Abstract
In the United States, breast cancer affects one in eight women, with mortality that is second only to lung cancer. Although chemotherapy is widely used in breast cancer treatment, its side effects remain a challenge. One way to address this problem is through drug delivery by the internalization of cell type-specific probes. Although nucleic acid aptamers are excellent probes for molecular recognition, only a few reports have demonstrated that aptamers can be internalized into living cells. Therefore, in this work, we report the development of a cancer cell-specific DNA aptamer probe, KMF2-1a. Using the cell-SELEX method, this aptamer was selected against breast cancer cell line MCF-10AT1. Our results showed that KMF2-1a was internalized efficiently and specifically to the endosome of target breast cancer cells. These results indicate that KMF2-1a is a promising agent for cell type-specific intracellular delivery with both diagnostic and therapeutic implications.
Keywords: aptamer, breast cancer, internalization, MCF-10AT1
Introduction
Breast cancer is by far the most frequently diagnosed cancer among women, with an estimated 1.38 million new cancer cases in 2008 (23% of all cancers) and 458,503 deaths worldwide (13.7% of cancer mortality in women). It is now the most common cancer with an estimated 690,000 new cases in both developed and developing countries.[1]
Among the newest tools in the fight against cancer, aptamers have gained increasing research interest. Aptamers are single-stranded oligonucleic acids or peptides that bind to specific targets with high affinity and specificity. Aptamers are derived from an iterative process called SELEX (Systematic Evolution of Ligands by Exponential enrichment).[2,3] Compared with the antibody approach, aptamers have high affinity, excellent specificity and low toxicity. Moreover, they are stable and easy to synthesize, modify and manipulate.[4–7] Although aptamers have been very effective in vitro, most aptamers cannot be directly taken up by cells without external assistance.[8] Up to now, only a few aptamers have been reported to be successfully internalized by cells, including anti-PSMA, targeting PSMA in prostate cancer cells, and Sgc8, targeting PTK7 in acute leukemia cells.[9–11] Obviously, internalization ability is very important for the application of aptamers in vivo, especially in targeted drug delivery.
A key challenge confronting aptamer internalization is the limited number of aptamer candidates available for cell-specific recognition. Our research group has developed the cell-SELEX method to generate a panel of aptamers against several types of cancer cells, including lymphocytic leukemia, myeloid leukemia, liver cancer, small cell lung cancer, ovarian cancer and colon cancer.[12–15] These aptamers were selected using whole intact cells as targets, while a related cell line was used as a negative control. This method enables the generation of multiple aptamers for molecular recognition of the target cells, and these aptamers could be used as excellent molecular recognition probes.
We recently used the cell-SELEX method to generate molecular probes for specific recognition of breast cancer cells. Two cell lines were used: breast cancer cell line MCF-10AT1, as target cells, and a non-cancer cell line, MCF-10A1, as negative control.[16,17] We have now identified a panel of aptamers (KMF2-1a, KMF3, and KMF9b) that specifically recognize the cancer cell line MCF-10AT1 with Kd’s in the nanomolar range. One of these aptamers, KMF2-1a, binds specifically to target cells, and its binding ability was shown to be very stable in our study. Furthermore, this aptamer can bind to target cells at both 4°C and 37°C, extending itsuse for invivo experimentation.
In this paper, we present the results ofan internalization study of dye-labeled KMF2-1a by flow cytometry and confocal imaging. The results show that this aptamer can be specifically internalized to target breast cancer cells. Thus, it is anticipated that aptamer KMF2-1a will be a suitable candidate for further study in targeted drug delivery, both in vitro and in vivo.
