Abstract
Detection of disease progression is an important and challenging component of glaucoma management. Optical coherence tomography (OCT) has proved to be valuable in the detection of glaucomatous damage. With its high resolution and proven measurement reproducibility, OCT has the potential to become an important tool for glaucoma progression detection. This manuscript presents the capabilities of the OCT technology pertinent for detection of progressive glaucomatous damage and provides a review of the current knowledge on the device’s clinical performance.
INTRODUCTION
Glaucoma is an optic neuropathy characterized by progressive loss of retinal ganglion cells and optic nerve damage that may result in visual field loss and irreversible blindness.1 The rate of functional and structural progression can be highly variable among subjects. Early identification of progression is of utmost importance because appropriate treatment can slow disease progression and preserve vision.
Because damage to the retinal ganglion cells cannot be directly detected in a clinical setting, clinicians have to rely on indirect methods of retinal ganglion cell evaluation. Retinal function can be measured with psychophysical techniques such as standard automated perimetry, short-wavelength automated perimetry, and frequency-doubling technology.2 Some subjects, however, show structural changes in the optic nerve head and/or retinal nerve fiber layer (RNFL) before any evidence of glaucomatous damage can be detected with automated perimetry.3,4 Assessment of posterior segment ocular structures is therefore a crucial step in glaucoma diagnosis and progression detection.
Clinical practice has shown that identification of progression is often challenging because glaucoma is a slowly progressing disease and its time course is often variable. Moreover, it is often difficult to discriminate between true disease-related changes and measurement variability or natural age-related decreases in visual function. Furthermore, there are no widely accepted gold standard criteria for establishing glaucoma progression. In clinical practice, progression is traditionally assessed by serial evaluation of visual fields and stereo disc photographs. However, it is well established that structural and functional progression often occurs at different times over the course of the disease. Therefore, the agreement between functional and structural progression is often poor.5,6
MEASURING GLAUCOMA PROGRESSION
A variety of strategies to determine glaucoma progression have been used clinically, ranging from subjective assessment based on clinical judgment to statistical analyses of many measurements collected over time. In this article, we will focus on methods to detect progression using information provided by OCT.
The statistical approaches used in assessing glaucoma progression can be divided into event based and trend based. In event analysis, progression is identified when a follow-up measurement exceeds a preestablished threshold for change from baseline. It is assumed that any change below this threshold is due to natural age-related loss and/or measurement variability, whereas changes exceeding the threshold represent true progression. The threshold for a change can be determined from an individual subject’s variability or from variability in a normal reference group. A higher threshold would result in greater specificity because only situations with marked change would be detected. However, this would reduce the sensitivity for detecting less dramatic changes. Conversely, setting the threshold to a lower level will increase sensitivity while simultaneously reducing specificity. Event analysis is intended to identify a gradual change over time that eventually crosses a threshold or to detect an acute event that exceeds a threshold. However, a confirmatory test is always recommended, particularly in the latter case, to prevent an artifactual measurement from being labeled as an actual event.
A trend analysis identifies progression by monitoring the behavior of a parameter over time. A regression analysis or mixed effect analysis of a dependent variable (ie, RNFL thickness) is performed on follow-up measurements, providing a rate of progression over time. This method is less sensitive to sudden change and the variability among consecutive tests because it is neutralized by the overall rate of change. This method also offers an advantage allowing the extrapolation of the rate of progression, which makes it possible to predict the time required to reach certain milestones.
PROGRESSION WITH TIME-DOMAIN OCT (TD-OCT)
Stratus OCT (Carl Zeiss Meditec, Dublin, CA), the commercially available TD-OCT, produces cross-sectional images at a scanning speed of 400 axial scans per second, with an axial resolution of 8 to 10 µm and a transverse resolution of approximately 20 µm. The device can acquire a variety of linear and circular scan patterns. The most commonly used TD-OCT scan for glaucoma evaluation is the “Fast RNFL scan.” This is a 3.4-mm diameter circular scan centered on the optic nerve head. RNFL thickness is automatically determined and reported as an overall mean, by quadrants, and by clock hours. The macula and optic nerve head are traditionally used as secondary targets for glaucoma diagnosis with TD-OCT. Both regions are scanned using six radial scans equally spaced 30 degrees apart. Quantitative information is provided for the total thickness of the macula and for the optic nerve head structures.
TD-OCT RNFL thickness measurements have been shown to discriminate well between normal and glaucomatous eyes.7–11 They have also been shown to have good reproducibility in detecting both diffuse and localized glaucomatous RNFL defects.12–17 Because of the relatively low intra-test and inter-test variability of peripapillary RNFL thickness measurements, they can be useful for observing patients with glaucoma over time and therefore are potentially valuable for detecting glaucomatous progression. Based on the published data on the repeatability of mean RNFL thickness measurements, any decrease in thickness not exceeding 6.4 to 8 µm can be considered to be within normal limits of test–retest variability with 95% tolerance.14,15,18 Any reproducible decrease in the mean RNFL thickness exceeding this range might indicate progression of the disease. These values should be used only for mean peripapillary RNFL thickness and not for quadrants and clock hours because the variability within the smaller sectors is higher. This is because the differences in the measured RNFL thickness resulting from shifts in the scan location are averaged out in the overall mean.
