Abstract
Acetylcholinesterase inhibitors are commonly used to treat patients with dementia with Lewy bodies. Hippocampal atrophy on magnetic resonance imaging and amyloid-β load on positron emission tomography are associated with the Alzheimer’s disease-related pathology in patients with dementia with Lewy bodies. To date, few studies have investigated imaging markers that predict treatment response in patients with dementia with Lewy bodies. Our objective was to determine whether imaging markers of Alzheimer’s disease-related pathology such as hippocampal volume, brain amyloid-β load on 11C Pittsburgh compound B positron emission tomography predict treatment response to acetylcholinesterase inhibitors in patients with dementia with Lewy bodies. We performed a retrospective analysis on consecutive treatment-naive patients with dementia with Lewy bodies (n = 54) from the Mayo Clinic Alzheimer’s Disease Research Centre who subsequently received acetylcholinesterase inhibitors and underwent magnetic resonance imaging with hippocampal volumetry. Baseline and follow-up assessments were obtained with the Mattis Dementia Rating Scale. Subjects were divided into three groups (reliable improvement, stable or reliable decline) using Dementia Rating Scale reliable change indices determined previously. Associations between hippocampal volumes and treatment response were tested with analysis of covariance adjusting for baseline Dementia Rating Scale, age, gender, magnetic resonance field strength and Dementia Rating Scale interval. Seven subjects underwent 11C Pittsburgh compound B imaging within 12 weeks of magnetic resonance imaging. Global cortical 11C Pittsburgh compound B retention (scaled to cerebellar retention) was calculated in these patients. Using a conservative psychometric method of assessing treatment response, there were 12 patients with reliable decline, 29 stable cases and 13 patients with reliable improvement. The improvers had significantly larger hippocampi than those that declined (P = 0.02) and the stable (P = 0.04) group. An exploratory analysis demonstrated larger grey matter volumes in the temporal and parietal lobes in improvers compared with those who declined (P < 0.05). The two patients who had a positive 11C Pittsburgh compound B positron emission tomography scan declined and those who had a negative 11C Pittsburgh compound B positron emission tomography scan improved or were stable after treatment. Patients with dementia with Lewy bodies who do not have the imaging features of coexistent Alzheimer’s disease-related pathology are more likely to cognitively improve with acetylcholinesterase inhibitor treatment.
Keywords: dementia with Lewy bodies, acetylcholinesterase inhibitors, MRI, PiB, PET, amyloid
Introduction
Dementia with Lewy bodies (DLB) is the second most common cause of degenerative dementia after Alzheimer’s disease. DLB is characterized by a progressive cognitive decline with deficits in attention/executive, and visuospatial ability associated with fluctuations, visual hallucinations and parkinsonism. Other characteristic features include rapid eye movement sleep behaviour disorder and sensitivity to antipsychotics (McKeith et al., 2005; Ferman et al., 2011). Many patients with DLB have coexistent Alzheimer’s disease pathology (Galasko et al., 1994; Gomez-Isla et al., 1999; Schneider et al., 2007). The overlap of the two pathologies is further demonstrated in imaging studies. For example, 11C Pittsburgh compound B (PiB) binding is often present in subjects with DLB consistent with presence of amyloid-β deposition (Edison et al., 2008; Maetzler et al., 2009; Foster et al., 2010; Kantarci et al., 2011, 2012). Clinically diagnosed patients with DLB on average have larger hippocampal volumes than patients with Alzheimer’s disease (Whitwell et al., 2007; Firbank et al., 2010; Kantarci et al., 2011), and hippocampal atrophy on MRI distinguishes patients with pathologically confirmed DLB and Alzheimer’s disease (Burton et al., 2009). Furthermore, hippocampal atrophy on MRI is associated with the neurofibrillary tangle pathology of Alzheimer’s disease in patients with Lewy body-related pathology (Kantarci et al., 2012).
Both DLB and Alzheimer’s disease are frequently treated with acetylcholinesterase inhibitors to improve cognitive function. Similarly, both disorders demonstrate loss of cholinergic neurons in the nucleus basalis of Meynert, but the loss appears to occur earlier in DLB and is far greater than in Alzheimer’s disease (Whitehouse et al., 1982; Forstl et al., 1993; Perry et al., 1993, 1994; Tiraboschi et al., 2002). Thus, patients with DLB would be expected to respond better to acetylcholinesterase inhibitors than Alzheimer’s disease (McKeith et al., 2000). This raises the possibility that neuroimaging markers of Alzheimer’s disease-related pathology in patients with DLB may predict response to acetylcholinesterase inhibitor treatment.
Our objective was to determine whether imaging markers of Alzheimer’s disease-related pathology such as hippocampal atrophy and brain amyloid-β load on PiB PET are associated with cognitive treatment response to acetylcholinesterase inhibitors in patients with DLB. We hypothesized that patients who respond to acetylcholinesterase inhibitors would have larger hippocampal volumes and be less likely to have PiB uptake on PET. We assessed treatment response based on reliable change scores in the Mattis Dementia Rating Scale (DRS) during ∼1 year of treatment with acetylcholinesterase inhibitors. Finally, we performed an exploratory analysis to demonstrate the topographic differences in grey matter volume among treatment response groups.
