Abstract
The objective of this study is to synthesize antibacterial methacrylate and methacrylamide monomers and formulate antibacterial fluoride-releasing dental composites. Three antibacterial methacrylate or methacrylamide monomers containing long-chain quaternary ammonium fluoride, 1,2-methacrylamido-N,N,N-trimethyldodecan-1-aminium fluoride (monomer I), N-benzyl-11-(methacryloyloxy)-N,N-dimethylundecan-1-aminium fluoride (monomer II), and methacryloxyldecylpyridinium fluoride (monomer III) have been synthesized and analyzed by nuclear magnetic resonance (NMR) and mass spectrometry (MS). The cytotoxicity test and bactericidal test against Streptococcus mutans indicate that antibacterial monomer II is superior to monomers I and III. A series of dental composites containing 0–6% of antibacterial monomer II have been formulated and tested for degree of conversion (DC), flexure strength, water sorption, solubility, and inhibition of S. mutans biofilms. An antibacterial fluoride-releasing dental composite has also been formulated and tested for flexure strength and fluoride release. The dental composite containing 3% of monomer II has a significant effect against S. mutans biofilm formation without major adverse effects on its physical and mechanical properties. The new antibacterial monomers can be used together with the fluoride-releasing monomers containing a ternary zirconiun- fluoride chelate to formulate a new antibacterial fluoride- releasing dental composite. Such a new dental composite is expected to have higher anticaries efficacy and longer service life.
Keywords: synthesis, antibacterial monomers, dental composites, biofilm, mechanical properties
INTRODUCTION
Dental caries is still a major health problem worldwide. According to the National Health and Nutrition Examination Survey from 1999 to 2004,1 59% of adolescents (12 to 19 years old) and 92% of adults (20- to 64-years-old) have dental caries. Resin-based dental composites (RBC) have been widely used to restore carious teeth.2–5 However, composite restorations have limited service life. The leading cause of failure of RBC is secondary (recurrent) caries,6,7 which is caused by proliferation of cariogenic bacteria such as Streptococcus mutans into the microgaps (microleakage) between the composite and tooth structure.8–11 Besides, the residual bacteria in the restoration margin after incomplete carious removal can cause severe dental pulpal pathosis.12 Fluoride is well documented as the most effective and widely used anticariogenic agent. Fluoride-releasing dental materials may reduce secondary caries.13 The anticariogenic effect of fluoride is mainly attributed to its ability to enhance remineralization and the formation of acid-resistant fluorapatite.14 Fluoride can also interfere with glycolytic activity and reduce acid production.15 Fluoride ions at high concentration (>0.12%) may have bactericidal effect,16 but the level of fluoride released from fluoride-releasing dental composites is usually too low to have any antibacterial effect.17
In recent years, we have been developing novel fluoride-releasing dental monomers containing ternary (three-component) zirconium-fluoride complexes.18–21 Since such complexes are present as anions, cations such as protons or quaternary ammonium ions are needed to maintain neutrality. For example, tetrabutylammonium fluoride (TBAF) has been used in synthesis of a fluoride-releasing monomer, which has better solubility in dental monomers than the acid form of the monomer. However, the TBA+ cation has a strong tendency to form a tight ion-pair with F− ion and such ion-pairs can leach out, which leads to increased water sorption, solubility, and decreased mechanical properties with time.20
Conversely, monomers and their polymers containing long-chain quaternary ammonium have antimicrobial effects.22–25 Antimicrobial polymers have also been used in health care products, wound-healing, food, textiles, and water treatment.25–28 In dental materials, an antibacterial monomer methacryloyloxydodecyl pyridinium bromide (MDPB) has been synthesized. Its antibacterial activity has been shown when immobilized in a resin-based material and it has been used in dental bonding agents.29–31 Other methacrylate monomers containing quaternary ammonium chloride salts have shown antibacterial effects on several bacteria associated with oral infections, including Streptococcus mutans, Staphylococcus aureus, and Lactobacillus casei.32
Incorporation of antimicrobial agents in dental restoration materials is of clinical importance. Oral bacterial biofilms can accumulate and grow on resin-based composites, even if they contain fluoride. Current dental composites have little, if any, inhibitory effect against cariogenic bacteria and thus little resistance to secondary caries.33 In fact, certain dental monomers (e.g., TEGDMA) have shown a stimulating effect on the growth of cariogenic bacteria.34 Therefore, antibacterial dental composites and bonding agents that can kill cariogenic bacteria and inhibit the growth of their biofilms are highly desirable.29–32 The materials containing soluble antibacterial agents such as chlorhexidine usually have only short-term antibacterial effects and poor mechanical properties due to the high water sorption and the formation of porous structures after elution of the antibacterial agent.35–40 In contrast, the materials with an immobilized antibacterial agent have more desirable long-term antibacterial effects and minimal negative effects on their mechanical properties.30
The objective of this study is to synthesize new methacrylate and methacrylamide antibacterial monomers containing long-chain quaternary ammonium fluoride moieties. The cation part of these monomers can function as both an antibacterial monomer and the counter ion in fluoride-releasing monomers while the anion (fluoride ion) can provide a source of fluoride. Experimental dental composites containing different amounts of synthesized antibacterial monomers have been formulated. The antibacterial effect, fluoride release, degree of conversion (DC), and physical and mechanical properties of the dental composites have been investigated.
EXPERIMENTAL
Reagents and analysis
Benzyl bromide, dodecane-1,12-diamine, methacrylic anhydride, iodomethane, silver(I) fluoride, methacryloyl chloride, 11-bromoundecan-1-ol, glucose, sucrose, and solvents were purchased from Aldrich. 2,2-bis[4-(2-hydroxy-3-methacroyloxypropoxy) phenyl]-propane (BisGMA) was purchased from Polysciences. Ethoxylated bisphenol-A dimethacrylate (EBPADMA) and 1,6-hexanediol dimethacrylate (HDDMA) were provided by Esstech (Essington, PA). Camphorquinone (CQ), ethyl 4-dimethylaminobenzoate (4E), and bis(2,4,6-trimethylbenzoyl)- phenyl-phosphine oxide (PO) were purchased from Aldrich. The silanized fluoride-releasing filler (mean particle size 1.3 μm) was provided by Caulk/Dentsply (York, PA). A fluoride-releasing dimethacrylate monomer was synthesized as previously reported.18
High resolution electrospray ionization mass spectrometry (ESI–MS) and tandem mass spectrometry (MS/MS) were carried out on a Synapt HD mass spectrometer (Waters, Milford, MA), which is a quadrapole-ion-mobility-time-of-flight (Q-IM-TOF) instrument with a resolution of 10,000 and a mass accuracy of 2 ppm (V mode). 1H- and 13C-nuclear magnetic resonance (NMR) spectra were acquired on a Varian Unity-400 NMR spectrometer (Varian, Santa Clara, CA), 1H at 400 MHz, and 13C at 100 MHz in CDCl3 or CD3OD. Fourier transformed near infrared spectroscopy (FT-NIR) was carried out using Nicolet Nexus 670 FTIR spectrometer (Thermo Scientific, West Palm Beach, FL). The ChemDraw Ultra 9.0 software (CambridgeSoft, Cambridge, MA) was used to draw the structures, generate the names, and calculate the exact masses of the synthesized monomers and their intermediates.
