Abstract
Stem cells self-renew and give rise to all differentiated cell types of the adult body. They are classified as toti-, pluri- or multi-potent based on the number of different cell types they can give rise to. Recently it has become apparent that chromatin regulation plays a critical role in determining the fate of stem cells and their descendants. In this review we will discuss the role of chromatin regulators in maintenance of stem cells and their ability to give rise to differentiating cells in both the animal and plant kingdom. We will highlight similarities and differences in chromatin-mediated control of stem cell fate in plants and animals. We will consider possible reasons why chromatin regulators play a central role in pluripotency in both kingdoms given that multicellularity evolved independently in each.
Keywords: Chromatin remodeling, Histone modification, Stem cells, Pluripotency, Differentiation
Introduction
In mammals, the fate of pluripotent embryonic stem cells (ESCs) is regulated by pluripotency transcription factors. These include the POU domain transcription factor Oct4, the divergent homeodomain protein Nanog, and the high mobility group (HMG)-box transcription factor Sox2 [1; see Table 1 for a list of pluripotency and chromatin regulators discussed in this review]. These pluripotency factors activate each other as well as other ESC-specific genes and repress expression of developmental regulators in ESCs [2]. Pluripotent cell fate can be induced in differentiating cells, such as fibroblasts, by expressing these and additional transcription factors to give rise to induced pluripotent stem cells [3].
Table 1.
Stem cell regulators in animals and plants
| Category | Mammals | Arabidopsis | Activity | Role in stem cells |
|---|---|---|---|---|
| Pluripotency regulators |
Sox2, Oct4, Nanog |
WUS, KNOX (STM, KNAT1, KNAT2, KNAT6); PLT1, PLT2; SHR, SCR, WOX5 |
Transcription factors | Activate pluripotency genes, proliferation genes, repress developmental regulator genes |
| SWI2/SNF2 remodeling ATPases |
Brg1 | SYD, BRM | Alteration of nucleosome conformation, presence, and position |
Mammals: repress developmental regulator genes, fine- tune (downregulate) pluripotency genes Plants: maintain expression of pluripotency genes |
| KAT | p300 Tip60 |
AtGCN5 | Histone lysine acetylation |
Both: activate expression of some pluripotency genes in stem cells |
| Co-repressor complex/HDACs |
Gro/TLE | TPL (WSIP1), WSIP2 |
Histone lysine deacetylation |
Both: repress developmental regulator genes in stem cells |
| PRC2 polycomb complex |
Ezh2, Ezh1; Suz12; EeD |
CLF, SWN; EMF2; FIE |
H3K27methylation | Mammals: repress developmental regulator genes in ESCs, activate developmental regulator genes during differentiation Both: repress pluripotency genes during differentiation |
| PRC1 polycomb complex |
Polycomb; Ring1A/B; etc. |
LHP1; AtRING1a/b |
Chromatin compaction | Mammals: repress developmental genes in ESCs Plants: repress pluripotency genes during differentiation |
| Trithorax group (TrxG) proteins |
MLL | ATX1, ATX2 |
H3K4 methylation | Mammals: activate pluripotency genes Plants: unknown |
| Histone chaperones |
CAF-1; NAP1; HirA |
FAS1, FAS2; NRP1, NRP2; HirA |
Chromatin assembly/disassembly |
Both: required for condensed chromatin in ESCs Plants: repress pluripotency genes during differentiation |
Histone lysine demethylases (KDMs), and enzymes controlling DNA methylation were not included here, despite their critical roles in pluripotency and differentiation. Sufficient information for across kingdom comparisons is not yet available for these chromatin regulators.
Plant stem cell populations reside in the growing tips of the shoot and the root in meristems. As for adult stem cells in animals, maintenance of the two pluripotent plant stem cell populations is entirely dependent on the stem cell niche [4]. Pluripotency transcription factors also regulate maintenance of stem cells in plants; they exercise this role from within the shoot or root stem cell niche.
In Arabidopsis thaliana, the pluripotency regulators and homeodomain transcription factors WUSCHEL (WUS) and SHOOT MERISTEMLESS (STM) maintain the shoot apical meristem stem cells. Consistent with this role, ectopic WUS and STM expression induces formation of ectopic stem cell pools [5]. However, only a subset of the co-expressing cells is responsive and the ectopic stem cells are not properly maintained [5], suggesting a requirement for additional pluripotency regulators. In contrast to animal pluripotency transcription factors, WUS and STM cannot activate each other [6]. WUS expression levels are positively correlated with the size of the stem cell population and restricted via negative feedback by the CLAVATA (CLV) signaling pathway [7,8]. STM promotes cell division and inhibits differentiation of the stem cells in the shoot apical meristem [6,9,10] together with other KNOTTED1-LIKE HOMEOBOX (KNOX) transcription factors, such as KNOTTED in ARABIDOPSIS THALIANA1 (KNAT1), KNAT2 and KNAT6 [11]. During differentiation, expression of the KNOX genes is repressed by the MYB-domain transcription factor ASYMMETRIC LEAVES 1 (AS1) together with the LATERAL ORGAN BOUNDARIES (LOB) domain transcription factor AS2 [12-14].
Stem cell populations in the root meristem are controlled by the pluripotency regulators and double AP2 domain transcription factors PLETHORA1 (PLT1) and PLT2, together with the GRAS transcription factors SCARECROW (SCR) and SHORTROOT (SHR) [15-17]. A local maximum of the plant hormone auxin induces PLT1 and PLT2 expression [18], while SHR induces SCR expression in the stem cell niche [19]. In agreement with their role as pluripotency regulators, PLT and SCR can induce formation of ectopic root stem cell pools [15,18]. A WUS-like regulator, WOX5 has also been implicated as a root stem cell pluripotency regulator [20,21].
