Abstract
Background
We assessed the contribution of endothelium-derived hyperpolarizing factors (EDHFs) to resting and agonist-stimulated vasodilator tone in health and disease. Tetraethylammonium chloride (TEA) was employed to inhibit K+Ca channel activation and fluconazole to inhibit cytochrome P450 2C9-mediated epoxyeicosatrienoic acid synthesis. We hypothesized that 1) EDHFs contribute to resting vascular tone by K+Ca channel activation and epoxyeicosatrienoic acid release, and 2) EDHFs compensate for reduced nitric oxide bioavailability at rest and with endothelium-dependent vasodilators.
Methods and Results
In 103 healthy subjects and 71 non-hypertensive subjects with multiple risk factors, resting forearm blood flow (FBF) was measured using venous occlusion plethysmography before and after intra-arterial infusions of NG-monomethyl-L-arginine (L-NMMA), TEA, fluconazole, and their combination. The effects of these antagonists on resting FBF, and on bradykinin- and acetylcholine-mediated vasodilation was studied.
Resting FBF decreased with TEA and L-NMMA in all subjects (P<0.001), however, the vasoconstrictor response to L-NMMA was greater (p=0.04) and to TEA lower (p=0.04) in healthy subjects compared to those with risk factors. Fluconazole decreased resting FBF in all subjects and addition of TEA further reduced FBF after fluconazole, suggesting that cytochrome P450 metabolites and other hyperpolarizing factor(s) activate K+Ca channels.
Both L-NMMA and TEA attenuated bradykinin-mediated vasodilation in healthy and hypercholesterolemic subjects (P<0.001). In contrast, acetylcholine-mediated vasodilation remained unchanged with TEA in healthy subjects, but was significantly attenuated in hypercholesterolemia (P<0.04).
Conclusions
Firstly, EDHFs by activating TEA inhibitable K+Ca channels together with NO contribute to resting microvascular dilator tone. The contribution of K+Ca channel activation compared to NO is greater in those with multiple risk factors compared to healthy subjects. Second, activation of K+Ca channels is only partly through epoxyeicosatrienoic acid release, indicating presence of other hyperpolarizing mechanisms. Third, bradykinin, but not acetylcholine stimulates K+Ca channel-mediated vasodilation in healthy subjects, whereas in hypercholesterolemia, K+Ca channel-mediated vasodilation compensates for the reduced NO activity. Thus, enhanced EDHF activity in conditions of NO deficiency contributes to maintenance of resting and agonist-stimulated vasodilation.
Clinical Trial Registration Information: http://clinicaltrials.gov/, Identifier: NCT00166166
Keywords: endothelium-derived factors, vasodilation, endothelial function, endothelium-derived hyperpolarizing factor, nitric oxide
Introduction
The endothelium contributes to vasodilation by releasing a variety of paracrine factors. Persistent endothelium-dependent vasodilation after inhibition of both nitric oxide (NO) and prostacyclin has been attributed to endothelium derived hyperpolarizing factor (EDHF) activity.1-4 Tonic release of NO contributes to resting vasodilator tone5-7 and to physiologic vasodilation during exercise,2 and its activity is significantly impaired in individuals with cardiovascular risk factors.7-10 In contrast, the contribution of EDHF to vasodilator tone in the human circulation, and the impact of risk factors that impair NO bioavailability on EDHF activity in vivo remain unknown.6, 11, 12
In the human vasculature, endothelium-dependent hyperpolarization is at least partly attributed to the release of epoxyeicosatrienoic acids (EETs) from cytochrome P450 (CYP450)-dependent metabolism of arachidonic acid. EETs promote vasodilation by stimulation of small and large calcium-dependent potassium channels (K+Ca) on endothelial cells, with subsequent membrane hyperpolarization and vasodilation.13, 14 Agonists such as bradykinin stimulate endothelial G-protein-coupled receptors provoking increase in intracellular calcium leading to opening of endothelial K+Ca channels and triggering the EDHF phenomena that includes: (1) synthesis of EETs, (2) transmission of endothelial cell hyperpolarization to the vascular smooth muscle via gap junctions, and/or (3) release of K+ from the endothelial cells, that in turn induces smooth muscle vasodilation by activating several other K+ channels.15-17 Experimentally, a combination of apamin plus charybdotoxin is used to specifically inhibit EDHF responses due to K+Ca activation, whereas in clinical studies, tetraethylammonium chloride (TEA) has been used as a K+Ca channel inhibitor.18 19, 20 The role of EETs as potential EDHFs has been studied in humans using azoles such as miconazole, fluconazole and sulphaphenazole that selectively inhibit epoxidation (EET generation) of arachidonic acid.11, 13, 21
In this study, we measured the contribution of EDHF to resting vasomotor tone in the human forearm microcirculation in health and disease, both in the presence and absence of NO, with the hypothesis that activation of K+Ca channels and/or cytochrome P450 metabolites contributes to endothelium-dependent hyperpolarization, and that this contribution is altered in disease states with endothelial dysfunction. Second, we explored the contribution of EDHF to endothelium-dependent vasodilation, both in the presence and absence of NO, with the hypothesis that activation of K+Ca channels in response to acetylcholine and bradykinin contributes to forearm microvascular vasodilation and that this contribution is altered in hypercholesterolemic compared to the healthy circulation.
Methods
Subjects
Healthy and non-hypertensive subjects with multiple risk factors aged between 21 and 60 years were enrolled into 4 separate protocols that explored 1) the contribution of EDHF to resting tone in healthy subjects and those with cardiovascular risk factors that included hypercholesterolemia and diabetes, 2) the contribution of EETs to resting tone, 3) the differential contribution of EETs and K+Ca channel activation to resting tone, and 4) the contribution of EDHF to agonist-stimulated vasodilation in healthy and hypercholesterolemic subjects. All subjects were non-smokers and free from hypertension, cardiovascular disease, and systemic disorders. Subjects with risk factors (Protocols 1 to 3) were older, had similar blood pressure, a greater body mass index (BMI), higher fasting levels of glucose, total cholesterol, low density lipoprotein cholesterol, and triglycerides, and lower levels of high density lipoprotein cholesterol compared to healthy subjects (Table 1). Subjects in Protocol 4 had similar significant differences in cholesterol and its subfractions compared to healthy subjects, but were matched for all other factors. All healthy subjects and those with hypercholesterolemia were not taking any medications; diabetics discontinued their insulin or metformin the night before, and one subject on sulphonylureas discontinued it before enrolment. Pregnant females were excluded. The study was approved by the Emory University Institutional Review Board and all subjects yielded informed consent.
