Abstract
We present a simple and efficient method for expressing cDNAs in Purkinje neurons (PNs) present in heterogeneous mouse cerebellar cultures. The method combines the transfection of freshly-dissociated cerebellar cells via nucleofection with the use of novel expression plasmids containing a fragment of the L7 (Pcp2) gene that, within the cerebellum, drives PN-specific expression. The efficiency of PN transfection (determined 13 days post nucleofection) is approximately 70%. Double and triple transfections are routinely achieved at slightly lower efficiencies. Expression in PNs is obvious after one week in culture and still strong after three weeks, by which time these neurons are well-developed. Moreover, high-level expression is restricted almost exclusively to the PNs present in these mixed cultures, which greatly facilitates the characterization of PN-specific functions. As proof of principle, we used this method to visualize (1) the morphology of living PNs expressing mGFP, (2) the localization and dynamics of the dendritic spine proteins PSD-93 and Homer-3a tagged with mGFP and (3) the interaction of live PNs expressing mGFP with other cerebellar neurons expressing mCherry from a β-Actin promoter plasmid. Finally, we created a series of L7-plasmids containing different fluorescent protein cDNAs that are suited for the expression of cDNAs of interest as N- and C-terminally tagged fluorescent fusion proteins. In summary, this procedure allows for the highly efficient, long-term, and specific expression of multiple cDNAs in differentiated PNs, and provides a favorable alternative to two procedures (viral transduction, ballistic gene delivery) used previously to express genes in cultured PNs.
Keywords: cerebellar culture, Purkinje cells, dendritic spines, L7 (Pcp2), Amaxa nucleofection, transfection, live cell microscopy
1. Introduction
Purkinje neurons (PNs) are key players in the circuitry of the cerebellum in that they provide the sole output from the cerebellar cortex (Hansel et al., 2001). These neurons integrate excitatory input from the axons of cerebellar granule neurons (GNs) (parallel fibers) and the axons of neurons present in the inferior olive (climbing fibers). Methods for the preparation of dissociated cerebellar cultures containing PNs from the cerebellar primordium of embryonic or newborn mice/rats have been developed (Cohen-Cory et al., 1991; Furuya et al., 1998; Gruol and Franklin, 1987; Hockberger et al., 1989; Okubo et al., 2001; Schilling et al., 1991; Tabata et al., 2000; Weber and Schachner, 1984; Yuzaki and Mikoshiba, 1992). These primary cultures are very heterogeneous, containing GNs, inhibitory interneurons and glial cells as well as PNs. Notably, the survival and development of PNs in culture depends on the presence of GNs (Baptista et al., 1994; Hirai and Launey, 2000; Hisatsune et al., 2006; Morrison and Mason, 1998). Various aspects of PN biology, including their morphological development, electrophysiological properties, sub-cellular organization, and relationships with other cell types have been investigated using these dissociated cerebellar cultures (Cohen-Cory et al., 1991; Dunn et al., 1998a, b; Gruol and Franklin, 1987; Hirai and Launey, 2000; Hirano and Hagiwara, 1988; Hisatsune et al., 2006; Hockberger et al., 1989; Kuroyanagi et al., 2009; Linden, 1997; Mashimo et al., 2008; Matsuda et al., 2010; Matsuda et al., 2006; Nagata et al., 2006; Okubo et al., 2001; Schilling et al., 1991; Stepanova et al., 2003; Tanaka et al., 2006; Tu et al., 1998; Uemura et al., 2010). Moreover, the existence of extensive afferent synapses on the dendritic spines, dendritic shafts and cell bodies of the cultured PNs has been documented (Dunn et al., 1998a, b; Hirano and Kasono, 1993; Ito-Ishida et al., 2008), and the molecular mechanisms of synaptic plasticity at the GN-PN synapse has been elucidated with the help of these mixed cerebellar cultures (Chung et al., 2003; Eto et al., 2002; Hirai et al., 2003; Kawaguchi and Hirano, 2007; Launey et al., 2004; Leitges et al., 2004; Linden, 1996; Linden and Ahn, 1999; Linden and Connor, 1991; Linden et al., 1991; Lonart et al., 2003; Matsuda et al., 2000; Simsek-Duran et al., 2004; Smith-Hicks et al., 2010; Steinberg et al., 2006; Takamiya et al., 2008; Wang and Linden, 2000; Yawata et al., 2006).
The ability to transfect and express exogenous cDNAs in cultured cells is a central tool in cell biological research. In the case of PNs, the procedures that have mainly been used to deliver exogenous DNAs are ballistic gene delivery via the ‘gene gun’ (Chung et al., 2003; Eto et al., 2002; Leitges et al., 2004; Linden and Ahn, 1999; Lonart et al., 2003; Simsek-Duran et al., 2004; Smith-Hicks et al., 2010; Steinberg et al., 2006; Tu et al., 1998) and viral transduction (Gimenez-Cassina et al., 2007; Mashimo et al., 2008; Matsuda et al., 2006; Okubo et al., 2001; Stepanova et al., 2003). Both of these techniques possess significant disadvantages, however. Gene gun-mediated DNA delivery suffers from low transfection efficiency (Biewenga et al., 1997) and physical damage to the cells, while viral transduction is hampered by the time-consuming preparation of viral stocks, possible adverse effects on cellular physiology (Warren et al., 2006; Yedowitz et al., 2005), limitations in DNA size in the case of some viral vectors (Gimenez-Cassina et al., 2007; Lundstrom et al., 2001; Takayama et al., 2008), and safety concerns.
Recently, a highly-efficient electroporation technique called nucleofection has been developed that circumvents many of the disadvantages of gene gun- and virus-mediated gene transfer. Nucleofection has been applied successfully to the transfection of several types of primary neurons, including hippocampal neurons and cerebellar GNs (Dityateva et al., 2003; Gartner et al., 2006; Zeitelhofer et al., 2007), but not to PNs. Since nucleofection is performed with cells in suspension, it is applied in the case of primary neurons to freshly-dissociated cells before they are plated. Primary neurons, including PNs, take weeks in culture to mature, i.e. to develop axons, dendrites, and synapses. Therefore, the successful application of nucleofection to achieve expression of exogenous cDNAs in differentiated PNs requires the use of a promoter element that is not only sufficiently active to generate detectable amounts of protein in cells, but also active long enough (i.e. weeks) for the PNs to fully develop.
The heterogeneous nature of dissociated cerebellar cultures represents an additional challenge for the characterization of expressed cDNAs in PNs. For example, the uniform expression of a GFP-tagged protein in all of the cell types present in these densely populated, heterogeneous cultures would make it difficult to characterize the distribution of the protein within just PNs, especially since they are sparsely distributed. Furthermore, certain experimental questions require the ability to express cDNAs in PNs, but not, for example, in GNs.
Here, we provide an in-depth description and characterization of a simple and highly-efficient method that allows for the high-level, long-term expression of exogenous cDNAs specifically in PNs present in heterogeneous dissociated cerebellar cultures. This method was key to our recent efforts to define the role of myosin-Va in the targeting of endoplasmic reticulum to the dendritic spines of PNs (Wagner et al., 2011).
2. Materials and methods
2.1. Preparation and transfection of dissociated cerebellar cultures from C57BL/6 mice
Dissociated cerebellar cultures were prepared from mice essentially as described (Linden, 1996; Linden et al., 1991; Schilling et al., 1991; Tabata et al., 2000), except that the cells were transfected by nucleofection just before plating (see below). Every embryo was treated separately, resulting in one culture from one cerebellum. This allows the simultaneous processing and transfection of cerebellar cells from embryos with different genotypes (followed by genotyping after the fact). Briefly, day E17 or E18 mouse embryos were obtained from timed pregnant C57BL/6 females that had been anesthetized using isoflurane (Forane, Baxter; 1-chloro-2,2,2-trifluoroethyl difluoromethyl ether) and euthanized via cervical dislocation. Routinely, two pregnant females were processed in parallel. Sterile scissors and forceps were used. Immediately after euthanasia, the uteri with the embryos were removed and transferred into a sterile 140 mm tissue culture dish (Nunc #168381) that was kept on ice and filled with ~50 ml ice-cold, modified Hank's Balanced Salt Solution (MHS; 5.333 mM KCl, 0.441 KH2PO4, 137.931 mM NaCl, 0.336 mM Na2HPO4-7H2O, and 5.556 mM D-glucose (HBSS, Invitrogen 14185-052), 20 μg/ml gentamycin (Invitrogen 15710-064), pH adjusted to 7.2 with NaOH, sterile-filtered). All reagents for cell culture, including MHS and culture medium (DFM; see below), were prepared using sterile-filtered, deionized and UV-treated water (KD Medical RGF-3410). Single embryos were isolated from the uteri and placed separately into the wells of a sterile six-well plate (BD Falcon #353046) containing 3 ml ice-cold MHS per well and kept on ice. Each embryo was then decapitated and the body was removed from the well. Next, each head was dissected and the cerebellar primordium was transferred into a sterile 1.5 ml tube containing 250 μl ice-cold MHS (one cerebellum per tube) and kept on ice. After all heads were processed, the isolated cerebellar primordia were minced in the tubes using a scalpel (handle #3 with blade #11) to obtain chunks of ~ 1 mm size. Subsequently, the cerebellar tissues were digested by adding 250 μl of freshly prepared, ice-cold papain solution (MHS containing 20 U/ml papain, Sigma P-5306) to each tube, followed by incubation in a 33°C water bath for 30 min.
