Laparoscopic adnexectomy, bagging, and colpotomy are suggested to be desirable goals for patients with benign ovarian masses in the 8-cm to 13-cm range.
Keywords: Early ovarian cancer, Laparoscopy, Treatment, Minimally invasive surgery
Abstract
Objective:
Guidelines for referring women with pelvic masses suspicious for ovarian cancer to gynecologic oncologists have been developed by the American College of Obstetrician Gynecologists (ACOG). We set out to evaluate the negative predictive value of these guidelines and to assess a modified algorithm involving minimally invasive surgery in the treatment of women with masses suspected to be benign.
Methods:
257 consecutive patients with adnexal masses of 8cm to 13cm on preoperative ultrasound examination meeting Triage Criteria set forth in ACOG Committee Opinion 280. Patients meeting the selection criteria were scheduled for operative laparoscopy, washings, adnexectomy, bagging, and colpotomy. A total of 240 patients successfully completed intended treatment (93.38%), and 234 of these did not require admission (97.5%). There was a low incidence of significant complications: 97.50% of women were successfully treated as outpatients, 97.92% of surgeries lasted <136 minutes, and 97.08% had blood loss <200mL. The negative predictive value of ACOG Committee Opinion 280 Triage Criteria as a deselector for having invasive ovarian malignancy in our population was 95.57% for premenopausal and 90.91% for postmenopausal women.
Conclusions:
Laparoscopic adnexectomy, bagging, and colpotomy is a desirable goal for patients with ovarian masses in the 8cm to 13cm range meeting selection criteria affording a minimally invasive approach with attendant benefits including outpatient treatment (97.5%), few complications, low likelihood of iatrogenic rupture of the ovarian capsule (1.25%), and low necessity for reoperation after final pathology is evaluated (6.03%). Negative predictive value of ACOG Committee Opinion 280 is confirmed in a community gynecology practice and is recommended to form the basis of a new treatment algorithm for women with adnexal masses.
INTRODUCTION
Because of the relatively high incidence of adnexal masses in the female population and because most United States hospitals do not have gynecologic oncologists readily available in the operating room, we set out to test the safety of a modified algorithm for treating adnexal masses. Our proposed management algorithm assures both adherence to standard of care recommendations regarding referral of adnexal masses likely to represent ovarian cancer to gynecologic oncologists and treatment of adnexal masses felt to be benign using minimally invasive surgical techniques while carefully observing standard principals of oncologic surgery. We selected masses in this size range, because just as with larger uteri, many gynecologic surgeons view increasing size of an adnexal mass as an important deselector for laparoscopic surgery. It is our belief that the days of laparotomy in mainstream gynecology should be over. Various organizations have expressed positions on the benefits of minimally invasive surgery in hysterectomy.1–3 We advocate the same position for ovarian masses.
Adnexal masses are relatively common, contributing to gynecologists' office volume and surgical case load. Conversely, ovarian cancer has a relatively low prevalence. Ovarian cancer has nonspecific symptoms and is usually silent in its early stages.4 Presently, we have no reliable screening test for ovarian cancer, and we have a limited ability to detect it using current diagnostic strategies.5,6
Various studies have addressed the likelihood of malignancy within an ovarian mass. This likelihood ranges from 0.38% to 18.67% (Table 1) and is population dependent.7–19
Table 1.
Likelihood of Malignancy in Adnexal Masses
| Study | Patients | Malignancy | % Affected |
|---|---|---|---|
| Mage et al7 1990 | 433 | 9 | 2.08 |
| Mecke et al8 1992 | 773 | 11 | 1.42 |
| Nezhat et al9 1992 | 1011 | 4 | 0.40 |
| Hulka et al10 1992 | 13793 | 411 | 2.98 |
| Canis et al11 1994 | 757 | 19 | 2.51 |
| Marzana et al12 1994 | 527 | 2 | 0.38 |
| Wenzl et al13 1996 | 16601 | 108 | 0.65 |
| Childers et al14 1996 | 138 | 19 | 13.77 |
| Canis et al15 1997 | 230 | 15 | 6.52 |
| Hidlebaugh et al16 1997 | 405 | 8 | 1.98 |
| Malik et al17 1998 | 292 | 11 | 3.77 |
| Mettler et al18 2001 | 493 | 8 | 1.62 |
| Valentin et al19 2006 | 1066 | 199 | 18.67 |
| Present study, 2012 | 257 | 15 | 5.84 |
| Total | 36776 | 839 | 2.28 |
Contemporary preoperative workup for an adnexal mass involves history and physical examination, labs including CA 125, and imaging studies usually including transvaginal ultrasound. The risk of encountering an unexpected ovarian malignancy after modern preoperative screening is 0.9% to 13%.20 Dating to Jacobs et al 199021 and Sassone et al 1991,22 scoring systems have related ultrasound characteristics, CA 125, family history, and other variables in predicting the likelihood of ovarian malignancy.23–43 By 2002, the American College of Obstetricians & Gynecologists evaluated the various predictors of ovarian malignancy and published ACOG Committee Opinion 280 setting forth criteria to refer both premenopausal and postmenopausal women for care by gynecologic oncologists.44 These referral criteria are summarized in Table 2. Postmenopausal criteria include a lower CA 125 threshold or a nodular or fixed pelvic mass for referral. Importantly, only one referral criterion must be met for a patient to be referred to a gynecologic oncologist.
Table 2.
ACOG Committee Opinion 280 Criteria For GYN Oncology Referral44
| Premenopausal Women |
| CA 125 >200U/mL |
| Ascites |
| Evidence of abdominal or distant metastases |
| Family history first-degree relative(s) with breast or ovarian cancer |
| Postmenopausal Women |
| Elevated CA 125 |
| Ascites |
| Nodular or fixed pelvic mass |
| Evidence of abdominal or distant metastases |
| Family history first-degree relative(s) with breast or ovarian cancer |
Two prior studies have looked at the operational characteristics of ACOG Committee Opinion 280 and how it functions in actual practice. In 2005, Im et al45 demonstrated in a multi-center study that using criteria in ACOG Committee Opinion 280 in a referral population to identify women at high risk for ovarian cancer yielded a positive predictive value of 33.8% in premenopausal women and 59.5% in postmenopausal women. Data were not uniform from each of the 7 centers, and at some centers pelvic masses were only identified retrospectively.45 These investigators conceded their data did not address how ACOG Committee Opinion 280 would operate in a general population.
In a more elegant study, Dearking et al in 200746 demonstrated in a prospectively enrolled cohort that using criteria set forth in ACOG Committee Opinion 280 in a nonreferred population to identify women at elevated risk for ovarian cancer yielded a positive predictive value of 13.6% in premenopausal women and 44.9% in postmenopausal women. In their referral population applying the same selection criteria lead to a positive predictive value of 47.3% in premenopausal women and 90.5% in postmenopausal women.46 The referral population had a demonstrably higher prevalence of disease, positively influencing assessment of the selection criteria.
By whatever method, once located, the prognosis of an ovarian tumor is determined by surgical staging, histologic subtype, and grade of tumor differentiation.47 Overall, only approximately 25% of patients diagnosed with ovarian cancer have early ovarian cancer (EOC) or Stage 1. These patients have 5-year survival approaching 90%.48,49 About 20% of EOC actually have microscopic metastatic disease on staging.50 This is why thorough staging is crucial irrespective of the initial surgical perception.
