Abstract
Serotonin (5-HT)-induced long-term facilitation (LTF) of the Aplysia sensorimotor synapse depends on enhanced gene expression and protein synthesis, but identification of the genes whose expression and regulation are necessary for LTF remains incomplete. In this study, we found that one such gene is synapsin, which encodes a synaptic vesicle-associated protein known to regulate short-term synaptic plasticity. Both synapsin mRNA and protein levels were increased by 5-HT. Upregulation of synapsin protein occurred in presynaptic sensory neurons at neurotransmitter release sites. To investigate the molecular mechanisms underlying synapsin regulation, we cloned the promoter region of Aplysia synapsin, and found that the synapsin promoter contained a cAMP response element (CRE), raising the possibility that the transcriptional activator CRE-binding protein 1 (CREB1) mediates 5-HT-induced regulation of synapsin. Indeed, binding of CREB1 to the synapsin promoter was increased following treatment with 5-HT. Furthermore, increased acetylation of histones H3 and H4 and decreased association of histone deacetylase 5 near the CRE site are consistent with transcriptional activation by CREB1. RNA interference (RNAi) targeting synapsin mRNA blocked the 5-HT-induced increase in synapsin protein levels and LTF; in the absence of 5-HT treatment, basal synapsin levels were unaffected. These results indicate that the 5-HT-induced regulation of synapsin levels is necessary for LTF and that this regulation is part of the cascade of synaptic events involved in the consolidation of memory.
Introduction
The consolidation of long-term memory (LTM) is dependent upon changes in gene transcription and translation. Previous work has focused primarily on the identification and regulation of transcription factors important for LTM (Alberini, 2009), but the identification of effector proteins that participate in the modulation of synaptic transmission necessary for LTM remains incomplete. Accumulating evidence supports a role in learning and memory for synapsin, a protein associated with synaptic vesicles (for review, see Cesca et al., 2010). Synapsins have been implicated in the regulation of neurotransmitter release and synaptic plasticity (Gitler et al., 2004; Fioravante et al., 2007; Cesca et al., 2010) as well as synaptogenesis and neurite outgrowth (Han et al., 1991; Lu et al., 1992). During long-term potentiation in the rat hippocampus, increased synapsin mRNA and protein levels are correlated with persistent enhancement of synaptic transmission (Lynch et al., 1994; Morimoto et al., 1998; Sato et al., 2000), and downregulation of synapsin is observed during long-term depression (Fioravante et al., 2008). Nevertheless, the regulators of synapsin expression during LTM are currently unknown, as is the causal role of synapsin expression in LTM.
To examine these questions, we exploited the technical advantages of the marine mollusc Aplysia. Long-term sensitization (LTS) of the withdrawal reflexes, a simple form of nonassociative memory, is accompanied by long-term synaptic facilitation (LTF) of the connections between sensory neurons (SNs) and motor neurons (MNs) (Frost et al., 1985; Cleary et al., 1998). Both LTS and LTF are associated with the formation of new synaptic connections (Bailey and Chen, 1988, 1989; Glanzman et al., 1990; Wainwright et al., 2002; Kim et al., 2003). Treatment with serotonin (5-HT) mimics behavioral training and induces LTF (Montarolo et al., 1986; Zhang et al., 1997; Mauelshagen et al., 1998; Liu et al., 2008). 5-HT also leads to increased levels of cAMP (Bernier et al., 1982; Ocorr and Byrne, 1985) and cAMP-induced transcriptional regulation through the phosphorylation of the transcriptional activator cAMP response element-binding protein 1 (CREB1) (Dash and Moore, 1996; Bartsch et al., 1998; Liu et al., 2008). Behavioral training also leads to CREB1 up-regulation (R.-Y. Liu and J. H. Byrne, unpublished observations). CREB1-mediated gene induction is necessary for the consolidation of LTF and involves binding of CREB1 to the cAMP response element (CRE) sequence in the promoter region of target genes (Dash et al., 1990; Dash and Moore, 1996; Mohamed et al., 2005; Liu et al., 2008).
In mammals, the synapsin I promoter region includes a CRE (Sauerwald et al., 1990; Südhof, 1990). If the Aplysia synapsin promoter also contains a CRE, it would be a likely target of regulation by CREB1. The subsequent increased expression of synapsin may be part of or may trigger a cascade of events critical for the induction and expression of LTF.
Materials and Methods
Quantitative PCR analysis of synapsin mRNA.
For each measurement of mRNA, pleural ganglia were surgically removed from two anesthetized animals. Ganglia were rinsed with modified artificial sea water (ASW) [L15:ASW, 1:1 (v/v)] and rested at 18°C for 1–2 h. Ganglia from the right side of the first animal and the left side of the second animal were pooled into a single sample. The two remaining ganglia were pooled as well. One pair of ganglia was treated with five 5 min pulses of vehicle (Veh; L15:ASW), and the other with 50 μm 5-HT with an interstimulus interval (ISI) of 20 min. Either immediately or 1, 2, 5, 12, or 24 h following treatment, ganglia were rinsed with L15:ASW, frozen on dry ice, and stored at −80°C for further processing. Total RNA was isolated from frozen ganglia using Trizol (Invitrogen) and treated with RNase-free DNase I. Quantification of mRNA was done by quantitative PCR (qPCR), conducted in the Quantitative Genomics Core Laboratory of the Department of Integrative Biology and Pharmacology, University of Texas Medical School, Houston, using a 7700 Sequence Detector (Applied Biosystems) and following published procedures (Mohamed et al., 2005). One hundred nanograms of total RNA was loaded into each well, and the following gene-specific primers were used for synapsin: 231CGCCTTATTGCAGAGCTG248, 305GGCTTTGCTCATGGAAGTC323, and 256CCAAGATGCAAGCCATGTGTAAACC280 (probe sequence). The average threshold cycle (Ct) was ∼20. For each time point, nine experiments were performed. For each experiment, synapsin mRNA was normalized to the level of 18S ribosomal RNA (rRNA) in the same sample, providing normalized synapsin mRNA values (Mohamed et al., 2005). To examine the magnitude of the 5-HT effect at each time point, the 5-HT-treated group was compared with the vehicle-treated group using paired two-tailed Student's t tests with SigmaStat software (Systat Software). To examine the effect of time on 5-HT-induced changes in synapsin mRNA, a one-way ANOVA on the ratio of 5-HT/Veh was performed. In addition, a one-way ANOVA was performed on the 18S rRNA-normalized values of vehicle-treated group to examine fluctuations in basal synapsin levels in the absence of 5-HT over time.
Western blot analysis.
For each experimental group, pleural–pedal ganglia were surgically removed from one anesthetized animal so that the left and right ganglia were randomly assigned to receive either control (vehicle) or 5-HT treatment (Liu et al., 2008). Pleural–pedal ganglia were lysed at various time points after the end of treatment. Lysate protein concentrations were determined using a Bio-Rad protein assay. Thirty micrograms of protein from each lysate were resolved by SDS-PAGE and transferred to nitrocellulose membrane. After confirmation of equal loading with Ponceau staining (Bio-Rad) and blocking in 5% nonfat dry milk, blots were incubated overnight at 4°C with a previously characterized, custom-made rabbit polyclonal antibody raised against recombinant Aplysia synapsin (Cocalico Biologicals) (Angers et al., 2002; Fioravante et al., 2007, 2008). A single major immunoreactive band was recognized by this antibody. After incubation of the membrane with peroxidase-conjugated anti-rabbit secondary antibody (Thermo Scientific) for 1 h at room temperature (RT), immunoreactive bands were visualized by ECL (GE Healthcare) and measured by densitometry (ImageQuant 5.0 software, GE Healthcare Life Sciences). As a loading control, membranes were reprobed using a rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (catalog #IMG-5143A, IMGENEX). Immunoreactive bands were quantified as described above. Synapsin immunoreactive bands were normalized to GAPDH immunoreactive bands. Six time points were examined (0, 1, 2, 5, 12, and 24 h), with each time point using six animals. As described in the previous section, to examine the magnitude of the 5-HT effect, comparisons were made between 5-HT-treated and vehicle-treated ganglia using paired two-tailed Student's t tests at each time point. To examine the effect of time after 5-HT treatment, a one-way ANOVA was performed on the ratio of 5-HT/Veh. In addition, a one-way ANOVA was performed on the normalized values of vehicle-treated ganglia to examine fluctuations in basal synapsin levels over time.
Immunofluorescence.
SNs from pleural ganglia were cultured on coverslips in dishes for 5 d as described previously (Angers et al., 2002). For each time point (2, 12 or 24 h after treatment), one dish of cultured SNs was treated with 5-HT, and the other was treated with vehicle as described in the previous section. Again, to examine the magnitude of the 5-HT effect, comparisons were made between 5-HT-treated and vehicle-treated cultures using paired two-tailed Student's t tests. The number of samples (n) reported in the Results indicates the number of independent pairs. Cells were fixed with 4% paraformaldehyde in PBS containing 30% sucrose and blocked for 30 min at RT in Superblock blocking buffer (Pierce) supplemented with 0.2% Triton X-100 and 3% normal goat serum as previously described (Liu et al., 2008). Fixed cells were incubated with a primary anti-synapsin antibody (see above) followed by goat anti-rabbit secondary antibody conjugated to cyanine-3 (catalog #111-165-144, Jackson ImmunoResearch Laboratories). A z-series of optical sections was obtained with a Zeiss LSM 510 confocal microscope using a 63× oil-immersion lens. Initially, at low power, a single field was chosen that contained the maximum number and length of neurites for each neuron. At high power, confocal image stacks through ∼10 μm were collected at 0.5 μm increments and projected into a single image (MetaMorph Offline software, Universal Imaging). Intensity of synapsin immunoreactivity was measured within individual varicosities, which were defined as swellings along the SN neurite that were >1.5 times the diameter of the neurite. For each sample, four to eight neurons on each coverslip (one 5-HT treated, the other vehicle treated) were analyzed in this manner, and measurements from neurons on the same coverslip were averaged. The observer was blind to treatment to eliminate potential bias during imaging and analysis.
Cloning the synapsin promoter region.
Aplysia genomic DNA was isolated, digested, and ligated with the Genome-Walker adaptor DNA (Universal GenomeWalker kit, Clontech). The genomic fragments were used as templates for PCR with an adaptor primer (AP1, 5′-GTAATACGACTCACTATAGGGC-3′; or AP2, 5′-ACTATAGGGCACGCGTGGT-3′) and a synapsin gene-specific primer (1096CCGAACAATTGGTGAACCATTCCTTGAAACC1126 and 1009CATGTTGCCC TCTCAGTTCCACTCTCAGG1137). After electrophoresis, the PCR product was extracted from agarose gel and subcloned into pCR 2.1 TOPO TA vector (Invitrogen). The cloned fragment was sequenced by SEQRIGHT (GenBank accession number JN846835), using M13 universal primers. Predicted regulatory elements were identified using the Transcription Element Search System software (TESS; http://www.cbil.upenn.edu/cgi-bin/tess/tess) using default settings. Annotated sequences of the promoter region were obtained using a minimum log-likelihood ratio (ta) of 6.0 and a maximum log-likelihood deficit (td) of 8.0 (default; combined query option).
