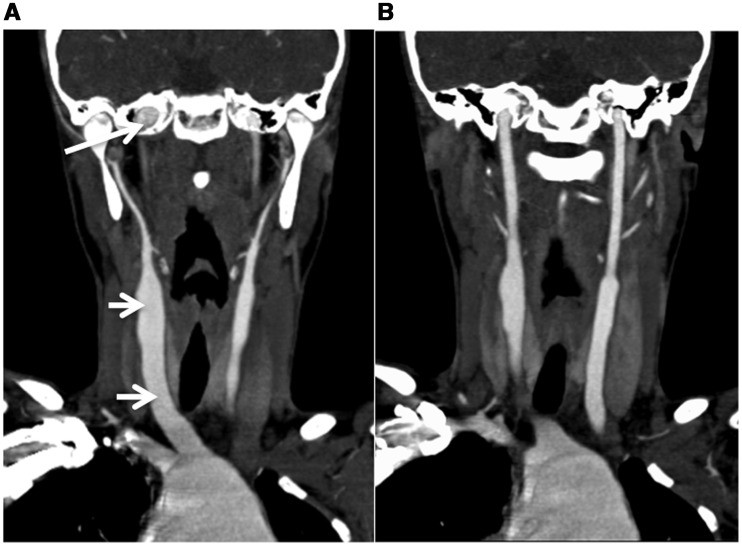
Figure 2.
2D reconstruction of CT angiography of the neck of Patient 1 showing (A) dilatation of the right common carotid artery within the neck to its origin (short arrows) and marked dilatation of the aortic arch. The petrous component of both internal carotid arteries can be seen to be dilated (long arrow). (B) A more posterior coronal slice showing the left common carotid artery, entire left internal carotid artery and aortic root dilatation.
The posterior circulation was involved in all cases. However, due to the limitations of the imaging studies available, visualization of the posterior circulation was poorer than for the anterior circulation. Abnormal arterial calibre was observed in 11 cases. When abnormal, vertebral arteries were dilated in four cases (Fig. 1T) and stenosed in another four (Fig. 1I). When transition in calibre from dilated to stenosis/normal was observed, this was between vertebral and basilar arteries in one case and basilar artery and proximal posterior cerebral artery in one case. The basilar artery was stenosed in seven cases, dilated in two cases and normal in four cases. The posterior cerebral arteries appeared narrow in 5 of 12 cases (two on catheter angiography and three on magnetic resonance angiography). None of the patients had a persistent foetal morphology of the posterior circulation, with persistent posterior communicating arteries, nor collaterals from branches of the posterior cerebral arteries, both of which are commonly seen in moyamoya disease.
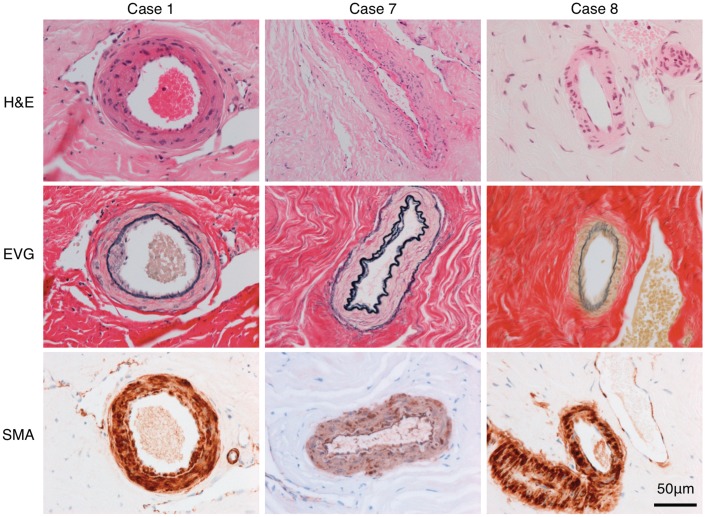
Histological specimens of dura and dural vessels were available from Patients 1, 7 and 8, who had had dural biopsy at the time of surgical revascularization. The biopsied vessels all appeared normal on review of pre-surgical catheter cerebral angiography. All the cases had focal abnormalities of the dural arteries, characterized by intimal thickening with elastin deposition (Fig. 3). These areas formed small indentations into the lumen or short crescents of thick intima but did not occlude the vessels. There was occasional reduplication of the internal elastic lamina. The remainder of the vessel wall structure was comparatively preserved.
