SUMMARY
The PI3K-AKT, mTOR-p70S6 kinase and AMPK pathways play distinct and critical roles in metabolic regulation. Each pathway is necessary for leptin's anorexigenic effects in the hypothalamus. Here we show that these pathways converge in an integrated phosphorylation cascade to mediate leptin action in the hypothalamus. We identify serine491 on α2AMPK as the site of convergence and show that p70S6 kinase forms a complex with α2AMPK, resulting in phosphorylation on serine491. Blocking α2AMPK-serine491 phosphorylation increases hypothalamic AMPK activity, food intake, and body weight. Serine491 phosphorylation is necessary for leptin's effects on hypothalamic α2AMPK activity, neuropeptide expression, food intake, and body weight. These results identify an inhibitory AMPK kinase, p70S6 kinase, and demonstrate that AMPK is a substrate for mTOR-p70S6 kinase. This discovery has broad biologic implications since mTOR-p70S6 kinase and AMPK have multiple, fundamental and generally opposing cellular effects that regulate metabolism, cell growth, and development.
INTRODUCTION
Obesity has reached epidemic proportions worldwide and increases the risk for diabetes, cardiovascular disease, and early mortality. Maintaining normal body weight requires tight control of energy homeostasis, which necessitates a constant flow of metabolic input to the hypothalamus in the form of nutrients and hormones. Specific signal transduction pathways have evolved to process afferent hypothalamic input into adaptive changes in food intake and energy expenditure (Morton et al., 2006). Leptin, an adipocyte-derived anorexigenic factor, relays the status of fat stores to the hypothalamus through multiple signaling pathways resulting in regulation of food intake and body weight (Friedman and Halaas, 1998; Halaas et al., 1995; Morton et al., 2006). Binding of leptin to its cytokine-like receptor activates Jak2, which phosphorylates the receptor on tyrosine residues that have been specifically mapped to effects on food intake, energy expenditure, and reproduction (Myers et al., 2009; Villanueva and Myers, 2008). While Stat3-regulated gene transcription is a well-established mechanism mediating many of leptin's actions (Hill et al., 2008; Niswender et al., 2001), the PI3K-AKT, mTOR-p70S6 kinase (p70S6K), and AMPK pathways mediate important, rapid effects of leptin. How these pathways integrate to produce leptin's anorexigenic effects is a critical question for understanding energy balance.
The heterotrimeric AMPK complex is composed of an α catalytic subunit and two regulatory subunits, β and γ. AMPK is a cellular “energy gauge” that responds to increases in the AMP:ATP ratio (Hardie et al., 2003) and plays a fundamental role in cell growth and metabolism. Alterations in the AMPK pathway cause diseases ranging from cardiac arrhythmias to cancer (Hardie, 2007; Steinberg and Kemp, 2009). In the hypothalamus, AMPK acts as a systemic energy sensor. Inhibition of hypothalamic α2AMPK activity is necessary for leptin to reduce food intake and body weight (Minokoshi et al., 2004). AMPK activation requires phosphorylation at threonine172 of the α catalytic subunit by upstream kinases (Hardie, 2007). Recently, other phosphorylation sites on the α and β subunits have been shown to alter kinase activity (Djouder et al., 2010; Horman et al., 2006; Hurley et al., 2006; Soltys et al., 2006; Warden et al., 2001), thereby broadening the potential mechanisms for regulating AMPK activity and its biologic effects. Phosphorylation of AMPK α subunits on serine485/491 inhibits AMPK threonine172 phosphorylation and AMPK activity (Horman et al., 2006; Hurley et al., 2006). Here we show that phosphorylation of α2AMPK on serine491 in the hypothalamus inhibits its activity and is critical for leptin action on food intake and body weight. We further show that p70S6K is an inhibitory AMPK kinase that forms a complex with the α2AMPK catalytic subunit and directly phosphorylates it on serine491. This is a critical integration point where multiple signaling pathways converge in the hypothalamus to mediate leptin action.
RESULTS
Leptin and Refeeding Decrease α2AMPK Activity and Induce AMPK Serine485/491 Phosphorylation in the Hypothalamus
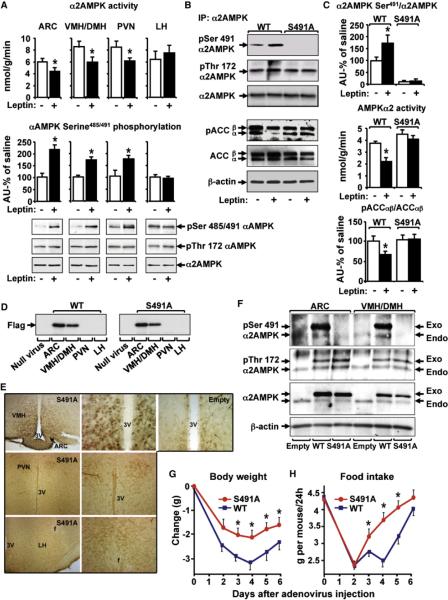
To determine the mechanisms by which leptin inhibits hypothalamic α2AMPK activity and regulates body weight, we investigated the phosphorylation of serine491 on α2AMPK in response to leptin intracerebroventricularly (i.c.v.). We studied this site because increased serine485/491 phosphorylation is associated with decreased AMPK activity (Hurley et al., 2006; Pulinilkunnil et al., 2011). We focused on α2AMPK because leptin alters the activity of α2 and not α1AMPK (Minokoshi et al., 2002, 2004). In normal mice, leptin acutely decreases α2AMPK activity in arcuate (ARC), ventromedial (VMH)/dorsomedial (DMH), and paraventricular (PVN) hypothalamus while stimulating AMPK serine485/491 phosphorylation (Figure 1A, also analyzed in VMH and DMH separately–Figure S1A available online). While we did not see a change in acetyl-CoA carboxylase (ACC) phosphorylation at this time point (data not shown), we did see increased phosphorylation of p70S6Kinase, another AMPK target and its downstream target, S6 (Figure S1B). In lateral hypothalamus (LH), where α2AMPK activity is not inhibited by leptin, AMPK serine phosphorylation is not increased (Figure 1A). To further understand the physiologic regulation of αAMPK serine485/491, we assessed the effects of fasting and re-feeding. αAMPK serine485/491 phosphorylation was low in hypothalamus of overnight fasted mice and increased 75% with refeeding (6 hr) in conjunction with inhibition of α2AMPK activity (Figure S1C).
