Abstract
Mitosis is tightly regulated and any errors in this process often lead to aneuploidy, genomic instability, and tumorigenesis. Deregulation of mitotic kinases is significantly associated with improper cell division and aneuploidy. Because of their importance during mitosis and the relevance to cancer, mitotic kinase signaling has been extensively studied over the past few decades and, as a result, several mitotic kinase inhibitors have been developed. Despite promising preclinical results, targeting mitotic kinases for cancer therapy faces numerous challenges, including safety and patient selection issues. Therefore, there is an urgent need to better understand the molecular mechanisms underlying mitotic kinase signaling and its interactive network. Increasing evidence suggests that tumor suppressor p53 functions at the center of the mitotic kinase signaling network. In response to mitotic spindle damage, multiple mitotic kinases phosphorylate p53 to either activate or deactivate p53-mediated signaling. p53 can also regulate the expression and function of mitotic kinases, suggesting the existence of a network of mutual regulation, which can be positive or negative, between mitotic kinases and p53 signaling. Therefore, deciphering this regulatory network will provide knowledge to overcome current limitations of targeting mitotic kinases and further improve the results of targeted therapy.
1. Introduction
Mitosis involves a highly orchestrated and fine-tuned sequence of events to properly transfer genetic information to the next generation by cell division [1, 2]. It is usually divided into five phases (prophase, prometaphase, metaphase, anaphase, and telophase) based on structure and behavior of the spindle and chromosomes, and cytokinesis begins at the end of mitosis [1, 3]. This whole process must be tightly regulated to prevent improper segregation of chromosomes [4, 5]. For this reason, cells employ a surveillance mechanism, known as the “spindle checkpoint” to ensure high fidelity of chromosome segregation in mitosis by sending a “wait signal” and thus delaying anaphase until all the chromosomes are properly aligned on the spindle apparatus (reviewed in [6]). When cells fail to delay anaphase in response to activation of spindle checkpoint, it will lead to an earlier anaphase onset, possibly causing chromosome instability, aneuploidy, and tumorigenesis [7–11]. Aneuploidy, an abnormal number of chromosomes, is a characteristic feature of cancer cells and a common cause of many genetic diseases [12, 13]. Aneuploid cells occur by an improper segregation of the chromosomes during cell division [12, 13]. The most common cause of aneuploidy is mitotic errors due to defects in “proper” mitotic kinase signaling in multiple cell cycle checkpoints, resulting in unfaithful chromosome segregation [12, 14, 15].
Multiple phosphorylation and proteolysis events play important roles in the regulation of mitotic progression and cytokinesis [1, 2]. Numerous proteins involved in these posttranslational events have been identified, including kinases and cysteine proteases [16–18]. One of the best understood kinases in the regulation of mitosis is cyclin-dependent kinase 1 (Cdk1) [2]. Cdks are highly conserved serine/threonine protein kinases that regulate cell cycle progression and subsequent cell division in eukaryotic cells and ubiquitously expressed throughout the cell cycle (reviewed in [19]). Among all Cdk family members, only five of them, Cdk1, Cdk2, Cdk3, Cdk4, and Cdk6, have been implicated in controlling cell cycle [20, 21]. While other Cdks are mainly involved in the early phase of cell division, Cdk1 plays a key role in several mitotic processes [2, 21, 22]. The regulation of Cdk1 has been extensively reviewed elsewhere [23–25]. Briefly, during the G2/M transition, the activation of the mitotic kinase Cdk1/Cyclin B phosphorylates a variety of substrates, such as a kinesin-related motor protein Eg5 [26], lamin [27], and condensin [28], to initiate mitotic entrance and control its progression and mitotic exit [2, 26, 27, 29]. The kinase activity appears in late G2 and peaks at metaphase [30]. At the end of the metaphase, the anaphase promoting complex (APC) (also known as cyclosome, APC/C), which is an E3 ubiquitin ligase [31], recruits cyclin B for ubiquitination and degradation to allow mitosis to proceed [32, 33]. Therefore, it is undoubtful that the perfect regulation of Cdk1/cyclin B activity is critical for normal mitotic progression. Since the discovery of Cdks, much attention has been given to the other mitotic kinases, such as Aurora kinases, Polo-like kinases (Plks), monopolar spindle 1 (Mps1), benzimidazoles 1 homolog (Bub1), and Bub1-related kinase 1 (BubR1), due to their pivotal roles in mitosis [16] as well as the relevance to cancer. Studies indicate that Aurora kinases and Plks are mainly involved in regulating the centrosome cycle and mitotic spindle formation, while Mps1, Bub1, and BubR1 regulate the spindle assembly checkpoint [34, 35]. Therefore, the tight regulation of their kinase activities is required for proper mitotic progression, which is essential for maintaining genomic integrity [5].
Many studies have reported that deregulation of these mitotic kinases causes mitotic failure and aneuploidy and is closely associated with genomic instability and tumorigenesis [2, 36–38]. To defend against tumorigenesis caused by mitotic failure and guard genome stability, cells have utilized tumor suppressors, such as p53 [39] and BRCA1 [40] in a mitotic regulatory network. Because of its importance, tremendous efforts have been made to better understand the role of the functional crosstalk between mitotic kinases and tumor suppressors during mitosis. The p53 is one of the most frequently mutated or deleted genes in human cancers and plays a role in many cellular processes, including cell growth, differentiation, senescence, and DNA repair (reviewed in [41]). In addition, p53 is a key decision maker between cell cycle arrest and apoptosis in response to DNA damage [42, 43]. The loss-of-function of p53 can trigger an increase in genome instability and cancer predisposition, suggesting that p53 is essential for the maintenance of genome stability (reviewed in [44]). The human p53 is located on chromosome 17 (17 p13) and consists of an N-terminal transactivation domain, a central specific DNA-binding domain and a C-terminal domain, containing a tetramerization domain and regulatory region [45]. At least 20 phosphorylation sites exist in human p53 [46] and importantly, several N-terminal phosphorylation sites, such as Ser-15 [47], Thr-18 [48], and Ser-20 [49] are critical for preventing oncogenic E3 ligase MDM2-mediated p53 ubiquitination and degradation [50]. On the other hand, phosphorylation at C-terminal and a few N-terminal sites, such as Ser-362/366 [51] and Thr-55 [52] often suppresses its tumor suppressive function by destabilizing p53. These findings suggest that phosphorylation events may play significant roles in regulating p53 protein stability and function.
Under normal circumstances, cells induce the p53-dependent transcriptional activation, cell cycle arrest, and apoptosis in response to mitotic defects or DNA damage [53, 54]. However, cells lacking functional p53 due to deregulation of mitotic kinases, such as Aurora A [55], Plk1 [56], and Bub1 [57], do not undergo these cellular events and thus lead to genome instability, resulting in aneuploidy [15]. Phosphorylation of p53 by Mps1 [58] and BubR1 [59] stabilizes p53 and appears to antagonize the function of Aurora A, Plk1, and Bub1 in p53 signaling. Studies have shown that p53 can also regulate the expression and function of these kinases [60–64], suggesting that there may be mutual regulatory interactions between mitotic kinases and p53 in a mitotic signaling network (Figure 1).
Figure 1.
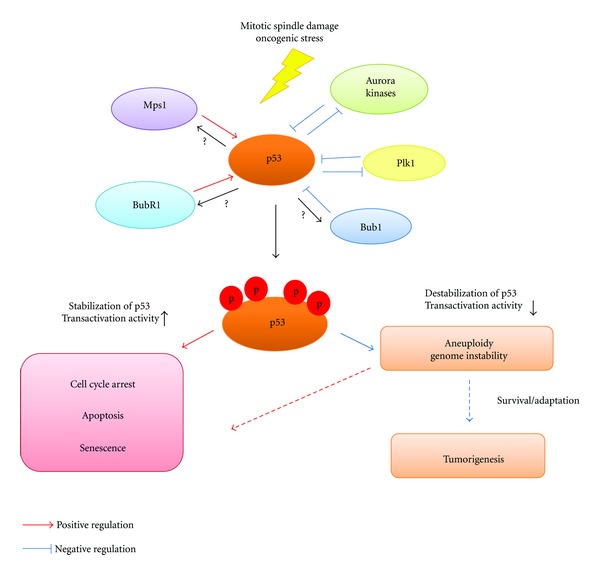
A model for regulatory networks of mitotic kinases controlling p53 signaling.
In this paper, we will specifically focus on the classic mitotic kinases, including Aurora kinases, Plks, Bub1, Mps1, and BubR1, and their roles in regulating p53 protein stability and activity.
2. Negative Regulation of p53
2.1. Aurora Kinases
Aurora kinases belong to a highly conserved family of serine/threonine kinases crucial for chromosome segregation, condensation, and spindle assembly [1]. The first Aurora kinase was discovered in Drosophila melanogaster mutants having defects in mitotic spindle-pole formation [65]. Subsequently, homologues of Aurora kinases have been identified in various species. In budding yeast, there is a single Aurora kinase, known as increase-in-ploidy 1 (Ipl1) [66]. The Ipl1 gene is essential for maintaining genome stability through its roles in chromosome segregation, spindle checkpoint, mitotic spindle disassembly, and cytokinesis [67, 68]. Caenorhabditis elegans has two Aurora kinases, Aurora/Ipl1-related-1 and -2 (AIR-1 and AIR-2), and they are thought to be key regulators of mitotic spindle assembly and dynamics [69, 70]. Three members of Aurora kinase family, Aurora A, B, and C, have been identified in mammalian cells [1]. The Aurora kinase family share a highly conserved C-terminal catalytic domain and a short N-terminal domain [71], and function in the regulation of mitosis and cytokinesis [72]. Deregulation of Aurora kinases causes a defect in spindle assembly, checkpoint function, and cell division, leading to chromosome missegregation or polyploidization [73]. Not surprisingly, overexpression of Aurora kinases is often found in a variety of human cancers [74–76]. Since the discovery of Aurora kinases, many efforts have been made to improve our understanding of their biological and physiological function in mitosis and the regulatory mechanisms relevant to cancer.
