Abstract
Background
Sickle cell disease (or simply, SCD) is an inherited hemoglobinopathy which is mostly prevalent among persons of African descent. SCD results from a monogenic (Hemoglobin, beta) point-mutation (substitution of the base Adenine with Thymine at position six) that leads to replacement of the amino acid glutamic acid (E) with valine (V). Management of SCD within resource-poor settings is largely syndromic, since the option of cure offered by bone-marrow transplantation (BMT) is risky and unaffordable by most affected individuals. Despite previous reports of repair and inhibition of the sickle beta-globin gene and messenger ribonucleic acids (mRNAs), respectively in erythrocyte precursor cells via gene-targeting using an oligomer-restriction enzyme construct and either ribozyme- or RNA-DNA chimeric oligonucleotides (or simply third strand binding), gene-therapy to treat SCD still remains largely preclinical. In the wake of the advances in target- gene- mutagenesis and repair wrought by zinc finger nuclease (ZFN) technology, it was hypothesized that SCD may be cured by the same. The goal of this study thus, was constructing a database of zinc finger arrays (ZFAs) and engineering ZFNs, that respectively bind and cleave within or around specific sequences in the sickle hemoglobin, beta (−βS) gene.
Methods and results
First, using the complete 1606 genomic DNA base pair (bp) sequences of the normal hemoglobin-beta (βA) chain gene, and the ZiFiT-CoDA-ZFA software preset at default, 57 three-finger arrays (ZFAs) that specifically bind 9 base-pair sequences within the normal hemoglobin-beta chain, were computationally assembled. Second, by serial linkage of these ZFAs to the Flavobacterium okeanokoites endonuclease Fok I― four ZFNs with unique specificity to >24 bp target-sequences at the genomic contextual positions 82, 1333, 1334, and 1413 of the βA chain-gene were constructed in-silico. Third, localizing the point-mutation of SCD at genomic contextual position −69-70-71- bp (a position corresponding to the 6th codon) of the βA chain-gene, inspired the final design of five more ZFNs specific to >24 bp target-sequences within the 8,954 bp that are genomically adjacent to the 5′ end of the βA chain-gene.
Conclusions
This set of 57 ZFAs and 9 ZFNs offers us gene-therapeutic precursors for the targeted mutagenesis and repair of the SCD mutation or genotype.
Background
Sickle cell diseases (SCD) or sickle cell anemia (SCA) is a hemoglobinopathy that is mostly common among persons of African descent [1]. SCD arises from a single, point-mutation (base-substitution of Adenine with Thymine in the sixth codon: CAG → CTG) of the gene coding for the beta chain of the Hemoglobin molecule [2]. The phenotypic consequence of this substitution is a replacement of the amino acid glutamic acid (E) with valine (V) [1,2]. Homozygous expression of this mutant globin genotype (SS) causes SCD, while the heterozygous genotype (AS) is termed the “sickle cell trait” [1,2]. Unlike the case observed in most normal adult humans where the commonest hemoglobin type (hemoglobin A or Hb A) is a tetramer (which contains 4 subunit proteins- α2β2A that are non-covalently bound together), patients with SCD have an adult hemoglobin type with two mutant β subunits (called βS) called hemoglobin S (or simply, Hb S) [3,4]. Hb S has a high predilection to crystallize under conditions of low oxygen-pressure such as may occur following physical or pathological-stress. Specifically, formation of intracellular S crystals causes polymerization of red blood cells, reduced oxygen uptake and or carriage, a as well as clogging of small blood vessels [5,6]. Overall, although the clinical syndrome of SCD is diversely-wide, its hallmark is a devastating group of symptoms and signs that are collectively known as a ‘sickle cell crisis’ [7,8]. About 200,000 new born babies within Africa recessively inherent the double autosomal-sickle cell genotype each year— a figure that constitutes approximately 66.6% of the children born with haemoglobinopathies worldwide [9]. Previously studies have shown that persons with heterozygosity for βS (Hb SA) or the sickle cell trait are protected against infection by malaria causing protozoa [10]. This, together with findings of an equally high-incidence or common-distribution of the sickle cell trait within the malaria-belt, has led to the proposition that this trait emerged as an evolutionary adaptation of the human-host to infection with plasmodia [11]. The medical management of SCD remains an area of particular challenge [12]. Specifically, despite advances made towards curing SCD through bone-marrow transplantation [13,14], the resource-intensive nature of this approach has made it impossible for the most affected populations of Africa to access. Thus, care for affected individuals (homozygous SS) here still mostly revolves around syndromic management, with or without agents that increase fetal hemoglobin (Hb F) [15]. Improvements are obviously sought here.
By virtue of its monogenic, point-mutant origin, SCD has attracted several attempts for gene therapy. For instance, as early as 1991, Shesely ED, et al.[16] described a technique for the correction of a human βS globin gene to the normal βA allele by homologous recombination in the mouse-human hybrid cell line BSM using an oligomer-restriction enzyme construct. In 1998, Lans N, et al.[17,18] reported ribozyme mediated deletion and augmentation of the sickle-cell (βS) mutation with fetal haemoglobin levels in the red cells. Selective inhibition of beta-globin RNA transcripts by antisense RNA molecules has equally been tried as a strategy to reduce levels of Hb S polymerization in red blood cells and the symptoms associated with SCD [19-21]. Pace BS, et al.[21] specifically identified antisense RNA targets in the beta-globin gene other than the homologous regions in gamma-globin, proposing that gene therapy strategies which combine gamma-globin induction along with beta-globin inhibition using antisense vectors may yield more favorable anti-sickling effects longterm. Amosova O, et al.[22],on the other hand, reported third-strand directed repair of the sickle cell mutation using RNA-DNA chimeric oligonuceotides (COs) [23,24] achieved by shortening the psoralen linker to enhance the specificity of photoadduct formation at the desired mutant T residue site. Despite these notable advances, the place for gene-replacement or repair therapy in SCD has remained rather experimental [25], with no clinical trials of any of the above approaches in human populations yet reported.