To stop the digestion, 1 ml MHS/FBS (84% v/v MHS, 16% v/v fetal bovine serum [FBS; Invitrogen 10082-139]) prewarmed to room temperature (RT) was added to each tube. After gentle mixing by inverting the tube, the cells were harvested by centrifugation at RT for 4 min at 0.6 × g. All subsequent steps were carried out at RT under the hood. After all supernatants were removed, 300 μl of freshly-prepared DNase solution (MHS containing 11.86 mM MgSO4 [Sigma M-2643] and 5 U/ml DNase I, RNase-free [Roche 10776785001]) was added to each of the harvested cerebellar cell pellets. Each cell pellet was then triturated carefully by pipetting up and down ~40 times using a Gilson Pipetman P1000 equipped with a sterile 1000 μl tip (Rainin RT-200S). The triturated cells were then passed through a 210 μm nylon mesh (Small Parts, Inc. CMN-0210-A; sterilized by submerging in 100% ethanol, followed by air-drying under the hood), collected in a fresh 1.5 ml tube, and harvested by centrifugation (RT for 4 min at 0.6 × g). The cells were then washed twice by resuspending them (via inverting the tube several times) in 1 ml of RT MHS and harvesting them by centrifugation (RT for 4 min at 0.6 × g).
The transfection of the cells was performed using the Amaxa Mouse Neuron Nucleofector Kit (Lonza VPG-1001) according to the manufacturer's instructions. The next steps (nucleofection, plating) were followed through to the end for each pellet before proceeding to the next pellet. Immediately after removing the supernatant of the second wash, 100 μl of the nucleofection solution was mixed with the plasmid DNA to be transfected. This mix was then used to resuspend the cerebellar cell pellet (~ 1.5 × 106 cells). The resulting cell suspension was transferred into one of the cuvettes provided in the kit and subjected without delay to nucleofector program O-03 (or another program, if indicated). Immediately after nucleofection, one of the pipettes provided in the Amaxa kit was used to dilute the cell suspension with 300 μl of a mix containing 90% v/v DFM (see below) and 10% v/v FBS and to transfer the mixture into the 14 mm diameter well of a glass bottom culture dish (MatTek P35G-1.0-14-C) that had been coated with poly-L-ornithine (see below). The dish was then placed in a 37°C incubator (5% CO2, 97% humidity) before proceeding with the next cell pellet. 1.5 to 2.0 hours after nucleofection, 1.8 ml DFM at 37°C was added to each dish. 24 to 36 hours after nucleofection, 1.5 ml of culture medium was exchanged with fresh, prewarmed DFM. After that, cells were fed 8, 15, and 22 days after plating by exchanging 1 ml of culture medium with fresh, pre-warmed DFM.
An unsupplemented culture medium stock solution corresponding to 1x D-MEM/F-12 (Sigma D2906-10X1L) containing 1.2 g/L NaHCO3 (Sigma S-5761), 100 μM putrescine (Sigma P-5780), 30 nM Na2SeO3 (Sigma S-5261), 1.4 mM additional L-glutamine (Gibco 25030-081), 5 μg/ml gentamycin (Gibco 15710-064), pH adjusted 7.0 using NaOH, was sterile filtered and stored at 4°C. To obtain DFM, 50 ml of the unsupplemented medium were supplemented with a final concentration of 2 μM cytosine β-D-arabinofuranoside (Sigma C-6645), 40 nM progesterone (Sigma P-6149), 0.5 ng/ml tri-iodothyronine (Calbiochem 64245), 200 μg/ml transferrin (Sigma T-1147), 100 μg/ml bovine serum albumin (Sigma A-3156), and 20 μg/ml insulin (Sigma I-6634). The supplemented medium was then sterile filtered through a 0.22 μm filter (Millipore SLVG 025 LS) and added to cells within 3 days. The following stock solutions were stored at -20 °C: 300 μM Na2SeO3, 2 mM cytosine β-D-arabinofuranoside, 200 μM progesterone, and 2.5 mg/L tri-iodothyronine.
To coat the sterile glass-bottom culture dishes (MatTek P35G-1.0-14-C), 2 ml 100% ethanol was added to each dish. The glass bottom and the well's borders were then cleaned by rubbing them with a sterile cotton swab. Subsequently, the ethanol was aspirated off, the dishes were air-dried in a sterile hood, and 300 μl of poly-L-ornithine solution (PLO; 0.5 mg/ml; Sigma P-4638; freshly dissolved in sterile water) was pipetted onto the cover glass of the 14 mm well. The dishes were then incubated overnight in a 37°C incubator (5% CO2, 97% humidity). Immediately before culture preparation, the PLO was aspirated off, and the dishes were rinsed three times with 2 ml of sterile water and air dried.
2.2. DNA plasmids
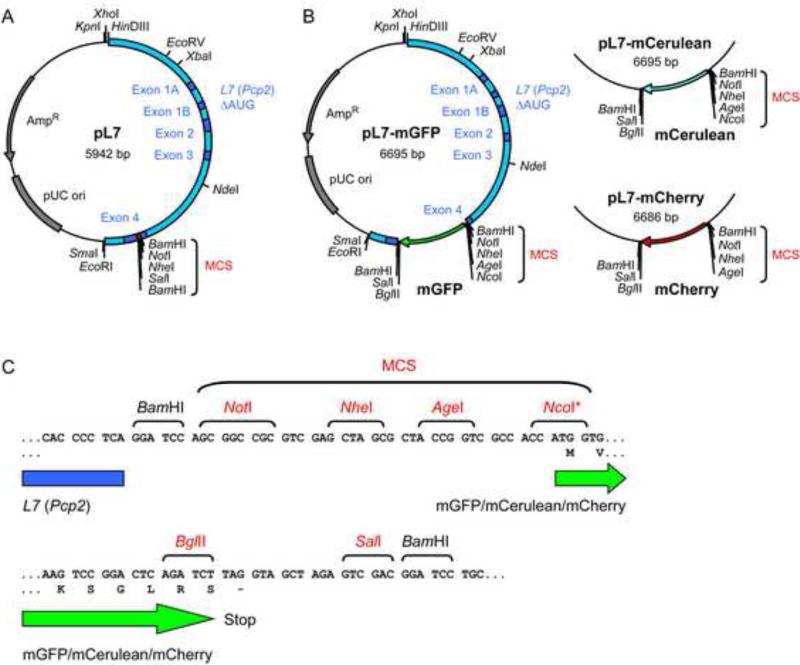
We constructed pL7 (Fig. 1a), an expression vector containing the L7 (Pcp2) promoter, as follows. First, the SalI site in pBluescript SK+ was destroyed by cutting the plasmid with SalI, followed by fill-in of the 3’-recessive ends using T4 DNA polymerase and religation. To remove the restriction sites NotI, BamHI, SpeI, and XbaI, the pBluescript SK+ lacking the SalI site was then digested with NotI and BamHI. The 3’-recessive ends were filled-in using T4 DNA polymerase, and the plasmid backbone was religated, creating pBluescript SK+ ΔSalI, NotI, BamHI, SpeI, XbaI. Subsequently, a 3.0 kb fragment of this plasmid generated by HinDIII/EcoRI digestion was ligated with a 3.0 kb L7 fragment released using HinDIII/EcoRI from pGEM3-L7ΔAUG/1B (Serinagaoglu et al., 2007; Smeyne et al., 1995), generating pBS-L7ΔAUG/1B. This L7 fragment corresponds to a piece of mouse genomic DNA comprising 0.9 kb of the L7 promoter sequence, as well as L7 exon and intron sequences. This genomic fragment was previously modified by site directed mutagenesis to create a BamHI site in exon 4 and to destroy all potential start codons in exons 1A, 1B, 2, 3, and 4 (to ensure that translation begins at the start codon of the ORFs inserted into the BamHI site) (Serinagaoglu et al., 2007; Smeyne et al., 1995; Zhang et al., 2002). Numerous transgenic mouse lines carrying transgenes containing this L7 fragment with cDNAs inserted at the BamHI site were found to specifically express these cDNAs in cerebellar PNs (Baader et al., 1998; Barski et al., 2000; Buffo et al., 1997; De Zeeuw et al., 1998; McEvoy et al., 2007; Oberdick et al., 1993; Oberdick et al., 1990; Serinagaoglu et al., 2007; Smeyne et al., 1995; Smeyne et al., 1991; Tomomura et al., 2001; Wang et al., 2006; Wulff et al., 2007; Zhang et al., 2001). In order to facilitate the insertion of cDNAs into the L7 fragment in pBS-L7ΔAUG/1B, we introduced a multiple cloning site (MCS) into the BamHI site in exon 4 by inserting a DNA linker generated by BamHI digestion of two annealed oligonucleotides with the sequence CGCGGATCCAGCGGCCGCGTCGAGCTAGCATTAGCTGTCGACGGATCCCGC and its reverse complement, thereby generating plasmid pL7 (Fig. 1).
Figure 1. Plasmids for the expression of exogenous cDNAs in cultured Purkinje neurons.
(A) Schematic of plasmid pL7. The L7 DNA fragment (shown in blue) contains a promoter sequence, as well as all of the exons (dark blue) and introns that comprise the small L7 structural gene. All of the AUG codons present in exons 1A, 1B, 2, 3, and 4 were mutated (Serinagaoglu et al., 2007; Smeyne et al., 1995) to ensure that translation starts within the cDNA inserted into the multiple cloning site (MCS; red). The indicated restriction sites are present only once in the vector (except for BamHI). (B) Schematic maps of plasmids pL7-mGFP, pL7-mCerulean and pL7-mCherry, all of which allow fusion of a protein of interest to either the N- or C-terminus of the fluorescent protein. In addition to the L7 DNA fragment (blue) and the L7 exons (dark blue), the cDNA encoding the fluorescent protein is depicted (mGFP, green; mCerulean, blue; mCherry, red). (C) DNA sequences immediately upstream and downstream of the fluorescent protein cDNAs. These sequences are identical in pL7-mGFP, pL7-mCerulean and pL7-mCherry. Also shown are the reading frame and the first two amino acids of the fluorescent proteins and the reading frame at the end of the fluorescent proteins. Restriction sites that occur only once in these plasmids are shown in red. Note that NcoI is not a single-cutter in pL7-mCherry. N-terminal fusions (i.e. the protein of interest precedes the fluorescent protein) are made by inserting cDNAs into the MCS that precedes the fluorescent protein coding sequence. C-terminal fusions (i.e. the protein of interest follows the fluorescent protein) are made by inserting cDNAs using the BglII site or the BglII and SalI-sites that follow the fluorescent protein coding sequence (note that the SalI site cannot be used by itself for cDNA insertion because of an in frame stop codon between the BglII and SalI sites).