Table 1. Characteristics of Study Subjects in Protocol 1.
| Healthy | Risk factors | |
|---|---|---|
| 71 | ||
| Age (years) | 34±11 | 46±12* |
| Male/Female | 54/49 | 39/32 |
| White | 46 (45%) | 20 (29%) |
| African American | 47 (46%) | 50 (70%) |
| Other | 10 (9%) | 1 (1%) |
| Hypercholesterolemia | 0 | 48 |
| Diabetes | 0 | 23 |
| Systolic BP (mm Hg) | 115±12 | 121±14 |
| Diastolic BP (mm Hg) | 69±11 | 72±10 |
| Height (cm) | 171±10 | 172±10 |
| Weight (Kg) | 76±15 | 89±18* |
| Body mass index | 26.0±5 | 29.9±5* |
| Glucose (mg/dL) | 85±10 | 134±86* |
| Triglycerides (mg/dL) | 87±63 | 151±91* |
| Total Cholesterol (mg/dL) | 168±28 | 226±43* |
| High Density Lipoprotein Cholesterol (mg/dL) | 55±11 | 49±13* |
| Low Density Lipoprotein Cholesterol (mg/dL) | 96±22 | 149±41* |
Data are mean ± SD.
Indicates a statistically significant (p < 0.05; t test) difference between groups.
Measurement of forearm blood flow
Measurements were performed after an overnight fast in a quiet temperature-controlled (22 to 24°C) room. Subjects refrained from exercise, alcohol and caffeine for at least 24 hours beforehand. After insertion of an intra-brachial arterial cannula for arterial pressure monitoring and delivery of drug infusions, subjects received oral aspirin (975mg) to inhibit prostacyclin synthesis at least 1 hour prior to the study.22 Simultaneous forearm blood flow (FBF) measurements were obtained in both arms using a dual-channel venous occlusion strain gauge plethysmograph (model EC6, DE Hokanson, Bellevue, WA) as described previously.2 Flow measurements were recorded for approximately 7 seconds, every 15 seconds up to eight times and a mean FBF value in mL-min-1·100 mL-1 was computed. Forearm vascular resistance (FVR) was calculated as the mean arterial pressure ÷ FBF and expressed as mmHg per mL-min-1·100 mL-1.
Protocol 1: Contribution of NO and K+Ca channel activation to resting vasodilator tone
All agents were administered intra-arterially after resting measurements were made during saline infusion (2.5 ml/min). In 37 healthy and 25 subjects with risk factors, FBF was measured during inhibition of NO synthesis during a 5- minute infusion of L-NMMA (Clinalfa, Switzerland) given at 8 μmol/min, that was shown to attenuate resting and agonist-stimulated FBF previously.2 While continuing L-NMMA, intra-arterial TEA (Clinalfa, Switzerland) at 1 mg/min was infused for 5 minutes to investigate the effect of combined blockade of NO and K+Ca channels on resting vessel tone. When given at 0.25 to 1 mg/min, TEA selectively inhibits K+Ca channels, but reduces FBF with bradykinin only at 1 mg/min (<0.6μmol/min).6, 12 19, 20 In separate experiments in 41 healthy subjects and 32 subjects with risk factors, TEA was infused first in order to investigate the effects of blockade of K+Ca channels without prior NO inhibition. This was followed by combined infusions of TEA and L-NMMA. Arterial blood pressure and FBF were measured in the last 2 minutes of each intervention.
Protocol 2: Contribution of cytochrome P450 metabolites to resting vasodilator tone
In 26 healthy and 7 subjects with risk factors, FBF was measured at rest and after fluconazole (Pfizer, New York, NY) given at 0.4 μmol/min for 5 minutes in order to investigate the effects of epoxide inhibition without prior NO blockade, and after combined infusions of fluconazole and L-NMMA 8 μmol/min in order to investigate the effects of combined blockade of NO and cytochrome P450 epoxide synthesis. In separate experiments in 8 healthy and 7 subjects with risk factors, FBF was measured at rest and after L-NMMA 8 μmol/min for 5 minutes, and after combined infusion of L-NMMA and fluconazole.
Protocol 3: Comparative contribution of cytochrome P450 metabolites and K+Ca channels to resting vasodilator tone
In 19 healthy subjects, the differential contribution of cytochrome P450 derived epoxides and K+Ca channel activation to resting vasodilator tone was investigated. FBF was measured at rest and after infusion of fluconazole (0.4 μmol/min) for 5 minutes, and finally after combined infusions of fluconazole and TEA at 1 mg/min for 5 mins.
Protocol 4: Contribution of K+Ca channel activation to bradykinin and acetylcholine-mediated vasodilation in healthy and hypercholesterolemic vasculature
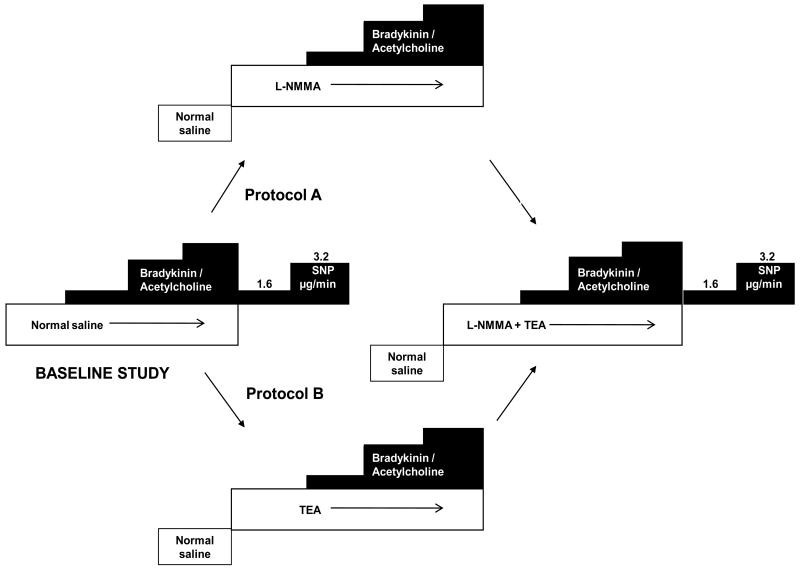
Fifty-four healthy and 44 subjects with hypercholesterolemia participated in this protocol. In 27 healthy and 24 hypercholesterolemic subjects FBF was measured at rest, after intra-arterial infusion of the endothelium-dependent vasodilators, bradykinin (Clinalfa, Switzerland) at 100, 200 and 400ng/min, or acetylcholine (Novartis, East Hanover, NJ) at 7.5, 15 and 30 μg/min, followed by intra-arterial infusion of the endothelium-independent vasodilator, sodium nitroprusside at 1.6 and 3.2 mg/min for 5 minutes each (Figure 1). This was followed by infusion of L-NMMA (8 μmol/min) for 5 minutes after which escalating doses of either bradykinin or acetylcholine were repeated. After a 30-minute rest period, TEA at 1 mg/min was commenced for 5 minutes, and while continuing L-NMMA and TEA infusions, bradykinin or acetylcholine infusions, and sodium nitroprusside infusions were repeated. This protocol enabled measurement of the contribution of NO and combined NO and K+Ca channel activation to endothelium-dependent and -independent vasodilation.
Figure 1. Study Design for Protocol 4.