Recent investigations have shown that pluripotency transcription factors are regulated by and act together with chromatin regulators [1,22,23]. The basic unit of chromatin, the nucleosome, is formed by 147 base pairs of DNA wrapped around the histone octamer: two copies each of histone H2A, H2B, H3 and H4. Nucleosomes are separated by linker DNA associated with the linker histone H1. The initial positioning of nucleosomes on the DNA is partially genome encoded [24]. Nucleosomes are progressively folded into higher order chromatin [25], which is increasingly inhibitory to processes that require access to genomic DNA, such as transcription, replication, and DNA repair. However, DNA accessibility in the context of chromatin is regulated by the activities of several protein complexes including ATP-dependent chromatin remodeling enzymes, enzymes mediating covalent modifications of histones or the DNA, and chromatin assembly factors [22].
Chromatin regulation of pluripotency and differentiation
Chromatin remodeling
SWI2/SNF2 chromatin remodeling ATPases in both mammals and plants directly control expression of pluripotency transcription factors. These chromatin remodeling complexes utilize the energy released by ATP hydrolysis to disrupt contacts between histones and DNA, resulting in changes in nucleosome conformation, position, composition and occupancy [26]. Those changes have been shown to alter DNA accessibility, regulating access of gene-specific or general transcription factors to the DNA to activate or repress gene expression [27]. In mammalian ESCs, a unique SWI2/SNF2 complex called esBAF has been described, which contains the chromatin remodeling ATPase BRAHMA related gene 1 (Brg1; also called Smarca 4) as enzymatic subunit [28]. esBAF contains a unique subset of associated regulatory factors (BAFs) critical for its function in ESCs [28]. Brg1 regulates maintenance of pluripotency and initiation of differentiation [22]; loss of Brg1 leads to loss of self-renewal in ESCs [28,29]. Brg1 binds to the regulatory regions of the pluripotency genes Oct4, Sox2, and Nanog as well as to their direct targets [30]. In addition, Brg1 physically interacts with the Oct4 and Sox2 transcription factors [28]. Surprisingly, Brg1 not only represses expression of developmental regulator genes in ESCs but also that of puripotency genes [30]. It has been proposed that downregulation of pluripotency gene expression by Brg1 may fine-tune their expression level to facilitate exit from self-renewal during differentiation [30].
Mutations in the homologous Arabidopsis SWI2/SNF2 chromatin remodeling ATPases SPLAYED (SYD) and BRAHMA (BRM) cause defects in the maintenance of the stem cell population in the shoot apical meristem [31-33]. The molecular mechanism that underlies shoot meristem phenotype of brm mutants is currently not understood. SYD is required to maintain expression of the pluripotency regulator WUS in the shoot apical meristem during reproductive development and occupies WUS regulatory regions [33]. Arabidopsis BRCA-1 ASSOCIATED RING DOMAIN 1 (BARD1) represses WUS expression outside of the stem cell niche [34]. Since BARD1 associates with SYD it was proposed that BARD1 may regulate WUS expression by inhibiting SYD complex activity [34]. Both SYD and BRM also play a role in embryonic shoot apical meristem formation by controlling organ boundary gene expression [35]. The composition of SYD/BRM complex in stem cells or differentiating cells is not yet known, although many SWI2/SNF2 BAFs are conserved between plants and animal [36].
A different type of chromatin remodeling ATPase, the chromodomain-containing remodeling factor PICKLE/GYMNOS (PKL) controls differentiation in Arabidopsis [37]. PKL mutations enhance as1 and as2 phenotypes and cause ectopic meristem formation due to de-repression of KNOX gene expression [10].
Histone acetylation
Histone acetylation plays a role in pluripotency regulator gene expression in both mammals and plants. In addition, histone acetyl transferases (KATs) and histone deacetylates (HDACs) are important cofactors for pluripotency regulators. KATs add acetyl groups to lysines in the amino-terminal tails or in the core of histones. This modification generally promotes activation of transcription by reducing strength of the interaction between histones and DNA [22]. In ESCs, the p300 KAT acts as a transcriptional coactivator of pluripotency transcription factors [38] and directly activates Nanog expression [39]. In addition, recruitment of p300 to its target genes depends on the presence of one or more of the pluripotency factors, Nanog, Oct4 and Sox2 [38]. Another KAT, Tip60 is also required to maintain stem cell identity and regulates similar target genes as Nanog [40]. The KAT activity of the Tip60-p400 complex appears to be required to repress developmental regulator gene expression in ESCs. One possible explanation for the unexpected repressive role of Tip60-p400 is that Tip60-p400 activates a repressor of developmental regulator genes [40].
In Arabidopsis, the AtGCN5 KAT restricts WUS expression to the stem cell niche; in atgcn5 mutants WUS is ectopically expressed [41]. As for Tip-60 in mammals (above), it has been proposed that AtGCN5 activates a negative regulator of WUS expression [41]. AtGCN5 has an apparent opposite role in root stem cells, where it is required for root stem cell maintenance and activates the pluripotency regulator PLT [42,43]. KATs have not yet been implicated as pluripotency transcription factor coactivators in Arabidopsis.
HDACs, by contrast, create a closed chromatin conformation that represses transcription. The Nucleosome Remodeling Deacetylase (NuRD) complex contains both ATP-dependent chromatin remodeling and deacetylase activities [44]. Mbd3, a core subunit of NuRD complex is required for pluripotency. ESCs lacking Mbd3 are slow growing and have differentiation defects [45]. An ESC-specific co-repressor complex containing many NuRD subunits called NODE interacts with Nanog and Oct4 and represses developmental regulators in ESCs [46].