To construct plasmids for producing monomeric GFP (mGFP) or monomeric Cerulean (mCerulean) under the control of the L7 promoter, we generated pL7-mGFP and pL7-mCerulean (Fig. 1b). First, 0.8 kb fragments containing the fluorescent protein ORFs were released from plasmids pCMV-mGFP (corresponding to a pEGFP-C1 plasmid from Clontech, modified to encode a GFP with the L221K mutation (Zacharias et al., 2002)) and pmCerulean-C1 (corresponding to a modified pCerulean-C1 encoding Cerulean with the A206K mutation (Rizzo et al., 2004)) using restriction enzymes NheI and SalI. Each of these fragments was then ligated with the 5.9 kb fragment of pL7 generated by NheI/SalI digestion, thereby generating plasmids pL7-mGFP-latestop and pL7-mCerulean-latestop. Subsequently, the 30 bp BglII/SalI portion of these plasmids was replaced by a linker fragment generated by BglII/SalI digestion of two annealed oligonucleotides with the sequence GCTTACAGATCTTAGGTAGCTAGAGTCGACGCTTAC and its reverse complement. This linker inserts a stop codon in between the BglII and SalI sites and in frame with the fluorescent protein ORFs. The resulting plasmids are termed pL7-mGFP and pL7-mCerulean. To create pL7-mCherry (Fig. 1b), a 0.8 kb fragment generated by AgeI/BglII digestion of pmCherry-C1 (a plasmid corresponding to pEGFP-C1, but containing the mCherry ORF AY678264 (Shaner et al., 2004)) was ligated with a 6 kb fragment generated by AgeI/BglII digestion of pL7-mGFP. The correct sequence of the oligonucleotide-based linkers and the fluorescent protein ORFs was verified by DNA sequencing.
Plasmids for the expression of Homer-3aii and PSD-93-C3,5 under the control of the L7 promoter were constructed as follows. The Homer-3aii ORF was PCR amplified using a plasmid containing a mouse Homer-3 cDNA (Clone ID 3602414; Open Biosystems MMM1013-64736) as a template and oligonucleotides TACATATGCGGCCGCTGACCAATGTCCACAGCCAGGGAACAG and TAAGTACCATGGGTGCTGCCTCTGCCAGGCGTGCCAG. The PSD-93-C3,5 ORF was amplified using a plasmid containing a rat PSD-93-C3,5 cDNA (El-Husseini et al., 2000), and oligonucleotides TACATATGCGGCCGCCACCATGATTTGCCACTGCAAAGTTG and GAAGTACCATGGCTAACTTCTCCTTTGAGGGAATCCAGATG. The Homer-3 and PSD-93 PCR fragments were cut with NcoI and NotI and ligated with a 6.7 kb fragment generated by NcoI/NotI digestion of pL7-mGFP, generating pL7-PSD-93-C3,5-mGFP or pL7-Homer-3aii-mGFP. The Homer-3aii and PSD-93-C3,5 ORF sequences were verified by DNA sequencing.
To create a plasmid for the expression of mCherry under control of the β-Actin promoter (pβ-Actin-mCherry), a 0.8 kb fragment containing the mCherry ORF that was generated by NheI/XbaI digestion of pmCherry-C1, was ligated with a 6.0 kb fragment generated by SpeI/XbaI digestion of pBact16 (Ludin et al., 1996).
Plasmid DNAs used for nucleofection were prepared using QIAfilter midi kits (Qiagen #12243). DNAs were dissolved in TE (10 mM Tris HCl, 0.15 mM EDTA, pH8.0) at a final concentration of 1-4 μg/μl. Plasmid maps were generated with Clone Manager Professional Suite (Scientific & Educational Software).
2.3. Immunofluorescence staining of cerebellar cultures
Immunofluorecence labeling was used to determine the percentage of PNs in dissociated cerebellar cultures that were transfected with pL7-mGFP. All steps were carried out at RT and the volumes used per dish are indicated. First, cultures were rinsed with 2 ml of PBS (154 mM NaCl, 5 mM Na2HPO4, 1.7 mM KH2PO4, pH7.4; KD Medical RGF-3210) containing 4% w/v paraformaldehyde (Electron Microscopy Sciences #15710), incubated in fresh 2 ml of the PBS/paraformaldehyde solution for 30 min, and washed three times with PBS. Fixed cells were then incubated for 25 min in 3 ml of Blocking solution (PBS containing 10% v/v normal goat serum, 1% w/v bovine serum albumin, and 0.5% Triton-X100), followed by a 40 min incubation in 1.3 ml anti-Calbindin-D-28K antibody (Sigma C-9848; diluted 1:500 in Blocking solution). The samples were then washed three times by rinsing with 2 ml PBS, and incubated for 45 min in 1.2 ml Alexa Fluor 568-labeled goat anti-mouse secondary antibody (Molecular Probes A11031; diluted 1:1000 in Blocking solution). After three rinses with 2 ml PBS, the cells were covered with 2 ml PBS and imaged.
2.4. Confocal microscopy of cerebellar cultures
Live and fixed cerebellar cultures were imaged using a laser scanning confocal microscope Zeiss (LSM 510, Carl Zeiss, Inc.) equipped with a 40x (N.A. 1.3) or 100x (N.A. 1.4) objective. During observation of live cells, culture dishes were mounted in a chamber (Heating Insert P, Incubator S; PeCon GmbH) kept at 37°C and supplied with air containing 5% CO2. If not indicated otherwise, stacks of 0.7 to 1.2 μm thick Z-planes covering the entire height of the fluorescent cells were collected and are shown as a maximum projection or 3D reconstruction (‘transparent projection’) generated with the Zeiss LSM software. For time-lapse recordings, images were acquired every 2 seconds.
The number of mGFP-positive cells in live cultures transfected with pL7-mGFP was determined by screening the indicated number of dishes using wide field fluorescence microscopy with a 40x objective. To determine the percentage of PNs that express mGFP, cultures that had been transfected with pL7-mGFP and stained for Calbindin-D-28K to detect all PNs were used to count the number of Calbindin- and mGFP-positive PNs. To determine the percentage of PNs that express CMV-driven mGFP in cultures transfected with pCMV-mGFP and pL7-mCherry, the first 100 randomly-encountered PNs identified by mCherry-expression and characteristic morphology were analyzed for mGFP expression. Standard deviations are indicated.
3. Results
3.1. A plasmid series for expressing cDNAs in cultured Purkinje neurons
We reasoned that a DNA fragment derived from the gene L7 (Pcp2), which mediates the PN-specific expression of transgenes in mice (Baader et al., 1998; Barski et al., 2000; Buffo et al., 1997; Burright et al., 1995; De Zeeuw et al., 1998; McEvoy et al., 2007; Oberdick et al., 1993; Oberdick et al., 1990; Serinagaoglu et al., 2007; Smeyne et al., 1995; Smeyne et al., 1991; Tomomura et al., 2001; Vandaele et al., 1991; Wang et al., 2006; Wulff et al., 2007; Zhang et al., 2001; Zhang et al., 2002; Zu et al., 2004), might be useful for the long-term, PN-specific expression of cDNAs in PNs present in mixed cerebellar cultures. Therefore, we constructed plasmid pL7 (Fig. 1a), which contains this previously-generated, 3.0 kb fragment of L7 that includes a 5’ promoter element and the entire transcribed portion of L7 (with all ATGs mutated) (Serinagaoglu et al., 2007; Smeyne et al., 1995). To build plasmids that mediate the L7-controlled production of proteins of interest fused to fluorescent proteins, we inserted cDNAs encoding mGFP (Zacharias et al., 2002), a monomeric version of the blue-shifted mGFP-variant Cerulean (Rizzo et al., 2004), or the red fluorescent protein mCherry (Shaner et al., 2004) into a multiple cloning site within the L7 element, thereby creating plasmids pL7-mGFP, pL7-mCerulean and pL7-mCherry, respectively (Fig. 1b). Multiple restriction sites are available to allow the insertion of cDNAs of interest that are either N- or C-terminally fused to the fluorescent protein (Fig. 1c).
3.2. Nucleofection of pL7-mGFP leads to expression of mGFP in both immature and differentiated Purkinje neurons in culture
To test the ability of plasmid pL7-mGFP to drive the expression of mGFP in PNs present in mixed cerebellar cultures, we made use of Lonza's Amaxa nucleofection technique to deliver the plasmid into freshly-dissociated cerebellar cells obtained from E18 mouse embryos (see Materials and Methods for details). The nucleofected cells were plated on poly-L ornithine-coated cover glass and allowed to develop in vitro. Observation of live cultures using wide field and confocal fluorescence microscopy at 8 to 22 days in vitro (DIV) after plating showed that mGFP-expressing PNs are present at all time points (Fig. 2 and data not shown). PNs were recognized by their characteristic morphology (Dunn et al., 1998a; Tabata et al., 2000). Nevertheless, we also confirmed the identity of mGFP-expressing PNs by immunofluorescence staining using an antibody against the PN-specific protein Calbindin-D-28K (Fig. 3).
