Abstract
RELT is a recently identified Tumor Necrosis Factor Receptor that posesses two homologues in humans named RELL1 and RELL2. We investigated whether RELT and its homologues could induce cellular death when transiently transfected into HEK 293 epithelial cells. Transfection of RELT family members into HEK 293 epithelial cells induced cell death characterized by rounding and lifting of cells accompanied by DNA fragmentation, characteristics that are consistent with the activation of an apoptotic pathway. Overexpression of RELT in COS-7 cells resulted in cell rounding and lifting without DNA fragmentation, suggesting that the effects of RELT signaling may vary among different cell types. In summary, we report that overexpression of RELT or its homologues RELL1 and RELL2 in HEK 293 epithelial cells results in cell death with morphological characteristics consistent with the activation of an apoptotic pathway.
INTRODUCTION
Tumor Necrosis Factors (TNF) are signaling molecules originally characterized by their ability to induce apoptosis of tumor cells. Signaling by TNF members has also been shown to be required for many other processes such as development, differentiation, proliferation and inflammation [1,2,3]. Different forms of TNFs can exist as either secreted or membrane bound ligands, and exert their effects through binding to membrane bound Tumor Necrosis Factor Receptors (TNFRs). TNFR signaling can be extremely complex; the final outcome of signaling is often dependent on the health and metabolic state of the cell, as well as the nature and magnitude of other integrated signaling pathways.
Apoptosis or programmed cell death has been classically described as resulting from both an extrinsic pathway initiated by TNFR signaling, and an intrinsic pathway initiated by release of pro-apoptotic molecules from the mitochondria. Although traditionally considered as independent pathways, recent appreciation for how the extrinsic and intrinsic pathways are integrated has grown considerably [4]. Apoptotic pathways result in the activation of proteases termed caspases which in turn cleave a variety of substrates such as cytoskeletal scaffold proteins and inhibitors of DNA cleavage (ICAD) that lead to the cell rounding and DNA laddering characteristics of apoptosis respectively [5,6].
RELT is a recently identified TNF receptor family member that is expressed in hematological tissues and can stimulate the proliferation of T-cells [7]. We previously reported the identification of two RELT homologues; RELL1 and RELL2, that contain significant homology to RELT yet lack extensive extracellular sequences. We have previously demonstrated that RELT, RELL1 and RELL2 bind to each other in vitro and co-localize with one another at the plasma membrane, and suggested that RELL1 and RELL2 be referred to as RELT family members [8]. In this study, we attempted to shed more light on the function of RELT by determining whether overexpression of RELT or its two homologues could induce cell death when transiently transfected into HEK 293 epithelial cells.
MATERIALS AND METHODS
Reagents
The HEK 293 and COS-7 cell lines were purchased from ATCC. Mammalian expression vector constructs for RELT, RELL1 and RELL2 were generated as previously described [8]. The mammalian expression vector constructs for TRAF2 were kindly provided by Dr. Hong-Bing Shu (Wuhan University). TUNEL stain was purchased from Roche Diagnostics (Roche, Mannheim, Germany). Anti FLAG (Sigma, St. Louis, MO) and anti-HA antibodies (Covance, Berkeley, CA) were purchased from the indicated sources. Anti-RELT antibody was purchased from R&D Systems (Minneapolis, MN).
Transfections and Cellular Death Assays
Transfections for cell death assays were performed using FuGENE 6 Transfection Reagent (Roche Applied Science). For X-gal morphology staining, cells (≈1 × 105) were plated in 6 well plates with DMEM medium containing 2% FBS and 1 mM sodium pyruvate for HEK 293 cells or 10% FBS and 1mM sodium pyruvate for COS-7 cells. The cells were transfected the next day with 2.0 µg of RELT, RELL1, RELL2, TNFR1, Caspase 8 or empty vector control expression plasmids together with 0.2 µg of an expression plasmid for the enzyme β-galactosidase. After the indicated amount of time cells were fixed by adding a PBS solution containing 0.06% glutaraldehyde. After fixing for 5 minutes at room temperature, cells were washed twice with PBS to remove fixative and then stained overnight at 37 °C with X-gal staining solution; 50 mM Tris-HCl (pH 7.4), 15 mM NaCl, 1 mM MgCl2, 5 mM potassium ferricyanide and 5 mM potassium ferrocyanide with fresh X-gal added to a final concentration of 0.5 mg/ml. A minimum of 5 viewing fields containing at least 20 cells each were quantified under 20 X magnification for each individual data point. Time courses for X-gal morphology staining were performed a minimum of 3 times for either HEK 293 or COS-7 cells, a representative time course for an experiment conducted in HEK 293 cells or COS-7 cells is shown in Figures 1B and 4B respectively. Cells that had become condensed and rounded were counted as a dead or dying cell. Data is expressed as the percentage of β-galactosidase positive cells that also possess apoptotic-like morphology.