Monomer synthesis
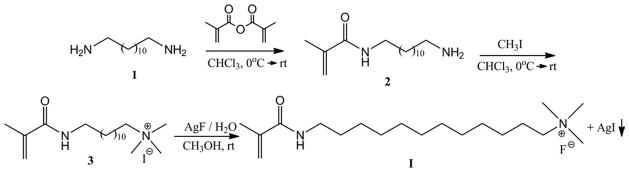
N-(12–diaminododecyl) methacrylamide (2)
A 250-mL round-bottomed flask equipped with a magnetic stirring bar was charged with 1,12-diaminododecane (1) (10 g, 50 mmol) and chloroform (150 mL) and placed in an ice bath. After the reaction flask was cooled for 15 min, methacrylic anhydride (50 mmol) was added via syringe over 10 min. The reaction mixture was stirred at 0°C to room temperature for overnight. The reaction mixture was quenched by adding saturated aqueous K2CO3 (150 mL). The aqueous layer was extracted with chloroform (50 mL × 3). The combined organic extracts were washed sequentially with saturated aqueous NaHCO3 (100 mL × 2) and brine (100 mL × 2), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure by a rotary evaporator. The crude product was purified on silica gel column with CH3COOC2H5:CH3OH (3:1) as mobile phase. It produced 10.72 g (40 mmol) of white solid (yield: 80%). HRMS analysis (in methanol, positive ions): m/z = 269.2597 (C16H33N2O+ ([M+H]+), calculated: 269.2515). MS/MS (269.3, positive ions): 269.3(18), 241.4(19), 224.3(50), 182.3(100). 1H-NMR (400 MHz, CDCl3) δ: 1.239–1.334(m, 16H), 1.46–1.526 (m, 4H), 1.936–1.938 (d, 3H), 2.65–2.68 (t, 2H), 3.247–3.297 (m, 2H), 5.276 (d, 1H), 5.637 (s, 1H). 13C-NMR (100 MHz, CDCl3) δ: 18.875, 27.044, 27.135, 29.412, 29.458, 29.625, 29.671, 29.701, 29.747, 33.436, 39.919, 42.091, 119.071, 140.631, 168.660.
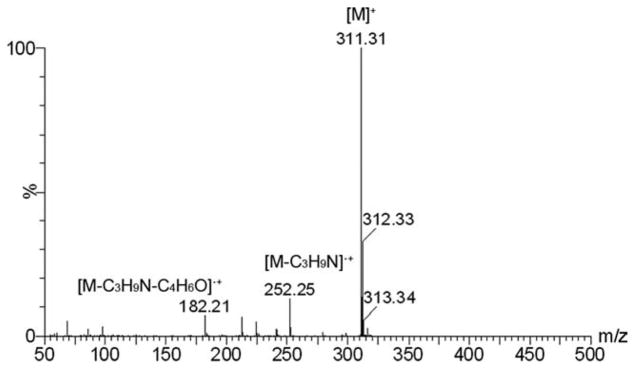
12-methacrylamido-N,N,N- trimethyldodecan-1-aminium iodide (3)
N-(12–diaminododecyl)methacrylamide (2) (2 mmol) and chloroform (30 mL) were added to a 100 mL round bottomed flask equipped with a magnetic stirring bar. The solution was cooled to 0°C in an ice bath. CH3I (7 mmol) was added by syringe over 15 min. The reaction was stirred at 0°C for 4 h and then at room temperature for 10 h. After the reaction was completed, the solvent was removed by rotary evaporation. The residual mixture was purified on silica gel column with CH3COOC2H5:CH3OH (1:5) as mobile phase. Removal of solvent and drying under vacuum generated 12.6 g (28.8 mmol) of pale yellow solid (yield: 72%). HRMS analysis (in methanol, positive ions): m/z = 311.3065 (C19H39N2O+ ([M]+), calculated: 311.3062). MS/MS (311.3, positive ions): 311.3(100), 252.2(15), 182.2(9). 1H-NMR (400 MHz, CDCl3) δ: 1.24–1.42 (m, 16H), 1.460–1.526 (m, 4H), 1.94 (s, 3H), 3.173 (s, 9H), 3.214–3.243 (t, 2H), 3.375–3.409 (m, 2H), 5.347 (s, 1H), 5.668 (s, 1H). 13C-NMR (100 MHz, CDCl3) δ: 19.065, 24.200, 27.550, 28.217, 29.257, 30.374, 30.662, 30.668, 30.730, 30.824, 40.860, 53.907, 53.953, 68.163, 120.100, 141.662, 171.80.
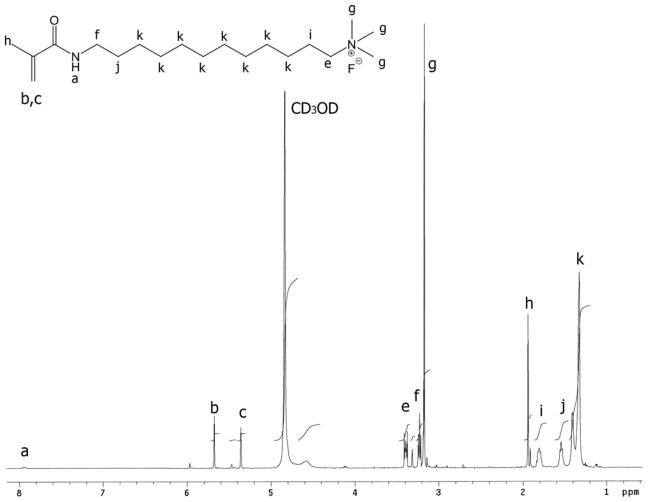
12-methacrylamido-N,N,N-trimethyldodecan-1-aminium fluoride (I)
12-methacrylamido-N,N,N-trimethyldodecan-1-aminium iodide (3) (0.877 g, 2 mmol) was dissolved in CH3OH (48 mL)/H2O (20 mL) and was divided equally into six tubes (15 mL polypropylene conical tube). Totally, 2 mmol of AgF aqueous solution (1.0M) was added dropwise under dimmed light. The tube was shaken and centrifuged to remove the AgI precipitate. All supernatant solutions were combined into one, which was then concentrated by rotary evaporation and purified through a silica gel column using CH2Cl2:CH3OH (9:1) as mobile phase, (yield: 75%, 1.5 mmol). HRMS analysis (in 1:1 methanol/H2O, positive ions): m/z = 311.3064 (C19H39N2O+ ([M]+, cation part), calculated: 311.3062). MS/MS (311.3, positive ions): 311.3(100), 252.3(13), 182.2(8). 1H-NMR (400 MHz, CD3OD) δ: 1.29–1.42 (m, 18H), 1.553–1.555 (m, 2H), 1.94 (s, 3H), 3.173 (s, 9H), 3.214–3.243 (t, 2H), 3.375–3.409 (m, 2H), 5.36 (s, 1H), 5.681 (s, 1H). 13C-NMR (100 MHz, CDCl3) δ: 19.06, 24.01, 27.42, 28.13, 30.32, 30.52, 30.62, 30.68, 30.73, 40.76, 53.87, 53.91, 68.01, 123.95, 141.49, 171.30.
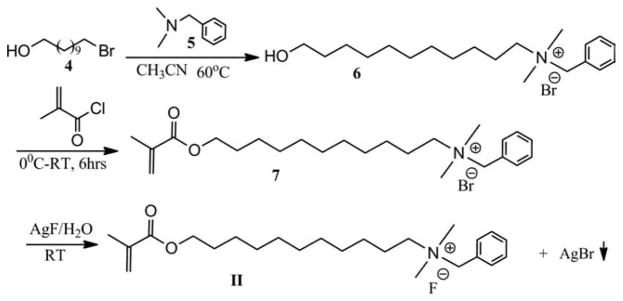
N-benzyl-11-hydroxy-N,N-dimethylundecan-1-aminium bromide (6)
A 250-mL, round-bottomed flask equipped with a magnetic stirring bar was charged with 11-bromoundecan-1-ol (10 g, 40 mmol) and acetonitrile (150 mL). The solution was placed in an oil bath. N,N-dimethyl-1-phenylmethanamine (40 mmol) was added via syringe. The reaction mixture was stirred at 60°C for 6 h. When the mixture was cooled to room temperature, a white solid precipitated. The white solid was filtered, washed with cold diethyl ether, and dried in a vacuum oven yielding 13.03 g. HRMS analysis (in methanol, positive ions): m/z =306.2800 (C20H36NO+ ([M]+, cation part), calculated: 306.2791). MS/MS (306.3, positive ions): 306.3(26), 214.2(100), 91.0(98). 1H-NMR (400 MHz, CDCl3) δ: 1.29–1.37(m, 14H), 1.577–1.613 (m, 2H), 1.85 (s, 2H), 3.322 (s, 6H), 3.587–3.663 (m, 4H), 5.086 (s, 2H), 7.444–7.582 (m, 3H), 7.712–7.728 (d, 2H). 13C-NMR (100 MHz, CDCl3) δ: 23.12, 25.91, 26.43, 29.33, 29.38, 29.42, 29.47, 29.55, 32.95, 49.78, 62.89, 64.07, 67.54, 127.58, 129.40, 130.91, 133.49.