Synapsin-driven enhanced green fluorescent protein reporter.
To estimate transcriptional activation of synapsin, an EGFP reporter vector was constructed to contain a region of the synapsin promoter that included the CRE site, as well as the CBP and nuclear factor κ-light-chain-enhancer of activated B cell (NF-κB) sites. The vector was constructed from a pNEX3–EGFP vector (kind gift from B. Kaang, Seoul National University, Seoul, South Korea). The promoter region of the pNEX3–EGFP vector containing the AP-1 enhancer element and the Rous sarcoma virus region was replaced by a 622 bp region of the cloned synapsin promoter using restriction enzyme digestion with flanking HindIII sites. The synapsin promoter–EGFP vector was sequenced by SEQWRIGHT. SNs were cultured as described previously (Angers et al., 2002). On culture day 3, the pNEX3–synapsin promoter–EGFP plasmid (1 μg/μl) was pressure injected into the nucleus of SNs using an Eppendorf InjectMan NI 2 coupled with FemtoJet microinjection system. Dextran-conjugated Texas Red fluorescent dye (70 kDa; 2.5 mg/ml in 100 mm KCl; Invitrogen) was coinjected to monitor the efficacy and locus of injection. On day 5, SNs were treated with 5-HT or vehicle as described above. Two hours after the end of the treatment, cells were fixed with 4% paraformaldehyde solution and processed for confocal imaging. Optical sections through the middle of the cell body were obtained with a 63× oil-immersion lens and analyzed for mean fluorescence intensity. Green fluorescence emitted by EGFP in the cytoplasm and red fluorescence emitted by injection dye in the nucleus were determined after tracing the outline of the cell body and nucleus using MetaMorph Offline software. Mean fluorescence intensity for EGFP in the cytoplasm was normalized to the intensity of fluorescent dye injected into the nucleus. The fluorescence intensities from three to five injected neurons on each coverslip were analyzed, and measurements from neurons on the same coverslip were averaged as described in the previous section.
Chromatin immunoprecipitation assay.
Chromatin immunoprecipitation (ChIP) assays were performed as previously described (Mohamed et al., 2005; Fioravante et al., 2008). For each assay, pairs of pleural–pedal ganglia were surgically removed from four anesthetized animals, and the eight ganglia were divided into two groups. Each group included four ganglia: two ganglia from the right side of two animals; and two ganglia from the left side of the other two animals. Each group was treated with either vehicle or 5-HT. Following treatment, ganglia were treated with 1% paraformaldehyde for 30 min at RT with rotation to cross-link proteins bound to DNA. The reaction was quenched with the addition of glycine (final concentration, 0.125 m). The pleural ganglia were isolated in ice-cold PBS containing protease inhibitors (protease inhibitor cocktail, Sigma) and processed using a ChIP Assay Kit (Millipore) according to the manufacturer's instructions. To measure association of CREB1 with the synapsin promoter, six experiments were performed using CREB1 antibody (Genemed) (Mohamed et al., 2005) to precipitate DNA–protein complexes. For the histone acetylation assay, five experiments were performed using the following antibodies: anti-acetyl-histone H3 and anti-acetyl-histone H4 (catalog #06-599 and #06-866, respectively, Millipore), and anti-histone deacetylase 5 (HDAC5) (catalog #SC-5250, Santa Cruz Biotechnology). DNA isolated from the assay was analyzed by PCR. The PCR primer sequences (5′ to 3′ direction) for the synapsin promoter were as follows: forward primer, 5′-CATGTTGCCCTCTCAGTTCCACTCTC-3′; and reverse primer, 5′-GAGTACAAAGCAACAAGGTTGAGTG)-3′ (Integrated DNA Technologies). A 272 bp fragment containing the CRE sequence of the synapsin promoter was amplified by PCR and resolved on a 1% agarose gel followed by densitometry measurements using ImageQuant 5.0 software. The PCR product from antibody-precipitated DNA (anti-CREB1, anti-acetyl-H3, anti-acetyl-H4, and anti-HDAC5 antibodies) was normalized to input control. Ganglia treated with 5-HT were compared with vehicle-treated ganglia, and results were analyzed with a two-tailed Student's t test.
RNA interference.
Small interfering RNA (siRNA) was designed and prepared by Dharmacon to specifically target Aplysia synapsin mRNA. The Custom SmartPool contained four unique siRNAs to target specific areas of the synapsin sequence that lack similarity to any other genes in the human, rat and mouse BLAST databases. The synapsin siRNAs were designed against the following sequences: 1299CGATATCCACGTTCAGAAA1317; 1537CCAATGAGAGCGCCAGGTA1555; 1659GGTTAGGCAAAGAGTCGTT1677; and 2018TCAAGTTGTGGGTGGACGA2036. Control siRNA (scrambled siRNA, also provided by Dharmacon) or synapsin siRNA (5 μm) was injected into the cytoplasm of cultured SNs, together with a fluorescent marker (2.5 mg/ml Alexa Fluor 488-dextran, 10 kDa, Invitrogen) in a 100 mm KCl solution 2.5 h before treatment with vehicle or 5-HT. Two hours after treatment, cells were fixed and processed for immunostaining (see above, Immunofluorescence). Immunoreactivity measurements from control siRNA-injected, 5-HT-treated SNs and synapsin siRNA-injected, 5-HT-treated SNs were normalized to control siRNA-injected, vehicle-treated SNs and were compared using a two-tailed Student's t test. To assess the effects of siRNA on basal synapsin protein levels, SNs were injected with either control or synapsin siRNA and fixed 6 h (n = 3), 28 h (n = 5) or 52 h (n = 5) after injection. Immunoreactivity measurements in synapsin siRNA-injected SNs were compared with control siRNA-injected SNs at each time point using a paired two-tailed Student's t test. An additional experiment was performed to examine whether siRNA was functional at 24 h after injection. SNs were injected with control or synapsin siRNA, treated 20.5 h after injection with vehicle or 5-HT, and fixed 2 h after treatment (24 h after injection), and then were compared using a two-tailed Student's t test (n = 4).
Electrophysiology.
Sensorimotor cocultures were prepared as described previously (Angers et al., 2002). Synapsin or control siRNA (5 μm) in injection solution (100 mm KCl, 0.1% Alexa Fluor 488-dextran 10 kDa) was pressure injected into the cytoplasm of SNs using an Eppendorf microinjection system 2.5 h before the pretest. The efficacy of the injection was monitored by fluorescence of the Alexa Fluor 488-conjugated dextran. Before treatment with 5-HT, basal synaptic strength (pretest) was assessed by evoking an EPSP in the MN by extracellular stimulation of the presynaptic SN using a patch electrode filled with L15:ASW (50%:50% volume) placed next to the SN cell body. EPSPs were recorded from MNs with 10–15 MΩ sharp electrodes filled with 3 m potassium acetate. MNs with a resting membrane potential more positive than −40 mV and input resistance <10 MΩ were excluded. The resting membrane potential of MNs was current-clamped at −90 mV. Responses were recorded using an Axoclamp-2B amplifier and pCLAMP 8.2 software (Molecular Devices). EPSP amplitude was measured off-line using Clampfit 9.0 (Molecular Devices). If the pretest EPSP was <5 mV, the culture was excluded from further use. Twenty-four hours after the end of treatment, a post-test EPSP was recorded. For statistical analysis, the ratio of post-test/pretest EPSP amplitudes was calculated (control siRNA, vehicle treatment: n = 9; control siRNA, 5-HT treatment: n = 7; synapsin siRNA, vehicle treatment: n = 8; synapsin siRNA, 5-HT treatment: n = 8). In addition, changes in the MN input resistance and resting membrane potential were assessed. Data were analyzed by a two-way ANOVA followed by post hoc analysis with Student–Newman–Keuls tests.
In a separate set of experiments, short-term synaptic depression and facilitation after depression were assessed in cocultures injected with either control (n = 5) or synapsin siRNA (n = 6). Twenty-eight hours after siRNA injection, a train of 10 stimuli at 0.05 Hz (i.e., 1 stimulus every 20 s) was delivered. The first eight EPSPs, which resulted from eight stimuli, were designed to examine the magnitude of short-term synaptic depression. Depression was assessed by comparing the amplitude of the eighth EPSP (EPSP8) to that of the first EPSP (EPSP1). Immediately after the eighth stimulus, a bolus of 5-HT (50 μm final concentration) was applied with a pipette to the center of the culture dish, close to the cells, to examine the extent of facilitation after depression. Facilitation of the depressed synapse was assessed by comparing the amplitude of the tenth EPSP (40 s after 5-HT application) to EPSP8 (before 5-HT application). Results were analyzed using a repeated-measures two-way ANOVA followed by post hoc analysis with Student–Newman–Keuls tests.
