Abstract
Migraine is twice as common in females as in males, but the mechanisms behind this difference are still poorly understood. We used high-field magnetic resonance imaging in male and female age-matched interictal (migraine free) migraineurs and matched healthy controls to determine alterations in brain structure. Female migraineurs had thicker posterior insula and precuneus cortices compared with male migraineurs and healthy controls of both sexes. Furthermore, evaluation of functional responses to heat within the migraine groups indicated concurrent functional differences in male and female migraineurs and a sex-specific pattern of functional connectivity of these two regions with the rest of the brain. The results support the notion of a ‘sex phenotype’ in migraine and indicate that brains are differentially affected by migraine in females compared with males. Furthermore, the results also support the notion that sex differences involve both brain structure as well as functional circuits, in that emotional circuitry compared with sensory processing appears involved to a greater degree in female than male migraineurs.
Keywords: migraine, headache, pain, sex differences, fMRI, morphometry, precuneus, insula
Introduction
Pain disorders are more prevalent in females than in males (Unruh, 1996; Fillingim et al., 2009). Gender differences are also observed in migraine, where the incidence is about twice as high in females compared with males (Russell et al., 1995; Brandes, 2006; Le et al., 2011). Despite evidence for sex differences in pain neurobiology, treatment efficacy and experimental pain responses, the mechanisms underlying these differences are still poorly understood (Greenspan et al., 2007). Recent functional MRI studies have observed differences in pain processing between the sexes in healthy subjects (Paulson et al., 1998; Derbyshire et al., 2002; Moulton et al., 2006; Straube et al., 2009; Kong et al., 2010). However, little information is available regarding sex differences in brain structure and function in clinical states (Gong et al., 2011; Koepp, 2011) including migraine. Given that migraine has a significant sex disparity in prevalence, by combining both structural and functional approaches, the data provide novel insights suggesting that brain systems are differentially affected in males and females.
A number of sex differences in migraineurs may be salient including: (i) the increased prevalence of migraine in females (Stewart et al., 1992); (ii) the potential role of oestradiol fluctuations in migraine onset (Martin et al., 2007); (iii) sex-related responses to treatments such as triptans (Ferrari et al., 2011); (iv) higher incidence of cutaneous allodynia in female migraineurs (Bigal et al., 2008); (v) reversal from chronic migraine to episodic migraine in females (∼60% of individuals) by hormonal preventives (Calhoun and Ford, 2008); (vi) increased levels of depression and anxiety (Guidetti et al., 2009); and (vii) higher mean scores for psychic and somatic anxiety in female migraineurs (Hedborg et al., 2011). Thus, a preponderance of evidence clearly suggests that the female and male migraine brain may be different. If so, this would have significant implications on the nature of how future migraine studies should be conducted and potentially have an impact on how we evaluate and treat the disease. Such specific functional and structural brain changes may contribute to our understanding of the ‘sex phenotype’ in migraine including differences in emotionality, depression or cognition.
Earlier studies have reported structural differences in migraine versus normal brains (Table 1), but no study has addressed the role of sex differences in brain structure in migraine. In these structural studies, male migraineurs have been less frequently studied with studies reporting an average male to female ratio of 1:8. This is not representative of the sex distribution in the migraine population, which is reported to be around 1:2 (Russell et al., 1995; Brandes, 2006; Le et al., 2011). Although whole-brain volume is greater in males than females, when controlling for total volume, sex-related regional volume differences are less consistent in the literature (Cosgrove et al., 2007). Genetic differences may explain some morphometric and functional differences in migraineurs versus non-migraineurs (although not well understood; Shyti et al., 2011), but many disease modifiers are different in females and males and may be a component of potential sex differences in the disease. Moreover, sex differences in resting state networks of migraine sufferers have recently been reported in chronic migraine where females were noted to have more dysfunctional organization of their resting state networks (Liu et al., 2011a). Intermittent pain attacks in migraine are a major manifestation in most migraineurs, although other significant symptoms and behaviours are also manifested (e.g. nausea/vomiting, autonomic changes and drowsiness). We and others have reported significant differences in measures of the brain’s response to pain in the interictal state in migraineurs (Mailis-Gagnon et al., 2003; Moulton et al., 2008; Burstein et al., 2010; Tessitore et al., 2011; Russo et al., 2012) but also these cohorts have included mostly females. Experimental pain stimuli may be used to evaluate alterations in a chronic pain state as has previously been reported (Apkarian et al., 2001; Becerra et al., 2006). It seems reasonable to hypothesize that by using a pain stressor, gender differences in pain processing circuits may be observed in migraineurs.
Table 1.
Structural differences associated with migraine reported in previous imaging studies
| Patients | Controls | |||||
|---|---|---|---|---|---|---|
| (M/F) | (M/F) | Method | Results | Patients versus controls | Sex difference | Reference |
| 28 (2/26) | 28 (2/26) | 2 T VBM | no sign diff | no sign diff | NR | Matharu et al. (2003) |
| 35 (3/32) | 31 (0/31) | 1.5 T VBM | < | ACC, Ins, PCC | NR | Schmidt-Wilcke et al. (2008) |
| 24 (0/24) | 24 (0/24) | 3 T VBM | < | IPL, MFG | - | Schmitz et al. (2008) |
| 27 (6/21) | 27 (7/20) | 1 T VBM | < | IFG, ParOper, PCG, STG, TTG | NR | Valfre et al. (2008) |
| 20 (3/17) | 33 (4/29) | 1.5 T VBM | < | ACC, IFG, Ins, IPG, LG, MFG, | NR | Kim et al. (2008) |
| PCC, PCG, SFG, SPG | ||||||
| 16 (1/15) | 15 (2/13) | 3 T VBM | < | ACC, IFG, ITG, MFG, MTG, PCG, | NR | Rocca et al. (2006) |
| SFG, STG, Unc | ||||||
| > | PAG, Pons | |||||
| 28 (0/28) | 28 (0/28) | 3 T VBM | < | IPL SFG | – | Schmitz et al. (2008) |
| 24 (8/16) | 12 (3/9) | 3 T Cor. Th | > | SSC, V3A, MT | NR | DaSilva et al. (2007) |
| Total | ||||||
| (23/179) | (18/180) | |||||
VBM = voxel based morphometry; Cor. Th = cortical thickness; ACC = anterior cingulate cortex; IFG = inferior frontal gyrus; Ins = insula; IPG = inferior parietal gyrus; IPL = inferior parietal lobule; ITG = inferior temporal gyrus; LG = lingual gyrus; MFG = middle frontal gyrus; MT = middle temporal visual area; NR = ; PAG = periaqueductal grey; ParOper = parietal operculum; PCC = posterior cingulate cortex; PCG = precentral gyrus; SFG = superior frontal gyrus; SPG = superior parietal gyrus; SSC = somatosensory cortex; STG = superior temporal gyrus; TTG = transverse temporal gyrus; Unc = uncus; V3A = visual area 3a.
In this study we evaluated the effect of sex on migraine and possible interactions between sex and the disease in brain structure. Healthy controls were matched for sex and age, and female and male migraineurs were matched for age, migraine frequency, migraine intensity, mediation type and medication use in order to control for disease burden. In addition to structural alterations, we evaluated functional differences in experimental pain responses within the male and female migraineur cohorts, and in female healthy controls, and assessed if the observed sex-related structural differences in migraineurs translated to any specific sex-related differences in functional measures. We hypothesized that pain processing in migraineurs, assessed by functional responses to noxious heat, would map onto observed sex-related structural differences.
Materials and methods
Subjects
A total of 44 subjects were scanned for this study (n = 22 healthy and n = 22 migraine patients). A total of 11 male and 11 female migraineurs, matched for age, age of onset, medication type and frequency of migraine attacks, were selected from screening a cohort of 60 patients with migraine. The migraine subjects (i) met the criteria for episodic migraine as classified in the International Classification for Headache (ICHD-2; http://www.i-h-s.org/upload/ct_clas/ihc_II); (ii) had Beck Depression Inventory II (BDI-II) scores <25; (iii) suffered from episodic migraine for ≥3 years; and (iv) had no migraine 72 h prior to the scan and no symptoms of developing a migraine during or 24 h after the scans. Three females took oral contraceptives, one of which did not take oral contraceptives for a week preceding the scan. One female had an intrauterine device and the rest were not taking oral contraceptives or hormone treatments. All studies were performed in the interictal state.
