Abstract
Despite the need for more effective treatments for psychiatric disorders, development of new medications has stalled. Here we discuss the promise of personalized medicine in developing more efficacious and individualized pharmacotherapies that take into account genetic variation and target groups of patients who share biology, not just symptoms.
Introduction: Pharmacogenomics, pharmacogenetics, and “personalized medicine”
Medication development for mental disorders has stalled over the past three decades. After the serendipitous discovery of antipsychotic and antidepressant medications in the 1950’s and 1960’s, and the development of more selective and better tolerated compounds in the 1970’s and 1980’s, the field has come to rely on “me-too” compounds and aggressive marketing. This approach has led to robust sales of medication but little evidence of greater efficacy. One exception is the development of clozapine, an antipsychotic that appears more effective than other compounds but is under-prescribed because of rare adverse hematologic events. Recently, many major pharmaceutical companies have all but abandoned drug discovery efforts for mental illness. We may have left behind the era of blockbuster drugs designed to treat large segments of the population. We now need to identify new drug targets and refocus our drug discovery efforts to search -- as Munos [2009] put it -- for breakthroughs rather than blockbusters.
The need for better treatments is undeniable. Mental illness is now the leading cause of healthy life lost in the developed world, and is rising rapidly in developing countries [WHO, 2006]. Existing antipsychotics fail to address the cognitive symptoms of schizophrenia, such as executive dysfunction, which have been increasingly recognized as highly disabling [Hyman and Fenton, 2003]. Available antidepressants act slowly and still fail to bring about remission in more than half of patients with depression. Lithium remains highly effective for some people with bipolar disorder, but most do not enjoy sufficient benefit from lithium or a range of more recently developed mood stabilizers. PTSD and other combat-related mental illness have reached crisis levels among recent veterans and yet no medication has proven effective. Suicide, usually related to mental illness, is a major cause of death, with a rate that is now twice the homicide rate and even surpasses traffic fatalities in the US [Centers for Disease Control; http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf).
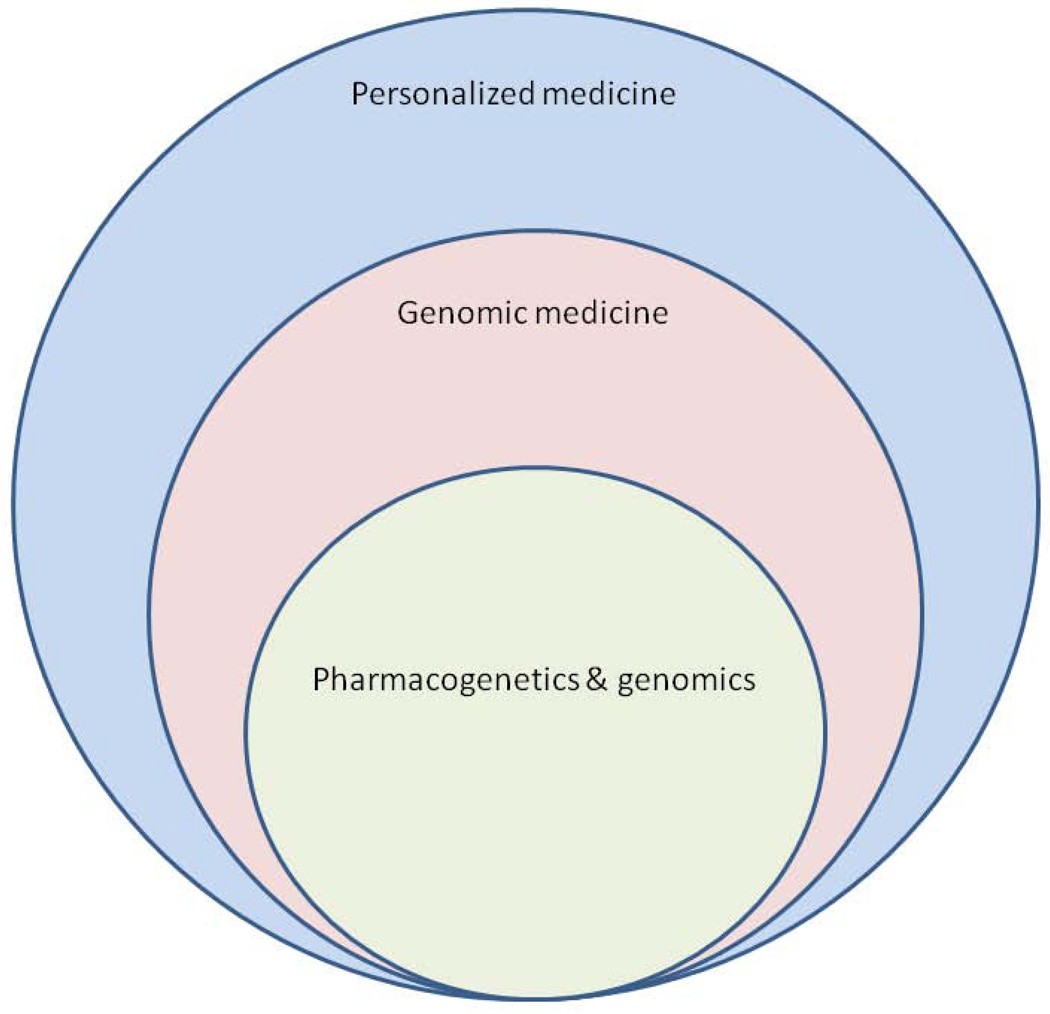