Aurora A is ubiquitously expressed in proliferating cells and its activity is tightly regulated through the cell cycle [77]. Both the expression level and kinase activity of Aurora A are significantly increased from the late G2 through the M phase [74, 78] and become low during interphase [79]. Aurora A plays a key role in mitotic spindle formation, centrosome maturation [80], and activation of cell cycle regulators, such as Plk1 [81, 82] and Cdk1 [83]. Deregulated expression and activity of Aurora A can generate aneuploidy phenotype due to centrosome amplification and spindle multipolarity [84]. Numerous substrates of Aurora A have been identified, including p53 [85], human enhancer of filamentation 1 (HEF1) [86], TPX2 [87], Ajuba [88], Plk1 [81], BRCA1 [89], and transforming acidic coiled-coil 3 (TACC3) [90]. Human p53 is directly phosphorylated by Aurora A at two sites, Ser-215 [85] and Ser-315 [55], in vitro and in vivo. Phosphorylation of Ser-215 but not Ser-315 inhibits p53 DNA binding and its transactivational activity [85], whereas phosphorylation of Ser-315 induces MDM2-mediated p53 ubiquitination and subsequent degradation [55]. These findings suggest that Aurora A-mediated phosphorylation of p53 plays a negative regulatory role in p53 protein stability and its downstream signaling pathways. In response to DNA damage, p53 interacts with the heterogeneous nuclear ribonucleoprotein K (hnRNPK), a transcriptional coactivator of p53, and induces the p53 signaling pathway [91]. hnRNPK is phosphorylated on Ser-379 by Aurora A and this phosphorylation disrupts its interaction with p53 [92], suggesting that Aurora A can indirectly/negatively regulate p53 function via hnRNPK phosphorylation. Interestingly, a recent study shows that Aurora A can positively regulate p53 protein expression levels and vice versa [60]. In addition, Xenopus p53 can block Xenopus Aurora A's ability to transform cells [61], further supporting the existence of crosstalk between Aurora A and p53.
Aurora B is a member of the chromosome passenger complex (CPC), a key regulator of chromosome segregation, histone modification, and cytokinesis during mitosis [93, 94]. The CPC is composed of Aurora B and its nonenzymatic regulatory subunits inner centromere protein (INCENP), Borealin and Survivin [94], required for the activity, localization, and stability of Aurora B [93]. Aurora B governs the spindle assembly checkpoint and manages the correct chromosome segregation and cytokinesis during mitosis [72, 95]. Inhibition of Aurora B results in a failure of mitosis due to defects in chromosome segregation and microtubule dynamics [96], leading to endoreduplication and further polyploidization [97, 98]. Aurora B phosphorylates p53 on Ser-183, Ser-269, and Thr-284, all located within the p53 DNA binding domain; however, phosphorylation on these sites does not lead to degradation of p53, instead, phosphorylation on Ser-269 and Thr-284 inhibits its transcriptional activity [46]. These findings suggest that the hyperactivation or overexpression of Aurora A and B may compromise p53's tumor suppressive function via its destabilization and inactivation.
In contrast to Aurora A and B, the biological function of Aurora C has not been well-defined. Aurora C was first discovered in mouse sperm and eggs using a kinase screen [99]. While Aurora A and B are ubiquitously expressed in many different tissues and cells, especially actively dividing cells [98, 100, 101], Aurora C is predominantly expressed in the testis [99, 102], but not in other normal mouse somatic tissues and cell lines and mitotic spermatogonia [103]. In addition, its loss-of-function leads to a failure of meiosis [103, 104], indicating that Aurora C plays a critical role in meiosis. Recent studies show that Aurora B and C have similar structural and functional properties [105]. Inhibition of Aurora C causes aneuploidy, just like Aurora B, and furthermore, simultaneous inhibition of Aurora B and C causes a higher frequency of aneuploidy [105]. Aurora C can also support mitotic progression in the absence of Aurora B [105]. Moreover, overexpression of Aurora C causes abnormal cell division due to amplified centrosomes and micronucleation [101, 106], suggesting that Aurora C may be involved in mitosis as well. Unlike Aurora A and B, the role of Aurora C in the regulation of p53 protein stability and function has not been reported yet.
2.2. Polo-Like Kinase 1 (Plk 1)
Plks are a family of highly conserved serine/threonine protein kinases [107] named after the polo gene of Drosophila melanogaster, whose mutation causes a high frequency of abnormal mitosis and meiosis [108]. Subsequently, its homologues have been found in other species, including Cdc5 in Saccharomyces cerevisiae, [109], Plo1p in Schizosaccharomyces pombe [110], Plc1, Plc2, and Plc3 in Caenorhabditis elegans [111, 112], and Plx1, Plx2, and Plx3 in Xenopus laevis [113–115]. In mammals, five Plks have been identified: Plk1 (also known as serine/threonine-protein kinase 13, STPK13), Plk2 (also known as serum-inducible kinase, SNK), Plk3 (also known as fibroblast-growth-factor-inducible kinase, FNK; proliferation-related kinase, PRK; or cytokine-inducible kinase, CNK), Plk4 (also known as SNK akin kinase, SAK or serine/threonine-protein kinase 18, STK18), and Plk5 [116–125]. All Plks are abundantly expressed in tissues exhibiting high levels of mitotic activity [120] and share two conserved domains, an N-terminal Ser/Thr kinase domain and a C-terminal polo-box domain (PBD) [107, 126].
It is now widely recognized that Plks are key regulators of mitosis, meiosis, and cytokinesis [107, 127, 128] as well as DNA damage response [107, 123, 126]. Deregulation of Plks leads to centrosome abnormalities, aneuploidy, and genomic instability [129], possibly leading to cancer development [130]. This may explain why deregulated expression of Plks is often detected in many types of cancer (reviewed in [37]).
Plk1 reaches peak expression during G2/M phase and kinase activity during mitosis [128, 129]. Plk1 is the best characterized family member among others and plays an essential role in centrosome maturation and separation [131], spindle assembly and formation [110], G2 checkpoint recovery through activating cyclin-dependent kinase [132], mitotic exit [113], and cytokinesis [133]. Studies have shown that cancer cells display a higher dependency on Plk1 for cell proliferation and mitosis [134, 135] than primary cells [136]. Deregulated expression and activity of Plk1 generate abnormal centrosomes [129] and initiate malignant transformation [137]. Not surprisingly, deregulation of Plk1 is often found in many types of cancer, including melanoma [138, 139], lung [140], head and neck [141, 142], breast [143], and ovarian cancer [144] with poor prognosis. Mounting evidence suggests that Plk1 negatively regulates p53 through direct and indirect mechanisms [145]. p53 is phosphorylated by Plk1 in vitro and its transcriptional activity and proapoptotic function are inhibited by direct interaction and phosphorylation of Plk1 [146]. Plk1 can also inhibit p53 phosphorylation at Ser-15, which is required for blocking p53-MDM2 interaction, thereby facilitating p53's degradation [56]. Plk1 phosphorylates topoisomerase I-binding protein (Topors) at Ser-718 [145]. Topors is a p53 and topoisomerase I binding protein [147]and functions as both ubiquitin and SUMO-1 E3 ligase for p53 [148, 149]. Phosphorylation of Topors on Ser-718 by Plk1 inhibits sumoylation of p53, whereas ubiquitination and subsequent degradation of p53 is enhanced, thereby suppressing p53 function [145]. G2 and S-phase-expressed 1 (GTSE1) is critical for G2 checkpoint recovery [150, 151] and negatively regulates transactivational and apoptotic activity of p53 [150, 152]. Phosphorylation of GTSE1 on Ser-435 by Plk1 promotes its nuclear localization and subsequently, shuttles p53 out from the nucleus to the cytoplasm [151, 152], leading to p53 degradation and inactivation during G2 checkpoint recovery [151]. Plk1, p53, and Cdc25C have shown to form a complex [56, 153]. Plk1 phosphorylates Cdc25C on Ser-198 [132, 154] and presumably, this phosphorylation may contribute to p53 destabilization [56, 153]. Interestingly, there is evidence that p53 can serve as a negative regulator of Plk1 by binding to the promoter of Plk1 and thus inhibiting its activity [62, 63].
The Plk2 and Plk3 are serum-inducible immediate early response genes [155] and activated near the G1/S phase transition [118, 156]. Evidence suggested that both Plk2 and Plk3 function as tumor suppressors in the p53-mediated signaling pathways to protect cell from DNA damage or oxidative stress (reviewed in [157]). Activation of Plk2 is required for centrosome duplication [156] and may have an important role in replication stress checkpoint signaling through the interaction with Chk1, Chk2, and p53 [158]. Plk2 appears to be a transcriptional target of p53 and its expression is induced after DNA damage in a p53-dependent manner [159]. Promoter analysis has shown the possible existence of p53 binding homology element (p53RE) in the basal promoter of Plk2 and furthermore, Plk2 is transcriptionally regulated by p53RE in human thyroid cells [160].
Plk3 plays an important role in the regulation of mitosis and DNA damage checkpoint [161, 162]. Its kinase activity peaks during late S and G2 phase [116]. The gene expression signature of Plk3 has shown deregulated expression of Plk3 in various types of cancers [122, 163], such as head and neck squamous cell carcinomas [164] and colon cancer [165]. Overexpression of Plk3 suppresses cell proliferation [166] and induces chromosome condensation [167]. In response to DNA damage, Plk3 is activated in an ATM-dependent manner [162] and subsequently, mediates ATM-dependent Chk2 phosphorylation and activation [161, 162]. Plk3 also inhibits entry into mitosis by phosphorylating Cdc25C [168, 169] and induces p53-dependent apoptosis [169]. In addition, Plk3 interacts with and phosphorylates p53 at Ser-20 [169], thereby preventing the interaction between p53 and MDM2, with the effect of stabilizing p53.
Plk4 shares relatively little sequence homology with other members of Plks [170]. Plk4 is essential for centrosome duplication [171, 172] and mouse embryonic development [173]. Its protein expression peaks during mitosis [174]. The loss-of-function of Plk4 causes a failure of cell division, possibly leading to aneuploidy and polyploidy, which may in turn contribute to tumorigenesis [171]. Plk4 interacts with proteins involved in the cellular response to DNA damage, such as p53 [175], Cdc25C [176], and Chk2 [177], suggesting that Plk4 may play an important role in the DNA damage response signaling [178]. Plk4 also binds to and phosphorylates p53 [173, 175, 178], possibly affecting protein stability and activity of p53 [178], although phosphorylation site(s) are currently unknown. Overexpression of Plk4 promotes centriole overduplication [172] and is found in human colon cancer [179].
A fifth member of the Plk family, Plk5, is mainly expressed in differentiated tissues, such as the brain, eye, and ovary [180], whereas it is undetectable in proliferating tissues [181]. Plk5 is involved in the process of neurite formation [181] and DNA damage response [123], rather than mitotic process. Nucleotide sequence analysis of Plk5 shows that the promoter region of Plk5 contains several p53 binding motifs; however, no such regulatory mechanisms have yet been found [123]. Interestingly, recent studies demonstrated that Plk5 is significantly downregulated by promoter hypermethylation in human brain tumors and its overexpression suppresses cell proliferation and malignant transformation by Ras oncogene, suggesting that Plk5 may function as a tumor suppressor gene in brain cancer [123, 181].
