Abstract
Waterpipe (hookah, narghile, shisha) use has become a global phenomenon, with numerous product variations. One variation is a class of products marketed as “tobacco-free” alternatives for the “health conscious user”. In this study toxicant yields from waterpipes smoked using conventional tobacco-based and tobacco-free preparations were compared. A human-mimic waterpipe smoking machine was used to replicate the puffing sequences of 31 human participants who completed two double-blind ad libitum smoking sessions in a controlled clinical setting: once with a tobacco-based product of their choosing and once with a flavor-matched tobacco-free product. Outcome measures included yields of carbon monoxide, nitric oxide, volatile aldehydes, nicotine, tar, and polycyclic aromatic hydrocarbons. Smoke from both waterpipe preparations contained substantial quantities of toxicants. Nicotine yield was the only outcome that differed significantly between preparations. These findings contradict advertising messages that “herbal” waterpipe products are a healthy alternative to tobacco products.
Keywords: hookah, narghile, shisha, nicotine, carbon monoxide, tobacco
1. Introduction
Waterpipe (hookah, narghile, shisha) tobacco smoking, long associated with the Eastern Mediterranean Region (Maziak et al., 2004), is becoming increasingly common among adolescents and young adults worldwide (e.g., Combrink et al., 2010; Dugas et al., 2010; Jackson & Aveyard, 2008; Parna et al., 2008). With a tobacco waterpipe, lit charcoal is used to heat sweetened and flavored tobacco that is placed in the “head”. When users inhale through the waterpipe mouthpiece, charcoal-heated air and associated charcoal combustion products are drawn through the tobacco, producing the mainstream smoke through a process of distillation and pyrolysis. The smoke, in turn, is drawn into the waterpipe body, bubbles through water in the bowl, and is then carried through the hose to the user (Shihadeh, 2003). This method of tobacco use is an important public health concern, given the toxicant content of waterpipe tobacco smoke.
As with cigarette smoke, waterpipe tobacco smoke contains toxicants like polycyclic aromatic hydrocarbons (PAH) that cause cancer, volatile aldehydes (VA) that cause lung disease, carbon monoxide (CO) that contributes to cardiovascular disease, and nicotine that causes dependence (Al Rashidi et al., 2008; Sepetdjian et al., 2008; Shihadeh, 2003). Depending on the toxicant in question (e.g. lead, chromium, benzo(a)pyrene), the amount in waterpipe smoke can be up to two orders of magnitude greater than the amount found in the smoke of a single cigarette (Shihadeh, 2003; Sepetdjian et al., 2008), suggesting that even occasional waterpipe users may be exposed to high toxicant levels. Furthermore, blood CO and nicotine levels rise during waterpipe use (Blank et al., 2011; Cobb et al., 2011), and metabolites of PAH and tobacco specific nitrosamines can be measured in the urine of waterpipe smokers (Jacob et al., 2011). Thus, waterpipe tobacco smoke contains toxicants to which users are exposed systemically.
Besides tobacco, some sweetened and flavored non-tobacco products are marketed for waterpipe use. Labeling of these products suggests reduced toxicant yield. For example, “Red Royal” manufactures “herbal shisha” in Canada that is marketed as “a healthier alternative to hookah molasses tobacco” (www.redroyal.ca). Also, “Soex” is a product line made in India that includes waterpipe preparations made from sugar cane and advertised for the “health conscious” user (soex.com/e/herbalmolasses.html) to provide “the same flavorful smoke found in other shisha without the harmful effects of tobacco” (www.texashookah.com/soex.html). Other companies marketing non-tobacco waterpipe products include Al Baraka (Australia), Bee Tobacco (Germany), and Zero N Zero (United States). Package labels for these products often include claims such as “0% nicotine, 0% tar, and 0% tobacco”.