Basing on the more-recent developments in targeted mutagenesis (genome-editing) and gene-repair wrought by zinc finger nuclease (ZFN) technology [26-32], it was hypothesized that the single, point mutation responsible for SCD can be abrogated using similar approaches. Specifically, Zinc finger nucleases-ZFNs, which are artificial, hybrid restriction enzymes created by covalently linking a DNA-binding zinc finger (Zif) domain (composed of three to six finger-arrays) to the non-specific DNA cleavage domain (or simply FN) of the Flavobacterium okeanokoites bacteria restriction endonuclease, FokI [33]; have recently become a powerful tool for primarily editing host genomes. Following induction of a double strand break (DSB) within the target DNA, repair occurs, either by homologous recombination (HR) or non-homologous end-joining (NHEJ) [26-32]. Perez et E al. [30]-using engineered ZFNs targeting human CCR5, previously demonstrated establishment of HIV-1 resistance in CD4+ T cells through generation of a doublestrand break (DSB) at predetermined sites in the CCR5 coding region upstream of the natural CCR5D32 mutation. Holmes N et al. [31] have demonstrated control of HIV-1 infection within NSG-mice transplanted with human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeting CCR5. Most recently, Wilen CB, et al.[32] successfully engineered HIV-Resistant Human CD4+ T Cells using CXCR4-Specific Zinc-Finger Nucleases (ZFN). This evidence, along with on-going improvements in the design and engineering of lentiviral [33,34] and parvovirus [35] vectors (LV and PV, respectively) for ex-vivo or in-vivo gene-delivery and transduction of erythroid precursors, suggests that the sickle cell mutation may be abrogated in erythroid bone marrow precursor with appropriate ZFNs. Indeed, we are aware that Sangamo Biosciences (http://www.sangamo.com/index.html)--one of the leading industries in ZFN-technology, has already focused its ZFN-mediated gene-editing technology to providing a unique solution for the treatment of monogenic diseases like hemophilia and SCA. Their ZFAs or ZFNs are, however, not publically available.
Thus, the specific goal of this study was to construct a database of zinc finger arrays (ZFAs) and engineer ZFNs that respectively specifically bind and cleave within or around the sickle hemoglobin beta (−βS) gene mutation.
Methods
Identification of HBB gene-specific ZFAs and ZFNs
No in-vivo or in-vitro experiments accompanied this bioinformatics study, and thereby no ethical approval and Consent was sought from the author’s institutional IRB.
Design
In-silico informatics
Materials and software
FASTA format of the nucleotide sequences of the entire Hemoglobin, beta-gene (provided in Additional file 1; the NCBI accession number provided at end) and the Zinc-Finger Nuclease-Consortium’s software CoDA-ZiFiT-ZFA and CoDA-ZiFiT-ZFN [36,37] (see software and availability section for URL link).
Interventions
The FASTA format of the nucleotide sequences of the hemoblobin, beta gene were separately fed into the user interfaces of CoDA-ZiFiT-ZFA and the CoDA-ZiFiT-ZFN, both of which were pre-set at default, with a spacer-option of 5–9 bp selected for the latter.
Measured variables
Lists of ZFAs and ZFNs, inclusive of graphic maps of their action in the genomic context of HB, beta, were generated as per the user protocol [36,37]. Another array of five ZFNs specific to >24 bp target-sequences within the adjacent 8,954 bp to the 5′ end of the βA chain-gene; was also engineered.
Software and database availability
The ZFN consortium CoDA-ZiFiT-ZFA/ZFN software and algorithms used are available at the following url: http://www.zincfingers.org/scientific-background.htm
The NCBI gene database hosting the HBB gene, is available at the following url: http://www.ncbi.nlm.nih.gov/gene/3043
Results
Zinc Finger Arrays (ZFAs) targeting hemoglobin, beta (βA) gene sequences
First, using the 1,606 genomic nucleotide base pair (bp) sequences (inclusive of introns) encoding the normal hemoglobin-beta (βA) chain gene (seeAdditional file 1), and the ZiFiT-CoDA-ZFA [36,37] software preset at default, 57 three-finger arrays (ZFAs) that specifically bind 9 base-pair (bp) sequences within the normal hemoglobin-beta chain (seeAdditional file 2) were computationally assembled. Overall, there were ZFA binding within the first 3/5 and last 1/5 of the genomic contextual bp- sequences at 5′ and 3′ regions of the HB, beta gene (Figure 1). Zinc fingers (Zif or ZF)—such as those specific to the genomic nucleotide sequences of the HBB-gene (provided in Additional file 1), are protein motifs capable of targeted DNA-binding [26-32]. Each individual zinc finger usually recognizes three nucleotide bases, but many zinc fingers can be combined to generate an array capable, as in the case of our listed ZFAs of three fingers, of recognizing nine nucleotides [36,37]. Residues −1 to 6 (numbered relative to the start) of the alpha-helix of the ZFAs are responsible for the specific recognition of triplets of DNA sequences through the formation of base-specific contacts in the major groove of the double-stranded target DNA (see Additional file 2) [26-32,36,37]. Therefore, residues −1 to 6 within the ZFs’ alpha helixes are denoted as ‘recognition’ residues and are listed in N- to C-terminal direction; while all the other residues in the ZF are called the ‘backbone’ [36,37]. As a consequence, the recognition sequences of the ZFAs bind target DNA sites through amino acids −1 to 6 of the ‘recognition’ alpha helix in the 3' to 5' direction. The afore going reverse-pattern of target DNA recognition and binding can be confusing as the DNA target site is always referred to in the 5' to 3' direction, whereas amino acid sequences are referred to from the N to C terminus. In this section, ZFAs were assembled that are capable of cleaving at loci located within and adjacent to the 6th or mutant codon of the sickle hemoglobin, beta (βS) gene, which is localized at genomic contextual positions of −69-70-71- bp (shown in colors, italics or bold in Additional file 1 and Additional file 2: one reverse or left ZFA alpha helix binding at position 51/41, and two forward or right ZFAs alpha helices binding at positions 70/80 and 73/83; respectively). ZFAs can recombinantly be tagged to a non-specific nuclease in-vivo, a process that renders the non-specific nuclease specific to the binding sites of the ZFAs [38].