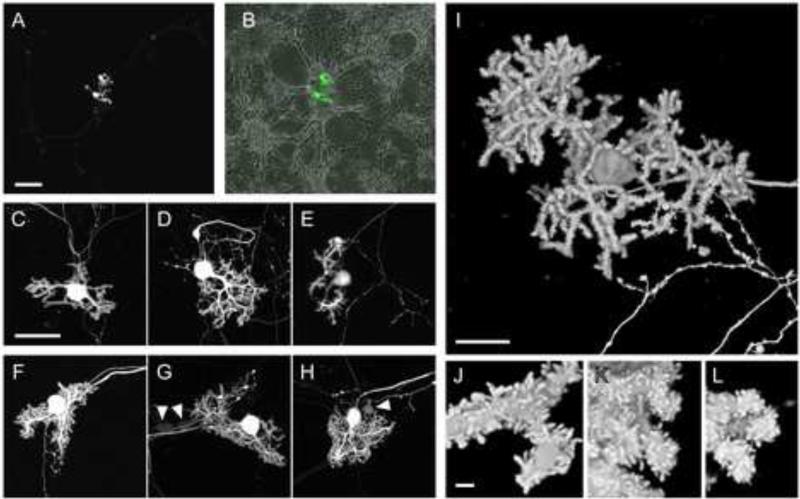
Figure 2. Live PNs in dissociated cerebellar culture expressing mGFP following nucleofection with pL7-mGFP.
Dissociated cerebellar cells from E18 mouse embryos were nucleofected with 10 μg of pL7-mGFP via nucleofection program O-03, cultured for 13 DIV (A-E) or 22 DIV (F-L), and imaged using confocal microscopy. Image stacks of Z-planes covering the entire thickness of the PNs were projected into a single plane as a maximum projection (A-H) or were processed using the ‘transparent projection’ function of the Zeiss LSM Image software to obtain a 3D reconstruction (I-L). (B) An overlay of the low magnification image shown in (A) with the corresponding transmitted light image is shown. The arrowheads in (G) and (H) indicate examples of neurons other than PNs that are expressing mGFP at low levels. Size bars: 100 μm (A,B); 50 μm (C-H); 20 μm (I); 2 μm (J-L).
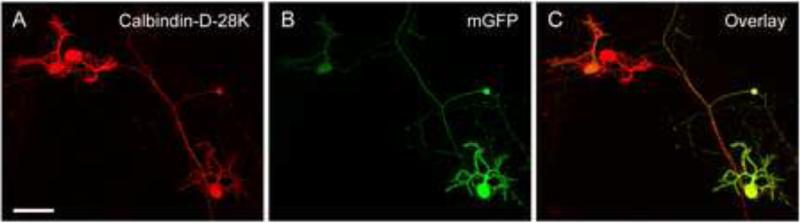
Figure 3. Immunofluorescence staining of mGFP-expressing PNs in dissociated cerebellar culture using anti-Calbindin-D-28K antibody.
Dissociated cerebellar cells were nucleofected with 10 μg of pL7-mGFP, cultured for 13 DIV, and subjected to immunofluorescence staining using an antibody against the PN-specific protein Calbindin-D-28K. Images show maximum projections of stacks of Z-planes acquired using confocal microscopy and correspond to the anti-Calbindin immunofluorescence signal (A), the mGFP signal (B), and the superimposition of these images (C). Size bar, 50 μm.
Confocal microscopy analyses of live cerebellar cultures nucleofected with pL7-mGFP showed that free mGFP localizes diffusely throughout the PN-cytoplasm, i.e. in the axon, cell body, and dendrites (Fig. 2). As observed previously for untransfected PNs (Tabata et al., 2000), the dendritic arbor of the mGFP-expressing PNs was more complex at 22 DIV (Fig. 2f-g) than at 13 DIV (Fig. 2c-e). At 8 DIV, mGFP-expressing PNs were essentially devoid of dendrites, but they do display short proto-dendrites and an extended axon (data not shown). The strong signal for mGFP made it possible to observe the detailed morphology of living PNs at high magnification. For example, numerous spine-like structures covering the dendrites of PNs at 13 and 22 DIV are clearly visible (Fig. 2i-l).
To compare different nucleofection programs, we counted the number of mGFP-expressing PNs per 14 mm diameter well containing the cells from one cerebellar primordium and nucleofected with 10 μg of pL7-mGFP DNA. Using nucleofection program O-03, 21 +/- 5.2 (n=5) mGFP-expressing PNs per well were detected at DIV 13. Using the same conditions, but programs G-13 or O-05, we obtained an average of 2 (n=3) and 11.5 (n=2) mGFP-expressing PNs per well, respectively. Nucleofection programs A-33 or C-13 did not yield any mGFP-expressing PNs. Therefore, we used program O-03 for all following transfections.
To measure directly the percentage of total PNs in the dish that are expressing mGFP at DIV 13 after nucleofection of pL7-mGFP using program O-03, we visualized all PNs in the nucleofected cerebellar cultures by immunofluorescence staining with the anti-Calbindin-D-28K antibody (Fig. 3). Strikingly, 70.9% +/- 8.0% (n=3) of the total PNs in the dish were also mGFP-positive. Therefore, in terms of the efficiency of PN transfection, this approach is highly efficient.
Regarding the specificity of mGFP expression from the pL7-mGFP plasmid, the brightest cells we routinely observe are PNs. However, on occasion mGFP-expressing cells are observed that are not PNs by shape or calbindin staining. These cells are usually quite dim and appear by shape to be neurons (see Fig. 2g,h for examples). Visual inspection of live cultures reveals the presence of 269 (+/-75, n=2) such cells per dish at 13 DIV. Given a rough estimate of 10,000 total neurons per dish (based on immunofluorescence staining using an antibody against neuron-specific enolase, data not shown), we estimate that pL7-mGFP nucleofection leads to mGFP-expression in ~2-3% of these neurons, as compared to ~70% of PNs. Together, these characteristics of the pL7/nucleofection-based cDNA expression system make it highly PN-specific.
3.3. The L7 plasmid, but not plasmids containing a CMV- or β-Actin promoter, mediate the PN-specific expression of exogenous cDNAs
Gene gun-mediated transfection of mixed cerebellar cultures has employed CMV promoter-based plasmids (see e.g. (Chung et al., 2003)), and the expression of exogenous cDNAs in cultured hippocampal neurons is routinely performed with plasmids containing either a β-Actin or the CMV promoter (El-Husseini et al., 2000; Gartner et al., 2006; McCroskery et al., 2006; Zeitelhofer et al., 2007). We therefore compared plasmids containing either the L7 promoter, the CMV promoter, or the β-Actin promoter for their ability to drive cDNA expression in dissociated cerebellar cultures following nucleofection.
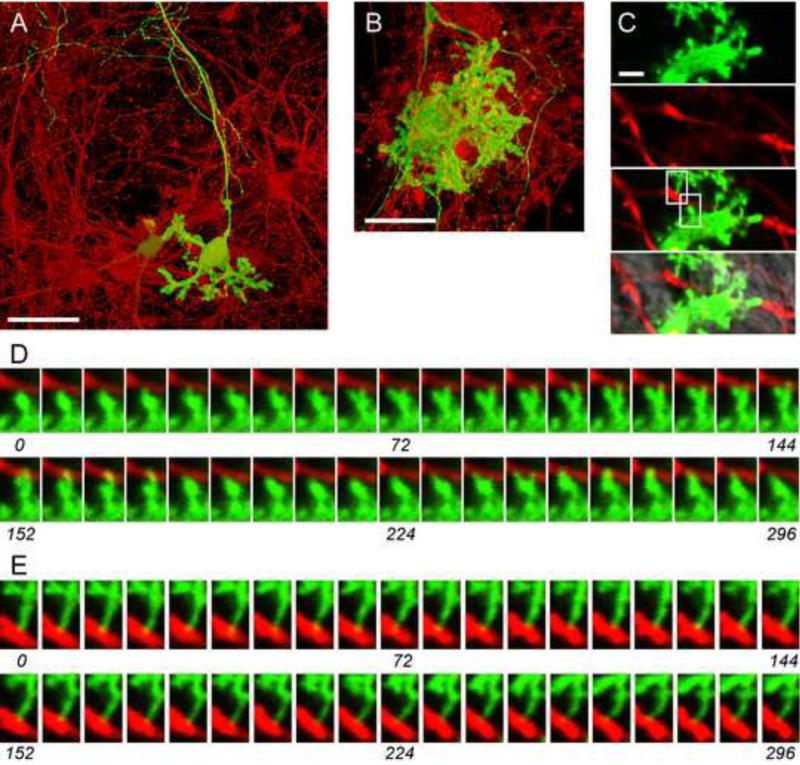
Figure 4 shows live cultures that were co-nucleofected with pL7-mGFP and a β-Actin promoter plasmid driving the expression of mCherry. At both DIV 13 and 22, numerous cells resembling GNs are expressing mCherry at high levels, while high-level expression of mGFP is detected only in PNs (Fig. 4a, b). Weak mCherry fluorescence was also detectable in PNs, as is evident in the split images (Fig. 4c). Therefore, following nucleofection, cDNA expression from the β-Actin promoter plasmid in these mixed cultures is not PN-specific, and high level expression from this plasmid occurs in neurons other than PNs.
Figure 4. Comparison of fluorescent protein cDNA expression from β-Actin- and L7-promoter plasmids in cerebellar cultures.