Longitudinal changes can be evaluated using three different options of the Stratus OCT software (version 5.0 and beyond). The summary tables and plots of RNFL thickness profiles for baseline and follow-up visits allow observation of the change over time and help pinpoint the location of focal changes, but they do not provide information about the significance of these changes. The third option, the guided progression analysis, is a trend-based analysis employing a linear regression to report change in overall mean RNFL thickness over time. The significance of the change is also provided. It should be noted that statistical significance is reported by Stratus if the rate of change in mean RNFL thickness is significantly different than zero rather than different from the rate of normal age-related loss. Therefore, some normal age-related changes may be reported as significant even though they do not represent true disease progression. Cross-sectional data indicate that the population average age-related RNFL loss is expected to be between 0.16 and 0.31 µm/year.19–21
There are relatively few studies that have evaluated the utility of OCT in assessing the progression of glaucoma. This is due to constantly evolving technology with resulting hardware and software changes, which combined with the fact that glaucoma is a slowly progressing disease, makes longitudinal assessments challenging. The first OCT longitudinal study evaluated 64 eyes of 37 subjects with glaucoma and suspected glaucoma who were observed for a median of 4.7 years.5 OCT progression was determined using an event-based analysis. The threshold for change in mean RNFL thickness was set to a change from baseline that exceeded 20 µm and was based on two times the reproducibility error of the device (10 µm). Visual-field criterion for progression was defined as a reduction in mean deviation of 2 dB from baseline in two of three consecutive visits. The study showed that 22% of the eyes were found to have progression by OCT compared with 9% by visual field mean deviation. Only 3% of the eyes progressed by both OCT and visual field. The higher rate of progression on OCT compared with standard automated perimetry suggests that OCT might have higher sensitivity to change. The higher sensitivity of the OCT may be due to structural changes preceding functional loss as measured by standard automated perimetry, or it may represent a high false-positive rate.
Another study used event analysis to determine the sensitivity and specificity of Stratus OCT for detection of glaucoma progression in 27 patients with glaucoma showing localized progressive loss of retinal nerve fibers in red-free fundus photographs and 62 healthy controls.18 The test–retest variability at the 95% level for this study was set at 6.4 µm for the average RNFL thickness. Progression was defined by red-free photography as clearly visible widening of preexisting localized defects or the development of new localized defects (but not deepening of the existing defect, which is undetectable on photograph assessment). The sensitivity of Stratus OCT RNFL measurements to detect progression ranged from 14.8% (for average RNFL thickness) to 85.2% (for clock hour thickness). The specificity was approximately 95% for average RNFL thickness, but decreased to 59.7% for clock hour and 77.4% for quadrant thickness. Specificity of the sectoral measurements increased when it was calculated based on two consecutive follow-up examinations.
Excellent topographic agreement was found between changes in RNFL thickness measured by OCT and progressive atrophy of the RNFL evidenced on red-free photographs. The clock-hour criterion was shown to have the highest sensitivity to detect progression despite having higher test–retest variability than that of the overall average and quadrant RNFL thickness measurements. To explain these results, the authors hypothesized that in the subjects who had expansion of a localized RNFL defect, progression occurred focally without a significant effect on average and quadrant RNFL thickness. It should be noted that the study results provided information regarding the ability of OCT to detect progressive RNFL atrophy only in subjects who have localized RNFL defects and not those with diffuse RNFL damage.
A trend-based approach was tested in a study where red-free fundus photography was used as the reference standard to determine progression.22 The study demonstrated that eyes showing progression of localized RNFL defects on red-free fundus photographs (76 of a total of 153 glaucomatous eyes) had significantly higher rates of RNFL loss over time, as measured by OCT trend analysis, than eyes that were stable. The rate of progression was highest in affected clock-hours, possibly reflecting the widening of a small, early defect and/or further loss of the nerve fibers within the existing defect. The rates of localized thickness change were shown to have higher discriminating ability between progressors and non-progressors than the global RNFL thinning rate, indicating that focal RNFL loss may not always result in a detectable change in global RNFL thickness. The study results underscored the importance of analyzing both global and sectoral (quadrant and clock-hour) RNFL thicknesses. However, it should be noted that, similar to the previous study,18 the subjects evaluated had localized defects only; thus, the implications of the results of this study should not be extended to subjects with diffuse RNFL atrophy. The study showed no difference in baseline RNFL thickness between progressors and non-progressors. This was in disagreement with previous observations of higher rates of RNFL thinning associated with higher baseline RNFL thickness and studies showing that progression defined as RNFL thickness loss is more evident than visual field progression in early glaucoma, whereas the opposite is true in the advanced stages of the disease.23–25
In another study employing a trend-based analysis, it was demonstrated that the guided progression analysis included in the latest version of the Stratus OCT was able to detect progressive RNFL loss and provide the rate of change in RNFL thickness in subjects with glaucoma.23 In this study, 21 and 22 of 116 glaucomatous eyes showed progression as measured by guided progression analysis of average RNFL thickness and two adjacent clock hours, respectively. The rate of average RNFL thickness loss was −1.2 to −15.4 µm/year. Of the 22 eyes that progressed by guided progression analysis in two adjacent clock hours, 8 eyes did not progress by guided progression analysis of average RNFL thickness, again indicating that focal RNFL loss may not always result in a detectable change in global RNFL thickness. The inferotemporal (7 o’clock) sector was the most frequent location that showed progression, suggesting that this location is not only important in discriminating glaucomatous from healthy eyes10,11,26 but it should also play an important role in detecting glaucomatous progression. The study also showed that at a comparable level of specificity, guided progression analysis of average RNFL thickness and trend analysis of the visual field index had a poor agreement for detection of progression, consistent with the well-recognized knowledge that the agreement between functional and structural progression is limited.5,6
In the studies described above, the use of TD-OCT was focused on assessment of the changes in the RNFL thickness. Stratus OCT is also capable of providing reproducible measurements of optic nerve head topography and macular thickness.15,27 The utility of these measurements in detecting glaucoma progression was evaluated and compared with RNFL parameters in a cohort of patients with glaucoma and suspected glaucoma.28 Visual field guided progression analysis and expert assessment of optic disc stereophotographs were used as reference standards to determine progression. The study showed that eyes progressing by visual field and/or optic disc stereophotographs had significantly higher rates of RNFL loss over time than the non-progressing eyes. For the global RNFL thickness, mean rate of change was −0.72 µm/year for progressors and 0.14 µm/year for non-progressors. The rates of change were widely variable among the eyes.