Similarly, WUS may recruit HDAC activities to repress expression of genes that control differentiation. This is thought to occur via interaction with two WUS-interacting proteins called WSIP1 and WSIP2 that bind to the conserved EAR motif in the C-terminal domain of WUS [47]. WSIP1, also called TOPLESS, and WSIP2 are similar to Gro/TLE co-repressors, which recruit HDACs to target genes to repress transcription [48]. Hence both animal and plant HDACs repress developmental regulator gene expression in stem cells.
Histone Methylation
Histone lysine methyltransferases (KMTs) exhibit high substrate specificity with respect to the lysine residue modified and the type of methylation added (mono-, di- or tri-methylation). Methylation of two lysines of histone H3 (lysine 4 and lysine 27) plays an important role in regulation of pluripotency. H3K27 KTMs are components of the Polycomb Repressive complex 2 (PRC2), while H3K4 KTMs are Trithorax Group (TrxG) proteins [49,50]. Trimethylation of H3K27 (H3K27me3) by PRC2 and subsequent activity of the larger PRC1 complex represses expression of differentiation genes in ESCs and pluripotency gene expression in differentiating cells in mammals. Thus far direct evidence is only available for the latter role in plants.
The mammalian PRC2 has three core subunits, the EZH2 KMT, SUZ12, and EED (Table1). Loss of activity in any of these causes similar but not identical defects including upregulation of developmental genes in ESCs, as well as failure to repress pluripotency regulators and to activate developmental regulators in differentiating cells [51]. The EZH1 KMT acts together with EZH2 to repress expression of developmental regulators in ESCs [52]. The PRC1 complex, composed of ca. 10 subunits including POLYCOMB, RING1A and RING1B, also plays a role in maintaining stem cell identity and is required for proper differentiation [53]. PRC1 promotes higher order chromatin compaction to further repress the expression of target genes [49]. Consistently, ESCs depleted of RING1B showed de-repression of developmental regulator genes and abnormal differentiation [54,55].
The Arabidopsis PRC2 complex prevents pluripotency gene transcription during differentiation. A homolog of the EZH2 H3K27 KMT, CURLY LEAF (CLF), directly binds to the STM promoter and deposits H3K27me3 modifications at this locus [56]. Both STM and KNAT2 expression is de-repressed in clf mutants [57]. A second EZH2 homolog, SWINGER (SWN), acts partly redundantly with CLF in inhibiting STM expression [58]. Other PRC2 complex components also play roles in repressing KNOX transcription; silencing of the Arabidopsis PRC2 core subunit and EED homolog FERTILIZATION INDEPENDENT ENDOSPERM (FIE) causes ectopic STM, KNAT2, and KNAT6 expression [57]. Interestingly, the moss Physcomitrella patens FIE (PpFIE) is required to maintain pluripotency of the apical daughter cells [59]. Although it is not yet clear what the direct targets of PpFIE are, the observed phenotype is consistent with a possible role for PCR2 in repression of differentiation gene expression in pluripotent cells in plants. The plant PRC1 complex is divergent from its metazoan counterpart and apparently contains TFL2/LHP1, a protein similar to metazoan HETEROCHROMATIN PROTEIN1 (HP1), in lieu of POLYCOMB [60,61]. The Arabidopsis genome does not contain a POLYCOMB ortholog. Notably, like POLYCOMB and unlike HP1, LHP1 binds to H3K27me3, the histone modification generated by PRC2 [62]. Recently, a double mutant in the Arabidopsis PRC1 complex components atring1a and atring1b was shown to cause ectopic shoot meristem formation and de-repression of KNOX gene expression [61], suggesting that PRC2 and PRC1 jointly repress pluripotency gene expression in differentiating cells in Arabidopsis.
Methylation of H3K4 by TrxG KTMs is an activating histone modification [63]. In human ESCs, many genes marked with H3K4me3 encode proteins involved in proliferation [64] including Oct4, Sox2 and Nanog [64-67]. Hence TrxG proteins activate pluripotency regulator gene expression. The Arabidopsis TrxG proteins ATX1 and ATX2 are responsible for H3K4me3 and H3K4me2 methylation, respectively [68]. Thus far no direct role in stem cell maintenance has been described for either protein, perhaps because of functional redundancy with related H3K4 KMTs [69].
Despite their opposing roles, H3K27me3 and H3K4me3 co-occupy many regulatory elements in ESCs in mammals [70]. It has been proposed that these ‘bivalent histone modifications’ keep developmental genes poised for activation in ESCs to resolve to either H3K27me3 (repressed state) or H3K4me3 (activated state) upon differentiation [63,70; Fig. 1]. By contrast, recent genome-wide analyses suggest that the H3K4me2 and not H3K4me3 is associated with H3K27me3 in bivalent domains in Arabidopsis [71]. Furthermore, the H3K27 and H3K4 methylation status is not only controlled by KMTs, but also by histone lysine demethylases (KDMs), which like the KMTs have high substrate specificity. An H3K4me3 KDM represses developmental gene expression in ESC together with the PRC2 complex [72]. Conversely, an H3K27me3 KDM is required for differentiation [73] and this type of KDM is frequently associated with TrxG complexes. These combined activities are well suited to coordinately regulate the balance between H3K4me3 and H3K27me3 [51].