Aspirin (975mg) was administered 1hour prior to commencement of the study. FBF was measured with bradykinin (100, 200 and 400 ng/min) or acetylcholine (7.5, 15 and 30 μg/min), followed by sodium nitroprusside (1.6, 3.2 μg/min). Measurements were repeated after L-NMMA (A), K+Ca channel blockade with TEA (B), and after combined blockade with L-NMMA and TEA.
Finally, to investigate the effect of inhibition of K+Ca channels on agonist-mediated vasodilation without prior blockade of NO, we studied another 27 healthy and 20 hypercholesterolemic subjects. After measurement of FBF during bradykinin or acetylcholine infusions, TEA was infused followed by repeat infusions of bradykinin or acetylcholine. Finally, combined infusions of TEA and L-NMMA were given followed by repeat infusions of bradykinin or acetylcholine, and sodium nitroprusside (Figure 1). This enabled assessment of the contribution of K+Ca channel activation without blockade of NO to agonist-stimulated endothelium-dependent and -independent vasodilation.
Statistical analysis
In protocol 1, 2 and 3, to assess the treatment effect of TEA, L-NMMA, Fluconazole and their combination within healthy and risk factor groups, paired t-test was used to compare single blockade vs. baseline, and to compare combined blockade vs. single blockade. Then, to compare the magnitude of treatment effects between the healthy and risk factor groups (in protocol 1 and 2), unpaired t-test was used to compare the changes of single blockade upon baseline, as well as the changes of combined blockade upon single blockade. Multivariable regression analysis was performed by fitting regression models with each of the factors in Table 1 (age, gender, body mass index, blood pressure, glucose and lipid levels) for possible confounding effects. Pearson's correlation coefficient was used to assess the correlation between the percentage changes in FBF with L-NMMA versus TEA.
In protocol 4, the treatment effects in FBF and FVR are assessed within subjects for baseline vs. single blockade vs. combined blockade by repeated measures analysis of variance (ANOVA) with treatment levels as main effect. Then the magnitude of the treatment effects is compared between healthy and hypercholesterolemic subjects by repeated measures ANOVA with health status groups as main effect. Possible confounding effects were controlled for age, gender, blood pressure, body mass index and low density lipoprotein levels in a stepwise fashion. Reported p-values are based on main effects.
Results are presented as mean ±SD in the text, and as least squared means and standard errors in the figures. Bonferroni's method was used for adjustment for multiple comparisons for comparing baseline, TEA, L-NMMA, Fluconazole, and combinations. Reported p-values are p-values before adjustment.
Results
Relative contribution of K+Ca channel activation and NO to resting vasodilator tone
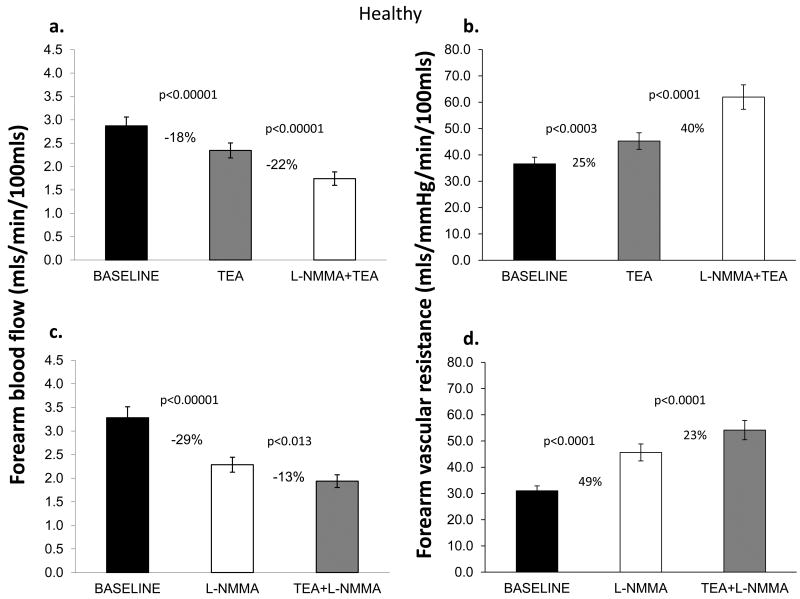
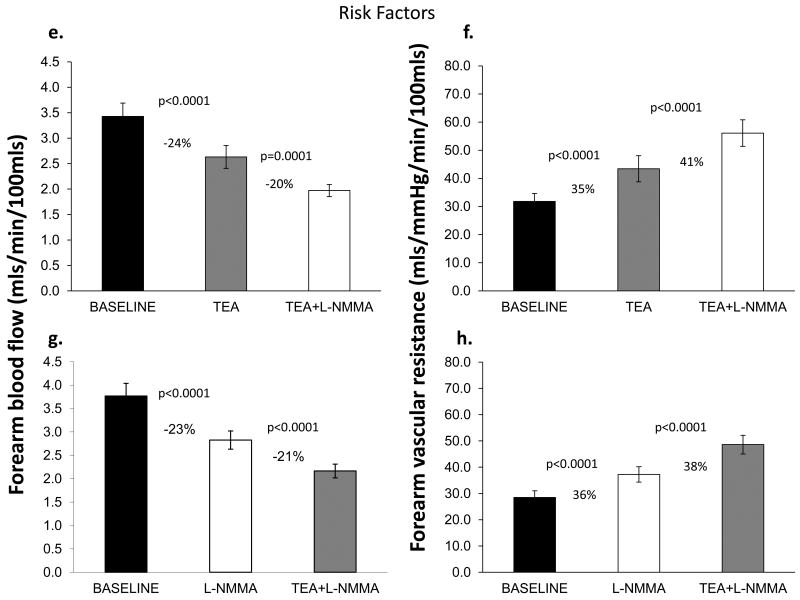
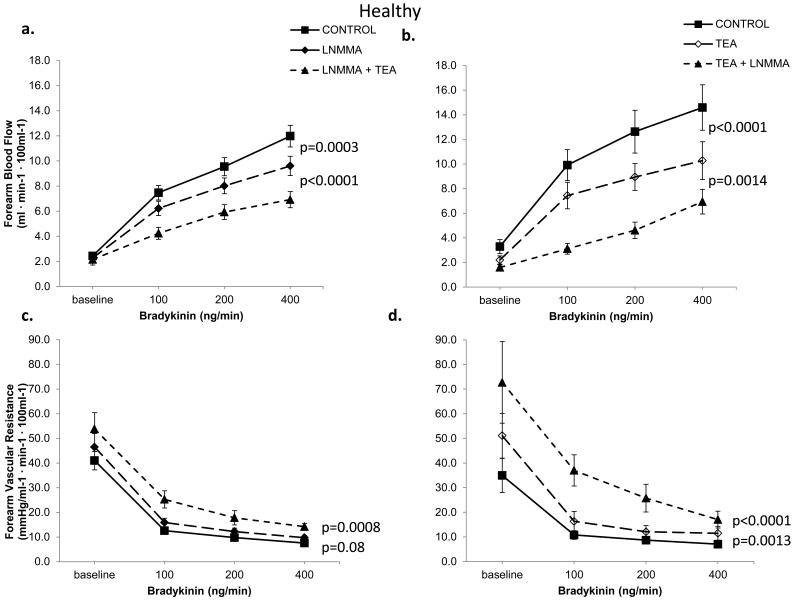
In Protocol 1, TEA infusion produced vasoconstriction; resting FBF decreased by 18±16% (P<0.0001) in healthy subjects and by 24±13% (P<0.0001) in those with risk factors, indicating significant contribution of K+Ca channel activation to resting microvascular vasodilator tone. The magnitude of vasoconstriction with TEA was lower in healthy subjects compared to those with risk factors (P=0.04). Addition of L-NMMA to TEA further reduced FBF in both groups (Figures 2a, 2b, 2e, 2f).