Figure 1. Leptin Induces Serine491 Phosphorylation of α2AMPK in Hypothalamus to Regulate Food Intake and Body Weight.
(A) α2AMPK αctivity, αAMPK serine485/491/α2AMPK, and AMPK threonine172 after saline or leptin injection (20 ng i.c.v.; 3 hr) in overnight fasted mice (n = 5–8/group). *p < 0.05 versus saline.
(B and C) GT1-7 neurons were transfected with Flag-WT or Flag-S491A-α2AMPK and treated with 0.5 μg/ml leptin (+) or vehicle (−) for 2 hr. Cell extracts were immunoprecipitated with α2AMPK antibody for (B and C, top panel) pSerine485/491/α2AMPK and pThreonine172 or (C, middle panel) α2AMPK activity or (B and C, lower panel) analyzed for pACCαβ/ACCαβ and actin. *p < 0.05 versus all other groups.
(D) Flag-WT and Flag-S491A α2AMPK expression in hypothalamic nuclei 8 days after adenovirus injection.
(E) Immunohistochemistry with Flag antibody. Low (left) and high (middle) magnification of ARC and VMH/DMH (top), PVN (middle row), and LH (bottom) after injection with Flag-S491A-α2AMPK adenovirus (columns 1 and 2) or null adenovirus (Empty) (top right, high magnification) are shown.
(F) Hypothalamic extracts were subjected to immunoprecipitation with α2AMPK antibody. Endogenous (Endo) and exogenous (Exo) (Flag-WT or Flag-S491A) α2AMPK phosphorylation was detected by immunoblotting.
(G and H) Changes in body weight and daily food intake after mediobasal hypothalamic injection of Flag-WT or Flag-S491A α2AMPK adenovirus (n = 22–24/group). *p < 0.05 versus mice expressing Flag-WT-α2AMPK.
Data in all panels are shown as means ± SEM. See also Figure S1.
Leptin Inhibits α2AMPK Activity by Phosphorylating It on Serine491
To determine whether serine491 phosphorylation is critical for leptin-induced inhibition of α2AMPK activity, we introduced a phospho-defective α2AMPK mutant (S491A) into GT1-7 neurons that normally express functional leptin receptors (Magni et al., 1999). Overexpression of WT α2AMPK in GT1-7 cells does not change α2AMPK activity (data not shown). Leptin stimulated serine491 phosphorylation of exogenous WT but not S491A α2AMPK (Figures 1B and 1C, top panel). Leptin inhibited α2AMPK activity (Figure 1C, middle panel) in cells expressing WT but not S491A α2AMPK, demonstrating that serine491 phosphorylation (Figure 1B) is necessary for α2AMPK inhibition by leptin. These changes in α2AMPK serine491 phosphorylation and the leptin-induced inhibition of α2AMPK activity were not associated with changes in AMPK threonine172 phosphorylation. A phosphomimetic α2AMPK mutant (S491D) mimicked the effect of leptin to inhibit α2AMPK activity (Figure S1D) underscoring the importance of this phosphorylation site. Blocking the leptin-induced α2AMPK serine491 phosphorylation inhibited ACC phosphorylation (Figures 1B and 1C, bottom panel). The mechanism by which phosphorylation of α2AMPK on serine491 inhibits AMPK activity does not involve changes in affinity for the β and γ subunits since we found the same amount of β1, γ1, and γ2 subunits complexed with wild-type and S491A α2 subunits (Figure S1E).
Hypothalamic α2AMPK Serine491 Phosphorylation Regulates Food Intake and Body Weight
To test the effect of hypothalamic serine491 phosphorylation on food intake and body weight, we introduced the WT and S491A mutant of α2AMPK into the mediobasal (ARC, VMH, DMH) hypothalamus of normal mice using recombinant adenoviruses. This hypothalamic region was chosen because of its critical role in regulation of food intake and body weight (Morton et al., 2006; Myers et al., 2009) and because leptin stimulates αAMPK serine485/491 phosphorylation in this region (Figure 1A).
Western blotting of individual hypothalamic nuclei showed expression of WT and S491A α2AMPK in the ARC and VMH/DMH but not in PVN or LH (Figure 1D). Consistent with these results, immunohistochemistry showed expression in ARC, VMH, and DMH but not in other hypothalamic areas (Figure 1E). While the exogenous and endogenous WT AMPK were phosphorylated on serine491, introduction of S491A α2AMPK completely blocked the phosphorylation of exogenous α2AMPK on serine491 in the in vivo experiments (Figure 1F, top panel). Obliterating the phosphorylation of α2AMPK on serine491 in ARC and VMH/DMH did not affect threonine172 phosphorylation (Figure 1F, second panel).
