This report stresses that a thorough knowledge of electrosurgical fundamentals by the entire operative team is essential for patient safety and recognizing potential complications.
Keywords: Electrosurgery, Electrosurgical safety, Laparoscopic electrosurgery
Abstract
Background:
Electrosurgical units are the most common type of electrical equipment in the operating room. A basic understanding of electricity is needed to safely apply electrosurgical technology for patient care.
Methods:
We reviewed the literature concerning the essential biophysics, the incidence of electrosurgical injuries, and the possible mechanisms for injury. Various safety guidelines pertaining to avoidance of injuries were also reviewed.
Results:
Electrothermal injury may result from direct application, insulation failure, direct coupling, capacitive coupling, and so forth.
Conclusion:
A thorough knowledge of the fundamentals of electrosurgery by the entire team in the operating room is essential for patient safety and for recognizing potential complications. Newer hemostatic technologies can be used to decrease the incidence of complications.
INTRODUCTION
A basic understanding of electricity is needed to safely apply electrosurgical technology for patient care.1 Electrosurgery is one of the most commonly used energy systems in laparoscopic surgery.2 The surgical team should have a good understanding of the principles of electrosurgery and tissue effects to avoid complications. The risk of complications is linked to the surgeon's fundamental knowledge of instruments, surgical technique, biophysics, relevant anatomy, and safe technical equipment. The risk of complications is linked to fundamental surgical knowledge of instruments, surgical technique, biophysics, and relevant anatomy. Appropriately applied, electrosurgery is safe and effective. Electrothermal injury may result from direct application, insulation failure, direct coupling, and capacitive coupling.3
History
The conception of electrosurgery began in the early 19th century when the French physicist Becquerel first used electrocautery. Rather than using boiled oil to achieve hemostasis, he passed direct current through a wire thereby heating it and effectively cauterizing tissue upon contact. In 1881, D. Arsonoval pioneered the use of alternating current.
It was not until the late 1920s that collaboration between the physicist, William T. Bovie and the neurosurgeon Harvey Cushing resulted in the predecessor of today's electrosurgical unit. This model was used until 1968 when a smaller model was developed by Valleylab, which has since produced today's platform of electrosurgical units.3
BASIC PRINCIPLES OF ELECTROSURGERY
Energy in wattage (power) is the product of current and voltage. Power is the amount of current times the voltage level at a given point measured in wattage or watts (W). It corresponds to the rate of work being performed, W=V×I.
Ohm's law, I=V/R, shows the relationship between the properties of electrosurgical energy.
Current (I) is what flows on a wire or conductor like water flowing down a river. Current flows from negative to positive on the surface of a conductor. Current is measured in amperes (A) or amps.
Voltage (V) is the difference in electrical potential between 2 points in a circuit. It is the push or pressure behind current flow through a circuit and is measured in volts (V).
Resistance determines how much current will flow through a component. Resistors are used to control voltage and current levels. A very high resistance allows a small amount of current to flow. A very low resistance allows a large amount of current to flow. Resistance is measured in Ω ohms.
Principles of Electrosurgery
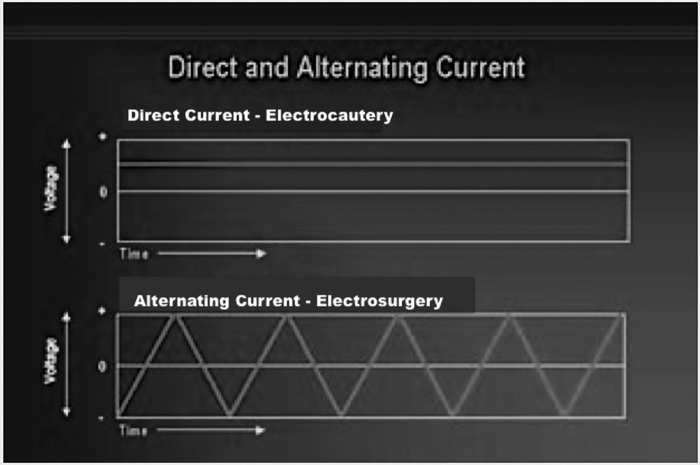
Often “electrocautery” is used to describe electrosurgery. This is incorrect. Electrocautery refers to direct current (electrons flowing in one direction), whereas electrosurgery uses alternating current (Figure 1). Modern day electrosurgery is the utilization of alternating current at radiofrequency levels. During electrocautery, current does not enter the patient's body. Only the heated wire comes in contact with tissue. In electrosurgery, the patient is included in the circuit and current enters the patient's body.
Figure 1.
Direct and alternating current.