Results
Synapsin mRNA and protein levels are dynamically regulated in ganglia for at least 24 h after treatment with 5-HT
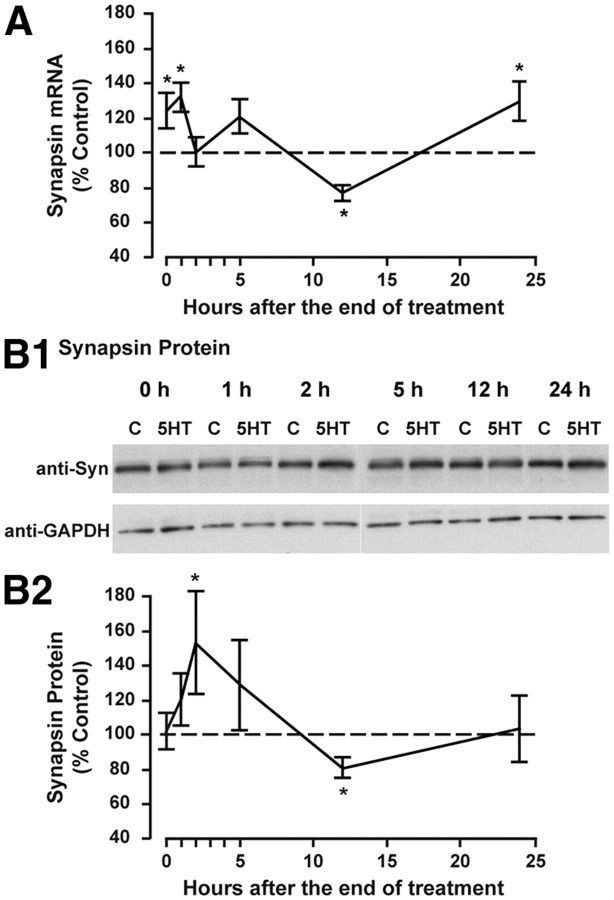
To examine whether synapsin is regulated during LTF, synapsin mRNA levels were measured in ganglia at various time points after treatment with 5-HT or vehicle. For each time point, synapsin mRNA levels in both groups were normalized to 18S rRNA (Mohamed et al., 2005; Liu et al., 2008). A one-way ANOVA on the ratio of 5-HT/vehicle (Fig. 1A) indicated a significant effect of time after 5-HT treatment on synapsin mRNA (F(5,53) = 5.57, p < 0.001). To examine the magnitude of the 5-HT effect at each individual time point, the 18S-normalized values from the 5-HT-treated group were compared with the 18S-normalized values from the vehicle-treated group using paired two-tailed Student's t tests for each time point. Both immediately and 1 h following 5-HT treatment, synapsin mRNA levels were significantly increased compared with vehicle-treated ganglia (mean percentage control ± SEM: immediately: 124.3 ± 10.3%, t(8) = 2.36, n = 9, p < 0.05; 1 h: 131.7 ± 8.70%, t(8) = 4.77, n = 9, p < 0.05). However, synapsin mRNA levels were not significantly different from control at either 2 or 5 h after 5-HT (2 h: 100.2 ± 8.20%, t(8) = 0.49, n = 9, p = 0.63; 5 h: 120.5 ± 9.72%, t(8) = 1.35, n = 9, p = 0.21). A significant decrease occurred at 12 h (77.0 ± 4.15%, t(8) = 3.59, n = 9, p < 0.05) followed by another significant increase at 24 h (129.3 ± 11.2%, t(8) = 2.86, n = 9, p < 0.05). In addition, a one-way ANOVA was also performed on the 18S rRNA-normalized values in vehicle-treated groups to examine fluctuations in basal synapsin mRNA levels in the absence of 5-HT over time. A one-way ANOVA on ranks indicated no significant effect of time on basal levels of synapsin mRNA (H5 = 8.310, p = 0.14), indicating that the changes we observed over time after 5-HT treatment were not due to basal fluctuations in synapsin.
Figure 1.
Synapsin mRNA and protein levels in ganglia after five pulses of 5-HT. A, Summary data from qPCR showing synapsin mRNA levels immediately (0 h), and 1, 2, 5, 12, and 24 h after 5-HT treatment and compared with vehicle-treated, time-matched controls. Synapsin levels in both vehicle- and 5-HT-treated ganglia were normalized to 18S rRNA levels. Synapsin mRNA levels were significantly elevated immediately, 1, and 24 h after 5-HT treatment but significantly decreased at 12 h after 5-HT treatment (*p < 0.05). These results indicate that 5-HT treatment regulates synapsin mRNA levels in ganglia. B1, Representative Western blots from ganglia treated with five pulses of vehicle (control, C) or 5-HT and lysed at the indicated times. Membranes were probed with anti-synapsin and anti-GAPDH antibodies. B2, Summary data from Western blot analysis of synapsin protein levels immediately (0 h), and 1, 2, 5, 12, and 24 h after 5-HT treatment and compared with vehicle-treated, time-matched controls. Synapsin protein was normalized to levels of GAPDH, which served as a loading control. Synapsin protein levels were significantly increased 2 h following 5-HT treatment, significantly decreased at 12 h and returned to baseline 24 h after treatment (*p < 0.05).
We next asked whether synapsin protein levels also changed after 5-HT treatment. To this end, we performed Western blots and quantified synapsin protein levels at the same time points as mRNA (Fig. 1B1,B2). Synapsin protein levels were normalized to GAPDH. A one-way ANOVA on the ratio of 5-HT/vehicle data (Fig. 1B) indicated a significant effect of time after 5-HT treatment for synapsin protein (H5 = 11.98, p < 0.05). We further examined the magnitude of the 5-HT effect at each individual time point (Fig. 1B). Compared with vehicle-treated ganglia, synapsin protein levels from 5-HT-treated ganglia were significantly increased 2 h after treatment and significantly decreased at 12 h (mean percentage control ± SEM: immediately: 102 ± 11%, t(5) = 0.01, p = 0.50; 1 h: 121 ± 15%, t(5) = 1.40, p = 0.11; 2 h, 153 ± 30%, t(5) = 2.02, p < 0.05; 5 h: 129 ± 26%, t(5) = 0.99, p = 0.18; 12 h: 81.0 ± 5.8%, t(5) = 3.67, p < 0.05; 24 h: 104 ± 20%, t(5) = 0.29, p = 0.39; for all the time points: n = 6). As with the mRNA analysis, a one-way ANOVA indicated no significant effect of time on basal levels of synapsin protein (F(5,30) = 1.18, p = 0.34). These results indicate that both synapsin mRNA and protein are dynamically regulated in ganglia after treatment with 5-HT.
Synapsin protein levels are increased in isolated SNs 2 and 24 h after 5-HT treatment
The experiments described above examined synapsin regulation in whole ganglia, which are comprised of various neuronal subtypes in addition to SNs. To determine whether synapsin is specifically regulated in SNs, the presynaptic site associated with LTF, we examined the effect of 5-HT on synapsin protein in isolated SNs using immunofluorescence. Because synapsin is a synaptic vesicle-associated protein that is primarily localized to synapses (Fletcher et al., 1991; Angers et al., 2002), we investigated the regulation of synapsin expression specifically in axonal varicosities, the presumed sites of neurotransmitter release (Bailey and Chen, 1988). Synapsin levels in SNs were examined at 2, 12, and 24 h after treatment with vehicle or 5-HT. Consistent with the results from ganglia, synapsin expression was increased in the varicosities of SNs 2 h after treatment with 5-HT (mean percentage of vehicle-treated cells ± SEM: 127 ± 4.9%, t(2) = 6.34, n = 3, p < 0.05) (Fig. 2A1,A2,B). However, synapsin levels returned to baseline at 12 h after 5-HT (105 ± 8.1%, t(4) = 0.43, n = 5, p = 0.69) (Fig. 2A3,A4,B). A second phase of increased synapsin immunoreactivity in varicosities of SNs was observed 24 h after treatment (119 ± 5.0%, t(4) = 2.92, n = 5, p < 0.05) (Fig. 2A5,A6,B). This result suggests that synapsin protein is also dynamically regulated in presynaptic neurons after treatment with 5-HT, and these changes in synapsin expression could be involved in the dynamics of synaptic transmission and LTF.
Figure 2.
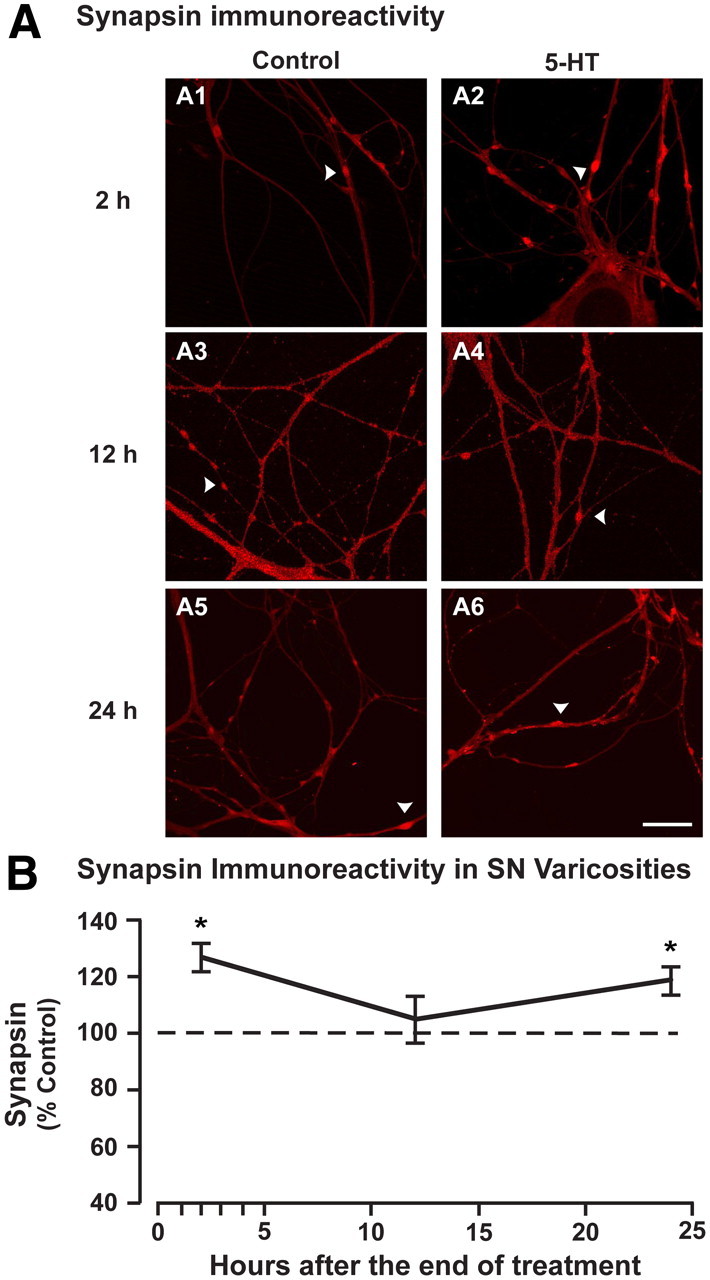
Synapsin protein levels are increased in cultured sensory neurons 2 and 24 h after treatment with 5-HT. A, Synapsin immunoreactivity in the varicosities of isolated, cultured sensory neurons treated with vehicle (control) or 5-HT and fixed 2 (A1, A2), 12 (A3, A4), or 24 h (A5, A6) after treatment. Arrowheads point to synapsin-immunoreactive varicosities along neurites. A single field was chosen that contained the maximum number and length of neurites. B, Summary data. Plot of average immunofluorescence intensity (±SEM) in the 5-HT-treated groups (normalized to control) assayed at 2, 12, and 24 h after treatment. A significant increase in synapsin immunoreactivity within varicosities was observed at both 2 and 24 h after 5-HT (*p < 0.05) but not at 12 h after 5-HT (p = 0.69). Scale bar, 25 μm.