A total of 11 male and 11 female age-matched healthy (no pain or headache disorders and not more than two non-migraine, non-cluster type headaches per year) control subjects were enrolled in the study. Informed consent forms were obtained from all subjects, the Institutional Review Board approved this study and it met the criteria of the Helsinki accord for experimentation of pain in human subjects (http://history.nih.gov/research/downloads/helsinki.pdf).
Quantitative heat sensory testing
In both migraineurs and healthy controls, quantitative heat sensory testing was performed using a 1.6 × 1.6 cm contact thermode (TSA-II, Medoc Advanced Medical Systems) prior to the scanning session in order to determine the pain threshold of each individual. The temperature increased from a 32°C baseline temperature at the 1°C/s rate until stopped by the subject at the first onset of pain. This test was repeated three times and the thresholds were averaged and the corresponding temperature was recorded as the pain threshold. Testing was performed on the dorsum of the hand on the predominant side of migraine attacks. If there was no predominant side the testing was performed on the left side.
Noxious thermal stimulation
Functional MRI was performed in the migraine cohorts and in the healthy female group. For stimulation during functional imaging, three blocks of stimulation [duration: 15 s, temperature: 1°C higher than the pain threshold temperature determined during the quantitative heat sensory testing (pain threshold +1°C)] were delivered from a baseline temperature of 32°C at 30 s intervals. The stimulations were delivered to the dorsum of the same hand that quantitative heat sensory testing was performed. The rate of temperature change was 4°C/s. The 15 s pain stimulation period did not include the ramp-up and ramp-down periods of the thermode from the baseline temperature (∼2–3 s). The ramps were modelled in defining the explanatory variables for functional MRI data analysis.
Imaging
All data were collected on a 3 T Siemens Trio scanner with an eight-channel phased array head coil. For structural data, high resolution, T1-weighted data sets were collected from each subject using a 3D MPRAGE pulse sequence (repetition time/echo time/inversion time = 2100/2.74/1100 ms, flip angle = 12, 128 sagittal slices, resolution = 1.33 × 1.0 × 1.0 mm3). For acquiring functional data, a gradient echo echo planar imaging sequence (repetition time/echo time = 30/2500, voxel size = 3.5 × 3.5 × 3.5 mm3, matrix = 64 × 64, 41 slices, 74 volumes) was used.
Data analysis
Structural analysis
We determined cortical thickness and volumes in the migraineurs and the healthy controls to elucidate disease and sex related morphometric differences.
Segmentation was performed with the automatic parcellation tools of the Freesurfer image analysis software (http://surfer.nmr.mgh.harvard.edu/). These tools enable labelling subcortical and cortical tissue classes using an atlas-based Bayesian segmentation procedure (Fischl et al., 2002). The processing steps included removal of non-brain tissue (Segonne et al., 2004), automated Talairach transformation and segmentation of the subcortical white matter and deep grey matter volumetric structures (Fischl et al., 2002, 2004). A univariate analysis of variance for the segmented volumes was then performed separately using IBM SPSS 19.0 statistics package to assess the differences between healthy subjects and male and female patients with migraine while accounting for the differences in the cranium size (Buckner et al., 2004) and age (Gonoi et al., 2010) as additional regressors. In order to account for the differences in cranium size, an estimate of the total intracranial volume was used as an additional regressor (Buckner et al., 2004), which is estimated based on a statistical relationship between the total intracranial volume computed from a manual segmentation and the Talairach transform.
For cortical thickness comparisons further steps were taken for tessellation of the grey/white matter boundary, automated topology correction (Fischl et al., 2001; Segonne et al., 2007), surface deformation (Fischl and Dale, 2000) and registration of the subjects’ brains to a common spherical atlas. Statistical surface maps were generated to assess the cortical thickness differences at each vertex for male versus female migraineurs and healthy control subjects. Surface data for each subject were smoothed with a 10 mm full-width at half-maximum kernel to improve inter-subject averaging. A general linear model was used to test for cortical thickness differences between disease state (migraine and healthy) and sex and for any interaction between these two factors at each vertex. To correct for multiple comparisons, spatial clusters of thickness differences were defined as contiguous clusters of vertices with P-values < 0.05 (two-tailed). The statistical surface maps were cluster-wise corrected for multiple comparisons using a Monte Carlo Simulation (10 000 iterations) and the P-values for these clusters were determined. Only clusters that survived this correction with P-values < 0.05 (two-tailed) were deemed significant. The average cortical thickness was measured for every cluster surviving the correction for multiple comparisons and plotted for comparison. Cortical thickness differences between the healthy controls (male versus female) and migraineurs (male versus female) were evaluated separately and also altogether to determine the main disease effect on the cortical thickness and also to assess the disease by sex interaction effect on the cortical thickness.
Functional analysis
Functional MRI analysis was carried out using FMRIB Software Library (FSL) (www.fmrib.ax.ac.uk/fsl), version 4.1.3. The pre-statistical processing for each subject consisted of skull stripping using a Brain Extraction Tool (BET) with bias field correction and motion correction. The volumes were spatially smoothed with a 5 mm full-width at half-maximum filter and a 60 s high-pass temporal filter was applied. First-level functional MRI analysis of single subject data was performed using functional MRI Expert Analysis Tool (FEAT) Version 5.98. The explanatory variables for thermal stimuli were entered using the recorded temperature traces for each subject. Subjects were spatially normalized to the MNI152 brain for group analysis. Spatial normalization was performed using FLIRT (FMRIB’s Linear Image Registration Tool) following a two-stage process. First a low-resolution image of the whole brain (which was acquired with the similar imaging parameters as to the functional MRI acquisition separately) was linearly registered to the high-resolution structural MPRAGE image. The degree of freedom (df) for this transformation was 6. Then the MPRAGE image was linearly (affine, df = 12) registered to the standard image (MNI 152 average brain). These two transformations were combined to transform the low-resolution functional MRI images (and the statistic images derived from the first-level analyses) straight into standard space and when applied later, during group analysis. Patients with right-sided migraines had their functional MRI data flipped along the y-axis to correspond with the majority of the patients with predominantly left-sided migraines. Group activation maps were generated by FEAT fMRIB’s Local Analysis of Mixed Effects (FLAME1). Statistical parametric maps were thresholded using a Gaussian mixture model technique (Pendse et al., 2009) and clusters were then thresholded with a minimum cluster criterion of 3 × 3 × 3 voxels in MNI space. In this approach, for all resulting activation and deactivation maps the threshold is determined at a posterior probability of P > 0.5. For each data set and contrast this posterior probability corresponds to different z-statistic values. Hence, the threshold is not determined by rejecting the null hypothesis, but instead by accepting the alternative hypothesis of activation. The minimum cluster criterion of 3 × 3 × 3 voxels was applied after thresholding. The reported clusters are thus clusters that met both criteria of Gaussian mixture model threshold and minimum cluster criterion. In order to define areas of overlap or common regions of activation (P < 0.01) for both male and female migraineurs, a conjunction analysis (Nichols et al., 2005) was performed using FSL tools where the minimum of the group average z-statistic images of the two cohorts were taken and thresholded at z = 2.3; clusters were then thresholded with a minimum cluster criterion of 3 × 3 × 3 voxels in MNI space.
Functional connectivity analysis
To further elucidate the observed sex differences in pain responses in migraineurs, a post hoc functional connectivity analysis was performed to compare female versus male migraineurs. The time series of each of the two seed regions of interest in insula and precuneus were correlated to the time series of all other voxels in the brain, during the pain task (Fox et al., 2005; Zhang et al., 2008). The regions of interest were defined based on the group results of the cortical thickness comparisons (see ‘Results’ section), which was first transformed (cortex to cortex registration) to each subject’s anatomical space and then transformed to each subject’s functional space. This transformation was calculated as the inverse of functional to structural data transformation with (df = 6) that was calculated during the spatial normalization steps described above. Preprocessing steps were similar to the steps described for functional analysis above. For each subject the white matter and CSF masks were created in anatomical space using Freesurfer tools (http://surfer.nmr.mgh.harvard.edu/). All time-courses in the brain were orthogonalized with respect to the eigen time-courses of white matter and CSF masks which were computed by singular value decomposition. We extracted all functional MRI time-courses from each seed region of interest and computed an Eigen time-course for the region of interest using singular value decomposition. After this step we normalized the region of interest Eigen time-course Z to unit norm Zn. Similarly, all time-courses in the brain will also be normalized to unit norm. The resulting normalized time-course Zn was entered into a general linear model analysis against the rest of the brain to assess connectivity with the chosen region of interest. It should be noted that because of normalization, the general linear model parameter estimates will be correlation coefficients. The general linear model parameter estimates (which are really correlation coefficients) were transformed into normally distributed quantities using a Fisher z-transform, registered to MNI space and entered into a mixed effects group analysis (FLAME1). Statistical parametric maps were thresholded using a Gaussian mixture model technique (Pendse et al., 2009) and then clusters were thresholded with a minimum cluster criterion of 3 × 3 × 3 voxels in MNI space.