Electrical current flows when electrons from one atom move to an adjacent atom through a circuit. Heat is produced when electrons encounter resistance. For current to flow, a continuous circuit is needed. In the operating room, the circuit is composed of the patient, the electrosurgical generator, the active electrode and the return electrodes. The electrosurgical unit is the source of the voltage.4–6
Electrical energy is converted to heat in tissue as the tissue resists the flow of current from the electrode. Three tissue effects are possible with today's electrosurgical units—cutting, desiccation, and fulguration.7 Achieving these effects depends on the following factors: current density, time, electrode size, tissue conductivity, and current waveform.6,8
1. Current density
As expected, the greater the current that passes through an area, the greater the effect will be on the tissue.4
2. Time
The length of time a surgeon uses an active electrode determines the tissue effect. Too long an activation will produce wider and deeper tissue damage. Too short an activation will result in absence of the desired tissue effect.9
3. Electrode Size
With respect to electrode size, smaller electrodes provide a higher current density and result in a concentrated heating effect at the site of tissue contact. Following the same principle, the patient return electrode used in monopolar electrosurgery is large in relation to the active electrode in order to disperse the current returning to the electrosurgical unit and minimize heat production at this return electrode site.6–8
4. Tissue Conductivity
Various tissue types have a different electrical resistance, which affects the rate of heating. Adipose tissue and bone have high resistance and are poor conductors of electricity, whereas muscle and skin are good conductors of electricity and have low resistance.7,10
5. Current Waveforms
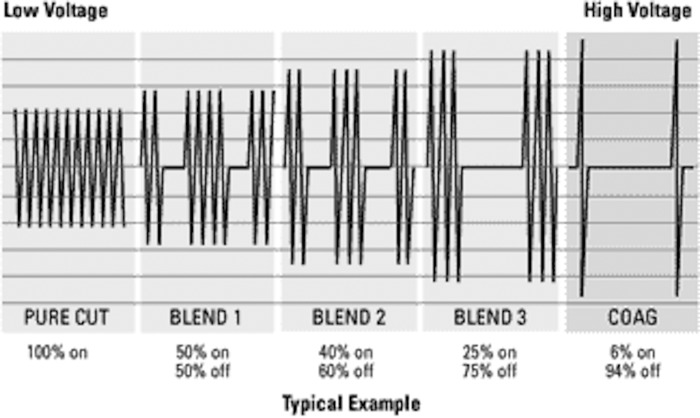
The final determinant of how tissue responds to electrosurgery is the current type. Electrosurgical units produce 3 different waveforms: cut, blend, and coagulation (Figure 2).9
Figure 2.
Wave forms of electrosurgical units with different tissue effects.
A pure cutting (vaporization) waveform is continuous, unmodulated, and undamped. A coagulation waveform is interrupted, modulated, and damped current.11,12 A blend waveform is a modification of the cutting waveform and is used when hemostasis is needed while cutting.5,6 This waveform type consists of a combination of both cutting and coagulation waveforms.4 Higher blend settings translate into more time between bursts of current and greater coagulation, as seen in the following examples: Blend 1 (80% cut, 20% coagulation); Blend 2 (60% cut, 40% coagulation); and Blend 3 (50% cut, 50% coagulation).9
A cutting current power setting must be between 50W and 80W to be effective. Ideally, the electrode is held slightly away from the tissue to create a spark gap or steam envelope through which the current arcs to the tissue. This spark gap results from heating up the atmosphere between the electrode and the tissue.13 The coagulation current is effective with the power settings in the range of 30W and 50W.6
Fulguration (Spray) is a noncontact coagulation that also utilizes spark gap to mediate tissue effects, which results in heating and necrosis as well as greater thermal spread. Desiccation (Deep) is another form of coagulation in which direct contact is made with the tissue, resulting in electrical energy being converted into heat within the tissue. The end result is deeper necrosis and greater thermal spread.9
Table 1 is giving an overview of the tissue effects of the two traditional and two innovative energy modalities.
Table 1.
Comparison of Tissue Effects of 4 Energy Modalities4
| Monopolar | Traditional Bipolar | Advanced Bipolar | Ultrasonic | |
|---|---|---|---|---|
| Tissue Effect | Cutting, Coagulation | Coagulation | Cutting, coagulation | Cutting, coagulation |
| Power Setting | 50–80 W | 30–50W | DEFAULT | 55,000 Hz frequency |
| Thermal Spread | Not well assessed | 2–6mm | 1–4mm | 1–4mm |
| Maximum Temperature | >100°C | >100°C | Not well assessed | <80°C |
| Vessel Sealing Ability | Not applicable | Not applicable | Seals vessels ≤7mm | Seals vessels ≤5mm |
| Technique | Not applicable | Not applicable | Tension free application | Tension free application |
In monopolar electrodes, radiofrequency current flows from the generator through the active electrode, into the target tissue, through the patient, the dispersive electrode and then returns to the generator.13 The most common site of injury is at the patient return electrode. The return electrode must be of low resistance with a large enough surface area to disperse the electrical current without generating heat. If the patient's return electrode is not large enough or is not completely in contact with the patient's skin, then the current exiting the body can have enough density to produce unintended burns. Excessive hair, adipose tissue, bony prominences, and the presence of fluid and scar tissue compromise the quality of contact. To avoid this type of injury, contact quality monitoring systems were introduced in 1981. This system inactivates the generator if a condition develops at the patient return electrode site that could result in a burn (Figure 3 and Table 1).
Figure 3.