Figure 2. Contribution of NO and K+Ca channel activation to resting FBF and vascular resistance.
Change in resting FBF and vascular resistance in healthy subjects and those with risk factors; Upper panels depict responses to TEA alone and combined TEA and L-NMMA infusions. Lower panels show responses with L-NMMA alone followed by L-NMMA and TEA infusions. Data presented as mean ± SEM.
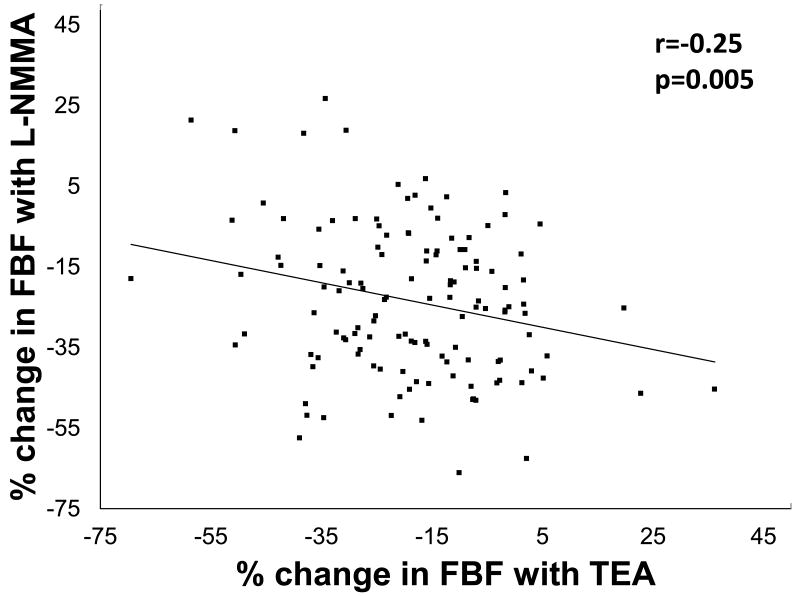
When L-NMMA was infused first, resting FBF decreased by 29±17% (P<0.0001) in healthy subjects and by 23±15% (P<0.0001) in those with risk factors. Vasoconstriction with L-NMMA was greater in healthy subjects than in those with risk factors (P=0.04). Addition of TEA to L-NMMA further reduced FBF in both groups indicating a significant contribution of K+Ca channel activation to resting vasodilator tone, even after NO blockade (Figure 2c, 2d, 2g, 2h). Thus, there was greater contribution of K+Ca channel activation and a lower contribution of NO to resting microvascular vasodilator tone in those with risk factors compared to healthy subjects. Moreover, in the entire population there was an inverse relationship between the constrictor responses to L-NMMA and TEA, indicating that reduced NO bioavailability is accompanied by higher K+Ca channel activity, and vice versa (Figure 3). The reduction in FBF after dual blockade was similar in healthy subjects and those with risk factors (38±17% vs. 39±17% reduction in FBF, P=0.78).
Figure 3. Relationship between contribution of NO and K+Ca channel activation to resting vasodilator tone.
Correlation between the percent change in FBF with L-NMMA and TEA (n=131).
Multivariate analysis was performed to determine whether demographic/risk factors including age, sex, body mass index, total, low density, and high density cholesterol, glucose, or systolic blood pressure were determinants of the flow responses to L-NMMA and TEA. The multivariate determinants of the constrictor response with L-NMMA were low density cholesterol (P=0.0002), total cholesterol (P=0.0003), systolic blood pressure (P=0.0016), and fasting glucose (P=0.003), and multivariate determinants of the vasoconstrictor response to TEA were LDL levels (P=0.05) and the L-NMMA response (P=0.006).
Contribution of cytochrome P450 metabolites to resting vasodilator tone
In Protocol 2, infusion of fluconazole decreased FBF by 13±16% (P=0.001) in healthy subjects and by 17±13% (P=0.04) in those with risk factors, indicating a significant contribution of cytochrome P450 metabolites to resting vasodilator tone. Infusion of L-NMMA after fluconazole further reduced FBF by an additional 25±13% (P=0.02) in healthy subjects, changes that were similar to those observed in subjects with risk factors. When L-NMMA was infused before fluconazole, resting FBF decreased by 37±12% (P=0.002) in healthy subjects and by 31±14% (P=0.009) in subjects with risk factors. In the presence of L-NMMA, infusion of fluconazole further reduced FBF by 26±22% (P=0.03) in both groups. Reduction in FBF with fluconazole tended to be greater after inhibition of NO than when it was given in the presence of NO (26±22% vs. 13±16% reduction in FBF, P=0.07).
Comparative contribution of cytochrome P450 metabolites and K+Ca channel activation
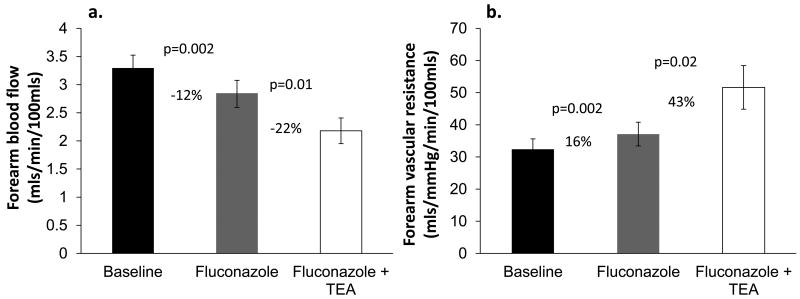
To assess the relative contribution of the two potential hyperpolarization mechanisms (cytochrome P450 metabolites and K+Ca channel activation) to resting vasodilator tone, individual and combined blockade with fluconazole and TEA were studied in Protocol 3. In healthy subjects, fluconazole infusion reduced FBF by 12±13% (P=0.002) and addition of TEA further reduced FBF by 22±23% (P=0.01), indicating that hyperpolarizing factor(s) beyond cytochrome P450 metabolites are activating K+Ca channels at rest (Figures 4a, 4b).