RNFL parameters performed well in discriminating eyes that progressed by visual fields and/or optic disc stereophotographs from eyes that did not. Consistent with the previous studies, inferior quadrant RNFL thickness had the best discriminatory performance. The RNFL parameters performed significantly better than optic nerve head and macular thickness parameters for detection of change. None of the macular thickness parameters were able to discriminate progressors from non-progressors. The poor sensitivity of the optic nerve head scan to detect small focal changes occurring over time can be partially explained by the lack of sectoral analysis of the optic nerve head by TD-OCT. Moreover, TD-OCT optic nerve head parameters are obtained using only six radial scans, which necessitates heavy interpolation between the radial tissue sampling.
A large amount of interpolation between scans is also a likely explanation for the poor performance of the macular thickness parameters in detecting progression (similarly to the optic nerve head scan, the macular scan pattern consists of only six radial scans). Moreover, the macular parameters were based on total retinal thickness rather than specific retinal layers that are affected in glaucoma. Previous studies have shown that the glaucoma diagnostic ability of TD-OCT parameters measuring total retinal thickness was found to be inferior to the RNFL thickness parameters,8,9,29–31 and therefore the same is likely to be true for detection of longitudinal changes.
PROGRESSION WITH SPECTRAL-DOMAIN OCT (SD-OCT)
SD-OCT offers higher scanning rates (up to 50,000 axial scans per second) and improved resolution (axial resolution: 3 to 6 µm, transverse resolution: 20 µm) compared with TD-OCT. These improvements have resulted in the development of novel scanning patterns that enable the acquisition of three-dimensional data from areas of interest. This allows post-processing of the data in desired locations and enables registration of consecutive images. The substantial increase in SD-OCT scanning speed over TD-OCT makes scans less prone to eye movement artifacts. Image registration minimizes misalignment between consecutive images, potentially decreasing the variability in RNFL thickness measurements, which was one of the main factors limiting the ability to detect true structural changes over time using TD-OCT. Indeed, recent studies have demonstrated excellent intra-visit and inter-visit measurement reproducibility for SD-OCT,32–38 superior to TD-OCT,32,38–40 indicating this instrument’s potential utility in monitoring glaucoma progression.
Several studies evaluated the glaucoma diagnostic ability of SD-OCT compared with TD-OCT.39,41–46 The different SD-OCT devices evaluated were found to have a good glaucoma diagnostic ability, but these studies showed no statistically significant difference between SD-OCT and TD-OCT. In most of these studies, the subject population had significant glaucomatous damage, making it difficult to evaluate the superiority of one imaging device versus another. However, when the diagnostic ability of SD-OCT and TD-OCT to detect focal RNFL defects was compared in patients with pre-perimetric glaucoma, the study failed to detect a statistically significant difference between the best performing parameters from each device.47 It has to be noted that although a new method of acquiring data is used by SD-OCT, peripapillary data for RNFL thickness come from the same location as in TD-OCT (a 3.4-mm diameter peripapillary circle centered on the optic disc). This similarity makes it easy to compare the data from the two devices, but the potential advantage offered by three-dimensional volumetric data is not fully being used, thus leading to a similar level of diagnostic ability. Indeed, some localized RNFL defects were demonstrated on the SD-OCT deviation map and were not evident on TD-OCT.47
Several studies are currently underway evaluating the utility of SD-OCT RNFL thickness measurements in detecting glaucoma progression but, considering the relatively recent launch time of commercially available SD-OCT devices and the slowly progressive nature of glaucoma, these results are not yet available.
Another advantage of SD-OCT related to the increased resolution and three-dimensional rendering is the ability to perform precise measurement, segmentation, and mapping of the retinal layers and delineation of the layers affected by glaucoma. For example, RTVue’s (Optovue, Fremont, CA) ganglion cell complex selectively measures the inner retinal layers within the macular region that are specifically susceptible to glaucomatous damage. The ganglion cell complex is composed of the macular nerve fiber layer, ganglion cell layer, and inner plexiform layer. Assessment of these layers has been shown to have an improved ability to detect glaucoma compared with using full retinal thickness. Ganglion cell complex diagnostic accuracy for detecting glaucoma has been shown to be similar to that of peripapillary RNFL thickness,48–52 making it potentially valuable for monitoring glaucoma progression. The intra-session and inter-session variability of the ganglion cell complex measurements was investigated in a recent study on 37 healthy subjects with ocular hypertension and glaucoma experienced in imaging examinations and 40 screening trial participants without such experience.32 The test–retest variability of the ganglion cell complex parameters evaluated in this study did not exceed 4.51 µm. This value may serve as reference for setting a threshold for change in future longitudinal studies.