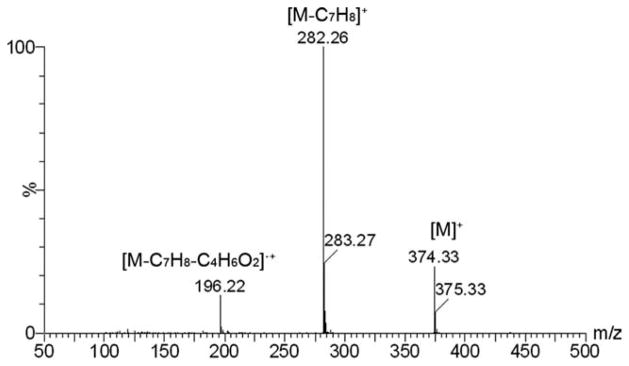
N-benzyl-11-(methacryloyloxy)-N,N-dimethylundecan-1-aminium bromide (7)
A 250-mL round-bottomed flask equipped with a magnetic stirring bar was charged with N-benzyl- 11-hydroxy-N,N-dimethylundecan-1-aminium bromide (6) (11.55 g, 30 mmol) and dichloromethane (100 mL) and was placed in an ice bath. After the reaction flask was cooled for 15 min, methacryloyl chloride (31 mmol) was added via syringe over 10 min. The reaction mixture was stirred at 0°C for 2 h and at room temperature for 6 h. The solution was poured into a 250-mL separation funnel and organic layer was washed by aqueous Na2CO3, water and brine, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure in a rotary evaporator. The crude product was purified by a silica gel column using CH2Cl2:CH3OH (9:1) as mobile phase. Removal of solvent and drying under vacuum gave a colorless oil (8.14 g, 20.7 mmol, yield: 69%). HRMS analysis (in methanol/acetone 1:3, positive ions): m/z = 374.3051 (C24H40NO2+ ([M]+), calculated: 374.3054). MS/MS (374.3, positive ions): 374.5(19) 282.4(100), 196.3 (16), 91.1 (28). 1H-NMR (400 MHz, CDCl3) δ: 1.347–1.413 (m, 14H), 1.663–1.698 (t, 2H), 1.900–1.929 (m, 5H), 3.054 (s, 6H), 3.328–3.370 (m, 2H), 4.122–4.155 (t, 2H), 4.828 (s, 2H), 5.608–5.617 (t, 1H), 6.075 (s, 1H), 7.528–7.609 (m, 5H) 13C-NMR (100 MHz, CDCl3) δ: 18.661, 23.936, 27.271, 27.674, 29.924, 30.389, 30.482, 30.668, 30.730, 50.680, 66.084, 69.109, ,125.995, 128.565, 130.431, 131.998, 134.232, 138.132, 168.568.
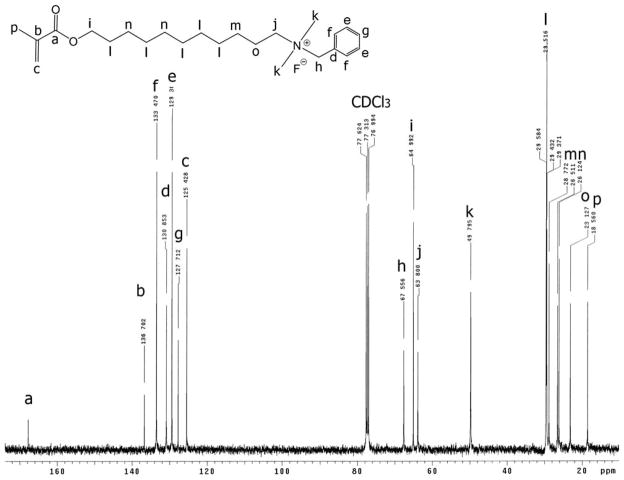
N-benzyl-11-(methacryloyloxy)-N,N-dimethylundecan-1-aminium fluoride (II)
N-benzyl-11-(methacryloyloxy)-N,Ndimethylundecan-1-aminium bromide (7) (1.05g, 2.6 mmol) was dissolved in CH3OH (48 mL)/H2O (20 mL) and was poured into 6 tubes (15-mL polypropylene conical tube) and then aqueous AgF (0.055g, 0.43 mmol) was added to each tube. The resulting AgBr precipitate was removed by decanting after centrifugation. The separate solutions were combined and concentrated by rotary evaporator. The residue was purified by a silica gel column using CH2Cl2:CH3OH (9:1) as mobile phase, (yield: 77%, 9.04 g, 23 mmol). HRMS analysis (in methanol/acetone 1:3, positive ions): m/z = 374.3051, (C24H40NO2+ ([M]+, cation part), calculated: 374.3054). MS/MS (374.3, positive ions): 374.3 (22), 282.3 (100), 196.2 (15). 1H-NMR (400 MHz, CDCl3) δ: 1.266–1.339 (m, 14H), 1.649–1.719 (m, 2H), 1.816 (s, 2H), 1.958 (s, 3H), 3.325 (s, 6H), 3.514–3.556 (m, 2H), 4.133–4.166 (t, 2H), 5.077 (s, 2H), 5.562–5.569 (t, 1H), 6.111 (s, 1H), 7.424–7.482 (m, 3H), 7.676–7.696 (m, 2H). 13C-NMR (100 MHz, CDCl3) δ: 18.560, 23.127, 26.124, 26.511, 28.772, 29.371, 29.432, 29.516, 29.584, 49.795, 63.800, 64.992, 67.556, 125.428, 127.712, 129.366, 130.853, 133.470, 136.702, 167.786.
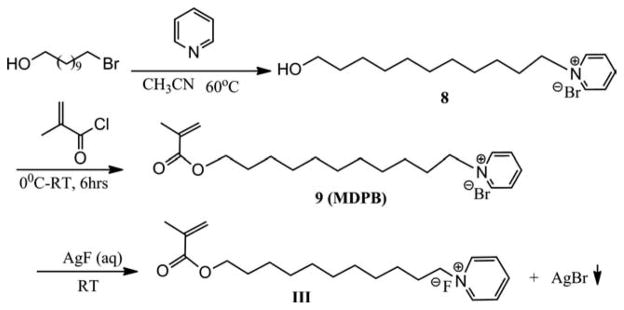
1-(11-hydroxyundecyl)pyridinium bromide (8)
A 50-mL round-bottomed flask was charged with 11-bromo-1-undecanol (1.0048 g, 4 mmol) and 5-mL pyridine, stirred over night at room temperature. A solid was formed. The excess pyridine was removed by rotary evaporation and the residue was washed with hexane, filtered and dried under vacuum to give a white solid (1.24 g, 3.77 mmol, yield: 94%). HRMS analysis (in methanol/acetone 1:3, positive ions): m/z = 250.2183 (C16H28NO+ ([M]+, cation part), calculated 250.2165). MS/MS (250.2, positive ions): 250.2 (94), 80.1 (100).
1-(11-(methacryloyloxy)undecyl)pyridinium bromide (MDPB) (9)
A 50-mL round-bottomed flask was charged with 8 (1.24 g, 3.77 mmol) and CHCl3 (18 mL). After cooling the solution to 0°C, methacryloyl chloride is added dropwise via a syringe. The reaction mixture is stirred at 0°C for 2 h and at room temperature overnight. The solution was poured into a 100 mL separation funnel and the organic layer was washed by aqueous Na2CO3, water and brine (35 mL × 2), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure in a rotary evaporator. The crude product was purified by a silica gel column using CH2Cl2:CH3OH (9:1) as mobile phase. Removal of the solvent gives a yellow oil (1.05 g, 2.6 mmol, yield: 70%). HRMS analysis (in methanol/acetone 1:3, positive ions): m/z = 318.2430 (C20H32NO2+ ([M]+), calculated: 318.2428).