Results and Discussion
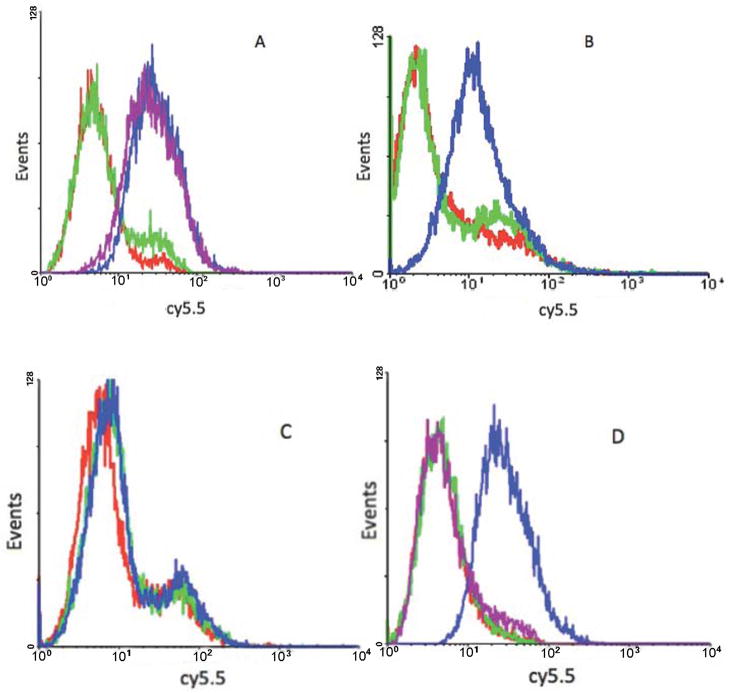
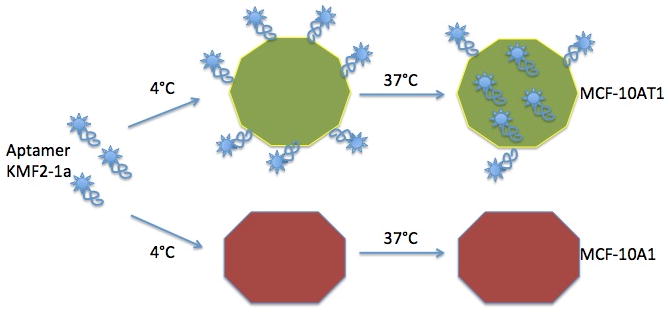
We first tested the binding ability of aptamer KMF2-1a under different time and temperature conditions. As shown in Figure 1a, after incubation with aptamer KMF2-1a for 30 mins at 4°C, MCF-10AT1 cells were washed twice with washing buffer to remove the free aptamers, and analyzed by flow cytometry. After further incubation at 4°C for 2h in binding buffer without aptamer, flow cytometry confirmed identical results, indicating that the time elapsed after removal of the aptamer did not affect the binding ability. It is well known that the secondary structure of DNA aptamers can change under different temperatures, explaining why some aptamers have lost their binding ability at 37°C. Therefore, aptamer KMF2-1a was incubated with the target cell line at 37°C, and it showed binding ability similar that at 4°C (Figure 1b), suggesting that KMF2-1a has potential for in vivo studies. Importantly, MCF-10A1 cells, which were the negative control, could not bind to KMF2-1a at 4°C, indicating that KMF2-1a could specifically bind to target cells (Figure 1c).
Figure 1. Flow cytometry results of aptamer binding with MCF-10AT1 cells under different conditions.
All the aptamers were labeled with biotin. A: KMF2-1a shows stable binding to target cells at 4°C. Red line: MCF-10AT1 cells. Green line: library aptamers as negative control. Blue line: 30mins after KMF2-1a was incubated with target cells at 4°C. Purple line: 2h after KMF2-1a was incubated with target cells at 4°C. B: KMF2-1a can still bind to target cells at 37°C. Red line: MCF-10AT1 cells. Green line: library aptamers as negative control. Blue line: 30mins after KMF2-1a was incubated with target cells at 37°C. C: Negative control MCF-10A1 cells could not bind with KMF2-1a. Red line: MCF-10A1 cells. Green line: library aptamers. Blue line: MCF-10A1 cells incubated with KMF2-1a at 4°C for 30mins. D: Trypsin removed specific binding of KMF2-1a. Red line: MCF-10AT1 cells. Green line: library (negative control) incubated with target cells without trypsin treatment at 4°C. Blue line: KMF2-1a incubated with target cells without trypsin treatment at 4°C. Purple line: KMF2-1a-target cell incubation after trypsin treatment at 4°C.