Figures 4. Contribution of cytochrome P450 metabolites and K+Ca channel activation to resting FBF and vascular resistance.
Change in (a) resting FBF and (b) vascular resistance with fluconazole alone and combined infusions of fluconazole and TEA. Mean ± SEM.
Contribution of K+Ca channel activation and NO to bradykinin responses
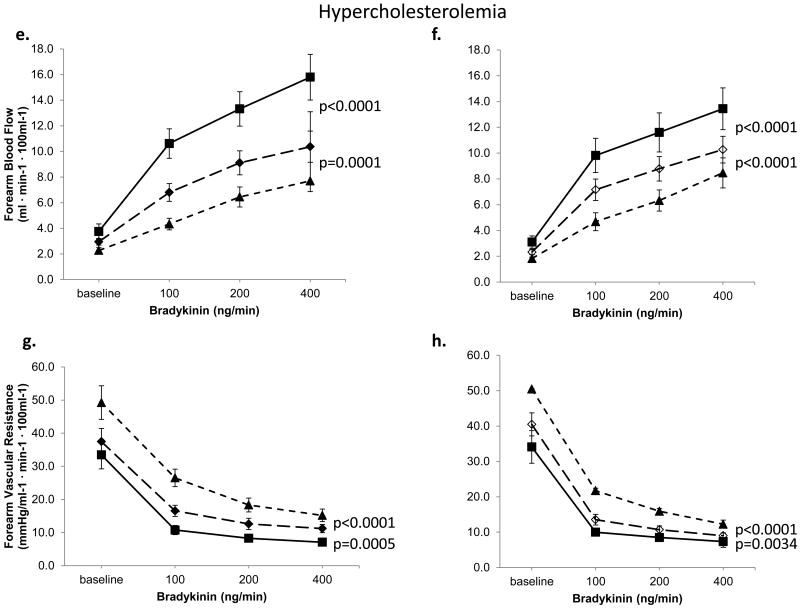
Studies were performed in healthy and hypercholesterolemic subjects in Protocol 4. Bradykinin infusion resulted in dose-related forearm vasodilation that was similar in magnitude in healthy (n=29) and hypercholesterolemic (n=26) subjects, (both P<0.0001, Figure 5). L-NMMA lowered FBF by 17%, P=0.0003 in healthy subjects (Figure 5a, 5c), and by 33%, P<0.0001 in hypercholesterolemic subjects (Figure 5e, 5g). Bradykinin-mediated vasodilation was also attenuated by TEA in the absence of L-NMMA in both groups. Thus, in healthy subjects, FBF was 25%, P<0.0001 lower (Figure 5b, 5d) after TEA and by 25%, P<0.0001 in those with hypercholesterolemia (Figure 5f, 5h). Bradykinin-induced vasodilation was further attenuated after addition of L-NMMA to TEA in both groups.
Figure 5. Contribution of NO and K+Ca channel activation to bradykinin-mediated vasodilation.
FBF and vascular resistance in response to bradykinin in healthy and hypercholesterolemic subjects. Left panels demonstrate effect of L-NMMA, and combined blockade with L-NMMA and TEA, in 17 healthy and 16 hypercholesterolemic subjects. Right panels depict effects of TEA and combined infusions of TEA and L-NMMA, in 12 healthy and 10 hypercholesterolemic subjects. Mean ± SEM
Contribution of K+Ca channel activation and NO to acetylcholine responses
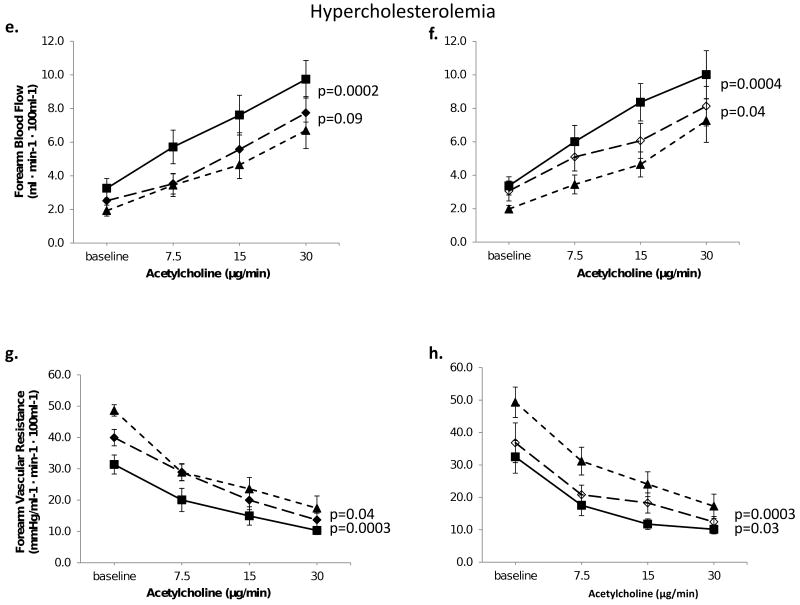
Acetylcholine produced dose-related forearm vasodilation that was significantly lower in hypercholesterolemic compared to healthy subjects (P=0.05), as previously described.2 L-NMMA attenuated acetylcholine-stimulated FBF increase by 28%, P=0.0006 (Figure 6a, 6c) in healthy subjects and by 26%, P=0.0002 in those with hypercholesterolemia (Figure 6e, 6g). Addition of TEA to L-NMMA produced no further inhibition of acetylcholine-induced vasodilation (P=0.19) in healthy subjects (Figure 6a, 6c), but in those with hypercholesterolemia, there was a 10% reduction in FBF, P=0.09 and 21% increase in FVR, P=0.04 (Figure 6e, 6g).
Figure 6. Contribution of NO and K+Ca channel activation to acetylcholine-stimulated vasodilation in healthy and hypercholesterolemic forearm vasculature.
FBF and vascular resistance in response to acetylcholine in healthy and hypercholesterolemic subjects. Left panels demonstrate effect of L-NMMA, and combined blockade with L-NMMA and TEA, in 10 healthy and 8 hypercholesterolemic subjects. Right panels depict effects of TEA and combined infusions of TEA and L-NMMA, in 15 healthy and 10 hypercholesterolemic subjects. Mean ± SEM
Infusion of TEA alone did not change acetylcholine-mediated vasodilation in healthy subjects (p=0.97, Figure 6b, 6d). In contrast, in hypercholesterolemic subjects, acetylcholine-stimulated FBF was lowered by 18%, P=0.04 (Figure 6f, 6h). Addition of L-NMMA to TEA significantly attenuated acetylcholine-stimulated vasodilation in both groups. Thus, unlike bradykinin, there was no contribution of K+Ca channel activation to acetylcholine-mediated vasodilation in healthy subjects; however, there was a significant K+Ca channel activation with acetylcholine in hypercholesterolemia. Moreover, the magnitude of constriction with NO blockade in the presence of K+Ca channel blockade was lower in the hypercholesterolemic group compared to healthy subjects (9% vs. 37%, P=0.023).