Dissociated cerebellar cells were nucleofected with pL7-mGFP and pβ-Actin-mCherry and cultured for 13 DIV (A, C-E) or 22 DIV (B) before imaging live cultures using confocal microscopy. (A, B) The mGFP (green) and mCherry (red) fluorescence image stacks of Z-planes were processed using the ‘transparent projection’ function of the Zeiss LSM Image software to obtain 3D reconstructions. The superimposed mGFP and mCherry images are shown. (C) The panels show images of a single confocal plane of (from top to bottom) the mGFP fluorescence signal, the mCherry fluorescence signal, their overlay, and an overlay with the corresponding transmitted light image. (D, E) The images of mGFP (green) and mCherry (red) fluorescence signals depict the regions indicated in (C), and were taken from a time series in which images were recorded every two seconds. Time (sec) is indicated; every fourth recorded image is shown. See also Supplemental Movie 1. Size bars: 50 μm (A, B); 2 μm (C).
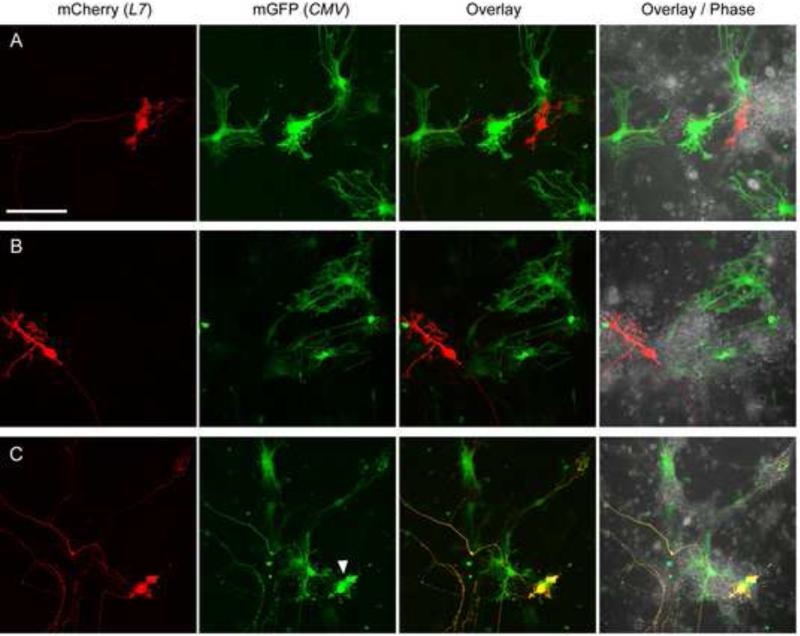
Figure 5 shows live cultures that were co-nucleofected with pL7-mCherry and a CMV promoter plasmid driving the expression of mGFP. Interestingly, at 13 DIV (Fig. 5a, b), as well as at 10 DIV (Fig. 5c), strong mGFP expression was detected in cells that are morphologically distinct from PNs and might represent glia. High level expression mCherry was seen only in PNs, as expected (Fig. 5). Notably, expression of mGFP from pCMV-mGFP was rarely detected in PNs (in 12 +/- 3% of the mCherry-expressing PNs, n=2; see Fig. 5c for an example). Together, these results show that, following nucleofection, cDNA expression from the CMV promoter plasmid is not PN-specific and occurs mainly in cells other than PNs.
Figure 5. Comparison of fluorescent protein cDNA expression from CMV- and L7-promoter plasmids in cerebellar cultures.
Dissociated cerebellar cells were nucleofected with pL7-mCherry and pCMV-mGFP and cultured for 13 (A, B) or 10 DIV (C) before imaging live cultures. The mCherry and mGFP fluorescence image stacks of Z-planes were projected into a single plane as a maximum projection and are shown in red (mCherry (L7)), green (mGFP (CMV)), and superimposed on each other (Overlay). Also shown is the superimposition of the mCherry, mGFP and the corresponding phase contrast image (Overlay / Phase). The arrowhead indicates a PN expressing mGFP from pCMV-mGFP. Size bar, 100 μm.
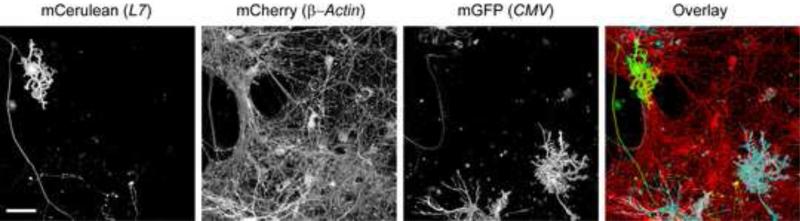
Finally, we co-nucleofected cerebellar cultures with three different plasmids each encoding a distinct fluorescent protein under control of either the L7-, β-Actin-, or CMV-promoters. As shown in Figure 6, this permits the simultaneous visualization of three distinct populations of cells in these cultures, i.e. PNs (Fig. 6; mCerulean (L7)), neurons resembling GNs (Fig. 6; mCherry (β-Actin)), and cells resembling glia (Fig. 6; mGFP(CMV)).
Figure 6. Comparison of fluorescent protein cDNA expression from L7-, β-Actin-, and CMV- and promoter plasmids in cerebellar cultures.
Dissociated cerebellar cells were nucleofected with pL7-mCerulean, pβ-Actin-mCherry and pCMV-mGFP and cultured for 15 DIV before imaging live cultures. The fluorescence image stacks of Z-planes were processed using the ‘transparent projection’ function of the Zeiss LSM Image software to obtain 3D reconstructions. The mCerulean, mCherry, and mGFP images, as well as their superimposition (Overlay) are shown. Size bar, 50 μm.
The differential expression properties of the β-Actin-, CMV- and L7 promoter plasmids provides an opportunity to observe in detail the interaction of PNs with other cell types in the culture. Figure 4c shows a PN dendrite covered with spine-like extensions and labeled with mGFP (expressed from pL7-mGFP), and putative granule neuron axons labeled with mCherry (expressed from pβ-Actin-mCherry). Time-lapse analysis reveals instances where a PN spine interacts dynamically with a putative granule neuron axon (Fig. 4d, Supplemental Movie 1) or makes more stable contacts with an axon (Fig. 4e, Supplemental Movie 1). Therefore, it should be possible to use our methods to examine in detail the dynamic interactions of PNs with other cell types in the mixed cerebellar cultures.
3.4. Visualization of GFP-tagged PSD-93 and Homer-3a in live Purkinje neurons
We next sought to determine if our novel expression system can be used to co express within PNs two or more cDNAs simultaneously off of multiple plasmids so that we can visualize, for example, fluorescently-tagged dendritic spine proteins in differentiated, living PNs. Towards this end, we co-nucleofected dissociated cerebellar cells with pL7-mCherry to label the cell volume and a second pL7-based plasmid encoding an mGFP-tagged version of either the membrane-associated guanylate kinase family protein PSD-93 (Brenman et al., 1996; El-Husseini et al., 2000; McGee et al., 2001; Petralia et al., 2001) or the adaptor protein Homer-3a (Shiraishi-Yamaguchi and Furuichi, 2007; Shiraishi et al., 2004; Xiao et al., 1998). Both PSD-93 and Homer-3a localize to PN spines in vivo and are components of the postsynaptic densitiy (PSD), a protein structure that is essential for synaptic signaling at excitatory synapses (Sheng and Hoogenraad, 2007).
Here, we introduced a cDNA corresponding to an alternatively spliced isoform of PSD-93 that comprises the 5’a N-terminus (also known as C3,5 or β N-terminus) (Brenman et al., 1996; El-Husseini et al., 2000) into pL7-mGFP. Confocal microscopy of cultured PNs expressing mCherry as a volume marker and mGFP-PSD-93-C3,5 shows that, consistent with the known localization of endogenous PSD-93 (El-Husseini et al., 2000; Petralia et al., 2001), mGFP-PSD-93-C3,5 accumulates in dots that are located in the spine-like structures as well as close to the plasma membrane within the dendrite shafts of the PNs in DIV 13 and 22 cultures (Figure 7a,b).
Figure 7. Expression of cDNAs encoding mGFP-tagged PSD-93-C3,5 or Homer-3 aii in cultured PNs.
Dissociated cerebellar cells from E18 mouse embryos were nucleofected with plasmids pL7-mCherry and pL7-PSD-93-C3,5-mGFP (A, B) or pL7-Homer-3aii-mGFP (C, D) and cultured for 13 DIV (A, C) or 22 DIV (B, D) before imaging live cultures. Fluorescence image stacks of Z-planes spanning the entire height of the PN were processed to obtain a 3D reconstruction. The resulting PSD-93-C3,5-mGFP (PSD-93-C3,5) and Homer-3aii-mGFP (Homer-3aii) images are shown, as well as the superimposition of the respective mGFP images with the corresponding mCherry image (Overlay). The inserts show images from a single confocal plane of the fluorescence signal from PSD-93-C3,5-mGFP (PSD-93-C3,5) and Homer-3aii-mGFP (Homer-3aii), as well as the superimposition of the respective images with the corresponding mCherry image (Overlay). The inserts are taken from time series recordings (see Supplemental Movie 2 and 3). Size bar, 20 μm (A-D) and 2 μm (inserts).
Alternative splicing creates short and long isoforms of Homer-3, of which the latter is known to localize to the PSD in PN spines (Shiraishi-Yamaguchi and Furuichi, 2007). Here we introduced a cDNA corresponding to a long isoform of Homer-3a that contains two alternatively-spliced, 9 base pair exons (referred to as Homer-3aii (Shiraishi-Yamaguchi and Furuichi, 2007)) into pL7-mGFP. We find that mGFP-Homer-3aii accumulates within the spines of PNs, although it also localizes diffusely throughout the cytoplasm at DIV 13 and 22 (Fig. 7c,d). This pattern of Homer-3aii-mGFP localization is strikingly similar to what has been seen in cultured PNs stained for endogenous Homer-3a/b (Shiraishi et al., 2004).