2.3. Budding Uninhibited by Benzimidazoles 1 Homolog (Bub1)
Bub1 belongs to a small group of serine/threonine kinases that play multiple roles in chromosome segregation and spindle checkpoint during mitosis [182]. Bub1 was originally identified in genetic screens of Saccharomyces cerevisiae along with mitotic arrest-deficient 1, 2, and 3 (Mad1, Mad2, and Mad3 (BubR1) in mammals), Bub3, and Mps1 [183, 184]. All of these proteins play critical roles in the mitotic checkpoint signaling [183, 184]. Deregulated Bub1 expression and its kinase activity have been associated with chromosomal instability, aneuploidy, and several forms of human cancer [185–187]. APC/C is involved in controlling sister chromatid separation and mitotic exit [188]. Bub1 ensures that activation of APC/C is delayed until all the chromosomes have achieved proper bipolar connections to the mitotic spindle, by phosphorylating Cdc20, a key regulator of APC/C activity [189]. Phosphorylation of H2A on Ser-121 by Bub1 in fission yeast prevents chromosome instability via maintenance and localization of Sgo1 (Shugoshin), a protector of centromeric cohesion [190–192]. Bub1 interacts with p53 at kinetochores in response to mitotic spindle damage and negatively regulates p53-mediated cell death [57]. It has shown that SV40 large T antigen (LT) phosphorylates p53 on Ser-37 in a Bub1-binding manner [193]. In addition, purified Bub1 directly phosphorylates p53 on Ser-37 in vitro, possibly inducing cellular senescence [193]. An interesting observation has been reported that the loss of both Bub1 and p53 causes a failure in p53-mediated cell death signaling, thereby leading to the accumulation of cells with aneuploidy and polyploidy [194].
3. Positive Regulation of p53 Activation
3.1. Monopolar Spindle 1 (Mps1)
Mps1 has an essential role in centrosome duplication, checkpoint signaling, cytokinesis, and development in organisms from yeast to mammalian [195–197]. Kinases structurally related to human Mps1 were identified in various organisms, including Mph1p in Schizosaccharomyces pombe [198], PPK1 in Arabidopsis thaliana [199], xMps1in Xenopus laevis [200] and mMps1 in mouse [201]. Mps1 acts as a dual-specificity protein kinase that can phosphorylate serine/threonine as well as tyrosine residues [198, 202] and is highly expressed during mitosis [203]. Deregulation of Mps1 causes a high frequency of chromosome missegregation and aneuploidy [203, 204] and fails to induce apoptosis in response to spindle damage [196]. The kinase activity of Mps1 is critical for maintaining chromosome stability by phosphorylating other protein substrates [205, 206]. For instance, Mps1 is crucial for Aurora B activity and chromosome alignment by phosphorylating Borealin/Dasra B, a member of CPC that regulates Aurora B [205]. In addition, Mps1 phosphorylates Blm, which is a bloom syndrome product and a member of the RecQ helicases [207], at Ser-144 [206]. Blm phosphorylation by Mps1 is important for the faithful chromosome segregation [206]. Mps1 phosphorylates p53 at Thr-18, and this phosphorylation is critical for the stabilization of p53 by interfering with MDM2 binding [58]. Mps1-mediated p53 phosphorylation is also required for the activation of p53-dependent postmitotic checkpoint [58]; thus, inhibition of Mps1 kinase activity causes a defective postmitotic checkpoint and chromosome instability [58, 208]. These findings suggest that Mps1-mediated phosphorylation and subsequent stabilization of p53 may play an important role in the activation of p53 after spindle damage as well as the prevention of aneuploidy/polyploidy [58, 208]. Interestingly, a recent study shows that increased expression of Mps1 is associated with an increased p53 mutation, a basal-like phenotype of breast cancer and a poor prognosis outcome [209]. These findings suggest that both the expression and function of Mps1 and p53 are highly correlated and critical for effective and faithful mitosis to maintain genome stability.
3.2. Bub1-Related Kinase 1 (BubR1)
BubR1 is the mammalian homolog of yeast Mad3 and Bub1 [185, 210]. It has shown to play an essential role in mitotic checkpoint activation and subsequent apoptotic events to prevent the adaptation of abnormal and unstable mitotic cells with chromosome instability [59, 211]. During mitotic checkpoint activation, BubR1 directly binds to APC/C and Cdc20 and subsequently, inhibits the E3 ligase activity of APC/C by blocking the binding of Cdc20 to APC [212], suggesting that BubR1 plays an essential role in stabilization of kinetochores-microtubule attachment [213]. Several studies have shown that BubR1 deficiency causes a loss of checkpoint control, abnormal mitosis, genomic instability, and tumorigenesis as well as a compromised response to DNA damage [214]. For instance, mice with BubR1 haploinsufficiency display a genetic instability phenotype due to underlying defects in DNA repair and chromosomal segregation [215]. Moreover, the complete loss of BubR1 leads to early embryonic lethality [216]. The reduced protein level of BubR1 promotes cellular senescence in mouse embryonic fibroblasts [217]. Increasing evidence suggests that a positive regulatory loop between p53 and BubR1 exits [218]. BubR1 interacts with and phosphorylates p53, thereby stabilizing p53 in response to spindle damage [59]. The expression level of p53 protein is reduced in BubR1-deficient cells, possibly leading to malignant transformation [214]. In p53-null cells, inhibition of BubR1 expression enhances chromosomal instability and polyploidy; conversely, overexpression of BubR1 restores the checkpoint function, suppresses centrosome amplification, and selectively eliminates cells with amplified centrosomes [64]. Interestingly, BubR1 transcription and expression are largely controlled by p53 [64].Despite of its important function, mutations of BubR1 in cancers are very rare [1, 219].
4. Conclusions
Thanks to advances in proteomics technology, many of the substrates for mitotic kinases have been identified, such as those listed above; however, the functional significance of these phosphorylation events has not been explored thoroughly. Therefore, dissecting the functional consequences of mitotic kinase-mediated phosphorylation should be given high priority to better understand their roles in mitosis.
It appears that there is a very well-organized interactive feedback loop between p53 and mitotic kinases in cell cycle progression. p53 tightly and negatively regulates the expression and activity of mitotic kinases, such as Aurora A, Plk1, and Bub1, thereby inhibiting cell proliferation and survival signaling in normal mitosis [61–64]. Protein stability and transcriptional and apoptotic activity of p53 can be also negatively regulated by mitotic kinases-mediated phosphorylation of p53 (summarized in Table 1) [55, 56, 85, 146]. On the other hand, Mps1 and BubR1 are thought to be positive regulators of p53 and may have an important role in antagonizing the function of Aurora kinases, Plk1, and Bub1 in the regulation of p53 signaling during mitosis [209, 220]. When this critical feedback loop is disrupted (e.g., by mutation of p53 or deregulation of mitotic kinases), p53 cannot be activated when damage occurs to the mitotic spindle, thereby inducing mitotic slippage and preventing apoptosis (Figure 1) [221, 222]. Based on these studies, we speculate that the status of both mitotic kinases and p53 may be critical for cell fate decisions in mitotic cells.
Table 1.
Mitotic kinases-mediated p53 phosphorylation and the possible consequences.
| Mitotic kinases | Phosphorylation sites | Outcome | References |
|---|---|---|---|
| Aurora kinases | |||
| Aurora A | Ser-215 | Inhibition of DNA binding and transcriptional activity | [85] |
| Ser-315 | Protein destabilization | [55] | |
| Aurora B | Ser-183 | Unknown | [46] |
| Ser-269/Thr284 | Inhibition of transcriptional activity | ||
| Aurora C | Unknown | ||
|
| |||
| Polo-like kinases | |||
| Plk1 | Unknown | Inhibition of transcriptional and proapoptotic activity | [146] |
| Plk2 | Unknown | ||
| Plk3 | Ser-20 | Protein stabilization | [169] |
| Plk4 | Unknown | Possibly affecting protein stabilization and transcriptional activation | [178] |
| Plk5 | Unknown | ||
|
| |||
| SAC kinases | |||
| Bub1 | Ser-37 | Possibly inducing cellular senescence | [193] |
| Mps1 | Thr-18 | p53 stabilization | [58] |
| p53-dependent postmitotic checkpoint activation | |||
| BubR1 | Unknown | p53 stabilization | [59] |
Despite promising preclinical data of targeting mitotic kinases for cancer therapy, many challenges still remain to be overcome, such as safety issues and selection of patient population. Studies have demonstrated that current mitotic inhibitors that target mitotic kinases have major side effects because mitotic kinases are mainly expressed in actively proliferating cells (both normal dividing cells and cancer cells) [2]. Therefore, selecting the right drugs and doses for right patients may be the key to successful cancer therapy.
Studies have shown that depletion/inhibition of Aurora A, Aurora B, Plk1, or Bub1 induces cellular senescence or cell death in a p53-dependent or -independent but p73-dependent manner in many different cell types [217, 223–231]. Importantly, p53-deficient/mutated cells are more sensitive to inhibitors targeting Aurora kinases or Plk1 than cells with wild-type p53, due to a significant increase in cellular senescence and cell death [227, 231, 232], suggesting that patients with p53 deficiency and mutations may benefit from inhibitors targeting Aurora kinases, Plk1, or Bub1. Mps1 and BubR1-mediated p53 phosphorylation are required for p53 activation to properly induce cell death in a p53-dependent manner in response to mitotic spindle damage [58, 59, 209]. Inhibition of Mps1 or BubR1 appears to be disabling a p53-mediated cell death signaling pathway, possibly leading to accumulation of aneuploid/polyploid cells in response to mitotic spindle damage or oncogene-induced DNA damage [59, 217]. Moreover, a recent study shows that depletion/inhibition of Mps1 fails to kill p53-deficient/mutated cells more efficiently than cells expressing wild-type p53 [233], suggesting that Mps1 or BubR1 inhibition may offer a better therapeutic benefit for cancer patients expressing wild-type p53. These finding suggest that the status of p53 is a very attractive maker capable of selecting patients who will benefit from these mitotic kinase inhibitors.
Acknowledgment
The authors thank Dr. M. Denning for helpful discussion and critical reading of the paper.