The route of definitive treatment of ovarian cancer has been changing. Initially reported in 1990 by Reich et al, laparoscopic surgery for staging of ovarian cancer has become more common.51 Laparoscopy has now become a standard treatment modality for patients with suspected benign adnexal masses.52,53 Laparoscopy and robot-assisted laparoscopy have equal efficacy in removal of adnexal masses, but masses were small with mean weight of 50 grams and size of 5.4cm (laparoscopic group) and 28 grams and size of 4.5cm (in the robotic group).54
Surgical treatment of adnexal masses suspected to be benign on preoperative ultrasound has also evolved over time. In each of these studies, one anesthesia was used, usually with conversion to laparotomy, if frozen section diagnosed malignancy. Definitive staging was performed at the time of initial operation by gynecologic oncologists.
Havrilesky et al55 reported on 396 patients treated over 7 years by laparoscopy for adnexal masses thought to be benign preoperatively at a teaching center. No clear statement of criteria used to discriminate benign from malignant masses preoperatively is contained in the article. Median preoperative mass size was 5.2cm (range, 0.5 to 17): <4cm 26%, 4 to 8cm 57.4%, >8cm 16.6%. If a mass was drained within a bag, it was not counted as ruptured. Ninety-seven percent of masses were benign on pathology. Intraoperative rupture of the capsule occurred in 25% of cases. Conversion to laparotomy occurred in 25% of cases. CA 125 levels were not predictive of malignancy, but levels were not stratified based on menopausal status.
Smorgick et al56 reported on 263 women undergoing laparoscopic adnexectomy or cystectomy between 2002 and 2006. No clear statement of criteria used preoperatively to discriminate benign from malignant masses is presented. Mean cyst size was 6.6cm. A collection bag was used if a cyst was suspected to contain irritating fluid or possible malignant cells. Of these 263 cases, 93.5% were benign. Cyst rupture occurred in 16.6% of cases, ranging from 7.4% in adnexectomy cases to 29.5% for cystectomy procedures.
Panici et al57 reported on 60 eligible patients aged 18 to 45 years, randomized to laparoscopy or laparoscopically directed mini-laparotomy for 7-cm to 18-cm adnexal cysts. Inclusion criteria were no ultrasonographic suspicion of endometriosis or malignancy, CA 125 within normal range, BMI<29kg/m2, ASA score 0 to 2, no previous laparotomy, and no contralateral cyst >7cm (amongst other criteria). A collection bag was used to containerize the mass if possible, during aspiration and removal. Frozen section was always performed, and the case was converted to laparotomy if definitive staging was required. Uncontrolled rupture occurred in 87% of the laparoscopy patients and in 29% of the laparoscopically assisted, mini-laparotomy patients. No data on malignancy rate was included.57
Sagiv et al58 reported on 21 patients with cystic or complex adnexal masses extending at least cephalad to the umbilicus with a “low probability of malignancy” managed laparoscopically. Inclusion criteria were no suspicion of malignancy on imaging, no enlarged pelvic lymph nodes, and CA 125 <130U/mL. Masses were aspirated without containerization. If frozen section was positive for malignancy, the gynecologic oncology team immediately performed laparotomy and definitive staging. One of 21 patients had malignancy (adenocarcinoma) or 4.76%. These investigators state they could not contain spillage from such large masses, so they proceeded to laparotomy at the same setting if cancer was found.58
An important consideration in adnexal mass surgery is inadvertent opening of the ovarian capsule. Likelihood of cyst rupture either during laparotomic or laparoscopic removal ranges from 10.5% to 41.8% in published studies.55,56,59–61
Spillage affects recurrence rate for some benign lesions including mucinous cystadenoma.62 In cases of ovarian malignancy where disease is confined to the ovary, rupture of the ovary increases the Stage to IC.
Vergote et al63 in 2001 reported on over 1500 patients with Stage I epithelial ovarian carcinoma and found intraoperative rupture worsened disease-free survival. Various other retrospective multicenter studies support intraoperative cyst rupture as an independent predictor of disease-free survival.64–66
Alternatively, another group of publications67–71 failed to demonstrate a difference in disease-free survival based on intraoperative cyst rupture. Limitations of both groups of older studies assessing long-term outcome of patients with inadvertent, intraoperative capsular rupture involve inclusion of nonstaged or incompletely staged cases, lack of consistent adjuvant treatments for women positive for malignancy, and lack of separate analysis of Stage IC cases.
In perhaps the most definitive work on this topic, Bakkum-Gamez et al61 reported a retrospective study to specifically address outcomes related to intraoperative capsule rupture (stage IC) in treatment of Stage I epithelial cancer between 1991 and 2007. Of 161 cases meeting inclusion criteria, intraoperative capsule rupture occurred in 61 or 38%. All patients were treated in one anesthesia with definitive staging performed based on positive frozen section results. For patients whose only Stage IC qualification was intraoperative capsular rupture, there was found to be significantly higher recurrence and mortality rates.61
ACOG Committee Opinion 280 sets forth the standard of care for preoperative discrimination of suspected malignant vs. suspected benign adnexal masses. But how successful is application of Committee Opinion 280 in selecting a population of women at low-risk for ovarian malignancy and how should these women be optimally treated?
Im et al45 in 2005 showed that strict adherence to ACOG Committee Opinion 280 in a referral population yields a negative predictive value (NPV) of 92.0% for all cases of ovarian cancer in premenopausal women and an NPV of 91.1% in postmenopausal women.
Dearking et al in 200746 demonstrated that strict adherence to ACOG Committee Opinion 280 in a nonreferred population arising from their primary catchment area yields an NPV of 97.7% for all cases of ovarian cancer in premenopausal women and an NPV of 95.0% in postmenopausal women. In their referral population, NPV was 91.0% in premenopausal and 90.5% in postmenopausal women, or essentially identical to the Im et al data.45 Of the women not referred based on ACOG Committee Opinion 280 who were later found to have ovarian cancer, 8 of 10 premenopausal and 11 of 14 postmenopausal women were found to have Stage I or II disease at the time of definitive surgery.
Virtually all women with adnexal masses thought to be benign are managed by general gynecologists. Some of these women are managed expectantly while others are taken directly to surgery. Regrettably, many women with adnexal masses are treated without an appropriate workup. Many gynecologists do not operate in medical centers where there is instant availability of gynecologic oncologists for intraoperative consultation and continuation of care with the patient under the same anesthesia. Because of the supposition that the adnexal mass is benign, washings are not obtained, appropriate care may not be exercised in removing the mass leading to spillage, frozen section is not ordered, and thorough examination of all peritoneal surfaces for disease is not accomplished. As a consequence, many adnexal masses are not found to be malignant until well after the procedure is concluded. Timely re-evaluation and definitive staging is required for these patients.72–76 Lehner74 examined reoperations before and after 17 days and found such a delay between laparoscopy and laparotomy may adversely affect the distribution of disease stage.
Definitive staging of ovarian cancer includes cytologic washings, total hysterectomy, bilateral salpingo-oophorectomy, peritoneal surface biopsies, total omentectomy, and retroperitoneal lymphadenectomy from the pelvis and paraaortic regions to the left renal vessel.77 Laparoscopy and laparotomy have equal efficacy in both early and advanced stage ovarian cancer.78
Because a final pathology report returning a diagnosis of invasive ovarian cancer is often a surprise, a subsequent surgical procedure is often performed for definitive staging of disease. Frequency of upstaging in patients with EOC at initial examination is in the range of 10% to 35.7% (Table 3).72,73,79–87
Table 3.