To exclude interference signals from outside the cell, the confirmation of aptamer internalization requires that aptamers bound to the cell surface be removed. In previous studies with aptamer sgc8, our group found that trypsin treatment is an easy method to achievethis goal.[18]Therefore, before flow cytometry and prior to the internalization study, trypsin was used to remove cell surface-bound aptamers. We found that trypsin treatment could also successfully eliminatespecific binding of KMF2-1a with target cells. Thus, at 4°C, the fluorescence signal which resulted from the binding of KMF2-1a-Biotin to the membrane of MCF-10AT1 cells was entirely eliminated after trypsin treatment (Figure 1d). Based on this finding, two conclusions can be drawn. First, the removal of specific binding of KMF2-1a from target cell surfaces by trypsin treatment indicates that the molecular target of this aptamer may be membrane protein(s). Second, once surface-bound aptamers have been removed, the remaining fluorescence signals should arise only from aptamers that have been taken up, i.e., internalized, by the target cell.
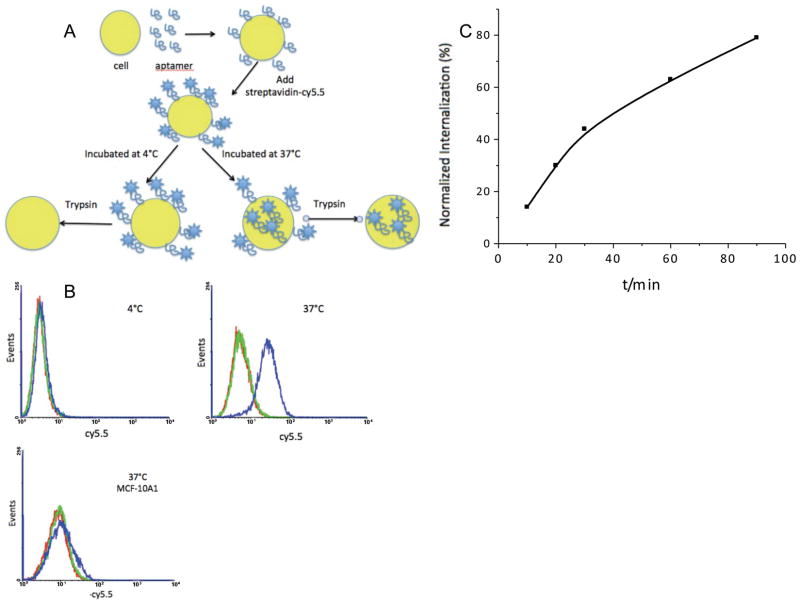
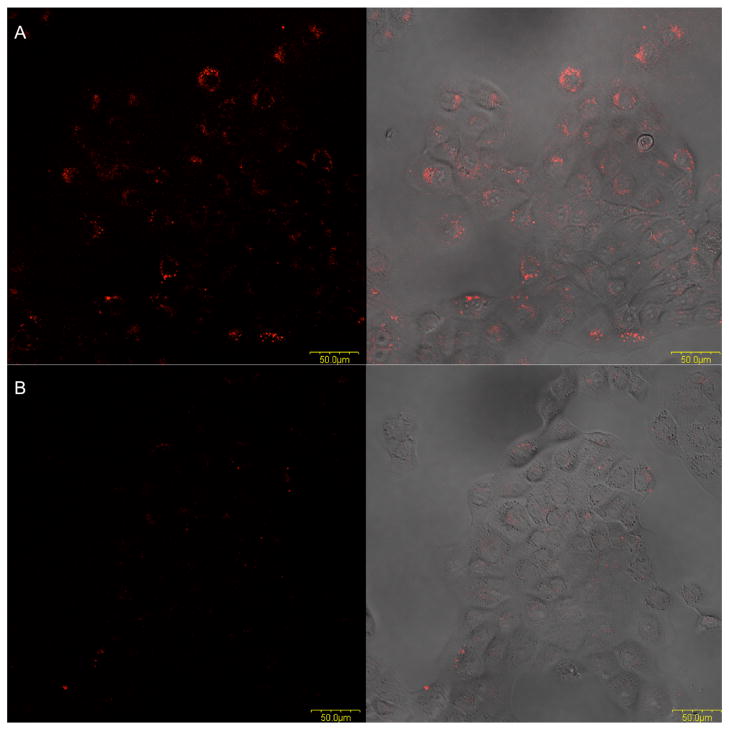
The internalization assay is shown schematically in Figure 2a. MCF-10AT1 is an adherent cell line. Before starting the internalization study, we split cells into a 6-well plate and cultured for another 24h to ensure that cell membrane proteins had recovered to a normal level. The aptamer internalization study was carried out at 4°C and 37°C. The flow cytometry results confirmed aptamer internalization. Even though the cells had been treated with trypsin after aptamer application at 37°C, we could still detect the shift signal from the 37°C group, but not the 4°C group, after incubation for 2h with aptamer KMF2-1a. Since trypsin had removed all the cell surface-bound aptamers, the signal detected from the 37°C group must have originated from aptamers inside the cell. Similar to KMF2-1a internalized by MCF-10AT1 assay, we used MCF-10A1 cells as a negative control, and no signal was detected from the 37°C group. Thus, we concluded that KMF2-1a could be internalized specifically into target MCF-10AT1 cells. We used confocal microscopy to further confirm aptamer internalization. As shown in Figure 3, after 2h incubation with KMF2-1a-TAMRA, we observed fluorescence signals from inside the MCF-10AT1 cells. On the other hand, no fluorescence was observed inside the cells incubated with Lib-TAMRA, and no fluorescence shift was observed from Lib-BIOTIN in flow cytometry under the same conditions as KMF2-1a-TAMRA/KMF2-1a-BIOTIN. These results provided strong evidence that KMF2-1a had been internalized by the MCF-10AT1 cells. We then studied the time dependence of internalization by flow cytometry. The intensity at each time interval was normalized to the fluorescence signal from the cell surface at 4°C. As shown in Figure 2c, the internalization of KMF2-1a into the cells was constantly increasing during the 90-min monitoring period.