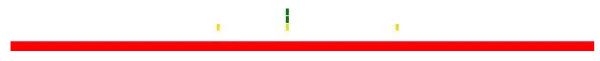
Figure 1.
Schematics of the ZFA- target binding sites within the hemoglobin, beta gene. This figure graphically illustrates the binding sites of the 57 ZFAs that specifically target sequences within the genomic context of the hemoglobin, beta gene. Hits (blue, green, and gold bars) represent targets along the gene (red bars). ZFA hits in the graphic are color-coded based on spacer size (5 bp = Blue; 6 bp = Green; 7 bp = Gold).
Zinc Finger Nucleases (ZFN) targeting hemoglobin, beta (βA) gene sequences
Second, by serial linkage of these ZFAs to the Flavobacterium okeanokoites endonuclease Fok I of the type IIS class as Kim YG, et al. [38] previously achieved in-vitro; four ZFNs with unique specificity to >24 bp target-sequences at the genomic contextual positions 82, 1333, 1334, and 1413 of the βA chain-gene were constructed in-silico (seeAdditional file 3 and Figure 2, respectively). Throughout the latter experiments, the description of the spacer regions was maintained at 5–7 base-pairs. Each specific ZFN has a left and right alpha-helical ‘recognition’ site bound to Fok I (see Table 1). Therefore, these ZFNs bind to their target >18 bp Hemoglobin, beta gene DNA sites as dimers, with each monomer using its zinc finger domain to recognize a ‘half-site’ of the targeted DNA sequence. In-vivo, dimerization of ZFNs is mediated by the FokI cleavage domain through cleavage of a five or six base pair ‘spacer’ sequence that separates the two inverted target ‘half sites’ [26-32]. Importantly, since the DNA-binding specificities of zinc finger domains can be re-engineered using various methods, customized ZFNs can, in principle, be constructed to specifically target almost any gene sequence [37]. A list of three ZFNs, inclusive of their −1 to 6 alpha-helical nucleotide binding domains (F1, F2, F3/F3, F2, F1) alongside the respective site specific sequence within the genomic context of the Hemoglobin, beta gene, are presented in Table 1. Graphic-analysis of the cleavage-pattern within the HB, beta gene induced by the four identified ZFNs revealed one of them to cleave at the 5′ end (position 82/106) while the other three cleaved at the 3′ end (positions 1,333/1,359, 1,334/1,359, and 1,413/1,439, respectively) (see, Figure 2).
Figure 2.
Schematics of ZFN-cleavage sites within the hemoglobin, beta gene. This figure graphically illustrates the cleavage sites of the four ZFNs targeting sequences within the human-genomic context of the hemoglobin, beta gene. Hits (blue, green, and gold bars) represent targets along the gene (red bars). ZFN hits in the graphic are color-coded based on spacer size (5 bp = Blue; 6 bp = Green; 7 bp = Gold).
Table 1.
ZFN targeting and cleaving within the hemoglobin, beta (βA)-gene
| Zinc Finger Nuclease (ZFN) | Left Fn; triplet- α-Helix | Right Fn; triplet- α-Helix |
|---|---|---|
| -targeting HBB (βA)-DNA at 5′ Sites | ||
|
ZFN-unknown-SP-5-1 |
|
|
| 5′_82 cCGTTACTGCCCTGTGGGGCAAGGt 106_′3 |
F1; RNITLVR; (ACG) |
F1; RNEHLKV; (AGG) |
| 5′_82 gGCAATGACGGGACACCCCGTTCCa 106_′3 |
F2; QRSSLVR; (GTA) |
F2; QSTTLKR; (GCA) |
| |
F3; QDNTLRR; (GCA) |
F3; RTEHLAR; (GGG) |
| -targeting HBB (βA)-DNA at 3′ Sites | ||
|
ZFN-unknown-SP-7-1 |
|
|
| 5′_1333 cTTCCTCCCACAGCTCCTGGGCAACGt 1359_′3 |
F1; QASNLLR; (GAA) |
F1; RSQTLAQ; (ACG) |
| 5′_1333 gAAGGAGGGTGTCGAGGACCCGTTGCa 1359_′3 |
F2; RQDNLGR; (GAG) |
F2; QSTTLKR; (GCA) |
| |
F3; RMDHLAG; (TGG) |
F3; RSDHLSL; (TGG) |
|
ZFN-unknown-SP-6-1 |
|
|
| 5′_1334 tTCCTCCCACAGCTCCTGGGCAACGt 1359_′3 |
F1; RTDRLIR; (GGA) |
F1; RSQTLAQ; (ACG) |
| 5′_1334 aAGGAGGGTGTCGAGGACCCGTTGCa 1359_′3 |
F2; QSAHLKR; (GGA) |
F2; QSTTLKR; (GCA) |
| F3; RNTALQH; (GTG) | F3; RSDHLSL; (TGG) | |
Zinc Finger Nucleases (ZFN) targeting within the adjacent 8,954 bp to the 5′ end of the βA chain-gene
Third, by localizing the point-mutation of SCD at genomic contextual position −69-70-71- bp of the βA chain-gene sequences used (that is, within 6th codon of the gene: shown by green italicized letters in Additional file 1), design of another array of five ZFNs specific to >18 bp target-sequences within the adjacent 8,954 bp to the 5′ end of the βA chain-gene (seeAdditional file 4 for 8,954 bp to the 5′ end of the βA chain-gene; and Additional file 5 for ZFN cleaving here) was inspired. Two of the ZFN cleaving closest to the 5′ end of the βA chain-gene are shown in Table 2. A graphic summary of the distribution of cleavage patterns therein is shown in Figure 3.