Characterization of the promoter region of Aplysia synapsin
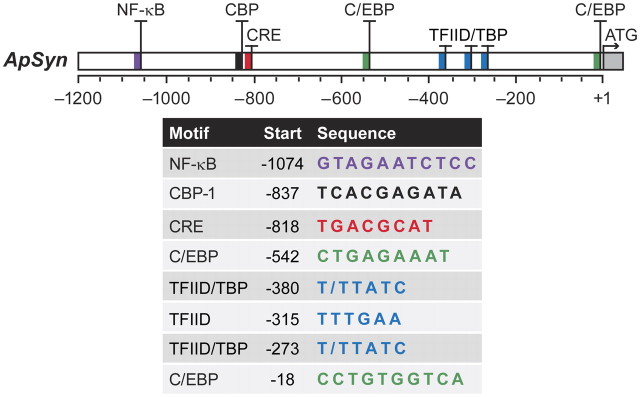
We cloned and characterized the promoter region of Aplysia synapsin to determine potential transcription factor binding sites that might contribute to the dynamic regulation of synapsin. A ∼1.3 kb region of the Aplysia synapsin promoter was analyzed using TESS software, which uses known consensus sequences to predict transcription factor binding sites in a given sequence of DNA. Figure 3 illustrates potential binding sites for transcription factors in the synapsin promoter. Locations are indicated as the number of base pairs from the translation start site (ATG) because the transcription start site is not known.
Figure 3.
Potential regulatory element-binding sites in the promoter region of Aplysia synapsin. A section of ∼1.3 kbp of the promoter region of the Aplysia synapsin gene was cloned and analyzed. Predicted regulatory elements were identified using TESS software. Potential binding sites for transcription factors were identified and labeled accordingly as base pairs from the translation start site (the ATG codon; +1) because the transcription start site remains unknown: NF-κB (purple, −1074), CBP (black, −837), CRE (red, −818), C/EBP (green, −542 and −18), and TFIID/TBP (blue, −380, −315 and −273).
Putative binding sites for CCAAT-enhancer-binding protein (C/EBP) were located at −542 bp (5′-CTGAGAAAT-3′) and at −18 bp (5′-CCTGTGGTCA-3′) from the ATG (Fig. 3, green). A putative site for NF-κBs was also observed at −1071 bp (5′-GTAGAATCTCC-3′) (Fig. 3, purple).
A single CRE variant 5′-TGACGCAT-3′ was found at −818 bp from the ATG (Fig. 3, red). The presence of a CRE site is required for CREB-dependent gene induction (Montminy et al., 1986; Mayr and Montminy, 2001; Mohamed et al., 2005), which is necessary for LTF (Dash et al., 1990; Liu et al., 2008). Nineteen base pairs upstream of the CRE site is a CREB-binding protein (CBP) motif (5′-TCACGAGATA-3′) at −837 bp from ATG (Fig. 3, black). CBP is a transcriptional coactivator that modulates CREB1-mediated gene expression through its actions as a histone acetyltransferase (Bannister and Kouzarides, 1996; Ogryzko et al., 1996) as well as through CREB acetylation (Lu et al., 2003). In addition, potential binding sites for transcription factor II D (TFIID) and TATA binding protein (TBP) are located at −380 bp (5′-T/TTATC-3′) and −273 bp (5′-T/TTATC-3′) from the translation start site (Fig. 3, blue). An additional TFIID potential binding site is located at −315 bp (5′-TTTGAA-3′) from the ATG (Fig. 3, blue).
5-HT treatment regulates the synapsin promoter
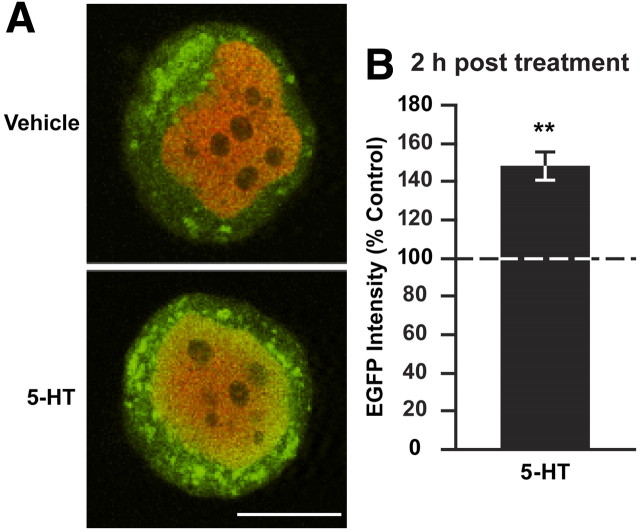
To assess whether synapsin promoter-mediated gene expression can be regulated by 5-HT, we modified a pNEX3–EGFP vector, which successfully drives EGFP expression in Aplysia (Kaang, 1996). The original promoter region of this vector was removed and replaced with the synapsin promoter encompassing the putative CRE site and inserted upstream of the EGFP gene. The synapsin promoter–EGFP expression vector was coinjected into the nucleus of cultured SNs with dextran-conjugated Texas Red 48 h before treatment (to allow for basal EGFP expression). Two hours after treatment with 5-HT or vehicle, cells were fixed and imaged using confocal microscopy (Fig. 4A). This time point corresponds to the largest increase in synapsin protein after 5-HT treatment observed in ganglia (Fig. 1B) and SNs (Fig. 2A1,A2,B). Mean fluorescence intensity for EGFP in the cytoplasm of the cell body was normalized to the amount of plasmid injected, indicated by the intensity of injected red fluorescent dye in the nucleus. 5-HT treatment significantly increased the normalized EGFP expression in neurons compared with those treated with vehicle only (expressed as a percentage of vehicle ± SEM: 149 ± 7.4%, t(5) = 3.95, n = 6, p < 0.02) (Fig. 4B). These results indicate that the cloned synapsin promoter is functional and can be regulated by 5-HT. Such regulation in vivo could support the increase in synapsin mRNA and protein 2 h after 5-HT treatment.
Figure 4.
The synapsin promoter is transcriptionally regulated in response to 5-HT. A, Synapsin promoter-driven EGFP expression in the cytoplasm of cultured sensory neurons 2 h after vehicle (top) or 5-HT treatment (bottom). SNs were coinjected with dextran-conjugated Texas Red fluorescent dye and the expression vector on the third day of culture, treated on day 5 and fixed 2 h after treatment for immunofluorescence analysis. EGFP intensity in the cytoplasm was normalized to red injection dye in the nucleus. B, Summary data. Average EGFP immunofluorescence intensity (±SEM) in 5-HT-treated SNs (normalized to vehicle-treated SNs). The significantly increased EGFP levels 2 h after 5-HT treatment (**p < 0.02) indicated that the synapsin promoter is regulated by 5-HT. Scale bar, 25 μm.
5-HT treatment enhances CREB1 association and histone acetylation at the CRE site of the synapsin promoter
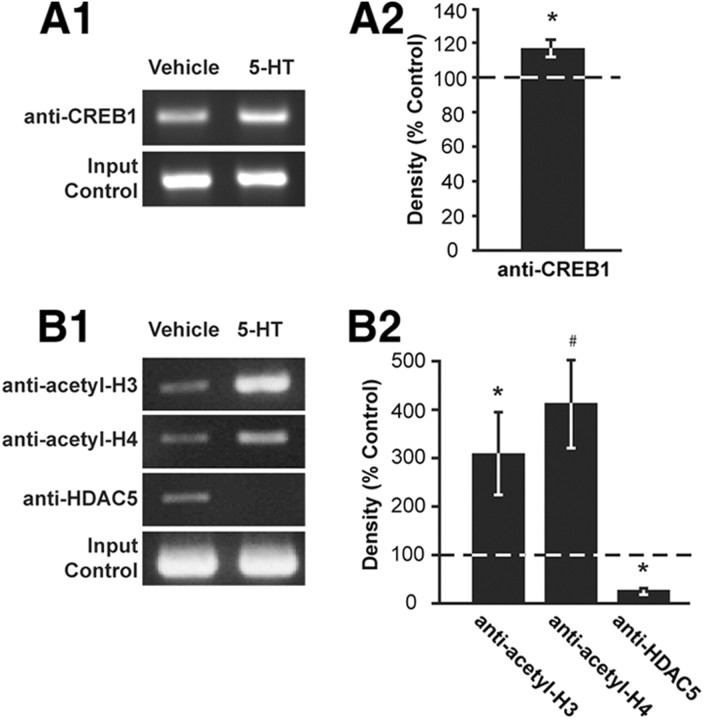
The CRE site in the synapsin promoter suggests that CREB1 may regulate synapsin expression. ChIP assays have been previously used in Aplysia to examine the association of CREB1 with the promoter region of various genes and to assess the state of histone acetylation (Guan et al., 2002; Mohamed et al., 2005; Fioravante et al., 2008). We performed ChIP assays to examine the association of CREB1 with the synapsin CRE under basal conditions (vehicle treated) and after treatment with 5-HT. Based on our findings that synapsin mRNA levels in ganglia were significantly increased immediately and 1 h following treatment with 5-HT (Fig. 1A), we assessed the association of CREB1 with the synapsin promoter region immediately (0 h) after the end of the treatment period. Densitometry measurements of the amplified PCR product from the ChIP assays revealed that under basal conditions, CREB1 associated with the synapsin promoter, and this association was significantly enhanced after treatment with 5-HT (Fig. 5A) (mean percentage vehicle ± SEM: 116.7 ± 4.97%, t(5) = 2.01, n = 6, p < 0.05) (values were normalized to input control). These results provide additional support that synapsin is regulated by CREB1 directly during LTF.
Figure 5.
Binding of CREB1 to Aplysia synapsin promoter is accompanied by histone acetylation after 5-HT treatment. A1, Representative DNA gel from ChIP assay. Ganglia were treated with vehicle or 5-HT to measure CREB1 association with a 272 bp region of the synapsin promoter that includes the CRE site. Densitometry measurements of bands from PCR-amplified DNA resulting from ChIP assays with an anti-CREB1 antibody were normalized to input control. CREB1 associates with the synapsin promoter under control conditions (left lane) and after treatment with 5-HT (right lane). A2, Summary data indicating that 5-HT treatment led to increased association of CREB1 to the synapsin promoter (*p < 0.05). No change was observed in input control. B1, Representative DNA gel from a ChIP assay assessing the state of histone acetylation of the synapsin promoter region containing the CRE. Ganglia were treated with 5-HT or vehicle. Densitometry measurements of bands from PCR-amplified DNA precipitated with antibodies against acetylated histone H3 (anti-acetyl-H3), acetylated histone H4 (anti-acetyl-H4) and anti-histone deacetylase 5 (anti-HDAC5) were normalized to input control. Basal acetylation of the synapsin promoter was observed with vehicle treatment (left lane) as well as basal association of HDAC5 (left lane). After treatment with 5-HT, association of histones H3 and H4 (right lane) and of HDAC5 (right lane) was modified compared to vehicle. B2, Summary data indicating that treatment with 5-HT led to a significant increase in acetylation of histones H3 and H4 and decreased association of HDAC5 surrounding the CREB1 binding site of the synapsin promoter region (compared with vehicle-treated ganglia) (*p < 0.05, #p < 0.01). No change in input control was observed.