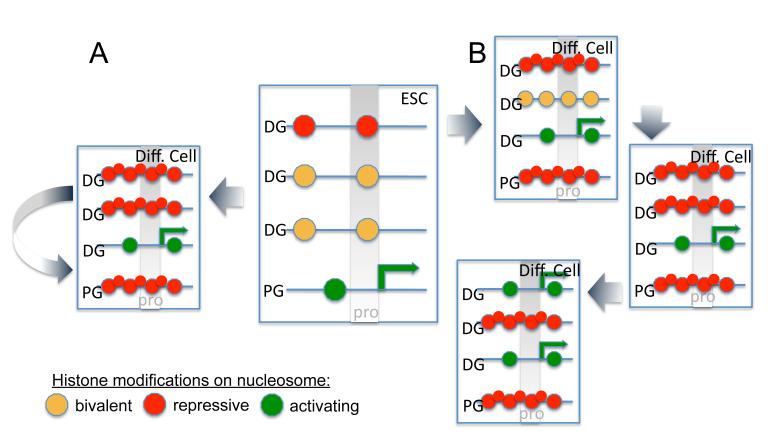
Figure 1. Dynamic chromatin in stem cells and differentiating cells.
Center: Stem cell chromatin is highly accessible as indicated by the low nucleosome density on three developmental regulator genes and one pluripotency gene. In differentiating cells the existing chromatin modifications and chromatin structure at each of the four loci is inherited or copied after replication for transcriptional memory and lineage fidelity (A). In addition to transcriptional memory, chromatin regulators direct transcriptional reprogramming of specific loci in differentiating cells via alteration of histone modifications and nucleosome density in response to developmental or environmental cues (B).
DG: developmental regulator gene, PG: pluripotency gene, pro: promoter, Diff. Cell: differentiating cell, ESC: embryonic stem cell.
Thus, two opposing histone methyl marks on H3K4 and on H3K27 play an important role in stem cell maintenance and differentiation in mammals by controlling expression of proliferation genes and developmental regulator genes. While H3K27me3 is required for repression of pluripotency gene expression in differentiating plant cells, it remains to be determined what role methylation of H3K4 or H3K27 plays in plant stem cell maintenance.
Histone chaperones
Histone chaperones have important roles in chromatin assembly and disassembly both during and outside of replication [74]. In both mammals and plants, histone chaperones are required to maintain repressed chromatin states. For example, components of the replication-coupled assembly factor CAF-1 complex are required for chromatin condensation and maintenance of repressive histone modifications in ESCs [75]. Mutations in the replication-independent histone chaperone HirA cause accelerated differentiation in ESCs [76], suggesting a role for this histone chaperone in stem cell maintenance.
In Arabidopsis, histone chaperones are also required to maintain condensed chromatin, in addition they repress pluripotency regulator gene expression in differentiating cells. CAF-1 subunit loss-of-function fasciata (fas) mutants exhibit ectopic foci of WUS and SCR expression, suggesting that CAF-1 is critical for restricting stem cell fate to the shoot and root meristem [77]. The ectopic SCR expression pattern in fas mutant roots varies among individual plants and among neighboring cells suggesting a stochastic collapse of transcriptionally repressed chromatin states [77,78]. Arabidopsis RETINOBLASTOMA-RELATED acts synergistically with CAF-1 to inhibit root stem cell proliferation by repressing expression of pluripotency transcription factors [79,80]. Similarly, loss of function of the NAP1 histone chaperone homolog NAP1-RELATED PROTEIN1 (NRP1) and NRP2 causes increased expression of the root pluripotency regulator PLT2 and NRP1 binds to the PLT2 locus [81]. In the shoot, AS1 recruits HIRA to the KNAT1 and KNAT2 loci [82]. Reduced HIRA activity causes KNAT1 and KNAT2 de-repression, suggesting that the AS1-AS2/HIRA complex maintains repressive chromatin at the KNAT1 and KNAT2 loci during differentiation [82].
DNA methylation
DNA methylation in mammals occurs on cytosine residues in CpG dinucleotides and represses transcription [83]. The promoters of Oct4 and Nanog as well as those of other ESC-specific genes are free of methylation in ESCs, yet are heavily methylated in differentiating cells [1,83]. Conversely, during induction of pluripotency, DNA methylation at Oct4 and Nanog promoters diminishes, suggesting a causal role for DNA demehylation in reprogramming [83]. DNA methylation has not yet been implicated in repression of pluripotency genes in differentiating cells in Arabidopsis.
Perspective
In summary, despite some of the differences described above, chromatin regulators clearly play an important role in stem cell self-renewal and in the ability of stem cells to give rise to differentiating cells in both animals and plants. One explanation for the evolutionary conserved role of chromatin regulators in pluripotency may be that stem cell chromatin is fundamentally different from that of differentiating cells and that maintenance of this unique chromatin state requires the activity of chromatin regulators.
Indeed, ESCs are characterized by highly accessible chromatin, hyperdynamic chromatin association of architectural proteins such as HP1 and histone H1, and a preponderance of activating histone modifications relative to differentiating cells [Fig. 1; 76,83,84]. In addition, ESCs are more transcriptionally active, both in genic and intergenic regions than differentiating cells, and expression of genes encoding for chromatin remodeling complex components is elevated [84]. The open chromatin and stochastic transcription may allow ESCs and their immediate descendants to readily assume one of many lineages-specific transcription programs [83,84]. It remains to be seen whether adult stem cells in animals or pluripotent plant stem cells likewise have highly accessible chromatin and increased transcription.
It has been proposed that in differentiating cells, by contrast, chromatin primarily maintains lineage fidelity [Fig. 1A; 85]. Indeed, chromatin regulators play an important role in transcriptional memory to maintain heritable alternate states of gene activity, the classical definition of epigenetic regulation. Consistently, certain chromatin modifications and chromatin architectural proteins are mitotically heritable including DNA methylation and presence of PRC1 [86,87].