Figure 3.
The images show representative small arterial dural vessels stained with haematoxylin and eosin (H&E), elastin Van Gieson (EVG) and smooth muscle actin (SMA). There is focal abnormality of the intima, which is most prominent in Patient 1 but subtle in Patients 7 and 8. There is focal intimal thickening with deposition of elastin (black on the elastin Van Gieson). There is focal reduplication of the internal elastic lamina in Patient 7.
Discussion
Here, we highlight the key distinctive neuroradiological features associated with mutations in ACTA2 that disrupt Arg179 to aid in the diagnosis of these patients. Recognition of these cerebrovascular features is important because other organs may be involved. In particular, affected patients should be monitored for aortic disease that can develop later (Disabella et al., 2011). Additionally, we have observed a relatively high rate of postoperative ischaemic stroke in children with this mutation who had revascularization surgery (two of three), compared with a much lower rate in our programme of revascularization for moyamoya (Ng et al., 2012). Patient 1 had severe lower limb ischaemia following femoral arterial puncture for cardiac catheterization; arterial instrumentation or surgery may be especially hazardous in these patients. Our data do not enable robust prediction of natural history, although they suggest some propensity to recurrent arterial ischaemic stroke and progressive brain injury. However, there is a likely referral bias for more severely affected patients to present to us.
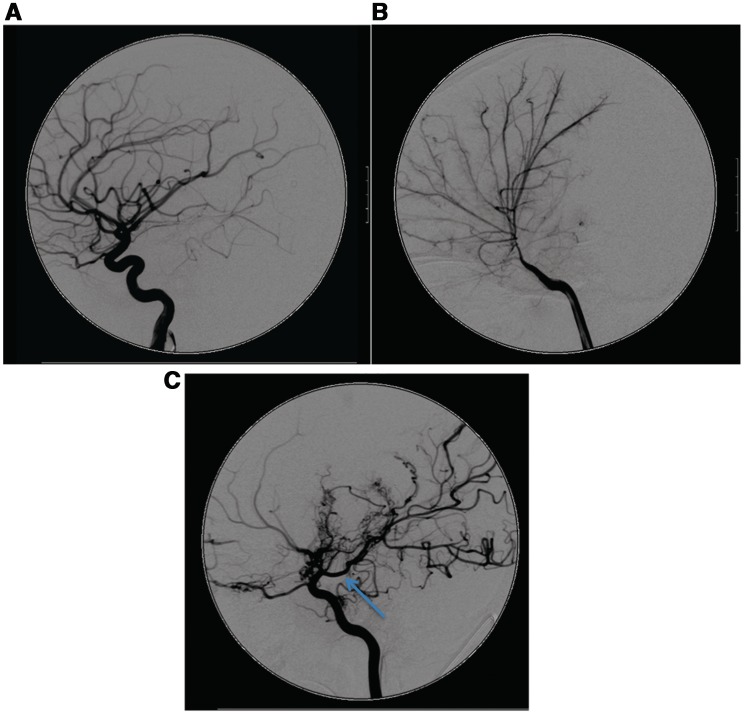
Images and description of some of these features have been published but they have not been synthesized into a comprehensive and consistent cerebrovascular phenotype. The Arg179His mutation was previously described as associated with moyamoya disease (Guo et al., 2009) but features distinguishing these cases from typical moyamoya disease, namely the combination of ectasia and stenosis, straight arterial course, absence of basal collaterals and more widespread cerebrovascular involvement were not emphasized (Fig. 4). Current paediatric arteriopathy classifications are not comprehensive and patients like these who fall out with currently recognized phenotypic entities are likely to prove informative in terms of genotype-phenotype correlations (Munot et al., 2011).
Figure 4.
The lateral view of the internal carotid artery injections of a cerebral angiogram of (A) an unaffected 3-year-old child, (B) a child with ACTA2 Arg179H is and (C) a child with idiopathic moyamoya disease. (B) Distinctive cerebrovascular features of ACTA2 mutations with dilatation of the proximal internal carotid artery, occlusive disease of distal intracranial circulation, an abnormally straight course of intracranial arteries and absence of ‘moyamoya’, other basal and leptomeningeal collaterals, which are prolific in (C), the child with moyamoya disease. A persistent posterior communicating artery (arrow) is a frequent feature in children with moyamoya disease, (C), but not seen in children with ACTA2 mutations.