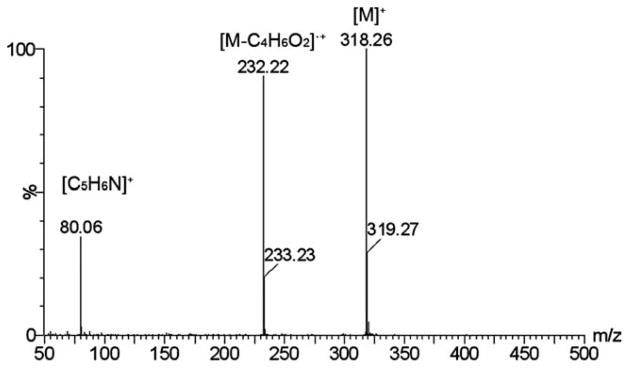
1-(11-(methacryloyloxy)undecyl)pyridinium fluoride (III)
1-(11-(methacryloyloxy)undecyl)pyridinium bromide (9) (1.05 g, 2.6 mmol) was dissolved in C2H5OH (48 mL)/H2O (20 mL) and poured into 6 tubes (15 mL polypropylene conical tube), and then a AgF aqueous solution (0.3299 g, 2.6 mmol AgF dissolved in 6 mL water) was added dropwise (1.0 mL/tube) under dimmed light. The AgBr precipitate was removed by decanting following centrifugation. The separate solution was combined and was concentrated by rotary evaporator. The residue was purified by a silica gel column using CH2Cl2:CH3OH (9:1) as mobile phase. HRMS analysis (in methanol/acetone 1:3, positive ions): m/z = 318.2424 (C20H32NO2+ ([M]+), calculated: 318.2428). MS/ MS (318.3, positive ions): 318.3 (100), 232.2 (90). 1H-NMR (400 MHz, CDCl3) δ: 1.321–1.392 (m, 14), 1.652–1.688 (q, 2H), 1.918–1.924 (q, 3H), 2.012–2.048 (t, 2H), 4.117–4.150 (t, 2H), 4.631–4.668 (t, 3H), 5.603–5.611 (t, 1H), 6.065–6.072 (q, 1H), 8.113–8.147 (t, 2H), 8.590–8.629 (t, 1H), 9.02–9.35 (d, 2H). 13C-NMR (100 MHz, CDCl3) δ: 18.528, 27.184, 27.308, 29.837, 30.193, 30.380, 30.353, 30.581, 30.643, 32.582, 63.298, 66.028, 125.892, 129.584, 137.992, 145.965, 146.912, 168.986.
Biocompatibility and bactericidal study
The cytotoxicity of monomers I, II, and III to L-929 mouse fibroblast cells was tested using agar overlay method according to the ISO standard 10993-5 (Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity). Triplicate wells were inoculated with 0.1 mL of the test article (10−4M of each monomer in 0.5% DMSO/medium solution) on a filter. High density polyethylene and latex were used as negative and positive controls, respectively. After incubating at 37°C in 5% CO2 for 24 h, the cell culture was examined macroscopically and microscopically (100×) for cell decolonization to determine the zone of cell lysis and the cell morphology.
The bactericidal (kill-time) effect of the monomers I, II, and III against Streptococcus mutans ATCC 35668 was tested as follows. Test tubes (n = 3) containing 10 mL solution of a serial dilution (10−3M through 10−6M in 0.5% DMSO/medium) of each antibacterial monomer and BisGMA (control) and planktonic S. mutans cells with starting colony-formingunits (CFU) of 1.14 × 105 were incubated for 24 h at 37°C, and the surviving bacteria cells were counted microscopically. The results were recorded as Log Reduction = LogB − LogA, where A = CFU/mL of the test tube containing the test article after incubation, B = CFU/mL of the starting population.
Formulation of experimental antibacterial dental composites
Based the preliminary results from the biocompatibility and bactericidal study, the antibacterial monomer II was selected to fabricate antibacterial experimental dental composites. Their compositions (wt %) are listed in Table II. Control and experimental composites PC-0.5 through PC-6 contain 0–6.0% of monomer II replacing equal amount of BISGMA and EBPADMA. All composites contain 70% silanized fluoride-releasing filler, 29% monomer mixture, and 1% photoinitiators. A fluoride-releasing antibacterial composite has also been formulated using the synthesized fluoride- releasing monomer containing a ternary zirconium-fluoride chelate.18 The uncured composite resins were blended using a SpeedMixer (FlackTek, Landrum, SC).
TABLE II.
Compositions of Experimental Antibacterial Dental Composites (wt %)
| Components | Control | PC-0.5 | PC-1 | PC-2 | PC-3 | PC-6 | F-RC |
|---|---|---|---|---|---|---|---|
| Antibacterial Monomer | 0.0 | 0.5 | 1.0 | 2.0 | 3.0 | 6.0 | 3.0 |
| BisGMA | 11.6 | 11.35 | 11.1 | 10.6 | 10.1 | 8.6 | 8.7 |
| EBPADMA | 11.6 | 11.35 | 11.1 | 10.6 | 10.1 | 8.6 | 5.8 |
| HDDMA | 5.8 | 5.8 | 5.8 | 5.8 | 5.8 | 5.8 | 5.8 |
| F-releasing monomer18 | – | – | – | – | – | – | 5.7 |
| F-releasing glass filler | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 | 70 |
| CQ | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 |
| 4E | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 |
| PO | 0.28 | 0.28 | 0.28 | 0.28 | 0.28 | 0.28 | 0.28 |
| Total | 100.0 | 100 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
BisGMA, 2,2-bis[4-(2-hydroxy-3-methacroyloxypropoxy)phenyl]-propane; EBPADMA, ethoxylated bisphenol-A dimethacrylate; HDDMA, 1,6- hexanediol dimethacrylate; CQ, camphorquinone; 4E, ethyl 4-dimethylaminobenzoate; PO, bis(2,4,6-trimethylbenzoyl)-phenyl-phosphine oxide.
Photopolymerization of experimental composites
The photopolymerization experiments were conducted using Fourier transformed near infrared spectroscopy (FT-NIR) as previously reported.20,41 The disk composite specimens (5 mm diameter, 2 mm thick, n = 5) were prepared using a rubber ring pressed between a pair of glass slides (22 × 22 × 0.17 mm3). The specimen was placed on top of a Smart NIR UpDRIFT, a top-loading diffuse reflection accessory, and the NIR spectrum for the uncured resin was acquired using a Nicolet Nexus 670 FTIR spectrometer (Thermo Scientific, West Palm Beach, FL). The specimen was then light-cured through the upper glass slides using an Optilux 501 curing light (Kerr, output > 500 mW/cm2) for 40 or 80 s. After each light curing, the NIR spectrum of the specimen was immediately acquired. All spectra were recorded using wavelength range 5500–8000 cm−1, resolution 4 cm−1, and scan number 128. The degree of conversion (DC) was calculated using the area of the first overtone of the vinyl (C=C) absorbance peak around 6163 cm−1 as follows:
where Ap is the peak area of the cured composite (polymer) and Am is the peak area of uncured resin (monomer). The average DC values of five specimens under each curing time were reported.
Characterization of experimental composites
Flexure strength specimens (25 × 2 × 2 mm3, n = 10) were prepared in stainless steel molds and light cured with an Optilux 501 curing light (Kerr, Orange, CA, output > 500 mW/cm2) for 40 s each in three segments on both surfaces. The cured specimens were polished with 600-grit SiC paper, stored in deionized water at 37°C for 24 h or 3 months, and tested on the Instron 5566 testing machine with a crosshead speed of 1 mm/min. Fluoride release from the cylindrical samples (φ 4 × 9 mm2, n = 5) of control, PC-3.0 and the F-releasing composite in 3 mL deionized water was analyzed daily for 17 days using an ion-selective electrode. The water sorption and solubility test were conducted using disk specimens (φ15 × 1 mm2, n = 5) according to the ISO standard 4049.
Microbial biofilm inhibition test
To evaluate the efficacy of the antibacterial composites to inhibit S. mutans biofilm formation, disk specimens (φ10 × 1 mm2, n = 5) of each composite were immersed in the 20 mL of properly diluted mid-exponential phase cultures of S. mutans (approximately 5 × 107 clone-forming-unit per mL) in semidefined biofilm medium containing 18 mM glucose and 2 mM sucrose, and bacteria were allowed to grow at 37°C in an aerobic incubator with 5% CO2.40,42 After 48 h, one specimen was used for biofilm analysis using a field emission-scanning electron microscope (FE-SEM) and the remaining four were used for microbiological analysis. For FE-SEM analysis, the biofilm samples were fixed in 2.5% glutaradehyde in pH 7.2 phosphate buffer overnight at 4°C, rinsed in sodium phosphate buffered solution, dehydrated with increasing concentrations of ethanol, and then subjected to critical point drying in a carbon dioxide critical point drier (Electron Microscopy Sciences, Hatfield, PA). The dried specimens were coated with carbon using a Carbon Coater 208C (Cressington Scientific Instruments, Watford, UK) and analyzed using a Hitachi 4800 FE-SEM (Hitachi, Tokyo, Japan). For microbiological analysis, samples were briefly rinsed in phosphate saline (pH 7.0) and microbial biofilms on the disks were dispersed by brief sonication.43 Serial dilutions were made and plated in triplicate on BHI agar plates.