Results
Psychophysical and biometric results
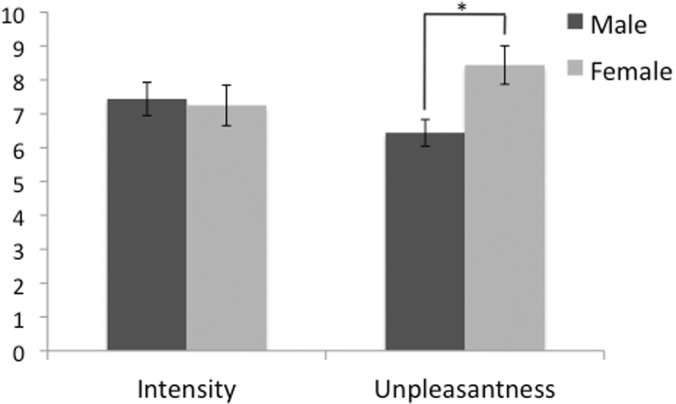
Participants included in the analysis were matched for age, age of onset, medication type and frequency of migraine attack. Specifically, all of the studied cohorts were age matched (migraine males: 42.7 ± 9.3 years, migraine females: 41.3 ± 10.1 years, healthy males: 43 ± 9.9 years, healthy females: 42.3 ± 10.3 years). There was no significant sex-difference in the patients’ headache intensity [males (mean ± SD): 7.4 ± 1.5, females: 7.2 ± 1.7] on a 0–10 subjective scale. However, the headache unpleasantness rating was significantly lower in males (mean ± SD, 6.4 ± 1.1) than in females (8.44 ± 1.7), (P < 0.05) (Fig. 1). Beck Depression Index ratings for subjects were <12 in all cohorts, 0 for the majority of the participants (migraine males: 3.2 ± 3.1, migraine females: 4.4 ± 4.9, healthy males: 2.5 ± 4.8, healthy females: 1.9 ± 2.8), and not significantly different. Estimated total number of migraine attacks (frequency × duration) experienced throughout life in males ranged from 204 to 3744 (average: 1243) and from 144 to 4056 (average: 1178) in females with no significant difference between the two cohorts. There was no significant difference between the frequency of migraine attacks (P < 0.36) or the estimate of the number of migraine attacks in a year (P < 0.3), or the total number of migraine attacks estimate between male versus female migraineurs (P < 0.37). The pain threshold was 46.9 ± 2.9°C in migraine males, 46.3 ± 3.8°C in migraine females, 47.1 ± 2.4°C in healthy males and 46.7 ± 2.8°C in healthy females, with no significant sex or disease differences or sex by disease interactions.
Figure 1.
Migraine pain intensity and unpleasantness ratings. A significant difference in the pain unpleasantness scores was observed between the two cohorts (*P < 0.05). The scores are based on a 0–10 subjective scale for migraine pain intensity and pain unpleasantness.
Magnetic resonance imaging measures
Structural analysis
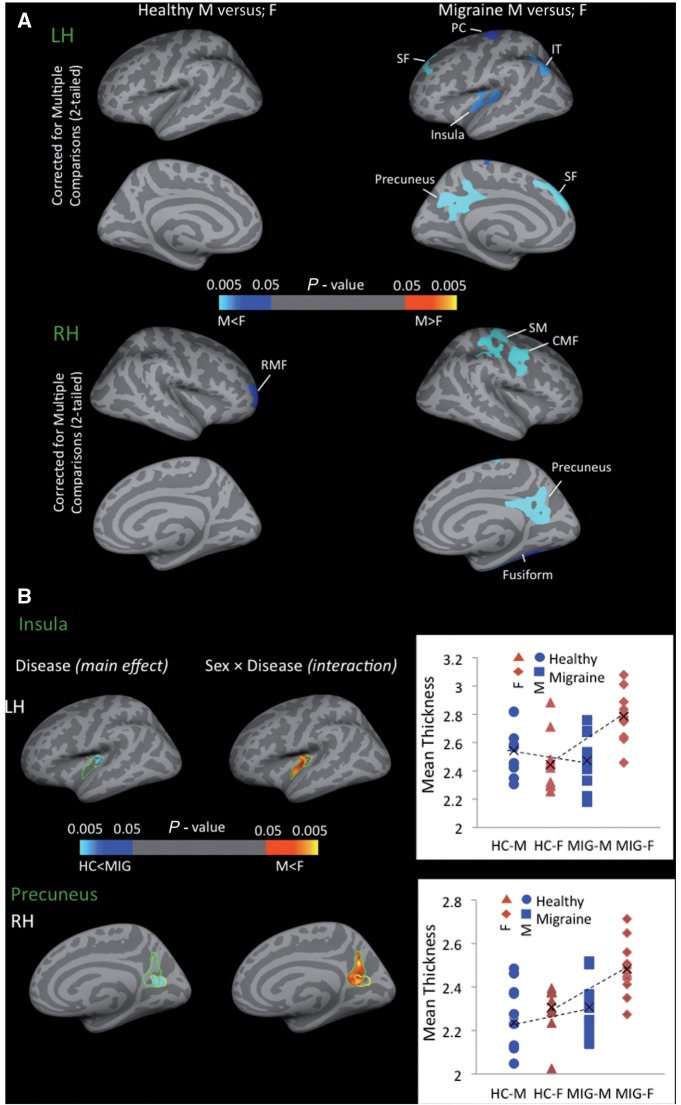
Cortical thickness comparisons results are presented in Fig. 2. The sex-related differences were determined first in each cohort separately, the result of this comparison is presented in Fig. 2A for female versus male comparisons in healthy control subjects and migraineurs. The only significant sex-related difference in healthy control subjects was in the right rostral middle frontal gyrus (P < 0.002). The same comparison in the migraineurs revealed multiple areas with significant cortical thickness differences between male versus female migraineurs including left superiorfrontal (P < 0.0001), right caudal middle frontal gyrus (P < 0.004), right supramarginal gyrus (P < 0.0001), left (P < 0.036) and right precentral gyrus (P < 0.0003), left insula (P < 0.017) and right (P < 0.0004) and left precuneus (P < 0.0001). In all of these areas cortex was thicker in females. Figure 2B shows significant clusters from vertex-wise cortical thickness comparisons conducted on all of the subjects to determine the main disease effect and sex by disease interactions. Significantly thicker cortex in the precuneus (P < 0.001) and posterior insula (P < 0.019) regions were observed in migraineurs versus healthy control subjects. Comparing migraineurs versus healthy control subjects revealed a sex × disease interaction in the left precuneus and right posterior insula with thicker cortex in female migraineurs in the precuneus (P < 0.02) and posterior insula (P < 0.017) that overlapped with the disease-only-related (main effect) differences between the healthy and migraine groups (independent of sex) (Table 2). Direct within sex (disease-only effect) comparisons were also determined and similarly corrected for multiple comparisons (Supplementary Fig. 1). While there were no significant differences in cortical thickness between male migrianeurs versus male healthy control subjects, the comparison in female subjects revealed significant thickening in migraine patients in the precuneus (bilateral, left hemisphere: P < 0.0001, right hemisphere: P < 0.015), posterior insula (bilateral, left hemisphere: P < 0.018, right hemisphere: P < 0.007), superiortemporal (left hemisphere: P < 0.011) and inferiortemporal (right hemisphere: P < 0.007). Comparing sex-related volumetric differences between healthy and migraineur subjects also revealed significant differences in the parahippocampal gyrus volume as a function of the disease state in males (P < 0.017) but not in females (Fig. 3).
Figure 2.