References
- 1.Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nature Reviews Molecular Cell Biology. 2001;2(1):21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- 2.Schmit TL, Ahmad N. Regulation of mitosis via mitotic kinases: new opportunities for cancer management. Molecular Cancer Therapeutics. 2007;6(7):1920–1931. doi: 10.1158/1535-7163.MCT-06-0781. [DOI] [PubMed] [Google Scholar]
- 3.Fidalgo JAP, Roda D, Roselló S, Rodríguez-Braun E, Cervantes A. Aurora kinase inhibitors: a new class of drugs targeting the regulatory mitotic system. Clinical and Translational Oncology. 2009;11(12):787–798. doi: 10.1007/s12094-009-0447-2. [DOI] [PubMed] [Google Scholar]
- 4.Janssen A, Kops GJPL, Medema RH. Elevating the frequency of chromosome mis-segregation as a strategy to kill tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(45):19108–19113. doi: 10.1073/pnas.0904343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu LY, Yu X. The balance of Polo-like kinase 1 in tumorigenesis. Cell Division. 2009;4, article no. 4 doi: 10.1186/1747-1028-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner RD, Burke DJ. The spindle checkpoint: two transitions, two pathways. Trends in Cell Biology. 2000;10(4):154–158. doi: 10.1016/s0962-8924(00)01727-x. [DOI] [PubMed] [Google Scholar]
- 7.Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 8.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266(5192):1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 9.Basu J, Bousbaa H, Logarinho E, et al. Mutations in the essential spindle checkpoint gene bub1 cause chromosome missegregation and fail to block apoptosis in Drosophila . Journal of Cell Biology. 1999;146(1):13–28. doi: 10.1083/jcb.146.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nature Reviews Cancer. 2001;1(3):222–231. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- 11.Rajaraman R, Guernsey DL, Rajaraman MM, Rajaraman SR. Stem cells, senescence, neosis and self-renewal in cancer. Cancer Cell International. 2006;6, article no. 25 doi: 10.1186/1475-2867-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang X, Zhang P. Aneuploidy and tumorigenesis. Seminars in Cell and Developmental Biology. 2011;22(6):595–601. doi: 10.1016/j.semcdb.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen S. Aneuploidy and cancer. Current Opinion in Oncology. 2000;12(1):82–88. doi: 10.1097/00001622-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Chi YH, Jeang KT. Aneuploidy and cancer. Journal of Cellular Biochemistry. 2007;102(3):531–538. doi: 10.1002/jcb.21484. [DOI] [PubMed] [Google Scholar]
- 15.Ciciarello M, Mangiacasale R, Casenghi M, et al. p53 displacement from centrosomes and p53-mediated G1 arrest following transient inhibition of the mitotic spindle. Journal of Biological Chemistry. 2001;276(22):19205–19213. doi: 10.1074/jbc.M009528200. [DOI] [PubMed] [Google Scholar]
- 16.Warner SL, Gray PJ, Von Hoff DD. Tubulin-associated drug targets: Aurora kinases, polo-like kinases, and others. Seminars in Oncology. 2006;33(4):436–448. doi: 10.1053/j.seminoncol.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Hauf S, Waizenegger IC, Peters JM. Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science. 2001;293(5533):1320–1323. doi: 10.1126/science.1061376. [DOI] [PubMed] [Google Scholar]
- 18.Honda S, Marumoto T, Hirota T, et al. Activation of m-calpain is required for chromosome alignment on the metaphase plate during mitosis. Journal of Biological Chemistry. 2004;279(11):10615–10623. doi: 10.1074/jbc.M308841200. [DOI] [PubMed] [Google Scholar]
- 19.Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nature Reviews Molecular Cell Biology. 2008;9(11):910–916. doi: 10.1038/nrm2510. [DOI] [PubMed] [Google Scholar]
- 20.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends in Biochemical Sciences. 2005;30(11):630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Viera A, Rufas JS, Martínez I, Barbero JL, Ortega S, Suja JA. CDK2 is required for proper homologous pairing, recombination and sex-body formation during male mouse meiosis. Journal of Cell Science. 2009;122(12):2149–2159. doi: 10.1242/jcs.046706. [DOI] [PubMed] [Google Scholar]
- 22.Harvey SL, Enciso G, Dephoure N, Gygi SP, Gunawardena J, Kellogg DR. A phosphatase threshold sets the level of Cdk1 activity in early mitosis in budding yeast. Molecular Biology of the Cell. 2011;22(19):3595–3608. doi: 10.1091/mbc.E11-04-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloom J, Cross FR. Multiple levels of cyclin specificity in cell-cycle control. Nature Reviews Molecular Cell Biology. 2007;8(2):149–160. doi: 10.1038/nrm2105. [DOI] [PubMed] [Google Scholar]
- 24.Morgan DO. Principles of CDK regulation. Nature. 1995;374(6518):131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 25.Lindqvist A, Rodríguez-Bravo V, Medema RH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. Journal of Cell Biology. 2009;185(2):193–202. doi: 10.1083/jcb.200812045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blangy A, Lane HA, D’Hérin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83(7):1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 27.Peter M, Nakagawa J, Doree M, Labbe JC, Nigg EA. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990;61(4):591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- 28.Kimura K, Hirano M, Kobayashi R, Hirano T. Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science. 1998;282(5388):487–490. doi: 10.1126/science.282.5388.487. [DOI] [PubMed] [Google Scholar]
- 29.Hong KU, Kim HJ, Kim HS, et al. Cdk1-cyclin B1-mediated phosphorylation of tumor-associated microtubule-associated protein/cytoskeleton-associated protein 2 in mitosis. Journal of Biological Chemistry. 2009;284(24):16501–16512. doi: 10.1074/jbc.M900257200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salaun P, Rannou Y, Claude P. Cdk1, plks, auroras, and neks: the mitotic bodyguards. Advances in Experimental Medicine and Biology. 2008;617:41–56. doi: 10.1007/978-0-387-69080-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acquaviva C, Pines J. The anaphase-promoting complex/cyclosome: APC/C. Journal of Cell Science. 2006;119(12):2401–2404. doi: 10.1242/jcs.02937. [DOI] [PubMed] [Google Scholar]
- 32.Rudner AD, Murray AW. Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. Journal of Cell Biology. 2000;149(7):1377–1390. doi: 10.1083/jcb.149.7.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clute P, Pines J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nature Cell Biology. 1999;1(2):82–87. doi: 10.1038/10049. [DOI] [PubMed] [Google Scholar]
- 34.Malumbres M, Barbacid M. Cell cycle kinases in cancer. Current Opinion in Genetics and Development. 2007;17(1):60–65. doi: 10.1016/j.gde.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Lens SMA, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nature Reviews Cancer. 2010;10(12):825–841. doi: 10.1038/nrc2964. [DOI] [PubMed] [Google Scholar]
- 36.Ma HT, Poon RYC. How protein kinases co-ordinate mitosis in animal cells. Biochemical Journal. 2011;435(1):17–31. doi: 10.1042/BJ20100284. [DOI] [PubMed] [Google Scholar]
- 37.Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I. Polo-like kinases (Plks) and cancer. Oncogene. 2005;24(2):287–291. doi: 10.1038/sj.onc.1208272. [DOI] [PubMed] [Google Scholar]
- 38.Fu J, Bian M, Jiang Q, Zhang C. Roles of aurora kinases in mitosis and tumorigenesis. Molecular Cancer Research. 2007;5(1):1–10. doi: 10.1158/1541-7786.MCR-06-0208. [DOI] [PubMed] [Google Scholar]
- 39.Tritarelli A, Oricchio E, Ciciarello M, et al. p53 localization at centrosomes during mitosis and postmitotic checkpoint are ATM-dependent and require Serine 15 phosphorylation. Molecular Biology of the Cell. 2004;15(8):3751–3757. doi: 10.1091/mbc.E03-12-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin S, Gao H, Mazzacurati L, et al. BRCA1 interaction of centrosomal protein Nlp is required for successful mitotic progression. Journal of Biological Chemistry. 2009;284(34):22970–22977. doi: 10.1074/jbc.M109.009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nature Reviews Cancer. 2002;2(8):594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 42.Tang Y, Luo J, Zhang W, Gu W. Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Molecular Cell. 2006;24(6):827–839. doi: 10.1016/j.molcel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 43.Oren M. Decision making by p53: life, death and cancer. Cell Death and Differentiation. 2003;10(4):431–442. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- 44.Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harbor perspectives in biology. 2010;2(2, article a001107) doi: 10.1101/cshperspect.a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ko LJ, Prives C. p53: puzzle and paradigm. Genes and Development. 1996;10(9):1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 46.Wu L, Ma CA, Zhao Y, Jain A. Aurora B interacts with NIR-p53, leading to p53 phosphorylation in its DNA-binding domain and subsequent functional suppression. Journal of Biological Chemistry. 2011;286(3):2236–2244. doi: 10.1074/jbc.M110.174755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91(3):325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 48.Böttger V, Böttger A, Garcia-Echeverria C, et al. Comparative study of the p53-mdm2 and p53-MDMX interfaces. Oncogene. 1999;18(1):189–199. doi: 10.1038/sj.onc.1202281. [DOI] [PubMed] [Google Scholar]
- 49.Unger T, Juven-Gershon T, Moallem E, et al. Critical role for Ser20 of human p53 in the negative regulation of p53 by Mdm2. EMBO Journal. 1999;18(7):1805–1814. doi: 10.1093/emboj/18.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nature Reviews Cancer. 2006;6(12):909–923. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- 51.Xia Y, Padre RC, De Mendoza TH, Bottero V, Tergaonkar VB, Verma IM. Phosphorylation of p53 by IκB kinase 2 promotes its degradation by β-TrCP. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(8):2629–2634. doi: 10.1073/pnas.0812256106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li HH, Li AG, Sheppard HM, Liu X. Phosphorylation on Thr-55 by TAF1 mediates degradation of p53: a role for TAF1 in cell G1 progression. Molecular Cell. 2004;13(6):867–878. doi: 10.1016/s1097-2765(04)00123-6. [DOI] [PubMed] [Google Scholar]
- 53.Lanni JS, Jacks T. Characterization of the p53-dependent postmitotic checkpoint following spindle disruption. Molecular and Cellular Biology. 1998;18(2):1055–1064. doi: 10.1128/mcb.18.2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minn AJ, Boise LH, Thompson CB. Expression of Bcl-x(L) and loss of p53 can cooperate to overcome a cell cycle checkpoint induced by mitotic spindle damage. Genes and Development. 1996;10(20):2621–2631. doi: 10.1101/gad.10.20.2621. [DOI] [PubMed] [Google Scholar]