Frequency of Upstaging at Definitive Procedure
| Study | Patients | Upstaged | % Upstaged |
|---|---|---|---|
| Pomel et al79 1995 | 10 | 1 | 10.00 |
| Childers et al80 1995 | 14 | 5 | 35.70 |
| Stier et al81 1996 | 45 | 7 | 15.56 |
| Tozzi et al83 2004 | 24 | 5 | 20.80 |
| Leblanc et al84 2004 | 44 | 8 | 18.20 |
| Spirtos et al85 2005 | 58 | 6 | 11.00 |
| Chi et al86 2005 | 20 | 2 | 10.00 |
| Ghezzi et al87 2007 | 15 | 4 | 26.70 |
| Colomer et al72 2008 | 20 | 4 | 20.00 |
| Nezhat et al73 2009 | 36 | 7 | 19.44 |
| Present study | 9 | 4 | 44.44 |
| Total | 295 | 53 | 17.96 |
Stier et al in 1996 reported on 45 patients being re-staged by laparotomy 12 to 161 days (mean 56 days) after initial surgery. Prestaging workup showed no radiographic evidence of metastasis. Seven (15.56%) patients had their disease upstaged.81
Colomer et al72 in 2006 reported on 20 patients undergoing either primary treatment or completion of staging. The interval between the initial operation and the staging procedure was 4.7 weeks (range, 2 to 11.4). Nineteen cases (95%) had successful laparoscopic surgical staging with one converted to laparotomy. In this series, 4 (20%) patients were upstaged.72
Nezhat et al73 in 2009 reported on 36 patients laparoscopically staged for early-stage ovarian and fallopian tube cancers over a 12-year period. Nine were referred for staging and 27 with adnexal masses. In all cases, a specimen retrieval bag was used to remove tissue. All cases were accomplished laparoscopically. Seven (19.4%) patients were upstaged.73
The purpose of this study is 3-fold. First, to demonstrate the safety of minimally invasive surgical treatment of 8-cm to 13-cm adnexal masses felt to be benign. Second, to confirm the negative predictive value of ACOG Committee Opinion 280 for selecting women whose adnexal masses are unlikely to be malignant in this size range. Third, based on these data, to advocate for acceptance of a new treatment algorithm prominently featuring minimally invasive surgery in the treatment of these lesions.
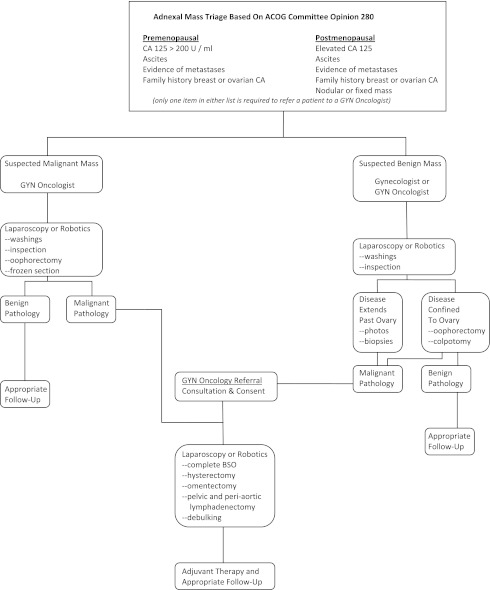
We propose a new treatment algorithm with initial triage based on ACOG Committee Opinion 280 (Figure 1). Women having adnexal masses suspected to be malignant are immediately referred to gynecologic oncologists, while women with masses not suspected to be malignant are treated laparoscopically with careful attention to standard oncologic surgical principals. If disseminated disease is encountered at initial laparoscopy in patients felt to have benign disease, pelvic washings, biopsies of appropriate surfaces, and photo documentation is conducted. Laparoscopy is then abandoned and the patient immediately referred to gynecologic oncologists for consultation and definitive surgical staging. If after the initial laparoscopic procedure for benign disease pathology, a woman has invasive ovarian cancer, she is referred to gynecologic oncologists for definitive staging and treatment.
Figure 1.
Adnexal Mass Triage Based On ACOG Committee Opinion 280.
Following this strategy, all women having adnexal masses will receive appropriate care and optimal attention will be focused on masses not initially suspected to be malignant.
MATERIALS AND METHODS
A prospective descriptive study was conducted on patients treated by a private gynecologic surgery practice in a community hospital setting from January 1, 2004 through April 30, 2011. Two-hundred fifty-seven consecutive patients with adnexal masses of 8cm to 13cm on preoperative ultrasound examination not meeting Triage Criteria set forth in ACOG Committee Opinion 280 for referral to gynecologic oncologists were treated with operative laparoscopy, adnexectomy, bagging, and colpotomy.
Patients either arose within the practice or were referred for pelvic pain, pelvic mass, or with an incidental finding on imaging studies performed for other indications. Ultrasound examinations were performed either by the gynecologic surgeons who have extensive experience in OB/GYN ultrasound or in hospital radiology units in the case of in-patients. When imaging was performed in a hospital radiology unit, images were independently interpreted by the investigators as part of the preoperative workup.
Conventional closed laparoscopy was performed with Veress needle insufflation. An 11-mm bladed trocar is placed at the base of the umbilicus. Location and size of additional ports is dictated by the clinical situation. Washings are obtained. Inspection of all peritoneal surfaces is conducted. If disseminated frank malignancy is encountered, biopsies are performed, photo documentation is conducted, and laparoscopy is terminated. The patient is referred immediately with her records to our gynecologic oncology associates for consultation, informed consent, and definitive surgery.
If inspection demonstrates an intact ovarian capsule and no evidence of other disease, oophorectomy (172 patients) or salpingo-oophorectomy (68 patients) is performed. The ovary or the tube and ovary are dissected free as necessary, infundibulopelvic ligament divided with a bipolar energy source (most often LigaSure from Covidien, requiring 5-mm or 10-mm port or Enseal from Ethicon Endo Surgery, requiring 5-mm port). When extensive adhesions of the ovary to the pelvic sidewall are present, extra attention is used to decrease the chance of capsular rupture. A conventional retroperitoneal dissection beginning by dividing the round ligament, identifying the pelvic ureter, isolating and transecting the infundibulopelvic ligament assists in mobilizing a fixed ovary when required. When present, adhesions of the ovary to bowel are meticulously lysed and any serosal denudation of bowel is oversewn with appropriate postprocedure follow-up.
Once free, the mass is containerized within the collection system (Endo Catch from Covidien Surgery, requiring a 10-mm port or Anchor Tissue Retrieval System from Anchor Products Company, requiring either 11-mm or 13-mm port). The collection system is closed, and the introducer is removed leaving the closed bag and string within the abdominal-pelvic cavity. The string end is held with a laparoscopic grasper. A 3-puncture laparoscopy is most often required with the largest incision of either 11mm or 13mm, depending on the tissue collection system used.
Colpotomy is performed in preference to extending an abdominal incision, because the vagina is known to be more distensible than rectus fascia and allows for a larger hole without impact on the likelihood of postoperative ileus or abdominal wall hernia formation. When the uterus is in place and the cul-de-sac is free, colpotomy is performed vaginally by elevating the posterior lip of the cervix, tenting the posterior vaginal mucosa on the midline, and transecting this tissue with Mayo scissors. When the uterus is in place and the cul-de-sac obstructed, meticulous lysis of adhesions is conducted laparoscopically to make the space accessible. Colpotomy incision can be created with electrocautery against a sponge stick inserted into the posterior vaginal fornix for additional control. When the uterus is absent, additional care is taken by performing colpotomy laparoscopically to ensure the bladder is not injured. A device (formerly a Heaney retractor handle, currently a Sacro-colpopexy Tip on the Rumi handle from Cooper Surgical) is used to distend the vaginal barrel. The bladder is filled with 300mL of sterile saline to demonstrate its location then drained. Parietal peritoneum posterior to the bladder reflection is incised with endoscopic scissors then forced caudad on the vaginal barrel with both blunt and sharp dissection. Once sufficient space is developed, a semi-circular incision is created by using the endoscopic scissors and 50 watts of cutting current at the vaginal apex.