Next, we tested the effect of S491A α2AMPK on body weight and food intake. Transient weight loss was seen as expected after intrahypothalamic (IHP) injections of adenoviral constructs. Expression of WT α2AMPK did not affect body weight or food intake compared to empty virus control (data not shown). However, mice expressing S491A α2AMPK maintained higher weight than controls (Figure 1G), which could at least partially be explained by greater food intake (Figure 1H). Thus, alteration of serine491 phosphorylation of hypothalamic α2AMPK is sufficient to regulate food intake and body weight.
Phosphorylation of α2AMPK on Serine491 in the Hypothalamus Is Necessary for Leptin's Effect on Food Intake and Body Weight
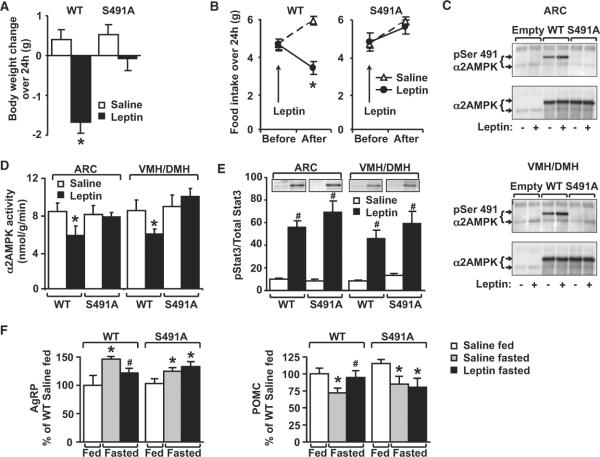
To determine whether phosphorylation of α2AMPK on serine491 is required for leptin's effect on food intake and body weight, we injected overnight-fasted mice expressing WT or S491A α2AMPK in the mediobasal hypothalamus with leptin. In WT mice, leptin decreased body weight by 1.67 g over 24 hr compared to a 0.38 g increase in body weight in saline-injected control mice (Figure 2A). In contrast, in mice expressing S491A α2AMPK, leptin did not reduce body weight (Figure 2A). Effects on food intake paralleled the effects on body weight. In saline-injected mice, food intake increased as expected after overnight fasting (Figure 2B). Leptin decreased 24 hr food intake from 5.97 ± 0.22 g to 3.43 g ± 0.35 in WT α2AMPK-expressing mice but had no effect in S491A AMPK-expressing mice (Figure 2B). This resistance to leptin's effects could be explained by defective AMPK serine491 phosphorylation since leptin increased serine491 phosphorylation (Figure 2C) and inhibited α2AMPK activity (Figure 2D) in ARC and VMH/DMH of mice injected with WT α2AMPK but not with S491A α2AMPK. Thus, phosphorylation of serine491 in mediobasal hypothalamus is necessary for leptin to inhibit α2AMPK activity in vivo and for leptin's anorexigenic and weight loss effects; failure to modulate phosphorylation on this site appears to cause resistance to leptin's effects on AMPK activity and food intake.
Figure 2. Expression of S491A-α2AMPK in hypothalamus blocks leptin's effect on food intake and body weight.
(A and B) Body weight change (A) and food intake (B) 24 hr after leptin (5 mg/kg X2 injections i.p.) (n = 12/group). *p < 0.05 versus all other groups.
(C) α2AMPK serine491 phosphorylation in hypothalamic nuclei after leptin (5 mg/kg i.p.) injected 24, 12 and 3 hr before sacrifice on day 8 after adenovirus injection. ARC and VMH/DMH were subjected to immunoprecipitation with α2AMPK antibody. Endogenous and exogenous (Flag-WT or Flag-S491A) α2AMPK were detected by immunoblotting.
(D) α2AMPK activity in hypothalamic nuclei used in (C) (n = 8/group). *p < 0.05 versus all other groups.
(E) pSTAT3 in hypothalamic nuclei used in (C) (n = 6/group). #p < 0.05 versus saline injection.
(F) S491A blocks leptin's effect on AgRP and POMC mRNA in ARC and VMH/DMH of fasted mice. *p < 0.05 versus fed for mice with same vector. #p < 0.05 versus fasted saline for mice with same vector.
Data in all panels are shown as means ± SEM. See also Figure S2.
We sought to determine whether serine491 phosphorylation is involved in chronic leptin resistance such as that induced by high-fat diet (HFD). In HFD, hypothalamic α2AMPK activity is already low, and leptin does not lower it further (Martin et al., 2006), indicating that modulation of α2AMPK activity is critical for leptin's anorexigenic effects. HFD increased AMPK serine485/491 phosphorylation and inhibited α2AMPK activity in basomedial hypothalamus and PVN (Figure S2). In lateral hypothalamus (LH), where serine485/491 phosphorylation is not increased by HFD, α2AMPK activity is not suppressed (Figure S2).
Interestingly, leptin-induced Stat3 phosphorylation was not impaired by S491A-αAMPK (Figure 2E), similar to observations with constitutively active AMPK (Minokoshi et al., 2004). Thus, biologic leptin resistance can be induced independent of signaling through Stat3. To explore the mechanism by which serine491 phosphorylation mediates the anorexigenic effect of leptin, we examined hypothalamic neuropeptide expression. In mice expressing WT α2AMPK, overnight fasting stimulated AgRP while suppressing POMC messenger RNA (mRNA) levels compared to ad libitum-fed mice. Leptin treatment of fasted mice reversed these effects (Figure 2F). In contrast, leptin failed to prevent the effect of fasting to increase AgRP and inhibit POMC mRNA expression in S491A α2AMPK-expressing mice (Figure 2F). Thus, the effects of leptin-induced αAMPK serine491 phosphorphorylation may be mediated by changes in hypothalamic neuropeptide expression.