Cortical thickness changes. (A) Significant clusters from vertex-wise cortical thickness comparisons conducted on female versus male healthy subjects (left column) and female versus male migraine patients (right column). Blue–light blue colours represent areas with thicker cortex in female versus male and red–yellow colours represent areas with thicker cortex in male versus male in each of the cohorts. (B) Significant clusters from vertex-wise cortical thickness comparisons conducted on all of the subjects (migraine male and female and healthy control male and female) to determine the main effect (disease) effect and interaction effect (sex × disease). The disease effect (blue–light blue colour map) and sex × disease interaction (red–yellow colour map) are shown for (A) insula and (B) precuneus. The results are Monte Carlo corrected for multiple comparisons. The plots show the average cortical thickness values corresponding to each subject of each cohort that are plotted for each of the clusters representing the main effect of migraine disease. CMF = caudal middle frontal; HC-F = female healthy control; HC-M = male healthy control; IT = inferior temporal; LH = left hemisphere; MIG-F = female migraine; MIG-M = male migraine; PC = precentral gyrus; RH = right hemisphere; RMF = rostral middle frontal; SF = superiorfrontal; SM = supramarginal.
Table 2.
Significant clusters from cortical thickness comparisons
| No | Max | VtxMax | Size (mm2) | TalX | TalY | TalZ | CWP | CWPLow | CWPHi | Annotation |
|---|---|---|---|---|---|---|---|---|---|---|
| Disease effect (HC < MIG) | ||||||||||
| 1 | −3.2062 | 100826 | 502.81 | −33.9 | −18.4 | 16.6 | 0.0193 | 0.0175 | 0.0211 | Insula |
| 2 | −4.4865 | 24097 | 379.56 | 21.8 | −65.4 | 12.9 | 0.0001 | 0 | 0.0002 | Cuneus |
| Sex-Disease Interaction (M < F) | ||||||||||
| 1 | 2.6266 | 155434 | 601.37 | −34.8 | −16.1 | 14.2 | 0.0178 | 0.0161 | 0.019 | Insula |
| 2 | 2.9927 | 25994 | 655.39 | 4.8 | −62.3 | 28.4 | 0.0212 | 0.0194 | 0.0231 | Precuneus |
Max = maximum −log10(P-value) in the cluster; VtxMax = vertex number at the maximum; size = surface area of cluster; Tal(XYZ) = the Talairach (MNI305) coordinate of the maximum; CWP = clusterwise P-value.
CWPLow and CWPHi: 90% confidence interval for CWP.
HC = healthy controls; MIG = migraine.
Figure 3.
Parahippocampal volume differences. Comparing sex-related volumetric differences between healthy and migraineur subjects revealed significant differences in the parahippocampal gyrus volume as a function of the disease or health state only in males (P < 0.017) but not in females.
Functional analysis
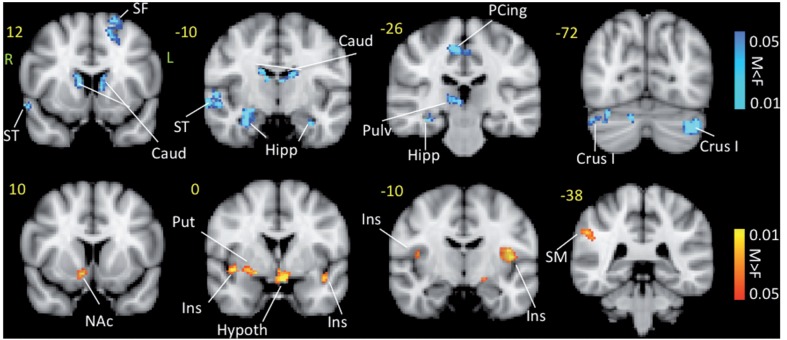
Contrast analysis of the male versus female migraine groups in response to the ‘pain threshold +1°C’ stimuli revealed significant differences between males and females (Fig. 4 and Table 3). Group average results for response to noxious stimulation for migraine males and migraine females alone was evaluated in parallel to the contrast result to determine the direction of response i.e. positive or negative correlation with the stimulus in the regions that showed contrast. Females showed stronger positive blood oxygen level-dependent responses in bilateral caudate, contralateral superior temporal, ipsilateral superior frontal and contralateral precuneus, contralateral posterior cingulate, and in regions corresponding to the contralateral main sensory nucleus and spinal trigeminal nucleus in the brainstem. Males, on the other hand, showed stronger responses in bilateral insula, contralateral S1 and contralateral putamen.
Figure 4.
Contrast maps for painful heat functional MRI activation. Contrast analysis of the male versus female migraine group in response to the ‘pain threshold +1°C’ stimuli revealed significant (P < 0.05, corrected) differences between the two groups. Caud = caudate; F = female; Hipp = hippocampus; Hypoth = hypothalamus; Ins = insula; L = left; M = male; NAc = nucleus accumbens; PCing = posterior cingulate; Pulv = pulvinar; Put = putamen; R = right; SF = superior frontal; SM = somatosensory cortex; ST = superior temporal.
Table 3.
Contrast analysis results for painful heat functional MRI activation
| Brain region | Lat. | z-stat | X (mm) | Y (mm) | Z (mm) | Vol (cm3) |
|---|---|---|---|---|---|---|
| Activations male > female | ||||||
| Insula | R | 1.8035 | 46 | 6 | 0 | 0.44 |
| Insula | R | 2.3373 | 40 | 0 | −6 | 0.464 |
| Rolandic operculum | R | 2.3857 | 48 | 4 | 10 | 0.632 |
| Rolandic operculum | L | 2.6726 | −42 | −8 | 10 | 2.24 |
| Rolandic operculum | L | 1.9971 | −46 | −30 | 22 | 0.8 |
| Putamen | R | 2.1527 | 28 | −2 | −8 | 0.656 |
| SupraMarginal | R | 2.1848 | 56 | −38 | 34 | 0.88 |
| Activations female > male | ||||||
| Superior frontal | L | 2.5885 | −18 | 16 | 46 | 0.544 |
| Precuneus | R | 3.3477 | 8 | −44 | 60 | 0.456 |
| Superior temporal | R | 3.0442 | 62 | −2 | −4 | 0.512 |
| Superior temporal | R | 2.7187 | 58 | −10 | 2 | 0.328 |
| Paracingulate | R | 2.5808 | 8 | 48 | 24 | 0.344 |
| Posterior cingulate | R | 2.6386 | 6 | −20 | 46 | 0.4 |
| Posterior cingulate | R | 2.9012 | 10 | −38 | 36 | 0.28 |
| Caudate | R | 2.7762 | 8 | 14 | 6 | 0.504 |
| Caudate | L | 2.3695 | −10 | 12 | 14 | 0.44 |
| MSN | R | 2.6017 | 12 | −36 | −40 | 0.392 |
| SpV | R | 1.912 | 0 | −40 | −52 | 2.296 |
| Cerebellum_Crus2 | L | 2.6213 | −34 | −68 | −40 | 0.224 |
| Cerebellum_Crus1 | L | 2.4506 | −44 | −72 | −32 | 1.16 |
| Deactivations male > female | ||||||
| NAc | R | −2.181 | 10 | 10 | −10 | 0.6 |
| Hypothalamus* | L | −2.5926 | −4 | 0 | −14 | 0.424 |
| Deactivations female > male | ||||||
| Hippocampus | R | −2.6758 | 30 | −14 | −18 | 0.512 |
| Amygdala | R | −2.5102 | 34 | 0 | −28 | 0.392 |
Contrast analysis of female versus male migraine patients in response to noxious heat (threshold + 1°C). *Activation in males, deactivation in females.
L = left; R = right; MSN = principle sensory trigeminal nucleus/main sensory nucleus of the trigeminal nerve; NAc = nucleus accumbens; SpV = trigeminovascular nucleus.
The contralateral nucleus accumbens, contralateral amygdala and the bilateral hippocampal region showed negative response to stimulus (deactivation). Moreover, the negative response to the stimulus in the amygdala and hippocampus was stronger (i.e. more deactivated) in female subjects and the contralateral nucleus accumbens was more deactivated in males.
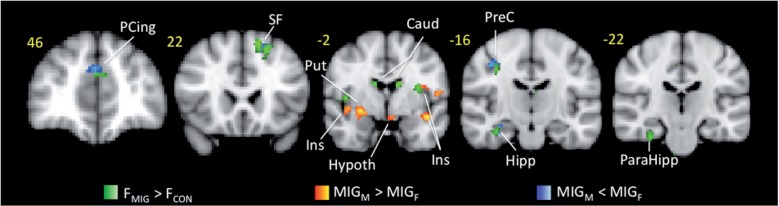
Moreover, contrast analysis of the functional differences in female migraineurs versus female healthy control subjects in response to the ‘pain threshold +1°C’ stimuli revealed significant disease related differences in paracingulate, ipsilateral superior frontal gyrus, contralateral hippocampus, parahippocampal and precentral gyrus (Fig. 6). There results overlap with sex-related differences in patients with migraine, which may support the specificity of the sex-related differences that were observed in migraineurs to migraine disease.