The key lesson of the past decade of clinical trials is the heterogeneity of psychiatric diagnoses. Diagnostic categories, such as schizophrenia, depression, or autism, though each defined by a broader set of observed symptoms, may individually comprise different biological entities with distinct pathophysiologies, requiring different treatments. What we need now are medications for targeted subgroups of patients within diagnostic categories who share biology, not just symptoms. This is the essence of personalized medicine or what has recently been called “precision medicine” [Committee on a Framework for Developing a New Taxonomy of Disease, 2011]. Personalized medicine overlaps [Fig. 1] with what is coming to be known as “genomic medicine,” which uses information from a patient’s genome for diagnosis, prognosis, and treatment planning, emphasizing uncommon or unique aspects of each patient [for review, see Feero et al 2010]. Emphasis on the unique aspects of a patient is, in fact, nothing new for psychiatry. Effective psychiatric care has always been challenging, in part, precisely because it has always been personalized. Every unhappy family may indeed be unhappy in its own way. That is why we need a much larger variety of treatments, each with a much narrower range of indications.
Figure 1.
The nested relationship between personalized medicine, genomic medicine, and pharmacogenetics and genomics.
Some pharmacogenomic “home runs”
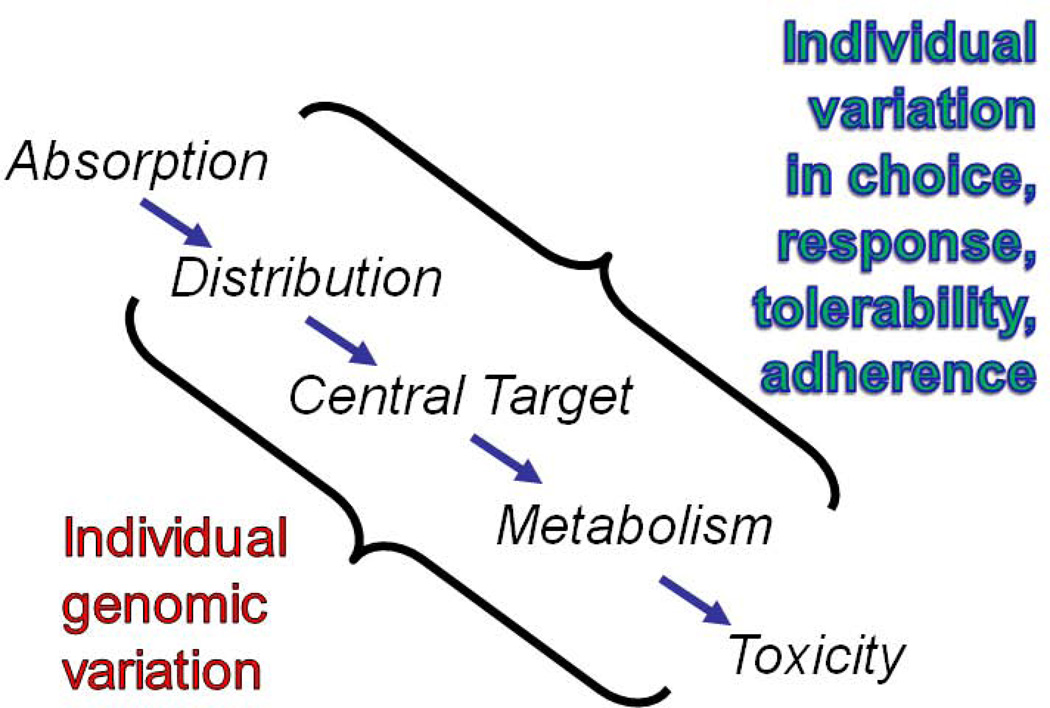
Traditional pharmacogenetics and genomics are forerunners of genomic medicine that use genetic methods to better match patients with treatments. The focus is on genetic markers that correlate with treatment response or adverse effects. Unlike clinical trials, which emphasize homogeneity of outcomes, pharmacogenetic studies emphasize heterogeneity. As such, the goal is to maximize efficacy while minimizing adverse events. Genetic variation can affect how individuals handle medications in a variety of ways, ranging from absorption to toxicity, all in the context of other individual variables, such as treatment adherence [Fig. 2]. Despite this complexity, several pharmacogenetic success stories have emerged in recent years. A few are highlighted here to illustrate how genetics can help reduce toxicity and adverse events – traditional aims of pharmacogenomics – but also help identify subgroups of patients with distinct pathophysiology that may be uniquely responsive to particular medications.
Figure 2.
Approaches to the pharmacogenomics of psychotropic medications. Individual variation reflects both genetic and non-genetic factors that converge on the absorption, distribution, central target, metabolism, and toxicity of medications.
Warfarin dosing polymorphisms