However, all known chromatin alterations are reversible, even those closely linked to transcriptional memory [88,89]. Furthermore, differentiating cells in both animals and plants have and can establish bivalent chromatin domains, thought to mark genes that are poised for activation or repression [Fig. 1B; 22,90]. In both kingdoms some lineage specific developmental regulator genes are only activated in late in morphogenesis and this activation requires a switch to activating chromatin configuration [Fig. 1B; 83,91]. Thus, in differentiating cells chromatin regulators are also required for transcriptional reprogramming [83].
Hence, the reason chromatin regulators have similar roles in pluripotency and differentiation in the plant and the animal kingdoms is perhaps not simply to maintain the highly accessible chromatin of stem cells. Instead we propose that the observed conserved role of chromatin regulators in pluripotency and differentiation is likely due to their unique ability to provide both stability and plasticity to transcriptional programs, key challenges for stem cells and their descendents as well as for differentiating cells in developing organisms [83]. In all of these cell types, chromatin regulators ensure faithful inheritance of transcriptional programs from mother to daughter cells, while being able to direct large scale transcriptional reprogramming in response to critical developmental and environmental cues. The challenge for the future is to elucidate the regulatory eventss that lead from perception of endogenous or exogenous cues to alteration of chromatin regulator activity from memory to reprogramming at relevant loci.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ng JH, Heng JC, Loh YH, Ng HH. Transcriptional and epigenetic regulations of embryonic stem cells. Mutat Res. 2008;647:52–58. doi: 10.1016/j.mrfmmm.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Daley GQ. Molecular basis of pluripotency. Hum Mol Genet. 2008;17:R23–27. doi: 10.1093/hmg/ddn050. [DOI] [PubMed] [Google Scholar]
- 3.Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136:509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheres B. Stem-cell niches: nursery rhymes across kingdoms. Nat Rev Mol Cell Biol. 2007;8:345–354. doi: 10.1038/nrm2164. [DOI] [PubMed] [Google Scholar]
- 5.Gallois JL, Woodward C, Reddy GV, Sablowski R. Combined SHOOT MERISTEMLESS and WUSCHEL trigger ectopic organogenesis in Arabidopsis. Development. 2002;129:3207–3217. doi: 10.1242/dev.129.13.3207. [DOI] [PubMed] [Google Scholar]
- 6.Lenhard M, Jurgens G, Laux T. The WUSCHEL and SHOOTMERISTEMLESS genes fulfil complementary roles in Arabidopsis shoot meristem regulation. Development. 2002;129:3195–3206. doi: 10.1242/dev.129.13.3195. [DOI] [PubMed] [Google Scholar]
- 7.Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science. 2000;289:617–619. doi: 10.1126/science.289.5479.617. [DOI] [PubMed] [Google Scholar]
- 8.Schoof H, Lenhard M, Haecker A, Mayer KF, Jurgens G, Laux T. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell. 2000;100:635–644. doi: 10.1016/s0092-8674(00)80700-x. [DOI] [PubMed] [Google Scholar]
- 9.Williams RW. Plant homeobox genes: many functions stem from a common motif. Bioessays. 1998;20:280–282. doi: 10.1002/(SICI)1521-1878(199804)20:4<280::AID-BIES2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 10.Ori N, Eshed Y, Chuck G, Bowman JL, Hake S. Mechanisms that control knox gene expression in the Arabidopsis shoot. Development. 2000;127:5523–5532. doi: 10.1242/dev.127.24.5523. [DOI] [PubMed] [Google Scholar]
- 11.Scofield S, Murray JA. KNOX gene function in plant stem cell niches. Plant Mol Biol. 2006;60:929–946. doi: 10.1007/s11103-005-4478-y. [DOI] [PubMed] [Google Scholar]
- 12.Byrne ME, Simorowski J, Martienssen RA. ASYMMETRIC LEAVES1 reveals knox gene redundancy in Arabidopsis. Development. 2002;129:1957–1965. doi: 10.1242/dev.129.8.1957. [DOI] [PubMed] [Google Scholar]
- 13.Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA. Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature. 2000;408:967–971. doi: 10.1038/35050091. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y, Li Z, Xu B, Li H, Wang L, Dong A, Huang H. Subcellular localizations of AS1 and AS2 suggest their common and distinct roles in plant development. J Integr Plant Biol. 2008;50:897–905. doi: 10.1111/j.1744-7909.2008.00693.x. [DOI] [PubMed] [Google Scholar]
- 15.Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature. 2007;449:1053–1057. doi: 10.1038/nature06206. [DOI] [PubMed] [Google Scholar]
- 16.Sabatini S, Heidstra R, Wildwater M, Scheres B. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev. 2003;17:354–358. doi: 10.1101/gad.252503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyer-Pascuzzi AS, Benfey PN. Transcriptional networks in root cell fate specification. Biochim Biophys Acta. 2009;1789:315–325. doi: 10.1016/j.bbagrm.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser MT, Benfey PN. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell. 2000;101:555–567. doi: 10.1016/s0092-8674(00)80865-x. [DOI] [PubMed] [Google Scholar]
- 20.Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature. 2007;446:811–814. doi: 10.1038/nature05703. [DOI] [PubMed] [Google Scholar]