- 55.Katayama H, Sasai K, Kawai H, et al. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nature Genetics. 2004;36(1):55–62. doi: 10.1038/ng1279. [DOI] [PubMed] [Google Scholar]
- 56.Chen J, Dai G, Wang YQ, et al. Polo-like kinase 1 regulates mitotic arrest after UV irradiation through dephosphorylation of p53 and inducing p53 degradation. FEBS Letters. 2006;580(15):3624–3630. doi: 10.1016/j.febslet.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 57.Gao F, Ponte JF, Levy M, et al. hBub1 negatively regulates p53 mediated early cell death upon mitotic checkpoint activation. Cancer Biology and Therapy. 2009;8(7) [PubMed] [Google Scholar]
- 58.Huang YF, Chang MDT, Shieh SY. TTK/hMps1 mediates the p53-dependent postmitotic checkpoint by phosphorylating p53 at Thr18. Molecular and Cellular Biology. 2009;29(11):2935–2944. doi: 10.1128/MCB.01837-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ha GH, Baek KH, Kim HS, et al. p53 activation in response to mitotic spindle damage requires signaling via BubR1-mediated phosphorylation. Cancer Research. 2007;67(15):7155–7164. doi: 10.1158/0008-5472.CAN-06-3392. [DOI] [PubMed] [Google Scholar]
- 60.Warnock LJ, Raines SA, Milner J. Aurora A mediates cross-talk between N- and C-terminal post-translational modifications of p53. Cancer Biology and Therapy. 2011;12(12):1059–1068. doi: 10.4161/cbt.12.12.18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eyers PA, Maller JL. Regulation of Xenopus Aurora A Activation by TPX2. Journal of Biological Chemistry. 2004;279(10):9008–9015. doi: 10.1074/jbc.M312424200. [DOI] [PubMed] [Google Scholar]
- 62.Kho PS, Wang Z, Zhuang L, et al. p53-regulated transcriptional program associated with genotoxic stress-induced apoptosis. Journal of Biological Chemistry. 2004;279(20):21183–21192. doi: 10.1074/jbc.M311912200. [DOI] [PubMed] [Google Scholar]
- 63.McKenzie L, King S, Marcar L, et al. p53-dependent repression of polo-like kinase-1 (PLK1) Cell Cycle. 2010;9(20):4200–4212. doi: 10.4161/cc.9.20.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oikawa T, Okuda M, Ma Z, et al. Transcriptional control of BubR1 by p53 and suppression of centrosome amplification by BubR1. Molecular and Cellular Biology. 2005;25(10):4046–4061. doi: 10.1128/MCB.25.10.4046-4061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81(1):95–105. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]
- 66.Chan CSM, Botstein D. Isolation and characterization of chromosome-gain and increase-in-ploidy mutants in yeast. Genetics. 1993;135(3):677–691. doi: 10.1093/genetics/135.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buvelot S, Tatsutani SY, Vermaak D, Biggins S. The budding yeast Ipl1/Aurora protein kinase regulates mitotic spindle disassembly. Journal of Cell Biology. 2003;160(3):329–339. doi: 10.1083/jcb.200209018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Biggins S, Murray AW. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes and Development. 2001;15(23):3118–3129. doi: 10.1101/gad.934801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schumacher JM, Ashcroft N, Donovan PJ, Golden A. A highly conserved centrosomal kinase, AIR-1, is required for accurate cell cycle progression and segregation of developmental factors in Caenorhabditis elegans embryos. Development. 1998;125(22):4391–4402. doi: 10.1242/dev.125.22.4391. [DOI] [PubMed] [Google Scholar]
- 70.Schumacher JM, Golden A, Donovan PJ. AIR-2: an Aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos. Journal of Cell Biology. 1998;143(6):1635–1646. doi: 10.1083/jcb.143.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glet R, Prigent C. The non-catalytic domain of the Xenopus laevis aurora A kinase localises the protein to the centrosome. Journal of Cell Science. 2001;114(11):2095–2104. doi: 10.1242/jcs.114.11.2095. [DOI] [PubMed] [Google Scholar]
- 72.Carmena M, Ruchaud S, Earnshaw WC. Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Current Opinion in Cell Biology. 2009;21(6):796–805. doi: 10.1016/j.ceb.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meraldi P, Honda R, Nigg EA. Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Current Opinion in Genetics and Development. 2004;14(1):29–36. doi: 10.1016/j.gde.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 74.Zhou H, Kuang J, Zhong L, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nature Genetics. 1998;20(2):189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 75.Sen S, Zhou H, White RA. A putative serine/threonine kinase encoding gene BTAK on chromosome 20q13 is amplified and overexpressed in human breast cancer cell lines. Oncogene. 1997;14(18):2195–2200. doi: 10.1038/sj.onc.1201065. [DOI] [PubMed] [Google Scholar]
- 76.Bischoff JR, Anderson L, Zhu Y, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO Journal. 1998;17(11):3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dar AA, Goff LW, Majid S, Berlin J, El-Rifai W. Aurora kinase inhibitors—rising stars in cancer therapeutics? Molecular Cancer Therapeutics. 2010;9(2):268–278. doi: 10.1158/1535-7163.MCT-09-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sasai K, Parant JM, Brandt ME, et al. Targeted disruption of Aurora A causes abnormal mitotic spindle assembly, chromosome misalignment and embryonic lethality. Oncogene. 2008;27(29):4122–4127. doi: 10.1038/onc.2008.47. [DOI] [PubMed] [Google Scholar]
- 79.Johnston CA, Hirono K, Prehoda KE, Doe CQ. Identification of an Aurora-A/PinsLINKER/ Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell. 2009;138(6):1150–1163. doi: 10.1016/j.cell.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kress E, Gotta M. Aurora A in cell division: kinase activity not required. Nature Cell Biology. 2011;13(6):638–639. doi: 10.1038/ncb2276. [DOI] [PubMed] [Google Scholar]
- 81.Macůrek L, Lindqvist A, Lim D, et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455(7209):119–123. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 82.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora A cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320(5883):1655–1658. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Horn RD, Chu S, Fan L, et al. Cdk1 activity is required for mitotic activation of Aurora A during G 2/M transition of human cells. Journal of Biological Chemistry. 2010;285(28):21849–21857. doi: 10.1074/jbc.M110.141010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meraldi P, Honda R, Nigg EA. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53-/- cells. EMBO Journal. 2002;21(4):483–492. doi: 10.1093/emboj/21.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Q, Kaneko S, Yang L, et al. Aurora-A abrogation of p53 DNA binding and transactivation activity by phosphorylation of serine 215. Journal of Biological Chemistry. 2004;279(50):52175–52182. doi: 10.1074/jbc.M406802200. [DOI] [PubMed] [Google Scholar]
- 86.Pugacheva EN, Golemis EA. HEF1-Aurora A interactions: points of dialog between the cell cycle and cell attachment signaling networks. Cell Cycle. 2006;5(4):384–391. doi: 10.4161/cc.5.4.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bayliss R, Sardon T, Vernos I, Conti E. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Molecular Cell. 2003;12(4):851–862. doi: 10.1016/s1097-2765(03)00392-7. [DOI] [PubMed] [Google Scholar]
- 88.Hirota T, Kunitoku N, Sasayama T, et al. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114(5):585–598. doi: 10.1016/s0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- 89.Ouchi M, Fujiuchi N, Sasai K, et al. BRCA1 phosphorylation by Aurora-A in the regulation of G2 to M transition. Journal of Biological Chemistry. 2004;279(19):19643–19648. doi: 10.1074/jbc.M311780200. [DOI] [PubMed] [Google Scholar]
- 90.Kinoshita K, Noetzel TL, Pelletier L, et al. Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis. Journal of Cell Biology. 2005;170(7):1047–1055. doi: 10.1083/jcb.200503023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moumen A, Masterson P, O’Connor MJ, Jackson SP. hnRNP K: an HDM2 target and transcriptional coactivator of p53 in response to DNA damage. Cell. 2005;123(6):1065–1078. doi: 10.1016/j.cell.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 92.Hsueh K-W, Fu S-L, Huang C-YF, Lin C-H. Aurora-A phosphorylates hnRNPK and disrupts its interaction with p53. FEBS Letters. 2011;585(17):2671–2675. doi: 10.1016/j.febslet.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 93.Vader G, Medema RH, Lens SMA. The chromosomal passenger complex: guiding Aurora-B through mitosis. Journal of Cell Biology. 2006;173(6):833–837. doi: 10.1083/jcb.200604032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruchaud S, Carmena M, Earnshaw WC. The chromosomal passenger complex: one for all and all for one. Cell. 2007;131(2):230–231. doi: 10.1016/j.cell.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 95.Mistry HB, MacCallum DE, Jackson RC, Chaplain MAJ, Davidson FA. Modeling the temporal evolution of the spindle assembly checkpoint and role of Aurora B kinase. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(51):20215–20220. doi: 10.1073/pnas.0810706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kallio MJ, McCleland ML, Stukenberg PT, Gorbsky GJ. Inhibition of Aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Current Biology. 2002;12(11):900–905. doi: 10.1016/s0960-9822(02)00887-4. [DOI] [PubMed] [Google Scholar]
- 97.Ditchfield C, Johnson VL, Tighe A, et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. Journal of Cell Biology. 2003;161(2):267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hauf S, Cole RW, La Terra S, et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. Journal of Cell Biology. 2003;161(2):281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tseng TC. Protein kinase profile of sperm and eggs: cloning and characterization of two novel testis-specific protein kinases (AIE1, AIE2) related to yeast and fly chromosome segregation regulators. DNA and Cell Biology. 1998;17(10):823–833. doi: 10.1089/dna.1998.17.823. [DOI] [PubMed] [Google Scholar]
- 100.Katayama H, Brinkley WR, Sen S. The Aurora kinases: role in cell transformation and tumorigenesis. Cancer and Metastasis Reviews. 2003;22(4):451–464. doi: 10.1023/a:1023789416385. [DOI] [PubMed] [Google Scholar]