Table 2.
ZFN targeting and cleaving within the adjacent 8,954 bp to the 5′ end of the hemoglobin, beta (βA)-gene
| Zinc Finger Nuclease (ZFN) | Left Fn; triplet- α-Helix | Right Fn; triplet- α-Helix |
|---|---|---|
|
ZFN-unknown-SP-6-1 |
|
|
| 5′_4231 aGCCACCACCTTCT GATAGGCAGCCt 4256_′3 |
F1; VPSKLKR; (GGC) |
F1; DPSTLRR; (GCC) |
| 5′_4231 tCGGTGGTGGAAGA CTATCCGTCGGa 4256_′3 |
F2; EAHHLSR; (GGT) |
F2; QSTTLKR; (GCA) |
| |
F3; IRHHLKR; (GGT) |
F3; RRDGLAG; (TAG) |
|
ZFN-unknown-SP-6-2 |
|
|
| 5′_4234 cACCACCTTCTGAT AGGCAGCCTGCa 4259_′3 |
F1; MKHHLAR; (GGT) |
F1; RGRNLEM; (TGC) |
| 5′_4234 gTGGTGGAAGA CTATCCGTCGGACGt 4259_′3 |
F2; EAHHLSR; (GGT) |
F2; DSSVLRR; (GCC) |
| F3; QDGNLTR; (GAA) | F3; QGGTLRR; (GCA) |
Figure 3.
Schematics of ZFN-cleavage sites within the adjacent 8,954 bp to the 5′ end of the hemoglobin, beta gene. This figure graphically illustrates the cleavage sites of five ZFNs targeting sequences within 8,954 bp located to the 5′ end of the βA chain gene. Two of the ZFNs cleaving closest to the 5′ end of the βA chain-gene are shown in Table 2. Hits (blue, green, and gold bars) represent targets along the gene (red bars). ZFN hits in the graphic are color-coded based on spacer size (5 bp = Blue; 6 bp = Green; 7 bp = Gold).
Discussion and conclusions
I present here a novel set of 57 zinc finger arrays (ZFAs) and 9 zinc finger nucleases (ZFNs) ― that constitute gene-therapeutic precursors for the targeted mutagenesis and repair of the SCD mutation or genotype. Specifically, although SCD results from a monogenic (Hemoglobin, beta) point-mutation (substitution of A with T) that has attracted extensive interest for target gene-therapy [16-25], the place for gene-replacement or repair therapy in SCD has remained rather experimental [25], and no clinical trials of any of the above approaches in human populations are yet to be reported. Basing on the more-recent developments in targeted mutagenesis (genome-editing) and gene-repair wrought by zinc finger nuclease (ZFN) technology [26-32], it was hypothesized that the single, point mutation responsible for SCD can be abrogated using similar approaches. To this end, we sought to construct a database of zinc finger arrays (ZFAs) and engineer ZFNs that specifically bind and cleave within or around the sickle hemoglobin, beta (−βS) gene. Now, we present a database of 57 ZFAs (see, Additional file 2 and Figure 1) specific to the βA gene (see,Additional file 1 for genomic contextual sequences). Using these ZFAs, we also constructed four ZFNs (shown in Additional file 3 and Figure 2) cleaving specifically within the same βA gene. Three of these four ZFN are shown in Table 1. Because the point-mutation of SCD is located at genomic contextual position −69-70-71- bp of the βA chain-gene sequences used (that is, within the 6th codon of the gene), we were equally inspired to design another array of five ZFNs (shown in Additional file 5) specific to >24 bp target-sequences within the adjacent 8,954 bp to the 5′ end of the βA chain-gene (seeAdditional file 4 for the 8,954 bp localized to the 5′ end of the βA chain-gene). Two of the ZFN cleaving within these- 8,954 bp but closest to the 5′ end of the βA chain-gene, are shown in Table 2, while Figure 3 offers a graphic summary of their distribution.