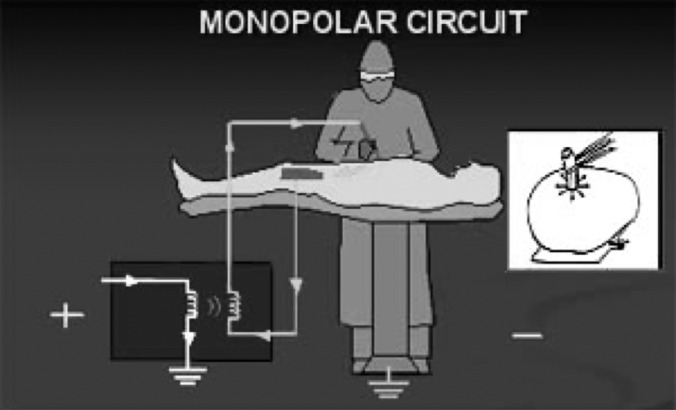
Monopolar circuit.
In bipolar electrosurgery the active and return electrodes are located at the site of surgery, typically within the instrument tip. The classical example is the 2 tines of forceps that are the active and return electrode and represent the entire circuit.9 Most bipolar units use a lower voltage waveform to achieve hemostasis and avoid collateral tissue damage.4 Bipolar electrosurgery has a more limited area of thermal spread compared with that of monopolar electrosurgery, and is similar to that of a laser.14,15 The maximal lateral thermal spread is within 5mm and the depth limited to the serosal layer (Figure 4 and Table 1).15
Figure 4.
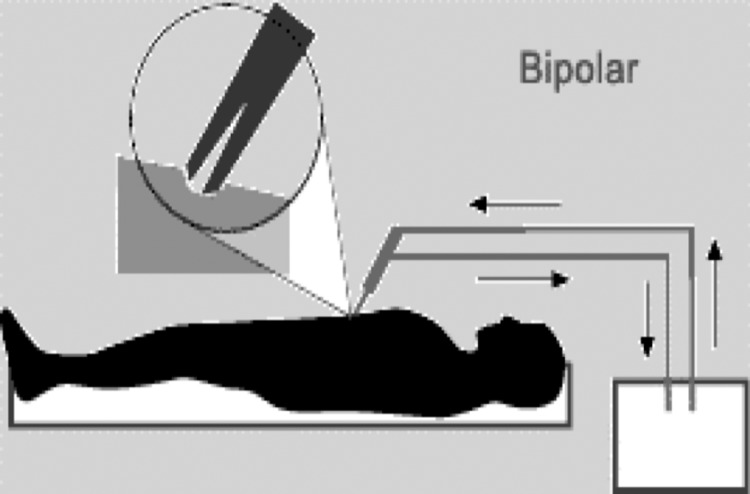
In bipolar electrosurgery, the 2 tines of the forceps form the active and return electrode functions.
Disadvantages of bipolar electrosurgery include the increased time needed for coagulation due to a low power setting, charring, and tissue adherence with incidental tearing of adjacent blood vessels (Figure 5).16
Figure 5.
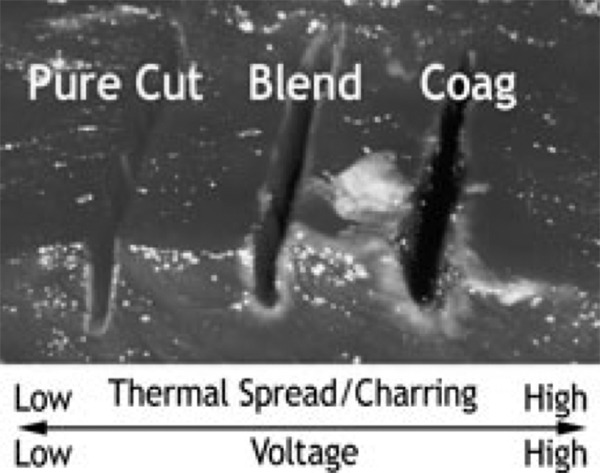
Tissue charring and thermal spread are inversly related to the voltage setting.
Incidence of Electrothermal Injuries
Injury from inadvertent energy transfer has a reported incidence of 1 to 5 recognized injuries per 1,000 cases.17,18
Complications of Electrosurgery
Electrothermal injury may result from the following situations: direct application, direct coupling, insulation failure, capacitive coupling, and so forth.
Direct Application
This may be due to unintended activation of the electrosurgical probe, eg, moving from the intended operating area to an iliac artery or vein on the pelvic sidewall, or operating on a moving ovarian cyst.17
Direct Coupling
Direct coupling occurs when the electrosurgical unit is accidentally activated while the active electrode is in close proximity to another metal instrument. Current from the active electrode flows through the adjacent instrument through the pathway of least resistance, and potentially damages adjacent structures or organs not within the visual field that are in direct contact with the secondary instrument.6 It can be prevented with visualization of the electrode in contact with the target tissue and avoiding contact with any other conductive instruments prior to activating the electrode (Figure 6).4
Figure 6.