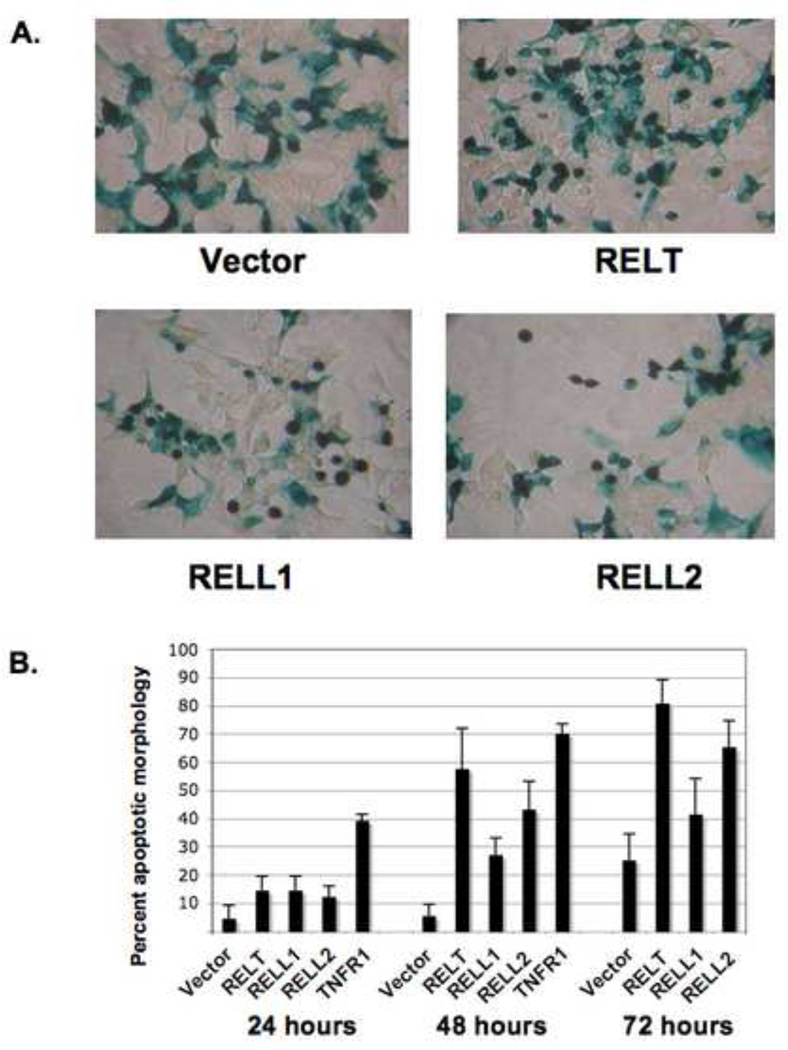
Fig. 1. Expression of RELT family members induces cell death in HEK 293 cells. HEK 293.
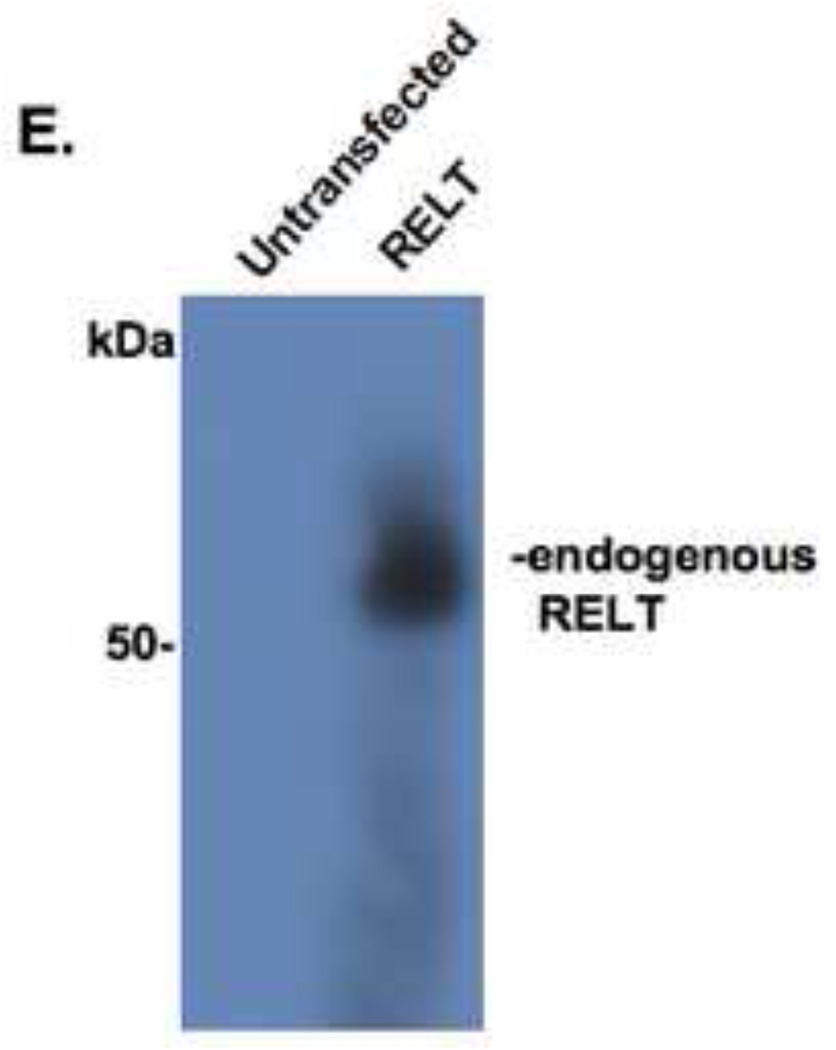
cells (≈1 × 105) were plated in 6 well plates and transfected with a mixed solution of 2 µg of the indicated expression plasmid plus 0.2 µg of an expression plasmid for β-galactosidase. Cells were fixed and stained with X-gal as described in Materials and Methods. Fig. 1A; morphology of cells fixed and stained 48 hours cells after transfection. Rounded cells are observed in cells transfected with RELT family members, consistent of cell death by an apoptotic pathway. Fig. 1B; experiment performed as in Fig. 1A, except a time course was performed with cells fixed and stained with X-gal at the indicated times. Data is expressed as a percentage of cells containing both β-galactosidase activity and apoptotic-like morphology versus cells that possess only β-galactosidase activity. A minimum of 5 viewing fields containing 20 cells each was quantified for each individual data point. A representative of one of three time course experiments is shown. Fig. 1C; Representative Western Blot demonstrating RELT family member overexpression from transient transfections in HEK 293 cells. Western blot was performed on protein lysates with anti-Flag and anti-HA antibodies to examine expression of RELL2 (Flag-tagged), RELT (HA-tagged) and RELL1 (HA-tagged). Fig. 1D; Western blot of HEK 293 cells using anti-RELT polyclonal antibody (R&D Systems #AF1385). Expression of endogenous RELT was compared between untransfected HEK 293 cells and HEK 293 cells transfected with RELT expression plasmid.