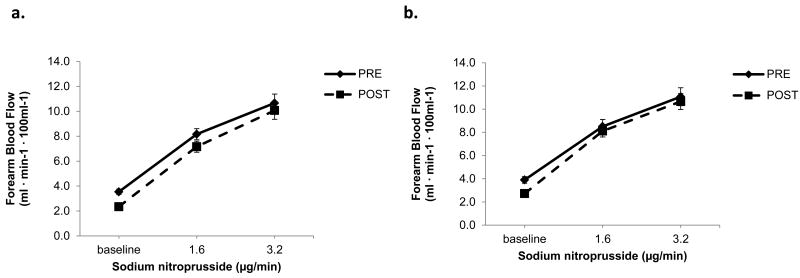
Contribution of K+Ca channel activation and NO to sodium nitroprusside responses
Vasodilation with sodium nitroprusside was similar in the healthy and hypercholesterolemic subjects (at the peak dose, FBF 10.4±4 versus 10.9±5 mL min-1·100 mL-1, P=0.63, respectively). Importantly, infusions of L-NMMA and TEA did not alter the vasodilator responses to sodium nitroprusside in either group (Figure 7).
Figure 7. Contribution of NO and K+Ca channel activation to nitroprusside-stimulated vasodilation.
FBF in response to sodium nitroprusside alone and after combined blockade with L-NMMA and TEA in healthy (n=42) and hypercholesterolemic (n=38) subjects. Mean ± SEM.
Throughout each experiment, there was no change in mean arterial blood pressure or the FBF in the control, non-infused arm.
Discussion
Herein we report firstly that EDHFs contribute to resting vasodilator tone in vivo via activation of K+Ca channels. The contribution of NO to resting tone in the human forearm microcirculation is greater compared to activation of K+Ca channels in healthy subjects, but in those with multiple risk factors, K+Ca channel activation contributes equally as much as NO. Thus, there is an inverse relationship between tonic basal NO and EDHF activities. Second, a candidate EDHF, the cytochrome P450-derived EETs, also contribute to resting vasodilator tone in the healthy microcirculation, and in the presence of NO deficiency, EET-mediated vasodilation is up-regulated. Third, sources other than EETs activate K+Ca channels because there was additional constriction with TEA after fluconazole. Fourth, bradykinin-stimulated vasodilation is mediated by both NO and activation of K+Ca channels in healthy and hypercholesterolemic subjects. Finally, in contrast to bradykinin, K+Ca channel activation does not contribute to acetylcholine-stimulated vasodilation in health, but in hypercholesterolemia, there is reduced NO release and significant contribution of K+Ca channel activation to the acetylcholine response. Thus, enhanced EDHF activity in conditions of NO deficiency contributes to maintenance of resting and agonist-stimulated vasodilation.
Contribution of NO and K+Ca channel activation to resting microcirculatory tone
We confirm previous findings that basal NO contributes substantially to vasodilator tone of the microcirculation.7, 21 For the first time we demonstrate that presence of multiple risk factors, including raised low density lipoprotein cholesterol, hyperglycemia, and higher systolic blood pressure, even within the normal range, are all associated with reduced basal NO activity in the microcirculation, findings previously described in conductance vessels and with agonists such as acetylcholine.4, 23, 24 In our study, the most important determinants of TEA responses were serum LDL and the amount of basal NO activity.
Previous controversies regarding the contribution of EDHF in the human vasculature resulted largely from the use of non-specific antagonists for inhibition of endogenous EDHFs.6, 12 We found that activation of K+Ca channels plays an important role in maintenance of resting vasodilator tone, contributes less than NO in healthy subjects, but contributes as much as NO in those with multiple cardiovascular risk factors. Thus, in the presence of endothelial dysfunction, typified by decreased NO activity, preservation of residual endothelium-dependent vasodilation is via K+Ca channel signaling in humans (Figure 3), a finding previously demonstrated in experimental animals.13, 25
Contribution of endothelium-derived cytochrome P450 metabolites to basal tone
Endothelium-derived cytochrome P450 metabolites of arachidonic acid hyperpolarize membranes primarily by activating the K+Ca channels, although the identity of the specific EETs remains controversial.26-28 EETs may also act in an autocrine fashion promoting amplification of endothelial cell hyperpolarization, the initial step in the EDHF phenomenon. Using fluconazole, we have demonstrated an important contribution of cytochrome P450-dependent EET synthesis to microvascular dilator tone in vivo, both in the presence and absence of NO, and in both the healthy vasculature and in those with risk factors. We found no difference in the magnitude of inhibition with fluconazole between healthy subjects and those with risk factors, while greater K+Ca channel activation was present in the latter. This suggests that EDHFs other than EETs that activate K+Ca channels are upregulated in subjects with risk factors. Previous studies investigating the contribution of EETs to resting tone have been contradictory, partly due to differences in inhibitors utilized or the small numbers of subjects studied. For example, sulphaphenazole and miconazole had no effect on resting blood flow in small groups of subjects. Moreover, both decrease and no change in conductance vessel diameter and flow have been reported previously with cytochrome P450 inhibition.11, 21, 29, 30 Other studies have demonstrated a significant contribution of cytochrome P450-derived metabolites to resting vasodilator tone in coronary and renal arteries from several species, and in the human mammary artery, forearm vasculature, and skeletal muscle circulations.31, 32
Because inhibition of EETs had a lesser effect than inhibition of K+Ca channels on resting tone, and that further inhibition of vasodilator tone was observed after TEA, it is clear that sources of K+Ca channel activation other than EETs are contributing to the EDHF phenomenon, which in experimental studies has been attributed to hydrogen peroxide, gap junctions or elevations in K+ release from the endothelial cells.13, 17, 18 Another possibility is that stores of EETs in the endothelial layer may be released once the cell is activated, independent of cytochrome P450 epoxygenases.33
Contribution of NO and EDHF to bradykinin- and acetylcholine -mediated vasodilation
Having confirmed our previous findings that the response to bradykinin is similar between healthy and hypercholesterolemic subjects, we now demonstrate that both K+Ca channel activation and NO contribute to the bradykinin response.2 In contrast, the response to acetylcholine is diminished in hypercholesterolemia, which in experimental studies is believed to be associated with defects in NO bioavailability, no change in prostaglandins, and increased contribution of EDHF.2 25, 34 35 36 We now demonstrate that not only is there no contribution of K+Ca channel activation to acetylcholine-mediated vasodilation in healthy subjects, but that there is increased signaling via K+Ca channel activation in hypercholesterolemia. Similar findings of increased contribution of cytochrome P450 2C9 products with acetylcholine have been reported in hypertension.37
Limitations
Our findings are limited to the forearm microcirculation, thus other vascular beds including conductance arteries warrant further investigation. Nevertheless, it is known that the contribution of EDHF is less in conductance vessels than in the microvessels.13, 14, 38 Second, L-NMMA, TEA, and fluconazole are competitive inhibitors and may not completely inhibit NO, K+Ca channels, and cytochrome P450 pathways, and thus may underestimate the importance of these mediators in vivo. Third, other endothelium-derived vasoactive mediators may also play a compensatory role after blockade of NO, K+Ca channels and cytochrome P450 epoxygenase, and need to be investigated. Finally, our investigations were conducted on a background of cyclooxygenase inhibition. Although we have previously demonstrated that vasodilator prostanoids contribute to resting vasodilator tone, we found that cyclooxygenase products did not contribute to the endothelial dysfunction of hypertensive or hypercholesterolemic patients.36
Implications
Because of the known protective role of NO on the vessel wall that not only promotes blood flow but impedes thrombosis and atherosclerosis, research has focused predominantly on developing strategies that enhance NO bioavailability. Understanding the pathophysiology of endothelial dysfunction beyond NO, and in particular with respect to EDHF in these disease states is crucial in understanding both the pathophysiology of atherosclerosis and developing novel therapies.