After the colpotomy is developed, the collection system's string is passed out the colpotomy defect and into the vagina using a laparoscopic grasper manipulated by an assistant. The surgeon, positioned at the vagina, uses retractors to visualize the laparoscopic grasper and string. A Kelly clamp is used to secure the string and the laparoscopic instrument is withdrawn by the assistant. The mouth of the bag is delivered out the colpotomy defect and out the vagina. Traction is maintained on the bag both to preserve the pneumoperitoneum (laparoscopic surveillance of debulking helps ensure the bag is not perforated) and to deliver the bag once the volume of the lesion has been sufficiently decreased to allow it to pass through the colpotomy defect. This continuous caudad traction creates a seal that helps assure no fluid or tissue goes back into the peritoneal cavity. A second layer of protection from the positive pressure gradient created by the pneumoperitoneum further protects against fluid or tissue falling back into the peritoneal cavity.
In principally cystic masses, wall suction is used to aspirate fluid. In principally solid masses, ring forceps are used to disrupt the mass and withdraw it piece meal. Traction is maintained on the bag, facilitating its delivery once the volume of the lesion has been sufficiently decreased to allow it to pass through the colpotomy defect. The colpotomy is then closed in one layer encompassing both peritoneum and vaginal mucosa. Closure in all cases is performed vaginally.
New gloves are donned, and after inspection and copious irrigation, the laparoscopy is terminated. Thirty mL of 0.25% Marcaine without epinephrine is instilled to assist in postoperative analgesia. A Carter-Thomason closure system (Cooper Surgical) is used to close the fascial defect for all large ports (≥10mm).88
Patients were assessed postprocedure and discharged home if no complications were noted and pain status was amenable to treatment with oral analgesics. Otherwise, patients were admitted for pain control and re-assessed regularly.
Patients with findings of ovarian cancer are referred immediately to our collaborating gynecologic oncologists with all required reports and intraoperative photos. Consultation, appropriate informed consent, and speedy reoperation within 7 days to 10 days follows.
RESULTS
Of 257 consecutive cases with stated inclusion criteria, 6 were found to have disseminated ovarian malignancy at the time of laparoscopy (Table 4). Eleven cases were judged not to be candidates for inclusion in this study at the time of laparoscopy (Table 5). A total of 240 patients successfully completed intended treatment (93.38%). Of patients successfully completing treatment, 234 did not require admission (97.5%). One patient had an inadvertent bowel injury secondary to adhesiolysis requiring reoperation during the admission. One patient developed deep vein thrombosis well after discharge. Nine patients (3.75%) required reoperation by gynecologic oncologists after final pathology was available.
Table 4.
Main Outcome Data
| Total patients in study period (N=257) | n | Percentage (%) |
|---|---|---|
| Surgery ends with laparoscopya | 6 | 2.33 |
| Failed inclusion at laparoscopyb | 11 | 4.28 |
| Total patients successfully completing | 240 | 93.38 |
| Duration of surgery | ||
| ≤45 minutes | 52 | 21.67 |
| 46 to 90 minutes | 145 | 60.42 |
| 91 to 135 minutes | 38 | 15.83 |
| >135 minutes | 5 | 2.08 |
| Age Range | ||
| ≤20 years | 1 | 0.42 |
| 21 to 40 years | 37 | 15.42 |
| 41 to 60 years | 126 | 52.50 |
| 61 to 80 years | 68 | 28.33 |
| ≥81 years | 8 | 3.33 |
| Menopausal Status | ||
| Premenopausal | 138 | 57.50 |
| Postmenopausal | 102 | 42.50 |
| Blood Loss (mL) | ||
| <100 | 138 | 57.50 |
| 101 to 200 | 95 | 39.58 |
| 201 to 300 | 4 | 1.67 |
| 301 to 400 | 2 | 0.83 |
| >400 | 1 | 0.42 |
| Duration of Admission | ||
| outpatient only | 234 | 97.50 |
| 1 hospital day | 5 | 2.08 |
| 2 or more hospital days | 2 | 0.84 |
| Intraoperative Complications | ||
| Inadvertent rupture of mass | 3 | 1.25 |
| Cuff cellulitis | 0 | 0.00 |
| Febrile morbidity | 9 | 3.75 |
| Injury to bowel | 1 | 0.42 |
| Injury to bladder | 0 | 0.00 |
| Injury to ureter | 0 | 0.00 |
| Injury to major vessels | 0 | 0.00 |
| Postoperative Complications | ||
| Deep vein thrombosis | 1 | 0.42 |
| Pulmonary embolism | 0 | 0.00 |
| Port site hernia | 0 | 0.00 |
| Vaginal dehiscence | 0 | 0.00 |
| Re-operation this admission | 1 | 0.42 |
| Death | 0 | 0.00 |
| Final Results | ||
| Washings positive for malignancy | 6 | 2.50 |
| Re-operated later by Gyn Oncology | 9 | 3.75 |
Washings, directed biopsies of peritoneal surfaces and photo documentation are obtained.
See Table 5.
Table 5.
Patients Not Meeting Inclusion Criteria (11 Patients)
| Patients | % Patients | |
|---|---|---|
| Adhesions prevent laparoscopic visualization | 3 | 27.27 |
| Frozen pelvis | 2 | 18.18 |
| Fallopian tube cyst/hydrosalpinx | 3 | 27.27 |
| Fallopian tube cancer | 1 | 9.09 |
| Fibroid uterus/pedunculated myomas | 1 | 9.09 |
| GI malignancy | 1 | 9.09 |
Laparoscopic surgery combined with posterior colpotomy has a low incidence of significant complications. Outcome data show that by observing the principals of minimally invasive surgery, 97.50% of women were successfully treated as outpatients: 97.92% of surgeries lasted <136 minutes; 97.08% had blood loss <200mL, and there were few consequential postoperative complications.
Intraoperative rupture of the ovarian capsule was extremely uncommon in our series. Capsular rupture was noted in just 1.25% of cases.
Distribution of pathologic results is not surprising (Table 6). The most common lesions were cystadenomas, endometriomas, cysts, and mature teratomas accounting for 85% of all cases. Borderline tumors accounted for 5% of lesions, while invasive ovarian malignancy represented 3.75% of the specimens.
Table 6.
Pathology Results
| Patients | % Patients | |
|---|---|---|
| Ovarian cystadenoma | 64 | 26.67 |
| Functional cyst | 47 | 19.58 |
| Endometrioma | 34 | 14.17 |
| Simple cyst | 32 | 13.33 |
| Mature teratoma | 27 | 11.25 |
| Ovarian fibroma | 5 | 2.08 |
| Other benign ovarian lesions | 10 | 4.17 |
| Borderline ovarian tumor | 12 | 5.00 |
| Invasive ovarian cancer | 9 | 3.75 |
Reoperation, when required, occurred usually between 6 and 10 days following the initial laparoscopy (average 7.2 days, range 4 to 19 days).
Laparoscopies abandoned based on presumption of disseminated disease had significant findings at the time of definitive staging by gynecologic oncologists: Stage I-0%, Stage II-16.67%, Stage III-50%, and Stage IV-33.33% (Table 7). Of the 9 cases we treated that turned out to be invasive carcinoma on final pathology, all were believed to be Stage I disease at the time of referral to gynecology oncology. No capsules had excrescences, no disease was noted elsewhere in the abdomen and pelvis, but 6 had positive washings. Definitive staging upstaged 4 of the 9 lesions or 44.44% (Table 7).