Figure 4. Effect of overexpressing RELT in COS-7 cells.
Figure 4A and 4B; effect of RELT family member overexpression on morphology of COS-7 cells. COS-7 cells (≈1×105) were plated in 6 well plates and transfected with a mixed solution of 2 µg of the indicated expression plasmid plus 0.2 µg of an expression plasmid for β-galactosidase. Cells were fixed and stained with X-gal as described in Materials and Methods. Fig. 4A: morphology of COS-7 cells fixed and stained 48 hours cells after transfection. Fig. 4B; experiment performed as in Fig. 4A, except a time course was performed with cells fixed and stained with x-gal at the indicated times. Data is expressed as the percentage of cells containing both β-galactosidase activity and apoptotic-like morphology versus cells that possess only β-galactosidase activity. A minimum of 5 viewing fields under 20 X magnification was quantified for each individual data point. A representative of one of three separate time course experiments is shown. Fig. 4C and 4D; effect of RELT overexpression on TUNEL staining in COS-7 cells. COS-7 cells (≈5 ×104) were grown on coverslips in 12 well plates and cotransfected with 0.5 µg of a GFP expression vector and 0.5 µg of the indicated expression vector and assayed for TUNEL staining as described in Materials and methods. Fig. 4C; representative images of COS-7 cell TUNEL staining 18 hours after cells were transfected with Vector, RELT or Caspase-8. Fig. 4D; Cells were cotransfected with 0.5 µg of a GFP expression vector and 0.5 µg of the indicated expression vector and assayed for TUNEL staining at either 18 or 24 hours after transfection. Data is expressed as the percentage of cells that are both TUNEL and GFP positive versus cells that are only GFP positive. At least five viewing fields under 40 X magnification were utilized to obtain one data point, and the results of three separate experiments were averaged and normalized to the TUNEL staining produced by empty vector control. Figure 4E; Expression of RELT in COS-7 cells. Untransfected and RELT transfected COS-7 cell lysates were analyzed by Western blotting utilizing an anti-RELT polyclonal antibody (R&D Systems #AF1385).
For TUNEL staining to observe DNA fragmentation, HEK 293 or COS-7 cells (≈5×104) were plated on coverslips in 12 well plates the day prior to transfection. HEK 293 cells were grown in DMEM containing 2% FBS and 1mM sodium pyruvate, COS-7 cells were grown in DMEM containing 10% FBS with no sodium pyruvate added. Cells were transfected with the indicated amount of RELT, RELL1, RELL2, TNFR1, Caspase-8 or empty vector control expression plasmids in combination with Green Fluorescent Protein (GFP) expression vector. Coverslips containing cells were fixed and the TUNEL assay was performed at the indicated time periods after transfection. The percentage of GFP positive cells in a viewing field that were also TUNEL positive was quantified. At least five viewing fields containing at least 20 cells each were quantified under 40 X magnification to obtain each data point. The percentage of GFP positive cells that were also TUNEL positive was normalized to the vector control.
Co-immunoprecipitations and Western Blotting
HEK 293 cells (≈5×106) were transfected with 10 mg of each of the expression plasmids as indicated. 293 cells were harvested 16 hours following transfection and centrifuged at 2,000 rpm for 5 minutes. The supernatants were removed and pellets were resuspended in 0.7 ml of lysis buffer (20mM Tris-HCl pH 7.4, 150mM NaCl, 1mM EDTA, 1% Triton X-100) containing the protease inhibitors aprotinin, leupeptin, and PMSF, and subsequently rotated at 4°C for at least 20 minutes to lyse the cells. The lysates were centrifuged at 14,000rpm for 10 minutes at 4°C to remove cell debris. A 30µl aliquot of sample was removed for analysis of expression of proteins in the lysate by western blot. The remainder of the sample was divided into two tubes and 30µl of protein-G sepharose beads was added to each tube. For co-immunoprecipitations either 0.3µg of anti-Flag or anti-HA antibody where appropriate was added to one tube, and 0.3µg of mouse IgG was added to the other tube as a control. The tubes were rotated at 4°C for at least 2 hours. The solutions were spun at 14,000rpm for 20 seconds and washed three times with lysis buffer containing 0.5mM NaCl. The beads remaining after the last wash were reconstituted in 30µl of 2×SDS PAGE sample buffer and boiled at 100°C for 5 minutes. Samples were analyzed by Western blotting as described elsewhere [9].