Materials and methods
Subjects
We performed a retrospective analysis on consecutive treatment-naive patients with DLB (n = 54) from the Mayo Clinic Alzheimer Disease Research Centre. Individuals participating in the Mayo Clinic Alzheimer Disease Research Centre undergo approximately annual clinical examinations, MRI, routine laboratory tests and neuropsychological evaluation. A consensus committee meeting is held annually involving behavioural neurologists, neuropsychologists and nurses to assign a clinical diagnosis. All 54 patients in this study eventually received a consensus clinical diagnosis of probable DLB based on published criteria (McKeith et al., 2005).
We included patients with probable DLB who underwent neuropsychological assessment with the DRS (Jurica et al., 2001) before and after treatment with acetylcholinesterase inhibitors. The DRS is a brief test of global cognition that yields a maximum score of 144 points based on performance in five subtests. Our group has extensive experience with the DRS and developed normative data as part of Mayo’s Older Americans Normative Studies (Lucas et al., 1998). All patients with DLB had MRI within 2 years of treatment. Seven of the patients with DLB also underwent PiB PET imaging within a week of the MRI, with the exception of one subject who underwent PET 16 weeks after the MRI scan. Exclusion criteria were as follows: patients with DLB who were on cholinesterase inhibitors prior to undergoing DRS testing, patients with DLB who did not receive or were intolerant of acetylcholinesterase inhibitors and patients with DLB who did not participate in the MRI study.
Mattis dementia rating scale reliable change
We used published criteria for normative rates of change on the DRS (Pedraza et al., 2007) to determine reliable change. The reliable change index was generated using Mayo’s Older Americans Normative Studies data (Ivnik et al., 1992; Lucas et al., 1998). Reliable change indices take into account measurement error and practice effect to estimate whether an individual’s performance over time represents reliable improvement or decline versus intra-individual chance fluctuation (Pedraza et al., 2007). Patients were divided into three groups based on their reliable DRS change scores: reliable improvement, stability or reliable decline. Reliable improvement was defined as an increase of at least nine DRS points for a test–retest interval <15 months and an increase of at least 10 points for a test–retest interval >15 months. Reliable decline was defined as a decrease of at least six DRS points for a test–retest interval <15 months and a decrease of at least seven points for a test–retest interval >15 months. Stability was defined as any DRS change that did not exceed these reliable change index cut-off values.
Magnetic resonance imaging
MRI examinations were performed at 1.5 or 3 T (GE Healthcare). At 1.5 T, a 3D high-resolution spoiled gradient recalled acquisition with repetition time/echo time/inversion time = 7/3/900 ms; flip angle 8°; in plane resolution of 1.0 mm and a slice thickness of 1.2 mm was performed for the automated segmentation of hippocampal volumes. At 3 T, a 3D high-resolution MPRAGE acquisition with repetition time/echo time/inversion time = 7/3/900 ms; flip angle, 8°; in-plane resolution of 1.0 mm, and a slice thickness of 1.2 mm was performed for the automated segmentation of hippocampal volumes and for the anatomical segmentation and labelling of PiB PET images in patients who underwent PiB PET.
The normalized volumes of the hippocampi were computed by combining the right and left hippocampal volumes. Each subject’s high resolution T1-weighted MRI scan was spatially normalized to the custom template with the in-house modified anatomical labelling atlas labels and segmented into grey matter, white matter and CSF using the unified segmentation model of SPM5, giving a discrete cosine transformation. Then for each subject, inverse of this transformation was applied to warp the hippocampal regions of interest to the subject’s native anatomical space to determine the grey matter volume in the segmented T1-weighted MRIs.
We used 3D Structural Abnormality due to Neurodegeneration maps to explore the differences between the reliable decline and reliable improvement groups. Structural Abnormality due to Neurodegeneration maps represent 3D intracranial volume and age-adjusted regional grey matter Z-score information in 92 hemispheric regions estimated using individual subject’s scans relative to a bank of MRI scans from cognitively normal subjects with a similar MRI acquisition sequence and field strength (Vemuri et al., 2011). The Structural Abnormality due to Neurodegeneration maps between the two groups of patients with DLB were compared using a two sample t-test and the significant regions at P < 0.05 uncorrected for multiple comparisons.
11C Pittsburgh compound B positron emission tomography
PiB PET imaging was performed in seven of the patients within 16 weeks of the MRI examination. Subjects were injected with PiB (average, 596 MBq; range, 292–729 MBq). Following a 40-min PiB uptake period, a 20-min PiB scan was obtained. PiB PET acquisition consisted of four 5-min dynamic frames, acquired from 40 to 60 min after injection. PiB PET image volumes of each subject were co-registered to his/her own T1-weighted MRI scan with the in-house modified anatomical labelling atlas labels, using a 6° of freedom affine registration with mutual information cost function. Atlas-based parcellation of PiB PET images into regions of interest was therefore performed in subject’s T1-weighted MRI space. Partial volume correction for tissue and CSF compartments was applied using the two compartment model to remove atrophy effects PiB uptake on PET images (Meltzer et al., 1999). Cerebellar uptake was used as an internal reference region of interest for PiB PET normalization. Statistics on image voxel values were extracted from modified Automated Anatomical Labelling atlas regions of interest. The global cortical PiB retention ratio was calculated by averaging the PiB retention ratio from the bilateral parietal (including posterior cingulate and precuneus), temporal, prefrontal, orbitofrontal and anterior cingulate grey matter regions where the average is weighted by region of interest size (Jack et al., 2008; Lowe et al., 2009).