- 21.Stahl Y, Wink RH, Ingram GC, Simon R. A signaling module controlling the stem cell niche in Arabidopsis root meristems. Curr Biol. 2009;19:909–914. doi: 10.1016/j.cub.2009.03.060. [DOI] [PubMed] [Google Scholar]
- 22.Keenen B, de la Serna IL. Chromatin remodeling in embryonic stem cells: regulating the balance between pluripotency and differentiation. J Cell Physiol. 2009;219:1–7. doi: 10.1002/jcp.21654. [DOI] [PubMed] [Google Scholar]
- 23.Shen WH, Xu L. Chromatin remodeling in stem cell maintenance in Arabidopsis thaliana. Molecular Plant. 2009;2:600–609. doi: 10.1093/mp/ssp022. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan N, Moore IK, Fondufe-Mittendorf Y, Gossett AJ, Tillo D, Field Y, LeProust EM, Hughes TR, Lieb JD, Widom J, et al. The DNA-encoded nucleosome organization of a eukaryotic genome. Nature. 2009;458:362–366. doi: 10.1038/nature07667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horn PJ, Peterson CL. Heterochromatin assembly: a new twist on an old model. Chromosome Res. 2006;14:83–94. doi: 10.1007/s10577-005-1018-1. [DOI] [PubMed] [Google Scholar]
- 26.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Sif S. ATP-dependent nucleosome remodeling complexes: enzymes tailored to deal with chromatin. J Cell Biochem. 2004;91:1087–1098. doi: 10.1002/jcb.20005. [DOI] [PubMed] [Google Scholar]
- 28.Ho L, Ronan JL, Wu J, Staahl BT, Chen L, Kuo A, Lessard J, Nesvizhskii AI, Ranish J, Crabtree GR. An embryonic stem cell chromatin remodeling complex, esBAF, is essential for embryonic stem cell self-renewal and pluripotency. Proc Natl Acad Sci U S A. 2009;106:5181–5186. doi: 10.1073/pnas.0812889106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Efroni S, Melcer S, Nissim-Rafinia M, Meshorer E. Stem cells do play with dice: a statistical physics view of transcription. Cell Cycle. 2009;8:43–48. doi: 10.4161/cc.8.1.7216. [DOI] [PubMed] [Google Scholar]
- 30.Ho L, Jothi R, Ronan JL, Cui K, Zhao K, Crabtree GR. An embryonic stem cell chromatin remodeling complex, esBAF, is an essential component of the core pluripotency transcriptional network. Proc Natl Acad Sci U S A. 2009;106:5187–5191. doi: 10.1073/pnas.0812888106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurtado L, Farrona S, Reyes JC. The putative SWI/SNF complex subunit BRAHMA activates flower homeotic genes in Arabidopsis thaliana. Plant Mol Biol. 2006;62:291–304. doi: 10.1007/s11103-006-9021-2. [DOI] [PubMed] [Google Scholar]
- 32.Wagner D, Meyerowitz EM. SPLAYED, a novel SWI/SNF ATPase homolog, controls reproductive development in Arabidopsis. Curr Biol. 2002;12:85–94. doi: 10.1016/s0960-9822(01)00651-0. [DOI] [PubMed] [Google Scholar]
- 33.Kwon CS, Chen C, Wagner D. WUSCHEL is a primary target for transcriptional regulation by SPLAYED in dynamic control of stem cell fate in Arabidopsis. Genes Dev. 2005;19:992–1003. doi: 10.1101/gad.1276305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han P, Li Q, Zhu YX. Mutation of Arabidopsis BARD1 causes meristem defects by failing to confine WUSCHEL expression to the organizing center. Plant Cell. 2008;20:1482–1493. doi: 10.1105/tpc.108.058867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon CS, Hibara K, Pfluger J, Bezhani S, Metha H, Aida M, Tasaka M, Wagner D. A role for chromatin remodeling in regulation of CUC gene expression in the Arabidopsis cotyledon boundary. Development. 2006;133:3223–3230. doi: 10.1242/dev.02508. [DOI] [PubMed] [Google Scholar]
- 36.Kwon CS, Wagner D. Unwinding chromatin for development and growth: a few genes at a time. Trends Genet. 2007;23:403–412. doi: 10.1016/j.tig.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Ogas J, Kaufmann S, Henderson J, Somerville C. PICKLE is a CHD3 chromatin-remodeling factor that regulates the transition from embryonic to vegetative development in Arabidopsis. Proc Natl Acad Sci U S A. 1999;96:13839–13844. doi: 10.1073/pnas.96.24.13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 39.Zhong X, Jin Y. Critical roles of coactivator p300 in mouse embryonic stem cell differentiation and Nanog expression. J Biol Chem. 2009;284:9168–9175. doi: 10.1074/jbc.M805562200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fazzio TG, Huff JT, Panning B. An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity. Cell. 2008;134:162–174. doi: 10.1016/j.cell.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertrand C, Bergounioux C, Domenichini S, Delarue M, Zhou DX. Arabidopsis histone acetyltransferase AtGCN5 regulates the floral meristem activity through the WUSCHEL/AGAMOUS pathway. J Biol Chem. 2003;278:28246–28251. doi: 10.1074/jbc.M302787200. [DOI] [PubMed] [Google Scholar]
- 42.Kornet N, Scheres B. Members of the GCN5 Histone Acetyltransferase Complex Regulate PLETHORA-Mediated Root Stem Cell Niche Maintenance and Transit Amplifying Cell Proliferation in Arabidopsis. Plant Cell. 2009 doi: 10.1105/tpc.108.065300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stockinger EJ, Mao Y, Regier MK, Triezenberg SJ, Thomashow MF. Transcriptional adaptor and histone acetyltransferase proteins in Arabidopsis and their interactions with CBF1, a transcriptional activator involved in cold-regulated gene expression. Nucleic Acids Res. 2001;29:1524–1533. doi: 10.1093/nar/29.7.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denslow SA, Wade PA. The human Mi-2/NuRD complex and gene regulation. Oncogene. 2007;26:5433–5438. doi: 10.1038/sj.onc.1210611. [DOI] [PubMed] [Google Scholar]