RESULTS
Induction of cellular death by RELT members
X-gal morphology staining of HEK 293 cells
HEK 293 cells are human embryonic kidney cells that are relatively easy to transfect and are widely used to study a diverse number of biological processes. To determine the effect of overexpressing RELT family members on the cellular morphology of epithelial cells, RELT expression plasmids were co-transfected with an expression plasmid for β-galactosidase into HEK 293 cells followed by X-gal staining as described in Materials and Methods. RELT expression in HEK 293 cells resulted in cells rounding and eventually lifting, indicative of a cell dying by apoptosis. Assays detecting β-galactosidase positive cells rounding and lifting off have been used to demonstrate induction of apoptosis by ICE-like proteases [10], par-4 [11] and PKC [12]. An increased incidence of apoptotic morphology was observed when RELT transfected cells were grown in medium varying between 0.5 and 10% FBS, yet this morphological change was maximally enhanced between RELT and empty vector control when cells were grown at a concentration of 2% FBS (data not shown). Therefore medium containing 2% FBS was utilized for all future experiments utilizing HEK 293 cells. Figure 1A shows characteristic morphology of cells transfected with either RELT family members or vector control for 48 hours followed by X-gal staining. Expression of RELT family members induced the condensation and rounding of cells followed by eventual detachment (Figure 1A).
The time course of cellular death induced by RELT family members was examined and compared with TNFR1 (Figure 1B). Data is expressed as a percentage of cells containing both β-galactosidase activity and possessing condensed and rounded morphology versus cells that have β-galactosidase activity and display projections typical of an attached differentiated epithelial cell in tissue culture. Each data point represents the average of five viewing fields containing at least 20 cells per viewing field, a representative of three separate time course experiments is shown. Induction of apoptotic-like morphology by RELT, RELL1 or RELL2 was observed to be significantly enhanced compared with vector control within 48 hours of transfection (p<0.05). RELT transfected cells exhibited a significantly higher incidence of cell death at 48 and 72 hours (p<0.05) when compared to RELL1 transfected cells at these time points (Figure 1B). Conversely, there was no statistically significant difference between RELT and RELL2 transfected cells or between RELL1 and RELL2 transfected cells at any of the time points (p>0.05). Transfection of TNFR1 into HEK 293 cells induced apoptotic morphology that was significantly higher than vector control or any of the RELT members at 24 hours (p<0.05). The increased rate by which TNFR1 could induce cellular death in 293 cells was also evidenced by the rapid rate of detachment of TNFR1 transfected cells compared to cells transfected with RELT family members. This made it difficult to quantify data for X-gal positive TNFR1 transfected cells 72 hours after transfection. Western blots utilizing anti-FLAG and anti-HA antibodies demonstrated the expected overexpression of RELT family members in HEK 293 cells after transient transfections (Figure 1C). Western blots utilizing an antibody directed against human-RELT (R&D Systems) indicate that HEK 293 cells do not express detectable amounts of endogenous RELT (Figure 1D),
TUNEL staining of HEK 293 cells
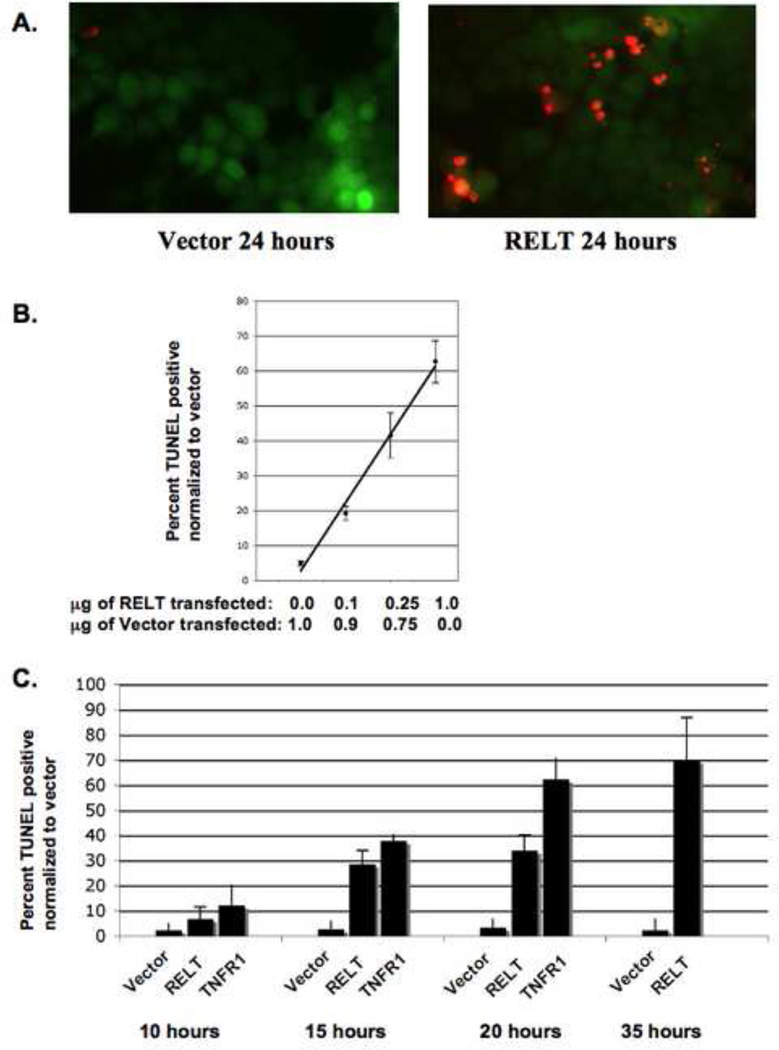