To our knowledge only one study has addressed the smoke toxicants associated with using non-tobacco products in a waterpipe, and results showed that, relative to a tobacco product, smokers using a non-tobacco product (Soex) had equivalent exposure to CO but no nicotine exposure (Blank et al., 2011). Given that both product types are heated with charcoal, these results might be expected: while a non-tobacco product would likely not contain and therefore not expose users to nicotine, charcoal is the main source of waterpipe-delivered CO and PAH (Monzer et al., 2008). Also, the “molasses” added to the products results in a large fraction of the preparation being composed of sugars (Rees et al., 2007), which have been shown to form VA at the temperatures characteristic of waterpipe tobacco during smoking (Monzer et al., 2008; Bassilakis et al., 2001). Thus, while using a waterpipe to smoke sweetened and flavored non-tobacco products produces smoke with no nicotine, we hypothesize that the smoke contains other toxicants such as CO, NO, PAH, and VA. The purpose of this study was to test this hypothesis by comparing toxicant intake for waterpipe users when smoking tobacco and non-tobacco waterpipe products. To do so, we used a machine to reproduce the exact puffing sequences of 31 waterpipe users who smoked each product type, and analyzed the resulting nicotine, CO, NO, carcinogenic PAH, and VA content of the smoke produced.
2. Materials and Methods
This study involved recording the puffing behavior (i.e. puff topography) of participants who smoked a waterpipe under controlled conditions in a clinical laboratory, once using a tobacco-based product, and once using a flavor-matched tobacco-free product. The recordings were then used to reproduce the smoke in the analytical laboratory using a digitally controlled waterpipe smoking machine. Smoke components were then analyzed.
2.1 Clinical laboratory procedure
Complete details of the clinical laboratory procedure, carried out at Virginia Commonwealth University, are reported elsewhere (Blank et al., 2011). Briefly, smoking behavior was recorded from 33 healthy waterpipe smokers (three African-American, six Asian, 17 Caucasian, one Hawaiian/Pacific Islander and six mixed/other ethnicity) who were between the ages of 18–50 (mean ±standard error of the mean (SEM)=20.2±1.8 years) and reported using a waterpipe to smoke tobacco 2–5 times/month (3.7±1.0) for ≥six months (20.7±13.5). Each participant completed two smoking sessions (separated by ≥48 hours) that differed by the product placed in the waterpipe head: 10 g preferred brand/flavor of tobacco or 10 g flavor-matched Soex™ non-tobacco preparation.
Participants were given a minimum of 45 minutes to smoke the waterpipe ad libitum (for other clinical laboratory details see Blank et al., 2011). In each session, smoking topography was measured via an orifice integrated into the waterpipe hose and connected to a pressure transducer whose signal is automatically acquired and digitized (Shihadeh et al., 2005). Instantaneous puff velocity (ml/s) is computed from the logged pressure transducer signal, and the saved record is used subsequently to reproduce the smoking session in the analytical laboratory using a human mimic smoking machine (Shihadeh & Azar, 2006). Importantly, there was no statistically significant difference observed across waterpipe preparation (tobacco; non-tobacco) on any topography measure including puff number (tobacco, mean±SEM = 66.3±7.3; non-tobacco, 71.2±8.5); total puff volume (tobacco, 57.0±7.9 l; non-tobacco, 55.7±5.6 l); and interpuff interval (tobacco 47.5±3. s; non-tobacco 45.8±4.9 s; Blank et al., 2011).
2.2 Analytical laboratory methods
A digitally controlled puff-replicating waterpipe smoking machine (Shihadeh & Azar, 2006) at the American University of Beirut was used to reproduce the flow data recorded for 31 of 33 participants in the clinical laboratory (two records could not be reproduced due to technical limitations; these two records were for participants who had smoked Nakhla double apple and its flavor-matched non-tobacco product). Each of the 62 smoking records (31 participants × 2 sessions per participant) was reproduced one time. Procedures identical to those reported by Blank and colleagues (Blank et al., 2011) were followed to prepare each waterpipe prior to connecting it to the smoking machine. Tobacco and non-tobacco preparations were from the same batches used in the clinical laboratory and were stored in the dark at -4 C until 24 hours prior to their use, at which time they were placed in a darkened 22-23 C environment at 50-60% relative humidity. Three Kings™ (Holland) quick-light charcoal briquettes (33 mm diameter) identical to those used in the clinical laboratory were used. Waterpipe hoses in the clinical and analytical studies were of identical design and material (leather), and their porous wall infiltration rates varied between 1.0 and 1.8 lpm at a waterpipe mouthpiece flow rate of 12.2 lpm when connected to a waterpipe, as determined by the method described in Saleh & Shihadeh (2008).