Statistical analysis
We analysed hippocampal volumes after scaling them by the subject’s total intracranial volume. To test for a group effect, we used analysis of covariance with reliable change group treated as the main effect, and age at the time of the baseline DRS, sex, the time from treatment to the second DRS date and field strength treated as covariates. We used contrasts to perform pair-wise comparisons of the groups. Contrasts are a method of comparing linear functions of observations, in this case, differences of group means, using F-tests. We tested for group effects in the patient characteristics using Kruskal–Wallis tests for the continuous variables and chi-square tests for the categorical variables. All analyses were performed with SAS version 9.2 and R statistical software version 2.13.2 (http://www.R-project.org). This study was approved by the Mayo Clinic Institute Review Board and informed consent was obtained from the subjects and/or a proxy.
Results
Patient characteristics
Using a conservative psychometric method of assessing treatment response, 12 patients demonstrated reliable decline, 29 patients remained stable and 13 patients showed reliable improvement on the DRS. Table 1 demonstrates the clinical features of the three cognitive response groups at the time of MRI. Groups were similar with respect to age, gender, education, MRI interval, apolipoprotein E status, auditory verbal learning test delayed recall Mayo’s Older Americans Normative Studies, Global Deterioration Scale and Mini-Mental State Examination. Clinical features of DLB were also similar amongst the cognitive response groups. DRS scores were lower at baseline in the group with reliable improvement. DRS subscale analysis revealed worse attention and conceptualization scores and a trend towards worse memory score in the reliable improvement group. Although the proportion of patients who underwent 1.5 T examinations was similar across the cognitive response groups, we still included field strength as a covariate in statistical analysis.
Table 1.
Patient’s characteristics at the time of MRIa
| Reliable decline (n = 12) | Stable (n = 29) | Reliable improvement (n = 13) | P-valueb | |
|---|---|---|---|---|
| Age (years) | 72 (63 to 84) | 73 (64 to 84) | 75 (61 to 82) | 0.40 |
| No. of females | 3 (25) | 7 (24) | 3 (23) | 0.99 |
| No. of APOE ε4 carriers | 7 (58) | 15 (52) | 6 (46) | 0.83 |
| Education (years) | 15 (12 to 20) | 14 (8 to 20) | 12 (8 to 18) | 0.13 |
| Baseline DRS total | 129 (115 to 140) | 125 (97 to 137) | 114 (64 to 126) | 0.001 |
| Follow-up DRS total | 114 (28 to 133) | 129 (97 to 137) | 130 (110 to 137) | 0.002 |
| DRS change | −16 (−89 to −7) | 1.0 (−6 to 7) | 13 (9 to 62) | <0.001 |
| Attention | 35.5 (32 to 37) | 35 (29 to 37) | 31 (21 to 37) | 0.003 |
| Initiation | 32 (23 to 37) | 31 (19 to 37) | 25 (17 to 36) | 0.15 |
| Construction | 6 (4 to 6) | 6 (3 to 6) | 5 (1 to 6) | 0.11 |
| Conceptualization | 36 (31 to 39) | 35 (22 to 39) | 31 (14 to 39) | 0.02 |
| Memory | 20 (16 to 24) | 20 (11 to 25) | 17 (7 to 22) | 0.053 |
| Time between DRS tests (years) | 1.0 (0.9 to 1.7) | 1.1 (0.6 to 2.2) | 1.1 (0.5 to 1.6) | 0.64 |
| Time between treatment and second DRS test (years) | 0.8 (0.2 to 1.2) | 1.0 (0.3 to 2.0) | 1.0 (0.5 to 1.6) | 0.09 |
| Time between treatment and MRI scan (years) | 1.0 (−0.1 to 1.7) | 1.0 (−.5 to 1.8) | 1.1 (0.0 to 1.9) | 0.51 |
| No. of subjects with fluctuations | 5 (71) | 9 (50) | 3 (38) | 0.42 |
| No. of subjects with visual hallucinations | 5 (42) | 11 (38) | 6 (46) | 0.88 |
| No. of subjects with rapid eye movement sleep behaviour disorder | 8 (73) | 17 (74) | 12 (100) | 0.14 |
| Total unified Parkinson’s disease rating scale | 17.5 (3 to 20) | 10 (0 to 25) | 10 (0 to 17) | 0.23 |
| Mini-mental state examination | 22 (9.0 to 28) | 24 (12 to 30) | 24 (10 to 28) | 0.41 |
| Global deterioration scale | 4 (3 to 6) | 4 (2 to 5) | 4 (2 to 6) | 0.55 |
| Clinical Dementia Rating sum of boxes | 4.8 (1.0 to 17) | 3.5 (0.5 to 8.0) | 3.5 (1.0 to 12) | 0.31 |
| Auditory verbal learning test delayed recall age-corrected scaled scores | 6 (2 to 10) | 7 (2 to 15) | 6 (3 to 15) | 0.37 |
| No. of 1.5 T magnetic resonance scans | 8 (67) | 20 (69) | 8 (62) | 0.89 |
a Median (min, max) reported for the continuous variables and count (%) for the categorical variables.
b The P-values reported for the continuous variables are from the Kruskal–Wallis test while those for categorical variables are from the chi-squared test.