HEK 293 cells were transfected with RELT family members and after 24 hours were assayed for cellular death utilizing the TUNEL In Situ Cell Death Detection kit as described in Materials and Methods. TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) is a technique that utilizes a transferase enzyme to fluorescently label the free 3' -OH termini of DNA [13]. Cells in the later stages of apoptosis are preferentially labeled by TUNEL due to the extensive amount of fragmented DNA in cells at the later stages of apoptosis. Transient overexpression of RELT induced DNA fragmentation in HEK 293 cells as evidenced by enhanced TUNEL staining, representative photographs comparing TUNEL staining in vector and RELT transfected cells are shown in Figure 2A. The amount of TUNEL staining observed after 24 hours was directly proportional to the amount of RELT expression plasmid used in transfections (Figure 2B). At least five viewing fields were utilized to obtain each data point, and a representative of three individual dose dependent experiments is shown in Figure 2B. A time course experiment demonstrated that RELT was able to significantly (p<0.05) increase TUNEL staining over vector control within 15 hours after transfection and at later time points (Figure 2C). Additionally, TNFR1 was able to significantly increase the amount of TUNEL staining in comparison to RELT within 20 hours after transfection (p<0.05), but the differences between RELT members and TNFR1 were not statistically significant 24 hours after transfection (Figure 2D). It was difficult to quantitate TUNEL staining of TNFR1 transfected cells at 35 hours, since by this time the vast majority of TUNEL positive cells were not GFP positive, presumably due to the plasma membrane being compromised during later stages of cell death, allowing GFP to leak out of the TUNEL positive cell. At least five viewing fields were utilized to obtain each data point, and a representative of three individual time course experiments is shown in Figure 2C.
Figure 2. Increased TUNEL staining in HEK 293 cells expressing RELT family members.
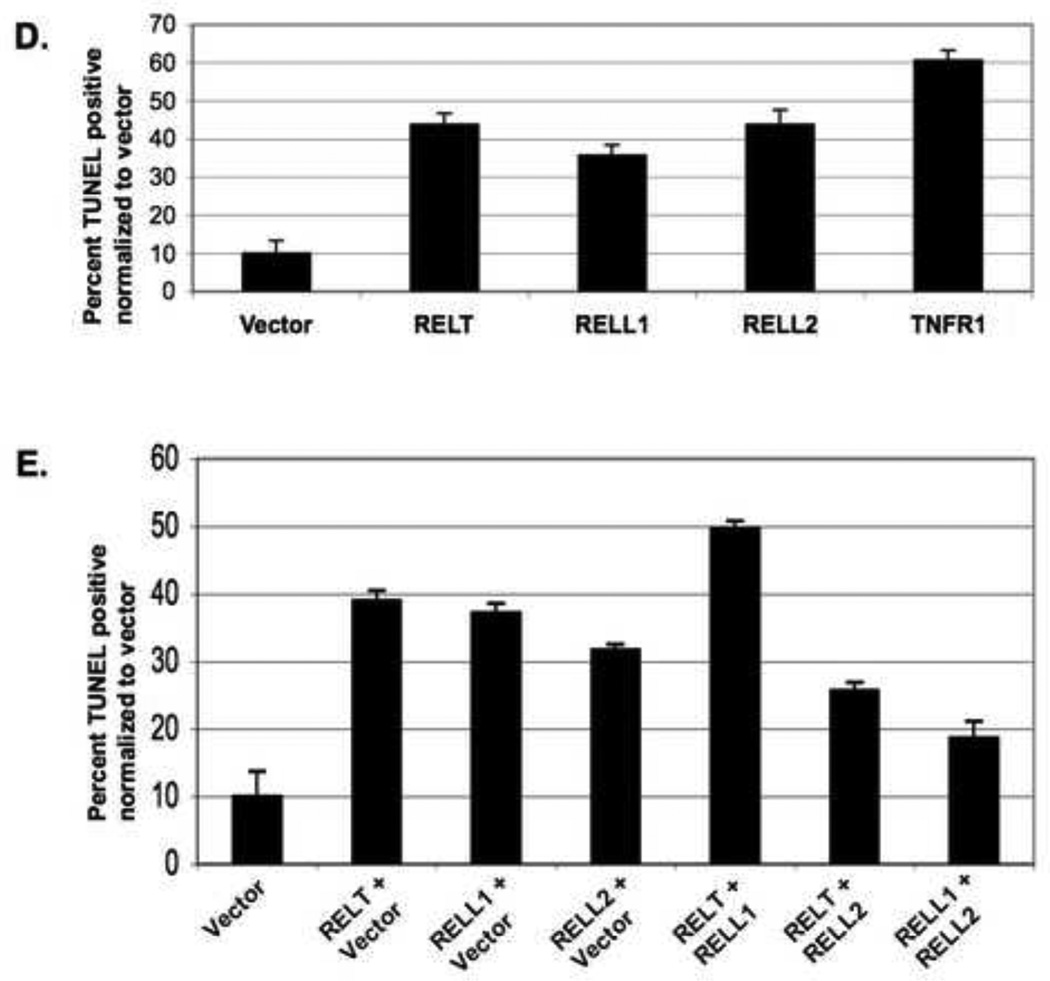
293 cells (≈ 5×104) were grown on coverslips in 12 well plates and fixed for TUNEL staining as described in Materials and Methods. Fig. 2A; Representative images of TUNEL staining 24 hours after transfection of HEK 293 cells with either empty vector control or a RELT expression plasmid. Fig. 2B; Dose dependence of TUNEL staining as a function of amount of RELT expression plasmid used for transfection. A total of 1 µg of DNA was utilized for each data point. At least five viewing fields were utilized to obtain each data point, and a representative of three individual dose dependent experiments is shown. Data is expressed as the percentage of GFP positive cells that are also TUNEL positive. Fig. 2C; Time course of TUNEL staining produced by transfecting 0.5 µg of GFP with 0.5 µg of Vector, RELT or TNFR1. At least five viewing fields were utilized to obtain each data point, and a representative of three individual time course experiments is shown. Data is expressed as the percentage of GFP positive cells that are also TUNEL positive. Figure 2D; Cells were cotransfected with 0.5 µg of a GFP expression vector and 0.5 µg of the indicated expression vector and assayed for TUNEL staining 24 hours after transfection. Data is expressed as the percentage of cells that are both TUNEL and GFP positive versus cells that are only GFP positive. At least five viewing fields under 40 X magnification were utilized to obtain each data point, and the results of three separate experiments were averaged and normalized to the TUNEL staining produced by empty vector control. Figure 2E; 1.0 µg of GFP plasmid was combined with 0.5µg of each of the indicated expression plasmids for a total of 2.0 µg of DNA, and TUNEL staining was performed after 24 hours as described in Materials and Methods. Data is expressed as the percentage of cells that are both TUNEL and GFP positive versus cells that are only GFP positive. At least five viewing fields containing at least 20 cells were utilized to obtain one data point, and the results of three separate experiments were averaged and normalized to the TUNEL staining produced by empty vector control.