- 45.Kaji K, Caballero IM, MacLeod R, Nichols J, Wilson VA, Hendrich B. The NuRD component Mbd3 is required for pluripotency of embryonic stem cells. Nat Cell Biol. 2006;8:285–292. doi: 10.1038/ncb1372. [DOI] [PubMed] [Google Scholar]
- 46.Liang J, Wan M, Zhang Y, Gu P, Xin H, Jung SY, Qin J, Wong J, Cooney AJ, Liu D, et al. Nanog and Oct4 associate with unique transcriptional repression complexes in embryonic stem cells. Nat Cell Biol. 2008;10:731–739. doi: 10.1038/ncb1736. [DOI] [PubMed] [Google Scholar]
- 47.Kieffer M, Stern Y, Cook H, Clerici E, Maulbetsch C, Laux T, Davies B. Analysis of the transcription factor WUSCHEL and its functional homologue in Antirrhinum reveals a potential mechanism for their roles in meristem maintenance. Plant Cell. 2006;18:560–573. doi: 10.1105/tpc.105.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Z, Karmarkar V. Groucho/Tup1 family co-repressors in plant development. Trends Plant Sci. 2008;13:137–144. doi: 10.1016/j.tplants.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Pietersen AM, van Lohuizen M. Stem cell regulation by polycomb repressors: postponing commitment. Curr Opin Cell Biol. 2008;20:201–207. doi: 10.1016/j.ceb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–443. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- 51.Pasini D, Bracken AP, Agger K, Christensen J, Hansen K, Cloos PA, Helin K. Regulation of stem cell differentiation by histone methyltransferases and demethylases. Cold Spring Harb Symp Quant Biol. 2008;73:253–263. doi: 10.1101/sqb.2008.73.009. [DOI] [PubMed] [Google Scholar]
- 52.Shen X, Liu Y, Hsu YJ, Fujiwara Y, Kim J, Mao X, Yuan GC, Orkin SH. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32:491–502. doi: 10.1016/j.molcel.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 54.Leeb M, Wutz A. Ring1B is crucial for the regulation of developmental control genes and PRC1 proteins but not X inactivation in embryonic cells. J Cell Biol. 2007;178:219–229. doi: 10.1083/jcb.200612127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Endoh M, Endo TA, Endoh T, Fujimura Y, Ohara O, Toyoda T, Otte AP, Okano M, Brockdorff N, Vidal M, et al. Polycomb group proteins Ring1A/B are functionally linked to the core transcriptional regulatory circuitry to maintain ES cell identity. Development. 2008;135:1513–1524. doi: 10.1242/dev.014340. [DOI] [PubMed] [Google Scholar]
- 56.Schubert D, Primavesi L, Bishopp A, Roberts G, Doonan J, Jenuwein T, Goodrich J. Silencing by plant Polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. Embo J. 2006;25:4638–4649. doi: 10.1038/sj.emboj.7601311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katz A, Oliva M, Mosquna A, Hakim O, Ohad N. FIE and CURLY LEAF polycomb proteins interact in the regulation of homeobox gene expression during sporophyte development. Plant J. 2004;37:707–719. doi: 10.1111/j.1365-313x.2003.01996.x. [DOI] [PubMed] [Google Scholar]
- 58.Chanvivattana Y, Bishopp A, Schubert D, Stock C, Moon YH, Sung ZR, Goodrich J. Interaction of Polycomb-group proteins controlling flowering in Arabidopsis. Development. 2004;131:5263–5276. doi: 10.1242/dev.01400. [DOI] [PubMed] [Google Scholar]
- 59.Mosquna A, Katz A, Decker EL, Rensing SA, Reski R, Ohad N. Regulation of stem cell maintenance by the Polycomb protein FIE has been conserved during land plant evolution. Development. 2009;136:2433–2444. doi: 10.1242/dev.035048. [DOI] [PubMed] [Google Scholar]
- 60.Sanchez-Pulido L, Devos D, Sung ZR, Calonje M. RAWUL: a new ubiquitin-like domain in PRC1 ring finger proteins that unveils putative plant and worm PRC1 orthologs. BMC Genomics. 2008;9:308. doi: 10.1186/1471-2164-9-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu L, Shen WH. Polycomb silencing of KNOX genes confines shoot stem cell niches in Arabidopsis. Curr Biol. 2008;18:1966–1971. doi: 10.1016/j.cub.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 62.Turck F, Roudier F, Farrona S, Martin-Magniette ML, Guillaume E, Buisine N, Gagnot S, Martienssen RA, Coupland G, Colot V. Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet. 2007;3:e86. doi: 10.1371/journal.pgen.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwartz YB, Pirrotta V. Polycomb complexes and epigenetic states. Curr Opin Cell Biol. 2008;20:266–273. doi: 10.1016/j.ceb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 64.Zhao XD, Han X, Chew JL, Liu J, Chiu KP, Choo A, Orlov YL, Sung WK, Shahab A, Kuznetsov VA, et al. Whole-genome mapping of histone H3 Lys4 and 27 trimethylations reveals distinct genomic compartments in human embryonic stem cells. Cell Stem Cell. 2007;1:286–298. doi: 10.1016/j.stem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan G, Tian S, Nie J, Yang C, Ruotti V, Wei H, Jonsdottir GA, Stewart R, Thomson JA. Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell. 2007;1:299–312. doi: 10.1016/j.stem.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 68.Saleh A, Alvarez-Venegas R, Yilmaz M, Le O, Hou G, Sadder M, Al-Abdallat A, Xia Y, Lu G, Ladunga I, et al. The highly similar Arabidopsis homologs of trithorax ATX1 and ATX2 encode proteins with divergent biochemical functions. Plant Cell. 2008;20:568–579. doi: 10.1105/tpc.107.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Avramova Z. Evolution and pleiotropy of TRITHORAX function in Arabidopsis. Int J Dev Biol. 2009;53:371–381. doi: 10.1387/ijdb.082664za. [DOI] [PubMed] [Google Scholar]