Summary and clinical relevance.
The endothelium maintains vasodilator tone by releasing paracrine factors including nitric oxide (NO), prostaglandins and endothelium-derived hyperpolarizing factors (EDHFs). Although the former have been widely studied, the role of EDHFs in the human circulation remains unknown. In the human forearm circulation, we have investigated the role of EDHF by employing antagonists of K+Ca channels and epoxyeicosatrienoic acid synthesis, both purported EDHF pathways. Our results demonstrate firstly, that EDHFs by activating K+Ca channels together with NO contribute to resting vasodilator tone. The contribution of EDHF compared to NO is greater in those with atherosclerosis risk factors compared to healthy subjects. Second, activation of K+Ca channels is only partly through epoxyeicosatrienoic acid release, indicating presence of other EDHFs. Third, endothelium-dependent vasodilation with bradykinin is by stimulation of both NO and EDHF, whereas with acetylcholine, vasodilation is not EDHF-mediated in healthy subjects, but there is a clear contribution of EDHF in those with hypercholesterolemia. Thus, enhanced EDHF activity in conditions of NO deficiency contributes to maintenance of resting and agonist-stimulated vasodilation.
Acknowledgments
Funding sources: National Institutes of Health Research Grant RO1 HL79115, and in part by PHS Grant UL1 RR025008 from the Clinical and Translational Science Award Program, and PHS Grant M01 RR00039 from the General Clinical Research Center program, National Institutes of Health, National Center for Research Resources, British Cardiovascular Society Research Fellowship, and the National Blood Foundation
Footnotes
Disclosures: none
References
- 1.Quyyumi AA, Dakak N, Andrews NP, Gilligan DM, Panza JA, Cannon RO., 3rd Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation. 1995;92:320–326. doi: 10.1161/01.cir.92.3.320. [DOI] [PubMed] [Google Scholar]
- 2.Gilligan DM, Guetta V, Panza JA, Garcia CE, Quyyumi AA, Cannon RO., 3rd Selective loss of microvascular endothelial function in human hypercholesterolemia. Circulation. 1994;90:35–41. doi: 10.1161/01.cir.90.1.35. [DOI] [PubMed] [Google Scholar]
- 3.Joannides R, Richard V, Haefeli WE, Linder L, Luscher TF, Thuillez C. Role of basal and stimulated release of nitric oxide in the regulation of radial artery caliber in humans. Hypertension. 1995;26:327–331. doi: 10.1161/01.hyp.26.2.327. [DOI] [PubMed] [Google Scholar]
- 4.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res. 2001;88:145–151. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- 5.Bellien J, Joannides R, Iacob M, Arnaud P, Thuillez C. Calcium-activated potassium channels and NO regulate human peripheral conduit artery mechanics. Hypertension. 2005;46:210–216. doi: 10.1161/01.HYP.0000165685.83620.31. [DOI] [PubMed] [Google Scholar]
- 6.Inokuchi K, Hirooka Y, Shimokawa H, Sakai K, Kishi T, Ito K, Kimura Y, Takeshita A. Role of endothelium-derived hyperpolarizing factor in human forearm circulation. Hypertension. 2003;42:919–924. doi: 10.1161/01.HYP.0000097548.92665.16. [DOI] [PubMed] [Google Scholar]
- 7.Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468–1474. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- 8.Panza JA, Garcia CE, Kilcoyne CM, Quyyumi AA, Cannon RO., 3rd Impaired endothelium-dependent vasodilation in patients with essential hypertension. Evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation. 1995;91:1732–1738. doi: 10.1161/01.cir.91.6.1732. [DOI] [PubMed] [Google Scholar]
- 9.Quyyumi AA, Dakak N, Mulcahy D, Andrews NP, Husain S, Panza JA, Cannon RO., 3rd Nitric oxide activity in the atherosclerotic human coronary circulation. J Am Coll Cardiol. 1997;29:308–317. doi: 10.1016/s0735-1097(96)00472-x. [DOI] [PubMed] [Google Scholar]
- 10.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- 11.Bellien J, Joannides R, Iacob M, Arnaud P, Thuillez C. Evidence for a basal release of a cytochrome-related endothelium-derived hyperpolarizing factor in the radial artery in humans. Am J Physiol Heart Circ Physiol. 2006;290:H1347–1352. doi: 10.1152/ajpheart.01079.2005. [DOI] [PubMed] [Google Scholar]
- 12.Honing ML, Smits P, Morrison PJ, Rabelink TJ. Bradykinin-induced vasodilation of human forearm resistance vessels is primarily mediated by endothelium-dependent hyperpolarization. Hypertension. 2000;35:1314–1318. doi: 10.1161/01.hyp.35.6.1314. [DOI] [PubMed] [Google Scholar]
- 13.Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca(2+)-activated K(+) channels. Circulation. 2001;103:1992–1998. doi: 10.1161/01.cir.103.15.1992. [DOI] [PubMed] [Google Scholar]
- 14.Miura H, Gutterman DD. Human coronary arteriolar dilation to arachidonic acid depends on cytochrome P-450 monooxygenase and Ca2+-activated K+ channels. Circ Res. 1998;83:501–507. doi: 10.1161/01.res.83.5.501. [DOI] [PubMed] [Google Scholar]
- 15.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 16.Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vase Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- 17.Fleming I, Busse R. Endothelium-derived epoxyeicosatrienoic acids and vascular function. Hypertension. 2006;47:629–633. doi: 10.1161/01.HYP.0000208597.87957.89. [DOI] [PubMed] [Google Scholar]
- 18.Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarizations: past beliefs and present facts. Annals of medicine. 2007;39:495–516. doi: 10.1080/07853890701491000. [DOI] [PubMed] [Google Scholar]
- 19.Stanfield PR. Tetraethylammonium ions and the potassium permeability of excitable cells. Rev Physiol Biochem Pharmacol. 1983;97:1–67. doi: 10.1007/BFb0035345. [DOI] [PubMed] [Google Scholar]
- 20.Langton PD, Nelson MT, Huang Y, Standen NB. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. The American journal of physiology. 1991;260:H927–934. doi: 10.1152/ajpheart.1991.260.3.H927. [DOI] [PubMed] [Google Scholar]