Figure 2. Internalization study showed that KMF2-1a can be internalized specifically into target MCF-10AT1 cells.
A: Schematic of internalization assay. MCF-10AT1 cells were split into 6-well plate. After binding with KMF2-1a-Biotin at 4°C, streptavidin-cy5.5 dye was added. After washing away all the free aptamers and dye and adding medium, one group was incubated at 4°C for 2h and another group was incubated at 37°C for 2h. Trypsin was used to detach and treat cells. B: Internalization of KMF2-1a aptamer. Red line: MCF-10AT1 cells. Green line: library aptamers (negative control) were not internalized. Blue line: Trypsin was applied to remove the membrane-bound aptamers; fluorescence shift existed with KMF2-1a incubated cells at 37°C, but not at 4°C. However, KMF2-1a could not bind to negative control cell MCF-10A1 and could not be internalized by MCF-10A1 cells. C: Quantitative analysis of KMF2-1a internalization with time. The fluorescence intensities of cells that had internalized aptamers were normalized to those of cells at 4°C. Flow cytometry data showed increasing fluorescence intensity inside target cells, indicating increasing internalization of aptamers.
Figure 3. Internalization study of KMF2-1a-TAMRA into MCF-10AT1 cells by confocal microscopy.
(Left: fluorescence signal; Right: overlay of transmitted and fluorescence signal.) A: fluorescence signals were seen from inside the cells after incubation of KMF2-1a-TAMRA with cells for 2 hours at 37°C. B: Lib-TAMRA could not be internalized into cells at 37°C, even after an incubation of two hours.
In our internalization study, streptavidin-cy5.5 was used to monitor aptamer behavior. Streptavidin is a 52,800 Dalton tetrameric protein purified from the bacterium Streptomyces avidinii, and it can bind to biotin with an extraordinarily high affinity.[19] Our results showed that the entirecomplex of streptavidin and biotin-labeled KMF2-1a could be delivered into target breast cancer cells. This advantage could be further used for targeted delivery of large molecules. Therefore, KMF2-1a may have the potential for aptamer-based targeted drug delivery in breast cancer theragnosis. One aptamer that has already been successfully used for targeted delivery is the anti-PSMA aptamer, which specifically binds to prostate cancer cells. This aptamer has been used to deliver chemotherapy and other cytotoxic agents, including gelonin, a ribosomal toxin that causes cell death by disrupting protein synthesis,[20]and a quantum dot-doxorubicin conjugate.[21] Doxorubicin is the most used drug in breast cancer chemotherapy, but it can cause many side effects. To overcome this limitation, we have future interest in conjugating KMF2-1a with doxorubicin for breast cancer targeted chemotherapy.