COMMERCIALLY AVAILABLE PROGRESSION SD-OCT SOFTWARE
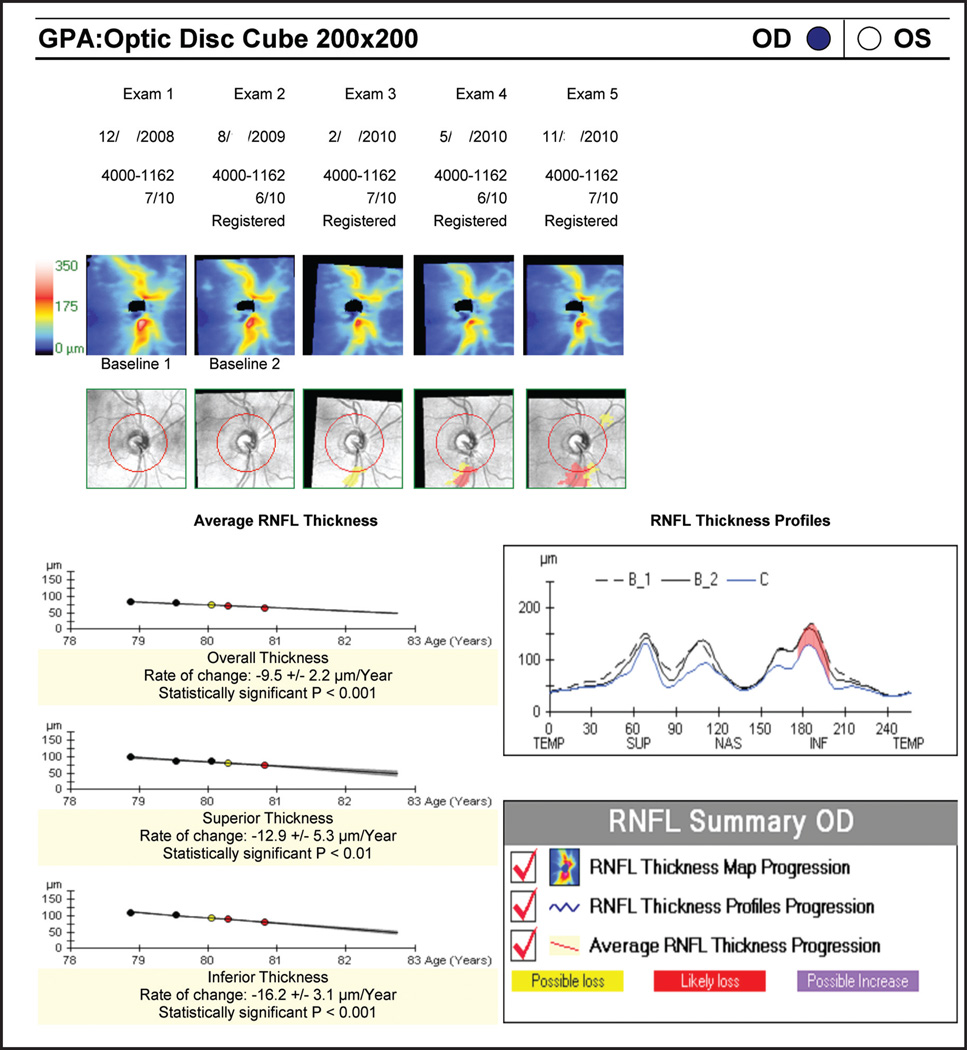
SD-OCT devices are manufactured by several companies, but only two machines currently offer progression analysis as part of their commercially available software: Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA) and RTVue (Optovue, Fremont, CA). Of these two devices, only the Cirrus HD-OCT provides statistical analyses for progression detection. Cirrus HD-OCT offers a glaucoma progression algorithm based on both event and trend analyses. Data sampling of the RNFL is obtained from the 3.4-mm diameter peripapillary circle. The software also displays RNFL thickness changes from baseline for each pixel in the scanned area. As mentioned above, this enables the detection of structural changes outside the confines of the circumpapillary scan, such as a new or expanding wedge defect. Possible or likely RNFL thickness loss is reported if change exceeds the expected test–retest variability in a single or two consecutive follow-up examinations, respectively. Additionally, linear regression is performed to determine the rate of change, confidence limits, and statistical significance of the trend. Figure 1 illustrates a case of glaucoma progression detected by SD-OCT RNFL thickness maps and profiles along with a significant rate of RNFL thickness reduction.
Figure 1.
Cirrus Spectral-Domain Optical Coherence Tomography Retinal Nerve Fiber Layer (RNFL) Guided Progression Analysis (GPA) (Carl Zeiss Meditec, Dublin, CA). RNFL Thickness Maps (top panel) show graduate progression in the inferotemporal region (yellow and red sectors). The inferotemporal progression is also notable in the RNFL Thickness Profiles (red; right panel). Average RNFL Thickness plots (left panel) show statistically significant thinning in the overall, superior and inferior RNFL thickness. OD = right eye; OS = left eye.
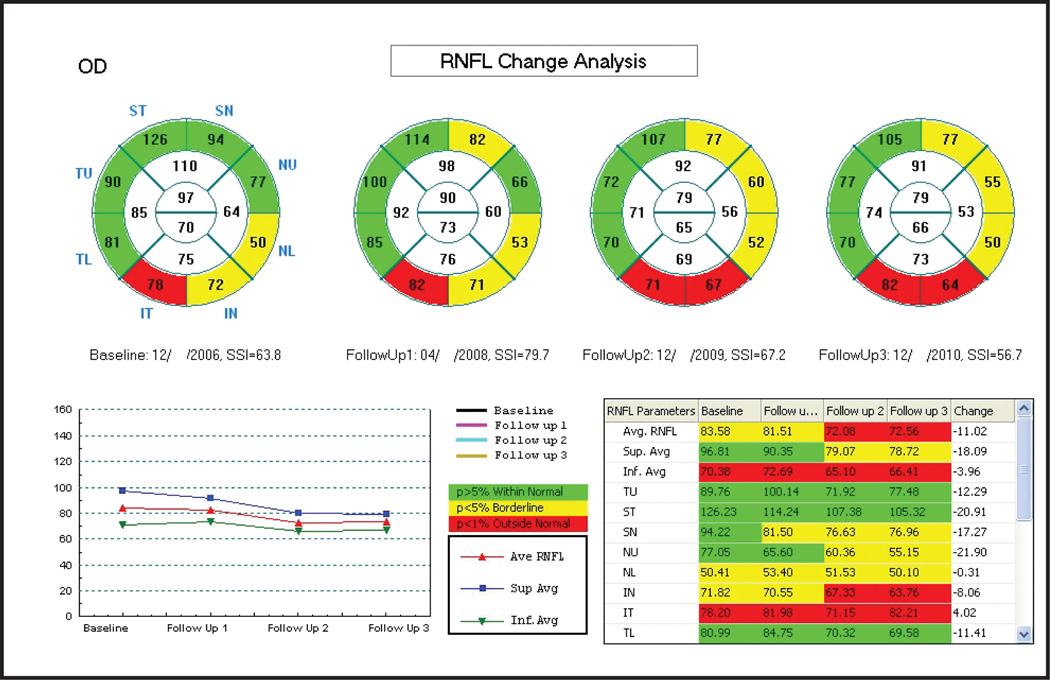
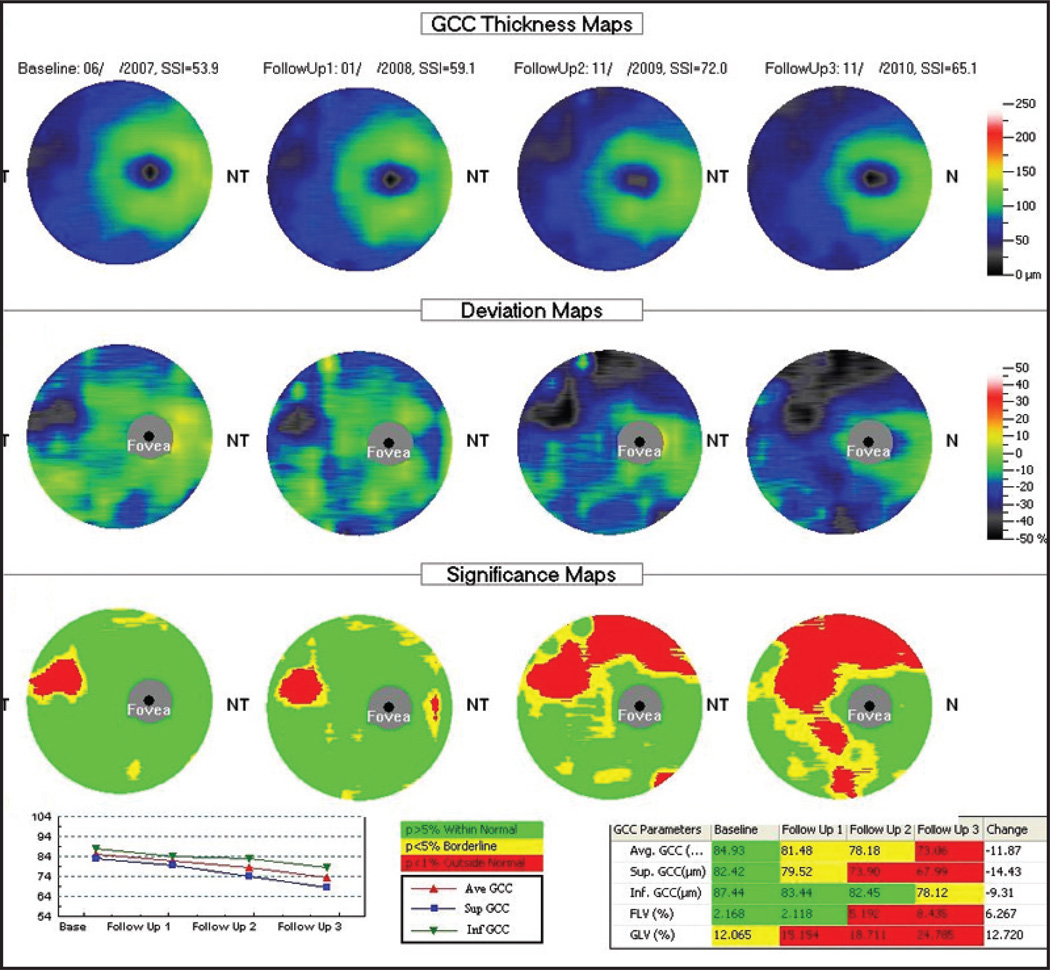