Acetylcholinesterase inhibitors
The breakdown of acetylcholinesterase use among all subjects was as follows: donepezil (n = 47), galantamine (n = 3) and rivastigmine (n = 4). Six were using concomitant memantine. The breakdown among the three subjects groups was as follows: (i) reliable improvement: donepezil (n = 13), galantamine (n = 0), rivastigmine (n = 0) and concomitant memantine (n = 2); (ii) stable: donepezil (n = 24), galantamine (n = 2), rivastigmine (n = 3) and concomitant memantine(n = 3); and (iii) reliable decline: donepezil (n = 10), galantamine (n = 1), rivastigmine (n = 1) and concomitant memantine (n = 1).
Neuropsychiatric and anti-parkinsonian medications
The breakdown of neuropsychiatric and anti-parkinsonian medications among the three subjects groups was as follows: (i) reliable improvement: mirtazapine (n = 2), citalopram (n = 1), carbidopa/levodopa (n = 6), quetiapine (n = 3); (ii) stable: citalopram (n = 3), trazodone (n = 2), sertraline (n = 2), venlafaxine (n = 1), fluoxetine (n = 1), carbidopa–levodopa (n = 9), pramipexole (n = 2), quetiapine (n = 3), risperidone (n = 1); and (iii) reliable decline citalopram (n = 1) sertraline (n = 1), fluoxetine (n = 1), buproprion (n = 1), paroxetine (n = 1), trazodone (n = 1), carbidopa/levodopa (n = 4) and quetiapine (n = 2).
Volumetric magnetic resonance imaging findings and acetylcholinesterase inhibitor response
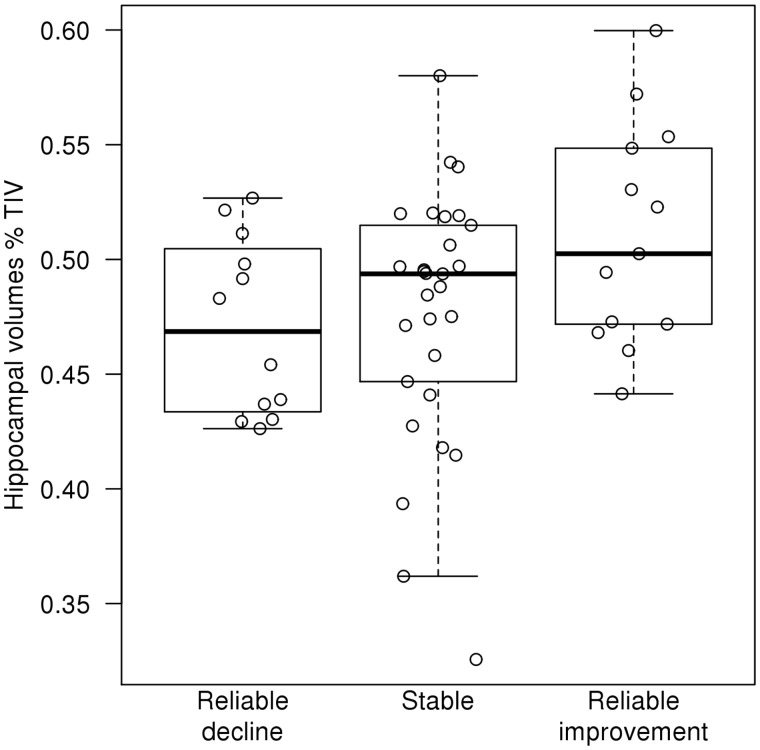
After adjusting for age, sex, the time from treatment to the second DRS and field strength, we found differences among the three cognitive response groups (analysis of covariance; P = 0.04). Specifically, patients with DLB with reliable cognitive improvement had larger hippocampal volumes than those that declined (P = 0.02) or remained cognitively stable (P = 0.04) (Fig. 1).
Figure 1.
Hippocampal volumes (%TIV) in reliable cognitive improvement, stable and reliable cognitive decline groups. TIV = total intracranial volume.
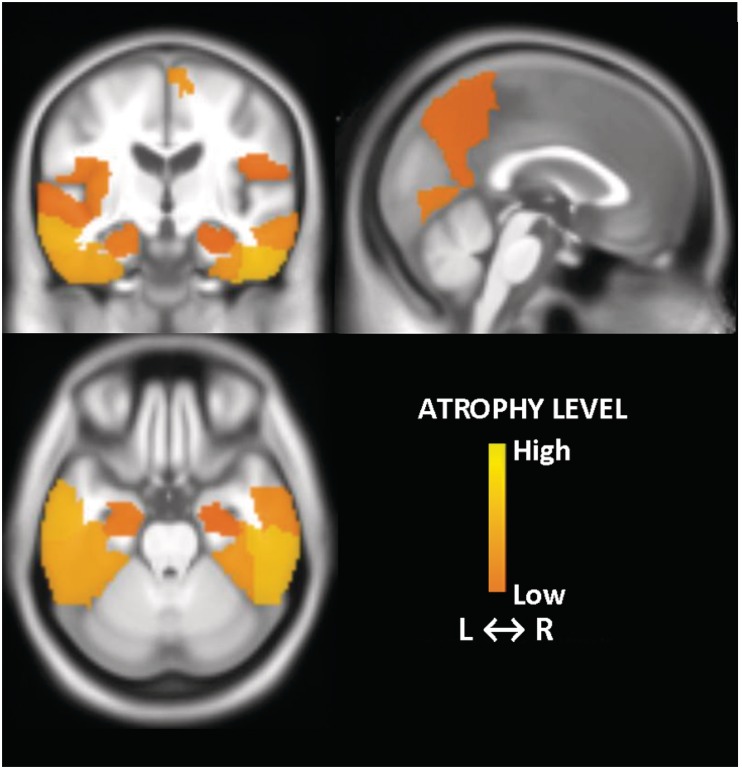
In an exploratory analysis, we found larger grey matter volumes in predominantly the temporal and parietal lobe regions in patients with DLB with reliable cognitive improvement than those that declined (P < 0.05) (Fig. 2). The topographic pattern of smaller grey matter volumes in patients with DLB with reliable cognitive decline closely resembled the pattern of grey matter atrophy observed in patients with pathologically confirmed Alzheimer’s disease (Vemuri et al., 2011).