Figure 6.
Overlap of disease-related and sex-related functional differences. Contrast analysis of the functional differences in female migraineurs versus female healthy control subjects (in green) in response to the ‘pain threshold +1°C’ stimuli revealed significant disease related differences that overlap with sex related differences in migraine patients (in red and blue). Caud = caudate; Hypoth = hypothalamus; Ins = insula; MIG-F = female migraine; MIG-M = male migraine; PCing = paracingulate; PreC = precuneus; Put = putamen; SF = superior frontal.
Conjunction analysis
A conjunction of male and female migraineurs pain response maps was calculated to determine areas involved in pain processing that commonly and similarly activated in both males and females. The results of this analysis are shown in Fig. 5 and Table 4.
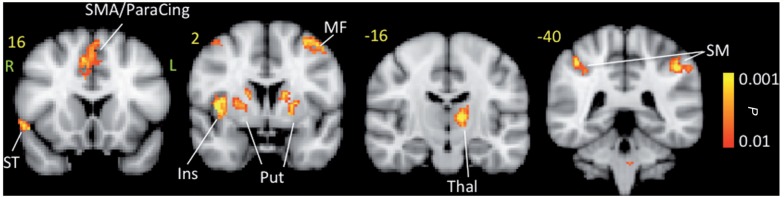
Figure 5.
Conjunction analysis maps for noxious heat response. The maps show common regions of activation for both male and female migraineurs. The active areas are commonly active in both groups but show no significance difference in pain response. Ins = insula; MF = middle frontal; ParaCing = paracingulate; SMA = supplementary motor area; Put = putamen; SM = somatosensory cortex; ST = superior temporal; Thal = thalamus.
Table 4.
Conjunction analysis
| Brain region | Lat. | z-stat | X (mm) | Y (mm) | Z (mm) | Vol (cm3) |
|---|---|---|---|---|---|---|
| Supramarginal | L | 3.0794 | −52 | −24 | 34 | 0.352 |
| Supramarginal | R | 3.4531 | 64 | −30 | 28 | 0.712 |
| Supramarginal | R | 3.2471 | 54 | −34 | 48 | 1.256 |
| Supramarginal | R | 2.9036 | 56 | −36 | 30 | 1.104 |
| SMA/Paracingulate | R | 3.3667 | 4 | 4 | 54 | 1.616 |
| Insula_anterior | R | 3.6828 | 40 | 4 | 6 | 3.272 |
| Thalamus | L | 3.156 | −10 | −16 | 8 | 1.128 |
| Thalamus | L | 2.9265 | −16 | −22 | 8 | 0.248 |
| Putamen | R | 2.9635 | 20 | 14 | 0 | 0.664 |
| Putamen | L | 2.9668 | −24 | 2 | 6 | 0.696 |
| Putamen | R | 3.1208 | 26 | 0 | 6 | 0.64 |
| Caudate | L | 3.1483 | −14 | 0 | 16 | 0.64 |
| Middle frontal | L | 3.0228 | −42 | 4 | 54 | 0.552 |
| Superior frontal | L | 2.9196 | −14 | 10 | 68 | 0.728 |
| Temporal pole superior | R | 3.0409 | 54 | 16 | −6 | 0.36 |
Conjunction analysis of response to noxious heat (threshold +1°C) in female versus male migraine patients.
SMA = Supplementary Motor Area.
Functional connectivity analysis
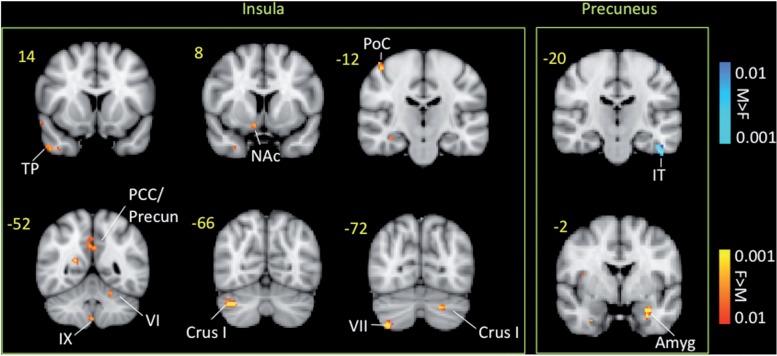
Based on the results of the cortical thickness comparisons, functional connectivity was performed for seed regions of interest that were created from the insular cluster and the precuneus cluster that showed significant cortical thickness differences between male and female migraineurs. The results of the functional connectivity analysis for these seed regions of interest are presented in Fig. 7 and Table 5. Female migraineurs showed significantly stronger negative functional connectivity between the insular region of interest and brain areas such as S1, posterior cingulate, precuneus and temporal pole. Significant differences in the functional connectivity of the precuneus region of interest were observed with amygdala and S1 (stronger in female migraineurs). Results of the connectivity analysis for both male and female migraine subjects are presented in Supplementary Fig. 2.
Figure 7.
Functional connectivity contrast maps of the insula and precuneus regions of interest. The insula and precuneus regions of interest were defined for each subject by transforming the cluster of significant cortical thickness differences between migraine and healthy subjects to each subject’s anatomical space. Amyg = amygdala; F = female; M = male; NAc = nucleus accumbens; PoC = postcentral gyrus; Precun = precuneus; SM = somatosensory cortex; TP = temporal pole.
Table 5.
Functional connectivity analysis results
| Brain region | Lat. | z-stat | X (mm) | Y (mm) | Z (mm) | Vol (cm3) |
|---|---|---|---|---|---|---|
| Functional connectivity for insula | ||||||
| Deactivations female > male | ||||||
| Superior frontal | R | −3.179 | 10 | 28 | 52 | 0.216 |
| Postcentral | R | −3.1995 | 46 | −20 | 58 | 0.32 |
| Precuneus | R | −3.0444 | 20 | −54 | 14 | 0.28 |
| Temporal pole | R | −2.6689 | 48 | 12 | −38 | 0.272 |
| Posterior cingulate | L | −2.7418 | 0 | −54 | 32 | 0.536 |
| Cerebellum Crus1 | R | −3.2979 | 38 | −66 | −36 | 0.376 |
| Cerebellum Crus1 | L | −3.1669 | −22 | −70 | −34 | 0.304 |
| Functional connectivity for precuneus | ||||||
| Activations female > male | ||||||
| Amygdala | L | −2.565 | −32 | −8 | −20 | 0.296 |
| Supramarginal | R | −2.5423 | 50 | −30 | 38 | 0.32 |
Functional connectivity analysis results of female versus male migraine patients in response to noxious heat (threshold ±1°C).
Discussion
Relatively few reports are available on sex differences in brain function in disease states (Gong et al., 2011). Like many chronic pain conditions (Unruh, 1996; Fillingim et al., 2009), migraine is a disease predominantly affecting females (Russell et al., 1995; Brandes, 2006; Le et al., 2011). The current study investigated sex-related differences in the interictal migraine brain. We controlled for multiple aspects of disease burden by evaluating females and males who had a history of migraine that were matched for age, age of onset, medication type and frequency of migraine attacks. There were three major findings of this study: (i) disease-related structural changes in insula and precuneus regions were specific to female migraineurs; (ii) disease-related structural changes in parahippocampal gyrus were specific to male migraineurs; and (iii) within the migraine group, functional changes in response to noxious heat showed more pronounced responses in female migraineurs in regions such as the amygdala and parahippocampus.
Structural and functional alterations in the insula and precuneus differentiate female from male migraineurs
One of the most notable findings in this study was that the left posterior insula was thicker in female migraineurs compared with both male migraineurs and healthy controls of both sexes. As previous studies on cortical thickness in healthy subject have not found sex differences in the posterior insula (Im et al., 2006; Sowell et al., 2007), the present finding may indicate some specificity of altered function in this region in female migraineurs. The basis for the observed differences is unknown but there is multiple evidence for insular involvement in interoception (Craig, 2009), emotional processing (Wiebking et al., 2011) and pain perception (Apkarian et al., 2009). As such, the observed alterations may be consistent with the higher pain unpleasantness experienced by female migraineurs in our data. Further, changes in insula processing are associated with menstrual cycle (de Leeuw et al., 2006; Tu et al., 2010) and there are sex differences in default mode network that includes insular processing (Lopez-Larson et al., 2011). Insular networks may also be altered by migraine (Maleki et al., 2012) in a similar manner to how these systems are changed in neuropathic pain (Cauda et al., 2010).