The progression analysis offered by the RTVue includes side-by-side RNFL thickness measurements and overlay of the RNFL profiles for the consecutive scans. Similar reports are also provided for ganglion cell complex thickness along with thickness change plots. Changes are presented in a similar fashion for the ganglion cell complex of the macula. However, a formal statistical analysis of change over time is not currently included in the latest version of the software for this device (version 6.1). Figure 2 shows an example of the RNFL Change Analysis report showing nasal expansion of peripapillary RNFL defect. Figure 3 is an example of the ganglion cell complex progression analysis report with notable expansion of superior macular defect.
Figure 2.
RTVue Spectral-Domain Optical Coherence Tomography Retinal Nerve Fiber Layer (RNFL) Change Analysis (Optovue, Fremont, CA). RNFL sectoral thickness measurements in comparison with normative data indicate nasal expansion of an inferior RNFL defect. Thickness plot (bottom left) shows progressive decreases in average (red), superior (blue) and inferior (green) RNFL thicknesses over time.
Figure 3.
RTVue Spectral-Domain Optical Coherence Tomography Ganglion Cell Complex (GCC) Progression Analysis (Optovue, Fremont, CA). Deviation maps show a focal damage to the GCC in the temporal region progressively expanding superiorly along with a new localized damage in the inferior region of the macula. Significance maps in comparison with a normative database reveal a gradually increasing area of damaged GCC. Thickness plot (bottom left) shows progressive decreases in average (red), inferior (green), and superior (blue) GCC thicknesses over time.
FUTURE DEVELOPMENTS
Several studies are currently underway assessing longitudinal RNFL thickness and segmented macular thickness data using SD-OCT. More sophisticated approaches for optic disc and macular evaluation are also under development. New retinal segmentation algorithms are being developed and tested by those SDOCT manufacturers who currently do not offer such a capability in their software. These algorithms should soon be incorporated in the future software releases.