Figure 2.
Topographic pattern of smaller grey matter volume in patients with DLB who cognitively declined (reliable decline) compared with patients with DLB who cognitively improved (reliable improvement) on acetylcholinesterase treatment. Regions with greater atrophy (P < 0.05; uncorrected for multiple comparisons) are displayed with a colour scale representing the atrophy level. L = left; R = right.
11C Pittsburgh compound B positron emission tomography findings in patients with dementia with Lewy bodies
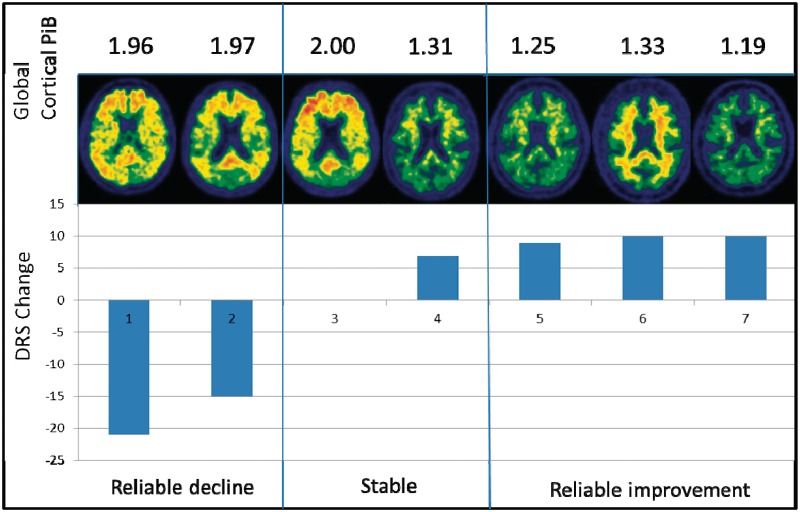
According to the typically used cut-off of 1.50 (Rowe et al., 2007; Jack et al., 2008), both subjects with reliable decline after acetylcholinesterase inhibitors were PiB positive, whereas all three subjects with reliable improvement were PIB negative. Stable subjects also showed a similar trend. Of the two patients with stable DRS after acetylcholinesterase inhibitors, one patient was PiB positive (global cortical PiB retention ratio = 2.00) and had no change in DRS after acetylcholinesterase inhibitor treatment. The other patient was PiB negative (global cortical PiB retention ratio = 1.31) and had a DRS increase of seven points, but this increase was within the range of chance fluctuation and did not represent reliable improvement (Fig. 3).
Figure 3.
Global cortical PiB retention ratio images in reliable cognitive decline, stable and reliable cognitive improvement groups. The change in dementia rating scale is displayed for each of the seven patients with DLB. Note the non-specific PiB retention in the white matter in Subject 6, which does not contribute to the global cortical PiB ratio.
Discussion
This study showed that larger hippocampal volumes on MRI are associated with cognitive improvement on acetylcholinesterase inhibitor treatment in patients with DLB. Furthermore, the topography of grey matter volume loss in patients with DLB who declined compared with those who improved was consistent with the pattern of atrophy observed in patients with pathologically confirmed Alzheimer’s disease (Vemuri et al., 2011). In agreement with the MRI findings, we also found evidence that a negative PiB PET examination may indicate a favourable cognitive response to acetylcholinesterase inhibitor treatment in DLB.
Many patients with DLB have both Alzheimer’s disease and Lewy body-related pathology at autopsy (Galasko et al., 1994; Gomez-Isla et al., 1999; Schneider et al., 2007). Hippocampal volumes are typically preserved in clinically diagnosed and pathologically confirmed patients with DLB (Whitwell et al., 2007; Sabattoli et al., 2008; Burton et al., 2009; Kantarci et al., 2011). Recently, in a cohort of cases with mixed Alzheimer’s disease and DLB pathology, we showed that ante-mortem hippocampal atrophy correlates with coexisting neurofibrillary tangle pathology of Alzheimer’s disease regardless of the extent of Lewy body-related pathology (Kantarci et al., 2012). In this study, hippocampal atrophy and an Alzheimer’s disease-like pattern of grey matter atrophy was associated with poor response to acetylcholinesterase inhibitor treatment. Furthermore, patients with DLB with a negative PiB PET scan improved cognitively on acetylcholinesterase inhibitors, whereas patients with DLB with a positive PiB PET scan tend to decline. Our findings suggest that patients with DLB with imaging features of Alzheimer’s disease-related pathology do not respond as well to acetylcholinesterase inhibitors compared with those who do not have imaging evidence of Alzheimer’s disease.