Both the anterior and the posterior insula were activated in response to painful stimuli, which was greater in male migraineurs than in female migraineurs. Previous functional MRI studies on sex differences in healthy subjects had found that males have greater pain activation of the insular cortex (Paulson et al., 1998; Derbyshire et al., 2002; Moulton et al., 2006; Straube et al., 2009; Kong et al., 2010), but sex-similar insula activation to pain has also been observed (Paulson et al., 1998; Moulton et al., 2006). Our results further suggest an inverse structural–functional relationship, i.e. decreased activation in a thicker insula in females. Such decreased activation and increased thickness have been observed in the basal ganglia in migraine (Maleki et al., 2011). In support of this functional/structural change, the functional connectivity analysis indicated that female migraineurs displayed a significant negative connectivity between the (thick) posterior insula and the primary somatosensory area, posterior cingulate, precuneus and temporal pole that was not present in male migraineurs. This may be in line with a recent resting state functional connectivity study indicating that female migraineurs may be more vulnerable to the disease in terms of network robustness, nodal centrality, and functional connections (Liu et al., 2011a).
We also observed sex differences of the precuneus in migraine. Female migraineurs displayed significant thickening in the dorsal and ventral portion of the precuneus in comparison to male migraineurs and healthy controls (Fig. 2). The precuneus region can be divided into three functional regions (Margulies et al., 2009): the anterior precuneus involved in sensorimotor processing (functionally connected with the superior parietal cortex, paracentral lobule and motor cortex); the central precuneus involved in cognition and associative processing (functionally connected with the dorsolateral prefrontal, dorsomedial prefrontal and multimodal lateral inferior parietal cortex); and the posterior cuneus involved in visual processing (functionally connected with adjacent visual cortical regions). The segregation may provide a model for precuneal involvement in migraine as alterations in particularly sensorimotor and associative/cognitive processing have been reported. For example, behavioural changes in patients with migraine include disturbances of body image and physical sensations (Spitzer, 1988), including alteration of time sense (Golden, 1979), indicative of alteration of self-perception and issues related to expected pain as may be the case in a disease that is recurrent (Koyama et al., 2005). Are these processes more involved in females? Brain imaging studies have indicated higher perfusion in the precuneus in females (Liu et al., 2011b). The region has one of the highest resting metabolic rates that decreases during engagement in non-self referential goal-directed actions and is part of the default mode network that is active at rest and task-independent introspection (Greicius et al., 2003). As assessed by diffusion tensor imaging, the precuneus forms part of a ‘rich club’ hub interconnected organization that includes superior frontal and superior parietal cortex, as well as the subcortical hippocampus, putamen and the thalamus (van den Heuvel and Sporns, 2011), indicative of its involvement in global integration of information. Perhaps a compelling potential function of the precuneus may be that active inhibition of arousal systems by migraine in some cortical regions leads to cortical deactivation in other cortical areas (Danielson et al., 2011). A recent study (Tomasi and Volkow, 2011) in healthy adults has found that while precuneus is the main resting state functional connectivity hub for both males and females, the degree of connectivity is 14% higher in females. In comparing disease-only differences in male and female subjects (Supplementary Fig. 1), we found significant difference in the cortical thickness of the female migraineurs in female subjects but not male subjects. In support of this, Liu et al. (2011a) found significant alterations of cuneus resting state connectivity in female, but not male, migraineurs. Taken together, this suggests there may be a network of brain regions that includes posterior insula and the precuneus that may play a dominant role in sex-related brain differences in migraine.
Female migraineurs show greater activation in brain regions involved in emotional processing
While the thermal pain thresholds were similar across both disease and sexes, female migraineurs reported similar intensity but higher unpleasantness of their headache, a difference that may relate to the observed sex differences in brain regions involved in emotional processing; the amygdala, parahippocampus, basal ganglia and posterior cingulate cortex.
Sex differences in migraineurs were also present in the parahippocampus, a region involved in numerous behaviours including stress and anxiety (Sauro and Becker, 2009) and the integration of complex information (Eichenbaum et al., 1992). The parahippocampal grey matter volume was smaller in male migraineurs compared with female migraineurs, consistent with some (Good et al., 2001), but not all (Pletzer et al., 2010) of the literature on sex differences in healthy populations. Because male migraineurs had lower parahippocampal volumes than did both female migraineurs and healthy males, we interpret these results as a true migraine-related decrease in males. The potential reasons for the observed changes include differences in the response to intermittent stress (migraine attacks), differential effects of gonadal (testosterone versus oestradiol or progesterone) on hippocampal function (Shors et al., 2001; Craft et al., 2004), and as noted above, differential effects of treatments (triptans) on brain systems (neurotransmitter availability and response). In male rats, chronic foot-shock stress leads to reduced hippocampal neurogenesis whereas female rats show no change or even increased hippocampal neurogenesis (Westenbroek et al., 2004; McLaughlin et al., 2009). Moreover, our functional studies showed a larger blood oxygen level-dependent response to pain in female versus male migraineurs. In our prior studies we reported that applying noxious heat resulted in an increased response in the same region in migraineurs (five females, three males) compared with healthy controls (Moulton et al., 2011).
The observed differences (female > male) in activation of the spinal trigeminal nucleus (shown to be activated in prior pain imaging studies in humans; DaSilva et al., 2002; Borsook et al., 2003), may relate to the differences in sensitivity of the trigeminal system in females versus males due to hormonal (Martin et al., 2007; Bolay et al., 2011; Tashiro et al., 2012) or yet undefined processes. Given the activated region in the brainstem and its extent and the landmarks that we use to locate spinal trigeminal nucleus and the differences observed may be relevant in migraine pathogenesis that contribute to the migraine gender differences observed in higher brain.
While this study does not provide data on functional differences in healthy male versus female subjects, there is a wealth of literature (Fillingim et al., 2009) on examining sex differences in brain activation to experimental pain in healthy subjects using various imaging modalities as summarized in Table 6 (Paulson et al., 1998; Derbyshire et al., 2002; Naliboff et al., 2003; Hobson et al., 2005; Berman et al., 2006; Chen et al., 2006; Moulton et al., 2006; Henderson et al., 2008; Fillingim et al., 2009; Straube et al., 2009; Kong et al., 2010; Ozawa et al., 2011). The evidence regarding sex differences from these studies is mixed and inconsistent in terms of the regions found and the direction of the differences (males > females or females > males). However, independent of the direction of the differences there are a couple of regions that are common to most of these studies and show differences between male versus female subjects including the anterior mid cingulate, S1, anterior insula and dorsolateral prefrontal cortex. In this study’s conjunction analysis of pain activation in both male and female migraineurs, we found common activation in contra-lateral anterior insula and bilateral S1 (Fig. 5 and Table 4). The male versus female migraineurs contrast analysis revealed differences in regions that have not commonly been observed in healthy subjects, i.e. the hippocampus, basal ganglia (caudate, putamen and nucleus accumbens), hypothalamus and posterior insula. Moreover, in contrasting healthy females with female migraineurs (Fig. 6), we found disease-related differences that overlapped with the sex-related differences in migraine patients in regions such as paracingulate, superior frontal gyrus, hippocampus, parahippocampal and precentral gyrus. This may suggest that these differences could be specific to female migraine.
Table 6.
Sex differences in evoked brain response to pain in healthy subjects
| M/F | Modality | Stimulation | Differences | Author |
|---|---|---|---|---|
| 28/33 | fMRI | Heat | M > F ACC, Insula, S2, Thalamus, DLPFC, MPFC | Kong et al. (2010) |
| 7/6 | fMRI | Visceral pressure | M > F Insula | Berman et al. (2006) |
| F > M Amygdala, Cingulate (mid) | ||||
| 11/11 | fMRI | Saline | M > F Cerebellum | Henderson et al. (2008) |
| F > M DLPFC, Cingulate (mid) | ||||
| 12/12 | fMRI | Electrical | F > M MPFC | Straube et al. (2009) |
| 11/17 | fMRI | Heat | F > M Anterior Insula, S1, DLPFC | Moulton et al. (2006) |
| 19/23 | fMRI | Pressure | M > F Insula, PAG, DLPFC | Naliboff et al. (2003) |
| F > M MPFC, ACC, Amygdala | ||||
| 8/8 | MEG | Electrical | No sex differences | Hobson et al. (2005) |
| 10/10 | PET | Heat | F> M PFC, Insula, Thalamus | Paulson et al. (1998) |
| 11/10 | PET | Heat | M > F PFC, S2, S1, Insula | Derbyshire et al. (2002) |
| F > M Cingulate | ||||
| 12/16 | ERP | Intranasal trigeminal stimulation | F > M, P3 component | Fillingim et al. (2009) |
| 21/19* | NIRS | Venous puncture | No sex differences | Ozawa et al. (2011) |
| 16/19 | EP | Heat | No sex differences | Chen et al. (2006) |
| 26/34 | fMRI | Heat | M > F PAG connectivity to amygdala and BG. | Linnman et al. (2011) |
*Neonates.