The OCT imaging technology continues to rapidly evolve. Research is focused on developing devices with higher scanning speeds (such as swept-source OCT), higher lateral resolution (OCT with adaptive optics), and devices that account for the optical polarization properties of the scanning area (polarization-sensitive OCT). These technologies will offer further improvements in resolution and scanning speeds, which should lead to improved measurement reliability and might improve the chances of detecting longitudinal changes.
Future studies will also evaluate the effect of potential confounding factors such as cataract formation and cataract surgery. It has been shown that both TD-OCT and SD-OCT RNFL and retinal thickness measurements are affected by media opacities, but the effect on the ability of OCT to detect glaucoma progression remains to be determined.53–56
CONCLUSION
Detection of glaucoma progression remains one of the most challenging aspects in the management of the disease. This is due to the slowly progressive and variable nature of the disease, the inherent measurement variability of devices used in serial assessments, and the lack of a commonly acceptable reference standard that can be used to definitively indicate progressive glaucomatous change. To date, only a few studies on the application of OCT in detecting glaucoma progression have been published, all of them on TD-OCT. These studies demonstrated that TD-OCT is a sensitive measure of glaucoma progression. Analyzing both overall average and sectoral RNFL thicknesses is important in maximizing the detection of progression. SD-OCT with its increased resolution, image registration capabilities, higher reproducibility, and three-dimensional rendering capabilities offers potential advantages over TD-OCT. Its application in the detection of glaucoma progression is currently being evaluated. The limited agreement between functional and structural tests emphasizes the importance of assessing both structure and function when making clinical decisions regarding glaucoma progression.
Acknowledgments
Supported in part by National Institute of Health grants R01-EY13178, and P30-EY08098 (Bethesda, MD), The Eye and Ear Foundation (Pittsburgh, PA), and an unrestricted grant from Research to Prevent Blindness (New York, NY).
Dr. Wollstein received research funding from Carl Zeiss Meditec and Optovue. Dr. Schuman received royalties for intellectual property licensed by the Massachusetts Institute of Technology to Carl Zeiss Meditec. The remaining authors have no financial or proprietary interest in the materials presented herein.
Dr. Schuman did not participate in the editorial review of this manuscript.
REFERENCES
- 1.American Academy of Ophthalmology. Preferred Practice Pattern: Primary Open-Angle Glaucoma. San Francisco: Author; 2003. pp. 1–40. [Google Scholar]
- 2.Johnson CA, Samuels SJ. Screening for glaucomatous visual field loss with frequency-doubling perimetry. Invest Ophthalmol Vis Sci. 1997;38:413–425. [PubMed] [Google Scholar]
- 3.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 4.Quigley HA, Katz J, Derick RJ, Gilbert D, Sommer A. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology. 1992;99:19–28. doi: 10.1016/s0161-6420(92)32018-4. [DOI] [PubMed] [Google Scholar]
- 5.Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123:464–470. doi: 10.1001/archopht.123.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strouthidis NG, Scott A, Peter NM, Garway-Heath DF. Optic disc and visual field progression in ocular hypertensive subjects: detection rates, specificity, and agreement. Invest Ophthalmol Vis Sci. 2006;47:2904–2910. doi: 10.1167/iovs.05-1584. [DOI] [PubMed] [Google Scholar]
- 7.Medeiros FA, Zangwill LM, Bowd C, Weinreb RN. Comparison of the GDx VCC scanning laser polarimeter, HRT II confocal scanning laser ophthalmoscope, and stratus OCT optical coherence tomograph for the detection of glaucoma. Arch Ophthalmol. 2004;122:827–837. doi: 10.1001/archopht.122.6.827. [DOI] [PubMed] [Google Scholar]
- 8.Wollstein G, Ishikawa H, Wang J, Beaton SA, Schuman JS. Comparison of three optical coherence tomography scanning areas for detection of glaucomatous damage. Am J Ophthalmol. 2005;139:39–43. doi: 10.1016/j.ajo.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Medeiros FA, Zangwill LM, Bowd C, Vessani RM, Susanna R, Jr, Weinreb RN. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol. 2005;139:44–55. doi: 10.1016/j.ajo.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 10.Budenz DL, Michael A, Chang RT, McSoley J, Katz J. Sensitivity and specificity of the StratusOCT for perimetric glaucoma. Ophthalmology. 2005;112:3–9. doi: 10.1016/j.ophtha.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 11.Bowd C, Zangwill LM, Berry CC, et al. Detecting early glaucoma by assessment of retinal nerve fiber layer thickness and visual function. Invest Ophthalmol Vis Sci. 2001;42:1993–2003. [PubMed] [Google Scholar]
- 12.Blumenthal EZ, Williams JM, Weinreb RN, Girkin CA, Berry CC, Zangwill LM. Reproducibility of nerve fiber layer thickness measurements by use of optical coherence tomography. Ophthalmology. 2000;107:2278–2282. doi: 10.1016/s0161-6420(00)00341-9. [DOI] [PubMed] [Google Scholar]
- 13.Budenz DL, Chang RT, Huang X, Knighton RW, Tielsch JM. Reproducibility of retinal nerve fiber thickness measurements using the stratus OCT in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2005;46:2440–2443. doi: 10.1167/iovs.04-1174. [DOI] [PubMed] [Google Scholar]