During each machine smoking session, the smoke exiting the waterpipe mouthpiece (Figure 1) was split into four parallel streams and each stream drawn through a 47 mm glass fiber filter pad (Gelman type A/E). Because puffing behavior varied widely across participants, the total particulate matter generated at any point in time also varied widely when replicating the puffs of these participants. Therefore we could not predict at what point in time or at what puff number filters would need replacement to avoid overload during a particular smoking session. To determine when filter changes were needed, an automatic filter friction coefficient (= pressure drop/flow rate) monitoring system was implemented in the smoking machine software and hardware; this involved installing pressure taps upstream and downstream of the filter assembly, and continuously monitoring the pressure drop across the filter assembly using the smoking machine data acquisition system. As particulate matter accumulates on a filter surface, the friction coefficient increases.
Figure 1.
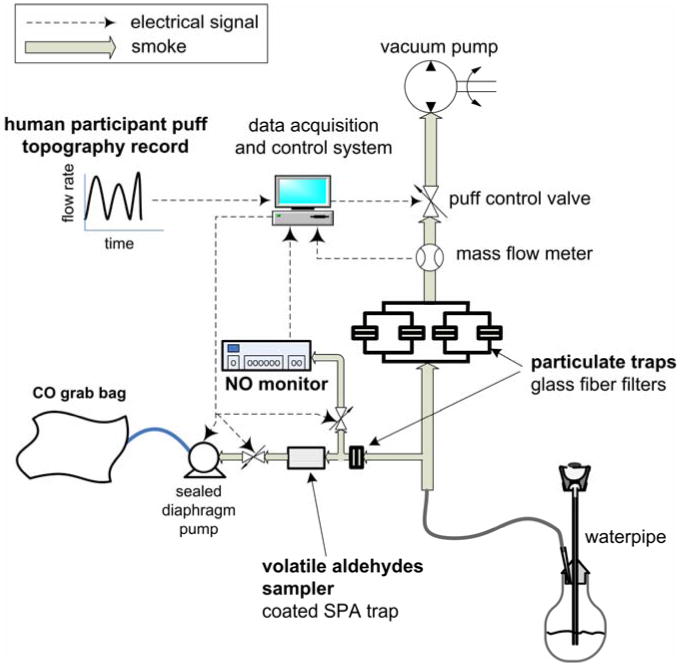
Smoking machine and smoke sampling configuration.
Prior to commencing the study, multiple experiments were conducted to correlate friction coefficient to particulate matter loading. A numerical value of the coefficient was determined which indicates a filter load approaching the 150 mg maximum allowed by the ISO standard smoking machine method (ISO 4387:1991). Thus during the study, when the friction coefficient approached this threshold the smoking session was paused for up to 15 s and the filters changed. Not more than two mid-session filter changes were needed during any of the smoking sessions.
NO, CO, nicotine, tar, VA, and PAH were quantified for each smoking session. NO was determined using a rapid-response EcoChem CLD 70S chemiluminescence analyzer. As shown in Figure 1, a small fraction of the smoke drawn during each puff was diverted from the mouthpiece into the NO analyzer, and the resulting instantaneous NO volume concentration signal was automatically logged by the smoking machine data acquisition system. NO yield was then computed as the average of the instantaneous NO concentration times the total drawn volume. The setup was validated by connecting the smoking machine inlet to a Teflon bag containing calibration gas with a NO concentration of 50 ppm, and allowing the machine to “puff” from the bag using widely varying puff topography parameters.