The state of histone acetylation has been used previously as an indicator of transcriptional regulation (Levenson and Sweatt, 2005). Hyperacetylation of histone tails, accompanied by a decrease in the association of histone deacetylases, leads to transcriptional activation (Guan et al., 2002; Mohamed et al., 2005) (but see Shahbazian and Grunstein, 2007; Fioravante et al., 2008). Therefore, to determine whether the enhanced association of CREB1 with the synapsin promoter following 5-HT treatment is associated with transcriptional initiation, changes in histone acetylation and the association of HDAC5 in the vicinity of the CRE site were investigated in ganglia treated with 5-HT or vehicle. ChIP assays were performed with antibodies directed against the acetylated forms of histones H3 and H4 and HDAC5 (Fig. 5B). These antibodies have previously been used to describe CREB2-dependent activation of transcription of ubiquitin C-terminal hydrolase (Fioravante et al., 2008). Densitometry measurements of the amplified PCR product from the ChIP assays revealed that 5-HT treatment significantly induced acetylation of histones H3 (mean percentage vehicle ± SEM: 308 ± 84%, t(4) = 3.15, n = 5, p < 0.05) and H4 (411 ± 89%, t(4) = 4.99, n = 5, p < 0.01), and decreased association of HDAC5 (27 ± 12%, t(4) = 3.24, n = 5, p < 0.05) (values were normalized to input control). These results suggest that transcriptional activation of the synapsin gene following 5-HT treatment may be regulated through the CRE site in the synapsin promoter.
5-HT-induced increase in synapsin expression is necessary for LTF
Elevated synapsin levels are correlated with enhanced synaptic plasticity (Morimoto et al., 1998; Sato et al., 2000) and learning and memory (Gómez-Pinilla et al., 2001; Velho and Mello, 2008; Rapanelli et al., 2009). In addition, basal levels of synapsin are important for several, although not all, types of learning and memory (Silva et al., 1996; Gitler et al., 2004; Godenschwege et al., 2004; Corradi et al., 2008; Knapek et al., 2010; Michels et al., 2011). However, the functional significance of enhanced synapsin expression associated with learning remains unclear. Therefore, we investigated the functional significance of synapsin regulation for LTF by using siRNA to specifically block the increase in synapsin protein after treatment with 5-HT.
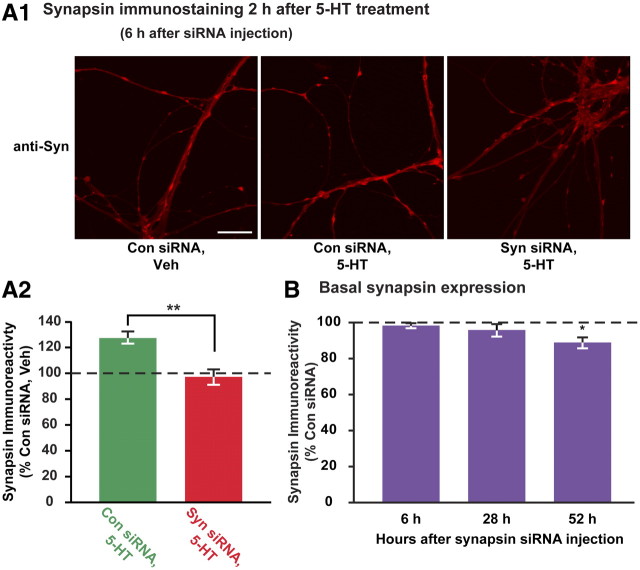
We first established a time point at which injection of siRNA could block the 5-HT-induced increase in synapsin expression without affecting basal levels of expression. Scrambled siRNA (control siRNA) or pooled synapsin siRNA was injected into the cytoplasm of cultured SNs 2.5 h before treatment, and cells were fixed 2 h after treatment. Compared with control siRNA-injected, vehicle-treated SNs, cells injected with control siRNA and treated with 5-HT showed a 27 ± 5% increase in synapsin levels, whereas synapsin levels in cells treated with 5-HT but injected with synapsin siRNA were −3 ± 6% of control siRNA-injected, vehicle-treated SNs (Fig. 6A2, normalized data are shown). The difference between the levels of synapsin in these two 5-HT-treated groups was statistically significant (t(4) = 3.86, n = 5, p < 0.02). These results support the effectiveness of synapsin siRNA in blocking increases in synapsin expression. Moreover, the effect of the synapsin siRNA was specific to newly synthesized synapsin, because basal synapsin levels were unaffected by synapsin siRNA injection (percentage of control siRNA-injected, vehicle-treated cells: 99.9 ± 1.63%, t(2) = 0.46, n = 3, p = 0.69) (Fig. 6B). At 28 h postinjection, which corresponds to 24 h after treatment, basal synapsin levels were not significantly changed by synapsin siRNA (96.0 ± 3.5%, t(4) = 1.08, n = 5, p = 0.34). However, 52 h after injection, synapsin protein levels were significantly decreased by synapsin siRNA injection (percentage of control-siRNA-injected cells: 89 ± 3.0%, t(4) = 3.65, n = 5, p < 0.05) (Fig. 6B), indicating that synapsin siRNA eventually leads to a decrease in basal synapsin expression after an extended period of time. Additional evidence for the stability and effectiveness of RNAi in the intracellular milieu is provided by the finding that the synapsin siRNA blocked the increase in synapsin protein in cells treated with 5-HT 20.5 h postinjection and measured 2 h after treatment (expressed as the percentage of control siRNA-injected, vehicle-treated cells: control siRNA+5-HT: 124.8 ± 2.4%; synapsin siRNA+5-HT: 99.3 ± 5.7%, t(3) = 7.05, n = 4, p < 0.05). These results validate the use of synapsin siRNA as a tool to block 5-HT-induced changes in synapsin protein levels without affecting basal levels for at least 24 h after treatment.
Figure 6.
Synapsin siRNA blocks the 5-HT-induced increase in synapsin immunoreactivity 2 h after 5-HT treatment. A1, Synapsin immunostaining in SN varicosities from cells injected with control or synapsin siRNA 2.5 h before treatment and fixed 2 h after treatment with vehicle or 5-HT. Examples of SNs injected with control siRNA and treated with vehicle (left), injected with control siRNA and treated with 5-HT (middle), and injected with synapsin siRNA and treated with 5-HT (right). Scale bar, 25 μm. A2, Summary data from A1. Average fluorescence intensity (±SEM) of synapsin immunostaining in varicosities 2 h after 5-HT treatment in control siRNA- and synapsin siRNA-injected SNs normalized to synapsin intensity in control siRNA-injected, vehicle-treated cells. The difference between the levels of synapsin in the two 5-HT-treated groups was statistically significant (**p < 0.02). B, Plot of basal synapsin immunoreactivity (average fluorescence intensity ± SEM) from synapsin siRNA-injected SNs (normalized to control siRNA) 6, 28, and 52 h after injection. No significant effect of synapsin siRNA injection on basal synapsin levels was observed at 6 h (p = 0.69) and 28 h (p = 0.34), but a significant reduction was observed at 52 h after injection (*p < 0.05).
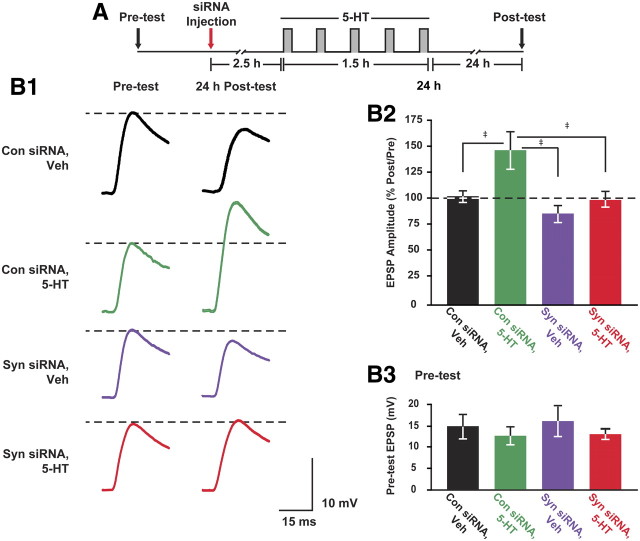
We next examined the effect of synapsin siRNA on LTF. After an assessment of basal synaptic strength in sensorimotor cocultures (pretest), either control siRNA or synapsin siRNA was injected into the cytoplasm of SNs. Two-and-one-half hours after the siRNA injections, cocultures were treated with five pulses of 5-HT or vehicle, and the extent of LTF was assessed 24 h after treatment. A two-way ANOVA revealed a significant effect of siRNA injection (F(1,28) = 9.31, p < 0.01) and 5-HT treatment (F(1,28) = 8.63, p < 0.01) (injection × treatment interaction) (F(1,28) = 2.43, p = 0.13) 24 h after treatment (Fig. 7B1,B2) (mean percentage post-treatment/pretreatment ± SEM: control siRNA+vehicle: 103.1 ± 5.2%, n = 9; control siRNA+5-HT: 148.4 ± 18.0%, n = 7; synapsin siRNA+vehicle: 88.1 ± 8.0%, n = 8; synapsin siRNA+5-HT: 102.0 ± 7.8%, n = 8). To determine the source of differences, Student–Newman–Keuls post hoc tests were performed. This analysis indicated that 5-HT induced significant facilitation in the control siRNA-injected cocultures (control siRNA: vehicle vs 5-HT, q = 4.48, p < 0.005). Synapsin siRNA blocked the facilitatory effect of 5-HT on synaptic transmission (5-HT: control siRNA vs synapsin siRNA, q = 4.47, p < 0.005). Although a slight increase was observed in the mean amplitude of 5-HT-treated cells compared with vehicle-treated cells injected with synapsin siRNA, this increase was not statistically different (synapsin siRNA: vehicle vs 5-HT, q = 1.39, p = 0.34). Furthermore, there was no significant difference between control siRNA- and synapsin siRNA-injected cells treated with vehicle (vehicle: control siRNA vs synapsin siRNA, q = 1.54; p = 0.29), indicating that injection of synapsin siRNA did not significantly affect synaptic transmission over a 28 h period. The observed differences in synaptic strength following treatments were not due to differences in the initial strength of the sensorimotor synapses, as indicated by a one-way ANOVA on ranks on pretest EPSP amplitudes (H1 = 0.007, p = 0.94) (Fig. 7B3). Moreover, two-way ANOVAs indicated that the passive properties of the postsynaptic MNs, assessed during both before and after tests, were not significantly different among groups (input resistance: injection, F(1,28) = 2.43, p = 0.13; treatment, F(1,28) = 0.17, p = 0.68; injection × treatment interaction, F(1,28) = 0.37, p = 0.55; resting potential: injection, F(1,28) = 2.78, p = 0.11; treatment; F(1,28) = 0.002, p = 0.96; injection × treatment interaction, F(1,28) = 0.39, p = 0.56). Overall, these results indicate that the 5-HT-induced increase of synapsin levels in SNs is necessary for LTF.