Leptin Stimulates αAMPK Serine485/491 Phosphorylation through PI3K-AKT Signaling
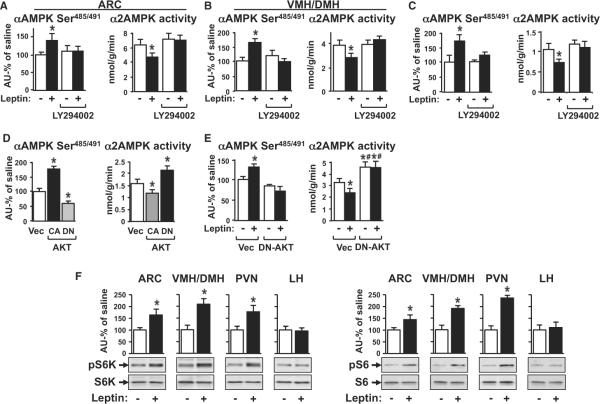
Because of the important biologic effects, we aimed to identify the kinase(s) that mediates leptin-induced serine491 phosphorylation. Both PKA and AKT can induce phosphorylation of α1 and α2AMPK on serine485 and serine491, respectively, in cultured cells, perfused heart, or BAT (Horman et al., 2006; Hurley et al., 2006; Mankouri et al., 2010; Pulinilkunnil et al., 2011). We first investigated the PI3K-AKT pathway in the hypothalamus because it plays a major role in regulation of energy balance and is necessary for leptin's anorexigenic action (Hill et al., 2008; Niswender et al., 2001; Xu et al., 2010). i.c.v. injection (30 min) of the PI3K inhibitor LY294002, which diminished the anorexigenic effect of leptin in previous studies (Morrison et al., 2005; Niswender et al., 2001), blocked the effect of leptin to stimulate αAMPK serine485/491 phosphorylation and to inhibit α2AMPK activity in the ARC (Figure 3A) and VMH/DMH (Figure 3B).
Figure 3. Leptin Stimulates αAMPK Serine485/491 Phosphorylation through PI3K-AKT Signaling.
(A and B) i.c.v. injection of vehicle (DMSO) or LY294002 (0.1 nmol; 30 min) followed by saline (−) or leptin (20 ng; 3 hr) (+) in overnight-fasted mice (n = 7/group). ARC (A) and VMH/DMH (B) were analyzed for α2AMPK activity and αAMPK serine485/491/α2AMPK.
(C) GT1-7 neurons were treated with DMSO or LY294002 (20 μM) and 0.5 μg/ml leptin (+) or vehicle (−) for 2 hr and analyzed for α2AMPK activity and αAMPK serine485/491/α2AMPK.
(D) GT1-7 neurons were transfected with CA- or DN-AKT or vector (Vec) and analyzed for α2AMPK activity and αAMPK serine485/491/α2AMPK.
(E) GT1-7 neurons were transfected with DN-AKT or vector and treated with 0.5 μg/ml leptin (+) or vehicle (−) for 2 hr. Cell extracts were analyzed for α2AMPK activity and αAMPK serine485/491/α2AMPK. *p < 0.05 versus empty vector control. #p < 0.05 versus empty vector leptin.
(F) pS6K/S6K and pS6/S6 in hypothalamic nuclei after saline or leptin injection (20 ng i.c.v.; 3 hr) in overnight fasted mice (n = 6/group). *p < 0.05 versus saline.
Data in all panels are shown as means ± SEM. See also Figure S3.
To determine whether PI3K stimulates serine485/491 phosphorylation directly, we studied the effects in GT1-7 neurons. The P13K inhibitor LY294002 blocked the effect of leptin to stimulate serine485/491 phosphorylation and to inhibit α2AMPK activity (Figure 3C). This effect of PI3K was confirmed with a second inhibitor, wortmannin, which also blocked leptin's effects (data not shown). These effects are consistent with genetic studies showing that disruption of PI3K signaling in hypothalamic neurons in vivo blocks leptin action on food intake and body weight (Al-Qassab et al., 2009).
To further elucidate the pathway involved in leptin-induced serine491 phosphorylation of AMPK, we investigated the downstream target of PI3K, AKT (Manning and Cantley, 2007). Constitutively active AKT (CA-AKT) increased αAMPK serine485/491 phosphorylation and inhibited α2AMPK activity in GT1-7 neurons while DN-AKT decreased phosphorylation and increased α2AMPK activity (Figure 3D). Furthermore, DN-AKT blocked leptin-induced αAMPK serine485/491 phosphorylation and inhibition of α2AMPK activity (Figure 3E), indicating that AKT activation is necessary for leptin-induced αAMPK serine485/491 phosphorylation. A PKA inhibitor did not block the effect of leptin on serine485/491 phosphorylation (Figure S3A) or α2AMPK activity (Figure S3B), and a PKA activator had no effect on α2AMPK activity (Figure S3C).