On review of the cerebrovascular images in Guo et al. (2007) of patients with missense mutations in ACTA2 that disrupt Arg258, the proximal dilatation and lack of basal collaterals, but not the straight arterial course, which we observed in the Arg179His patients, are also apparent. Thus it would appear that there is overlap in the cerebrovascular phenotype associated with these ACTA2 mutations, with none of the cases demonstrating features typical of the radiological definition of moyamoya disease (Fukui, 1997). Mutations disrupting Arg179 appear to show the most severe cerebrovascular disease, and multi-system phenotype. We searched PubMed (1990–2011) using the keywords ‘moyamoya’, ‘persistent ductus arteriosus’, ‘iris’, ‘mydriasis’ and ‘ectasia’ as well as the reference lists from the references cited in order to identify other published cases of potential ACTA2 Arg179 mutations where neuroimaging was provided. The cases of Yamada et al. (1985) and Hanakita et al. (1986) have some (proximal ectasia, basal collaterals) but not all the features we describe here (no stenotic disease/normal arterial course/ectasia of more distal vessels). The patient reported by Kato et al. (1999) had persistent ductus arteriosus and similar radiological features to those seen in the Arg179 patients, and seems likely to have had this mutation. Thus these previously described cases appear likely to represent a range of underlying mutations.
The brain imaging findings reflect ischaemic injury, both vaso-occlusive (focal arterial territory infarction) and haemodynamic (borderzone ischaemia). However, what is striking is the degree of periventricular and subcortical white matter leukoariotic change, suggesting concurrent angiographically occult small vessel disease (Patel and Markus, 2011). This is a distinctive feature of ischaemic brain injury in patients with ACTA2 mutations if present and is unusual in typical moyamoya disease. The absence of white matter disease in the youngest child also raises the possibility that this is an age related phenomenon. We were not able to demonstrate a statistical association with age as our group was largely composed of older children and adults.
Milewicz et al. (2010b) previously proposed that the diverse vascular effects (both dilatation and occlusive disease) of ACTA2 mutations could be due to a differential effect of the mutations on the smooth muscle cells based on whether the artery is elastic or muscular. Occlusive arterial disease observed in the smaller diameter muscular arteries could be due to the mutation causing increased smooth muscle cell proliferation. In contrast, the elastin in large elastic arteries may inhibit smooth muscle cell proliferation but decreased contractility resulting from mutant actin may underlie the observed arterial dilatation or dolichoectasia. This seems a more likely mechanism than dilatation being secondary to occlusive disease because the enlarged proximal arterial calibre observed in these patients was apparent even where distal arterial calibre was relatively normal. Patients with focal intracranial occlusive disease of other aetiologies do not commonly manifest proximal dilatation that is so marked and extensive. The internal carotid artery is a muscular artery, with a medial layer predominantly composed of smooth muscle cells; in its proximal segment there are two elastic laminae, the internal elastic lamina (between the intima and media) and external elastic lamina (between media and adventitia). Our analysis of the angiographic images suggests that the change in vessel calibre from dilated to stenosed occurs around the cavernous internal carotid artery, which is known to be where the external elastic lamina becomes absent from the arterial wall (Masuoka et al., 2010), providing in vivo support for Milewicz et al.’s (2010b) hypothesis.
The abnormally straight arterial course in these patients is unusual; this seems to predominantly affect the arteries that make up the circle of Willis. The underlying mechanism is not clear but the appearance is distinctive; the arteries look abnormally ‘stretched’ and the middle cerebral artery has a particularly straight course over the insula (Fig. 1). It seems likely that this relatively straight course represents a mismatch between relative rates of growth of the vessels and surrounding brain; of note in their case report of a likely Arg179 case, Kato et al. (1999) comment on the appearance ‘like arteries in the foetus’.