CO and nicotine were determined using electrochemical sensing and GC-MS, respectively, as described in Shihadeh & Saleh (2005). Tar, defined as the nicotine-free dry particulate matter, was computed as the total particulate matter (TPM) mass collected on the filters from a smoking session minus the mass of the water and nicotine found in the TPM. Thus “tar” includes PAH measured in the trapped particulate matter but excludes volatile aldehydes which were measured in the gas phase downstream of the filters. Water content in the TPM was determined using Karl-Fischer titration, as described in Shihadeh & Saleh (2005).
VA concentration was determined as in Al Rashidi et al. (2008) with minor modifications. First, for convenience only the vapor phase of the smoke was analyzed for VA; as shown in Figure 1, the DNPH cartridge used to trap and derivatize the VA is installed downstream of an ordinary particulate filter. Second, the HPLC DAD described by Al Rashidi et al. was also connected to an MS detector (Agilent LC/MSD Trap XCP) to confirm the identity of individual VA compounds. The MS analysis was conducted using negative atmospheric pressure photo-ionization. It should be noted that although we have previously measured significant quantities of acrolein in machine-generated waterpipe smoke (Al Rashidi et al., 2008) we found with the current study that in most smoke samples (for both the tobacco and non-tobacco products), the acrolein content was not detected or was below the limit of quantification, although all the other previously reported VA compounds were quantifiable. We therefore do not report acrolein in the current study.
PAH concentration was determined as in Sepetdjian et al. (2010). Filters were spiked with deuterated internal standards (acenaphthene-D10, phenanthrene-D10, chrysene-D12, and perylene-D12) and then sonicated in 10 ml toluene for 2 hours. The resulting extracts were cleaned using SPE silica cartridges and then concentrated under nitrogen before injection on GC-MS (Thermo Trace GC-Ultra equipped with ITQ-900 ion trap MS). Quantification was done using calibration curves of PAH standards and deuterated PAH standards in the selected ion current profile. PAH of interest to this study were the 4-, 5-, and 6-ringed compounds which appear on the US EPA priority pollutants list and which are classified as “probable human carcinogens”: benz(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, indeno(1,2,3-cd)pyrene.
2.3 Data analysis
Mean and 95% confidence intervals were computed for all measures. Differences in means between measures for tobacco and non-tobacco preparations were analyzed for significance using a two-tailed, paired t-test.
3. Results
Results of all analyses are presented in Table 1. As can be seen from the table, there were substantial amounts of CO, NO, tar, PAH, and VA produced for both tobacco and non-tobacco waterpipe preparations, relative to those found in the smoke of a single cigarette. Furthermore, there were no statistically significant differences observed across waterpipe preparation for these smoke toxicants. However, for nicotine, the tobacco product yielded 1.04 mg, on average, while no nicotine was measurable in the smoke of the non-tobacco product. Data for cigarettes are included in Table 1 for perspective though it should be noted that typically cigarette smokers consume an order of magnitude greater number of cigarettes in a day than waterpipe users consume waterpipes.
Table 1.
Mean (± 95% CI) mainstream smoke toxicant emissions per waterpipe use session for tobacco and non-tobacco waterpipe preparations. Smoke was generated by a machine that played back digital recordings of individual puff topography records generated by 31 waterpipe smokers who each smoked a tobacco and a non-tobacco preparation under controlled conditions (topography records did not differ by preparation smoked, see Blank et al., 2011). Toxicant emissions from previous studies of Kentucky Reference cigarettes (2R4F and 3R4F) are provided for comparison. Cigarette emissions data are given per cigarette smoked using 35 ml puff volume of 2 s duration at a frequency of 1 puff/min.