Figure 7.
5-HT-induced synapsin expression is necessary for LTF. A, Experimental protocol. Immediately following a pretest to assess basal synaptic strength, sensory neurons were injected with either control or synapsin siRNA. Two-and-one-half hours after injection, cocultures were treated with either five 5 min pulses of vehicle or 5-HT (gray pulses) at an ISI of 20 min. Synaptic strength was again assessed 24 h after the end of treatment (post-test). B1, EPSPs recorded from sensorimotor cocultures injected with control or synapsin siRNA before (pretest) and 24 h after (post-test) treatment with 5-HT or vehicle. B2, Plot of average percentage post-treatment/pretreatment ratio (±SEM) of EPSP amplitudes. 5-HT induced significant facilitation in the control siRNA-injected cocultures (‡p < 0.005), but not in the synapsin siRNA-injected cocultures (p = 0.34). The 5-HT-induced facilitation observed in control siRNA-injected cocultures was blocked in 5-HT-treated, synapsin siRNA-injected cocultures (‡p < 0.005). A significant difference was also observed between control siRNA-injected cultures treated with 5-HT and synapsin siRNA-injected cultures treated with vehicle (‡p < 0.005). Injection of synapsin siRNA did not significantly affect synaptic transmission over a 28 h period (p = 0.29). B3, Plot of average pretest EPSP amplitudes (±SEM) from cocultures. No difference in basal synaptic strength was observed among groups before injection (p = 0.78).
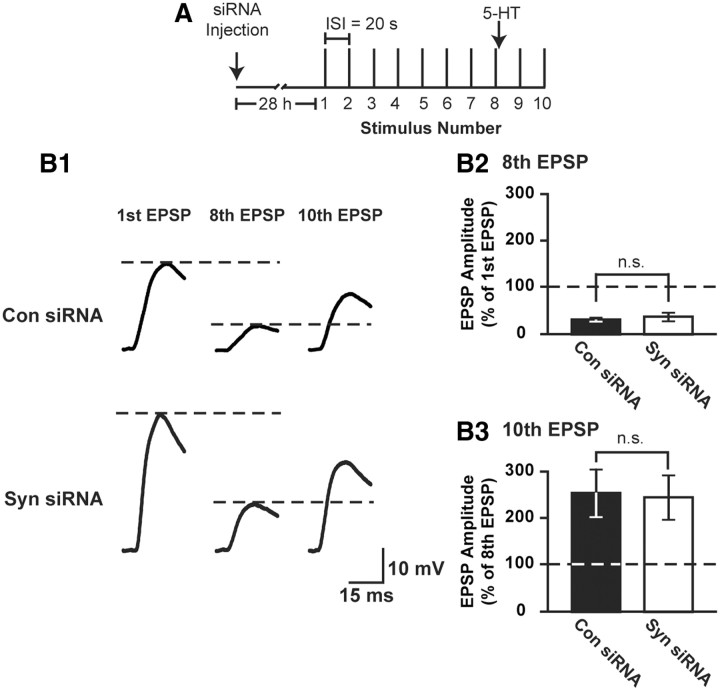
Data from immunofluorescence studies suggested that siRNA injection did not alter basal synapsin levels for at least 28 h (Fig. 6). Moreover, results from the electrophysiological recordings indicated that siRNA did not affect basal synaptic efficacy over a 28 h period (Fig. 7B2). Nonetheless, we performed additional tests of synaptic integrity based on the observation that altering basal synapsin levels affects short-term plasticity and the regulation of synaptic vesicle pools (Rosahl et al., 1995; Gitler et al., 2004; Fioravante et al., 2007). If synapsin siRNA perturbed basal protein levels and function, we might expect an impairment of short-term plasticity, such as short-term homosynaptic depression or 5-HT-induced facilitation of a depressed synapse (Castellucci and Kandel, 1976; Byrne, 1982; Emptage et al., 1996; Fioravante et al., 2007). Twenty-eight hours after siRNA injection (which corresponds to 24 h after treatment when LTF is measured; n = 5 for control siRNA and n = 6 for synapsin siRNA), a train of eight stimuli at 0.05 Hz was delivered to induce short-term synaptic depression. Immediately following the eighth EPSP, 5-HT was applied, followed by two additional stimuli delivered at 0.05 Hz to test for the extent of facilitation of the depressed synapse. We found that the eighth EPSP amplitude, compared with the first EPSP amplitude, depressed to 31.9 ± 1.5% in control siRNA-injected cultures and 37.6 ± 9.1% in synapsin siRNA-injected cultures (Fig. 8B2). Following treatment with 5-HT, the tenth EPSP amplitude was facilitated, compared with the eighth EPSP amplitude, by 250.4 ± 49.9% in control siRNA-injected cultures and 242.7 ± 46.7% in synapsin siRNA-injected cultures (Fig. 8B3). A two-way repeated-measures ANOVA on the first, eighth and tenth EPSP measurements after control or synapsin siRNA injection indicated that there was no effect of siRNA injection (F(1,18) = 1.181, p = 0.31) or siRNA injection × stimulus number interaction (F(2,18) = 0.67, p = 0.52), but there was an effect of stimulus number (F(2,18) = 16.76, p < 0.001). Post hoc analysis indicated that both siRNA-injected groups exhibited synaptic depression (comparing the eighth EPSP amplitude to the first EPSP amplitude: control siRNA: q = 4.40, p < 0.05; synapsin siRNA: q = 7.15, p < 0.05), but there was no difference in the extent of depression between control- or synapsin siRNA-injected cultures (q = 0.69, p = 0.63). Moreover, both siRNA-injected groups exhibited 5-HT-induced facilitation (comparing the tenth EPSP amplitude to the eighth EPSP amplitude control siRNA: q = 3.30, p < 0.05; synapsin siRNA: q = 4.16 p < 0.05), but there was no difference in the extent of facilitation between control- and synapsin siRNA-injected cultures (q = 1.03, p = 0.48). As previously observed (Fig. 7B2), no significant difference was found in basal synaptic strength between cocultures injected with control or synapsin siRNA (average amplitude of EPSP1 ± SEM: control siRNA: 14.8 ± 3.62 mV; synapsin siRNA: 21.5 ± 4.37 mV; q = 2.16, p = 0.15). The lack of effect of synapsin siRNA on short-term depression and subsequent facilitation of depressed synapses, compared with control siRNA, provides further evidence that injections did not affect basal synapsin levels and supports our assertion that the effect of synapsin siRNA is specific to LTF.
Figure 8.
siRNA does not affect short-term synaptic plasticity or facilitation of a depressed synapse. A, Experimental protocol. SNs were injected with control or synapsin siRNA. Twenty-eight hours later, a train of 10 stimuli was delivered at 0.05 Hz. Immediately following the eighth stimulus, a bolus of 5-HT was delivered to the coculture. B1, Representative traces of the first, eighth, and tenth EPSPs from sensorimotor cocultures injected with either control or synapsin siRNA. B2, Summary data showing the amplitude of the eighth EPSP normalized to the amplitude of the first EPSP for control and synapsin siRNA-injected cultures. No significant difference was observed in the extent of depression between cocultures injected with control or synapsin siRNA (p = 0.63). B3, Summary data showing the amplitude of the tenth EPSP (after 5-HT) normalized to the amplitude of the eighth EPSP (before 5-HT) for control and synapsin siRNA-injected cultures. No significant difference was observed in the extent of facilitation between cocultures injected with control or synapsin siRNA (p = 0.48).
Discussion
Increased synapsin levels have previously been associated with long-term plasticity and memory (Morimoto et al., 1998; Sato et al., 2000; Gómez-Pinilla et al., 2001; Rapanelli et al., 2009). However, these studies were correlative and did not demonstrate a causal role for synapsin regulation in long-term plasticity or memory. Using a combination of cellular, molecular, and electrophysiological approaches, our results support an essential role for the regulation of synapsin expression in the consolidation of LTF, a cellular model of LTM.
5-HT-induced regulation of synapsin expression
Treatment with 5-HT, which induces LTF of the sensorimotor synapse, regulates the expression of synapsin mRNA and protein (Figs. 1, 2). Levels of synapsin mRNA and protein exhibit similar, but not identical, regulatory patterns. These differences could be due to sample differences (mRNA and protein were collected from different specimens), small sample size (6 time points), and the fact that synthesis of protein follows synthesis of mRNA. In addition, it is possible that not all of the synapsin mRNA is translated, and that protein levels are further regulated post-translationally. Two hours after 5-HT treatment, synapsin protein was increased in both ganglia (Fig. 1B) and SNs (Fig. 2B), presumably in response to CREB1 activation of the synapsin promoter (Fig. 5) and mRNA upregulation (Fig. 1A). However, 12 h after 5-HT treatment, synapsin protein levels decreased (Figs. 1B2, 2B). This observation cannot be explained by a short half-life for synapsin. As suggested by our RNAi experiments (Fig. 6B), Aplysia synapsin has a half-life of at least 24 h, in agreement with estimates for mammalian synapsins (Daly and Ziff, 1997; Murrey et al., 2006). Rather, synapsin is probably degraded in a manner similar to 5-HT-induced degradation of the regulatory subunit of protein kinase A (PKA) (Hegde et al., 1993), C/EBP (Yamamoto et al., 1999), and the transcriptional repressor CREB1b (Upadhya et al., 2004). The mechanism and functional significance of synapsin degradation remain to be determined. The observed difference in magnitude of the 5-HT effect at the 12 and 24 h time points in ganglia and SNs (Figs. 1B2, 2B) is probably due to the fact that ganglia contain many cell types in addition to SNs.
The time course of synapsin expression is interesting in regard to processes related to LTF. In ganglia, 5-HT leads to an initial enhancement of synaptic strength that decays back to baseline 3 h after treatment, followed by an increase in strength beginning 10–15 h after treatment (Mauelshagen et al., 1996). These results are similar to the time course of PKA activity (Müller and Carew, 1998), morphological changes (Kim et al., 2003), and 5-HT-induced CREB1 expression (Liu et al., 2011b).
The regulation of synapsin by 5-HT is intrinsic and does not require the presence of a postsynaptic target. This result is consistent with previous studies showing that expression of the transcription factors CREB1, CREB2, and C/EBP, as well as translocation of MAPK, are dynamically regulated by 5-HT in the absence of follower MNs (Alberini et al., 1994; Martin et al., 1997; Liu et al., 2008, 2011a,b).