- 101.Khan J, Ezan F, Crémet J-Y, et al. Overexpression of active aurora-C kinase results in cell transformation and tumour formation. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0026512.e26512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bernard M, Sanseau P, Henry C, Couturier A, Prigent C. Cloning of STK13, a third human protein kinase related to Drosophila Aurora and budding yeast Ipl1 that maps on chromosome 19q13.3-ter. Genomics. 1998;53(3):406–409. doi: 10.1006/geno.1998.5522. [DOI] [PubMed] [Google Scholar]
- 103.Yang KT, Li SK, Chang CC, et al. Aurora-C kinase deficiency causes cytokinesis failure in meiosis I and production of large polyploid oocytes in mice. Molecular Biology of the Cell. 2010;21(14):2371–2383. doi: 10.1091/mbc.E10-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dieterich K, Zouari R, Harbuz R, et al. The Aurora Kinase C c.144delC mutation causes meiosis I arrest in men and is frequent in the North African population. Human Molecular Genetics. 2009;18(7):1301–1309. doi: 10.1093/hmg/ddp029. [DOI] [PubMed] [Google Scholar]
- 105.Slattery SD, Mancini MA, Brinkley BR, Hall RM. Aurora-C kinase supports mitotic progression in the absence of Aurora-B. Cell Cycle. 2009;8(18):2984–2994. [PubMed] [Google Scholar]
- 106.Chen HL, Tang CJC, Chen CY, Tang TK. Overexpression of an Aurora-C kinase-deficient mutant disrupts the Aurora-B/INCENP complex and induces polyploidy. Journal of Biomedical Science. 2005;12(2):297–310. doi: 10.1007/s11373-005-0980-0. [DOI] [PubMed] [Google Scholar]
- 107.Barr FA, Silljé HHW, Nigg EA. Polo-like kinases and the orchestration of cell division. Nature Reviews Molecular Cell Biology. 2004;5(6):429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 108.Sunkel CE, Glover DM. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. Journal of Cell Science. 1988;89:p. 1. doi: 10.1242/jcs.89.1.25. [DOI] [PubMed] [Google Scholar]
- 109.Kitada K, Johnson AL, Johnston LH, Sugino A. A multicopy suppressor gene of the Saccharomyces cerevisiae G1 cell cycle mutant gene dbf4 encodes a protein kinase and is identified as CDC5. Molecular and Cellular Biology. 1993;13(7):4445–4457. doi: 10.1128/mcb.13.7.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ohkura H, Hagan IM, Glover DM. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes and Development. 1995;9(9):1059–1073. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- 111.Chase D, Serafinas C, Ashcroft N, et al. The Polo-like kinase PLK-1 is required for nuclear envelope breakdown and the completion of meiosis in Caenorhabditis elegans . Genesis. 2000;26(1):26–41. doi: 10.1002/(sici)1526-968x(200001)26:1<26::aid-gene6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 112.Chase D, Golden A, Heidecker G, Ferris DK. Caenorhabditis elegans contains a third polo-like kinase gene. Mitochondrial DNA. 2000;11(3-4):327–334. doi: 10.3109/10425170009033251. [DOI] [PubMed] [Google Scholar]
- 113.Descombes P, Nigg EA. The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO Journal. 1998;17(5):1328–1335. doi: 10.1093/emboj/17.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kumagai A, Dunphy WG. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273(5280):1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- 115.Duncan PI, Pollet N, Niehrs C, Nigg EA. Cloning and characterization of Plx2 and Plx3, two additional Polo-like kinases from Xenopus laevis . Experimental Cell Research. 2001;270(1):78–87. doi: 10.1006/excr.2001.5333. [DOI] [PubMed] [Google Scholar]
- 116.Wang Q, Xie S, Chen J, et al. Cell cycle arrest and apoptosis induced by human Polo-like kinase 3 is mediated through perturbation of microtubule integrity. Molecular and Cellular Biology. 2002;22(10):3450–3459. doi: 10.1128/MCB.22.10.3450-3459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kauselmann G, Weiler M, Wulff P, et al. The polo-like protein kinases Fnk and Snk associate with a Ca2+- and integrin-binding protein and are regulated dynamically with synaptic plasticity. EMBO Journal. 1999;18(20):5528–5539. doi: 10.1093/emboj/18.20.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma S, Liu MA, Yuan YLO, Erikson RL. The serum-inducible protein kinase Snk is a G1 phase polo-like kinase that is inhibited by the calcium- and integrin-binding protein CIB. Molecular Cancer Research. 2003;1(5):376–384. [PubMed] [Google Scholar]
- 119.Fode C, Motro B, Yousefi S, Heffernan M, Dennis JW. Sak, a murine protein-serine/threonine kinase that is related to the Drosophila polo kinase and involved in cell proliferation. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(14):6388–6392. doi: 10.1073/pnas.91.14.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Golsteyn RM, Schultz SJ, Bartek J, Ziemiecki A, Ried T, Nigg EA. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. Journal of Cell Science. 1994;107(6):1509–1517. doi: 10.1242/jcs.107.6.1509. [DOI] [PubMed] [Google Scholar]
- 121.Hamanaka R, Maloid S, Smith MR, O’Connell CD, Longo DL, Ferris DK. Cloning and characterization of human and murine homologues of the Drosophila polo serine-threonine kinase. Cell Growth and Differentiation. 1994;5(3):249–257. [PubMed] [Google Scholar]
- 122.Li B, Ouyang B, Pan H, et al. prk, A cytokine-inducible human protein serine/threonine kinase whose expression appears to be down-regulated in lung carcinomas. Journal of Biological Chemistry. 1996;271(32):19402–19408. doi: 10.1074/jbc.271.32.19402. [DOI] [PubMed] [Google Scholar]
- 123.Andrysik Z, Bernstein WZ, Deng L, et al. The novel mouse Polo-like kinase 5 responds to DNA damage and localizes in the nucleolus. Nucleic Acids Research. 2010;38(9):2931–2943. doi: 10.1093/nar/gkq011.gkq011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Holtrich U, Wolf G, Brauninger A, et al. Induction and down-regulation of PLK, a human serine/threonine kinase expressed in proliferating cells and tumors. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(5):1736–1740. doi: 10.1073/pnas.91.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Holtrich U, Wolf G, Yuan J, et al. Adhesion induced expression of the serine/threonine kinase Fnk in human macrophages. Oncogene. 2000;19(42):4832–4839. doi: 10.1038/sj.onc.1203845. [DOI] [PubMed] [Google Scholar]
- 126.Lowery DM, Lim D, Yaffe MB. Structure and function of Polo-like kinases. Oncogene. 2005;24(2):248–259. doi: 10.1038/sj.onc.1208280. [DOI] [PubMed] [Google Scholar]
- 127.Eckerdt F, Strebhardt K. Polo-like kinase 1: target and regulator of anaphase-promoting complex/cyclosome-dependent proteolysis. Cancer Research. 2006;66(14):6895–6898. doi: 10.1158/0008-5472.CAN-06-0358. [DOI] [PubMed] [Google Scholar]
- 128.Hamanaka R, Smith MR, O’Connor PM, et al. Polo-like kinase is a cell cycle-regulated kinase activated during mitosis. Journal of Biological Chemistry. 1995;270(36):21086–21091. doi: 10.1074/jbc.270.36.21086. [DOI] [PubMed] [Google Scholar]
- 129.Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24(2):267–276. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]
- 130.Reagan-Shaw S, Ahmad N. Polo-like kinase (Plk) 1 as a target for prostate cancer management. IUBMB Life. 2005;57(10):677–682. doi: 10.1080/15216540500305910. [DOI] [PubMed] [Google Scholar]
- 131.Zhang W, Fletcher L, Muschel RJ. The role of polo-like kinase 1 in the inhibition of centrosome separation after ionizing radiation. Journal of Biological Chemistry. 2005;280(52):42994–42999. doi: 10.1074/jbc.M505450200. [DOI] [PubMed] [Google Scholar]
- 132.Roshak AK, Capper EA, Imburgia C, Fornwald J, Scott G, Marshall LA. The human polo-like kinase, PLK, regulates cdc2/cyclin B through phosphorylation and activation of the cdc25C phosphatase. Cellular Signalling. 2000;12(6):405–411. doi: 10.1016/s0898-6568(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 133.Carmena M, Riparbelli MG, Minestrini G, et al. Drosophila polo kinase is required for cytokinesis. Journal of Cell Biology. 1998;143(3):659–671. doi: 10.1083/jcb.143.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Renner AG, Dos Santos C, Recher C, et al. Polo-like kinase 1 is overexpressed in acute myeloid leukemia and its inhibition preferentially targets the proliferation of leukemic cells. Blood. 2009;114(3):659–662. doi: 10.1182/blood-2008-12-195867. [DOI] [PubMed] [Google Scholar]
- 135.Schmidt M, Hofmann HP, Sanders K, Sczakiel G, Beckers TL, Gekeler V. Molecular alterations after Polo-like kinase 1 mRNA suppression versus pharmacologic inhibition in cancer cells. Molecular Cancer Therapeutics. 2006;5(4):809–817. doi: 10.1158/1535-7163.MCT-05-0455. [DOI] [PubMed] [Google Scholar]
- 136.Raab M, Kappel S, Krämer A, et al. Toxicity modelling of Plk1-targeted therapies in genetically engineered mice and cultured primary mammalian cells. Nature Communications. 2011;2(1, article no. 395) doi: 10.1038/ncomms1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smith MR, Wilson ML, Hamanaka R, et al. Malignant transformation of mammalian cells initiated by constitutive expression of the Polo-like kinase. Biochemical and Biophysical Research Communications. 1997;234(2):397–405. doi: 10.1006/bbrc.1997.6633. [DOI] [PubMed] [Google Scholar]
- 138.Strebhardt K, Kneisel L, Linhart C, Bernd A, Kaufmann R. Prognostic value of pololike kinase expression in melanomas. Journal of the American Medical Association. 2000;283(4):479–480. doi: 10.1001/jama.283.4.479. [DOI] [PubMed] [Google Scholar]
- 139.Kneisel L, Strebhardt K, Bernd A, Wolter M, Binder A, Kaufmann R. Expression of polo-like kinase (PLK1) in thin melanomas: a novel marker of metastatic disease. Journal of Cutaneous Pathology. 2002;29(6):354–358. doi: 10.1034/j.1600-0560.2002.290605.x. [DOI] [PubMed] [Google Scholar]
- 140.Wolf G, Elez R, Doermer A, et al. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene. 1997;14(5):543–549. doi: 10.1038/sj.onc.1200862. [DOI] [PubMed] [Google Scholar]
- 141.Knecht R, Elez R, Oechler M, Solbach C, Von Ilberg C, Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Research. 1999;59(12):2794–2797. [PubMed] [Google Scholar]
- 142.Knecht R, Oberhauser C, Strebhardt K. PLK (polo-like kinase), a new prognostic marker for oropharyngeal carcinomas. International Journal of Cancer. 2000;89(6):535–536. [PubMed] [Google Scholar]
- 143.Wolff G, Hildenbrand R, Schwar C, et al. Polo-like kinase: a novel marker of proliferation: correlation with estrogen-receptor expression in human breast cancer. Pathology Research and Practice. 2000;196(11):753–759. doi: 10.1016/S0344-0338(00)80107-7. [DOI] [PubMed] [Google Scholar]
- 144.Weichert W, Denkert C, Schmidt M, et al. Polo-like kinase isoform expression is a prognostic factor in ovarian carcinoma. British Journal of Cancer. 2004;90(4):815–821. doi: 10.1038/sj.bjc.6601610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang X, Li H, Zhou Z, et al. Plk1-mediated phosphorylation of topors regulates p53 stability. Journal of Biological Chemistry. 2009;284(28):18588–18592. doi: 10.1074/jbc.C109.001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ando K, Ozaki T, Yamamoto H, et al. Polo-like kinase 1 (Plk1) inhibits p53 function by physical interaction and phosphorylation. Journal of Biological Chemistry. 2004;279(24):25549–25561. doi: 10.1074/jbc.M314182200. [DOI] [PubMed] [Google Scholar]