The above ZFAs and ZFNs may be applied towards the target mutagenesis or repair of the sickle cell mutation, in various ways. Firstly, splicing out the entire genomic region located between positions 5′-82/106, and 3′ -1,333/1,359 or −1,334/1,359 of the βS globin gene may be achieved using the ZFNs shown in Table 1. This alone― when followed by the process of non-homologous end-joining (NHEJ) [26-32] of the residual double strand DNA break (DSB)’s edges could lead to deletion of over 80% bp-sequences of the defective βS globin gene. Depending on the efficiency of the gene-delivery and transduction achieved by the vectors [34,35] used within erythroid precursors, therefore, this strategy alone offers the possibility of reducing the intracellular (either mature red blood cell, RBC or erythroid precursor) expression of the βS globin gene, and ultimately curtailing the symptomatology resulting from S polymerization. Note that, because this approach does not involve deletion of the SCD point-mutation which is located at genomic contextual position −69-70-71- bp of the βA chain-gene sequences used (that is, within the 6th codon of the gene), it is only a means to functionally irreversibly inactivate the βS globin gene. Thus, as a second option, another may purpose to delete the 5′ region before position −82/106 of the βS globin gene, which contains the point-mutation of SCD. This can be achieved using the ZFN in Table 1 that cleaves at position 82/106 and any of the two ZFNs in Table 2 that cleave within the 8,954 bp localized to the 5′ end of the βA chain-gene. Alternatively, however, secondary therapeutic events, including say (i) either repair of the target βS globin gene cleavage-site by either recruiting the homologous recombination (HR) pathway through providing a normal, non-mutant β globin gene template for repair of the spliced ‘mutant (disease causing)’ region [26-32,36,37] (ii) or supplementary replacement of this pathogenic-spliceon with a corresponding 5′ exon of γ-globin in a similar way as Lans N, et al.[17] and Weatherall DJ. [18] did with a 3′ exon, may be sought. Pace BS, et al.[21] has proposed that gene therapy strategies which combine gamma-globin induction along with beta-globin inhibition may yield more favorable anti-sickling effects in the longterm. Lastly, perhaps completely novel gene-therapeutic strategies devised in future may equally explore the target-DNA-binding mechanisms inherent in our ZFAs or ZFN to repair the mutant codon of the βS globin gene.
Our study presents a number of limitations that need to be taken into account. First, it is vital to note that the work is limited to only sequence analyses and accompanied by no in-vitro proof of concept studies. This should be attributed to the apparent limited resource-capacity in our Lab, although the same may be ratified by the fact that these very methods [36,37] have previously been used to successfully assemble ZFAs and engineer ZFNs that are experimentally safe and effective [26-32]. That said, it is imperative that further optimization be incorporated, including say: (i) modular analysis and assembly [39] to add on one, two or even three other ZFA onto our currently 3 finger-arrays so as to enhance specificity and avoid off-target host-genome toxicity; and (ii) in-vivo assembly and testing for efficacy, say by using either a bacteria-one hybrid (B1H) or yeast one-hybrid (Y1B) system to further inform the best ZFNs to use [40]. Modifications to the cleavage domain in order to generate a hybrid capable of functionally interrogating the ZFN dimer interface so as to prevent homodimerization, while-still enhancing the efficiency of cleavage [41], is also possible. Second, additional pre-clinical experiments employing either humanized-mouse cell models [16] or erythroid precursors cells, are still required to assess the efficacy and safety of these ZFNs and their transducing vectors [34,35,42]. Perhaps, those experiments that Wilen CB, et al.[32] recently conducted to assess the safety and efficacy of the Ad5/F35 vector carrying CXCR4-Specific Zinc-Finger Nucleases they used to engineer HIV-resistant human CD4+ T cells, may suffice. Specifically, it is important to (i) compare genome profiles and the hemoglobin, beta gene- sequence-profiles of the erythroid precursors targeted, and (ii) evaluate biophysical profiles of the mutagenic or repair resultant, presumably adult hemoblogin (HBβS→A) produced, including analyzing its crystal structure (see Figure 4 for crystal structure of the normal adult hemoglobin A) [43-45]Third, its reasonable for one to question how the lentiviral or parvovirus vectors carrying and transducing a diploid (or pair) copy of these ZFN will be used in the clinics within resource limited settings where SCD is most prevalent [25,46]. Our translational projection or proposition is to have this attempted via in-vivo gene-delivery and transduction, rather than the expensive ex-vivo manipulation. Therefore, sub-dermal, intravenous or intra-osseous routes of injection or infusion for in-vivo use of these vectors as a less-Labour-intensive and affordable gene-delivery and transduction alternative to ex-vivo manipulation must seriously be considered and tried despite the evidence of low efficiency of gene-delivery and transduction offered by the in-vivo routes, when compared to ex-vivo gene delivery [34,35]. Fourth, zinc finger nucleases targeting host-genes have recently been found to cleave off-target loci [47], and these off-targets-though minimized by reducing the binding energy of ZFN, could not be predicted by in-silico methods [48].
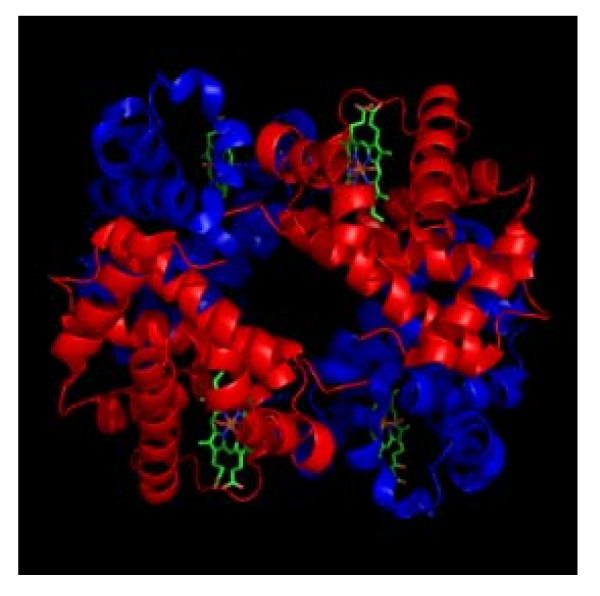
Figure 4.