Transfection of all three RELT family members into HEK 293 cells significantly increased the level of TUNEL staining in comparison to the empty vector control 24 hours after transfection (p <0.05), although the differences observed among individual RELT family members were not statistically significant (p>0.05) (Figure 2D). We sought to examine the outcome of transfecting different combinations of RELT family members into HEK 293 cells simultaneously by co-transfecting 0.5 µg of the indicated expression plasmids into HEK 293 cells simultaneously (Figure 2E). Transfection of 0.5µg of RELT combined with 0.5µg of RELL1 demonstrated a slight increase in TUNEL staining in comparison to cells transfected with either 0.5µg of RELT or RELL1 individually, although this result was not statistically significant (p>0.05). Co-transfection of cells with 0.5µg each of RELL2 and either RELT or RELL1 expression plasmids resulted in a decrease of TUNEL staining in comparison to transfecting 0.5µg of either of the three expression plasmids individually (Figure 2E). This difference was statistically significant for RELL1-RELL2 co-transfection versus either RELL1 or RELL2 transfected individually (p<0.05), yet not statistically significant when comparing RELT-RELL2 co-transfections with either RELT or RELL2 transfected individually (p>0.05). For Figures 2D and 2E, at least five viewing fields under 40 X magnification were utilized to obtain each data point, and the results of three separate experiments were averaged and normalized to the TUNEL staining produced by empty vector control.
RELL2 is the only RELT member that binds TRAF2
Due to the lack of cysteine rich domains and extensive sequences in the extracellular domains of RELL1 or RELL2, we hypothesize that RELL1 and RELL2 serve to modulate signaling by RELT in response to an as of yet unidentified ligand. If this hypothesis is correct, then RELL1 and RELL2 should possess the ability to bind to unique signaling proteins. We tested the ability of the different RELT family members to physically interact with TRAF2 via an in-vitro co-immunoprecipitation assay using cell lysates from transiently transfected HEK 293 cells. TRAFS are Tumor Necrosis Factor Receptor-associated factors that can serve to modulate signaling by TNFR members [14]. We also included OSR1, a protein that has been shown to physically interact in-vitro with all three RELT members [8]. As shown in Figure 3A, RELL2 was precipitated with TRAF2, but RELL1 and OSR1 were not precipitated by TRAF2 despite adequate expression levels of TRAF2 and either RELL1 or OSR1 in the whole cell lysates. Furthermore, Figure 3B demonstrates that RELT does not physically interact with TRAF2 despite adequate expression levels of both TRAF2 and RELT. The inability of RELT to physically interact with TRAF2 has been previously reported by two separate groups [7,15].
Figure 3. RELL2, but not other RELT family members binds TRAF2.
Fig. 3A: RELL2, but not RELL1 or OSR1, binds to TRAF2. 293 cells (≈5×106) were transfected with 10 mg of an expression plasmid for HA-tagged TRAF2 together with 10 mg of Flag tags of RELL1, RELL2 or OSR1 as indicated. Co-immunoprecipitations were performed with anti-HA (αHA) antibody or control mouse IgG (C) antibody as indicated. Western blot of co-immunoprecipitations was performed with anti Flag antibody to visualize proteins pulled down with HA tagged TRAF2. Expression of proteins in the whole cell lysate was visualized using a western blot for both anti- Flag and anti-HA antibodies. Figure 3B. RELT does not interact with TRAF2. Experiments performed as in Fig. 3A. 293 cells transfected with 10 mg Flag-tagged TRAF2 together with 10 mg of HA-tagged RELT and co-immunoprecipitated with anti-Flag antibody or control mouse IgG (C) antibody as indicated. Western blot of co-immunoprecipitations was performed with anti HA antibody and expression of proteins in the whole cell lysate was visualized using both anti- Flag and anti-HA antibodies.