5-HT-induced transcriptional regulation of synapsin
To examine mechanisms that regulate 5-HT-induced synapsin expression, ∼1.3 kb of the Aplysia synapsin promoter region was cloned and analyzed for consensus binding sequences for various transcription factors. Several putative binding sites known to be involved in neuronal plasticity were identified. These include two consensus sequences for C/EBP binding. C/EBP is a CREB1-regulated transcription factor essential for LTF (Alberini et al., 1994; Guan et al., 2002). Whether synapsin is regulated by C/EBP and the functional significance of such regulation are potentially interesting questions for future studies. A consensus site for NF-κB was also identified. Activity of NF-κB plays a role in hippocampal synaptic plasticity (Albensi and Mattson, 2000), but Aplysia NF-κB activity does not appear to be affected by treatment with 5-HT (Povelones et al., 1997).
The promoter region of Aplysia synapsin contains a CRE motif, similar to that observed in the human and rat synapsin I promoter regions (Sauerwald et al., 1990; Südhof, 1990). Our experiments using an EGFP reporter construct driven by a synapsin promoter fragment containing the CRE sequence indicated that treatment with 5-HT activated the promoter (Fig. 4). Although the Aplysia synapsin CRE motif TGACGCAT varies from the canonical CRE motif TGACGTCA found in the human and rat synapsin I promoter, it contains the central CpG dinucleotide, which is important for strong CREB binding (Smith et al., 2007). Indeed, ChIP assays revealed that 5-HT enhances the association of CREB1 with the region of the synapsin promoter surrounding the CRE site (Fig. 5A). The enhanced binding was accompanied by an increase in associated acetylated histones and a decrease in associated HDAC (Fig. 5B). Acetylation of lysine residues on histone tails changes the structural conformation so that transcriptional regulators are able to access DNA and control gene expression. Similar modulation of chromatin structure has been associated with the regulation of gene expression during memory formation (Guan et al., 2002; Levenson and Sweatt, 2005; Chwang et al., 2006). Together, these results suggest that, upon treatment with 5-HT, CREB1 is recruited to the promoter region of synapsin and drives its expression.
In some cell types, regulation of synapsin is not affected by the cAMP cascade (Jüngling et al., 1994; Hoesche et al., 1995). This finding could be due to the fact that the neuroblastoma and PC12 cell lines used in those studies are transformed and, thus, are in a different cellular state than SNs. In addition, both of those studies used selective activation of the cAMP/PKA cascade rather than a more general agonist like 5-HT, which activates multiple second-messenger cascades. For example, in Aplysia and other systems (Karpinski et al., 1992; Jüngling et al., 1994; Vecsey et al., 2007), relief of basal repression is necessary for CREB-mediated transcription to proceed (Bartsch et al., 1995; Guan et al., 2002, 2003; Mohamed et al., 2005). 5-HT-induced recruitment of ERK appears necessary to remove repression mediated by the transcriptional repressor CREB2 (Bartsch et al., 1995; Martin et al., 1997). The combined activation of CREB1 and inhibition of CREB2-mediated repression leads to the rapid induction of immediate early genes necessary for LTF (Kandel, 2001). Therefore, it is probably the combined effect of activation of CREB1 and removal of inhibitory constraints that allows induction of synapsin, as well as of other immediate early genes essential for LTF.
Expression of LTF requires synapsin
The present study differs from previous investigations of the role of synapsin in long-term synaptic plasticity in that we acutely blocked the stimulus-induced increase in synapsin protein levels using RNAi. Three lines of evidence support our conclusion that the effect of synapsin siRNA is specific to LTF. First, during the time window of the electrophysiological experiments, basal synapsin protein levels were not altered in SNs injected with synapsin siRNA, indicating that synapsin turnover was not high enough to affect basal levels. Second, basal synaptic strength was not affected in injected cocultures, which indicated that the siRNA did not interfere with transmitter release mechanisms. Third, short-term plasticity was also not altered, which indicated that the effects of siRNA were associated only with a form of long-term plasticity that required new synapsin expression. Previously, elevated expression of synapsin has been used as a marker for enhancement of synaptic plasticity (Morimoto et al., 1998) and learning (Gómez-Pinilla et al., 2001; Rapanelli et al., 2009). However, our study reveals that elevated synapsin expression is more than a simple marker of plasticity: it is a necessary element of the signaling pathway mediating long-term synaptic facilitation.
Although increased expression of synapsin is necessary for LTF, the specific mechanism through which synapsin regulates LTF remains to be elucidated. One possibility is that the increased expression of synapsin is necessary for the synaptogenesis accompanying LTF (Glanzman et al., 1990). As a major component of presynaptic varicosities (Fornasiero et al., 2010), 5-HT-induced elevation in synapsin may be required for increased synapse formation. Consequently, blocking the 5-HT-induced increase in synapsin protein with RNAi would be expected to impair formation of new varicosities and thereby block LTF. If synapsin is found to be necessary for the morphological changes associated with LTF, a secondary question is whether it is sufficient. Evidence supporting this hypothesis comes from studies of neuroblastoma cells, which implicated synapsin in synaptogenesis (Han et al., 1991). In contrast, a previous study in Aplysia SNs suggested that an increase in synapsin may not be sufficient for synaptogenesis, because overexpression of synapsin did not increase the number of varicosities (Fioravante et al., 2007). However, this study was performed in isolated SNs devoid of postsynaptic targets. Also, in this study, the magnitude and time course of overexpression of synapsin, driven by a viral promoter, was unlikely to mimic the dynamic pattern that we observed for endogenous synapsin (Figs. 1, 2). Our study provides the first evidence that newly synthesized synapsin is required for long-term synaptic plasticity. Learning more about synapsin function at the synapse will provide insight into critical effector proteins that regulate the induction and consolidation of LTF.
Footnotes
This research was supported by NIH Grant NS019895. We thank J. Liu for preparing the cultures, W. Yao for assisting with some of the experiments, L. Morales for help with the illustrations, P. Smolen for helpful comments, and B. Kaang for providing plasmids.
References
- Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Angers A, Fioravante D, Chin J, Cleary LJ, Bean AJ, Byrne JH. Serotonin stimulates phosphorylation of Aplysia synapsin and alters its subcellular distribution in sensory neurons. J Neurosci. 2002;22:5412–5422. doi: 10.1523/JNEUROSCI.22-13-05412.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Chen M. Long-term memory in Aplysia modulates the total number of varicosities of single identified sensory neurons. Proc Natl Acad Sci U S A. 1988;85:2373–2377. doi: 10.1073/pnas.85.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Chen M. Time course of structural changes at identified sensory neuron synapses during long-term sensitization in Aplysia. J Neurosci. 1989;9:1774–1780. doi: 10.1523/JNEUROSCI.09-05-01774.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- Bernier L, Castellucci VF, Kandel ER, Schwartz JH. Facilitatory transmitter causes a selective and prolonged increase in adenosine 3′:5′-monophosphate in sensory neurons mediating the gill and siphon withdrawal reflex in Aplysia. J Neurosci. 1982;2:1682–1691. doi: 10.1523/JNEUROSCI.02-12-01682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne JH. Analysis of synaptic depression contributing to habituation of gill-withdrawal reflex in Aplysia californica. J Neurophysiol. 1982;48:431–438. doi: 10.1152/jn.1982.48.2.431. [DOI] [PubMed] [Google Scholar]
- Castellucci V, Kandel ER. Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science. 1976;194:1176–1178. doi: 10.1126/science.11560. [DOI] [PubMed] [Google Scholar]
- Cesca F, Baldelli P, Valtorta F, Benfenati F. The synapsins: key actors of synapse function and plasticity. Prog Neurobiol. 2010;91:313–348. doi: 10.1016/j.pneurobio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Chwang WB, O'Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary LJ, Lee WL, Byrne JH. Cellular correlates of long-term sensitization in Aplysia. J Neurosci. 1998;18:5988–5998. doi: 10.1523/JNEUROSCI.18-15-05988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi A, Zanardi A, Giacomini C, Onofri F, Valtorta F, Zoli M, Benfenati F. Synapsin-I- and synapsin-II-null mice display an increased age-dependent cognitive impairment. J Cell Sci. 2008;121:3042–3051. doi: 10.1242/jcs.035063. [DOI] [PubMed] [Google Scholar]
- Daly C, Ziff EB. Post-transcriptional regulation of synaptic vesicle protein expression and the developmental control of synaptic vesicle formation. J Neurosci. 1997;17:2365–2375. doi: 10.1523/JNEUROSCI.17-07-02365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Moore AN. Characterization and phosphorylation of CREB-like proteins in Aplysia central nervous system. Brain Res Mol Brain Res. 1996;39:43–51. doi: 10.1016/0169-328x(95)00350-2. [DOI] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Emptage NJ, Mauelshagen J, Mercer A, Carew TJ. Pharmacological dissociation of different forms of synaptic plasticity in the marine mollusc Aplysia. J Physiol Paris. 1996;90:385–386. doi: 10.1016/s0928-4257(97)87925-9. [DOI] [PubMed] [Google Scholar]
- Fioravante D, Liu RY, Netek AK, Cleary LJ, Byrne JH. Synapsin regulates basal synaptic strength, synaptic depression, and serotonin-induced facilitation of sensorimotor synapses in Aplysia. J Neurophysiol. 2007;98:3568–3580. doi: 10.1152/jn.00604.2007. [DOI] [PubMed] [Google Scholar]