A set of common genetic variants accounts for up to 40% of the variance in optimal dosage of warfarin, a common anticoagulant [for review, see Carlquist and Anderson, 2011]. This discovery has garnered much attention, since bleeding complications from warfarin are not rare and can be serious. In 2010, the FDA revised warfarin labeling to include dosage guidelines based on genotype – a first. However, it is not yet clear that the genetic tests bring additional clinical utility beyond what can be done by skillful monitoring of standard blood clotting assays, such as the INR.
HLA marker of Stevens Johnson Syndrome (SJS) with carbamazepine
SJS is a rare but serious inflammatory disorder of the skin that occurs in a variety of settings, but has long been associated with exposure to anticonvulsants such as lamotrigine, carbamazepine, and phenytoin. In 2004, Chung et al [2004] reported that patients of Han Chinese ancestry who developed SJS after exposure to carbamazepine were substantially more likely to carry the human leukocyte antigen (HLA) haplotype HLA-B*1502, which is common in persons of Asian ancestry. This finding has been confirmed in other Asian populations, but not in non-Asians, where HLA-B*1502 is rare.
Recently, the US Food & Drug Administration changed the carbamazepine labeling to highlight the potential value of HLA testing in patients of Asian ancestry being considered for carbamazepine treatment. This is an example of a strong genetic marker for a rare but serious adverse event. The clinical utility in patients of Asian ancestry seems clear, although it is not yet clear HLA-B*1502 screening is being widely adopted into clinical practice.
Ivacaftor Treatment for Uncommon Form of Cystic Fibrosis
Cystic fibrosis (CF) was one of the first diseases whose causative gene, CFTR, was identified by human genetic mapping. Subsequent work over two decades revealed that each of the disease mutations in CFTR affects the protein differently, making corrective therapy very challenging. A small molecule screening approach identified a compound that partially corrected the defect caused by the G551D mutation, present in about 4% of patients with CF. A version of this compound, known as ivacaftor, was later shown to improve health and lung function in patients over 5 years of age who received the drug over 48 weeks [Ramsey et al 2011]. Ivacaftor has not yet been shown to affect survival in G551D carriers, and apparently has no benefit for the majority of CF patients, who carry other mutations. Despite these limitations, ivacaftor is one of the first examples of an effective treatment that targets patients carrying a particular disease mutation.
How can this work in neuropsychiatry?