Finally, we followed both mGFP-PSD-93-C3,5 and mGFP-Homer-3aii dynamics in time-lapse recordings of live PNs at 13 DIV (Supplemental Movies 2 and 3). Therefore, the nucleofection of pL7-based plasmids readily facilitates the observation of fluorescently tagged PSD proteins in differentiated, living PNs. We note that triple-transfections, resulting in PNs that simultaneously express cDNAs from three different pL7-plasmids, are also feasible with this approach (Wagner et al., 2011).
4. Discussion
We have shown here that nucleofection of dissociated cerebellar cells with novel plasmids containing a DNA element comprising the promoter sequence of the gene L7 provides an efficient and simple method to achieve the expression of multiple exogenous cDNAs in cultured PNs. Dissociated cerebellar cultures are heterogeneous, containing granule neurons, interneurons and glia, in addition to PNs. Remarkably, the expression of cDNAs from nucleofected L7 plasmids is highly-specific for PNs. In contrast, expression from plasmids utilizing a β-Actin promoter is observed mainly in neuronal cells other than PNs (probably GNs). Similarly, CMV promoter-based plasmids led to high level cDNA expression in cells other than PNs (probably glia). While these differences highlight the value of our novel pL7-plasmids for studying PNs, they also suggest approaches for simultaneously labeling live PNs and other cerebellar cell types in different colors in order to visualize their interactions in culture (see Figure 4-6).
The use of nucleofection to achieve expression of cDNAs in differentiated PNs avoids many disadvantages associated with particle-mediated gene transfer via the ‘gene gun’ and with viral transduction. For example, nucleofection requires only the preparation of purified plasmid DNA, while gene gun transfection requires the additional step of coating gold particles with the DNA. With viral-mediated gene transfer, the generation of viral stocks is time consuming, and there are limitations in the size of cDNAs that can be delivered (4 kb for adeno-associated virus-based vectors, 5 to 8 kb for Semliki Forest virus, and less than 8 kb for lentiviral vectors (Lundstrom et al., 2001; Takayama et al., 2008), but see (Gimenez-Cassina et al., 2007)). Notably, we have successfully nucleofected ~12 kb pL7-plasmids to express 6.3 kb ORFs in PNs (Wagner et al., 2011). Safety issues associated with the use of viruses and the effect of viral infection on microtubule dynamics (Warren et al., 2006; Yedowitz et al., 2005) are other concerns that are avoided using our nucleofection method.
The combination of nucleofection-mediated DNA delivery with the use of our novel L7-based expression plasmids results in the highly efficient expression of cDNAs in differentiated PNs (i.e. ~70% of PNs are GFP-positive 13 days post nucleofection). Moreover, the expression levels of fluorescent proteins are usually high enough to allow their visualization in live PNs as early as 8 DIV. While viral gene delivery into post-mitotic neurons can also be highly efficient, the transfection efficiency obtained with the gene gun has not been reported for cultured PNs, but was found to be no more than 2% in the case of other cultured neurons (Biewenga et al., 1997). Moreover, unlike nucleofection, ballistic DNA delivery using the gene gun causes physical damage to the cells shortly (usually <36 hours) before they are to be analyzed.
In addition to allowing the highly-efficient and long-term expression of cDNAs in PNs, our pL7-based plasmids are almost entirely specific for the expression of cDNAs in PNs present in these heterogeneous cerebellar cultures. PN-specific expression in mixed cerebellar cultures has also been achieved using an HSV-1 derived viral vector, presumably because the DNA is preferentially delivered into PNs (Gimenez-Cassina et al., 2007). By contrast, the gene gun-mediated transfection of CMV promoter-based plasmids leads to the expression in these mixed cerebellar cultures of cDNAs not only PNs, but in other cell types as well (Linden and Ahn, 1999; Lonart et al., 2003; Simsek-Duran et al., 2004). Finally, we note that in a recent study, freshly-plated, dissociated cerebellar cells were transfected using a cationic lipid-based method (Lipofectamine 2000) to deliver an expression plasmid containing an L7 DNA fragment inserted downstream of a CMV promoter (Ohkawa et al., 2007). While this plasmid led to cDNA expression in PNs, neither the efficiency of transfection nor the specificity of expression were reported.
Since nucleofected pL7-based plasmids drive cDNA expression in differentiating PNs before dendrites and spines develop, the introduction of exogenous cDNAs that interfere with PN development or viability may be problematic. In this case, viral transduction to introduce the cDNA of interest into differentiated PNs might be useful. Another alternative that we are currently developing is the use of single cell axoporation (Tanaka et al., 2009) to introduce plasmids into mature PNs.
In summary, we have described a novel method for the transfection of PNs in dissociated cerebellar culture that represents an alternative to previously used procedures. This method is reliable, efficient, and easy to perform, and it leads to the expression of multiple cDNAs over long periods of time and with high specificity in PNs present within mixed cultures. We believe that this method will greatly facilitate the further study of PNs.
Supplementary Material
Highlights.
- simple and efficient method for expressing cDNAs in the Purkinje neurons of dissociated cerebellar cultures
- novel plasmids for Purkinje-specific expression of cDNAs as N- and C-terminally tagged fluorescent fusion proteins
- differential labeling and simultaneous visualization of Purkinje neurons and other cerebellar cell types in live cultures
Acknowledgments
We thank Roland Bock and David J. Linden (Johns Hopkins University, Baltimore) for teaching us cerebellar culture preparation, John Oberdick (The Ohio State University, Columbus) and David S. Bredt (University of California, San Francisco) for DNA constructs, and Xufeng Wu (NHLBI, NIH, Bethesda) for microscopy support.
Abbreviations
- DIV
days in vitro
- GN
granule neuron
- MCS
multiple cloning site
- ORF
open reading frame
- PLO
poly-L-ornithine
- PN
Purkinje neuron
- PSD
postsynaptic density
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baader SL, Sanlioglu S, Berrebi AS, Parker-Thornburg J, Oberdick J. Ectopic overexpression of engrailed-2 in cerebellar Purkinje cells causes restricted cell loss and retarded external germinal layer development at lobule junctions. J. Neurosci. 1998;18:1763–73. doi: 10.1523/JNEUROSCI.18-05-01763.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista CA, Hatten ME, Blazeski R, Mason CA. Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro. Neuron. 1994;12:243–60. doi: 10.1016/0896-6273(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000;28:93–8. [PubMed] [Google Scholar]
- Biewenga JE, Destree OH, Schrama LH. Plasmid-mediated gene transfer in neurons using the biolistics technique. J. Neurosci. Methods. 1997;71:67–75. doi: 10.1016/s0165-0270(96)00127-6. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Christopherson KS, Craven SE, McGee AW, Bredt DS. Cloning and characterization of postsynaptic density 93, a nitric oxide synthase interacting protein. J. Neurosci. 1996;16:7407–15. doi: 10.1523/JNEUROSCI.16-23-07407.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, Holtmaat AJ, Savio T, Verbeek JS, Oberdick J, Oestreicher AB, Gispen WH, Verhaagen J, Rossi F, Strata P. Targeted overexpression of the neurite growth-associated protein B-50/GAP-43 in cerebellar Purkinje cells induces sprouting after axotomy but not axon regeneration into growth-permissive transplants. J. Neurosci. 1997;17:8778–91. doi: 10.1523/JNEUROSCI.17-22-08778.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burright EN, Clark HB, Servadio A, Matilla T, Feddersen RM, Yunis WS, Duvick LA, Zoghbi HY, Orr HT. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell. 1995;82:937–48. doi: 10.1016/0092-8674(95)90273-2. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300:1751–5. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Dreyfus CF, Black IB. NGF and excitatory neurotransmitters regulate survival and morphogenesis of cultured cerebellar Purkinje cells. J. Neurosci. 1991;11:462–71. doi: 10.1523/JNEUROSCI.11-02-00462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zeeuw CI, Hansel C, Bian F, Koekkoek SK, van Alphen AM, Linden DJ, Oberdick J. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron. 1998;20:495–508. doi: 10.1016/s0896-6273(00)80990-3. [DOI] [PubMed] [Google Scholar]
- Dityateva G, Hammond M, Thiel C, Ruonala MO, Delling M, Siebenkotten G, Nix M, Dityatev A. Rapid and efficient electroporation-based gene transfer into primary dissociated neurons. J. Neurosci. Methods. 2003;130:65–73. doi: 10.1016/s0165-0270(03)00202-4. [DOI] [PubMed] [Google Scholar]
- Dunn ME, Schilling K, Mugnaini E. Development and fine structure of murine Purkinje cells in dissociated cerebellar cultures: dendritic differentiation, synaptic maturation, and formation of cell-class specific features. Anat. Embryol. (Berl) 1998a;197:31–50. doi: 10.1007/s004290050118. [DOI] [PubMed] [Google Scholar]