Leptin Inhibits α2AMPK Activity through p70S6K-Dependent AMPK Serine485/491 Phosphorylation
Examination of the sequence flanking the serine 491 site on α2AMPK revealed a proline in position −5 that renders this unfavorable for direct phosphorylation by ACG kinases including AKT and PKA (Kemp and Pearson, 1990; Manning and Cantley, 2007) but acceptable for phosphorylation by p70S6K (Richardson et al., 2004) (Figure S3D). Since Akt activates p70S6K through the TSC-mTOR pathway (Zoncu et al., 2011), we hypothesized that downstream to AKT, p70S6K is involved in the leptin signaling pathway that phosphorylates α2AMPK on serine491. To investigate this, we measured leptin-induced phosphorylation of p70S6K and its target S6 (Figure 3F) in hypothalamic nuclei. Leptin acutely increased phosphorylation of p70S6K and S6 in ARC, VMH/DMH, and PVN but not in the LH. (Figure 3F), suggesting that p70S6K could be mediating the effects of the PI3K-AKT pathway on leptin-induced serine phosphorylation of AMPK.
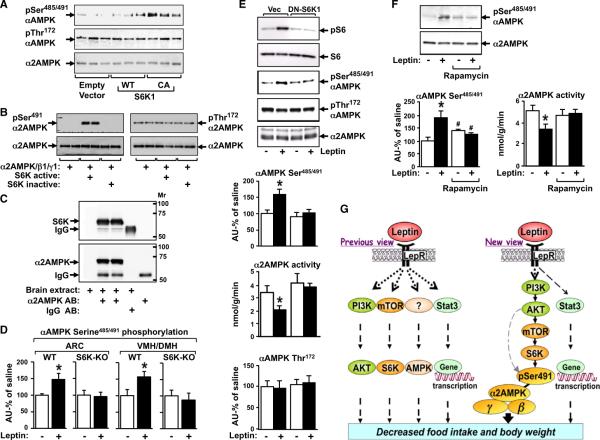
We tested whether p70S6K can alter AMPK phosphorylation directly. In GT1-7 neurons, CA-S6K1 phosphorylates αAMPK at serine485/491 and does not affect the phosphorylation on threonine172 (Figure 4A). An in vitro phosphorylation assay with naked proteins demonstrates that recombinant S6K directly phosphorylates recombinant α2AMPK/β1/γ1 complex on serine491 but not on threonine172 of α2AMPK (Figure 4B). This indicates that p70S6K is an AMPK kinase (AMPKK) and unlike the known AMPKKs, LKB1 and CamKK2, p70S6K phosphorylates αAMPK on serine and not threonine172. To determine whether p70S6K forms a complex with αAMPK similar to the complexes formed by LKB1 and CaMKKβ (Anderson et al., 2008; Shaw et al., 2004), we immunoprecipitated the endogenous α2AMPK subunit from brain extracts and identified p70S6K (Figure 4C). Since α1AMPK has the same S6K consensus site, we verified whether S6K1 could also phosphorylate α1AMPK. In addition to α2AMPK (Figures 4A and 4B), CA-S6K1 also phosphorylates α1AMPK (Figure S4A).
Figure 4. Leptin Inhibits α2AMPK Activity through p70S6K-Dependent AMPK Serine485/491 Phosphorylation.
(A) GT1–7 neurons were transfected with Empty, WT, or CA-S6K1 vectors. AMPK phosphorylation was analyzed by immunoblotting (n = 3/group).
(B) Recombinant α2AMPK/β1/γ1 complex was incubated with active and inactive recombinant S6K. Phosphorylation of α2AMPK on serine491 and threonine172 and total α2AMPK were analyzed by immunoblotting.
(C) Brain extracts were subjected to immunoprecipitation with α2AMPK antibody (AB) and blotted for p70S6K (top) or α2AMPK (bottom).
(D) αAMPK serine485/491/α2AMPK in hypothalamic nuclei after saline or leptin injection (i.p.) in overnight-fasted WT and S6K1 (−/−) mice (n = 5–8/group). *p < 0.05 versus saline.
(E) GT1–7 neurons were transfected with DN-S6K1 or vector (Vec), treated with 0.5 μg/ml leptin (+) or vehicle (−) for 2 hr and immunoblotted for pS6/S6, αAMPK serine485/491/α2AMPK, and pThreonine172/α2AMPK or analyzed for α2AMPK activity. *p < 0.05 versus all other groups.
(F) GT1–7 neurons were treated with rapamycin (7.5 nM; 30 min) followed by 0.5 μg/ml leptin (+) or vehicle (−) for 2 hr and analyzed for α2AMPK activity and αAMPK serine485/491/α2AMPK. p < 0.05 *versus all other groups; #versus no rapamycin (n = 8).
(G) The acute anorexigenic effect of leptin—from individual pathways to a unified signaling network in the hypothalamus. The gray dotted line indicates that although our data do not support a direct effect of AKT to phosphorylate α2AMPK on serine 491 in the hypothalamus in response to acute leptin stimulation, this site may be directly phosphorylated by AKT under other conditions or in response to other hormones or nutrients.
Data in all panels are shown as means ± SEM. See also Figure S4.