- Fioravante D, Liu RY, Byrne JH. The ubiquitin-proteasome system is necessary for long-term synaptic depression in Aplysia. J Neurosci. 2008;28:10245–10256. doi: 10.1523/JNEUROSCI.2139-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher TL, Cameron P, De Camilli P, Banker G. The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture. J Neurosci. 1991;11:1617–1626. doi: 10.1523/JNEUROSCI.11-06-01617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornasiero EF, Bonanomi D, Benfenati F, Valtorta F. The role of synapsins in neuronal development. Cell Mol Life Sci. 2010;67:1383–1396. doi: 10.1007/s00018-009-0227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost WN, Castellucci VF, Hawkins RD, Kandel ER. Mono-synaptic connections made by the sensory neurons of the gill-withdrawal and siphon-withdrawal reflex in Aplysia participate in the storage of long-term-memory for sensitization. Proc Natl Acad Sci U S A. 1985;82:8266–8269. doi: 10.1073/pnas.82.23.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D, Takagishi Y, Feng J, Ren Y, Rodriguiz RM, Wetsel WC, Greengard P, Augustine GJ. Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J Neurosci. 2004;24:11368–11380. doi: 10.1523/JNEUROSCI.3795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzman DL, Kandel ER, Schacher S. Target-dependent structural changes accompanying long-term synaptic facilitation in Aplysia neurons. Science. 1990;249:799–802. doi: 10.1126/science.2389145. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Reisch D, Diegelmann S, Eberle K, Funk N, Heisenberg M, Hoppe V, Hoppe J, Klagges BR, Martin JR, Nikitina EA, Putz G, Reifegerste R, Reisch N, Rister J, Schaupp M, Scholz H, Schwärzel M, Werner U, Zars TD, et al. Flies lacking all synapsins are unexpectedly healthy but are impaired in complex behaviour. Eur J Neurosci. 2004;20:611–622. doi: 10.1111/j.1460-9568.2004.03527.x. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F, So V, Kesslak JP. Spatial learning induces neurotrophin receptor and synapsin I in the hippocampus. Brain Res. 2001;904:13–19. doi: 10.1016/s0006-8993(01)02394-0. [DOI] [PubMed] [Google Scholar]
- Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, Schwartz JH, Thanos D, Kandel ER. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111:483–493. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- Guan Z, Kim JH, Lomvardas S, Holick K, Xu S, Kandel ER, Schwartz JH. p38 MAP kinase mediates both short-term and long-term synaptic depression in Aplysia. J Neurosci. 2003;23:7317–7325. doi: 10.1523/JNEUROSCI.23-19-07317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HQ, Nichols RA, Rubin MR, Bähler M, Greengard P. Induction of formation of presynaptic terminals in neuroblastoma cells by synapsin IIb. Nature. 1991;349:697–700. doi: 10.1038/349697a0. [DOI] [PubMed] [Google Scholar]
- Hegde AN, Goldberg AL, Schwartz JH. Regulatory subunits of cAMP-dependent protein kinases are degraded after conjugation to ubiquitin: a molecular mechanism underlying long-term synaptic plasticity. Proc Natl Acad Sci U S A. 1993;90:7436–7440. doi: 10.1073/pnas.90.16.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoesche C, Bartsch P, Kilimann MW. The CRE consensus sequence in the synapsin I gene promoter region confers constitutive activation but no regulation by cAMP in neuroblastoma cells. Biochim Biophys Acta. 1995;1261:249–256. doi: 10.1016/0167-4781(95)00014-8. [DOI] [PubMed] [Google Scholar]
- Jüngling S, Cibelli G, Czardybon M, Gerdes HH, Thiel G. Differential regulation of chromogranin B and synapsin I gene promoter activity by cAMP and cAMP-dependent protein kinase. Eur J Biochem. 1994;226:925–935. doi: 10.1111/j.1432-1033.1994.00925.x. [DOI] [PubMed] [Google Scholar]
- Kaang BK. Parameters influencing ectopic gene expression in Aplysia neurons. Neurosci Lett. 1996;221:29–32. doi: 10.1016/s0304-3940(96)13279-1. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Karpinski BA, Morle GD, Huggenvik J, Uhler MD, Leiden JM. Molecular cloning of human CREB-2: an ATF/CREB transcription factor that can negatively regulate transcription from the cAMP response element. Proc Natl Acad Sci U S A. 1992;89:4820–4824. doi: 10.1073/pnas.89.11.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Udo H, Li HL, Youn TY, Chen M, Kandel ER, Bailey CH. Presynaptic activation of silent synapses and growth of new synapses contribute to intermediate and long-term facilitation in Aplysia. Neuron. 2003;40:151–165. doi: 10.1016/s0896-6273(03)00595-6. [DOI] [PubMed] [Google Scholar]
- Knapek S, Gerber B, Tanimoto H. Synapsin is selectively required for anesthesia-sensitive memory. Learn Mem. 2010;17:76–79. doi: 10.1101/lm.1661810. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- Liu RY, Fioravante D, Shah S, Byrne JH. cAMP response element-binding protein 1 feedback loop is necessary for consolidation of long-term synaptic facilitation in Aplysia. J Neurosci. 2008;28:1970–1976. doi: 10.1523/JNEUROSCI.3848-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RY, Shah S, Cleary LJ, Byrne JH. Serotonin- and training-induced dynamic regulation of CREB2 in Aplysia. Learn Mem. 2011a;18:245–249. doi: 10.1101/lm.2112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RY, Cleary LJ, Byrne JH. The requirement for enhanced CREB1 expression in consolidation of long-term synaptic facilitation and long-term excitability in sensory neurons of Aplysia. J Neurosci. 2011b;31:6871–6879. doi: 10.1523/JNEUROSCI.5071-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Greengard P, Poo MM. Exogenous synapsin I promotes functional maturation of developing neuromuscular synapses. Neuron. 1992;8:521–529. doi: 10.1016/0896-6273(92)90280-q. [DOI] [PubMed] [Google Scholar]
- Lu Q, Hutchins AE, Doyle CM, Lundblad JR, Kwok RP. Acetylation of cAMP-responsive element-binding protein (CREB) by CREB-binding protein enhances CREB-dependent transcription. J Biol Chem. 2003;278:15727–15734. doi: 10.1074/jbc.M300546200. [DOI] [PubMed] [Google Scholar]
- Lynch MA, Voss KL, Rodriguez J, Bliss TV. Increase in synaptic vesicle proteins accompanies long-term potentiation in the dentate gyrus. Neuroscience. 1994;60:1–5. doi: 10.1016/0306-4522(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Martin KC, Michael D, Rose JC, Barad M, Casadio A, Zhu H, Kandel ER. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- Mauelshagen J, Parker GR, Carew TJ. Dynamics of induction and expression of long-term synaptic facilitation in Aplysia. J Neurosci. 1996;16:7099–7108. doi: 10.1523/JNEUROSCI.16-22-07099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauelshagen J, Sherff CM, Carew TJ. Differential induction of long-term synaptic facilitation by spaced and massed applications of serotonin at sensory neuron synapses of Aplysia californica. Learn Mem. 1998;5:246–256. [PMC free article] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Michels B, Chen YC, Saumweber T, Mishra D, Tanimoto H, Schmid B, Engmann O, Gerber B. Cellular site and molecular mode of synapsin action in associative learning. Learn Mem. 2011;18:332–344. doi: 10.1101/lm.2101411. [DOI] [PubMed] [Google Scholar]
- Mohamed HA, Yao W, Fioravante D, Smolen PD, Byrne JH. cAMP-response elements in Aplysia creb1, creb2, and Ap-uch promoters: implications for feedback loops modulating long term memory. J Biol Chem. 2005;280:27035–27043. doi: 10.1074/jbc.M502541200. [DOI] [PubMed] [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Montminy MR, Sevarino KA, Wagner JA, Mandel G, Goodman RH. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986;83:6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto K, Sato K, Sato S, Yamada N, Hayabara T. Time-dependent changes in rat hippocampal synapsin I mRNA expression during long-term potentiation. Brain Res. 1998;783:57–62. doi: 10.1016/s0006-8993(97)01154-2. [DOI] [PubMed] [Google Scholar]
- Müller U, Carew TJ. Serotonin induces temporally and mechanistically distinct phases of persistent PKA activity in Aplysia sensory neurons. Neuron. 1998;21:1423–1434. doi: 10.1016/s0896-6273(00)80660-1. [DOI] [PubMed] [Google Scholar]
- Murrey HE, Gama CI, Kalovidouris SA, Luo WI, Driggers EM, Porton B, Hsieh-Wilson LC. Protein fucosylation regulates synapsin Ia/Ib expression and neuronal morphology in primary hippocampal neurons. Proc Natl Acad Sci U S A. 2006;103:21–26. doi: 10.1073/pnas.0503381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr KA, Byrne JH. Membrane responses and changes in cAMP levels in Aplysia sensory neurons produced by serotonin, tryptamine, FMRFamide and small cardioactive peptideB (SCPB) Neurosci Lett. 1985;55:113–118. doi: 10.1016/0304-3940(85)90004-7. [DOI] [PubMed] [Google Scholar]
- Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- Povelones M, Tran K, Thanos D, Ambron RT. An NF-κB-like transcription factor in axoplasm is rapidly inactivated after nerve injury in Aplysia. J Neurosci. 1997;17:4915–4920. doi: 10.1523/JNEUROSCI.17-13-04915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapanelli M, Frick LR, Zanutto BS. Differential gene expression in the rat hippocampus during learning of an operant conditioning task. Neuroscience. 2009;163:1031–1038. doi: 10.1016/j.neuroscience.2009.07.037. [DOI] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Südhof TC. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- Sato K, Morimoto K, Suemaru S, Sato T, Yamada N. Increased synapsin I immunoreactivity during long-term potentiation in rat hippocampus. Brain Res. 2000;872:219–222. doi: 10.1016/s0006-8993(00)02460-4. [DOI] [PubMed] [Google Scholar]
- Sauerwald A, Hoesche C, Oschwald R, Kilimann MW. The 5′-flanking region of the synapsin I gene. A G+C-rich, TATA- and CAAT-less, phylogenetically conserved sequence with cell type-specific promoter function. J Biol Chem. 1990;265:14932–14937. [PubMed] [Google Scholar]
- Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Rosahl TW, Chapman PF, Marowitz Z, Friedman E, Frankland PW, Cestari V, Cioffi D, Südhof TC, Bourtchuladze R. Impaired learning in mice with abnormal short-lived plasticity. Curr Biol. 1996;6:1509–1518. doi: 10.1016/s0960-9822(96)00756-7. [DOI] [PubMed] [Google Scholar]
- Smith B, Fang H, Pan Y, Walker PR, Famili AF, Sikorska M. Evolution of motif variants and positional bias of the cyclic-AMP response element. BMC Evol Biol. 2007;7(Suppl 1):S15. doi: 10.1186/1471-2148-7-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. The structure of the human synapsin I gene and protein. J Biol Chem. 1990;265:7849–7852. [PubMed] [Google Scholar]
- Upadhya SC, Smith TK, Hegde AN. Ubiquitin-proteasome-mediated CREB repressor degradation during induction of long-term facilitation. J Neurochem. 2004;91:210–219. doi: 10.1111/j.1471-4159.2004.02707.x. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, Wood MA. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velho TA, Mello CV. Synapsins are late activity-induced genes regulated by birdsong. J Neurosci. 2008;28:11871–11882. doi: 10.1523/JNEUROSCI.2307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright ML, Zhang H, Byrne JH, Cleary LJ. Localized neuronal outgrowth induced by long-term sensitization training in Aplysia. J Neurosci. 2002;22:4132–4141. doi: 10.1523/JNEUROSCI.22-10-04132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Hegde AN, Chain DG, Schwartz JH. Activation and degradation of the transcription factor C/EBP during long-term facilitation in Aplysia. J Neurochem. 1999;73:2415–2423. doi: 10.1046/j.1471-4159.1999.0732415.x. [DOI] [PubMed] [Google Scholar]
- Zhang F, Endo S, Cleary LJ, Eskin A, Byrne JH. Role of transforming growth factor-beta in long-term synaptic facilitation in Aplysia. Science. 1997;275:1318–1320. doi: 10.1126/science.275.5304.1318. [DOI] [PubMed] [Google Scholar]