| Toxicant | Waterpipe preparation (mean ± 95% CI) | |||
|---|---|---|---|---|
| tobacco | non-tobacco | p | Ref. Cigarette | |
| Nicotine, mg | 1.04 ± 0.30 | < 0.01 | < 0.001 | 0.73a |
| Carbon monoxide, mg | 155 ± 49 | 159 ± 42 | n.s. | 12.0a |
| Nitric oxide, g | 437 ± 207 | 386 ± 116 | n.s. | 218.1c |
| Tar, mg | 464 ± 159 | 513 ± 115 | n.s. | 9.4a |
| TPM, mg | 770 ± 228 | 855 ± 192 | n.s. | 11a |
| Carcinogenic PAH, ng | ||||
| Benz (a)anthracene | 86.4 ± 15.2 | 113 ± 46 | n.s. | 14.1b |
| Chrysene | 106 ± 16 | 124 ± 36 | n.s. | 16.2b |
| Benzo(b+k)fluoranthenes | 64.7 ± 11.3 | 72.9 ± 12.6 | n.s. | 7.6b |
| Benzo(a)pyrene | 51.8 ± 12.9 | 66.1 ±17.8 | n.s. | 6.6b |
| Indeno(1,2,3-cd)pyrene | 47.3 ± 10.7 | 44.3 ± 10.4 | n.s. | 3.8b |
| Volatile aldehydes, μg | ||||
| Formaldehyde | 58.7 ± 21.6 | 117.6 ± 78.7 | n.s. | 20.6c |
| Acetaldehyde | 383 ± 121 | 566 ± 370 | n.s. | 587.4c |
| Acetone | 118 ± 36 | 163± 68 | n.s. | 270.4c |
| Propionaldehyde | 51.7 ± 15.3 | 98.4 ± 65.0 | n.s. | 49.0c |
| Methacrolein | 12.2 ± 4.4 | 20.4 ± 9.7 | n.s. | |
4. Discussion
The purpose of this study was to compare toxicant yields when waterpipe users smoked a tobacco-based and non-tobacco based product. To do so, puffing behavior was recorded in a clinical laboratory with each participant once smoking a tobacco-based and once smoking a flavor matched non-tobacco based product; the puffs produced by each participant in each condition were then subsequently replicated on a unique digitally controlled waterpipe smoking machine. NO was determined for the first time in waterpipe smoke, and found to be present in substantial quantities.
Results were unambiguous: while only the smoke from the tobacco preparation contained the dependence-causing drug nicotine, smoke from both preparations contained nearly equal amounts of toxicants known to contribute to the risk of tobacco-caused cancer, cardiovascular disease, and lung disease. Accordingly, while using the non-tobacco product presents no risk of nicotine exposure, there is no reason to believe that inhaling smoke from the non-tobacco preparation presents any less disease risk than smoke from a tobacco preparation.
The finding that the non-nicotine toxicant yields that we examined from the two types of products did not differ is consistent with previous results demonstrating that charcoal is the primary contributor to waterpipe smoke CO and PAH (Monzer et al., 2008). The lack of any observed difference in VA yield between the two products may also indicate that the sources of these smoke toxicants are the charcoal as well as chemical transformation of the sweeteners present in both the tobacco and non-tobacco preparation. If the charcoal and sweeteners are the primary source for CO, PAH, and VA, then smoke from any charcoal-heated, sweetened, waterpipe preparation might be expected to contain these toxicants.
An important limitation of this study is that the smoke yield of only one brand of non-tobacco preparation was examined, though it is likely that the findings presented here will be applicable to other brands. Another limitation owing to our current instrumentation is that the smoke was not examined for the presence of tobacco-specific nitrosamines, a group of potent carcinogens that has been found in waterpipe tobacco smoke, albeit at low levels (Schubert et al., 2011), and metabolites of these nitrosamines have been found in smokers' urine after a single waterpipe use (Jacob et al., 2011). Finally, the puff topography records used to program the smoking machine in this study were generated by occasional waterpipe users (see Blank et al., 2011). More experienced users might produce different puff topography (e.g., more puffs, greater volume, shorter interpuff interval as in Maziak et al., 2011 and Katurji et al., 2010); thus the resulting smoke toxicant yield for more experienced users might be expected to be greater than reported here (see Katurji et al. 2010, for CO, tar, and nicotine measured in smoke generated by café smokers in Beirut, Lebanon for comparison).