On the contrary, none of the DLB clinical features, demographics or apolipoprotein E status distinguished the response groups from each other. Specifically, parkinsonism severity, and presence of visual hallucinations, rapid eye movement sleep behaviour disorder or fluctuations did not distinguish between those who were more likely to improve or decline. Most patients fell into the moderate stage of dementia severity, based on their Global Deterioration Scale score and Clinical Dementia Rating sum of boxes score. The auditory verbal learning test is a neuropsychometric test for memory function that can help distinguish Alzheimer’s disease from DLB because memory is usually well preserved in DLB early in the disease compared with Alzheimer’s disease (Ferman et al., 2006). As expected, the auditory verbal learning test performance was mildly impaired to low average, but did not distinguish between the groups who were more likely to decline or improve with cholinesterase inhibitors. Lower baseline DRS in the reliable improvement group is consistent with the finding that response to acetylcholinesterase inhibitors is associated with worse cognitive performance in patients with dementia (Pakrasi et al., 2003). The reliable improvement subgroup performed worse on attention and conceptualization subscores of the DRS, which is consistent with patients with DLB having early difficulty with attention and visual perception on neuropsychological testing (Ferman et al., 2006).
The history of acetylcholinesterase inhibitor treatment in DLB can be traced back to initial clinical trials in Alzheimer’s disease. Drachman and Leavitt (1974) demonstrated that anticholinergic medications can produce memory impairment similar to dementias. Subsequent studies demonstrated decreased cholinergic neurons in the basal forebrain of patients with Alzheimer’s disease (Whitehouse et al., 1982). These findings led to the development of pharmacological agents aimed at targeting the cholinergic deficit. Tacrine is an acetylcholinesterase inhibitor that was one of the first agents to demonstrate efficacy in Alzheimer’s disease (Eagger et al., 1991; Davis et al., 1992). As studies on acetylcholinesterase inhibitors in Alzheimer’s disease were published in the early 1990s, the clinical diagnoses of DLB were not well recognized during enrolment. The first consensus clinical and pathological guidelines for DLB were published by McKeith et al. (1996) In fact, the authors of one of the original tacrine trials later noted that a small number of the patients had a large response to tacrine, which drove the trials positivity, and when three of the ‘responders’ came to autopsy, they had DLB pathology (Levy et al., 1994). As DLB was increasingly recognized and diagnosed in the early 1990s, investigators made several important observations, including that the cholinergic deficit in DLB was greater than that in Alzheimer’s disease (Forstl et al., 1993). In addition, choline acetyltransferase, the enzyme that synthesized acetylcholine, activity is consistently lower in DLB cases than Alzheimer’s disease cases (Perry et al., 1994). These findings led to clinical trials of acetylcholinesterase inhibitors in DLB. A randomized, double blind, placebo-controlled trial demonstrated acetylcholinesterase inhibitors improve cognition, apathy, delusions and hallucinations in subjects with DLB (McKeith et al., 2000). Therefore, as acetylcholinesterase inhibitor trials in Alzheimer’s disease were being published, knowledge of DLB was growing exponentially. It is likely that many subjects with DLB were included in the original trials, possibly accounting for some of the ‘responders’. For example, the clinical predictors of response to acetylcholinesterase inhibition include hallucinations and clinical diagnoses of DLB (Pakrasi et al., 2003). In fact, in early acetylcholinesterase inhibitor trials that used the neuropsychiatric inventory in Alzheimer’s disease, hallucinations and apathy were the subscores of the neuropsychiatric inventory that improved with treatment (Morris et al., 1998; Dubois et al., 1999). Later trials in Alzheimer’s disease did not find improvement in the neuropsychiatric inventory with acetylcholinesterase inhibitors (Winblad et al., 2001).
Investigations into the imaging predictors of treatment response in DLB have been limited. Treatment with acetylcholinesterase inhibition in a group of patients with DLB resulted in improved occipital perfusion on SPECT scans corresponding to improvement in hallucinations (Mori et al., 2006). On MRI, the volume of the substantia innominata correlated with response to acetylcholinesterase inhibition in a cohort of patients with dementia that included patients with DLB (Hanyu et al., 2007), but the imaging predictors of acetylcholinesterase inhibitor response were not evaluated specifically in DLB.
This study was conducted in a convenience sample and was not designed as a clinical trial; therefore, we could not compare the change in DRS with a group of patients with DLB who were not receiving treatment in a randomized setting. We used DRS reliable change indices, which allowed us to take into account measurement error and practice effect to make a more precise determination about cognitive decline versus improvement. It is important to note, however, that reliable change in a patient with DLB may be dissimilar from reliable change in cognitively normal older adults. For example, patients with DLB characteristically have fluctuations that may limit the reliability of neuropsychological testing at one point. Therefore, the range of scores for subgroups of reliable change in DLB may be wider than in cognitively normal subjects
Our data showed converging evidence that patients with probable DLB, who improve on the DRS during acetylcholinesterase inhibitor treatment, have larger hippocampal volumes, less grey matter loss in temporal and parietal regions typically involved with the neurodegenerative pathology of Alzheimer’s disease and less amyloid-β load compared with those who decline. Although MRI and PiB PET findings are consistent in this study, further investigation on the significance of amyloid-β load on acetylcholinesterase inhibitor response in larger samples is warranted. Further, any findings of the PIB PET imaging should be interpreted with caution because of the small sample size.