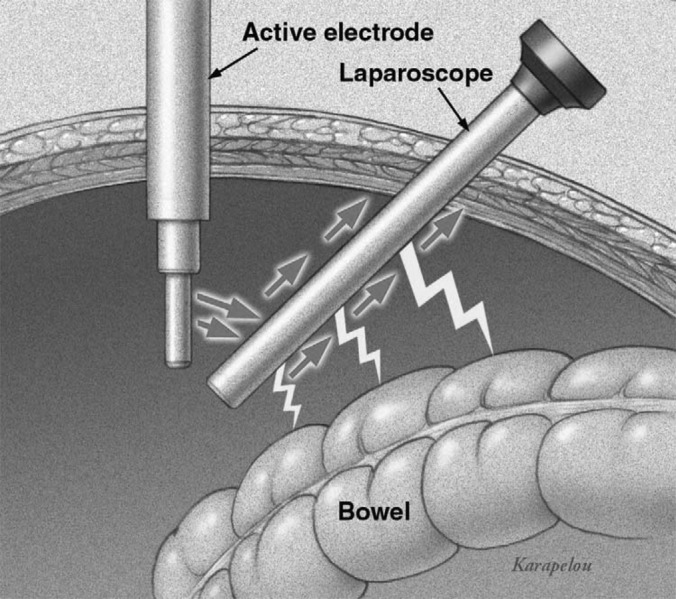
Direct coupling occurs when an active electrode makes an unintended contact with another electrode or conductive instrument.
Insulation Failure
This is now thought to be a main cause of laparoscopic electrosurgical injuries. It is defined as a break or defect in the insulation that coats the instrument. Insulation failure is caused by excessive use of reusable instruments, particularly with repetitive passage through trocars and frequent mechanized sterilization.19 By lowering the concentration of the current used, coagulation with cutting current and use of an active electrode monitoring system, the risk of accidental burns can be reduced.6
Eighteen percent of insulation defects are located in the section of the instrument most likely to create a catastrophic electrosurgical injury. Originally described as “Zone 2 ” by Voyles and Tucker, the location along the instrument, which is outside the view of the monitor but distal to the protective cannula, carries the highest risk for creating an injury that even the most attentive surgeon is unable to detect. Disposable instruments have a lower incidence of insulation failure compared with reusable instruments. The distal third of laparoscopic instruments is the most common site of insulation failure (Figure 7).20
Figure 7.
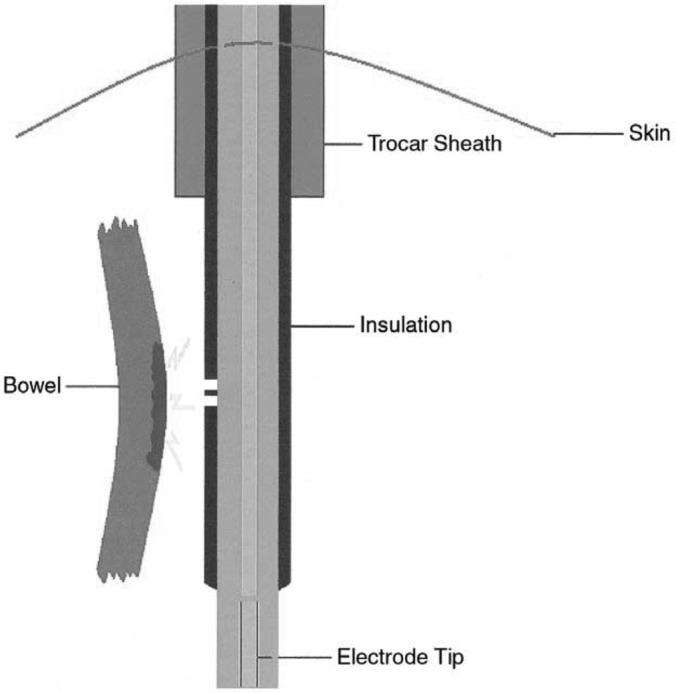
Insulation failures. Any break in the insulation may provide an alternate pathway for the flow of current.
Capacitive Coupling
Capacitive coupling is electrical current that is established in tissue or in metal instruments running parallel to but not directly in contact with the active electrode. This occurs when electric current is transferred from one conductor (the active electrode) through intact insulation and into adjacent conductive materials (eg, bowel) without direct contact.21
In monopolar mode, an alternate current flowing through an active monopolar electrode and back to the electrosurgical generator through the patient and the return pad induces an unintended current in any conductors in close proximity. The degree of current induced will depend on the proximity of the conductors, the voltage, and the insulation. Any conductor in the operating room is at risk of inheriting a stray current by becoming capacitively coupled to the current coming from the active electrode.
If an injury is to occur it is often away from the surgeons visual field and involves body structures. Ironically, the use of metal trocars can actually reduce this risk by allowing the stored energy from a capacitor to dissipate over the large surface area of the patient's skin, thereby making the electrical energy less concentrated and less dangerous. The use of an active electrode monitoring system and limiting the amount of time that a high voltage setting is used can also eliminate concerns about capacitive coupling (Figure 8).4,6,16,22
Figure 8.