In sum, the fact that smoke from a non-tobacco waterpipe preparation yields near identical amounts of CO, PAH, and VA suggests that advertising depicting that the product provides “the same flavorful smoke found in other shisha without the harmful effects of tobacco” (www.texashookah.com/soex.html) is, at best, misleading. These results, taken together with others addressing the tar and nicotine content of waterpipe tobacco smoke (Nakkash & Khalil, 2010; Vansickel et al., 2011) should be used to support much-needed regulation of labeling for all waterpipe products.
Research highlights.
Smoke toxicant content was compared for tobacco and non-tobacco products
Smoke from both categories of products contained substantial quantities of toxicants
Tobacco-free products did not exhibit reduced PAH, volatile aldehydes, CO, NO, or “tar” yields
Only the tobacco-based products produced a nicotine-containing smoke
Acknowledgments
The authors thank Barbara Kilgalen and Janet Austin for administering the clinical components of this study.
Funding: This work is supported by U.S. Public Health Service Grants R01CA120142, R01DA024876, and F31DA028102
Footnotes
Competing interests: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Rashidi M, Shihadeh A, Saliba NA. Volatile aldehydes in the mainstream smoke of the narghile waterpipe. Food Chem Toxicol. 2008;46(11):3546–3549. doi: 10.1016/j.fct.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassilakis R, Carangelo RM, Wójtowicz MA. TG-FTIR analysis of biomass pyrolysis. Fuel. 2001;80(12):1765–1786. [Google Scholar]
- Blank MD, Cobb CO, Kilgalen B, Austin J, Weaver MF, Shihadeh A, Eissenberg T. Acute effects of waterpipe tobacco smoking: A double-blind, placebo-control study. Drug Alcohol Depend. 2011;116(1-3):102–109. doi: 10.1016/j.drugalcdep.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb CO, Shihadeh A, Weaver MF, Eissenberg T. Water pipe tobacco smoking and cigarette smoking: a direct comparison of toxicant exposure and subjective effects. Nicotine Tob Res. 2011;13(2):78–87. doi: 10.1093/ntr/ntq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combrink A, Irwin N, Laudin G, Naidoo K, Plagerson S, Mathee A. High prevalence of hookah smoking among secondary school students in a disadvantaged community in Johannesburg. S Afr Med J. 2010;100(5):297–9. doi: 10.7196/samj.3965. http://www.ncbi.nlm.nih.gov/pubmed/20460022. [DOI] [PubMed] [Google Scholar]
- Dugas E, Tremblay M, Low NCP, Cournoyer D, O'Loughlin J. Water-pipe smoking among North American youths. Pediatrics. 2010;125(6):1184–1189. doi: 10.1542/peds.2009-2335. [DOI] [PubMed] [Google Scholar]
- Intorp M, Purkis S, Whittaker M, Wright W. Determination of “Hoffmann Analytes” in cigarette mainstream smoke. The Coresta 2006 Joint Experiment. Contrib Tob Res. 2009;23:161–202. [Google Scholar]
- Jackson D, Aveyard P. Waterpipe smoking in students: prevalence, risk factors, symptoms of addiction, and smoke intake. Evidence from one British university. BMC Public Health. 2008;8:174. doi: 10.1186/1471-2458-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, III, Raddaha AHA, Dempsey D, Havel C, Peng M, Yu L, Benowitz NL. Nicotine, Carbon Monoxide, and Carcinogen Exposure after a Single Use of a Waterpipe. Cancer Epidemiology Biomarkers and Prevention. 2011 Sep 9;:2011. doi: 10.1158/1055-9965.EPI-11-0545. OnlineFirst. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katurji M, Daher N, Sheheitli H, Saleh R, Shihadeh A. Direct measurement of toxicants inhaled by water pipe users in the natural environment using a real-time in situ sampling technique. Inhalation Toxicol. 2010;22(13):1101–1109. doi: 10.3109/08958378.2010.524265. [DOI] [PubMed] [Google Scholar]
- Liu C, Hu J, Mcadam KG. A feasibility study on oxidation state of arsenic in cut tobacco, mainstream cigarette smoke and cigarette ash by X-ray absorption spectroscopy. Spectrochim Acta B: Atom Spectr. 2009;64:1294–1301. [Google Scholar]