To the best of our knowledge, this study showed, for the first time, that acetylcholinesterase inhibitor treatment in patients with DLB is associated with the imaging markers of Alzheimer’s disease-related pathology. Our findings may have implications on treatment planning in patients with DLB. Patients with probable DLB appear to cognitively improve on acetylcholinesterase inhibitor treatment if they do not have imaging evidence of coexisting Alzheimer’s disease pathology. Therefore, imaging markers of Alzheimer’s disease in patients with DLB may be critical for treatment decisions as well as planning for clinical trials in probable DLB. Although high dose acetylcholinesterase inhibitor trials in Alzheimer’s disease have been disappointing (Farlow et al., 2010), future areas of research could include testing of higher dose acetylcholinesterase inhibition in patients with DLB who do not have imaging evidence of Alzheimer’s disease.
Funding
This work was supported by the National Institutes of Health: P50-AG16574/P1 and R01AG040042 (to K.K.), R01-AG015866 (to T.J.F.), P50-AG16574/P1 (to V.J.L.), R01-AG11378 to (C.R.J.), P50-AG16574 to (R.C.P.); Mangurian Foundation; and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program.
Glossary
Abbreviations
- DLB
dementia with Lewy bodies
- DRS
Mattis dementia rating scale
- PiB
11C Pittsburgh compound B
References
- Burton EJ, Barber R, Mukaetova-Ladinska EB, Robson J, Perry RH, Jaros E, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 2009;132:195–203. doi: 10.1093/brain/awn298. [DOI] [PubMed] [Google Scholar]
- Davis KL, Thal LJ, Gamzu ER, Davis CS, Woolson RF, Gracon SI, et al. A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer’s disease. The Tacrine Collaborative Study Group. N Engl J Med. 1992;327:1253–9. doi: 10.1056/NEJM199210293271801. [DOI] [PubMed] [Google Scholar]
- Drachman DA, Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30:113–21. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- Dubois B, McKeith I, Orgogozo JM, Collins O, Meulien D. A multicentre, randomized, double-blind, placebo-controlled study to evaluate the efficacy, tolerability and safety of two doses of metrifonate in patients with mild-to-moderate Alzheimer's disease: the MALT study. Int J Geriatr Psychiatry. 1999;14:973–82. [PubMed] [Google Scholar]
- Eagger SA, Levy R, Sahakian BJ. Tacrine in Alzheimer's disease. Lancet. 1991;337:989–92. doi: 10.1016/0140-6736(91)92656-m. [DOI] [PubMed] [Google Scholar]
- Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, et al. Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry. 2008;79:1331–8. doi: 10.1136/jnnp.2007.127878. [DOI] [PubMed] [Google Scholar]
- Farlow MR, Salloway S, Tariot PN, Yardley J, Moline ML, Wang Q, et al. Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer's disease: a 24-week, randomized, double-blind study. Clin Ther. 2010;32:1234–51. doi: 10.1016/j.clinthera.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferman TJ, Boeve BF, Smith GE, Lin SC, Silber MH, Pedraza O, et al. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology. 2011;77:875–82. doi: 10.1212/WNL.0b013e31822c9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferman TJ, Smith GE, Boeve BF, Graff-Radford NR, Lucas JA, Knopman DS, et al. Neuropsychological differentiation of dementia with Lewy bodies from normal aging and Alzheimer's disease. Clin Neuropsychol. 2006;20:623–36. doi: 10.1080/13854040500376831. [DOI] [PubMed] [Google Scholar]
- Firbank MJ, Blamire AM, Teodorczuk A, Teper E, Burton EJ, Mitra D, et al. High resolution imaging of the medial temporal lobe in Alzheimer’s disease and dementia with Lewy bodies. J Alzheimers Dis. 2010;21:1129–40. doi: 10.3233/jad-2010-100138. [DOI] [PubMed] [Google Scholar]
- Forstl H, Burns A, Luthert P, Cairns N, Levy R. The Lewy-body variant of Alzheimer's disease. Clinical and pathological findings. Br J Psychiatry. 1993;162:385–92. doi: 10.1192/bjp.162.3.385. [DOI] [PubMed] [Google Scholar]
- Foster ER, Campbell MC, Burack MA, Hartlein J, Flores HP, Cairns NJ, et al. Amyloid imaging of Lewy body-associated disorders. Mov Disord. 2010;25:2516–23. doi: 10.1002/mds.23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasko D, Hansen LA, Katzman R, Wiederholt W, Masliah E, Terry R, et al. Clinical-neuropathological correlations in Alzheimer's disease and related dementias. Arch Neurol. 1994;51:888–95. doi: 10.1001/archneur.1994.00540210060013. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Growdon WB, McNamara M, Newell K, Gomez-Tortosa E, Hedley-Whyte ET, et al. Clinicopathologic correlates in temporal cortex in dementia with Lewy bodies. Neurology. 1999;53:2003–9. doi: 10.1212/wnl.53.9.2003. [DOI] [PubMed] [Google Scholar]
- Hanyu H, Shimizu S, Tanaka Y, Hirao K, Iwamoto T, Abe K. MR features of the substantia innominata and therapeutic implications in dementias. Neurobiol Aging. 2007;28:548–54. doi: 10.1016/j.neurobiolaging.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, et al. Mayo’s Older Americans Normative Studies: WAIS-R, WMS-R and AVLT norms for ages 56 through 97. Clin Neuropsychol. 1992;6(Suppl):1–104. [Google Scholar]
- Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131:665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, Mattis S, editors. Dementia rating scale-2: Professional manual. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- Kantarci K, Dickson DW, Vemuri P, Ferman TJ, Boeve BF, Smith GE, et al. Ante mortem hippocampal atrophy predicts presence of Alzheimer’s disease pathology in pathologically proven Lewy body disease. Neurology. 2012 [Google Scholar]
- Kantarci K, Lowe VJ, Boeve BF, Weigand SD, Senjem ML, Przybelski SA, et al. Multimodality imaging characteristics of dementia with Lewy bodies. Neurobiol Aging. 2011;77:875–82. doi: 10.1016/j.neurobiolaging.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Yang C, Schneider JA, Senjem ML, Reyes DA, Lowe VJ, et al. Ante mortem amyloid imaging and beta-amyloid pathology in a case with dementia with Lewy bodies. Neurobiol Aging. 2012;33:878–85. doi: 10.1016/j.neurobiolaging.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Eagger S, Griffiths M, Perry E, Honavar M, Dean A, et al. Lewy bodies and response to tacrine in Alzheimer’s disease. Lancet. 1994;343:176. doi: 10.1016/s0140-6736(94)90966-0. [DOI] [PubMed] [Google Scholar]
- Lowe VJ, Kemp BJ, Jack CR, Jr, Senjem M, Weigand S, Shiung M, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med. 2009;50:878–86. doi: 10.2967/jnumed.108.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Kokmen E, et al. Normative data for the Mattis Dementia Rating Scale. J Clin Exp Neuropsychol. 1998;20:536–47. doi: 10.1076/jcen.20.4.536.1469. [DOI] [PubMed] [Google Scholar]
- Maetzler W, Liepelt I, Reimold M, Reischl G, Solbach C, Becker C, et al. Cortical PIB binding in Lewy body disease is associated with Alzheimer-like characteristics. Neurobiol Dis. 2009;34:107–12. doi: 10.1016/j.nbd.2008.12.008. [DOI] [PubMed] [Google Scholar]
- McKeith I, Del Ser T, Spano P, Emre M, Wesnes K, Anand R, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356:2031–6. doi: 10.1016/S0140-6736(00)03399-7. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–24. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Kinahan PE, Greer PJ, Nichols TE, Comtat C, Cantwell MN, et al. Comparative evaluation of MR-based partial-volume correction schemes for PET. J Nucl Med. 1999;40:2053–65. [PubMed] [Google Scholar]
- Mori T, Ikeda M, Fukuhara R, Nestor PJ, Tanabe H. Correlation of visual hallucinations with occipital rCBF changes by donepezil in DLB. Neurology. 2006;66:935–7. doi: 10.1212/01.wnl.0000203114.03976.b0. [DOI] [PubMed] [Google Scholar]
- Morris JC, Cyrus PA, Orazem J, Mas J, Bieber F, Ruzicka BB, et al. Metrifonate benefits cognitive, behavioral, and global function in patients with Alzheimer's disease. Neurology. 1998;50:1222–30. doi: 10.1212/wnl.50.5.1222. [DOI] [PubMed] [Google Scholar]
- Pakrasi S, Mukaetova-Ladinska EB, McKeith IG, O'Brien JT. Clinical predictors of response to acetyl cholinesterase inhibitors: experience from routine clinical use in Newcastle. Int J Geriatr Psychiatry. 2003;18:879–86. doi: 10.1002/gps.928. [DOI] [PubMed] [Google Scholar]
- Pedraza O, Smith GE, Ivnik RJ, Willis FB, Ferman TJ, Petersen RC, et al. Reliable change on the dementia rating scale. J Int Neuropsychol Soc. 2007;13:716–20. doi: 10.1017/S1355617707070920. [DOI] [PubMed] [Google Scholar]
- Perry EK, Haroutunian V, Davis KL, Levy R, Lantos P, Eagger S, et al. Neocortical cholinergic activities differentiate Lewy body dementia from classical Alzheimer’s disease. Neuroreport. 1994;5:747–9. doi: 10.1097/00001756-199403000-00002. [DOI] [PubMed] [Google Scholar]
- Perry EK, Irving D, Kerwin JM, McKeith IG, Thompson P, Collerton D, et al. Cholinergic transmitter and neurotrophic activities in Lewy body dementia: similarity to Parkinson’s and distinction from Alzheimer disease. Alzheimer Dis Assoc Disord. 1993;7:69–79. doi: 10.1097/00002093-199307020-00002. [DOI] [PubMed] [Google Scholar]
- Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–25. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- Sabattoli F, Boccardi M, Galluzzi S, Treves A, Thompson PM, Frisoni GB. Hippocampal shape differences in dementia with Lewy bodies. NeuroImage. 2008;41:699–705. doi: 10.1016/j.neuroimage.2008.02.060. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P, Hansen LA, Alford M, Merdes A, Masliah E, Thal LJ, et al. Early and widespread cholinergic losses differentiate dementia with Lewy bodies from Alzheimer disease. Arch Gen Psychiatry. 2002;59:946–51. doi: 10.1001/archpsyc.59.10.946. [DOI] [PubMed] [Google Scholar]
- Vemuri P, Simon G, Kantarci K, Whitwell JL, Senjem ML, Przybelski SA, et al. Antemortem differential diagnosis of dementia pathology using structural MRI: differential-STAND. Neuroimage. 2011;55:522–31. doi: 10.1016/j.neuroimage.2010.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–9. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer's disease. Brain. 2007;130:708–19. doi: 10.1093/brain/awl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489–95. doi: 10.1212/wnl.57.3.489. [DOI] [PubMed] [Google Scholar]