Statistical analysis of the data
The data were analyzed using multiway ANOVA and posthoc Tukey tests. The significance level was set at 95% (α = 0.05).
RESULTS AND DISCUSSION
Synthesis of monomers
The designing of the antibacterial monomers I and II containing long-chain quaternary ammonium fluoride salts is based on the following rationales: (1) the monomers must have at least one long aliphatic chain (eleven carbons or longer) to increase the lipophilicity, which has been shown to correlate with the bactericidal effect. Only quaternary ammonium salts containing an eight carbon or longer chain have shown significant antibacterial activity.27,32 Monomer II has an additional aromatic group to further increase its lipophilicity and bactericidal effect. (2) Usually chloride or bromide ions are used as the counter ions for antibacterial quaternary salts because they have a low tendency to form ion-pairs with quaternary cations. In this study, however, fluoride is purposely selected as the counter ion because the monomer will also serve as a fluoride source for fluoridereleasing dental materials. If other anions (Cl−, Br−, or I−) are used, the fluoride ion from the fluoride-releasing materials, the topical fluoride agents (>1500 ppm F−) or fluoride-containing tooth paste would exchange with them to form fluoride salts. In addition, bromide or iodide ions could also be oxidized to form colored molecules (Br2 or I2) and might reduce the color stability of the material. (3) Quaternary ammonium salts containing four large organic groups such as tetrabutylammonium (TBA) have a strong tendency to form tight ion-pairs with fluoride, which would reduce the efficacy of both antibacterial monomer and fluoride. To avoid or minimize the formation of such tight ion-pairs, the quaternary ammonium salts containing at least two small aliphatic (methyl) groups have been selected. On the other hand, the quaternary ammonium salts containing one or more protons (H) have also been excluded to minimize potential biological side effects. (4) The fluoride form of the previously reported MDP monomer has also been synthesized for comparison.
Monomers I and II were synthesized in three-steps (Schemes 1 and 2). The starting material of monomer I has two amino groups. When methacryloyl chloride was used as methacrylation agent, it was very difficult to control the reaction time so that only one amino group was reacted, and therefore, the yield of the mono-substitute product (2) was very low. We have found that using methacrylic anhydride instead of methacryloyl chloride can greatly increase the yield of 2 although the reaction time is longer (Scheme 1).
SCHEME 1.
Synthesis of monomer I.
SCHEME 2.
Synthesis of monomer II.
The third step (conversion of the iodide to fluoride) must be carried out with care to ensure that all iodide ions are removed while the excess of AgF should be avoided because it will also reduce the color stability. The reaction must also be carried out under dimmed or red light to avoid the formation of silver metal and molecular iodine by the photolysis of AgI, which could give the monomer a brownish color.
Scheme 3 shows the procedure for the synthesis of monomer III. In the initial synthesis of compound 9 (MDPB) we used the reported method,29,30 in which acetonitrile was used as the solvent and the yield was low. Later, we used pyridine as both the reactant and the solvent. As a result, the reaction was faster and the yield was greatly improved (94%).
SCHEME 3.
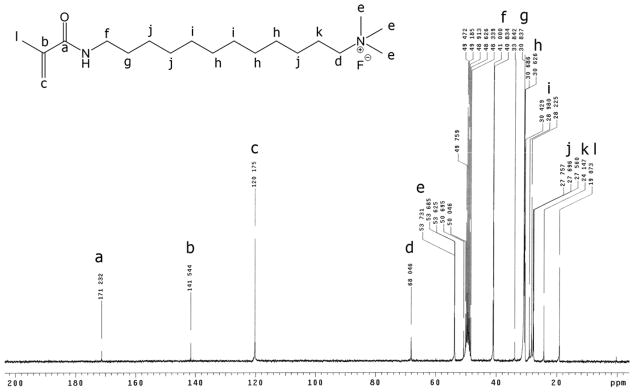
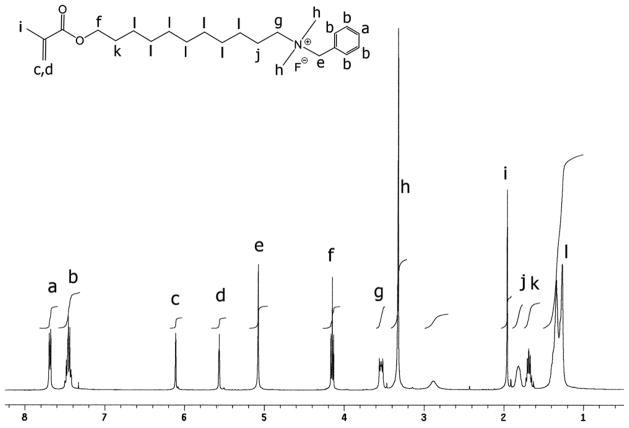
The positive ion tandem mass spectrometry (MS/MS) spectra of monomers I, II, and III are shown in Figures 1–3, respectively. Under the same collision induced fragmentation (CID) conditions (trap CE (collision energy) 30.0 V, transfer CE 4.0 V), monomer I has shown less fragment abundance (Figure 1) than monomer II (Figure 2) and monomer III (Figure 3). There are two reasons. First, monomers II and III have aromatic (benzyl and pyridinyl) groups, which can form more stable fragment radical ( , lost mass 92.06) or cation (C5H6N+, m/z = 80.05) than trimethylamine radical (C3H9N•, lost mass: 59.07) formed by monomer I. Second, monomer I is a methacrylamide, which is known to be chemically more stable than methacrylates (monomers II and III). Figures 2 and 3 show the fragments (m/z = 196.2 and m/z = 232.2) caused by the loss of methacrylic acid (C4H6O2, m = 86.04), which is more stable (easier to lose) than the methacrylaldehyde (C4H6O, m = 70.04), shown in Figure 1. Therefore, the methacrylamide monomer I may be more suitable for application in self-etching dental bonding agents, in which methacrylate monomers may be susceptible to acid-catalyzed hydrolytic degradation.44 The high-resolution electrospray mass spectrometry (HRMS), tandem mass spectrometry (MS/MS), and NMR data included in the experimental sections confirm the structures of the synthesized monomers and intermediates showing Schemes 1–3. The negative ion ES-MS spectrum (not shown) of the synthesized fluoride releasing monomer using the cation part of monomer II as the counter ion has shown only high abundance of m/z = 810.4, which corresponds to the anion of the monomer [L-3H+ZrF2]− (L is the chelating monomer).18 The anticipated ion-pairs [monomer II+F]−at m/z= 412.31 and [L-3H+ZrF2+monomer II]− at m/z = 1203.71 were not detected. This indicates that monomer II has a low tendency to form ion-pairs with fluoride. Monomer I has an even smaller quaternary ammonium cation and it should have even lower tendency to form ion-pairs. The 1H-NMR and 13C-NMR spectra of monomers I and II are shown in Figure 4–7, which confirm the structures of the monomers. The NMR data (spectra not shown) also confirm the structure of monomer III.
FIGURE 1.
Positive ion MS/MS spectrum of monomer I (M is the cation part of monomer I).
FIGURE 3.
Positive ion MS/MS spectrum of monomer III (M is the cation part of monomer III).
FIGURE 2.
Positive ion MS/MS spectrum of monomer II (M is the cation part of monomer II).
FIGURE 4.
1H-NMR spectrum of monomer I (the letter indicates the location of the protons in the structure).
FIGURE 7.
13C-NMR spectrum of monomer II (the letter indicates the location of the carbon in the structure).
Biocompatibility and antibacterial effects of monomers
A major concern for the biomedical applications for the antimicrobial polymers is their biocompatibility. The material should be able to kill certain bacteria (and fungi) or inhibit their growth without damaging the ecology of the normal flora and the host tissue.. The cytotoxicity test with L-929 mouse fibroblast cells using agar overlay method according to the ISO standard 10993-5 showed that monomer I and monomer II have grade 0 (no reactivity) while monomer III has grade 1 (slight reactivity). These results suggest that monomers I and II have even better biocompatibility than monomer III, but all antibacterial monomers synthesized in this study are biocompatible.