We were also very interested in the fate of KMF2-1a aptamer inside the target cells. By using Alexa633-labeled transferrin as a tracker, it has been shown that aptamer Sgc8 was taken up by endosomes in cells.[11] We also used this method to investigate the fate of the KMF2-1a aptamer inside breast cancer cells.
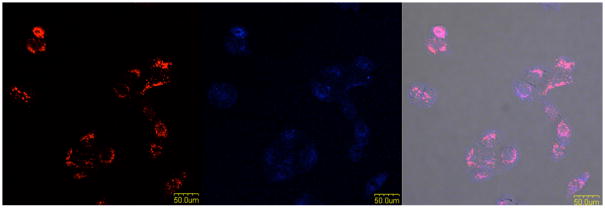
It has been reported that transferrin can be taken up by cells via receptor-mediated endocytosis, and Alexa633-labeled transferrin is commonly used to localize in the endosome.[22] Thus, co-localization of the aptamer with transferrin would indicate that most internalized KMF2-1a aptamers have been taken up by the process of endocytosis (Figure 4). In the context of drug delivery, the endosomal compartment is usually acidic, and many internalized extracellular molecules change their structures at low pH to release their bound substance. As such, internalized aptamers could potentially respond to these conditions to release their drug payloads. For example, one such acid-labile linkage was used by Yufen et al. for the sgc8c-Dox conjugate. This aptamer-drug conjugate was cleaved inside the acidic endosomal environment, resulting in Dox release.[23]
Figure 4. Co-localization of KMF2-1a and transferrin in endosomes.
From left to right: Images for KMF2-1a-TAMRA, transferrin-Alexa633, overlay of the fluorescence channels and bright-field channel.
Conclusion
In conclusion, we have shown that a cell-specific aptamer, KMF2-1a, which was generated against breast cancer cell line MCF-10AT1 by cell-SELEX, was specifically internalized into the target cells without external assistance and delivered to the endosome. This aptamer also delivered large molecules (streptavidin) into cells. As a molecular recognition probe, KMF2-1a can be conjugated with other agents of interest, such as chemotherapeutic drugs or siRNA, for specific intracellular delivery. This aptamer is anticipated to be widely used in breast cancer research.
Experimental Section
Cell lines and regents
The MCF-10AT1/MCF-10A1 cell lines were maintained in DMEM-F12 medium (ATCC) supplemented with 10μg/mL insulin, 0.5μg/mL hydrocortisone, 20ng/mL epidermal growth factor (EGF), 100ng/mL cholera enterotoxin, 5% horse serum and 20U/mL penicillin-streptomycin. Cells were washed after harvesting by using Non-enzymatic Cell Dissociation Solution (1×) (Sigma) with the washing buffer (4.5g/L glucose and 5mM MgCl2 in Dulbecco’s phosphate buffered saline with calcium chloride and magnesium chloride (Sigma)). The binding buffer used for aptamer selection was prepared by adding yeast tRNA (0.1 mg/mL) (Sigma) and BSA (1 mg/mL) (Fisher) to the washing buffer to reduce nonspecific binding.
DNA synthesis and labeling
All DNA synthesis reagents were from Glen Research (Sterling, VA). KMF2-1a: 5’-AGG CGG CAG TGT CAG AGT GAA TAG GGG ATG TAC AGG TCT GCA CCC ACT CGA GGA GTG ACT GAG CGA CGA AGA CCC C-3’. Library aptamers: 5’-ATG AGA GCG TCG GTG TGG TA-N40-TAC TTC CGC ACC CTC CTA CA-3’. All probes and DNAs were synthesized with an ABI3400 DNA/RNA synthesizer (Applied Biosystems, Foster City, CA). TAMRA, Biotin and CPG phosphoramidites were used for modification on aptamer KMF2-1a in theDNA synthesizer. HPLC purification was performed on a ProStar HPLC Station equipped with fluorescence and a photodioarray detector. A C-18 reverse phase column was used with acetonitrile-TEAA as the mobile phase.
Trypsin treatment of cells
Cells were first washed with washing buffer (500μL) to remove the FBS (Fetal Bovine Serum) in the binding buffer, which might quench the function of trypsin, and then incubated with Trypsin (Fisher Biotech, 500μL, 0.05%)/EDTA (0.53mM) in HBSS at 37°C for 15mins. After incubation, 50μL FBS was added, the cells were washed with washing buffer (500μL) once again, and suspended in binding buffer (200μL with 0.1% NaN3) for detection.