- 14.Budenz DL, Fredette MJ, Feuer WJ, Anderson DR. Reproducibility of peripapillary retinal nerve fiber thickness measurements with stratus OCT in glaucomatous eyes. Ophthalmology. 2008;115:661–666. doi: 10.1016/j.ophtha.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 15.Paunescu LA, Schuman JS, Price LL, et al. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Invest Ophthalmol Vis Sci. 2004;45:1716–1724. doi: 10.1167/iovs.03-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuman JS, Pedut-Kloizman T, Hertzmark E, et al. Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography. Ophthalmology. 1996;103:1889–1898. doi: 10.1016/s0161-6420(96)30410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurses-Ozden R, Teng C, Vessani R, Zafar S, Liebmann JM, Ritch R. Macular and retinal nerve fiber layer thickness measurement reproducibility using optical coherence tomography (OCT-3) J Glaucoma. 2004;13:238–244. doi: 10.1097/00061198-200406000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Lee EJ, Kim TW, Park KH, Seong M, Kim H, Kim DM. Ability of Stratus OCT to detect progressive retinal nerve fiber layer atrophy in glaucoma. Invest Ophthalmol Vis Sci. 2009;50:662–668. doi: 10.1167/iovs.08-1682. [DOI] [PubMed] [Google Scholar]
- 19.Budenz DL, Anderson DR, Varma R, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology. 2007;114:1046–1052. doi: 10.1016/j.ophtha.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parikh RS, Parikh SR, Sekhar GC, Prabakaran S, Babu JG, Thomas R. Normal age-related decay of retinal nerve fiber layer thickness. Ophthalmology. 2007;114:921–926. doi: 10.1016/j.ophtha.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Harwerth RS, Wheat JL, Rangaswamy NV. Age-related losses of retinal ganglion cells and axons. Invest Ophthalmol Vis Sci. 2008;49:4437–4443. doi: 10.1167/iovs.08-1753. [DOI] [PubMed] [Google Scholar]
- 22.Lee EJ, Kim TW, Weinreb RN, Park KH, Kim SH, Kim DM. Trend-based analysis of retinal nerve fiber layer thickness measured by optical coherence tomography in eyes with localized nerve fiber layer defects. Invest Ophthalmol Vis Sci. 2011;52:1138–1144. doi: 10.1167/iovs.10-5975. [DOI] [PubMed] [Google Scholar]
- 23.Leung CK, Cheung CY, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a study on optical coherence tomography guided progression analysis. Invest Ophthalmol Vis Sci. 2010;51:217–222. doi: 10.1167/iovs.09-3468. [DOI] [PubMed] [Google Scholar]
- 24.Schlottmann PG, De Cilla S, Greenfield DS, Caprioli J, Garway-Heath DF. Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2004;45:1823–1829. doi: 10.1167/iovs.03-0692. [DOI] [PubMed] [Google Scholar]
- 25.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanamori A, Nakamura M, Escano MF, Seya R, Maeda H, Negi A. Evaluation of the glaucomatous damage on retinal nerve fiber layer thickness measured by optical coherence tomography. Am J Ophthalmol. 2003;135:513–520. doi: 10.1016/s0002-9394(02)02003-2. [DOI] [PubMed] [Google Scholar]
- 27.Leung CK, Cheung CY, Lin D, Pang CP, Lam DS, Weinreb RN. Longitudinal variability of optic disc and retinal nerve fiber layer measurements. Invest Ophthalmol Vis Sci. 2008;49:4886–4892. doi: 10.1167/iovs.07-1187. [DOI] [PubMed] [Google Scholar]
- 28.Medeiros FA, Zangwill LM, Alencar LM, et al. Detection of glaucoma progression with stratus OCT retinal nerve fiber layer, optic nerve head, and macular thickness measurements. Invest Ophthalmol Vis Sci. 2009;50:5741–5748. doi: 10.1167/iovs.09-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guedes V, Schuman JS, Hertzmark E, et al. Optical coherence tomography measurement of macular and nerve fiber layer thickness in normal and glaucomatous human eyes. Ophthalmology. 2003;110:177–189. doi: 10.1016/s0161-6420(02)01564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung CK, Chan WM, Yung WH, et al. Comparison of macular and peripapillary measurements for the detection of glaucoma: an optical coherence tomography study. Ophthalmology. 2005;112:391–400. doi: 10.1016/j.ophtha.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Ojima T, Tanabe T, Hangai M, Yu S, Morishita S, Yoshimura N. Measurement of retinal nerve fiber layer thickness and macular volume for glaucoma detection using optical coherence tomography. Jpn J Ophthalmol. 2007;51:197–203. doi: 10.1007/s10384-006-0433-y. [DOI] [PubMed] [Google Scholar]
- 32.Garas A, Vargha P, Hollo G. Reproducibility of retinal nerve fiber layer and macular thickness measurement with the RTVue-100 optical coherence tomograph. Ophthalmology. 2010;117:738–746. doi: 10.1016/j.ophtha.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Garcia AO, Vizzeri G, Bowd C, Medeiros FA, Zangwill LM, Weinreb RN. Reproducibility of RTVue retinal nerve fiber layer thickness and optic disc measurements and agreement with Stratus optical coherence tomography measurements. Am J Ophthalmol. 2009;147:1067–1074. doi: 10.1016/j.ajo.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SH, Kim SH, Kim TW, Park KH, Kim DM. Reproducibility of retinal nerve fiber thickness measurements using the test-retest function of spectral OCT/SLO in normal and glaucomatous eyes. J Glaucoma. 2010;19:637–642. doi: 10.1097/IJG.0b013e3181ca7cbe. [DOI] [PubMed] [Google Scholar]
- 35.Li JP, Wang XZ, Fu J, Li SN, Wang NL. Reproducibility of RTVue retinal nerve fiber layer thickness and optic nerve head measurements in normal and glaucoma eyes. Chin Med J (Engl) 2010;123:1898–1903. [PubMed] [Google Scholar]