The antibacterial effects of the monomers are shown in Table I. The control monomer BisGMA (10−3M ~ 10−6M) has zero Log Reduction, meaning no bactericidal effect, which was expected. On the other hand, all three synthesized antibacterial monomers show significant bactericidal effect. At a concentration of 10−3M, monomers I, II, and III have Log Reductions of 2.15, 3.66, and 3.88, respectively. Monomers II and III are more effective than monomer I. At a concentration of 10−4M, however, only monomer II has a Log Reduction of 1.78 while monomer I and monomer III have no Log Reduction, indicating that monomer II has a lower minimum effective concentration than monomers I and III. To compare the antibacterial activities of the fluoride form of antimicrobial monomer II (AM2) and its bromide form (AM2Br), planktonic growth tests of Streptococcus mutans (S. m.) and Lactobacillus casei (L. c.) were also conducted using Bioscreen C™ (Oy Growth Curves Ab). The results indicate that the bromide form of antibacterial monomer II has very similar or slightly higher antibacterial activity than its fluoride form (See Supporting Information 2 for details). Considering both cytotoxicity and bactericidal effect, monomer II is superior to monomers I and III, and therefore, it has been selected to formulate experimental antibacterial dental composites.
TABLE I.
Bactericidal Effect of the Antibacterial Monomers Against S. mutans (Mean, n = 3)
| Monomer | Concentration | Log Reductiona |
|---|---|---|
| BisGMA | 10−3M~10−6M | No reduction |
| Monomer I | 10−3 M | 2.15 |
| Monomer I | 10−4M~10−6M | No reduction |
| Monomer II | 10−3 M | 3.66 |
| Monomer II | 10−4 M | 1.78 |
| Monomer II | 10−5 M~10−6 M | No reduction |
| Monomer III | 10−3 M | 3.88 |
| Monomer III | 10−4M~10−6 M | No reduction |
Log Reduction = LogB - LogA, where A and B are numbers of S. mutans (in CFU/mL) in test tubes before and after incubation with the test monomer, respectively.
Fabrication and photopolymerization of experimental composites
Six experimental dental composites and one control composite were formulated using monomer II and other dental monomers, fluoride-releasing glass fillers, and photoinitiators. Their compositions are listed in Table II. In this study, hexanediol dimethacrylate (HDDMA) instead of the traditional triethylene glycol dimethacrylate (TEGDMA) was used as a diluent monomer because our previous study21 indicated that replacing all TEGDMA (LogP 1.42) and part of BisGMA (LogP = 5.09) with more hydrophobic monomers HDDMA (LogP = 3.13) and EBPADMA (LogP = 5.54), respectively, could reduce water sorption and increase longterm mechanical properties. Control and experimental composites PC-0.5 through PC-6 contain 0–6.0% of monomer II, replacing equal amount of BisGMA and EBPADMA.
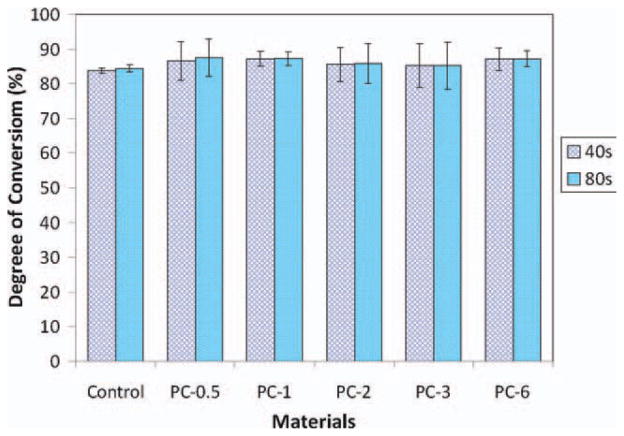
The degree of polymerization conversion (DC) of experimental and control composites after light cure for 40 and 80 s was measured using FT-NIR and the result is shown in Figure 8. There is no significant difference in DC between 40 and 80 s light cure for each composite (p > 0.05). There is also no significant difference in DC with different concentration of antibacterial monomer II (p > 0.05). This result indicates that the antibacterial monomer (within certain concentration range) does not affect the photopolymerization of the dental composites. Light cure with a traditional quartz-tungsten-halogen (QTH) dental curing light for 40 s is sufficient to polymerize the composites.
FIGURE 8.
Degree of polymerization conversion of experimental composites after light cure for 40 and 80 s. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Physical and mechanical properties
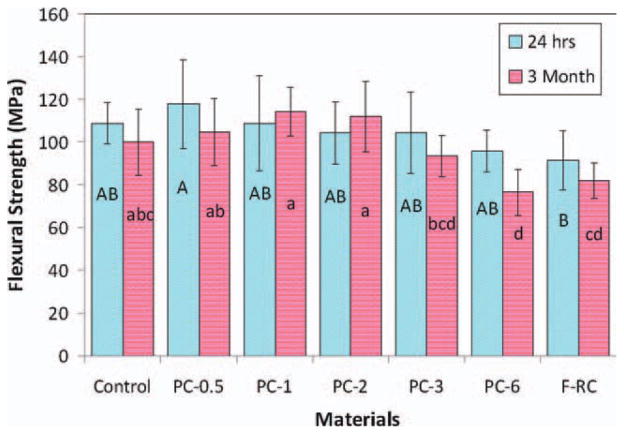
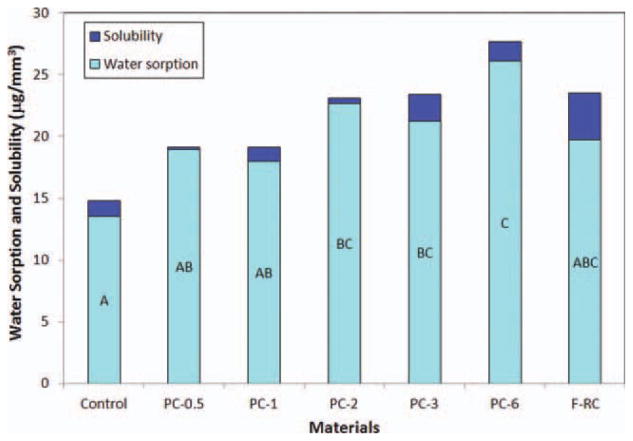
The flexural strengths of the experimental and control composites after immersion in water for 24 h and 3 months are shown in Figure 9. After 24-h immersion, the mechanical properties of all experimental composites containing up to 6% antibacterial monomer II (PC-0.6 - PC-3) are statistically not significantly different from that of control (p > 0.05) while the flexure strength of PC-6 decreased significantly (p < 0.05). After 3 months, PC-0.5 –PC-3 maintain the similar flexure strength to that of control (p > 0.05) but the strength of PC-6 decreases significantly (p = 0.004). The reduction of mechanical properties of PC-6 can be attributed to the increased monomer II content and increased water sorption of the composite as shown in Figure 10. Since the antibacterial monomer II is a long-chain quaternary ammonium salt (a highly polar molecule), it can increase water sorption of the composite as well as function as a plasticizer itself (increasing the distance and reducing the chain entanglement between polymer chains). Both effects can lead to the reduction of the mechanical properties of the composite. Therefore, the antibacterial monomer content in the composite should be limited (to ca. 3%). Figure 11 shows the appearance and colors of experimental and control composites after immersion in water for 7 days. It appears these experimental antibacterial dental composites have an acceptable color, similar to that of the control composite.
FIGURE 9.
Flexural strength after 24 h and 3 months. The groups with the same letter have no significant difference (p > 0.05). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
FIGURE 10.
Water sorption and solubility of experimental composites. The groups with the same letter have no significant difference (p > 0.05). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
FIGURE 11.