Pharmacogenomic studies have been underway for several years in neuropsychiatry, yet the field still seems in its infancy. Many early studies suffered from a lack of large study cohorts and high-throughput molecular technology, which only became available relatively recently. More recent studies have generated promising leads, but effect sizes remain small and replication studies in large samples are generally lacking.
Cytochrome P450
Most drugs are at least partly metabolized by the cytochrome P450 (CYP) system, a family of enzymes that seems to have evolved to help cope with environmental toxins. Variation in the genes encoding the cytochrome enzymes is extensive, and has long been known to affect metabolism of certain drugs, including psychotropics like olanzepine, sertraline, and several benzodiazepines. For these reasons, the CYP genes have been extensively studied in psychiatry and a gene-chip that captures most of the relevant functional variation is being promoted for use in the field [for review, see Black et al 2007]. So far, however, the clinical utility of such testing has not been proven for most patients [EGAPP Working Group, 2007]
Candidate gene and genome wide association studies of antidepressant outcome
Several candidate gene association studies have been carried out in recent years and have identified some promising markers of antidepressant outcome. Numerous studies have implicated SLC6A4 variation in antidepressant treatment outcome, although the outcome phenotypes have varied substantially and a recent meta-analysis found no overall effect [Taylor et al 2010]. Other promising leads include: FKBP5, which encodes a protein involved in glucocorticoid trafficking [Binder et al 2004]; HTR2A, encoding the serotonin 2A receptor [McMahon et al 2006]; and ABCB1, which encodes a p-glycoprotein that affects brain concentrations of some antidepressants [Uhr et al 2008]. All of these findings await robust replication in large samples.
Identification of treatment-responsive subgroups
Most of the common neuropsychiatric disorders probably represent a collection of less common – even rare -- diseases. We need to begin to think in terms of “lithium-responsive mood disorder” or “clozapine-responsive psychotic disorder.” Such treatment-responsive subgroups may share specific genes or other characteristics. Each of the current diagnostic categories may actually encompass several subgroups for which a new treatment needs to be designed, as underscored by the example of ivacaftor in CFTR therapy summarized above. Autism, which is likely a polygenic disorder, may serve as a good model in developing treatment strategies in the broader realm of neuropsychiatry. Recent work has identified several genomic anomalies associated with autism [reviewed in Malhotra and Sebat, 2012]. Each genetic alteration may therefore implicate a distinct molecular etiology, and hence a different potentially ‘druggable’ molecular target. Depending on the underlying molecular or neural substrates, which may differ even within the same diagnostic classification, effective treatment may require cognitive or behavioral treatments rather than medications. Most may require both.
Identification of patients at high risk for severe adverse events
As exemplified by SJS during carbamazepine treatment, we need to identify good predictive markers of severe adverse events arising during psychopharmacologic treatment. Such markers could enable much wider use of drugs such as clozapine that offer distinct advantages to the majority of patients, while preventing exposure of those at high risk for severe events. Recent suggestive data on genomic predictors of metabolic syndrome may be an early example of this approach [reviewed in Chowdhury et al. 2011).
Key challenges
Discovery requires large patient groups. The large number of hypotheses tested in a typical genome-wide experiment poses a substantial multiple-testing problem. Patients who suffer rare adverse events may not be represented in small clinical trials. Treatment-responsive subgroups may comprise only a minority of patients grouped by current diagnostic categories. Such problems can be overcome with large sample sizes, but these can be expensive to collect and study. The STAR*D, CATIE, and STEP*BD projects were the first to provide samples large enough for genome-wide searches. Each of these studies collected a large group of patients with a common diagnosis (major depression, schizophrenia, and bipolar disorder, respectively), and assessed outcomes prospectively after relatively standardized treatment with one or more established psychotropic agents. These studies were not designed as pharmacogenetic studies, but did collect DNA on many participants, thus enabling later pharmacogenetic studies that would not have otherwise been possible. However, we now need additional large samples. One approach might be to aggregate samples from the large numbers of ongoing clinical trials, as discussed further below.
Clinical and genetic sources of heterogeneity
Even the most valuable pharmacogenetic markers never tell the whole story. Treatment outcomes are always the result of a complex interplay of individual, social, and stochastic factors. In psychiatry, adherence is a serious and often overlooked problem. For complex disorders, the best treatment would be one that uniquely corrects a specific molecular defect. This is being achieved for occasional patients with rare diseases, such as dopa-responsive dystonia [Bainbridge et al 2011], but remains a major challenge, especially for neuropsychiatry.