To determine whether p70S6K is necessary for leptin-induced αAMPK serine485/491 phosphorylation in the hypothalamus in vivo, we injected S6K1 knockout (S6K1−/−) mice with saline or leptin. Previous studies have shown that S6K1−/− mice are resistant to leptin's anorectic action (Cota et al., 2008). Leptin increased AMPK serine phosphorylation by 47%–53% in ARC and VMH/DMH of WT mice but had no effect in the S6K1−/− mice (Figure 4D). This suggests that p70S6K is necessary for leptin-induced αAMPK serine485/491 phosphorylation in the hypothalamus. Consistent with the effects observed in S6K1−/− mice, suppression of p70S6K activity in the hypothalamus by DN-S6K1 blocks the effect of leptin to decrease food intake and body weight (Blouet et al., 2008). To further study the role of S6K in leptin signaling, we expressed DN-S6K1 in neurons followed by leptin treatment. We first verified the effectiveness of DN-S6K1. In cells transfected with empty vector, leptin stimulated p70S6K activity as indicated by increased phosphorylation of the substrate S6 (Figure 4E). DN-S6K1 blocked leptin-induced S6 phosphorylation, αAMPK serine485/491 phosphorylation, and inhibition of α2AMPK activity (Figure 4E). This indicates that p70S6K is necessary for leptin-induced serine491 phosphorylation of α2AMPK. These changes in AMPK serine485/491 phosphorylation and α2AMPK activity were independent of alterations in threonine172 phosphorylation (Figure 4E), consistent with our data in the hypothalamus (Figure 1F). This indicates that modulation of AMPK-serine491 phosphorylation can have a dominant role in regulation of AMPK activity. To reinforce the involvement of the mTOR-p70S6K pathway, we also tested the effect of rapamycin, an inhibitor of mTOR complex 1, which also blocked leptin-induced serine485/491 phosphorylation of αAMPK and inhibition of AMPK activity (Figure 4F).
DISCUSSION
The emerging role of AMPK not only in the regulation of metabolism, but also cancer and development, emphasizes the importance of understanding the molecular mechanisms that regulate its activity. We show that in the hypothalamus, phosphorylation of α2AMPK on serine491 inhibits its kinase activity and is sufficient to regulate food intake and body weight. Furthermore, phosphorylation on this site is necessary for leptin action on food intake and body weight. Threonine172 phosphorylation of αAMPK is critical for its activation (Hardie, 2007; Steinberg and Kemp, 2009). However, our studies demonstrate that αAMPK activity can be inhibited by phosphorylation on serine485/491 independent of changes in threonine172 phosphorylation. Therefore, serine485/491 phosphorylation is an “off switch” for AMPK activity in addition to AMPK phosphatases (Hardie, 2007; Sanders et al., 2007; Steinberg and Kemp, 2009).
Previous studies showed that leptin inhibits threonine172 phosphorylation in the hypothalamus (Gao et al., 2007; Andersson et al., 2004), and in some cell culture models, serine485/491 phosphorylation is associated with reduced threonine172 phosphorylation (Hurley et al., 2006; Soltys et al., 2006). The fact that we do not see this with leptin action in GT1-7 cells or in hypothalamus in vivo in this study and in previous studies (Martin et al., 2006; Minokoshi et al., 2004), may result from methodological differences. However, the immune complex assay demonstrates that leptin reduces AMPK activity (Figures 1A and 1C). The substrate for our AMP kinase assay consists of the 13 amino acid residues around serine 79 of ACC, which is the unique site on which AMPK phosphorylates ACC. Hence, the activity assay measures the ability of AMPK to phosphorylate ACC and this is reduced in response to leptin (Figures 1A and 1C).
mTOR and AMPK are both important nutrient sensors that have broad and mostly opposing effects on metabolic function. The current understanding is that AMPK is an upstream regulator of mTOR, inhibiting its activity. Our study demonstrates that p70S6K is also an AMPK kinase that directly phosphorylates αAMPK at serine485/491, thereby inhibiting its activity. A previous study showed activation of AMPK in muscle cells lacking S6K, although the authors' conclusion was that deletion of S6K stimulates AMPK indirectly by increasing cellular AMP and inorganic phosphate levels (Aguilar et al., 2007).
The mTOR-S6K pathway can play a role in both activation and inhibition of hypothalamic AMPK and food intake because some nutrient and hormonal signals such as feeding and branched chain amino acids (e.g., leucine) activate mTOR-S6K in the hypothalamus (Cota et al., 2006) while others suppress this pathway (e.g., fasting). Our data showing that the PI3K-AKT-mTOR-p70S6K pathway phosphorylates αAMPK on serine485/491 demonstrate that these previously individual pathways in leptin action form a unified signaling network in the hypothalamus to mediate the acute anorexigenic and body weight effects of leptin (Figure 4G). This signaling network, which is implicated in leptin's acute effects (Hill et al., 2008; Niswender et al., 2001), is likely to be complementary to the Stat3-mediated more long-term regulation of food intake and body weight (Bates et al., 2003; Myers et al., 2009).
Previous studies have shown that AKT can directly phosphorylate αAMPK on serine485/491 (Horman et al., 2006; Soltys et al., 2006). While this occurs under some conditions, our data show that AKT does not mediate the effect of leptin to phosphorylate αAMPK on serine485/491 because this phosphorylation is blocked when we inhibit S6K signaling with DN-S6K (Figure 4E) or rapamycin (Figure 4F). This strongly suggests that in the leptin signaling pathway, AKT is upstream of S6K and indirectly stimulates S6K-dependent αAMPK serine485/491 phosphorylation (see model, Figure 4G).
αAMPK serine485/491 phosphorylation may also be implicated in multiple peripheral signals such as insulin and nutrients that have previously been shown to modulate AMPK and mTOR in the hypothalamus (Minokoshi et al., 2004; Cota et al., 2006). Since PI3K, Akt, mTOR, and p70S6K have all been shown to be important in cancer biology (Manning and Cantley, 2007), this integration of these pathways may be important for cancer and other human diseases and could lead to improved therapeutic approaches.