Table 7.
Cancer staging from Gyn oncology
| Patients | % Patients | |
|---|---|---|
| Invasive Ovarian Cancer On Inspection—Laparoscopy Terminated | 6 | |
| Stage I | 0 | 0.00 |
| Stage II | 1 | 16.67 |
| Stage III | 3 | 50.00 |
| Stage IV | 2 | 33.33 |
| Invasive Ovarian Cancer—Ovary Removed and Later Directed To Gyn Oncology | 9 | |
| Stage I | 5 | 55.56 |
| Stage II | 3 | 33.33 |
| Stage III | 1 | 11.11 |
| Stage IV | 0 | 0.00 |
Finally, we examined the relationship of menopausal status and cancer Stage of all 15 patients found to have invasive ovarian malignancy (Table 8). Being postmenopausal conferred a greater likelihood of having any ovarian malignancy (8/88 or 9.09%) compared with premenopausal women (7/158 or 4.43%). The negative predictive value of ACOG Committee Opinion 280 Triage Criteria as a de-selector for having invasive ovarian malignancy in our population of women with 8-cm to 13-cm lesions was 95.57% for premenopausal and 90.91% for postmenopausal women.
Table 8.
Cancer Stage Based On Menopausal Status
| All Cases Of Invasive Ovarian Cancer (6 Initially excluded and 9 positive on final pathology). | ||
|---|---|---|
| Premenopausal | Postmenopausal | |
| Stage I | 3 | 2 |
| Stage II | 2 | 2 |
| Stage III | 1 | 3 |
| Stage IV | 1 | 1 |
| Total Patients | 7/158 (4.43%) | 8/88 (9.09%) |
| NPV ACOG 280 Triage Criteria | 151/158 (95.57%) | 80/88 (90.91%) |
DISCUSSION
It is not reasonable to think every woman with an adnexal mass can have surgery in a center affording immediate intraoperative consultation with gynecologic oncologists or that all women with adnexal masses should be referred to gynecologic oncologists for primary treatment. In this country, many women with adnexal masses are operated on by general gynecologists, and many cases of EOC are found only on final pathology after an inadequate initial surgery.
We suggest this new, staged treatment algorithm for adnexal lesions based on the unavailability of gynecologic oncologists at most United States hospitals and the knowledge that many ovarian masses are currently treated by general obstetrician/gynecologists without observance of ACOG Committee Opinion 280 Triage Criteria, without performing washings, without ordering frozen section, and without intraoperative availability of gynecology oncology consultation. Furthermore, many masses are purposefully opened or aspirated without bagging, because surgeons do not fully consider the possibility of malignancy preoperatively.
The goal of our new algorithm is to develop a Staged process focusing additional attention on adnexal masses not thought to be malignant and improving care within this subset of patients. Because many authorities have believed that increasing ovarian mass size is an important de-selector for minimally invasive surgical candidacy, we sought to evaluate outcomes of ovarian masses in this size range including performance of ACOG Committee Opinion 280 Triage Criteria and surgical outcomes.11,75 Laparoscopic adnexectomy, bagging, and colpotomy is a desirable goal for patients with adnexal masses meeting Triage Criteria for suspected benign lesions outlined in ACOG Committee Opinion 280 affording a minimally invasive approach with attendant benefits including outpatient treatment, decreased incidence of capsular rupture, few complications and low necessity for reoperation after final pathology is evaluated. Negative predictive value in our series is similar to reports in the literature,45,46 suggesting masses in this size range are no more or less likely to be malignant than previous series not selected for size. Outcomes including inadvertent rupture rate, surgical time, blood loss, intraoperative and postoperative complication also compare favorably to previous series. We believe this study convincingly extends the size range for adnexal masses safely treated laparoscopically to the 8-cm to 13-cm range.
Colpotomy is felt to be the ideal route to retrieve significant volumes of tissue from the pelvic cavity and has many advantages over mini-laparotomy: no visible abdominal incision, less postoperative ileus, decreased postoperative pain, and more rapid return to normal activities. In the days prior to widespread availability of laparoscopy, skilled gynecologic surgeons frequently used colpotomy for ready access to the pelvis. Unlike episiotomy that can cause dyspareunia, colpotomy does not transect muscles and, therefore, has less bleeding and negligible postoperative pain. It is our practice to insert the collection bag through a laparoscopic port, because it is easier to maintain the pneumoperitoneum during manipulation of the mass and insertion into the bag. Insertion of the collection system through the colpotomy requires only an 11-mm to 13-mm colpotomy defect that would then need to be extended to ultimately deliver the bag, particularly in the case of solid lesions. Some surgeons may point out the potential disadvantages of colpotomy, including incisional infection, peritonitis, and technical complexity, particularly in patients after hysterectomy. These surgeons may instead bring the opening of the collection bag out an anterior abdominal wall incision and will likely enjoy comparable results.
The negative predictive value of ACOG Committee Opinion 280 selection criteria for encountering malignancy in suspected benign cases are set forth in Table 9. Data from the present study is consistent with other reports in the literature. Although reoperation is required 6.09% of the time (15 out of 246 patients), in our algorithm for women with suspected benign lesions, this risk is substantially outweighed by saving laparotomy in 93.91% of patients having benign disease, in reducing 234 of 240 patients' treatment to a single outpatient encounter, to the clear reduction in anxiety in all women not having to sign informed consent for an unduly broad range of surgical options for the first procedure (ranging from laparoscopy to laparotomy to hysterectomy and castration) and to most women not having to travel to a referral center for the initial procedure. Informed consent for this algorithm necessarily needs to highlight the possibility of 2 anesthesias in women found to have ovarian cancer on pathology and compare that to the advantages of minimally invasive surgery enjoyed by the vast number of women with benign disease avoiding laparotomy.
Table 9.
Negative predictive value of ACOG Committee Opinion 280
The 2-step process for women found to have ovarian cancer inherent in our proposed algorithm is not a disservice to patients. Literature does not support the necessity for definitive surgery at the time of the initial operation. If appropriate steps are followed in the first surgery, a 2-step process permits an unrushed consultation with a gynecologic oncologist in which a patient's cytology and pathology reports can be discussed, concerns for future fertility can be addressed, and informed consent for definitive surgery can be thoroughly obtained.
Observing our treatment algorithm will increase the likelihood that women meeting ACOG Committee Opinion 280 Triage Criteria will be immediately referred to gynecologic oncologists, will assure appropriate pre- and intraoperative workup of patients with ovarian masses not felt to be malignant, promote access to minimally invasive surgery for more women with ovarian masses not felt to be malignant, and allow appropriate preoperative consent for the small subset of these women actually found to have ovarian cancer at the initial surgery.
Despite the reasonableness of these suggestions, numerous reports have suggested modifying referral criteria to increase sensitivity at the direct expense of specificity in selecting patients likely to have ovarian cancer.24,45 Changing the CA 125 cut-off to increase sensitivity of referral criteria identifying more cases of ovarian malignancy leads to more false-positives: women who are caused unnecessary anxiety, forced to travel to unfamiliar surroundings for care, and who may well be overtreated as a consequence of undergoing laparotomy in the hands of some gynecologic oncologists, only to hear postoperatively that frozen-section pathology was benign. Even worse is the scenario where oophorectomy is performed, either laparoscopically or laparotomically, frozen section is positive leading to abdominal hysterectomy, contralateral oophorectomy, omentectomy, and thorough staging only to find out final pathology returned benign disease. A recent study demonstrated frozen section was incorrect in 12% of ovarian cancer cases.89 Overtreatment is a real possibility in any treatment algorithm, the impact of which should not be underestimated.