FACS-based internalization assay
Flow cytometry analysis was performed on a FACScan (Becton Dickinson Immunocytometry Systems, San Jose, CA) by counting 20,000 events, and the data were analyzed with WinMDI software. For the KMF2-1a-Biotin binding experiment, cells were detached by using Non-enzymatic Cell Dissociation Solution. Cells were first washed twice with washing buffer (500μL) and then suspended in binding buffer (200μL, with 0.1% NaN3). Next, aptamer KMF2-1a-Biotin and Lib-Biotin (200nM) were added for 30mins incubation at 4°C and 37°C. After incubation, cells were washed twice with washing buffer (500μL); then dye streptavidin-cy5.5 was added in binding buffer (200μL) for another 15mins of incubation at 4°C. Before the internalization study was carried out, 1×106 cells were split into a 6-well plate for a 24-h cell culture to ensure that cell membrane proteins recovered to normal level. Cells were separated into two groups,for experiments at 4°C and 37°C, respectively. After binding both groups of cells with KMF2-1a at 4°C, free aptamers and dye were washed away, and then fresh medium was added. The 4°C cell group was incubated at 4°C for 2h in medium, and the 37°C cell group was incubated for 10, 20, 30mins, 1, 1.5, and 2h in medium. Internalization was stopped by placing cells on ice. In accordance with the trypsin (Fisher Biotech) treatment method described above, the two cell groups were analyzed by flow cytometry. All experiments were repeated three times.
Confocal imaging
All cellular fluorescent images were collected on the FV500-IX81 confocal microscope (Olympus America Inc., Melville, NY). Excitation wavelength and emission filters were as follows: TAMRA, 543nm laser line excitation, BP580±20nm emission filter; Alexa633, 633nm laser line excitation, LP650 emissionfilter. Cells ware split into poly-D-lysine-coated 35mm glass bottom dishes (Mat Tek Corp.) for a 24-h cell culture. Cells were washed with washing buffer and then incubated with KMF2-1a-TAMRA (200nM) in medium at 37°C for 2h. For the co-localization experiment, transferrin-Alexa633 (60nM) was added 30 minutes before the termination of incubation. Experiments were repeated three times and analyzed using the Fluoview analysis software.
Scheme 1.
Acknowledgments
Zhi Zhu, Da Han, Lu Peng, Mingxu You are acknowledged for technical assistance.This work was supported by the National Key Scientific Program of China (2011CB911001, 2011CB911003) and by grants awarded by the National Institutes of Health (GM066137, GM079359 and CA133086). We also acknowledge the Interdisciplinary Center for Biotechnology Research (ICBR) at the University of Florida.
Abbreviations
- SELEX
Systematic Evolution of Ligands by Exponential Enrichment
- PSMA
Prostate Specific Membrane Antigen
- PTK7
Protein Tyrosine Kinase 7
- DMEM
Dulbecco’s Modified Eagle Medium
- TAMRA
Tetramethy1-6-Carboxyrhodamine
- FBS
fetal Bovine Serum
- BSA
Bovine Serum Albumin
Footnotes
Authors’ contributions
Kejing Zhang performed laboratory experiments, data analysis and participated in the experimental design and drafting of the manuscript. Kwame Sefah, Zilong Zhaoand Guizhi Zhu participated in flow cytometry and confocal imaging experiments and interpretation of the results. Lili Tang contributed to the interpretation of the data and critical revision of the manuscript. Weijia Sun, Steve Goodison, and Weihong Tan participated in the design and coordination of the research, performed the statistical analysis and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Kejing Zhang, Email: kejingfl@gmail.com.
Prof. Lili Tang, Email: tlli77@medmail.com.cn.
Dr. Kwame Sefah, Email: skwame@ufl.edu.
Dr. Zilong Zhao, Email: zlzhao1002@hotmail.com.
Guizhi Zhu, Email: gzzhujoy@ufl.edu.
Prof. Weijia Sun, Email: sunweijia2009@126.com.
Prof. Steve Goodison, Email: Steven.Goodison@orlandohealth.com.