The colors of antibacterial dental composites and control after immersion in water for 7 days. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Fluoride release
A composite that has both high fluoride release capability and antibacterial effect would have enhanced caries–inhibitive efficacy, and therefore, highly desirable. As discussed before, the antibacterial monomers containing quaternary ammonium salts in this study are in the fluoride form instead of the usual chloride or bromide forms. After photo-polymerization, while the cation part of the monomer is copolymerized with other dental monomers and thus immobilized on the composite, the fluoride (anion) can be released through exchange with other anions (e.g., hydroxide, chloride, or phosphate ions) in water or saliva. Therefore, adding the fluoride form of antibacterial monomer II in a composite can have benefit of increasing fluoride release without significant reduction of its antibacterial effect (see Supporting Information 2).
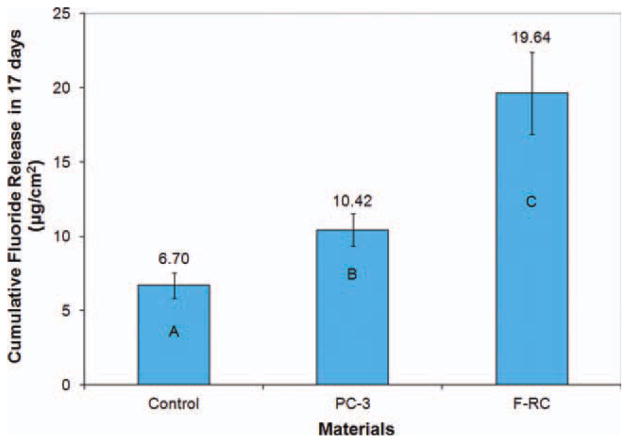
The cumulative fluoride release of the selected composites (control, PC-3, and F-RC) is shown in Figure 12. PC-3 has a significantly higher fluoride release than control (p = 0.015) due to additional fluoride in monomer II. The increased water sorption of PC-3 may have also contributed to the increase in fluoride release. The fluoride-releasing composite (F-RC) has significantly higher fluoride release than both PC-3 and control (p < 0.01) because it contains both monomer II and the fluoride-exchange monomer, which can enhance the transport of fluoride from the F-releasing glass filler inside the resin matrix to the surface of the composite through the ion exchange mechanism.18–21
FIGURE 12.
Cumulative fluoride release in 17 days. Different letters indicate significant difference (p < 0.05). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Efficacy against bacterial biofilms
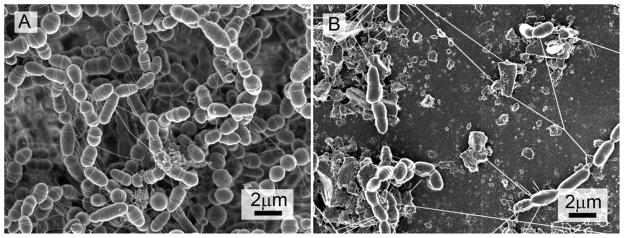
Results of the antibacterial polymer against S. mutans growth on the specimen surface are shown in Table III. In comparison, PC-3 and PC-6 had significantly lower CFU than the control and PC-0.5, PC-1, and PC-2. S. mutans developed a biofilm on the control specimen with a thickness of about 130 μm [Figure 13(A)]. However, inclusion of antibacterial monomer II, especially at concentrations higher than 3%, dramatically reduced the amount of biofilms by three orders (over 99.9% killing rate), as shown in Table III, and the biofilms on these composites contained a lot of deformed cells and debris of dead cells [Figure 13(B)]. In addition, the biofilms on the antibacterial composites appeared to have more “nanofiber-like” structures, although their exact nature and role in biofilm formation remain unclear. These results further suggest that dental composites containing 3% or higher concentration of antibacterial monomer II can significantly reduce the amount of S. mutans biofilm, and therefore, may have an enhanced anticaries effect. The novel antimicrobial monomer II in the composite is immobilized through photo-polymerization. Therefore, such antibacterial composite is expected to have a long-term antibiofilm and anticaries effect.
TABLE III.
S. mutans Biofilm on Experimental and Control Composites
| Composites (n = 4) | S. mutans Colony-Forming-Units (Mean ± SD)a |
|---|---|
| Control | (8.97 ± 2.75) × 107a |
| PC-0.5 | (8.17 ± 1.06) × 107a |
| PC-1 | (8.40 ± 1.03) × 107a |
| PC-2 | (12.0 ± 2.19) × 107a |
| PC-3 | (2.09 ± 0.16) × 104c |
| PC-6 | (1.04 ± 0.38) × 105b |
The groups with the same superscript letter have no significant difference (p > 0.05).
FIGURE 13.
FE-SEM images (× 10,000) of S. mutans biofilms on the surface of composites containing different concentration of antibacterial monomer II: (A) 0% (control), and (B) 3% (PC-3).
CONCLUSIONS
Antibacterial monomers I, II, III containing quaternary ammonium fluoride salts have been successfully synthesized. These monomers are biocompatible and have bactericidal effects against S. mutans at 10−3M concentration. Monomer II has lower minimum effective concentration (10−4M, thus higher bactericidal effect) than monomers I and III. The experimental F-releasing composite containing 3% of antibacterial monomer II has a significant inhibitive effect against the S. mutans biofilm growth on the composite surface. Such an antibacterial dental composite also has physical and mechanical properties similar to the conventional dental composite. Therefore, these new antibacterial monomers may find applications in antibacterial fluoride-releasing dental materials and other antibacterial biomaterials.
FIGURE 5.
13C-NMR spectrum of monomer I (the letter indicates the location of the carbon in the structure).
FIGURE 6.
1H-NMR spectrum of monomer II (the letter indicates the location of the protons in the structure).
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental & Craniofacial Research or the National Institutes of Health. The authors thank Esstech, Inc. for donation of dental monomers and Caulk/Dentsply for donation of F-releasing filler. The authors also thank Dr. Lian Chen for assistance in sample preparation, Ms. Corrine Gibb at University of New Orleans for NMR analysis, and Ms. Karen Huang at North American Science Associates (NAMSA) for cytotoxicity and bactericidal tests.
Contract grant sponsor: NIH/NCRR-COBRE; contract grant number: P20RR020160
Contract grant sponsor: NIH/NIDCR; contract grant number: R01DE019203
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.URL: Available at: http://www.nidcr.nih.gov/DataStatistics/FindDataByTopic/DentalCaries/
- 2.Yip KHK, Poon BKM, Chu FCS, Poon ECM, Kong FYC, Smales RJ. Clinical evaluation of packable and conventional hybrid resin-based composites for posterior restorations in permanent teeth—Results at 12 months. J Am Dent Assoc. 2003;134:1581–1589. doi: 10.14219/jada.archive.2003.0103. [DOI] [PubMed] [Google Scholar]
- 3.Casselli DSM, Martins LRM. Postoperative sensitivity in class I composite resin restorations in vivo. J Adhes Dent. 2006;8:53–58. [PubMed] [Google Scholar]
- 4.Lundin SA, Rasmusson CG. Clinical evaluation of a resin composite and bonding agent in Class I and II restorations: 2-year results. Quint Int. 2004;35:758–762. [PubMed] [Google Scholar]
- 5.Berkowitz GS, Horowitz AJ, Curro FA, Craig RG, Ship JA, Vena DA, Thompson VP. Research update: Postoperative hypersensitivity in class I resin-based composite restorations in general practice— Interim results. Compend Cont Ed Dent. 2009;30:356–363. [PMC free article] [PubMed] [Google Scholar]
- 6.Mjör IA, Moorhead JE, Dahl JE. Reasons for replacement of restorations in permanent teeth in general dental practice. Int Dent J. 2000;50:361–366. doi: 10.1111/j.1875-595x.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 7.Kidd EAM. Caries diagnosis within restored teeth. Adv Dent Res. 1990;4:10–13. doi: 10.1177/08959374900040010101. [DOI] [PubMed] [Google Scholar]
- 8.Kidd EAM, Beighton D. Prediction of secondary caries around tooth-colored restorations: A clinical and microbiological study. J Dent Res. 1996;75:1942–1946. doi: 10.1177/00220345960750120501. [DOI] [PubMed] [Google Scholar]
- 9.Heintz SD. Systematic reviews. I. The correlation between laboratory tests on marginal quality and bond strength. II: The correlation between marginal quality and clinical outcome. J Adhes Dent. 2007;9:77–106. [PubMed] [Google Scholar]