Effect of RELT overexpression in COS-7 cells
The observation that RELT expression in HEK 293 cells induces cell death led us to consider if RELT expression could induce cell death in other types of cells. COS-7 cells are an immortalized line of African green monkey kidney fibroblasts that are easy to transiently transfect and have been used in a wide number of biological studies. Representative images of COS-7 cells transfected with RELT family members or empty vector control are shown 48 hours after transfection (Fig. 4A). COS-7 cells transfected with RELT were observed to round and subsequently detach from the culture dish at a much more rapid rate than that observed with HEK 293 cells (Fig. 4B). Transient overexpression of RELT in COS-7 cells resulted in a statistically significant increase in apoptotic morphology compared with vector control, RELL1 or RELL2 within 14 hours of transfection (p<0.05). RELL2 transfected cells exhibited a statistically significant increase of apoptotic morphology compared with vector control at 48 hours (p<0.05), and RELL1 exhibited no statistical increase in apoptotic morphology at any time point in comparison to vector control (Figure 4B). Expression of Caspase-8 served as a positive control for apoptosis in COS-7 cells, and Caspase-8 expression resulted in virtually all transfected cells exhibiting apoptotic morphology for all time points as early as 14 hours after transfection (data not shown for time points other than 14 hours). Surprisingly, COS-7 cells overexpressing RELT did not exhibit an increase in TUNEL staining when compared with vector control despite adequate expression of RELT in COS-7 cells (Figure 4E). Representative images of TUNEL staining 18 hours after transfection are shown in Figure 4C, and the averages of three separate TUNEL experiments at 18 and 24 hours after transfection are shown in Figure 4D. An increase in TUNEL staining was not observed in comparing RELT transfected cells with vector control at time points later than 24 hours (data not shown). Additionally, overexpression of either RELL1 or RELL2 did not increase the incidence of TUNEL staining in COS-7 cells when compared with vector control (data not shown).
DISCUSSION
RELT is a recently described TNF receptor family member that is expressed at high levels in hematological tissues and can activate the proliferation of T cells [7], the p38 and JNK MAPK pathways [15], and serves as a substrate for the closely related kinases OSR1 and SPAK [8,15]. We previously identified two homologues of RELT; RELL1 and RELL2, which share significant amino acid homology with RELT [8]. The ligand for RELT has not been identified, and RELT was not reported to bind any known human or mouse TNF ligands in a comprehensive examination of interactions between known TNFs and TNFRs of mouse and human origin [16].
We sought to examine the function of RELT by transiently overexpressing the protein in cell lines amenable to transient transfections. We hypothesized that overexpression of RELT could mimic the oligomerization that occurs in the human body when RELT binds to an as of yet unidentified TNF ligand. Overexpression of other TNFR members has been reported to induce cell death presumably by promoting oligomerization in the absence of trimeric TNF ligands [17,18], yet other studies have demonstrated that TNFR members can exist as trimers in the absence of ligand [19]. The results presented in this manuscript characterize the important observation that RELT family members induce cell death with morphological characteristics consistent with an apoptotic pathway when transiently overexpressed in HEK 293 epithelial cells.
In apoptosis or programmed cell death, activated caspases cleave inhibitors of DNases in addition to cleaving cytoskeletal components. This action of caspases results in cells displaying DNA fragmentation and rounded morphology followed by eventual detachment of cells in tissue culture. Transient overexpression of RELT family members in HEK 293 cells resulted in cells displaying a rounded morphology characteristic of apoptotic cells (Figure 1), with cells eventually detaching as time progressed. Additionally, overexpression of RELT family members in HEK 293 cells also induces DNA fragmentation as evidenced by TUNEL staining (Figure 2). Furthermore, overexpression of RELT family members in HEK 293 cells also resulted in increased propidium iodine staining compared with transfecting vector control as measured by flow cytometry (data not shown), indicating that overexpression of RELT family members results in a compromised nuclear membrane. Future experiments will be required to determine if RELT induces cell death in HEK 293 cells through the action of caspases as described for other TNFR members in the activation of the extrinsic apoptotic pathway. The ability of RELT to induce cell death may depend on the type of cell used, as COS-7 cells exhibited cell rounding and lifting but not DNA fragmentation.
Interestingly, it was observed that RELL2 increases DNA fragmentation in HEK 293 cells when transfected alone, yet appears to decrease DNA fragmentation when co-transfected with either RELT or RELL1. This result was statistically significant for the difference between RELL1-RELL2 co-transfection and either RELL1 or RELL2 single transfections. This observed effect may be attributable to differing binding partners among the RELT family members, and the resulting differing signaling activities of heterotrimeric complexes versus homotrimeric complexes. We hypothesize that RELL1 and RELL2 serve as modulators for RELT signaling based on the lack of significant sequences or the presence of cysteine-rich regions in the extracellular domains of either RELL1 or RELL2. We identified TRAF2 as a protein that binds to RELL2, but not RELL1 or RELT via co-immunoprecipitation experiments. RELL2 and potentially RELL1 exhibiting differential binding capabilities from RELT may provide a possible mechanism to explain how a heterotrimeric RELT family member complex may possess different signaling capabilities than a homotrimeric complex consisting of RELT alone.