- 36.Mwanza JC, Chang RT, Budenz DL, et al. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with CirrusTM HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2010;51:5724–5730. doi: 10.1167/iovs.10-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menke MN, Knecht P, Sturm V, Dabov S, Funk J. Reproducibility of nerve fiber layer thickness measurements using 3D fourier-domain OCT. Invest Ophthalmol Vis Sci. 2008;49:5386–5391. doi: 10.1167/iovs.07-1435. [DOI] [PubMed] [Google Scholar]
- 38.Schuman JS. Spectral domain optical coherence tomography for glaucoma (an AOS thesis) Trans Am Ophthalmol Soc. 2008;106:426–458. [PMC free article] [PubMed] [Google Scholar]
- 39.Leung CK, Cheung CY, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology. 2009;116:1257–1263. doi: 10.1016/j.ophtha.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Kim JS, Ishikawa H, Sung KR, et al. Retinal nerve fibre layer thickness measurement reproducibility improved with spectral domain optical coherence tomography. Br J Ophthalmol. 2009;93:1057–1063. doi: 10.1136/bjo.2009.157875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park SB, Sung KR, Kang SY, Kim KR, Kook MS. Comparison of glaucoma diagnostic capabilities of Cirrus HD and Stratus optical coherence tomography. Arch Ophthalmol. 2009;127:1603–1609. doi: 10.1001/archophthalmol.2009.296. [DOI] [PubMed] [Google Scholar]
- 42.Chang RT, Knight OJ, Feuer WJ, Budenz DL. Sensitivity and specificity of time-domain versus spectral-domain optical coherence tomography in diagnosing early to moderate glaucoma. Ophthalmology. 2009;116:2294–2299. doi: 10.1016/j.ophtha.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 43.Vizzeri G, Balasubramanian M, Bowd C, Weinreb RN, Medeiros FA, Zangwill LM. Spectral domain-optical coherence tomography to detect localized retinal nerve fiber layer defects in glaucomatous eyes. Opt Express. 2009;17:4004–4018. doi: 10.1364/oe.17.004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreno-Montanes J, Olmo N, Alvarez A, Garcia N, Zarranz-Ventura J. Cirrus high-definition optical coherence tomography compared with Stratus optical coherence tomography in glaucoma diagnosis. Invest Ophthalmol Vis Sci. 2010;51:335–343. doi: 10.1167/iovs.08-2988. [DOI] [PubMed] [Google Scholar]
- 45.Cho JW, Sung KR, Hong JT, Um TW, Kang SY, Kook MS. Detection of glaucoma by spectral domain-scanning laser ophthalmoscopy/optical coherence tomography (SD-SLO/OCT) and time domain optical coherence tomography. J Glaucoma. 2011;20:15–20. doi: 10.1097/IJG.0b013e3181d1d332. [DOI] [PubMed] [Google Scholar]
- 46.Sehi M, Grewal DS, Sheets CW, Greenfield DS. Diagnostic ability of Fourier-domain vs time-domain optical coherence tomography for glaucoma detection. Am J Ophthalmol. 2009;148:597–605. doi: 10.1016/j.ajo.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeoung JW, Park KH. Comparison of Cirrus OCT and Stratus OCT on the ability to detect localized retinal nerve fiber layer defects in preperimetric glaucoma. Invest Ophthalmol Vis Sci. 2010;51:938–945. doi: 10.1167/iovs.08-3335. [DOI] [PubMed] [Google Scholar]
- 48.Garas A, Vargha P, Hollo G. Diagnostic accuracy of nerve fibre layer, macular thickness and optic disc measurements made with the RTVue-100 optical coherence tomograph to detect glaucoma. Eye (Lond) 2011;25:57–65. doi: 10.1038/eye.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim NR, Lee ES, Seong GJ, et al. Comparing the ganglion cell complex and retinal nerve fibre layer measurements by Fourier domain OCT to detect glaucoma in high myopia. Br J Ophthalmol. doi: 10.1136/bjo.2010.182493. [published online ahead of print October 17, 2010] [DOI] [PubMed] [Google Scholar]
- 50.Rao HL, Zangwill LM, Weinreb RN, Sample PA, Alencar LM, Medeiros FA. Comparison of different spectral domain optical coherence tomography scanning areas for glaucoma diagnosis. Ophthalmology. 2010;117:1692–1699. doi: 10.1016/j.ophtha.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 51.Tan O, Chopra V, Lu AT, et al. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology. 2009;116:2305–2314. doi: 10.1016/j.ophtha.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan O, Li G, Lu AT, Varma R, Huang D. Mapping of macular substructures with optical coherence tomography for glaucoma diagnosis. Ophthalmology. 2008;115:949–956. doi: 10.1016/j.ophtha.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Velthoven ME, van der Linden MH, de Smet MD, Faber DJ, Verbraak FD. Influence of cataract on optical coherence tomography image quality and retinal thickness. Br J Ophthalmol. 2006;90:1259–1262. doi: 10.1136/bjo.2004.097022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savini G, Zanini M, Barboni P. Influence of pupil size and cataract on retinal nerve fiber layer thickness measurements by Stratus OCT. J Glaucoma. 2006;15:336–340. doi: 10.1097/01.ijg.0000212244.64584.c2. [DOI] [PubMed] [Google Scholar]
- 55.Cheng CS, Natividad MG, Earnest A, et al. Comparison of the influence of cataract and pupil size on retinal nerve fibre layer thickness measurements with time domain and spectral domain optical coherence tomography. Clin Experiment Ophthalmol. doi: 10.1111/j.1442-9071.2010.02460.x. [published online ahead of print November 11, 2010] [DOI] [PubMed] [Google Scholar]
- 56.Mwanza JC, Bhorade AM, Sekhon N, et al. Effect of cataract and its removal on signal strength and peripapillary retinal nerve fiber layer optical coherence tomography measurements. J Glaucoma. 2011;20:37–43. doi: 10.1097/IJG.0b013e3181ccb93b. [DOI] [PubMed] [Google Scholar]