- 10.Frankenberger R, Krämer N, Lohbauer U, Nikolaenko SA, Reich SM. Marginal integrity: Is the clinical performance of bonded restorations predictable in vitro? J Adhes Dent. 2007;9:107–116. [PubMed] [Google Scholar]
- 11.Sarrett DC. Prediction of clinical outcomes of a restoration based on in vivo marginal quality evaluation. J Adhes Dent. 2007;9:117–120. [PubMed] [Google Scholar]
- 12.Maltz M, de Oliveira EF, Fontanella V, Bianchi R. A clinical, microbiologic, and radiographic study of deep caries lesions after incomplete caries removal. Quint Int. 2002;33:151–159. [PubMed] [Google Scholar]
- 13.Arends J, Dijkman GEHM, Dijkman AG. Review of fluoride release and secondary caries reduction by fluoridating composites. Adv Dent Res. 1995;9:367–376. [Google Scholar]
- 14.Driessens FCM, Verbeeck RMH. Biominerals. CRC Press; Boca Raton, Florida, USA: 1990. pp. 236–240. [Google Scholar]
- 15.Koo H. Strategies to enhance the biological effects of fluoride on dental biofilms. Adv Dent Res. 2008;20:17–21. doi: 10.1177/154407370802000105. [DOI] [PubMed] [Google Scholar]
- 16.Andres CJ, Shaeffer JC, Windeler AS., Jr Comparison of antibacterial properties of stannous fluoride and sodium fluoride mouth-washes. J Dent Res. 1974;53:457. doi: 10.1177/00220345740530024701. [DOI] [PubMed] [Google Scholar]
- 17.Maltz M, Emilson CG. Susceptibility of oral bacteria to various fluoride salts. J Dent Res. 1982;61:786–790. doi: 10.1177/00220345820610062701. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Ling L, Ding XZ, Burgess JO. Synthesis and characterization of a novel, fluoride-releasing dimethacrylate monomer and its dental composite. J Poly Sci A-Poly Chem. 2004;42:985–998. [Google Scholar]
- 19.Xu X, Ding XZ, Ling L, Burgess JO. Synthesis and characterization of novel fluoride-releasing monomers. II. Dimethacrylates containing bis(aminodiacetic acid) and their ternary zirconium fluoride complexes. J Poly Sci A-Poly Chem. 2005;43:3153–3166. [Google Scholar]
- 20.Xu X, Ling L, Wang R, Burgess JO. Formulation and characterization of a novel fluoride-releasing dental composite. Dent Mater. 2006;22:1014–1023. doi: 10.1016/j.dental.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Ling L, Xu X, Choi GY, Billodeaux D, Guo G, Diwan RM. Novel F-releasing composite with improved mechanical properties. J Dent Res. 2009;88:83–88. doi: 10.1177/0022034508328254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson RJ. United States Patent 5358688. Antimicrobial quaternary ammonium group-containing polymers, compositions thereof, and monomers used to produce said polymers. 1994
- 23.Dizman B, Elasri MO, Mathias LJ. Synthesis and antibacterial activities of water-soluble methacrylate polymers containing quaternary ammonium compounds. J Poly Sci A-Poly Chem. 2006;44:5965–5973. [Google Scholar]
- 24.Yudovin-Farber I, Golenser J, Beyth N, Weiss EI, Domb AJ. Quaternary ammonium polyethyleneimine: Antibacterial activity. J Nanomater. 2010:Article ID 826343. [Google Scholar]
- 25.Gregory J, Gabriel AS, Ahmad EM, Tarik E, Gregory NT. Infectious disease: Connecting innate immunity to biocidal polymers. Mater Sci Eng: R: Reports. 2007;57:28–64. doi: 10.1016/j.mser.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenawy ER, Worley SD, Broughton R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules. 2007;8:1359–1384. doi: 10.1021/bm061150q. [DOI] [PubMed] [Google Scholar]
- 27.Worley SD, Sun G. Biocidal polymers. Trends Polym Sci. 1996;4:364–370. [Google Scholar]
- 28.Kanazawa A, Ikeda T, Endo T. Polymeric phosphonium salts as a novel class of cationic biocides. III. Immobilization of phosphonium salts by surface photografting and antibacterial activity of the surface-treated polymer-films. J Poly Sci A-Poly Chem. 1993;31:1467–1472. [Google Scholar]
- 29.Imazato S, Torii Y, Takatsuka T, Inoue K, Ebi N, Ebisu S. Bactericidal effect of dentin primer containing antibacterial monomer methacryloyloxydodecylpyridinium bromide (MDPB) against bacteria in human carious dentin. J Oral Rehab. 2001;28:314–319. doi: 10.1046/j.1365-2842.2001.00659.x. [DOI] [PubMed] [Google Scholar]
- 30.Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dental Mater. 2003;19:449–457. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 31.Imazato S, Kinomoto Y, Tarumi H, Torii M, Russell RRB, McCabe JF. Incorporation of antibacterial monomer MDPB into dentin primer. J Dent Res. 1997;76:768–772. doi: 10.1177/00220345970760030901. [DOI] [PubMed] [Google Scholar]
- 32.Xiao YH, Chen JH, Fang M, Wang H, Wang YJ, Li F. Antibacterial effects of three experimental quaternary ammonium salt (QAS) monomers on bacteria associated with oral infections. J Oral Sci. 2008;50:323–327. doi: 10.2334/josnusd.50.323. [DOI] [PubMed] [Google Scholar]
- 33.Gilmour AS, Edmunds DH, Newcome RG. Prevalence and depth of artificial caries-like lesion adjacent to caries prepared in roots and restored with glass ionomer or a dentin-bonded composite material. J Dent Res. 1997;76:1854–1861. doi: 10.1177/00220345970760120801. [DOI] [PubMed] [Google Scholar]
- 34.Hansel C, Leyhause G, Mai UE, Geurtsen W. Effects of various resin composite (co)monomers and extracts on two caries-associated micro-organisms in vitro. J Dent Res. 1998;77:60–67. doi: 10.1177/00220345980770010601. [DOI] [PubMed] [Google Scholar]
- 35.Jedrychowski JR, Caputo AA, Kerper S. Anti-bacterial and mechanical- properties of restorative materials combined with chlorhexidines. J Oral Rehab. 1983;10:373–381. doi: 10.1111/j.1365-2842.1983.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 36.Takemura K, Sakamoto Y, Staninec M, Kobayashi S, Suehiro K, Tsuchitani Y. Antibacterial activity of a Bis–GMA based composite resin and antibacterial effect of chlorhexidine incorporation. Jpn J Conser Dent. 1983;26:540–547. [Google Scholar]
- 37.Addy M, Thaw M. In vitro studies into the release of chlorhexidine acetate, prednisolone sodium-phosphate, and prednisolone alcohol from cold cure denture base acrylic. J Biomed Mater Res. 1982;16:145–157. doi: 10.1002/jbm.820160207. [DOI] [PubMed] [Google Scholar]
- 38.Wilson SJ, Wilson HJ. The release of chlorhexidine from modified dental acrylic resin. J Oral Rehab. 1993;20:311–319. doi: 10.1111/j.1365-2842.1993.tb01613.x. [DOI] [PubMed] [Google Scholar]
- 39.Addy MJ. In vitro studies into the use of denture base and soft liner materials as carriers for drugs in the mouth. J Oral Rehab. 1981;8:131–142. doi: 10.1111/j.1365-2842.1981.tb00486.x. [DOI] [PubMed] [Google Scholar]
- 40.Wen ZT, Suntharaligham P, Cvitkovitch DG, Burne RA. Trigger factor in Streptococcus mutans is involved in stress tolerance, competence development, and biofilm formation. Infect Immun. 2005;73:219–225. doi: 10.1128/IAI.73.1.219-225.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stansbury JW, Dickens SH. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater. 2001;17:71–79. doi: 10.1016/s0109-5641(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 42.Wen ZT, Baker HV, Burne RA. Influence of BrpA on critical virulence attributes of Streptococcus mutans. J Bacter. 2006;188:2983–2992. doi: 10.1128/JB.188.8.2983-2992.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen ZT, Nguyen AH, Bitoun JP, Abranches J, Baker HV, Burne RA. Transcriptome analysis of LuxS-deficient Streptococcus mutans grown in biofilms. Mol Oral Microbiol. 2011;26:2–18. doi: 10.1111/j.2041-1014.2010.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, Van Meerbeek B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J Dent Res. 2005;84:18–132. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]