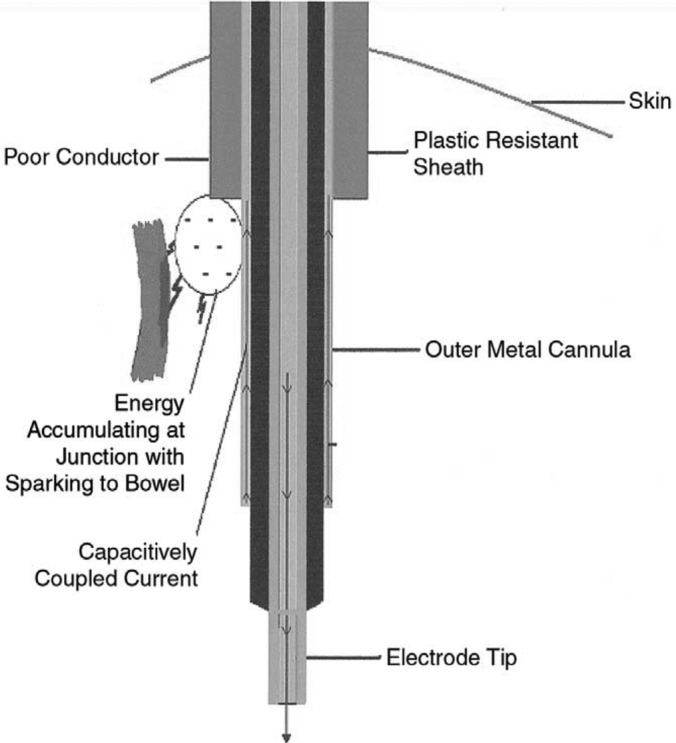
Capacitive Coupling. A capacitively coupled current typically returns through a metal trocar sheath to the grounding pad. If a plastic trocar sheath is used, the current will accumulate at the junction of the plastic and metal and seek an alternate path.
Clinicopathological Findings
Most electrothermal injuries to the bowel (approximately 75%) are unrecognized at the time of occurrence.16 The result of an unrecognized bowel injury is usually serious, often leading to long-term complications. The small bowel, especially the ileum, is most frequently involved, and the injury may not cause clear-cut or rapid symptoms and abnormal laboratory values.23 Generally speaking, symptoms of bowel perforation following electrothermal injury are usually seen 4 to 10 days after the procedure. With direct traumatic perforation, symptoms usually occur within 12 hours to 36 hours, although their occurrence up to 11 days later has been reported.24,25 The time delay from burn to perforation would appear to be related to the severity of the coagulation necrosis.26 Features of electrical injuries are distinguished by an area of coagulative necrosis, absence of capillary ingrowth of fibroblastic muscle coat reconstruction, and absence of white cell infiltration, except in focal areas at the viable borders of injury.24,25
Safety measures for prevention of electrosurgical complications:
Inspect insulation carefully
Use the lowest possible power setting
Use a low-voltage waveform (cut)
Use brief intermittent activation
Do not activate in open circuit
Do not activate in close proximity or direct contact with another instrument
Use bipolar electrosurgery when appropriate
Select an all metal cannula system as the safest choice
Utilize available technology (tissue response generator, active electrode monitoring) to eliminate concerns about insulation failure and capacitive coupling.27
NEWER TECHNOLOGIES
Active Electrode Monitoring Systems
In an effort to minimize the risks of insulation failure and capacitive coupling, active electrode monitoring systems now exist. When interfaced with electrosurgical units, these systems continuously monitor and shield against the occurrence of stray electrosurgical currents. Critical to the success of these systems are the integrated laparoscopic instruments that have a secondary conductor within the shaft that provides coaxial shielding.9
Tissue Response Generator
Tissue response generators are the next step in the evolution of electrosurgical generators. By using a computer-controlled tissue feedback system that senses tissue impedance or resistance, a consistent electrosurgical clinical effect is obtained through all tissue types.6,27
Vessel Sealing Technology
The most recent advancement in electrosurgery has been the introduction of vessel sealing technology. Core to this technology is the use of bipolar electrosurgery that relies on tissue response generators. This advanced electrical current is combined with optimal mechanical pressure delivery by the instruments to fuse vessel walls and create a seal. Specifically, high current and low voltage are delivered to the targeted tissue and denature the collagen and elastin in the vessel wall while the mechanical pressure from the instrument allows the denatured protein to form a coagulum.28 Vessels up to 7mm in diameter and large tissue bundles can now be surgically ligated. Additionally, thermal spread appears to be reduced compared to traditional bipolar electrosurgical systems. Unlike traditional electrosurgical instruments, these devices require a tension-free application to tissue bundles to successfully obtain the desired tissue effect. Valleylab, Gyrus ACMI, and SurgRx, Inc. are 3 companies that have developed devices for both open and laparoscopic applications.6,8,28–31
The LigaSure system produces supraphysiological seals with significantly higher bursting pressures than the plasma kinetics sealer (PK, Gyrus Medical, Maple Grove, MN) in vessels ranging from 4mm to 7mm. The plasma kinetics (PK) seal becomes progressively weaker while the LigaSure seal increases in strength as the vessel size increases.32
The LigaSure vessel sealing system is a safe alternative for securing pedicles in vaginal hysterectomy compared with conventional suture ligation.33 The LigaSure system reduces the operating time (by reducing pedicle-securing time) and blood loss without increasing the postoperative complication rates of vaginal hysterectomy. This beneficial effect was more pronounced in difficult procedures. Its higher cost is a significant handicap for its use in surgery in developing countries.