Crystal Structure of adult hemogblonin, A. This figure details the crystal structure of adult hemogblonin, A as deposited by Silva MM, Rogers PH, & Arnone A [43].
In conclusion, this set of 57 zinc finger arrays (ZFAs) and 9 zinc finger nucleases (ZFNs) ― offers us gene-therapeutic precursors for the targeted mutagenesis and repair of the SCD mutation or genotype. Specifically, the same may be used to either functionally or structurally abrogate the βS globin gene. Alternatively, supplementary replacement of this pathogenic-spliceon with a corresponding 5′ exon of γ-globin may be possible. Lastly, novel gene-therapeutic strategies devised in future may equally explore the target-DNA binding and cleaving mechanisms inherent in our ZFAs or ZFN to replace or repair the mutant codon of the βS globin gene.
Competing interests
There are no competing interests to declare.
Authors’ contributions
WM conceived the idea for this article, designed and undertook the experiments, and wrote the MS.
Accession numbers
The NCBI gene identity of the Hemoglobin, beta gene is |3043|; whereas the NCBI reference for the 8.95 kb region from base 5190000 to 5198951 is |NT_009237.18|
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
A detailed list of the 1,606 nucleotide bases within the Hemoglobin, beta gene. This file offers FASTA-format listing of the 1,606 nucleotide bases within the Hemoglobin, beta gene (gene identity |3043|).
A detailed list of the57ZFAs specific to the Hemoglobin, beta gene-sequences. This file lists the 57 ZFAs that specifically bind sequences within the Hemoglobin, beta gene
A detailed list of thefourZFNs specific to the Hemoglobin, beta gene-sequences. This file lists the four ZFNs that specifically bind and cleave sequences within the Hemoglobin, beta gene
A detailed list of the 8,954 bp located to the 5′ end of theβAchain gene. This file offers FASTA-format listing of the 8,954 bp located to the 5′ end of the βA chain gene. (NCBI reference sequence 5190000 to 5198951 is |NT_009237.18|)
A detailed list of thefiveZFNs specific to the 8,954 bp located to the 5′ end of theβAchain gene. This file lists the fives ZFNs that specifically bind and cleave sequences within the 8,954 bp located to the 5′ end of the βA chain gene.
Acknowledgements
WM is supported by Grant Number 5R24TW008886 OGAC, NIH and HRSA. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the supporting offices.
References
- Ballas SK, Lewis CN, Noone AM, Krasnow SH, Kamarulzaman E, Burka ER. Clinical, hematological, and biochemical features of Hb SC disease. Am J Hemat. 1982;13:37–51. doi: 10.1002/ajh.2830130106. [DOI] [PubMed] [Google Scholar]
- Blouin M-J, Beauchemin H, Wright A, De Paepe M, Sorette M, Bleau A-M, Nakamoto B, Ou C-N, Stamatoyannopoulos G, Trudel M. Genetic correction of sickle cell disease: insights using transgenic mouse models. Nature Med. 2000;6:177–182. doi: 10.1038/72279. [DOI] [PubMed] [Google Scholar]
- Herrick JB. Peculiar elongated and sickle-shaped red blood corpuscles in a case of severe anemia. Arch Intern Med. 1910;6:517–521. [PMC free article] [PubMed] [Google Scholar]
- Hebbel RP. Adhesive interactions of sickle erythrocytes with endothelium. J Clin Invest. 1997;99:2561–2564. doi: 10.1172/JCI119442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley-Koch A, Murphy CC, Khoury MJ, Boyle CA. Contribution of sickle cell disease to the occurrence of developmental disabilities: a population-based study. Genet Med. 2001;3:181–186. doi: 10.1097/00125817-200105000-00006. [DOI] [PubMed] [Google Scholar]
- Rees DC, Williams TN, Gladwin MT. Sickle cell disease. Lancet. 2010;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- Geller AK, O’Connor MK. The sickle cell crisis: a dilemma in pain relief. Mayo Clin Proc. 2008;83(3):320–323. doi: 10.4065/83.3.320. [DOI] [PubMed] [Google Scholar]
- Dunlop RJ, Bennett KC. Pain management for sickle cell disease. Cochrane Database Syst Rev. 2006;19(2):CD003350. doi: 10.1002/14651858.CD003350.pub2. [DOI] [PubMed] [Google Scholar]
- Serjeant GR. Mortality from sickle cell disease in Africa. BMJ. 2005;330(7489):432–433. doi: 10.1136/bmj.330.7489.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MJ, Trager W. The biochemistry of resistance to malaria. Sci Am. 1981;244(3):154–164. doi: 10.1038/scientificamerican0381-154. [DOI] [PubMed] [Google Scholar]
- Chui DHK. THAL for THAL? Blood. 2007;110(8):2788–2789. [Google Scholar]
- Luzzatto L, Goodfellow P. Sickle cell anemia: a simple disease with no cure. Nature. 1989;337:17–18. doi: 10.1038/337017a0. [DOI] [PubMed] [Google Scholar]
- Walters MC, Patience M, Leisenring W, Eckman JR, Scott JP, Mentzer WC, Davies SC, Ohene-Frempong K, Bernaudin F, Matthews DC, Storb R, Sullivan KM. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335(6):369–376. doi: 10.1056/NEJM199608083350601. [DOI] [PubMed] [Google Scholar]