To substantially increase the appropriateness of referrals to gynecologic oncologists, we will have to commensurately increase the precision of our diagnosis of ovarian cancer. This will require advent and testing of new technologies to boost diagnostic precision used in tandem with established modalities,21–24,90 or even development of high-sensitivity and high-specificity screening tests for early stage ovarian carcinoma.91
Until that day arrives, we propose acceptance and testing of our new treatment algorithm for adnexal masses using ACOG Committee Opinion 280 for initial triage of patients, focusing additional care and attention on women with suspected benign lesions while encouraging minimally invasive surgical care for all affected women. This systematic approach to evaluation and treatment of adnexal masses utilizing the skills of minimally invasive surgeons and gynecologic oncologists will lead to enhanced outcomes for women with both benign and malignant disease.
Contributor Information
Richard H. Demir, Arizona Regional Medical Center, Mesa, Arizona, USA..
Greg J. Marchand, Arizona Regional Medical Center, Mesa, Arizona, USA..
References:
- 1. AAGL AAGL Position Statement: Route of hysterectomy to treat benign disease. J Minim Invasive Gynecol. 2010;17:1–3 [DOI] [PubMed] [Google Scholar]
- 2. ACOG Committee Opinion No. 444 Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156–1158 [DOI] [PubMed] [Google Scholar]
- 3. Demir RH, Marchand GJ. Safe laparoscopic removal of a 3200 grams fibroid uterus. JSLS. 2010;14:600–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rossing MA, Wickland KG, Cushing-Haugen KL, Weiss NS. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst. 2010;102(4):222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menin U, Gentry-Maharaj A, Hallet R, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009;10(4):327–340 [DOI] [PubMed] [Google Scholar]
- 6. Manzo E, Bhatti S, McLachlan M, Al-Samarrai M. Outcomes of 207 laparoscopic procedures for suspected adnexal mass. J Minim Invasive Gynecol. 2009;16:S41 [Google Scholar]
- 7. Mage G, Canis M, Manhes H, Pouly JL, Wattiez A, Bruhat MA. Laparoscopic management of adnexal cystic masses. J Gynecol Surg. 1990;6:71–79 [DOI] [PubMed] [Google Scholar]
- 8. Mecke H, Lehmann-Wilenbrock E, Ibrahim M, et al. Pelviscopic treatment of ovarian cysts in premenopausal women. Gynecol Obstet Invest. 1992;34:36–42 [DOI] [PubMed] [Google Scholar]
- 9. Nezhat F, Nezhat C, Welander CE, Benigno B. Four ovarian cancers diagnosed during laparoscopic management of 1011 women with adnexal masses. Am J Obstet Gynecol. 1992;167:790–796 [DOI] [PubMed] [Google Scholar]
- 10. Hulka J, Parker W, Surrey M, Phillips JM. Management of ovarian masses. AAGL. 1990 survey. J Reprod Med. 1992;37:599–602 [PubMed] [Google Scholar]
- 11. Canis M, Mage G, Pouly JL, Wattiez A, Manhes H, Bruhat MA. Laparoscopic diagnosis of adnexal cystic masses: a 12-year experience with long-term follow-up. Obstet Gynecol. 1994;83:707–712 [PubMed] [Google Scholar]
- 12. Marana R, Vittori G, Campo S, Fanfani R, Montanino G, Casa A. Operative laparoscopy for adnexal cystic masses in patients under 40 years of age. J Am Asssoc Gynecol Laparosc. 1994;1:S20. [DOI] [PubMed] [Google Scholar]
- 13. Wenzl R, Lehner R, Husslein P, Sevelda P. Laparoscopic surgery in cases of ovarian malignancies: an Austria-wide survey. Gynecol Oncol. 1996;63:57–61 [DOI] [PubMed] [Google Scholar]
- 14. Childers JM, Masseri A, Surwit EA. Laparoscopic management of suspicious adnexal masses. Am J Obstet Gynecol. 1996;175:1451–1457 [DOI] [PubMed] [Google Scholar]
- 15. Canis M, Pouly JL, Wattiez A, Mage G, Manhes H, Bruhat MA. Laparoscopic management of adnexal masses suspicious at ultrasound. Obstet Gynecol. 1997;89:679–683 [DOI] [PubMed] [Google Scholar]
- 16. Hidlebaugh DA, Vulgaropoulos S, Orr RK. Treating adnexal masses: operative laparoscopy vs. laparotomy. J Reprod Med. 1997;42:551–558 [PubMed] [Google Scholar]
- 17. Malik E, Bohm W, Stoz F, Nitsch CD, Rossmanith WG. Laparoscopic management of ovarian tumors. Surg Endosc. 1998;12:1326–1333 [DOI] [PubMed] [Google Scholar]
- 18. Mettler L. The cystic adnexal mass: patient selection, surgical techniques and long-term follow-up. Curr Opin Obstet Gynecol. 2001;13:389–397 [DOI] [PubMed] [Google Scholar]
- 19. Valentin L, Ameye L, Testa A, et al. Ultrasound characteristics of different types of adnexal malignancies. Gynecol Oncol. 2006;102:41–48 [DOI] [PubMed] [Google Scholar]
- 20. Muzi L, Angioli R, Zullo M, Panici PB. The unexpected ovarian malignancy found during operative laparoscopy: incidence, management, and implications for prognosis. J Min Invasive Gynecol. 2005;12:81–89 [DOI] [PubMed] [Google Scholar]
- 21. Jacobs I, Oram D, Fairbanks J, Turner J, Frost C, Grudzinskas JG. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol. 1990;97:922–929 [DOI] [PubMed] [Google Scholar]
- 22. Sassone AM, Timor-Tritsch IE, Artner A, Westhoff C, Warren WB. Transvaginal sonographic characterization of ovarian disease: evaluation of a new scoring system to predict ovarian malignancy. Obstet Gynecol. 1991;78:70–76 [PubMed] [Google Scholar]
- 23. Geomini P, Kruitwagen R, Bremer G, Cnossen J, Mol WJ. The accuracy o disk scores in predicting ovarian malignancy. Obstet Gynecol. 2009;113:384–394 [DOI] [PubMed] [Google Scholar]
- 24. McDonald JM, Doran S, DeSimone CP, et al. Predicting risk of malignancy in adnexal masses. Obstet Gynecol. 2010;115:687–694 [DOI] [PubMed] [Google Scholar]
- 25. Mol BW, Boll D, DeKanter M, et al. Distinguishing the benign and malignant adnexal masses: an internal validation of prognostic models. Gynecol Oncol. 2001;80:162–167 [DOI] [PubMed] [Google Scholar]
- 26. Duponte A, Stergioti E, Messinis IE. Risk scoring for adnexal masses and endoscopic management. Int J Gynaecol Obstet. 2007;96:42–43 [DOI] [PubMed] [Google Scholar]
- 27. Aslam N, Tailor A, Lawton F, Carr J, Savvas M, Jurkovic D. Prospective evaluation of three different models for the pre-operative diagnosis of ovarian cancer. BJOG. 2000;107:1347–1353 [DOI] [PubMed] [Google Scholar]
- 28. Manjunath AP, kumar Pratap, Sujatha K, Vani R. Comparison of three risk of malignancy indices in evaluation of pelvic masses. Gynaecol Oncol. 2001;81:225–229 [DOI] [PubMed] [Google Scholar]
