Abstract
The 3' splice site of the second intron (I2) of the human apolipoprotein-AII gene, (GT)16GGGCAG, is unique in that, although fully functional, a stretch of alternating guanine and thymine residues replaces the polypyrimidine tract usually associated with 3' splice junctions. The transient expression of successive 5' deletion mutants has defined the minimum number of nucleotides at the 3' end of apo-AII I2 that are required to direct efficient splicing. Processing in two cell-types, representing apo-AII producing and non-producing tissue was identical; in both, only by removing all the GT repeats did the 3' splice site of apo-AII I2 become completely non-functional. Similar deletion analyses of "classic" 3' splice sites, which conform to the consensus sequence (Y)nNYAG, have indicated that a minimum of 14 nucleotides of the polypyrimidine tract are required for detectable levels of processing to take place. Here we report that the six nucleotides (GT)2GG, which directly replace this tract in a deletion mutant of the 3' splice site of apo-AII I2 are sufficient to direct the splicing process efficiently and correctly.
Full text
PDF


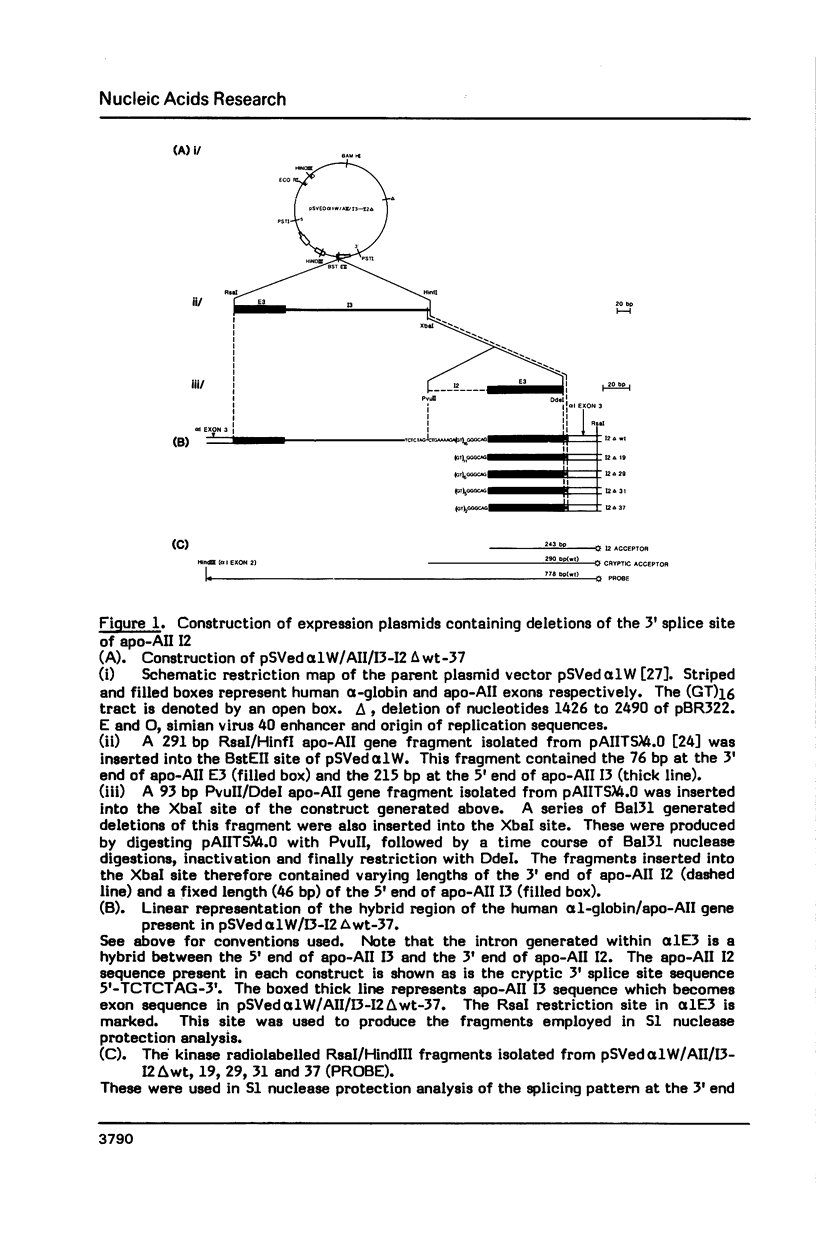

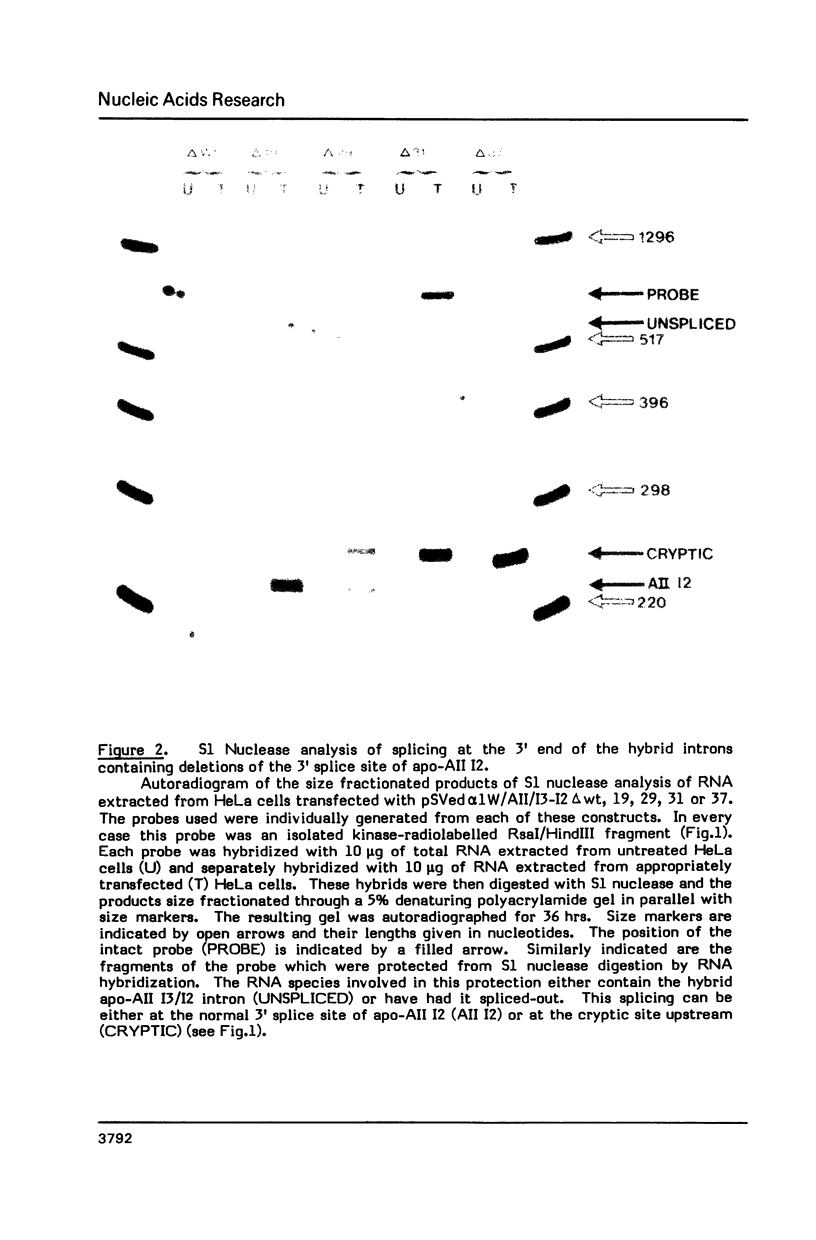

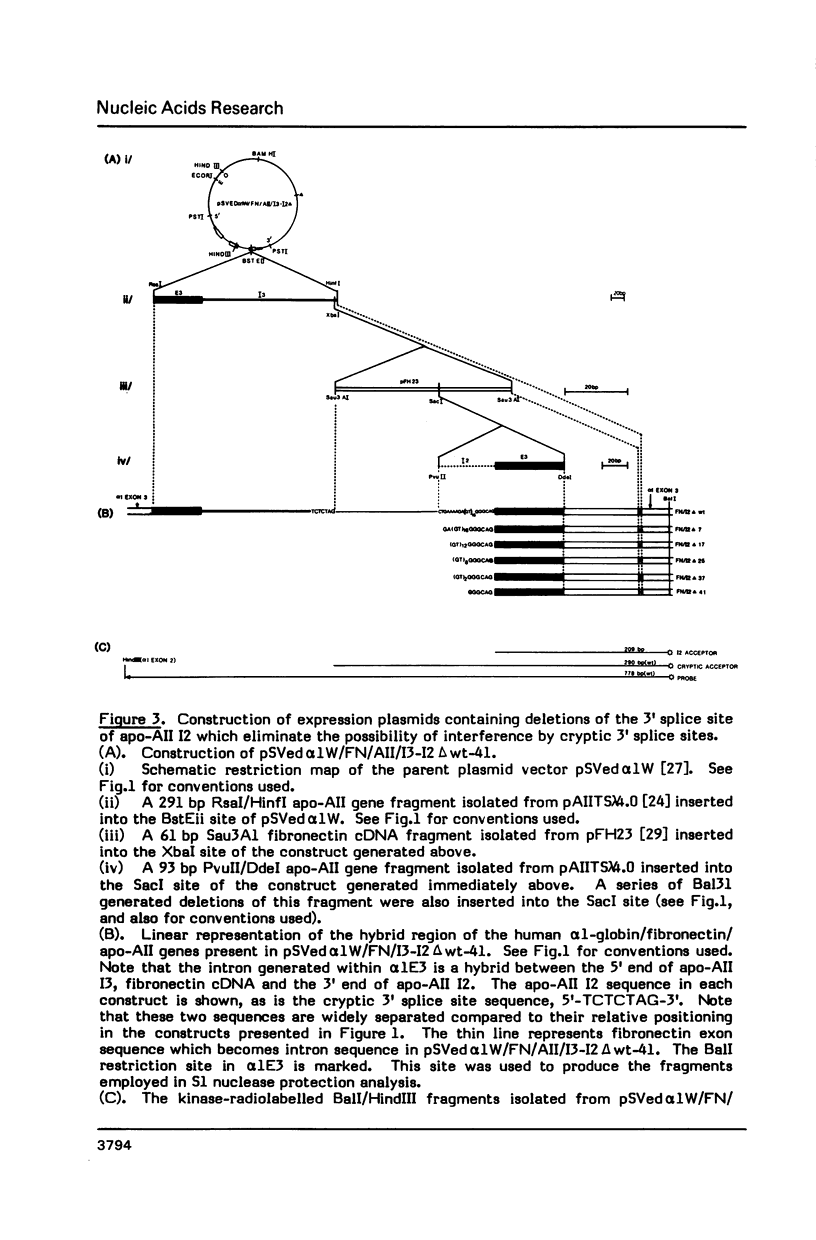



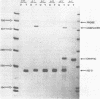