- Dunn ME, Schilling K, Mugnaini E. Development and fine structure of murine Purkinje cells in dissociated cerebellar cultures: neuronal polarity. Anat. Embryol. (Berl) 1998b;197:9–29. doi: 10.1007/s004290050117. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Topinka JR, Lehrer-Graiwer JE, Firestein BL, Craven SE, Aoki C, Bredt DS. Ion channel clustering by membrane-associated guanylate kinases. Differential regulation by N-terminal lipid and metal binding motifs. J. Biol. Chem. 2000;275:23904–10. doi: 10.1074/jbc.M909919199. [DOI] [PubMed] [Google Scholar]
- Eto M, Bock R, Brautigan DL, Linden DJ. Cerebellar long-term synaptic depression requires PKC-mediated activation of CPI-17, a myosin/moesin phosphatase inhibitor. Neuron. 2002;36:1145–58. doi: 10.1016/s0896-6273(02)01107-8. [DOI] [PubMed] [Google Scholar]
- Furuya S, Makino A, Hirabayashi Y. An improved method for culturing cerebellar Purkinje cells with differentiated dendrites under a mixed monolayer setting. Brain Res. Brain Res. Protoc. 1998;3:192–8. doi: 10.1016/s1385-299x(98)00040-3. [DOI] [PubMed] [Google Scholar]
- Gartner A, Collin L, Lalli G. Nucleofection of primary neurons. Methods Enzymol. 2006;406:374–88. doi: 10.1016/S0076-6879(06)06027-7. [DOI] [PubMed] [Google Scholar]
- Gimenez-Cassina A, Lim F, Diaz-Nido J. Gene transfer into Purkinje cells using herpesviral amplicon vectors in cerebellar cultures. Neurochem. Int. 2007;50:181–8. doi: 10.1016/j.neuint.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Gruol DL, Franklin CL. Morphological and physiological differentiation of Purkinje neurons in cultures of rat cerebellum. J. Neurosci. 1987;7:1271–93. doi: 10.1523/JNEUROSCI.07-05-01271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel C, Linden DJ, D'Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat. Neurosci. 2001;4:467–75. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- Hirai H, Launey T. The regulatory connection between the activity of granule cell NMDA receptors and dendritic differentiation of cerebellar Purkinje cells. J. Neurosci. 2000;20:5217–24. doi: 10.1523/JNEUROSCI.20-14-05217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, Launey T, Mikawa S, Torashima T, Yanagihara D, Kasaura T, Miyamoto A, Yuzaki M. New role of delta2-glutamate receptors in AMPA receptor trafficking and cerebellar function. Nat. Neurosci. 2003;6:869–76. doi: 10.1038/nn1086. [DOI] [PubMed] [Google Scholar]
- Hirano T, Hagiwara S. Synaptic transmission between rat cerebellar granule and Purkinje cells in dissociated cell culture: effects of excitatory-amino acid transmitter antagonists. Proc. Natl. Acad. Sci. U. S. A. 1988;85:934–8. doi: 10.1073/pnas.85.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Kasono K. Spatial distribution of excitatory and inhibitory synapses on a Purkinje cell in a rat cerebellar culture. J. Neurophysiol. 1993;70:1316–25. doi: 10.1152/jn.1993.70.4.1316. [DOI] [PubMed] [Google Scholar]
- Hisatsune C, Kuroda Y, Akagi T, Torashima T, Hirai H, Hashikawa T, Inoue T, Mikoshiba K. Inositol 1,4,5-trisphosphate receptor type 1 in granule cells, not in Purkinje cells, regulates the dendritic morphology of Purkinje cells through brain-derived neurotrophic factor production. J. Neurosci. 2006;26:10916–24. doi: 10.1523/JNEUROSCI.3269-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockberger PE, Tseng HY, Connor JA. Development of rat cerebellar Purkinje cells: electrophysiological properties following acute isolation and in long-term culture. J. Neurosci. 1989;9:2258–71. doi: 10.1523/JNEUROSCI.09-07-02258.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito-Ishida A, Miura E, Emi K, Matsuda K, Iijima T, Kondo T, Kohda K, Watanabe M, Yuzaki M. Cbln1 regulates rapid formation and maintenance of excitatory synapses in mature cerebellar Purkinje cells in vitro and in vivo. J. Neurosci. 2008;28:5920–30. doi: 10.1523/JNEUROSCI.1030-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi SY, Hirano T. Sustained structural change of GABA(A) receptor-associated protein underlies long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron. J. Neurosci. 2007;27:6788–99. doi: 10.1523/JNEUROSCI.1981-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroyanagi T, Yokoyama M, Hirano T. Postsynaptic glutamate receptor delta family contributes to presynaptic terminal differentiation and establishment of synaptic transmission. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4912–6. doi: 10.1073/pnas.0900892106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launey T, Endo S, Sakai R, Harano J, Ito M. Protein phosphatase 2A inhibition induces cerebellar long-term depression and declustering of synaptic AMPA receptor. Proc. Natl. Acad. Sci. U. S. A. 2004;101:676–81. doi: 10.1073/pnas.0302914101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitges M, Kovac J, Plomann M, Linden DJ. A unique PDZ ligand in PKCalpha confers induction of cerebellar long-term synaptic depression. Neuron. 2004;44:585–94. doi: 10.1016/j.neuron.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Linden DJ. Long-term potentiation of glial synaptic currents in cerebellar culture. Neuron. 1997;18:983–94. doi: 10.1016/s0896-6273(00)80337-2. [DOI] [PubMed] [Google Scholar]
- Linden DJ. A protein synthesis-dependent late phase of cerebellar long-term depression. Neuron. 1996;17:483–90. doi: 10.1016/s0896-6273(00)80180-4. [DOI] [PubMed] [Google Scholar]
- Linden DJ, Ahn S. Activation of presynaptic cAMP-dependent protein kinase is required for induction of cerebellar long-term potentiation. J. Neurosci. 1999;19:10221–7. doi: 10.1523/JNEUROSCI.19-23-10221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DJ, Connor JA. Participation of postsynaptic PKC in cerebellar long-term depression in culture. Science. 1991;254:1656–9. doi: 10.1126/science.1721243. [DOI] [PubMed] [Google Scholar]
- Linden DJ, Dickinson MH, Smeyne M, Connor JA. A long-term depression of AMPA currents in cultured cerebellar Purkinje neurons. Neuron. 1991;7:81–9. doi: 10.1016/0896-6273(91)90076-c. [DOI] [PubMed] [Google Scholar]
- Lonart G, Schoch S, Kaeser PS, Larkin CJ, Sudhof TC, Linden DJ. Phosphorylation of RIM1alpha by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell. 2003;115:49–60. doi: 10.1016/s0092-8674(03)00727-x. [DOI] [PubMed] [Google Scholar]
- Ludin B, Doll T, Meili R, Kaech S, Matus A. Application of novel vectors for GFP-tagging of proteins to study microtubule-associated proteins. Gene. 1996;173:107–11. doi: 10.1016/0378-1119(95)00899-3. [DOI] [PubMed] [Google Scholar]
- Lundstrom K, Schweitzer C, Rotmann D, Hermann D, Schneider EM, Ehrengruber MU. Semliki Forest virus vectors: efficient vehicles for in vitro and in vivo gene delivery. FEBS Lett. 2001;504:99–103. doi: 10.1016/s0014-5793(01)02707-7. [DOI] [PubMed] [Google Scholar]
- Mashimo M, Hirabayashi T, Murayama T, Shimizu T. Cytosolic PLA2{alpha} activation in Purkinje neurons and its role in AMPA-receptor trafficking. J. Cell Sci. 2008;121:3015–24. doi: 10.1242/jcs.032987. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Miura E, Miyazaki T, Kakegawa W, Emi K, Narumi S, Fukazawa Y, Ito-Ishida A, Kondo T, Shigemoto R, Watanabe M, Yuzaki M. Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science. 2010;328:363–8. doi: 10.1126/science.1185152. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Launey T, Mikawa S, Hirai H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO J. 2000;19:2765–74. doi: 10.1093/emboj/19.12.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Matsuda K, Yuzaki M. A new motif necessary and sufficient for stable localization of the delta2 glutamate receptors at postsynaptic spines. J. Biol. Chem. 2006;281:17501–9. doi: 10.1074/jbc.M600240200. [DOI] [PubMed] [Google Scholar]
- McCroskery S, Chaudhry A, Lin L, Daniels MP. Transmembrane agrin regulates filopodia in rat hippocampal neurons in culture. Mol. Cell. Neurosci. 2006;33:15–28. doi: 10.1016/j.mcn.2006.06.004. [DOI] [PubMed] [Google Scholar]
- McEvoy M, Cao G, Montero Llopis P, Kundel M, Jones K, Hofler C, Shin C, Wells DG. Cytoplasmic polyadenylation element binding protein 1-mediated mRNA translation in Purkinje neurons is required for cerebellar long-term depression and motor coordination. J. Neurosci. 2007;27:6400–11. doi: 10.1523/JNEUROSCI.5211-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Topinka JR, Hashimoto K, Petralia RS, Kakizawa S, Kauer FW, Aguilera-Moreno A, Wenthold RJ, Kano M, Bredt DS. PSD-93 knock-out mice reveal that neuronal MAGUKs are not required for development or function of parallel fiber synapses in cerebellum. J. Neurosci. 2001;21:3085–91. doi: 10.1523/JNEUROSCI.21-09-03085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison ME, Mason CA. Granule neuron regulation of Purkinje cell development: striking a balance between neurotrophin and glutamate signaling. J. Neurosci. 1998;18:3563–73. doi: 10.1523/JNEUROSCI.18-10-03563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata I, Ono K, Kawana A, Kimura-Kuroda J. Aligned neurite bundles of granule cells regulate orientation of Purkinje cell dendrites by perpendicular contact guidance in two-dimensional and three-dimensional mouse cerebellar cultures. J. Comp. Neurol. 2006;499:274–89. doi: 10.1002/cne.21102. [DOI] [PubMed] [Google Scholar]