EXPERIMENTAL PROCEDURES
Mice and Cells
Male FVB mice (age 8–9 weeks) were housed in a temperature-controlled environment with a 14 hr/10 hr light/dark cycle and free excess to chow diet (Formulab 5008; Farmer's Exchange) and water before beginning experiments. S6K1−/− mice were a kind gift from Sarah Kozma and George Thomas at the University of Cincinatti. Leptin (National Hormone and Peptide Program) was injected i.c.v. (20 ng; 3 hr) 1 week after cannula insertion or i.p. (5 mg/kg at 12 and 24 rh before sacrifice). Body weight and food intake were monitored daily. Mice were sacrificed by decapitation and hypothalamic nuclei were quickly dissected. All assays were performed on hypothalamic regions from individual mice. All aspects of animal care were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee.
GT1-7 cells were cultured at 37°C with 5% CO2 in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS). Cells were transfected with lipofectamine according to the manufacturer's protocol. For leptin studies, GT1-7 cells were serum starved overnight, exposed to 1 mM glucose DMEM without FBS for 6 hr and than treated with leptin (0.5 μg/ml) for 2 hr. For other studies, GT1-7 cells were serum starved overnight, and maintained in 5 mM glucose DMEM without FBS for 6 hr.
Surgery
i.c.v. cannulae were inserted in the third ventricle. Adenoviruses expressing WT, S491A α2AMPK or control-empty vector were injected bilaterally (anterior-posterior [AP] −1.40 mm, medial-lateral [ML] ± 0.5 mm from Bregma and dorsal-ventral [DV] −5.5 mm from the brain surface) in the mediobasal hypothalamus, consisting of the ARC, VMH, and DMH nuclei.
Perfusion and Sectioning
Mice were perfused transcardially with 0.9% saline, followed by 150 ml 10% formalin in 0.1 M phosphate-buffered saline (PBS) at pH 7.4. After overnight fixation, the whole brains were cryoprotected in a solution of 30% sucrose in 0.1 M PBS at pH 7.4. Serial coronal sections with 40 μm thickness containing the hypothalamus were cut with a microtome and collected in PBS.
Immunohistochemistry with Flag Antibody
Free-floating brain sections were incubated sequentially with (1) a primary mouse antibody directed against FLAG M2 (Sigma) at 1:2,000 dilution in 0.1 M PBS with 0.3% Triton X-100 for two nights at 4°C and (2) a secondary, biotinylated donkey anti-mouse antibody (1:300 dilution in PBS; Jackson Immunoresearch, West Grove, PA) for 2 and 3 hr, ABC Elite Kit (1:500; Vector Laboratories, Burlingame, CA) for 2 hr. Then the sections were processed with a mixture of diaminobenzidine (DAB; 0.2%), 0.05% hydrogen peroxide, and 0.6% nickel ammonium sulfate in 50 mM Tris buffer (pH 7.6).
After immunostaining, coronal brain sections were rinsed in PBS, mounted serially, dehydrated, and then coverslipped with Permount.
Dissection of Hypothalamic Regions
Each hypothalamic region was dissected from 1-mm-thick sagittal sections of fresh brain. ARC, VMH/DMH, and PVN were dissected from the first sections from the midline of the brain, and LH was dissected from the next lateral sections. Coordinates for each hypothalamic region are as follows: PVN, square area with anterior margin (posterior region of anterior commissure), dorsal margin (border with thalamus), ventral margin (1.5 mm ventral to the border with thalamus), and posterior margin (white matter separating PVN/anterior hypothalamus and VMH/DMH); VMH/DMH, triangular area with anterior margin (white matter separating PVH/anterior hypothalamus and VMH/DMH), posterior margin (border with mammillary body), and ventral margin (border with ARC); and ARC, ventral part of the medial hypothalamus with anterior and dorsal margin (border with ventral part of VMH and DMH, approximately 0.5 mm from the ventral surface of the medial hypothalamus) and posterior margin (border with mammillary body). VMH and DMH were collected from the triangular area with an anterior-dorsal margin of the white matter separating PVN and the anterior hypothalamus from the VMH and DMH, a ventral margin of the border with ARC.
α2AMPK Activity Measurement
α2AMPK activity was measured as described previously (Minokoshi et al., 2004). In brief, α2 isoform-specific AMPK was immunoprecipitated from individual hypothalamic nuclei and GT1-7 cell lysates with a specific α2AMPK antibody (Santa Cruz) bound to protein-G sepharose beads. The kinase activity of the immunoprecipitates was measured with “SAMS” peptide and γ-32P.
Immunoprecipitation and Western Blot Analysis
Individual hypothalamic nuclei and GT1-7 cell lysates were subjected to immunoprecipitation with α2AMPK antibody (Santa Cruz) and/or immunoblotted with antibodies against pSerine485/491 and pThreonine172 AMPK, pSerine473 AKT, pThreonine389 p70S6K, pSerine240/244 S6, pTyrosine705 STAT3, pACC, and ACC (all purchased from Cell Signaling). Protein levels were analyzed with α2AMPK, β-actin (Santa Cruz), AKT, p70S6K, S6, and STAT3 (Cell Signaling).