Demonstrating clinical utility
The initial discovery phases of pharmacogenetic studies typically emphasize statistical significance and replication. These yardsticks are necessary for establishing the scientific reliability of a finding, but tell us nothing about how valuable the information is for clinical decision making. Here, the well established concept of ‘Number Needed to Screen’ is valuable, since it incorporates both the frequency of a marker and the magnitude of its effect [Rembold 1998]. The NNS captures how many patients need to receive a test for every patient whose outcome is altered. Smaller NNS values are generally better, but there is no single threshold. If the goal is to avoid a severe adverse event, larger NNS might be reasonable, while quantitative improvements in response might require smaller NNS values to make sense clinically.
Physician education
The interpretation of genetic information is a new challenge for most physicians. Since the clinical utility of pharmacogenetic markers typically is probabilistic, increasing the odds of one outcome versus another, it is not always clear how best to use this information in clinical decision making [Khoury et al 2010]. As genetic information becomes more comprehensive, the competing odds become more difficult to judge. This will require a kind of actuarial decision making that is unfamiliar to many clinicians. Medical school curricula are becoming more genetically informed, but reaching residents and practicing physicians in ways that can alter their clinical practice is challenging [Winner et al 2010].
Conclusion: Some paths forward
Ultimately, better medications will follow from a better understanding of the biology of psychiatric disorders. This may take years, but there are several steps that can be taken now to make better use of what we already know and to position the field to capitalize quickly on new biologic insights, whenever they arise.
DNA collection in clinical trials
We have already explained why genetic discoveries require large samples, but these can be slow and expensive to collect. Volunteers in ongoing clinical trials offer an attractive alternative. Although they represent a heterogeneous group in terms of ascertainment, diagnosis, and treatments employed, the many ongoing clinical trials may collectively constitute a reasonably representative sample of the population, well suited to large-scale genetic studies. We need a coordinated effort by academia, industry, and government to begin collecting DNA in clinical trials and to send the samples and associated data -- in anonymous form -- to a central repository, where they can be used to fuel future large-scale studies.
Revisiting underused drugs that may be safe and effective in particular groups
The pharmacopeia is full of drugs that seem to have outlived their usefulness or never found wide application: Long-used medications known to be safe that have been superseded by drugs that are considered more efficacious; newer drugs that, while highly effective, were found to cause severe adverse events in some people. By use of genetic methods, it may be possible to ‘repurpose’ some of these medications for other indications. If good genetic markers of safety and efficacy can be established, such repurposed drugs could be helpful for targeted populations, where acceptable risk:benefit ratios can be more easily achieved. Systematic efforts along these lines are now being initiated in the National Center for Advancing Translational Sciences (NCATS). NCATS is a new entity within the NIH that aims to catalyze the generation of innovative methods and technologies to enhance the development, testing, and implementation of diagnostic tests and therapeutic agents across a wide range of human ills [www.ncats.nih.gov/].
Pharmacogenomics for identifying new drug targets
Traditional drug development pipelines are inefficient and expensive. Innovative strategies are needed, but innovation requires new perspectives. Genetics is providing some of these new perspectives. Genome-wide association studies have revealed a spectrum of common genetic markers for a number of traits, diseases, and treatment outcomes. At about the same time, a whole new class of genetic variation was discovered, known as copy number variants (CNVs): deletions and insertions of small chromosomal segments, containing from one to dozens of genes. CNVs have been shown to play a major role in autism, schizophrenia, and developmental disorders, and may also contribute to treatment outcomes [for review, see Malhotra & Sebat, 2012]. CNV’s often arise de novo as chromosomes are passed from parent to offspring, providing a dynamic source of genetic differences within every generation.
Large-scale sequencing of the genome is providing another new perspective. Thanks to this new technology, we now know that the average person harbors about 10,000 mutations that directly affect protein expression or structure, some 200 of which amount to total gene “knock-outs” [MacArthur et al 2012]. How big a role such dramatic variation will play in future pharmacogenomics findings we can only speculate – but it will probably be large.