- Maziak W, Rastam S, Ibrahim I, Ward KD, Shihadeh A, Eissenberg T. CO exposure, puff topography, and subjective effects in waterpipe tobacco smokers. Nicotine Tob Res. 2009;11(7):806–811. doi: 10.1093/ntr/ntp066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak W, Rastam S, Shihadeh A, Bazzi A, Ibrahim I, Zaatari GS, Ward KD, Eissenberg T. Nicotine exposure in daily waterpipe smokers and its relation to puff topography. Addictive Behaviors. 2011;36(4):397–399. doi: 10.1016/j.addbeh.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak W, Ward KD, Soweid Afifi RA, Eissenberg T. Tobacco smoking using a waterpipe: a re-emerging strain in a global epidemic. Tob Control. 2004;13(4):327–333. doi: 10.1136/tc.2004.008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzer B, Sepetdjian E, Saliba N, Shihadeh A. Charcoal emissions as a source of CO and carcinogenic PAH in mainstream narghile waterpipe smoke. Food Chem Toxicol. 2008;46(9):2991–2995. doi: 10.1016/j.fct.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Nakkash R, Khalil J. Health warning labeling practices on narghile (shisha, hookah) waterpipe tobacco products and related accessories. Tob Control. 2010;19(3):235–239. doi: 10.1136/tc.2009.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parna K, Usin J, Ringmets I. Cigarette and waterpipe smoking among adolescents in Estonia: HBSC survey results, 1994-2006. BMC Public Health. 2008;8:392. doi: 10.1186/1471-2458-8-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh R, Shihadeh A. Elevated toxicant yields with narghile waterpipes smoked using a plastic hose. Food Chem Toxicol. 2008;46(5):1461–1466. doi: 10.1186/1471-2458-8-392. [DOI] [PubMed] [Google Scholar]
- Schubert J, Hahn J, Dettbarn G, Seidel A, Luch A, Schulz T. Mainstream Smoke of the Waterpipe: Does This Environmental Matrix Reveal As Significant Source of Toxic Compounds? Toxicology Letters. 2011;205:279–284. doi: 10.1016/j.toxlet.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Sepetdjian E, Shihadeh A, Saliba NA. Measurement of 16 polycyclic aromatic hydrocarbons in narghile waterpipe tobacco smoke. Food Chem Toxicol. 2008;46(5):1582–1590. doi: 10.1016/j.fct.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Sepetdjian E, Saliba N, Shihadeh A. Carcinogenic PAH in waterpipe charcoal products. Food and Chemical Toxicology. 2010;48(11):3242–3245. doi: 10.1016/j.fct.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihadeh A. Investigation of mainstream smoke aerosol of the argileh water pipe. Food Chem Toxicol. 2003;41(1):143–152. doi: 10.1016/S0278-6915(02)00220-X. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Antonios C, Azar S. A portable, low-resistance puff topography instrument for pulsating, high-flow smoking devices. Behavior Research Methods. 2005;37(1):186–191. doi: 10.3758/bf03206414. http://www.ncbi.nlm.nih.gov/pubmed/16097360. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Azar S. A closed-loop control “playback” smoking machine for generating mainstream smoke aerosols. J Aerosol Med. 2006;19(2):137–147. doi: 10.1089/jam.2006.19.137. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Saleh R. Polycyclic aromatic hydrocarbons, carbon monoxide, “tar”, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food Chem Toxicol. 2005;43(5):655–661. doi: 10.1016/j.fct.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Tarrant JE, Mills K, Williard C. Development of an improved method for the determination of polycyclic aromatic hydrocarbons in mainstream tobacco smoke. J Chromatogr A. 2009;1216:2227–2234. doi: 10.1016/j.chroma.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Shihadeh A, Eissenberg T. Waterpipe tobacco products: nicotine labeling versus nicotine exposure. Tob Control. 2011 doi: 10.1136/tc.2010.042416. [DOI] [PMC free article] [PubMed] [Google Scholar]