In Vitro Phosphorylation
Recombinant proteins were purchased from Signal-Chem (Richmond, Canada). Recombinant α2AMPK/β1/γ1 complex was incubated with recombinant active p70S6K1 or with a negative control—recombinant inactive p70S6K2 in 50 mM Na-HEPES, 5 mM MgCl2, 500 μM ATP, 1 mM DTT for 30 min at 30°C.
qPCR Analysis of mRNA Levels of Neuropeptides
Total hypothalamic RNA was isolated with TriReagent according to the manufacturer's instructions (Molecular Research Center, Cincinnati, OH). mRNAs of AgRP and POMC were quantified by real-time reverse transcription-PCR (RT-PCR) with the TaqMan one-step RT-PCR Master mix (Applied Biosystems) and a Stratagene Mx3000P or Mx4000P system (Stratagene, La Jolla, CA) as described previously (Minokoshi et al., 2004).
Supplementary Material
ACKNOWLEDGMENTS
We thank A. Usheva for helpful advice and B.B. Lowell for comments on the manuscript and S. Kozma and G. Thomas for kindly providing the S6K1 knockout (S6K1−/−) mice. This work was supported by NIH P01 DK56116 (B.B.K.), R37 DK43051 (B.B.K.), and BADERC DK57521 (B.B.K.), a grant from the Picower and JPB Foundations (B.B.K.), NIH GM56203 (L.C.C.), CA120964 (L.C.C.), and NIH K99/R00-CA133245 (B.Z.).
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes four figures and can be found with this article online at doi:10.1016/j.cmet.2012.05.010.
REFERENCES
- Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudière E, Foretz M, Viollet B, et al. S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metab. 2007;5:476–487. doi: 10.1016/j.cmet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, et al. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 2009;10:343–354. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, Means AR. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008;7:377–388. doi: 10.1016/j.cmet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- Blouet C, Ono H, Schwartz GJ. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 2008;8:459–467. doi: 10.1016/j.cmet.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Cota D, Matter EK, Woods SC, Seeley RJ. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J. Neurosci. 2008;28:7202–7208. doi: 10.1523/JNEUROSCI.1389-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouder N, Tuerk RD, Suter M, Salvioni P, Thali RF, Scholz R, Vaahtomeri K, Auchli Y, Rechsteiner H, Brunisholz RA, et al. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J. 2010;29:469–481. doi: 10.1038/emboj.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Gao S, Kinzig KP, Aja S, Scott KA, Keung W, Kelly S, Strynadka K, Chohnan S, Smith WW, Tamashiro KL, et al. Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc. Natl. Acad. Sci. USA. 2007;104:17358–17363. doi: 10.1073/pnas.0708385104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J. Clin. Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, Schlattner U, Wallimann T, Carling D, Hue L, Rider MH. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J. Biol. Chem. 2006;281:5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- Hurley RL, Barré LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA. Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J. Biol. Chem. 2006;281:36662–36672. doi: 10.1074/jbc.M606676200. [DOI] [PubMed] [Google Scholar]
- Kemp BE, Pearson RB. Protein kinase recognition sequence motifs. Trends Biochem. Sci. 1990;15:342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Magni P, Vettor R, Pagano C, Calcagno A, Beretta E, Messi E, Zanisi M, Martini L, Motta M. Expression of a leptin receptor in immortalized gonadotropin-releasing hormone-secreting neurons. Endocrinology. 1999;140:1581–1585. doi: 10.1210/endo.140.4.6622. [DOI] [PubMed] [Google Scholar]
- Mankouri J, Tedbury PR, Gretton S, Hughes ME, Griffin SD, Dallas ML, Green KA, Hardie DG, Peers C, Harris M. Enhanced hepatitis C virus genome replication and lipid accumulation mediated by inhibition of AMP-activated protein kinase. Proc. Natl. Acad. Sci. USA. 2010;107:11549–11554. doi: 10.1073/pnas.0912426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J. Biol. Chem. 2006;281:18933–18941. doi: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Müller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferré P, Birnbaum MJ, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am. J. Physiol. Endocrinol. Metab. 2005;289:E1051–E1057. doi: 10.1152/ajpendo.00094.2005. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Myers MG, Jr., Münzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr., Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- Pulinilkunnil T, He H, Kong D, Asakura K, Peroni OD, Lee A, Kahn BB. Adrenergic regulation of AMP-activated protein kinase in BAT in vivo. J. Biol. Chem. 2011;11:8798–8809. doi: 10.1074/jbc.M111.218719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson CJ, Bröenstrup M, Fingar DC, Jülich K, Ballif BA, Gygi S, Blenis J. SKAR is a specific target of S6 kinase 1 in cell growth control. Curr. Biol. 2004;14:1540–1549. doi: 10.1016/j.cub.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem. J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys CL, Kovacic S, Dyck JR. Activation of cardiac AMP-activated protein kinase by LKB1 expression or chemical hypoxia is blunted by increased Akt activity. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2472–H2479. doi: 10.1152/ajpheart.01206.2005. [DOI] [PubMed] [Google Scholar]
- Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol. Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- Villanueva EC, Myers MG., Jr. Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes (Lond) 2008;32(Suppl 7):S8–S12. doi: 10.1038/ijo.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden SM, Richardson C, O'Donnell J, Jr., Stapleton D, Kemp BE, Witters LA. Post-translational modifications of the beta-1 subunit of AMP-activated protein kinase affect enzyme activity and cellular localization. Biochem. J. 2001;354:275–283. doi: 10.1042/0264-6021:3540275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Hill JW, Fukuda M, Gautron L, Sohn JW, Kim KW, Lee CE, Choi MJ, Lauzon DA, Dhillon H, et al. PI3K signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metab. 2010;12:88–95. doi: 10.1016/j.cmet.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.