The EnSeal instruments adjust dose energy simultaneously to various tissue types in a tissue bundle each with its own impedance characteristics. Less heat is required to accomplish fusion, as the tissue volume is minimized through compression; energy is focused on the captured segment; and the vessel walls are fused through compression, protein denaturation, and then renaturation.
Ultrasonic Technology
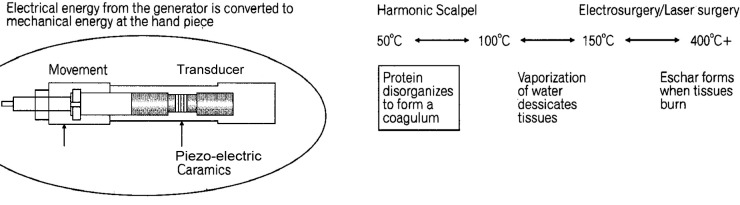
The Harmonic scalpel is an ultrasonic surgical instrument for cutting and coagulating tissue, operating at a frequency of 55.5 kHz/second or 55,500 cycles per second (Figure 9 and Table 1).34
Figure 9.
Principles of ultracision technology in surgery.
There is no electrosurgical current generated. The combination of mechanical energy and the heat that is generated causes protein denaturation and formation of a coagulum that seals small blood vessels. Typically, this energy modality is effective for blood vessels between 2mm and 3mm, although a newer device has demonstrated the ability to coagulate blood vessels up to 5mm in diameter with less heat, charring, and thermal injury to surrounding tissues.35–37
The 2 cutting mechanisms of the Harmonic scalpel are different from that observed with electrosurgery or laser surgery. The first mechanism is cavitational cutting and fragmentation. As the blade tip vibrates, it produces large transient pressure changes, which causes cellular water to vaporize at low temperature, rupturing cells, leading to very precise cutting and dissection. The second mechanism for cutting by Harmonic scalpel is the actual power cutting offered by a relatively large blade vibrating 55,500 times per second. The blade edge cuts tissue by stretching it beyond its elastic limit and on a more microscopic level, by breaking molecular bands. The heat generated from friction of tissue is typically <80°C. This minimizes tissue charring, desiccation, and the zone of thermal injury.34 Disadvantages of this technology are the formation of aerosolized fatty droplets from the tissue being treated, which can interfere with visualization through a laparoscope.28,38
Laparosonic Coagulating Shears (LCS)
Unsupported tissue, such as a transected bleeding vessel in a mesentry that cannot be compressed against a firm surface, is difficult to coagulate with a dissecting hook blade. To obviate this problem, the LCS was developed to include a vibrating blade with a sharp and blunt edge as well as passive tissue pad with which tissue is pressed against the active vibrating blade. This device allows unsupported tissue to be grasped and coagulated without difficulty, or cut and coagulated like a pair of scissors.34
With the advent of new technology, it is crucial to understand the mechanics of how instruments work to fully be able to utilize them and prevent injury. The LigaSure has the highest burst pressure and fastest sealing time and was the highest rated overall. The Harmonic scalpel produced the lowest thermal spread and smoke but had the lowest mean burst pressure. The Gyrus plasma kinetics had the highest smoke production and variable burst pressures. Despite using nanotechnology, the Enseal device was the slowest and had variable burst pressures.32
All of these partly or fully disposable instruments contribute to the quality of the surgery but also to the costs. These can be considerable; however, if we board a plane we expect the highest safety standards available and the same applies to surgical situations.
Thermal Injury Secondary to Fiberoptic Cables
Laparoscopy requires a reliable light source to provide adequate visualization. Thermal damage may however occur secondary to the fiberoptic light source.39
In a study conducted by Hindle et al,39 it was found that the maximum temperature at the optical cable was between 119.5° C and 268.6° C. They also found that when surgical drapes were exposed to the tip of the light source, the time to char was 3 seconds to 6 seconds, and significant injury was recorded with the optical cable 3mm from the skin. Hindle et al concluded that the temperature at the tip of the optical light cord can induce extreme damage and can produce immediate superficial tissue necrosis that can extend into the subcutaneous fat even when the optical tip is not in direct contact with the skin.39
Burns
Burns to the patient and perioperative personnel can occur when the cautery tip is not placed in its insulated container on the surgical field. Patients can also be burned at the site of the dispersive pad. Alternative site burns occur when the patient's skin is in contact with metal or other conductive materials and the electric currents return to the ground or the electrosurgical unit through this site.39
Shocks
Shocks from the electrosurgical unit have occurred, which are frequently mistaken for burns and usually occur when the surgeon is holding the instrument on the tissue to be cauterized. To prevent the shock, the active electrode should be placed on the region of interest before activation.40
Surgical Smoke
The National Institute of Occupational Safety and Health (NIOSH) and the Centers for Disease Control (CDC) have studied electrosurgical smoke at length. They state, “Research studies have confirmed that this smoke plume can contain toxic gases and vapors, such as benzene, hydrogen cyanide, formaldehyde, bioaerosols, dead and live cellular material and viruses.”