- 21.Halcox JPJ, Narayanan S, Cramer-Joyce L, Mincemoyer R, Quyyumi AA. Characterization of endothelium-derived hyperpolarizing factor in the human forearm microcirculation. Am J Physiol Heart Circ Physiol. 2001;280:H2470–2477. doi: 10.1152/ajpheart.2001.280.6.H2470. [DOI] [PubMed] [Google Scholar]
- 22.Heavey DJ, Barrow SE, Hickling NE, Ritter JM. Aspirin causes short-lived inhibition of bradykinin-stimulated prostacyclin production in man. Nature. 1985;318:186–188. doi: 10.1038/318186a0. [DOI] [PubMed] [Google Scholar]
- 23.Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88:2541–2547. doi: 10.1161/01.cir.88.6.2541. [DOI] [PubMed] [Google Scholar]
- 24.Quyyumi AA, Dakak N, Andrews NP, Husain S, Arora S, Gilligan DM, Panza JA, Cannon RO., 3rd Nitric oxide activity in the human coronary circulation. Impact of risk factors for coronary atherosclerosis. J Clin Invest. 1995;95:1747–1755. doi: 10.1172/JCI117852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandes RP, Behra A, Lebherz C, Boger RH, Bode-Boger SM, Phivthong-Ngam L, Mugge A. N(G)-nitro-L-arginine- and indomethacin-resistant endothelium-dependent relaxation in the rabbit renal artery: effect of hypercholesterolemia. Atherosclerosis. 1997;135:49–55. doi: 10.1016/s0021-9150(97)00145-7. [DOI] [PubMed] [Google Scholar]
- 26.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 27.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 28.Weston AH, Feletou M, Vanhoutte PM, Falck JR, Campbell WB, Edwards G. Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: involvement of potassium channel activation and epoxyeicosatrienoic acids. British journal of pharmacology. 2005;145:775–784. doi: 10.1038/sj.bjp.0706256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fichtlscherer S, Dimmeler S, Breuer S, Busse R, Zeiher AM, Fleming I. Inhibition of cytochrome P450 2C9 improves endothelium-dependent, nitric oxide-mediated vasodilatation in patients with coronary artery disease. Circulation. 2004;109:178–183. doi: 10.1161/01.CIR.0000105763.51286.7F. [DOI] [PubMed] [Google Scholar]
- 30.Passauer J, Bussemaker E, Lassig G, Pistrosch F, Fauler J, Gross P, Fleming I. Baseline blood flow and bradykinin-induced vasodilator responses in the human forearm are insensitive to the cytochrome P450 2C9 (CYP2C9) inhibitor sulphaphenazole. Clin Sci (Lond) 2003;105:513–518. doi: 10.1042/CS20030118. [DOI] [PubMed] [Google Scholar]
- 31.Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- 32.Hillig T, Krustrup P, Fleming I, Osada T, Saltin B, Hellsten Y. Cytochrome P450 2C9 plays an important role in the regulation of exercise-induced skeletal muscle blood flow and oxygen uptake in humans. The Journal of physiology. 2003;546:307–314. doi: 10.1113/jphysiol.2002.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weintraub NL, Fang X, Kaduce TL, VanRollins M, Chatterjee P, Spector AA. Potentiation of endothelium-dependent relaxation by epoxyeicosatrienoic acids. Circ Res. 1997;81:258–267. doi: 10.1161/01.res.81.2.258. [DOI] [PubMed] [Google Scholar]
- 34.Selemidis S, Cocks TM. Endothelium-dependent hyperpolarization as a remote anti-atherogenic mechanism. Trends Pharmacol Sci. 2002;23:213–220. doi: 10.1016/s0165-6147(02)01998-3. [DOI] [PubMed] [Google Scholar]
- 35.Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. Investigation of decreased availability of nitric oxide precursor as the mechanism responsible for impaired endothelium-dependent vasodilation in hypercholesterolemic patients. J Am Coll Cardiol. 1994;23:844–850. doi: 10.1016/0735-1097(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 36.Campia U, Choucair WK, Bryant MB, Quyyumi AA, Cardillo C, Panza JA. Role of cyclooxygenase products in the regulation of vascular tone and in the endothelial vasodilator function of normal, hypertensive, and hypercholesterolemic humans. The American journal of cardiology. 2002;89:286–290. doi: 10.1016/s0002-9149(01)02229-9. [DOI] [PubMed] [Google Scholar]
- 37.Taddei S, Versari D, Cipriano A, Ghiadoni L, Galetta F, Franzoni F, Magagna A, Virdis A, Salvetti A. Identification of a cytochrome P450 2C9-derived endothelium-derived hyperpolarizing factor in essential hypertensive patients. J Am Coll Cardiol. 2006;48:508–515. doi: 10.1016/j.jacc.2006.04.074. [DOI] [PubMed] [Google Scholar]
- 38.Urakami-Harasawa L, Shimokawa H, Nakashima M, Egashira K, Takeshita A. Importance of endothelium-derived hyperpolarizing factor in human arteries. J Clin Invest. 1997;100:2793–2799. doi: 10.1172/JCI119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005;289:F496–503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 40.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 41.Ai D, Fu Y, Guo D, Tanaka H, Wang N, Tang C, Hammock BD, Shyy JY, Zhu Y. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Natl Acad Sci U S A. 2007;104:9018–9023. doi: 10.1073/pnas.0703229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Omata K, Abraham NG, Escalante B, Schwartzman ML. Age-related changes in renal cytochrome P-450 arachidonic acid metabolism in spontaneously hypertensive rats. The American journal of physiology. 1992;262:F8–16. doi: 10.1152/ajprenal.1992.262.1.F8. [DOI] [PubMed] [Google Scholar]
- 43.Pfister SL, Falck JR, Campbell WB. Enhanced synthesis of epoxyeicosatrienoic acids by cholesterol-fed rabbit aorta. The American journal of physiology. 1991;261:H843–852. doi: 10.1152/ajpheart.1991.261.3.H843. [DOI] [PubMed] [Google Scholar]
- 44.Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- 45.Spiecker M, Darius H, Hankeln T, Soufi M, Sattler AM, Schaefer JR, Node K, Borgel J, Mugge A, Lindpaintner K, Huesing A, Maisch B, Zeldin DC, Liao JK. Risk of coronary artery disease associated with polymorphism of the cytochrome P450 epoxygenase CYP2J2. Circulation. 2004;110:2132–2136. doi: 10.1161/01.CIR.0000143832.91812.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu BN, Luo CH, Wang D, Wang A, Li Z, Zhang W, Mo W, Zhou HH. CYP2C9 allele variants in Chinese hypertension patients and healthy controls. Clin Chim Acta. 2004;348:57–61. doi: 10.1016/j.cccn.2004.04.028. [DOI] [PubMed] [Google Scholar]