- 29. Tingulstad S, Hagen B, Skjeldestad FE, et al. Evaluation of a risk malignancy index based on serum CA 125, ultrasound findings and menopausal status in the pre-operative diagnosis of pelvic masses. Br J Obstet Gynaecol. 1996;103:826–831 [DOI] [PubMed] [Google Scholar]
- 30. Van Holsbeke C, Van Calster B, Valentin L, et al. External validation of mathematical models to distinguish between benign and malignant adnexal tumors: a multicenter study by the International Ovarian Tumor Analysis Group. Clin Cancer Res. 2007;13:4440–4447 [DOI] [PubMed] [Google Scholar]
- 31. Asif N, Sattar A, Dawood MM, Rafi T, Aamir M, Anwar M. Pre-operative evaluation of ovarian mass: risk of malignancy index. J Coll Physicians Surg Pak. 2004;14:128–131 [PubMed] [Google Scholar]
- 32. Obeidat BR, Amarin ZO, Latimer JA, Crawford RA. Risk of malignancy index in the prospective evaluation of pelvic masses. Int J Gynaecol Obstet. 2004;85:255–258 [DOI] [PubMed] [Google Scholar]
- 33. Morgante G, la Marca A, Ditto A, De Leo V. Comparison of two malignancy risk indices based on serum CA 125, ultrasound score and menopausal status in the diagnosis of ovarian masses. Br J Obstet Gynaecol. 1999;106:524–527 [DOI] [PubMed] [Google Scholar]
- 34. Timmerman D, Verrelst H, Bourne TH, et al. Artificial neural network models for the preoperative discrimination between malignant and benign adnexal masses. Ultrasound Obstet Gynaecol. 1999;13:17–25 [DOI] [PubMed] [Google Scholar]
- 35. Davies AP, Jacobs I, Woolas R, Fish A, Oram D. The adnexal mass: benign or malignant? Evaluation of a malignancy index. Br J Obstst Gynaecol. 1993;100:927–931 [DOI] [PubMed] [Google Scholar]
- 36. Ulusoy S, Akbayir O, Numanoglu C, Ulsoy N, Odabas E, Gulkilik A. The risk of malignancy index in discrimination of adnexal masses. Int J Gynaecol Obstet. 2007;96:186–191 [DOI] [PubMed] [Google Scholar]
- 37. Yazbek J, Aslam N, Tailor A, Hillaby K, Raju KS, Jurkovic D. A comparative study of the risk of malignancy index and the ovarian crescent sign for the diagnosis of invasive ovarian cancer. Ultrasound Obstet Gynecol. 2006;28:320–324 [DOI] [PubMed] [Google Scholar]
- 38. Van Calster B, Timmerman D, Lu C, et al. Preoperative diagnosis of ovarian tumors using Bayesian kernel-based methods. Ultrasound Obstet Gynecol. 2007;29:496–504 [DOI] [PubMed] [Google Scholar]
- 39. Jacobs IJ, Skates SJ, MacDonald N, et al. Screening for ovarian cancer: a pilot randomized controlled trial. Lancet. 1999;353:1207–1210 [DOI] [PubMed] [Google Scholar]
- 40. Jacobs I, Davies AP, Bridges J, et al. Prevalence screening for ovarian cancer in postmenopausal women by CA 125 measurement and ultrasonography. BMJ. 1993;306:1030–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Drouin PD, Ehlen T, Ghatage P, et al. Guidelines for the laparoscopic management of the adnexal mass. J Soc Obsstet Gynaecol Can. 1998;20:983–989 [Google Scholar]
- 42. Shalev E, Eliyahu S, Peleg D, Tsabari A. Laparoscopic management of adnexal cystic masses in postmenopausal women. Obstet Gynecol. 1994;83:594–596 [DOI] [PubMed] [Google Scholar]
- 43. Falcone T. Adnexal masses: when to observe, when to intervene, and when to refer. Obstet Gynecol. 2010;115:680–681 [DOI] [PubMed] [Google Scholar]
- 44. The role of the generalist obstetrician-gynecologist in the early detection of ovarian cancer ACOG Committee Opinion No. 280. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2002;100:1413–1416 [DOI] [PubMed] [Google Scholar]
- 45. Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic mass. Obstet Gynecol. 2005;105:35–41 [DOI] [PubMed] [Google Scholar]
- 46. Dearking AC, Aletti GD, McGee ME, et al. How relevant are ACOG and SGO guidelines for referral of adnexal mass? Obstet Gynecol. 2007;110:841–848 [DOI] [PubMed] [Google Scholar]
- 47. Trimbos JB, Parmar M, Vergote I, et al. International collaborative ovarian neoplasm trial 1 and adjuvant chemotherapy in ovarian neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. J Natl Cancer Inst. 2003;95:105–132 [PubMed] [Google Scholar]
- 48. Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2006. CA Cancer J Clin. 2006;56:106–130 [DOI] [PubMed] [Google Scholar]
- 49. Petterson F. International Federation of Gynecology and Obstetrics Report, FIGO, 1991 [Google Scholar]
- 50. Brakat RR, Benjamin I, Lewis JL, et al. Platinum based chemotherapy for advanced-stage serous ovarian carcinoma of low malignant potential. Gynecol Oncol. 1995;59:390–393 [DOI] [PubMed] [Google Scholar]
- 51. Reich H, McGlynn F, Wilkie W. Laparoscopic management of Stage I ovarian cancer. J Reprod Med. 1990;35:601–605 [DOI] [PubMed] [Google Scholar]
- 52. Canis M, Rabischong B, Houlle C, et al. Laparoscopic management of adnexal masses: a gold standard? Curr Opin Obstet Gynecol. 2002;14:423–428 [DOI] [PubMed] [Google Scholar]
- 53. Medeiros LR, Fachel JM, Garry R, Stein AT, Furness S. Laparoscopy versus laparotomy for benign ovarian tumors. Cochrane Database Syst Rev. 2005;20:CD004751. [DOI] [PubMed] [Google Scholar]
- 54. Magrina J, Espada M, Munoz R, Noble BN, Kho RM. Robotic adnexectomy compared with laparoscopy for adnexal mass. Obstet Gynecol. 2009;114:581–584 [DOI] [PubMed] [Google Scholar]
- 55. Havrilesky LJ, Peterson BL, Dryden DK, Soper JT, Clark-Pearson DL, Berchuck A. Predictors of clinical outcomes in the laparoscopic management of adnexal masses. Obstet Gynecol. 2003;102:243–251 [DOI] [PubMed] [Google Scholar]
- 56. Smorgick N, Barel O, Halperin R, Schneider D, Pansky M. Laparoscopic removal of adnexal cyst: is it possible to decrease inadvertent intraoperative rupture rate? Am J Obstet Gynecol. 2009;200:237–238 [DOI] [PubMed] [Google Scholar]
- 57. Panici PB, Palaia I, Bellati F, Pernice M, Angioli R, Muzii L. Laparoscopy compared with laparoscopically guided minilaparotomy for large adnexal masses. Am J Obstet Gynecol. 2007;110:241–248 [DOI] [PubMed] [Google Scholar]
- 58. Sagiv R, Golan A, Glezerman M. Laparoscopic management of extremely large ovarian cysts. Obstet Gynecol. 2005;105:1319–1322 [DOI] [PubMed] [Google Scholar]
- 59. Fauvet R, Boccara J, Dufournet C, Poncelet C, Durai E. Laparoscopic management of borderline ovarian tumors: results of a French multi-center study. Ann Oncol. 2005;16:403–410 [DOI] [PubMed] [Google Scholar]