The Occupational Safety and Health Administration recommends that smoke evacuation systems be used to reduce potential acute and chronic health risks to patients and personnel.41
CONCLUSION
Principles of electrosurgery must be thoroughly understood by all operating room personnel. This forms the basis for patient safety and helps in early recognition of possible complications.42 The advantages and disadvantages of various forms of electrosurgery must be born in mind while using a particular modality. Newer technologies with more efficient hemostatic properties must be used whenever appropriate.
Contributor Information
Ibrahim Alkatout, Department of Obstetrics and Gynaecology, University Hospitals Schleswig-Holstein, Campus Kiel, Germany..
Thoralf Schollmeyer, Department of Obstetrics and Gynaecology, University Hospitals Schleswig-Holstein, Campus Kiel, Germany..
Nusrat A. Hawaldar, Department of Obstetrics and Gynaecology, University Hospitals Schleswig-Holstein, Campus Kiel, Germany..
Nidhi Sharma, Department of Obstetrics and Gynaecology, University Hospitals Schleswig-Holstein, Campus Kiel, Germany..
Liselotte Mettler, Department of Obstetrics and Gynaecology, University Hospitals Schleswig-Holstein, Campus Kiel, Germany..
References:
- 1. Christopher MJ, Ketsia BP, Ian BN, et al. Electro surgery. Cur Surg. 2006;63:458 [Google Scholar]
- 2. Wu MP, Ou CS, Chen SL, Yen EYT, Rowbotham R. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67–73 [DOI] [PubMed] [Google Scholar]
- 3. Nezhat CH, Nezhat F, Brill I, Nezhat C. Normal variations of abdominal and pelvic anatomy evaluated at laparoscopy. Obstet Gynecol. 1999;94:238–242 [DOI] [PubMed] [Google Scholar]
- 4. Jones CM, Pierre KB, Nicoud B, Stain SC, Melvin WV. Electrosurgery. Curr Surg. 2006;63:458–463 [DOI] [PubMed] [Google Scholar]
- 5. Van Way CW, Hinrichs CS. Technology focus: electrosurgery 201:basic electrical principles. Curr Surg. 2000;57:261–264 [DOI] [PubMed] [Google Scholar]
- 6. Valleylab Principles of electrosurgery. 1999;1–23 [Google Scholar]
- 7. Advincula AP, Wang K. The evolutionary state of electrosurgery: where are we now? Curr Opin Obstet Gynecol. 2008;20(4):353–358 [DOI] [PubMed] [Google Scholar]
- 8. Massarweh N, Cosgriff N, Slakey D. Electrosurgery: history, principles, and current and future uses. Am Coll Surg. 2006;202:520–530 [DOI] [PubMed] [Google Scholar]
- 9. Wang K, Advincula AP. Current thoughts in electrosurgery. Int J Gynaecol Obstet. 2007;97:245–250 [DOI] [PubMed] [Google Scholar]
- 10. Neufield GR. Principles and hazards of electrosurgery including laparoscopy. Surg Gynecol Obstet. 1978;147:705–710 [PubMed] [Google Scholar]
- 11. Miller CE. Basic equipment and room setup. In: Shirk GJ, Editor, The Video Encyclopedia of Endoscopic Surgery for the Gynecologist. St Louis: Medical Video Productions; 1994;11–19 [Google Scholar]
- 12. Phipps JH. Understanding electrosurgery: safety and efficiency. In: Lower A, Sutton Cand., Grudzinskas G, eds. Introduction to Gynecological Endoscopy. Oxford, UK: Iris Medical Media; 1996;39–56 [Google Scholar]
- 13. Tucker RD, Schimitt OH, Sievrt CE, et al. Demodulated low frequency currents from electrosurgical procedures. Surg Gynecol Obstet. 1984;159:39–43 [PubMed] [Google Scholar]
- 14. Martin DC, Soderstrom RM, Hulka JF, et al. Electrosurgery safety. Am Assoc Gynecol Laparosc Tech Bull. 1995;1–7 [Google Scholar]
- 15. Ryder RM, Hulka JF. Bladder and bowel injury after electrodesiccation with Kleppinger bipolar forceps: a clinicopathologic study. J Reprod Med. 1993;38:595–598 [PubMed] [Google Scholar]
- 16. Tucker RD, Volyes CR. Laparoscopic electrosurgical complications and their prevention. AORN. 1995;62:49–78 [DOI] [PubMed] [Google Scholar]
- 17. Nduka CC, Super PA, Monson JR, Darzi AW. Cause and prevention of electrosurgical injuries in laparoscopy. J Am Coll Surg. 1994;179:161–170 [PubMed] [Google Scholar]
- 18. Hulka JF, Levy BS, Parker WH, Philips JM. Laparoscopic-assisted vaginal hysterectomy: American Association of Gynecologic Laparoscopists 1995 membership survey. J Am Assoc Gynecol Laparosc. 1997;4:167–171 [DOI] [PubMed] [Google Scholar]
- 19. Vancaillie TG. Active electrode monitoring. How to prevent unintentional thermal injury associated with monopolar electrosurgery at laparoscopy. Surg Endosc. 1998;12:1009–1012 [DOI] [PubMed] [Google Scholar]