- 147.Weger S, Hammer E, Heilbronn R. Topors, a p53 and topoisomerase I binding protein, interacts with the adeno-associated virus (AAV-2) Rep78/68 proteins and enhances AAV-2 gene expression. Journal of General Virology. 2002;83(3):511–516. doi: 10.1099/0022-1317-83-3-511. [DOI] [PubMed] [Google Scholar]
- 148.Rajendra R, Malegaonkar D, Pungaliya P, et al. Topors functions as an E3 ubiquitin ligase with specific E2 enzymes and ubiquitinates p53. Journal of Biological Chemistry. 2004;279(35):36440–36444. doi: 10.1074/jbc.C400300200. [DOI] [PubMed] [Google Scholar]
- 149.Hammer E, Heilbronn R, Weger S. The E3 ligase Topors induces the accumulation of polysumoylated forms of DNA topoisomerase I in vitro and in vivo. FEBS Letters. 2007;581(28):5418–5424. doi: 10.1016/j.febslet.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 150.Monte M, Benetti R, Buscemi G, Sandy P, Del Sal G, Schneider C. The cell cycle-regulated protein human GTSE-1 controls DNA damage-induced apoptosis by affecting p53 function. Journal of Biological Chemistry. 2003;278(32):30356–30364. doi: 10.1074/jbc.M302902200. [DOI] [PubMed] [Google Scholar]
- 151.Liu XS, Li H, Song B, Liu X. Polo-like kinase 1 phosphorylation of G2 and S-phase-expressed 1 protein is essential for p53 inactivation during G2 checkpoint recovery. EMBO Reports. 2010;11(8):626–632. doi: 10.1038/embor.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Monte M, Benetti R, Collavin L, Marchionni L, Del Sal G, Schneider C. hGTSE-1 expression stimulates cytoplasmic localization of p53. Journal of Biological Chemistry. 2004;279(12):11744–11752. doi: 10.1074/jbc.M311123200. [DOI] [PubMed] [Google Scholar]
- 153.Schmit TL, Zhong W, Nihal M, Ahmad N. Polo-like kinase 1 (Plk1) in non-melanoma skin cancers. Cell Cycle. 2009;8(17):2697–2702. doi: 10.4161/cc.8.17.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Toyoshima-Morimoto F, Taniguchi E, Nishida E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Reports. 2002;3(4):341–348. doi: 10.1093/embo-reports/kvf069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Simmons DL, Neel BG, Stevens R, Evett G, Erikson RL. Identification of an early-growth-response gene encoding a novel putative protein kinase. Molecular and Cellular Biology. 1992;12(9):4164–4169. doi: 10.1128/mcb.12.9.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Warnke S, Kemmler S, Hames RS, et al. Polo-like kinase-2 is required for centriole duplication in mammalian cells. Current Biology. 2004;14(13):1200–1207. doi: 10.1016/j.cub.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 157.Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nature Reviews Drug Discovery. 2010;9(8):643–660. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- 158.Matthew EM, Yen TJ, Dicker DT, et al. Replication stress, defective S-phase checkpoint and increased death in Plk2-deficient human cancer cells. Cell Cycle. 2007;6(20):2571–2578. doi: 10.4161/cc.6.20.5079. [DOI] [PubMed] [Google Scholar]
- 159.Burns TF, Fei P, Scata KA, Dicker DT, El-Deiry WS. Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (Taxol)-exposed cells. Molecular and Cellular Biology. 2003;23(16):5556–5571. doi: 10.1128/MCB.23.16.5556-5571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shimizu-Yoshida Y, Sugiyama K, Rogounovitch T, et al. Radiation-inducible hSNK gene is transcriptionally regulated by p53 binding homology element in human thyroid cells. Biochemical and Biophysical Research Communications. 2001;289(2):491–498. doi: 10.1006/bbrc.2001.5993. [DOI] [PubMed] [Google Scholar]
- 161.Bahassi EM, Conn CW, Myer DL, et al. Mammalian Polo-like kinase 3 (Plk3) is a multifunctional protein involved in stress response pathways. Oncogene. 2002;21(43):6633–6640. doi: 10.1038/sj.onc.1205850. [DOI] [PubMed] [Google Scholar]
- 162.Bahassi EM, Myer DL, McKenney RJ, Hennigan RF, Stambrook PJ. Priming phosphorylation of Chk2 by polo-like kinase 3 (Plk3) mediates its full activation by ATM and a downstream checkpoint in response to DNA damage. Mutation Research - Fundamental and Molecular Mechanisms of Mutagenesis. 2006;596(1-2):166–176. doi: 10.1016/j.mrfmmm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 163.Winkles JA, Alberts GF. Differential regulation of polo-like kinase 1, 2, 3, and 4 gene expression in mammalian cells and tissues. Oncogene. 2005;24(2):260–266. doi: 10.1038/sj.onc.1208219. [DOI] [PubMed] [Google Scholar]
- 164.Dai W, Li Y, Ouyang B, et al. PRK, a cell cycle gene localized to 8p21, is downregulated in head and neck cancer. Genes Chromosomes and Cancer. 2000;27(3):332–336. doi: 10.1002/(sici)1098-2264(200003)27:3<332::aid-gcc15>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 165.Dai W, Liu T, Wang Q, Rao CV, Reddy BS. Down-regulation of PLK3 gene expression by types and amount of dietary fat in rat colon tumors. International journal of oncology. 2002;20(1):121–126. [PubMed] [Google Scholar]
- 166.Iida M, Sasaki T, Komatani H. Overexpression of Plk3 causes morphological change and cell growth suppression in ras pathway-activated cells. Journal of Biochemistry. 2009;146(4):501–507. doi: 10.1093/jb/mvp092. [DOI] [PubMed] [Google Scholar]
- 167.Conn CW, Hennigan RF, Dai W, Sanchez Y, Stambrook PJ. Incomplete cytokinesis and induction of apoptosis by overexpression of the mammalian polo-like kinase, Plk31. Cancer Research. 2000;60(24):6826–6831. [PubMed] [Google Scholar]
- 168.Ouyang B, Li W, Pan H, Meadows J, Hoffmann I, Dai W. The physical association and phosphorylation of Cdc25C protein phosphatase by Prk. Oncogene. 1999;18(44):6029–6036. doi: 10.1038/sj.onc.1202983. [DOI] [PubMed] [Google Scholar]
- 169.Xie S, Wu H, Wang Q, et al. Plk3 Functionally Links DNA Damage to Cell Cycle Arrest and Apoptosis at Least in Part via the p53 Pathway. Journal of Biological Chemistry. 2001;276(46):43305–43312. doi: 10.1074/jbc.M106050200. [DOI] [PubMed] [Google Scholar]
- 170.Sillibourne JE, Bornens M. Polo-like kinase 4: the odd one out of the family. Cell Division. 2010;5, article no. 25 doi: 10.1186/1747-1028-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, et al. SAK/PLK4 is required for centriole duplication and flagella development. Current Biology. 2005;15(24):2199–2207. doi: 10.1016/j.cub.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 172.Holland AJ, Lan W, Niessen S, Hoover H, Cleveland DW. Polo-like kinase 4 kinase activity limits centrosome overduplication by autoregulating its own stability. Journal of Cell Biology. 2010;188(2):191–198. doi: 10.1083/jcb.200911102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ko MA, Rosario CO, Hudson JW, et al. Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nature Genetics. 2005;37(8):883–888. doi: 10.1038/ng1605. [DOI] [PubMed] [Google Scholar]
- 174.Fode C, Binkert C, Dennis JW. Constitutive expression of murine Sak-a suppresses cell growth and induces multinucleation. Molecular and Cellular Biology. 1996;16(9):4665–4672. doi: 10.1128/mcb.16.9.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Swallow CJ, Ko MA, Siddiqui NU, Hudson JW, Dennis JW. Sak/Plk4 and mitotic fidelity. Oncogene. 2005;24(2):306–312. doi: 10.1038/sj.onc.1208275. [DOI] [PubMed] [Google Scholar]
- 176.Bonni S, Ganuelas ML, Petrinac S, Hudson JW. Human Plk4 phosphorylates Cdc25C. Cell Cycle. 2008;7(4):545–547. doi: 10.4161/cc.7.4.5387. [DOI] [PubMed] [Google Scholar]
- 177.Petrinac S, Ganuelas ML, Bonni S, Nantais J, Hudson JW. Polo-like kinase 4 phosphorylates Chk2. Cell Cycle. 2009;8(2):327–329. doi: 10.4161/cc.8.2.7355. [DOI] [PubMed] [Google Scholar]
- 178.Morettin A, Ward A, Nantais J, Hudson JW. Gene expression patterns in heterozygous Plk4 murine embryonic fibroblasts. BMC Genomics. 2009;10, article no. 319 doi: 10.1186/1471-2164-10-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Macmillan JC, Hudson JW, Bull S, Dennis JW, Swallow CJ. Comparative expression of the mitotic regulators SAK and PLK in colorectal cancer. Annals of Surgical Oncology. 2001;8(9):729–740. doi: 10.1007/s10434-001-0729-6. [DOI] [PubMed] [Google Scholar]
- 180.De Cárcer G, Manning G, Malumbres M. From Plk1 to Plk5: functional evolution of Polo-like kinases. Cell Cycle. 2011;10(14):2255–2262. doi: 10.4161/cc.10.14.16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.De Cárcer G, Escobar B, Higuero AM, et al. Plk5, a polo box domain-only protein with specific roles in neuron differentiation and glioblastoma suppression. Molecular and Cellular Biology. 2011;31(6):1225–1239. doi: 10.1128/MCB.00607-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Meraldi P, Sorger PK. A dual role for Bub1 in the spindle checkpoint and chromosome congression. EMBO Journal. 2005;24(8):1621–1633. doi: 10.1038/sj.emboj.7600641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hoyt MA, Totis L, Roberts BT. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66(3):507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- 184.Li R, Murray AW. Feedback control of mitosis in budding yeast. Cell. 1991;66(3):519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- 185.Cahill DP, Lengauer C, Yu J, et al. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392(6673):300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 186.Gemma A, Seike M, Seike Y, et al. Somatic mutation of the hBUB1 mitotic checkpoint gene in primary lung cancer. Genes Chromosomes and Cancer. 2000;29(3):213–218. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1027>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 187.Hon YR, Ron LC, We CL, Ji HC. hBUB1 defects in leukemia and lymphoma cells. Oncogene. 2002;21(30):4673–4679. doi: 10.1038/sj.onc.1205585. [DOI] [PubMed] [Google Scholar]
- 188.Díaz-Martínez LA, Yu H. Running on a treadmill: dynamic inhibition of APC/C by the spindle checkpoint. Cell Division. 2007;2, article no. 23 doi: 10.1186/1747-1028-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tang Z, Shu H, Oncel D, Chen S, Yu H. Phosphorylation of Cdc20 by Bub1 provides a catalytic mechanism for APC/C inhibition by the spindle checkpoint. Molecular Cell. 2004;16(3):387–397. doi: 10.1016/j.molcel.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 190.Tang Z, Sun Y, Harley SE, Zou H, Yu H. Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(52):18012–18017. doi: 10.1073/pnas.0408600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kawashima SA, Yamagishi Y, Honda T, Lshiguro KI, Watanabe Y. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science. 2010;327(5962):172–177. doi: 10.1126/science.1180189. [DOI] [PubMed] [Google Scholar]