- 60. Park JY, Bae J, Lim MC, et al. Laparoscopic and laparotomic staging in stage I epithelial ovarian cancer: a comparison of feasibility and safety. Int J Gynecol Cancer. 2008;15:2012–2019 [DOI] [PubMed] [Google Scholar]
- 61. Bakkam-Gamez JN, Richardson DL, Seamon LG, et al. Influence of intraoperative capsule rupture on outcomes in stage I epithelial ovarian cancer. Obstet Gynecol. 2009;113:11–17 [DOI] [PubMed] [Google Scholar]
- 62. Ben-Ami I, Smorgick N, Tovbin J, Fuchs N, Halperin R, Pansky M. Does intraoperative spillage of benign ovarian mucinous cystadenoma increase its recurrence rate? Am J Obstet Gynecol. 2010;202:142–147 [DOI] [PubMed] [Google Scholar]
- 63. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in Stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176–182 [DOI] [PubMed] [Google Scholar]
- 64. Sainz de la Cuesta R, Goff BA, Fuller AF, Nikrui N, Eichhorn JH, Rice LW. Prognostic importance of intraoperative rupture of malignant ovarian epithelial neoplasms. Obstet Gynecol. 1994;84:1–7 [PubMed] [Google Scholar]
- 65. Webb M, Decker DG, Mussey E, Williams TJ. Factors influencing survival in stage I ovarian cancer. Am J Obstet Gynecol. 1972;116:222–228 [DOI] [PubMed] [Google Scholar]
- 66. Mizuno M, Kikkawa F, Shibata K, et al. Long-term prognosis of stage I ovarian carcinoma. Prognosis importance of intraoperative rupture. Oncology. 2003;65:29–36 [DOI] [PubMed] [Google Scholar]
- 67. Ahmed FY, Wiltshaw E, A'Hern RP, et al. Natural history and prognosis of untreated stage I epithelial ovarian carcinoma. J Clin Oncol. 1996;14:2968–2975 [DOI] [PubMed] [Google Scholar]
- 68. Sjovall K, Nilsson B, Einhorn N. Diffferent types of rupture of the tumor capsule and the impact on survival in early ovarian carcinoma. Int J Gynecol Cancer. 1994;4:333–336 [DOI] [PubMed] [Google Scholar]
- 69. Sevelda P, Dittrich C, Salzer H. Prognostic value of the rupture of the capsule in stage I epithelial ovarian carcinoma. Gynecol Oncol. 1989;35:321–322 [DOI] [PubMed] [Google Scholar]
- 70. Dembo AJ, Davy M, Stenwig AE, Berle EJ, Bush RS, Kjorstad K. Prognostic factors in patients with early stage I epithelial ovarian cancer. Obstet Gynecol. 1990;75:263–273 [PubMed] [Google Scholar]
- 71. Kodama S, Tanaka K, Tokunaga A, Sudo N, Takahashi T, Matsui K. Multivariate analysis of prognostic factors in patients with ovarian cancer stage I and II. Int J Gynaecol Obstet. 1997;56:147–153 [DOI] [PubMed] [Google Scholar]
- 72. Colomer AT, Jimenez AM, Barcelo IB. Laparoscopic treatment and staging of early ovarian cancer. J Minim Invasive Gynecol. 2008;15:414–419 [DOI] [PubMed] [Google Scholar]
- 73. Nezhat FR, Ezzati M, Chuang L, Shamshirsaz AA, Rahaman J, Gretz H. Laparoscopic management of early ovarian and fallopian tube cancers: surgical and survival outcome. Am J Obstet Gynecol. 2009;200:83–85 [DOI] [PubMed] [Google Scholar]
- 74. Lehner R, Wenzl R, Heinzl H, Husslein P, Sevelda P. Influence of delayed staging laparotomy after laparoscopic removal of ovarian mass later found malignant. Obstet Gynecol. 1998;92:967–971 [DOI] [PubMed] [Google Scholar]
- 75. Maiman M, Seltzer V, Boys J. Laparoscopic excision of ovarian neoplasm subsequently found to be malignant. Obstet Gynecol. 1991;55:563–565 [PubMed] [Google Scholar]
- 76. Kindermann G, Maassen V, Kuhn W. Laparoscopic management of ovarian tumors subsequently diagnosed as malignant. J Pelvic Surg. 1996;2:245–251 [Google Scholar]
- 77. Moore DH. Primary surgical management of early epithelial ovarian carcinoma. In, Robin SC, Sutton GP, eds. Ovarian Cancer. 2nd ed Philadelphia: Lippincott Williams and Wilkins; 2001;201–218 [Google Scholar]
- 78. Liu CS, Nagarsheth NP, Nezhat FR. Laparoscopy and ovarian cancer: a paradigm change in the management of ovarian cancer? J Minim Invasive Gynecol. 2009;16:250–262 [DOI] [PubMed] [Google Scholar]
- 79. Pomel C, Provencher D, Dauplat J, Gauthier P, Le Boudee G, Drouin P. Laparoscopic staging of ovarian carcinoma. Gynecol Oncol. 1995;58:301–306 [DOI] [PubMed] [Google Scholar]
- 80. Childers J, Lang J, Surwit E, Hatch L. Laparoscopic surgical staging of ovarian cancer. Gynecol Oncol. 1995;59:25–33 [DOI] [PubMed] [Google Scholar]
- 81. Stier EA, Brakat RR, Curtin JP, et al. Laparotomy to complete staging of presumed early ovarian cancer. Obstet Gynecol. 1996;87:737–740 [DOI] [PubMed] [Google Scholar]
- 82. Young RC, Decker DG, Wharton JT, et al. Staging laparotomy in early ovarian cancer. JAMA. 1983;250:3072–3076 [PubMed] [Google Scholar]
- 83. Tozzi R, Koehler C, Ferrara A, Schneider A. Laparoscopic treatment of early ovarian cancer: surgical and survival outcomes. Gynecol Oncol. 2004;93:199–203 [DOI] [PubMed] [Google Scholar]
- 84. Leblanc E, Querleu D, Narducci F, et al. Laparoscopic restaging of early stage invasive adnexal tumors: a 10 year experience. Gynecol Oncol. 2004;94:624–629 [DOI] [PubMed] [Google Scholar]
- 85. Spirtos NM, Eisekop SM, Boike G, et al. Laparoscopic staging in patients with incompletely staged cancers of he uterus, ovary, fallopian tube, and primary peritoneum: a Gynecologic Oncology Group (GOG) study. Am J Obstet Gynecol. 2005;193:1645–1649 [DOI] [PubMed] [Google Scholar]
- 86. Chi DS, Abu-Rustum NR, Sonoda Y, et al. The safety and efficacy of laparoscopic surgical staging of apparent stage I ovarian and fallopian tube cancers. Am J Obstet Gynecol. 2005;192:1614–1619 [DOI] [PubMed] [Google Scholar]
- 87. Ghezzi F, Cromi A, Uccella S, et al. Laparoscopy versus laparotomy for the surgical management of apparent early stage ovarian cancer. Gynecol Oncol. 2007;105:409–413 [DOI] [PubMed] [Google Scholar]
- 88. Chiong E, Hegarty PK, Davis JW, Kamat AM, Pisters LL, Matin SF. Port site hernias occurring after the use of bladeless radially expanding trocars. Urology. 2010;75(3):574–580 [DOI] [PubMed] [Google Scholar]
- 89. Canis M, Mashiach R, Wattiez A, et al. Frozen section in laparoscopic management of macroscopically suspicious ovarian malignancy. J Am Assoc Gynecol Laparosc. 2004;11:365–369 [DOI] [PubMed] [Google Scholar]
- 90. Partridge E, Kreimer AR, Greenlee RT, et al. Results from our rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009;113:775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Muller CY. Doctor, should I get this new ovarian cancer test—OVA1? Obstet Gynecol. 2011;116:246–247 [DOI] [PubMed] [Google Scholar]