- Walters MC, Storb R, Patience M, Leisenring W, Taylor T, Sanders JE, Buchanan GE, Rogers ZR, Dinndorf P, Davies SC, Roberts IA, Dickerhoff R, Yeager AM, Hsu L, Kurtzberg J, Ohene-Frempong K, Bunin N, Bernaudin F, Wong WY, Scott JP, Margolis D, Vichinsky E, Wall DA, Wayne AS, Pegelow C, Redding-Lallinger R, Wiley J, Klemperer M, Mentzer WC, Smith FO, Sullivan KM. Impact of bone marrow transplantation for symptomatic sickle cell disease: an interim report. Multicenter investigation of bone marrow transplantation for sickle cell disease. Blood. 2000;95(6):1918–1924. [PubMed] [Google Scholar]
- de Montalembert M. Management of sickle cell disease. BMJ. 2008;337:a1397. doi: 10.1136/bmj.a1397. [DOI] [PubMed] [Google Scholar]
- Shesely EG, KiM H-K, Shehee RW, Papayannopoulou N, Smithies O, Popovicht BW. Correction of a human βS-globin gene by gene targeting. Proc Natl Acad Sci USA. 1991;88:4294–4298. doi: 10.1073/pnas.88.10.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N, Howrey RP, Lee SW, Smith CA, Sullenger BA. Ribozyme-mediated repair of sickle beta-globin mRNAs in erythrocyte precursors. Science. 1998;280(5369):1593–1596. doi: 10.1126/science.280.5369.1593. [DOI] [PubMed] [Google Scholar]
- Weatherall DJ. Gene therapy: repairing haemoglobin disorders with ribozymes. Curr Biol. 1998;8(19):R696–R698. doi: 10.1016/S0960-9822(98)70439-7. [DOI] [PubMed] [Google Scholar]
- Alami R, Gilman JG, Feng YQ, Marmorato A, Rochlin I, Suzuka SM, Fabry ME, Nagel RL, Bouhassira EE. Anti-beta s-ribozyme reduces beta s mRNA levels in transgenic mice: potential application to the gene therapy of sickle cell anemia. Blood Cells Mol Dis. 1999;25(2):110–119. doi: 10.1006/bcmd.1999.0235. [DOI] [PubMed] [Google Scholar]
- Xu L, Ferry AE, Monteiro C, Pace BS. Beta globin gene inhibition by antisense RNA transcripts. Gene Ther. 2000;7(5):438–444. doi: 10.1038/sj.gt.3301106. [DOI] [PubMed] [Google Scholar]
- Pace BS, Qian X, Ofori-Acquah SF. Selective inhibition of beta-globin RNA transcripts by antisense RNA molecules. Cell Mol Biol (Noisy-le-grand) 2004;50(1):43–51. [PubMed] [Google Scholar]
- Amosova O, Broitman SL, Fresco JR. Repairing the Sickle Cell mutation. II. Effect of psoralen linker length on specificity of formation and yield of third strand-directed photoproducts with the mutant target sequence. Nucleic Acids Research. 2003;31(16):4673–4681. doi: 10.1093/nar/gkg659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiec EB. Targeted gene repair. Gene Therapy. 1999;6(1):1–3. doi: 10.1038/sj.gt.3300789. [DOI] [PubMed] [Google Scholar]
- Lui L, Rice MC, Kmiec EB. In vivo gene repair of point and frameshift mutations directed by chimeric RNA/DNA oligonucleotides and modified single-stranded oligonucleotides. Nucleic Acids Res. 2001;29(20):4238–4250. doi: 10.1093/nar/29.20.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayengera M. Bone marrow transplantation (BMT) and gene replacement therapy (GRT) in sickle cell anemia. Niger J Med. 2008;17(3):251–256. doi: 10.4314/njm.v17i3.37390. [DOI] [PubMed] [Google Scholar]
- Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11(9):636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- Cathomen T, Joung JK. Zinc-finger nucleases: the next generation emerges. Mol Ther. 2008;16(7):1200–1207. doi: 10.1038/mt.2008.114. [DOI] [PubMed] [Google Scholar]
- Guo J, Gaj T, Barbas CF. Directed Evolution of an Enhanced and Highly Efficient FokI Cleavage Domain for Zinc Finger Nucleases. Journal of Molecular Biology. 2010;400(1):96. doi: 10.1016/j.jmb.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durai S, Mani M, Kandavelou K, Wu J, Porteus MH, Chandrasegaran S. Zinc finger nucleases: custom-designed molecular scissors for genome engineering of plant and mammalian cells. Nucl Acids Res. 2005;33(18):5978–5990. doi: 10.1093/nar/gki912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteus MH, Carroll D. Gene targeting using zinc finger nucleases. Nat Biotechnol. 2005;23(8):967–973. doi: 10.1038/nbt1125. [DOI] [PubMed] [Google Scholar]
- Perez E, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, Wang N, Lee G, Bartsevich VV, Lee YL, Guschin DY, Rupniewski I, Waite AJ, Carpenito C, Carroll RG, Orange JS, Urnov FD, Rebar EJ, Ando D, Gregory PD, Riley JL, Holmes MC, June CH. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26(7):808–816. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt N, Wang J, Kim K, Friedman G, Wang X, Taupin V, Crooks GM, Kohn DB, Gregory PD, Holmes MC, Cannon PM. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28(8):839–847. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilen CB, Wang J, Tilton JC, Miller JC, Kim KA, Rebar EJ, Sherrill-Mix SA, Patro SC, Secreto AJ, Jordan APO1, Lee G, Kahn J, Aye PP, Bunnell BA, Lackner AA, Hoxie JA, Danet-Desnoyers GA, Bushman FD, Riley JL, Gregory PD, June CH, Holmes MC, Doms RM. Engineering HIV-Resistant Human CD4+ T Cells with CXCR4-Specific Zinc-Finger Nucleases. PLoS Pathog. 