- 192.Kitajima TS, Kawashima SA, Watanabe Y. The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature. 2004;427(6974):510–517. doi: 10.1038/nature02312. [DOI] [PubMed] [Google Scholar]
- 193.Williams GL, Roberts TM, Gjoerup OV. Bub1: escapades in a cellular world. Cell Cycle. 2007;6(14):1699–1704. doi: 10.4161/cc.6.14.4493. [DOI] [PubMed] [Google Scholar]
- 194.Gao F, Ponte JF, Papageorgis P, et al. hBub1 deficiency triggers a novel p53 mediated early apoptotic checkpoint pathway in mitotic spindle damaged cells. Cancer Biology and Therapy. 2009;8(7):627–635. doi: 10.4161/cbt.8.7.7928. [DOI] [PubMed] [Google Scholar]
- 195.Fisk HA, Mattison CP, Winey M. Human Mps1 protein kinase is required for centrosome duplication and normal mitotic progression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(25):14875–14880. doi: 10.1073/pnas.2434156100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Stucke VM, Silljé HHW, Arnaud L, Nigg EA. Human Mps1 kinase is required for the spindle assembly checkpoint but not for centrosome duplication. EMBO Journal. 2002;21(7):1723–1732. doi: 10.1093/emboj/21.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fischer MG, Heeger S, Häcker U, Lehner CF. The mitotic arrest in response to hypoxia and of polar bodies during early embryogenesis requires Drosophila Mps1. Current Biology. 2004;14(22):2019–2024. doi: 10.1016/j.cub.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 198.He X, Jones MH, Winey M, Sazer S. mph1, a member of the Mps1-like family of dual specificity protein kinases, is required for the spindle checkpoint in S. pombe . Journal of Cell Science. 1998;111(12):1635–1647. doi: 10.1242/jcs.111.12.1635. [DOI] [PubMed] [Google Scholar]
- 199.Poch O, Schwob E, De Fraipont F, Camasses A, Bordonne R, Martin RP. RPK1, an essential yeast protein kinase involved in the regulation of the onset of mitosis, shows homology to mammalian dual-specificity kinases. Molecular and General Genetics. 1994;243(6):641–653. doi: 10.1007/BF00279573. [DOI] [PubMed] [Google Scholar]
- 200.Abrieu A, Magnaghi-Jaulin L, Kahana JA, et al. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 2001;106(1):83–93. doi: 10.1016/s0092-8674(01)00410-x. [DOI] [PubMed] [Google Scholar]
- 201.Fisk HA, Winey M. The mouse Mps1p-like kinase regulates centrosome duplication. Cell. 2001;106(1):95–104. doi: 10.1016/s0092-8674(01)00411-1. [DOI] [PubMed] [Google Scholar]
- 202.Lauze E, Stoelcker B, Luca FC, Weiss E, Schutz AR, Winey M. Yeast spindle pole body duplication gene MPS1 encodes an essential dual specificity protein kinase. EMBO Journal. 1995;14(8):1655–1663. doi: 10.1002/j.1460-2075.1995.tb07154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tardif KD, Rogers A, Cassiano J, et al. Characterization of the cellular and antitumor effects of MPI-0479605, a small-molecule inhibitor of the mitotic kinase Mps1. Molecular Cancer Therapeutics. 2011;10(12):2267–2275. doi: 10.1158/1535-7163.MCT-11-0453. [DOI] [PubMed] [Google Scholar]
- 204.Straight PD, Giddings TH, Winey M. Mps1p regulates meiotic spindle pole body duplication in addition to having novel roles during sporulation. Molecular Biology of the Cell. 2000;11(10):3525–3537. doi: 10.1091/mbc.11.10.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jelluma N, Brenkman AB, van den Broek NJF, et al. Mps1 phosphorylates borealin to control Aurora B activity and chromosome alignment. Cell. 2008;132(2):233–246. doi: 10.1016/j.cell.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 206.Leng M, Chan DW, Luo H, Zhu C, Qin J, Wang Y. MPS1-dependent mitotic BLM phosphorylation is important for chromosome stability. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(31):11485–11490. doi: 10.1073/pnas.0601828103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Selak N, Bachrati CZ, Shevelev I, et al. The Bloom’s syndrome helicase (BLM) interacts physically and functionally with p12, the smallest subunit of human DNA polymerase δ . Nucleic Acids Research. 2008;36(16):5166–5179. doi: 10.1093/nar/gkn498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dorer RK, Zhong S, Tallarico JA, Wong WH, Mitchison TJ, Murray AW. A small-molecule inhibitor of Mps1 blocks the spindle-checkpoint response to a lack of tension on mitotic chromosomes. Current Biology. 2005;15(11):1070–1076. doi: 10.1016/j.cub.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 209.Daniel J, Coulter J, Woo JH, Wilsbach K, Gabrielson E. High levels of the Mps1 checkpoint protein are protective of aneuploidy in breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(13):5384–5389. doi: 10.1073/pnas.1007645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ouyang B, Lan Z, Meadows J, et al. Human Bub1: a putative spindle checkpoint kinase closely linked to cell proliferation. Cell Growth and Differentiation. 1998;9(10):877–885. [PubMed] [Google Scholar]
- 211.Shin HJ, Baek KH, Jeon AH, et al. Dual roles of human BubR1, a mitotic checkpoint kinase, in the monitoring of chromosomal instability. Cancer Cell. 2003;4(6):483–497. doi: 10.1016/s1535-6108(03)00302-7. [DOI] [PubMed] [Google Scholar]
- 212.Sczaniecka M, Feoktistova A, May KM, et al. The spindle checkpoint functions of Mad3 and Mad2 depend on a Mad3 KEN box-mediated interaction with Cdc20-anaphase-promoting complex (APC/C) Journal of Biological Chemistry. 2008;283(34):23039–23047. doi: 10.1074/jbc.M803594200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lampson MA, Kapoor TM. The human mitotic checkpoint protein BubR1 regulates chromosome-spindle attachments. Nature Cell Biology. 2005;7(1):93–98. doi: 10.1038/ncb1208. [DOI] [PubMed] [Google Scholar]
- 214.Fang Y, Liu T, Wang X, et al. BubR1 is involved in regulation of DNA damage responses. Oncogene. 2006;25(25):3598–3605. doi: 10.1038/sj.onc.1209392. [DOI] [PubMed] [Google Scholar]
- 215.Dai W, Wang Q, Liu T, et al. Slippage of mitotic arrest and enhanced tumor development in mice with bubr1 haploinsufficiency. Cancer Research. 2004;64(2):440–445. doi: 10.1158/0008-5472.can-03-3119. [DOI] [PubMed] [Google Scholar]
- 216.Wang Q, Liu T, Fang Y, et al. BUBR1 deficiency results in abnormal megakaryopoiesis. Blood. 2004;103(4):1278–1285. doi: 10.1182/blood-2003-06-2158. [DOI] [PubMed] [Google Scholar]
- 217.Lee J, Lee CG, Lee KW, Lee CW. Cross-talk between BubR1 expression and the commitment to differentiate in adipose-derived mesenchymal stem cells. Experimental and Molecular Medicine. 2009;41(12):873–879. doi: 10.3858/emm.2009.41.12.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tomasini R, Mak TW, Melino G. The impact of p53 and p73 on aneuploidy and cancer. Trends in Cell Biology. 2008;18(5):244–252. doi: 10.1016/j.tcb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 219.Gupta A, Inaba S, Wong OK, Fang G, Liu J. Breast cancer-specific gene 1 interacts with the mitotic checkpoint kinase BubR1. Oncogene. 2003;22(48):7593–7599. doi: 10.1038/sj.onc.1206880. [DOI] [PubMed] [Google Scholar]
- 220.Suijkerbuijk SJE, Van Osch MHJ, Bos FL, Hanks S, Rahman N, Kops GJPL. Molecular causes for BUBR1 dysfunction in the human cancer predisposition syndrome mosaic variegated aneuploidy. Cancer Research. 2010;70(12):4891–4900. doi: 10.1158/0008-5472.CAN-09-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Blagosklonny MV. Mitotic arrest and cell fate: why and how mitotic inhibition of transcription drives mutually exclusive events. Cell Cycle. 2007;6(1):70–74. doi: 10.4161/cc.6.1.3682. [DOI] [PubMed] [Google Scholar]
- 222.Tsuiki H, Nitta M, Tada M, Inagaki M, Ushio Y, Saya H. Mechanism of hyperploid cell formation induced by microtubule inhibiting drug in glioma cell lines. Oncogene. 2001;20(4):420–429. doi: 10.1038/sj.onc.1204126. [DOI] [PubMed] [Google Scholar]
- 223.Niikura Y, Ogi H, Kikuchi K, Kitagawa K. BUB3 that dissociates from BUB1 activates caspase-independent mitotic death (CIMD) Cell Death and Differentiation. 2010;17(6):1011–1024. doi: 10.1038/cdd.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kitagawa K, Niikura Y. Caspase-independent mitotic death (CIMD) Cell Cycle. 2008;7(8):1001–1005. doi: 10.4161/cc.7.8.5720. [DOI] [PubMed] [Google Scholar]
- 225.Kreis NN, Sommer K, Sanhaji M, et al. Long-term downregulation of Polo-like kinase 1 increases the cyclin-dependent kinase inhibitor p21WAF1/CIP1. Cell Cycle. 2009;8(3):460–472. doi: 10.4161/cc.8.3.7651. [DOI] [PubMed] [Google Scholar]
- 226.Abbas T, Shibata E, Park J, Jha S, Karnani N, Dutta A. CRL4Cdt2 regulates cell proliferation and histone gene expression by targeting PR-Set7/Set8 for degradation. Molecular Cell. 2010;40(1):9–21. doi: 10.1016/j.molcel.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Degenhardt Y, Greshock J, Laquerre S, et al. Sensitivity of cancer cells to Plk1 inhibitor GSK461364A is associated with loss of p53 function and chromosome instability. Molecular Cancer Therapeutics. 2010;9(7):2079–2089. doi: 10.1158/1535-7163.MCT-10-0095. [DOI] [PubMed] [Google Scholar]
- 228.Liu X, Erikson RL. Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(10):5789–5794. doi: 10.1073/pnas.1031523100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kim H-J, Cho JH, Quan H, Kim J-R. Down-regulation of Aurora B kinase induces cellular senescence in human fibroblasts and endothelial cells through a p53-dependent pathway. FEBS Letters. 2011;585(22):3569–3576. doi: 10.1016/j.febslet.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 230.Huck JJ, Zhang M, McDonald A, et al. MLN8054, an inhibitor of Aurora A kinase, induces senescence in human tumor cells both in vitro and in vivo. Molecular Cancer Research. 2010;8(3):373–384. doi: 10.1158/1541-7786.MCR-09-0300. [DOI] [PubMed] [Google Scholar]
- 231.Dar AA, Belkhiri A, Ecsedy J, Zaika A, El-Rifai W. Aurora kinase A inhibition leads to p73-dependent apoptosis in p53-deficient cancer cells. Cancer Research. 2008;68(21):8998–9004. doi: 10.1158/0008-5472.CAN-08-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sur S, Pagliarini R, Bunz F, et al. A panel of isogenic human cancer cells suggests a therapeutic approach for cancers with inactivated p53. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(10):3964–3969. doi: 10.1073/pnas.0813333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Jemaa M, Vitale I, Kepp O, et al. Selective killing of p53-deficient cancer cells by SP600125. doi: 10.1002/emmm.201200228. EMBO Molecular Medicine. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