Moyamoya disease (idiopathic) and moyamoya syndrome (where the arteriopathy is associated with another condition) are radiological entities, defined by occlusive disease of the terminal internal carotid/proximal middle/anterior cerebral arteries and the presence of ‘abnormal vascular networks in the vicinity of the occlusive or stenotic lesions in the arterial phase’ (Fukui, 1997). This basal collateral circulation is a hallmark of moyamoya. It is plausible that the angiogenic response observed in classical moyamoya is absent in ACTA2 arteriopathy because α-actin is an important contributor to angiogenesis and has a role in lumen formation and vascular stabilization. The cerebrovascular disease in moyamoya is focal and occlusive, without the more widespread involvement of the carotid circulation and a combination of dilated and occlusive calibre change. Moyamoya disease in Japanese is often progressive and in its earliest and latest stages the characteristic basal collaterals are not apparent. However, the other features discussed above differentiate between ACTA2 associated cerebral arteriopathy and early moyamoya disease. The patients described here were generally evaluated with magnetic resonance angiography, rather than the gold standard investigation, catheter angiography, and thus it is possible that minor degrees of basal collateralization were not identified; however, these were also assessed on MRI and did not meet criteria for moyamoya disease.
The phenotypic spectrum associated with ACTA2 mutations continues to expand. We acknowledge that there is likely to be referral bias in these cases and it is likely that milder phenotypes are currently under-recognized. Two of the patients described here had hepatic dysfunction, which proved fatal in one. It is difficult to know whether to attribute this to the ACTA2 mutation but we highlight the liver as another potential organ that may be affected. Congenital mydriasis is rare and an important clinical flag for ACTA2 mutations. Iris flocculi were reported in association with ACTA2 mutations associated with thoracic aortic aneurysms (Disabella et al., 2011) but were also seen in two children with the multi-system phenotype described here, and are a further helpful clinical clue. Persistent ductus arteriosus is a common cardiac defect [1.4% live births (Ishikawa et al., 2011)]; an important clinical message is that the eyes should be examined in these patients. Intimal hyperplasia in clinically and angiographically unaffected arteries in three of our patients emphasizes the systemic nature of the vasculopathy associated with ACTA2 mutations and therefore potential involvement of any organ system dependent on smooth muscle cell function.
It is evident that the cerebrovascular disease associated with ACTA2 mutations has a variable natural history. Interventions that could be considered to prevent arterial ischaemic stroke include anti-platelet agents or surgical revascularization, as for moyamoya (Roach et al., 2008), but with the caveats noted above; ultimately, given the earlier discussion of disease mechanism, agents that could inhibit the abnormal smooth muscle cell proliferation (for example, imatinib) may have a role in preventing disease progression, making early diagnosis especially important. The identification of a distinctive radiological cerebrovascular phenotype in patients with ACTA2 mutations emphasizes the importance of precisely characterizing paediatric cerebral arteriopathies in order to identify novel associations and disease mechanisms and thus to develop targeted treatments.
Funding
V.G. is funded by Great Ormond Street Hospital Children’s Charity and D.M.M. by NIH RO1 HL62594.
References
- Ades LC, Davies R, Haan EA, Holman KJ, Watson KC, Sreetharan D, et al. Aortic dissection, patent ductus arteriosus, iris hypoplasia and brachytelephalangy in a male adolescent. Clin Dysmorphol. 1999;8:269–76. [PubMed] [Google Scholar]
- Amlie-Lefond C, Bernard TJ, Sebire G, Friedman NR, Heyer GL, Lerner NB, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation. 2009;119:1417–23. doi: 10.1161/CIRCULATIONAHA.108.806307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disabella E, Grasso M, Gambarin FI, Narula N, Dore R, Favalli V, et al. Risk of dissection in thoracic aneurysms associated with mutations of smooth muscle alpha-actin 2 (ACTA2) Heart. 2011;97:321–6. doi: 10.1136/hrt.2010.204388. [DOI] [PubMed] [Google Scholar]
- Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya’ disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg. 1997;99(Suppl 2):S238–40. [PubMed] [Google Scholar]
- Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics. 2007;119:495–501. doi: 10.1542/peds.2006-2791. [DOI] [PubMed] [Google Scholar]
- Ganesan V, Prengler M, McShane MA, Wade AM, Kirkham FJ. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol. 2003;53:167–73. doi: 10.1002/ana.10423. [DOI] [PubMed] [Google Scholar]
- Ganesan V, Prengler M, Wade A, Kirkham FJ. Clinical and radiological recurrence after childhood arterial ischemic stroke. Circulation. 2006;114:2170–7. doi: 10.1161/CIRCULATIONAHA.105.583690. [DOI] [PubMed] [Google Scholar]
- Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–93. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- Guo DC, Papke CL, Tran-Fadulu V, Regalado ES, Avidan N, Johnson RJ, et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am J Hum Genet. 2009;84:617–27. doi: 10.1016/j.ajhg.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakita J, Miyake H, Nagayasu S, Nishi S, Suzuki T. Surgically treated cerebral arterial ectasia with so-called moyamoya vessels. Neurosurgery. 1986;19:271–3. doi: 10.1227/00006123-198608000-00017. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Iwashima S, Ohishi A, Nakagawa Y, Ohzeki T. Prevalence of congenital heart disease assessed by echocardiography in 2067 consecutive newborns. Acta Paediatr. 2011;100:e55–60. doi: 10.1111/j.1651-2227.2011.02248.x. [DOI] [PubMed] [Google Scholar]
- Kato K, Tomura N, Takahashi S, Hirano H, Watarai J, Sawaishi Y, et al. A case of moyamoya-like vessels combined with brain anomaly. Radiat Med. 1999;17:373–7. [PubMed] [Google Scholar]
- Masuoka T, Hayashi N, Hori E, Kuwayama N, Ohtani O, Endo S. Distribution of internal elastic lamina and external elastic lamina in the internal carotid artery: possible relationship with atherosclerosis. Neurol Med Chir (Tokyo) 2010;50:179–82. doi: 10.2176/nmc.50.179. [DOI] [PubMed] [Google Scholar]
- Milewicz DM, Ostergaard JR, la-Kokko LM, Khan N, Grange DK, Mendoza-Londono R, et al. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet A. 2010a;52A:2437–43. doi: 10.1002/ajmg.a.33657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewicz DM, Kwartler CS, Papke CL, Regalado ES, Cao J, Reid AJ. Genetic variants promoting smooth muscle cell proliferation can result in diffuse and diverse vascular diseases: evidence for a hyperplastic vasculomyopathy. Genet Med. 2010b;12:196–203. doi: 10.1097/GIM.0b013e3181cdd687. [DOI] [PubMed] [Google Scholar]
- Munot P, Crow YJ, Ganesan V. Paediatric stroke: genetic insights into disease mechanisms and treatment targets. Lancet Neurol. 2011;10:264–74. doi: 10.1016/S1474-4422(10)70327-6. [DOI] [PubMed] [Google Scholar]
- Ng J, Thompson D, Lumley JP, Saunders DE, Ganesan V. Surgical revascularisation for childhood moyamoya. Childs Nerv Syst. 2012;28:1041–8. doi: 10.1007/s00381-012-1743-7. [DOI] [PubMed] [Google Scholar]
- Patel B, Markus HS. Magnetic resonance imaging in cerebral small vessel disease and its use as a surrogate disease marker. Int J Stroke. 2011;6:47–59. doi: 10.1111/j.1747-4949.2010.00552.x. [DOI] [PubMed] [Google Scholar]
- Perkins E, Stephens J, Xiang H, Lo W. The cost of pediatric stroke acute care in the United States. Stroke. 2009;40:2820–7. doi: 10.1161/STROKEAHA.109.548156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richer J, Milewicz DM, Gow R, de Nanassy J, Maharajh G, Miller E, et al. R179H mutation in ACTA2 expanding the phenotype to include prune-belly sequence and skin manifestations. Am J Med Genet A. 2012;158A:664–8. doi: 10.1002/ajmg.a.35206. [DOI] [PubMed] [Google Scholar]
- Roach ES, Golomb MR, Adams R, Biller J, Daniels S, Deveber G, et al. Management of stroke in infants and children: a scientific statement from a special writing group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the young. Stroke. 2008;39:2644–91. doi: 10.1161/STROKEAHA.108.189696. [DOI] [PubMed] [Google Scholar]
- Roder C, Peters V, Kasuya H, Nishizawa T, Wakita S, Berg D, et al. Analysis of ACTA2 in European Moyamoya disease patients. Eur J Paediatr Neurol. 2011;15:117–22. doi: 10.1016/j.ejpn.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Shimojima K, Yamamoto T. ACTA2 is not a major disease-causing gene for moyamoya disease. J Hum Genet. 2009;54:687–8. doi: 10.1038/jhg.2009.91. [DOI] [PubMed] [Google Scholar]
- Yamada K, Hayakawa T, Ushio Y, Mitomo M. Cerebral arterial dolichoectasia associated with moyamoya vessels. Surg Neurol. 1985;23:19–24. doi: 10.1016/0090-3019(85)90154-5. [DOI] [PubMed] [Google Scholar]