ACC = anterior cingulate cortex; BG = basal ganglia; DLPFC = dorsolateral prefrontal cortex; EP = echo planar; ERP = event-related evoked potentials; F = female; fMRI = functional MRI; MEG = magnetoencephalography; MPFC = medial prefrontal cortex; NIRS = near-infrared spectroscopy; PAG = periaqueductal grey; PFC = prefrontal cortex.
Study limitations
There are a number of caveats summarized here and discussed in detail in the Supplementary material. The number of subjects studied is relatively small for multiple groups that were matched; our results still show significance after correction for multiple comparisons. Functional data were not acquired in healthy male subjects, limiting the specificity of the functional data analysis. Instead, we have attempted to frame the results in comparison to other studies on sex-related differences in healthy subjects. Females were studied without assessing stage of the menstrual cycle. Menstrual cycle affects pain processing as shown by non-imaging and imaging studies (Goldstein et al., 2005, 2010; Choi et al., 2006; Pletzer et al., 2010). Contributions of other morphological changes such as measures of white matter abnormalities (DaSilva et al., 2007; Rocca et al., 2008) were not assessed. Clearly these may contribute to the understanding of how regions and pathways may affect function. Migraine is itself a form of recurrent painful attacks and although this study showed gender-specific differences in migraine it remains unclear if similar patterns could be seen in other pain conditions, which remain to be studied. Finally, the issue of the potential influence of medications (i.e. triptans and non-steroidal drugs) as a confounding variable, which is discussed in detail elsewhere (Maleki et al., 2011); it should be noted that the migraine cohorts were matched for medication use.
Conclusion
A substantial difference in both morphometric measures and functional activation in response a noxious stimulus is reported in female versus male migraineurs during their interictal state. The differences suggest a differential effect of the disease in female versus male migraineurs. Given this, it is important to define experimental approaches that take sex into account. In the migraine imaging literature, most of the participants are female. The present data may reflect sex-related differences in the underlying migraine pathology and justifies exploring sex-specific drug development and treatment approaches for migraine in order to facilitate better outcomes for patients.
Funding
The work was supported in by grants from NIH (K24 NS064050 (NINDS), R01-NS056195 (NINDS) and R01-NS073997 (NINDS) to D.B. C.L. received support from the IASP Early Career Grant and the Swedish Society for Medical Research (SSMF).
Supplementary material
Supplementary material is available at Brain online.
References
- Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Progress in neurobiology. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Krauss BR, Fredrickson BE, Szeverenyi NM. Imaging the pain of low back pain: functional magnetic resonance imaging in combination with monitoring subjective pain perception allows the study of clinical pain states. Neurosci Lett. 2001;299:57–60. doi: 10.1016/s0304-3940(01)01504-x. [DOI] [PubMed] [Google Scholar]
- Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, et al. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006;26:10646–57. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Bueller JA, et al. Sex differences in regional brain response to aversive pelvic visceral stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291:R268–76. doi: 10.1152/ajpregu.00065.2006. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Ashina S, Burstein R, Reed ML, Buse D, Serrano D, et al. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology. 2008;70:1525–33. doi: 10.1212/01.wnl.0000310645.31020.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolay H, Berman NE, Akcali D. Sex-related differences in animal models of migraine headache. Headache. 2011;51:891–904. doi: 10.1111/j.1526-4610.2011.01903.x. [DOI] [PubMed] [Google Scholar]
- Brandes JL. The influence of estrogen on migraine: a systematic review. JAMA. 2006;295:1824–30. doi: 10.1001/jama.295.15.1824. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–38. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010;68:81–91. doi: 10.1002/ana.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun A, Ford S. Elimination of menstrual-related migraine beneficially impacts chronification and medication overuse. Headache. 2008;48:1186–93. doi: 10.1111/j.1526-4610.2008.01176.x. [DOI] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Cocito D, Paolasso I, et al. Altered resting state attentional networks in diabetic neuropathic pain. J Neurol Neurosurg Psychiatry. 2010;81:806–11. doi: 10.1136/jnnp.2009.188631. [DOI] [PubMed] [Google Scholar]
- Chen IA, Hung SW, Chen YH, Lim SN, Tsai YT, Hsiao CL, et al. Contact heat evoked potentials in normal subjects. Acta Neurol Taiwan. 2006;15:184–91. [PubMed] [Google Scholar]
- Choi JC, Park SK, Kim YH, Shin YW, Kwon JS, Kim JS, et al. Different brain activation patterns to pain and pain-related unpleasantness during the menstrual cycle. Anesthesiology. 2006;105:120–7. doi: 10.1097/00000542-200607000-00021. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–55. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Danielson NB, Guo JN, Blumenfeld H. The default mode network and altered consciousness in epilepsy. Behav Neurol. 2011;24:55–65. doi: 10.3233/BEN-2011-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva AF, Granziera C, Tuch DS, Snyder J, Vincent M, Hadjikhani N. Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. Neuroreport. 2007;18:301–5. doi: 10.1097/WNR.0b013e32801776bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw R, Davis CE, Albuquerque R, Carlson CR, Andersen AH. Brain activity during stimulation of the trigeminal nerve with noxious heat. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:750–7. doi: 10.1016/j.tripleo.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Nichols TE, Firestone L, Townsend DW, Jones AK. Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. J Pain. 2002;3:401–11. doi: 10.1054/jpai.2002.126788. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. The hippocampus–what does it do? Behav Neural Biol. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Tiraferri I, Neri L, Sternieri E. Why pharmacokinetic differences among oral triptans have little clinical importance: a comment. J Headache Pain. 2011;12:5–12. doi: 10.1007/s10194-010-0258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden GS. The Alice in Wonderland syndrome in juvenile migraine. Pediatrics. 1979;63:517–9. [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci. 2010;30:431–8. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25:9309–16. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, He Y, Evans AC. Brain connectivity: gender makes a difference. Neuroscientist. 2011;17:575–91. doi: 10.1177/1073858410386492. [DOI] [PubMed] [Google Scholar]
- Gonoi W, Abe O, Yamasue H, Yamada H, Masutani Y, Takao H, et al. Age-related changes in regional brain volume evaluated by atlas-based method. Neuroradiology. 2010;52:865–73. doi: 10.1007/s00234-009-0641-5. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti V, Alberton S, Galli F, Salvi E. Gender, migraine and affective disorders in the course of the life cycle. Funct Neurol. 2009;24:29–40. [PubMed] [Google Scholar]
- Hedborg K, Anderberg UM, Muhr C. Stress in migraine: personality-dependent vulnerability, life events, and gender are of significance. Ups J Med Sci. 2011;116:187–99. doi: 10.3109/03009734.2011.573883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LA, Gandevia SC, Macefield VG. Gender differences in brain activity evoked by muscle and cutaneous pain: a retrospective study of single-trial fMRI data. Neuroimage. 2008;39:1867–76. doi: 10.1016/j.neuroimage.2007.10.045. [DOI] [PubMed] [Google Scholar]
- Hobson AR, Furlong PL, Worthen SF, Hillebrand A, Barnes GR, Singh KD, et al. Real-time imaging of human cortical activity evoked by painful esophageal stimulation. Gastroenterology. 2005;128:610–9. doi: 10.1053/j.gastro.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Im K, Lee JM, Lee J, Shin YW, Kim IY, Kwon JS, et al. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. NeuroImage. 2006;31:31–8. doi: 10.1016/j.neuroimage.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Kim JH, Suh SI, Seol HY, Oh K, Seo WK, Yu SW, et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia : Int J Headache. 2008;28:598–604. doi: 10.1111/j.1468-2982.2008.01550.x. [DOI] [PubMed] [Google Scholar]
- Koepp MJ. Gender and drug effects on neuroimaging in epilepsy. Epilepsia. 2011;52(Suppl 4):35–7. doi: 10.1111/j.1528-1167.2011.03150.x. [DOI] [PubMed] [Google Scholar]