2011;7(4):e1002020. doi: 10.1371/journal.ppat.1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, Down J, Denaro M, Brady T, Westerman K, Cavallesco R, Gillet-Legrand B, Caccavelli L, Sgarra R, Maouche-Chrétien L, Bernaudin F, Girot R, Dorazio R, Mulder GJ, Polack A, Bank A, Soulier J, Larghero J, Kabbara N, Dalle B, Gourmel B, Socie G, Chrétien S, Cartier N, Aubourg P, Fischer A, Cornetta K, Galacteros F, Beuzard Y, Gluckman E, Bushman F, Hacein-Bey-Abina S, Leboulch P. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467(7313):318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnazhagan S, Weigel KA, Raikwar SP, Mukherjee P, Yoder MC, Srivastava A. Recombinant human Parvovirus B19 vectors: erythroid cell-specific delivery and expression of transduced genes. J Virol. 1998;72:5224–5230. doi: 10.1128/jvi.72.6.5224-5230.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander JD, Maeder ML, Reyon D, Voytas DF, Joung JK, Dobbs D. ZiFiT (Zinc Finger Targeter): an updated zinc finger engineering tool. Nucl Acids Res. 2010;38:W462–W468. doi: 10.1093/nar/gkq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell JG, Barbas CF. Zinc finger tools: custom DNA-binding domains for transcription factors and nucleases. Nucl Acids Res. 2006;34(SI):W516–W523. doi: 10.1093/nar/gkl209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-G, Cha J, Chandrasegaran S. Hybrid restriction enzymes: Zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci USA. 1995;93:1156–1160. doi: 10.1073/pnas.93.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier B, Segal DJ, Barbas CF. Insights into the molecular recognition of the 5 '-GNN-3 ' family of DNA sequences by zinc finger domains. J Mol Biol. 2000;303(4):489–502. doi: 10.1006/jmbi.2000.4133. [DOI] [PubMed] [Google Scholar]
- Herrmann F, Garriga-Canut M, Baumstark R, Fajardo-Sanchez E, Cotterell J, Minoche A, Himmelbauer H, Isalan M. p53 Gene Repair with Zinc Finger Nucleases Optimised by Yeast 1-Hybrid and Validated by Solexa Sequencing. PLoS One. 2011;6(6):e20913. doi: 10.1371/journal.pone.0020913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon Y, Vo TD, Mendel MC, Greenberg SG, Wang J, Xia DF, Miller JC, Urnov FD, Gregory PD, Holmes MC. Enhancing zinc-finger-nuclease activity with improved obligate heterodimeric architectures. Nat Methods. 2011;8(1):74–79. doi: 10.1038/nmeth.1539. [DOI] [PubMed] [Google Scholar]
- Mátrai J, Chuah MK, VandenDriessche T. Recent advances in lentiviral vector development and applications. Mol Ther. 2010;18(3):477–490. doi: 10.1038/mt.2009.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MM, Rogers PH, Arnone A. A third quaternary structure of human hemoglobin A at 1.7-A resolution. J Biol Chem. 1992;267(24):17248–17256. [PubMed] [Google Scholar]
- Shaanan B. Structure of human oxyhaemoglobin at 2.1 A resolution. J Mol Biol. 1983;171(1):31–59. doi: 10.1016/S0022-2836(83)80313-1. [DOI] [PubMed] [Google Scholar]
- Fermi G, Perutz MF, Shaanan B, Fourme R. The crystal structure of human deoxyhaemoglobin at 1.74 A resolution. J Mol Biol. 1984;175(2):159–174. doi: 10.1016/0022-2836(84)90472-8. [DOI] [PubMed] [Google Scholar]
- Okwi AL, Byarugaba W, Ndugwa CM, Parkes A, Ocaido M, Tumwine JK. An up-date on the prevalence of sickle cell trait in Eastern and Western Uganda. BMC Blood Disord. 2010;10:5. doi: 10.1186/1471-2326-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanayak V, Ramirez CL, Joung KJ, Liu DR. Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nat Methods. 2011;8(9):765–770. doi: 10.1038/nmeth.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel R, Lombardo A, Arens A, Miller JC, Genovese P, Kaeppel C, Nowrouzi A, Bartholomae CC, Wang J, Friedman G, Holmes MC, Gregory PD, Glimm H, Schmidt M, Naldini L, von Kalle C. An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nat Biotechnol. 2011;29(9):816–823. doi: 10.1038/nbt.1948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A detailed list of the 1,606 nucleotide bases within the Hemoglobin, beta gene. This file offers FASTA-format listing of the 1,606 nucleotide bases within the Hemoglobin, beta gene (gene identity |3043|).
A detailed list of the57ZFAs specific to the Hemoglobin, beta gene-sequences. This file lists the 57 ZFAs that specifically bind sequences within the Hemoglobin, beta gene
A detailed list of thefourZFNs specific to the Hemoglobin, beta gene-sequences. This file lists the four ZFNs that specifically bind and cleave sequences within the Hemoglobin, beta gene
A detailed list of the 8,954 bp located to the 5′ end of theβAchain gene. This file offers FASTA-format listing of the 8,954 bp located to the 5′ end of the βA chain gene. (NCBI reference sequence 5190000 to 5198951 is |NT_009237.18|)
A detailed list of thefiveZFNs specific to the 8,954 bp located to the 5′ end of theβAchain gene. This file lists the fives ZFNs that specifically bind and cleave sequences within the 8,954 bp located to the 5′ end of the βA chain gene.