Prof. Weihong Tan, Email: tan@chem.ufl.edu.
References
- 1.Breast cancer incidence and mortality worldwide in 2008 summary. [ http://globocan.iarc.fr/factsheets/cancers/breast.asp]
- 2.Ellington AD, Szostak JW. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 3.Tuerk C, Gold L. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 4.Yang CJ, Jockusch S, Vicens M, Turro NJ, Tan W. Proc Nati Acad Sci USA. 2005;102:17278–17283. doi: 10.1073/pnas.0508821102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nutiu R, Li Y. Angew Chem Int ED Engl. 2005;44:1061–1065. doi: 10.1002/anie.200461848. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Lu Y. Angew Chem Int Ed Engl. 2005;45:90–94. doi: 10.1002/anie.200502589. [DOI] [PubMed] [Google Scholar]
- 7.Shangguan D, Tang Z, Mallikaratchy P, Xiao Z, Tan W. Chembiochem. 2007;8:603–606. doi: 10.1002/cbic.200600532. [DOI] [PubMed] [Google Scholar]
- 8.Rimmele M. Chembiochem. 2003;4:963–971. doi: 10.1002/cbic.200300648. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Rajasekaran AK, Moy P, Xia Y, Kim S, Navarro V, Rahmati R, Bander NH. Cancer Res. 1998;58:4055–4060. [PubMed] [Google Scholar]
- 10.Lupold SE, Hicke BJ, Lin Y, Coffey DS. Cancer Res. 2002;62:4029–4033. [PubMed] [Google Scholar]
- 11.Xiao Z, Shangguan D, Cao Z, Fang X, Tan W. Chemistry. 2008;14:1769–1775. doi: 10.1002/chem.200701330. [DOI] [PubMed] [Google Scholar]
- 12.Fang X, Tan W. Acc Chem Res. 2010;43:48–57. doi: 10.1021/ar900101s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sefah K, Tang ZW, Shangguan DH, Chen H, Lopez-colon D, Li Y, Parekh P, Martin J, Phillips JA, Kim YM, Tan WH. Leukemia. 2009;23:235–244. doi: 10.1038/leu.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen HW, Medley CD, Sefah K, Shangguan D, Tang Z, Meng L, Smith JE, Tan W. Chemmedchem. 2008;3:991–1001. doi: 10.1002/cmdc.200800030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Z, Shangguan D, Wang K, Shi H, Sefah K, Mallikratchy P, Chen HW, Li Y, Tan W. Anal Chem. 2007;79:4900–4907. doi: 10.1021/ac070189y. [DOI] [PubMed] [Google Scholar]
- 16.Heppner GH, Wolman SR. Breast J. 1999;5:122–129. doi: 10.1046/j.1524-4741.1999.00136.x. [DOI] [PubMed] [Google Scholar]
- 17.Dawson PJ, Wolman SR, Tait L, Heppner GH, Miller FR. Am J Pathol. 1996;148:313–319. [PMC free article] [PubMed] [Google Scholar]
- 18.Van Simaeys D, López-Colón D, Sefah K, Sutphen R, Jimenez E, Tan W. PLoS One. 2010;5:e13770. doi: 10.1371/journal.pone.0013770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green NM. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- 20.Chu TC, Marks JW, 3rd, Lavery LA, Faulkner S, Rosenblum MG, Ellington AD, Levy M. Cancer Res. 2006;66:5989–5992. doi: 10.1158/0008-5472.CAN-05-4583. [DOI] [PubMed] [Google Scholar]
- 21.Bagalkot V, Zhang L, Levy-Nissenbaum E, Jon S, Kantoff PW, Langer R, Farokhzad OC. Nano Lett. 2007;7:3065–3070. doi: 10.1021/nl071546n. [DOI] [PubMed] [Google Scholar]
- 22.Ciechanover A, Schwartz AL, Dautry-Varsat A, Lodish HF. J Biol Chem. 1983;258:9681–9689. [PubMed] [Google Scholar]
- 23.Huang YH, Shangguan D, Liu H, Phillips JA, Zhang X, Chen Y, Tan W. Chembiochem. 2009;10:862–868. doi: 10.1002/cbic.200800805. [DOI] [PMC free article] [PubMed] [Google Scholar]