- Oberdick J, Schilling K, Smeyne RJ, Corbin JG, Bocchiaro C, Morgan JI. Control of segment-like patterns of gene expression in the mouse cerebellum. Neuron. 1993;10:1007–18. doi: 10.1016/0896-6273(93)90050-2. [DOI] [PubMed] [Google Scholar]
- Oberdick J, Smeyne RJ, Mann JR, Zackson S, Morgan JI. A promoter that drives transgene expression in cerebellar Purkinje and retinal bipolar neurons. Science. 1990;248:223–6. doi: 10.1126/science.2109351. [DOI] [PubMed] [Google Scholar]
- Ohkawa N, Fujitani K, Tokunaga E, Furuya S, Inokuchi K. The microtubule destabilizer stathmin mediates the development of dendritic arbors in neuronal cells. J. Cell Sci. 2007;120:1447–56. doi: 10.1242/jcs.001461. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Kakizawa S, Hirose K, Iino M. Visualization of IP(3) dynamics reveals a novel AMPA receptor-triggered IP(3) production pathway mediated by voltage-dependent Ca(2+) influx in Purkinje cells. Neuron. 2001;32:113–22. doi: 10.1016/s0896-6273(01)00464-0. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Sans N, Worley PF, Hammer JA, 3rd, Wenthold RJ. Glutamate receptor targeting in the postsynaptic spine involves mechanisms that are independent of myosin Va. Eur. J. Neurosci. 2001;13:1722–32. doi: 10.1046/j.0953-816x.2001.01553.x. [DOI] [PubMed] [Google Scholar]
- Rizzo MA, Springer GH, Granada B, Piston DW. An improved cyan fluorescent protein variant useful for FRET. Nat. Biotechnol. 2004;22:445–9. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- Schilling K, Dickinson MH, Connor JA, Morgan JI. Electrical activity in cerebellar cultures determines Purkinje cell dendritic growth patterns. Neuron. 1991;7:891–902. doi: 10.1016/0896-6273(91)90335-w. [DOI] [PubMed] [Google Scholar]
- Serinagaoglu Y, Zhang R, Zhang Y, Zhang L, Hartt G, Young AP, Oberdick J. A promoter element with enhancer properties, and the orphan nuclear receptor RORalpha, are required for Purkinje cell-specific expression of a Gi/o modulator. Mol. Cell. Neurosci. 2007;34:324–42. doi: 10.1016/j.mcn.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004;22:1567–72. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu. Rev. Biochem. 2007;76:823–47. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8:206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi Y, Mizutani A, Yuasa S, Mikoshiba K, Furuichi T. Differential expression of Homer family proteins in the developing mouse brain. J. Comp. Neurol. 2004;473:582–99. doi: 10.1002/cne.20116. [DOI] [PubMed] [Google Scholar]
- Simsek-Duran F, Linden DJ, Lonart G. Adapter protein 14-3-3 is required for a presynaptic form of LTP in the cerebellum. Nat. Neurosci. 2004;7:1296–8. doi: 10.1038/nn1348. [DOI] [PubMed] [Google Scholar]
- Smeyne RJ, Chu T, Lewin A, Bian F, Sanlioglu S, Kunsch C, Lira SA, Oberdick J. Local control of granule cell generation by cerebellar Purkinje cells. Mol. Cell. Neurosci. 1995;6:230–51. doi: 10.1006/mcne.1995.1019. [DOI] [PubMed] [Google Scholar]
- Smeyne RJ, Oberdick J, Schilling K, Berrebi AS, Mugnaini E, Morgan JI. Dynamic organization of developing Purkinje cells revealed by transgene expression. Science. 1991;254:719–21. doi: 10.1126/science.1948052. [DOI] [PubMed] [Google Scholar]
- Smith-Hicks C, Xiao B, Deng R, Ji Y, Zhao X, Shepherd JD, Posern G, Kuhl D, Huganir RL, Ginty DD, Worley PF, Linden DJ. SRF binding to SRE 6.9 in the Arc promoter is essential for LTD in cultured Purkinje cells. Nat. Neurosci. 2010;13:1082–9. doi: 10.1038/nn.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg JP, Takamiya K, Shen Y, Xia J, Rubio ME, Yu S, Jin W, Thomas GM, Linden DJ, Huganir RL. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49:845–60. doi: 10.1016/j.neuron.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J. Neurosci. 2003;23:2655–64. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Sawada S, Araki K, Bono Y, Furuya S, Kano M. A reliable method for culture of dissociated mouse cerebellar cells enriched for Purkinje neurons. J. Neurosci. Methods. 2000;104:45–53. doi: 10.1016/s0165-0270(00)00323-x. [DOI] [PubMed] [Google Scholar]
- Takamiya K, Mao L, Huganir RL, Linden DJ. The glutamate receptor-interacting protein family of GluR2-binding proteins is required for long-term synaptic depression expression in cerebellar Purkinje cells. J. Neurosci. 2008;28:5752–5. doi: 10.1523/JNEUROSCI.0654-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K, Torashima T, Horiuchi H, Hirai H. Purkinje-cell-preferential transduction by lentiviral vectors with the murine stem cell virus promoter. Neurosci. Lett. 2008;443:7–11. doi: 10.1016/j.neulet.2008.07.058. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Yanagawa Y, Hirashima N. Transfer of small interfering RNA by single-cell electroporation in cerebellar cell cultures. J. Neurosci. Methods. 2009;178:80–6. doi: 10.1016/j.jneumeth.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Yanagawa Y, Obata K, Marunouchi T. Dendritic morphogenesis of cerebellar Purkinje cells through extension and retraction revealed by long-term tracking of living cells in vitro. Neuroscience. 2006;141:663–74. doi: 10.1016/j.neuroscience.2006.04.044. [DOI] [PubMed] [Google Scholar]
- Tomomura M, Rice DS, Morgan JI, Yuzaki M. Purification of Purkinje cells by fluorescence-activated cell sorting from transgenic mice that express green fluorescent protein. Eur. J. Neurosci. 2001;14:57–63. doi: 10.1046/j.0953-816x.2001.01624.x. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–26. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M, Taguchi R, Sakimura K, Mishina M. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell. 2010;141:1068–79. doi: 10.1016/j.cell.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Vandaele S, Nordquist DT, Feddersen RM, Tretjakoff I, Peterson AC, Orr HT. Purkinje cell protein-2 regulatory regions and transgene expression in cerebellar compartments. Genes Dev. 1991;5:1136–48. doi: 10.1101/gad.5.7.1136. [DOI] [PubMed] [Google Scholar]
- Wagner W, Brenowitz SD, Hammer JA., 3rd Myosin-Va transports the endoplasmic reticulum into the dendritic spines of Purkinje neurons. Nat. Cell Biol. 2011;13:40–8. doi: 10.1038/ncb2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Parris J, Li L, Morgan JI. The carboxypeptidase-like substrate-binding site in Nna1 is essential for the rescue of the Purkinje cell degeneration (pcd) phenotype. Mol. Cell. Neurosci. 2006;33:200–13. doi: 10.1016/j.mcn.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–47. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- Warren JC, Rutkowski A, Cassimeris L. Infection with replication-deficient adenovirus induces changes in the dynamic instability of host cell microtubules. Mol. Biol. Cell. 2006;17:3557–68. doi: 10.1091/mbc.E05-09-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Schachner M. Maintenance of immunocytologically identified Purkinje cells from mouse cerebellum in monolayer culture. Brain Res. 1984;311:119–30. doi: 10.1016/0006-8993(84)91404-5. [DOI] [PubMed] [Google Scholar]
- Wulff P, Goetz T, Leppa E, Linden AM, Renzi M, Swinny JD, Vekovischeva OY, Sieghart W, Somogyi P, Korpi ER, Farrant M, Wisden W. From synapse to behavior: rapid modulation of defined neuronal types with engineered GABAA receptors. Nat. Neurosci. 2007;10:923–9. doi: 10.1038/nn1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–16. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Yawata S, Tsuchida H, Kengaku M, Hirano T. Membrane-proximal region of glutamate receptor delta2 subunit is critical for long-term depression and interaction with protein interacting with C kinase 1 in a cerebellar Purkinje neuron. J. Neurosci. 2006;26:3626–33. doi: 10.1523/JNEUROSCI.4183-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedowitz JC, Kotsakis A, Schlegel EF, Blaho JA. Nuclear localizations of the herpes simplex virus type 1 tegument proteins VP13/14, vhs, and VP16 precede VP22-dependent microtubule reorganization and VP22 nuclear import. J. Virol. 2005;79:4730–43. doi: 10.1128/JVI.79.8.4730-4743.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzaki M, Mikoshiba K. Pharmacological and immunocytochemical characterization of metabotropic glutamate receptors in cultured Purkinje cells. J. Neurosci. 1992;12:4253–63. doi: 10.1523/JNEUROSCI.12-11-04253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–6. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- Zeitelhofer M, Vessey JP, Xie Y, Tubing F, Thomas S, Kiebler M, Dahm R. High-efficiency transfection of mammalian neurons via nucleofection. Nat Protoc. 2007;2:1692–704. doi: 10.1038/nprot.2007.226. [DOI] [PubMed] [Google Scholar]
- Zhang X, Baader SL, Bian F, Muller W, Oberdick J. High level Purkinje cell specific expression of green fluorescent protein in transgenic mice. Histochem. Cell Biol. 2001;115:455–64. doi: 10.1007/s004180100283. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang H, Oberdick J. Conservation of the developmentally regulated dendritic localization of a Purkinje cell-specific mRNA that encodes a G-protein modulator: comparison of rodent and human Pcp2(L7) gene structure and expression. Brain Res. Mol. Brain Res. 2002;105:1–10. doi: 10.1016/s0169-328x(02)00379-0. [DOI] [PubMed] [Google Scholar]
- Zu T, Duvick LA, Kaytor MD, Berlinger MS, Zoghbi HY, Clark HB, Orr HT. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J. Neurosci. 2004;24:8853–61. doi: 10.1523/JNEUROSCI.2978-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.