- Kong J, Loggia ML, Zyloney C, Tu P, Laviolette P, Gollub RL. Exploring the brain in pain: activations, deactivations and their relation. Pain. 2010;148:257–67. doi: 10.1016/j.pain.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci USA. 2005;102:12950–5. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le H, Tfelt-Hansen P, Russell MB, Skytthe A, Kyvik KO, Olesen J. Co-morbidity of migraine with somatic disease in a large population-based study. Cephalalgia: Int J Headache. 2011;31:43–64. doi: 10.1177/0333102410373159. [DOI] [PubMed] [Google Scholar]
- Linnman C, Beucke JC, Jensen KB, Gollub RL, Kong J. Sex similarities and differences in pain-related periaqueductal gray connectivity. Pain. 2011;153:444–54. doi: 10.1016/j.pain.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Qin W, Nan J, Li J, Yuan K, Zhao L, et al. Gender-related differences in the dysfunctional resting networks of migraine suffers. PLoS One. 2011a;6:e27049. doi: 10.1371/journal.pone.0027049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhu X, Feinberg D, Guenther M, Gregori J, Weiner MW, et al. Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magn Reson Med. 2011b doi: 10.1002/mrm.23286. epub ahead of print, doi:10/1002/mrm.23286. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, Anderson JS, Ferguson MA, Yurgelun-Todd D. Local Brain Connectivity and Associations with Gender and Age. Dev Cogn Neurosci. 2011;1:187–97. doi: 10.1016/j.dcn.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailis-Gagnon A, Giannoylis I, Downar J, Kwan CL, Mikulis DJ, Crawley AP, et al. Altered central somatosensory processing in chronic pain patients with "hysterical" anesthesia. Neurology. 2003;60:1501–7. doi: 10.1212/wnl.60.9.1501. [DOI] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Nutile L, Pendse G, Brawn J, Bigal M, et al. Migraine attacks the Basal Ganglia. Mol Pain. 2011;7:71. doi: 10.1186/1744-8069-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D, et al. Concurrent functional and structural cortical alterations in migraine. Cephalalgia. 2012;32:607–20. doi: 10.1177/0333102412445622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA. 2009;106:20069–74. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin VT, Lee J, Behbehani MM. Sensitization of the trigeminal sensory system during different stages of the rat estrous cycle: implications for menstrual migraine. Headache. 2007;47:552–63. doi: 10.1111/j.1526-4610.2007.00714.x. [DOI] [PubMed] [Google Scholar]
- Matharu MS, Good CD, May A, Bahra A, Goadsby PJ. No change in the structure of the brain in migraine: a voxel-based morphometric study. Eur J Neurol. 2003;10:53–7. doi: 10.1046/j.1468-1331.2003.00510.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Mol Neurobiol. 2009;40:166–82. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Becerra L, Maleki N, Pendse G, Tully S, Hargreaves R, et al. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb Cortex. 2011;21:435–48. doi: 10.1093/cercor/bhq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton EA, Burstein R, Tully S, Hargreaves R, Becerra L, Borsook D. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One. 2008;3:e3799. doi: 10.1371/journal.pone.0003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, Maitra R, Greenspan JD. Sex differences in the cerebral BOLD signal response to painful heat stimuli. Am J Physiol. 2006;291:R257–67. doi: 10.1152/ajpregu.00084.2006. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Chang L, Derbyshire SW, Suyenobu B, Vogt BA, et al. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–47. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Kanda K, Hirata M, Kusakawa I, Suzuki C. Effect of gender and hand laterality on pain processing in human neonates. Early Hum Dev. 2011;87:45–8. doi: 10.1016/j.earlhumdev.2010.09.371. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Minoshima S, Morrow TJ, Casey KL. Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain. 1998;76:223–9. doi: 10.1016/s0304-3959(98)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendse G, Borsook D, Becerra L. Enhanced false discovery rate using Gaussian mixture models for thresholding fMRI statistical maps. Neuroimage. 2009;47:231–61. doi: 10.1016/j.neuroimage.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer B, Kronbichler M, Aichhorn M, Bergmann J, Ladurner G, Kerschbaum HH. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Res. 2010;1348:55–62. doi: 10.1016/j.brainres.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Ceccarelli A, Falini A, Colombo B, Tortorella P, Bernasconi L, et al. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke. 2006;37:1765–70. doi: 10.1161/01.STR.0000226589.00599.4d. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Pagani E, Colombo B, Tortorella P, Falini A, Comi G, et al. Selective diffusion changes of the visual pathways in patients with migraine: a 3-T tractography study. Cephalalgia. 2008;28:1061–8. doi: 10.1111/j.1468-2982.2008.01655.x. [DOI] [PubMed] [Google Scholar]
- Russell MB, Rasmussen BK, Thorvaldsen P, Olesen J. Prevalence and sex-ratio of the subtypes of migraine. Int J Epidemiol. 1995;24:612–8. doi: 10.1093/ije/24.3.612. [DOI] [PubMed] [Google Scholar]
- Russo A, Tessitore A, Esposito F, Marcuccio L, Giordano A, Conforti R, et al. Pain processing in patients with migraine: an event-related fMRI study during trigeminal nociceptive stimulation. J Neurol. 2012 doi: 10.1007/s00415-012-6438-1. epub ahead of print, doi:10/1007/s.00415-012-6438-1. [DOI] [PubMed] [Google Scholar]
- Sauro KM, Becker WJ. The stress and migraine interaction. Headache. 2009;49:1378–86. doi: 10.1111/j.1526-4610.2009.01486.x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Ganssbauer S, Neuner T, Bogdahn U, May A. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia: Int J Headache. 2008;28:1–4. doi: 10.1111/j.1468-2982.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Admiraal-Behloul F, Arkink EB, Kruit MC, Schoonman GG, Ferrari MD, et al. Attack frequency and disease duration as indicators for brain damage in migraine. Headache. 2008;48:1044–55. doi: 10.1111/j.1526-4610.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Arkink EB, Mulder M, Rubia K, Admiraal-Behloul F, Schoonman GG, et al. Frontal lobe structure and executive function in migraine patients. Neurosci Lett. 2008;440:92–6. doi: 10.1016/j.neulet.2008.05.033. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–29. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–7. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyti R, de Vries B, van den Maagdenberg A. Migraine genes and the relation to gender. Headache. 2011;51:880–90. doi: 10.1111/j.1526-4610.2011.01913.x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cerebral Cortex. 2007;17:1550–60. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer M. [Perceptional disorders in migraine.] Schmerz. 1988;2:66–72. doi: 10.1007/BF02528677. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64–9. [PubMed] [Google Scholar]
- Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Sex differences in brain activation to anticipated and experienced pain in the medial prefrontal cortex. Hum Brain Mapp. 2009;30:689–98. doi: 10.1002/hbm.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Sex differences in brain activation to anticipated and experienced pain in the medial prefrontal cortex. Hum Brain Mapp. 2009;30:689–98. doi: 10.1002/hbm.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Bereiter DA. Rapid estrogenic effects on TMJ-responsive brainstem neurons. J Dent Res. 2012;91:210–4. doi: 10.1177/0022034511428156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Russo A, Esposito F, Giordano A, Taglialatela G, De Micco R, et al. Interictal cortical reorganization in episodic migraine without aura: an event-related fMRI study during parametric trigeminal nociceptive stimulation. Neurol Sci. 2011;32(Suppl 1):S165–7. doi: 10.1007/s10072-011-0537-0. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Gender differences in brain functional connectivity density. Hum Brain Mapp. 2011;33:849–60. doi: 10.1002/hbm.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu CH, Niddam DM, Chao HT, Chen LF, Chen YS, Wu YT, et al. Brain morphological changes associated with cyclic menstrual pain. Pain. 2010;150:462–8. doi: 10.1016/j.pain.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–67. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- Valfre W, Rainero I, Bergui M, Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48:109–17. doi: 10.1111/j.1526-4610.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J Neurosci. 2011;31:15775–86. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull. 2004;64:303–8. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Wiebking C, de Greck M, Duncan NW, Heinzel A, Tempelmann C, Northoff G. Are emotions associated with activity during rest or interoception? An exploratory fMRI study in healthy subjects. Neurosci Lett. 2011;491:87–92. doi: 10.1016/j.neulet.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol. 2008;100:1740–8. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.