- 20. Montero PN, Robinson TN, Weaver JS, Stiegmann GV. Insulation failure in laparoscopic instruments. Surg Endosc. 2010;24:462–465 [DOI] [PubMed] [Google Scholar]
- 21. Odell RC. Electrosurgery: biophysics, safety and efficacy. In: Mann WJ T, Stovall G, eds. Mann/Stovall Gynecologic Surgery. New York: Churchill Livingstone; 1996;55–67 [Google Scholar]
- 22. Vilos G, Latendresse K, Gan BS. Electrophysical properties of electrosurgery and capacitive induced current. Am J Surg. 2001;182:222–225 [DOI] [PubMed] [Google Scholar]
- 23. Soderstrom RM. Bowel injury litigation after laparoscopy. J Am Assoc Gynecol Laparosc. 1993;1:74–77 [DOI] [PubMed] [Google Scholar]
- 24. Levy BS, Soderstrom RM, Dail DH. Bowel injuries during laparoscopy: gross anatomy and histology. J Reprod Med. 1985;30:168–172 [PubMed] [Google Scholar]
- 25. Reich H. Laparoscopic bowel injury. Surg Laparosc Endosc. 1992;2:74–78 [PubMed] [Google Scholar]
- 26. Saltzstein EC, Schwartz SF, Levinson CJ. Perforation of the small intestine secondary to laparoscopic tubal cauterization. Ann Surg. 1973;178:34–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mayooran Z, Pearce S, Tsaltas J, et al. Ignorance of electrosurgery among obstetricians and gynaecologists. BJOG. 2004;111:1413–1418 [DOI] [PubMed] [Google Scholar]
- 28. Harold KL, Pollinger H, Matthews BD, Kercher KW, Sing RF, Heniford BT. Comparison of ultrasonic energy, bipolar thermal energy, and vascular clips for the hemostasis of small-, medium-, and large-sized arteries. Surg Endosc. 2003;17:1228–1230 [DOI] [PubMed] [Google Scholar]
- 29. Carbonell AM, Joels CS, Kercher KW, Matthews BD, Sing RF, Heniford BT. A comparison of laparoscopic bipolar vessel sealing devices in the hemostasis of small-, medium-, and large-sized arteries. J Laparoendosc Adv Surg Tech A. 2003;13:377–380 [DOI] [PubMed] [Google Scholar]
- 30. Pietrow PK, Weizer AZ, L'Esperance JO, Auge BK, Silverstein A, Cummings T, et al. Plasma kinetic bipolar vessel sealing: burst pressures and thermal spread in an animal model. J Endourol. 2005;19:107–110 [DOI] [PubMed] [Google Scholar]
- 31. Richter S, Kollmar O, Schilling MK, Pistorius GA, Menger MD. Efficacy and quality of vessel sealing: comparison of a reusable with a disposable device and effects of clamp surface geometry and structure. Surg Endosc. 2006;20:890–894 [DOI] [PubMed] [Google Scholar]
- 32. Lamberton GR, Hsi RS, Jin DH, Lindler TU, Jellison FC, Baldwin DD. Prospective comparison of four laparoscopic vessel ligation devices. J Endourol. 2008;22(10):2307–2312 [DOI] [PubMed] [Google Scholar]
- 33. Hefni MA, Bhaumik J, El-Toukhy T, et al. Safety and efficacy of using the LigaSure vessel sealing system for securing the pedicles in vaginal hysterectomy: randomised controlled trial. BJOG. 2005;112(3):329–333 [DOI] [PubMed] [Google Scholar]
- 34. Lee SJ, Park KH. Ultrasonic energy in endoscopic surgery. Yonsei Medical Journal. 1999;40:545–549 [DOI] [PubMed] [Google Scholar]
- 35. Shimi SM. Dissection techniques in laparoscopic surgery: a review. J R Coll Surg Edinb. 1995;40:249–259 [PubMed] [Google Scholar]
- 36. Goldstein SL, Harold KL, Lentzner A, et al. Comparison of thermal spread after ureteral ligation with the laparosonic ultrasonic shears and the ligasure system. J Laparoendosc Adv Surg Tech A. 2002;12:61–63 [DOI] [PubMed] [Google Scholar]
- 37. Heniford BT, Matthews BD, Sing RF, et al. Initial results with an electrothermal bipolar vessel sealer. Surg Endosc. 2001;15:799–801 [DOI] [PubMed] [Google Scholar]
- 38. Sutton C. Power sources in endoscopic surgery. Curr Opin Obstet Gynecol. 1995;7:248–56 [PubMed] [Google Scholar]
- 39. Hindle AK, Brody F, Hopkins V, Rosales G, Gonzalez F, Schwartz A. Thermal injury secondary to laparoscopic optic cables. Surg Endosc. 2008;23:1720–1723 [DOI] [PubMed] [Google Scholar]
- 40. Ulmer B. Electrosurgery Continuing Education Module. Valleylab Institute of Clinical Education, 2000 [Google Scholar]
- 41. AORN Recommended Practices for Electrosurgery, 2003
- 42. Randle Voyles C, Tucker RD. Safe use of electrosurgical devices during minimally invasive surgery. In: Wetter PA, ed. Prevention & Management of Laparoendoscopic Surgical Complications, 2nd Edition Miami, Florida, USA: Society of Laparoendoscopic Surgeons, Inc., 2005 [Google Scholar]