Genetics is not the whole answer, but offers a solid starting point. Further knowledge of the basic disease processes at the cellular and molecular level will be required to discover ideal, curative treatments for most patients with neuropsychiatric disorders, but much could be achieved by personalizing the existing pharmacopeia. Personalized medicine will bring new insights, more treatment options, and better outcomes to what psychiatrists have always strived for – caring for each patient as an individual.
Acknowledgements
Supported by the NIMH Intramural Research Program. We thank Gonzalo Laje for helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bainbridge MN, Wiszniewski W, Murdock DR, Friedman J, Gonzaga-Jauregui C, Newsham I, Reid JG, Fink JK, Morgan MB, Gingras MC, et al. Whole-genome sequencing for optimized patient management. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002243. 87re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Pütz B, Papiol S, Seaman S, Lucae S, Kohli MA, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Black JL, 3rd, O'Kane DJ, Mrazek DA. The impact of CYP allelic variation on antidepressant metabolism: A review. Expert Opin Drug Metab Toxicol. 2007;3:21–31. doi: 10.1517/17425255.3.1.21. [DOI] [PubMed] [Google Scholar]
- Carlquist JF, Anderson JL. Using pharmacogenetics in real time to guide warfarin initiation: A clinician update. Circulation. 2011;124:2554–2559. doi: 10.1161/CIRCULATIONAHA.111.019737. [DOI] [PubMed] [Google Scholar]
- Chowdhury NI, Remington G, Kennedy JL. Genetics of antipsychotic-induced side effects and agranulocytosis. Curr Psychiatry Rep. 2011;13:156–165. doi: 10.1007/s11920-011-0185-3. [DOI] [PubMed] [Google Scholar]
- Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, Wu JY, Chen YT. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486. doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- Committee on A Framework for Developing a New Taxonomy of Disease. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: Testing for cytochrome P450 polymorphisms in adults with nonpsychotic depression treated with selective serotonin reuptake inhibitors. Genet Med. 2007;9:819–825. doi: 10.1097/gim.0b013e31815bf9a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feero WG, Guttmacher AE, Collins FS. Genomic medicine--an updated primer. N Engl J Med. 2010;362:2001–2011. doi: 10.1056/NEJMra0907175. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Fenton WS. Medicine. What are the right targets for psychopharmacology? Science. 2003;299:350–351. doi: 10.1126/science.1077141. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Coates RJ, Evans JP. Evidence-based classification of recommendations on use of genomic tests in clinical practice dealing with insufficient evidence. Genet Med. 2010;12:680–683. doi: 10.1097/GIM.0b013e3181f9ad55. [DOI] [PubMed] [Google Scholar]
- MacArthur DG, Balasubramanian S, Frankish A, Huang N, Morris J, Walter K, Jostins L, Habegger L, Pickrell JK, Montgomery SB, et al. A systematic survey of loss-of-function variants in human protein-coding genes. Science. 2012;335:823–828. doi: 10.1126/science.1215040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra D, Sebat J. CNVs: Harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148:1223–1241. doi: 10.1016/j.cell.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon FJ, Buervenich S, Charney D, Lipsky R, Rush AJ, Wilson AF, Sorant AJ, Papanicolaou GJ, Laje G, Fava M, et al. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am J Hum Genet. 2006;78:804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munos B. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov. 2009;8:959–968. doi: 10.1038/nrd2961. [DOI] [PubMed] [Google Scholar]
- Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold CM. Number needed to screen: Development of a statistic for disease screening. BMJ. 1998;317:307–312. doi: 10.1136/bmj.317.7154.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Sen S, Bhagwagar Z. Antidepressant response and the serotonin transporter gene-linked polymorphic region. Biol Psychiatry. 2010;68:536–543. doi: 10.1016/j.biopsych.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhr M, Tontsch A, Namendorf C, Ripke S, Lucae S, Ising M, Dose T, Ebinger M, Rosenhagen M, Kohli M, et al. Polymorphisms in the drug transporter gene ABCB1 predict antidepressant treatment response in depression. Neuron. 2008;57:203–209. doi: 10.1016/j.neuron.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Winner JG, Goebert D, Matsu C, Mrazek DA. Training in psychiatric genomics during residency: A new challenge. Acad Psychiatry. 34:115–118. doi: 10.1176/appi.ap.34.2.115. (2101) [DOI] [PubMed] [Google Scholar]