- 70.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 71.Zhang X, Bernatavichute YV, Cokus S, Pellegrini M, Jacobsen SE. Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biol. 2009;10:R62. doi: 10.1186/gb-2009-10-6-r62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pasini D, Hansen KH, Christensen J, Agger K, Cloos PA, Helin K. Coordinated regulation of transcriptional repression by the RBP2 H3K4 demethylase and Polycomb-Repressive Complex 2. Genes Dev. 2008;22:1345–1355. doi: 10.1101/gad.470008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sen GL, Webster DE, Barragan DI, Chang HY, Khavari PA. Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes Dev. 2008;22:1865–1870. doi: 10.1101/gad.1673508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Henikoff S. Nucleosome destabilization in the epigenetic regulation of gene expression. Nat Rev Genet. 2008;9:15–26. doi: 10.1038/nrg2206. [DOI] [PubMed] [Google Scholar]
- 75.Houlard M, Berlivet S, Probst AV, Quivy JP, Hery P, Almouzni G, Gerard M. CAF-1 is essential for heterochromatin organization in pluripotent embryonic cells. PLoS Genet. 2006;2:e181. doi: 10.1371/journal.pgen.0020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meshorer E, Yellajoshula D, George E, Scambler PJ, Brown DT, Misteli T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev Cell. 2006;10:105–116. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaya H, Shibahara KI, Taoka KI, Iwabuchi M, Stillman B, Araki T. FASCIATA genes for chromatin assembly factor-1 in arabidopsis maintain the cellular organization of apical meristems. Cell. 2001;104:131–142. doi: 10.1016/s0092-8674(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 78.Ono T, Kaya H, Takeda S, Abe M, Ogawa Y, Kato M, Kakutani T, Scheid O Mittelsten, Araki T, Shibahara K. Chromatin assembly factor 1 ensures the stable maintenance of silent chromatin states in Arabidopsis. Genes Cells. 2006;11:153–162. doi: 10.1111/j.1365-2443.2006.00928.x. [DOI] [PubMed] [Google Scholar]
- 79.Wildwater M, Campilho A, Perez-Perez JM, Heidstra R, Blilou I, Korthout H, Chatterjee J, Mariconti L, Gruissem W, Scheres B. The RETINOBLASTOMA-RELATED gene regulates stem cell maintenance in Arabidopsis roots. Cell. 2005;123:1337–1349. doi: 10.1016/j.cell.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 80.Kornet N, Scheres B. Stem cell factors in plants: chromatin connections. Cold Spring Harb Symp Quant Biol. 2008;73:235–242. doi: 10.1101/sqb.2008.73.043. [DOI] [PubMed] [Google Scholar]
- 81.Zhu Y, Dong A, Meyer D, Pichon O, Renou JP, Cao K, Shen WH. Arabidopsis NRP1 and NRP2 encode histone chaperones and are required for maintaining postembryonic root growth. Plant Cell. 2006;18:2879–2892. doi: 10.1105/tpc.106.046490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Phelps-Durr TL, Thomas J, Vahab P, Timmermans MC. Maize rough sheath2 and its Arabidopsis orthologue ASYMMETRIC LEAVES1 interact with HIRA, a predicted histone chaperone, to maintain knox gene silencing and determinacy during organogenesis. Plant Cell. 2005;17:2886–2898. doi: 10.1105/tpc.105.035477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohn F, Schubeler D. Genetics and epigenetics: stability and plasticity during cellular differentiation. Trends Genet. 2009 doi: 10.1016/j.tig.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 84.Efroni S, Duttagupta R, Cheng J, Dehghani H, Hoeppner DJ, Dash C, Bazett-Jones DP, Le Grice S, McKay RD, Buetow KH, et al. Global transcription in pluripotent embryonic stem cells. Cell Stem Cell. 2008;2:437–447. doi: 10.1016/j.stem.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chi AS, Bernstein BE. Developmental biology. Pluripotent chromatin state. Science. 2009;323:220–221. doi: 10.1126/science.1166261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Francis NJ, Follmer NE, Simon MD, Aghia G, Butler JD. Polycomb proteins remain bound to chromatin and DNA during DNA replication in vitro. Cell. 2009;137:110–122. doi: 10.1016/j.cell.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- 88.Kia SK, Gorski MM, Giannakopoulos S, Verrijzer CP. SWI/SNF mediates polycomb eviction and epigenetic reprogramming of the INK4b-ARF-INK4a locus. Mol Cell Biol. 2008;28:3457–3464. doi: 10.1128/MCB.02019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gehring M, Reik W, Henikoff S. DNA demethylation by DNA repair. Trends Genet. 2009;25:82–90. doi: 10.1016/j.tig.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 90.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 91.Wagner D. Chromatin regulation of plant development. Curr Opin Plant Biol. 2003;6:20–28. doi: 10.1016/s1369526602000079. [DOI] [PubMed] [Google Scholar]