In summary, we report the interesting observation that overexpression of a recently identified Tumor Necrosis Factor Receptor in epithelial cells induces cell death by promoting cell rounding and DNA fragmentation. The observation that RELT induces cell death does not necessarily conflict with previous reports of RELT functioning to activate the proliferation of T-cells [7], as many TNFR members such as TNFR1 have been demonstrated to possess both pro-apoptotic and anti-apoptotic survival signaling capabilities [2]. Future studies will be required to both identify the ligand for RELT, the mechanism of RELT induction of cell death, the physiological role of this process and how it could potentially influence the function of hematopoietic tissues where RELT is expressed at high levels.
Acknowledgments
We thank R.A. Lagos, L.H. Bin, H.B. Shu and M.J. Humphries for their early contributions to this work. We thank R. Hallock for their assistance with statistical analysis and M.R. Pawlus and T. Adwan for the critical reading of this manuscript. This work was supported in part by grant # DEO15648 to M.E.R, #5 T32 AI00048 to John Kappler, #63067443 to David Quissell and #P30 DC04657 to Diego Restrepo.
The abbreviations used are
- RELT
Receptor Expressed in Lymphoid Tissues
- RELL1
RELt Like protein 1
- RELL2
RELt Like protein 2
- TNF
Tumor Necrosis Factor
- TNFR
Tumor Necrosis Factor Receptor
- TRAF2
TNF receptor-associated factor 2
- TUNEL
Terminal deoxynucelotidyl transferase dUTP nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF Receptor Superfamilies: Integrating Mammalian Biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB. Signalling Pathways of the TNF Superfamily: a Double-Edged Sword. Nat. Rev. Immunol. 2003;9:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 3.Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115:1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danial NN, Korsmeyer SJ. Cell Death: Critical Control Points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 5.Chang HY, Yang X. Proteases for Cell Suicide: Functions and Regulation of Caspases. Microbiol. Mol. Biol. Rev. 2000;64:821–846. doi: 10.1128/mmbr.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagata S. DNA Degradation in Development and Programmed Cell Death. Annu. Rev. Immunol. 2005;23:853–875. doi: 10.1146/annurev.immunol.23.021704.115811. [DOI] [PubMed] [Google Scholar]
- 7.Sica GL, Zhu G, Tamada K, Liu D, Ni J, Chen L. RELT, a new member of the tumor necrosis factor receptor superfamily, is selectively expressed in hematopoietic tissues and activates transcription factor NF-kappa B. Blood. 2001;97:2702–2707. doi: 10.1182/blood.v97.9.2702. [DOI] [PubMed] [Google Scholar]
- 8.Cusick JK, Xu LG, Bin LH, Han KJ, Shu HB. Identification of RELT homologues that associate with RELT and are phosphorylated by OSR1. Biochem. Biophys. Res. Commun. 2006;340:535–543. doi: 10.1016/j.bbrc.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 9.Sambrook J, Fritch EF, Maniatis A. Molecular Cloning: A Laboratory Manual. second ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 10.Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- 11.Díaz-Meco MT, Municio MM, Frutos S, Sanchez P, Lozano J, Sanz L, Moscat J. The product of par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell. 1996;86:777–786. doi: 10.1016/s0092-8674(00)80152-x. [DOI] [PubMed] [Google Scholar]
- 12.Reyland ME, Barzen KA, Anderson SM, Quissell DO, Matassa AA. Activation of PKC is sufficient to induce an apoptotic program in salivary gland acinar cells. Cell Death and Differentiation. 2000;7:1200–1209. doi: 10.1038/sj.cdd.4400744. [DOI] [PubMed] [Google Scholar]
- 13.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell. Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradley JR, Pober JS. Tumor necrosis factor receptor-associated factors (TRAFs) Oncogene. 2001;20:6482–6491. doi: 10.1038/sj.onc.1204788. [DOI] [PubMed] [Google Scholar]
- 15.Polek TC, Talpaz M, Spivak-Kroizman T. The TNF receptor, RELT, binds SPAK and uses it to mediate p38 and JNK activation. Biochem. Biophys. Res. Commun. 2006;343:125–134. doi: 10.1016/j.bbrc.2006.02.125. [DOI] [PubMed] [Google Scholar]
- 16.Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S, Ambrose C, Tschopp J, Schneider P. Interactions of Tumor Necrosis Factor (TNF) and TNF Receptor Family Members in the Mouse and Human. J. Biol. Chem. 2006;281:13964–13971. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- 17.Boldin MP, Mett IL, Varfolomeev EE, Chumakov I, Shemer-Avni Y, Camonis JH, Wallach D. Self-Association of the "Death Domains" of the p55 Tumor Necrosis Factor (TNF) Receptor and Fas/APO1 Prompts Signaling for TNF and Fas/APO1 Effects. J. Biol. Chem. 1995;270:387–391. doi: 10.1074/jbc.270.1.387. [DOI] [PubMed] [Google Scholar]
- 18.Vandevoorde V, Haegeman G, Fiers W. Induced Expression of Trimerized Intracellular Domains of the Human Tumor Necrosis Factor (TNF) p55 Receptor Elicits TNF Effects. J. Cell. Biol. 1997;137:1627–1638. doi: 10.1083/jcb.137.7.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan FK, Chun HJ, Zheng L, Siegel RM, Bui KL, Lenardo MJ. A Domain in TNF Receptors that Mediates Ligand-Independent Receptor Assembly and Signaling. Science. 2000;288:2351–2354. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]