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aden D. P., Fogel A., Plotkin S., Damjanov I., Knowles B. B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979 Dec 6;282(5739):615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- Atweh G. F., Anagnou N. P., Shearin J., Forget B. G., Kaufman R. E. Beta-thalassemia resulting from a single nucleotide substitution in an acceptor splice site. Nucleic Acids Res. 1985 Feb 11;13(3):777–790. doi: 10.1093/nar/13.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger M., Moschonas N., Flavell R. A. Beta + thalassemia: aberrant splicing results from a single point mutation in an intron. Cell. 1981 Dec;27(2 Pt 1):289–298. doi: 10.1016/0092-8674(81)90412-8. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Kopchick J. J., Stacey D. W. Effect of intron size on splicing efficiency in retroviral transcripts. Nucleic Acids Res. 1982 Oct 11;10(19):6177–6190. doi: 10.1093/nar/10.19.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D. S., Bahnak B. R. Human hepatoma cells secrete single chain factor X, prothrombin, and antithrombin III. Blood. 1984 Jul;64(1):194–204. [PubMed] [Google Scholar]
- Frendewey D., Keller W. Stepwise assembly of a pre-mRNA splicing complex requires U-snRNPs and specific intron sequences. Cell. 1985 Aug;42(1):355–367. doi: 10.1016/s0092-8674(85)80131-8. [DOI] [PubMed] [Google Scholar]
- Fukumaki Y., Ghosh P. K., Benz E. J., Jr, Reddy V. B., Lebowitz P., Forget B. G., Weissman S. M. Abnormally spliced messenger RNA in erythroid cells from patients with beta+ thalassemia and monkey cells expressing a cloned beta+-thalassemic gene. Cell. 1982 Mar;28(3):585–593. doi: 10.1016/0092-8674(82)90213-6. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Herr W. Diethyl pyrocarbonate: a chemical probe for secondary structure in negatively supercoiled DNA. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8009–8013. doi: 10.1073/pnas.82.23.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs D. R., Goodbourn S. E., Lamb J., Clegg J. B., Weatherall D. J., Proudfoot N. J. Alpha-thalassaemia caused by a polyadenylation signal mutation. Nature. 1983 Nov 24;306(5941):398–400. doi: 10.1038/306398a0. [DOI] [PubMed] [Google Scholar]
- Kaplan B. B., Bernstein S. L., Gioio A. E. An improved method for the rapid isolation of brain ribonucleic acid. Biochem J. 1979 Oct 1;183(1):181–184. doi: 10.1042/bj1830181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L. Protein structural domains in the Caenorhabditis elegans unc-54 myosin heavy chain gene are not separated by introns. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4253–4257. doi: 10.1073/pnas.80.14.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller E. B., Noon W. A. Intron splicing: a conserved internal signal in introns of animal pre-mRNAs. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7417–7420. doi: 10.1073/pnas.81.23.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W. The RNA lariat: a new ring to the splicing of mRNA precursors. Cell. 1984 Dec;39(3 Pt 2):423–425. doi: 10.1016/0092-8674(84)90449-5. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Grabowski P. J., Padgett R. A., Sharp P. A. Characterization of the branch site in lariat RNAs produced by splicing of mRNA precursors. Nature. 1985 Feb 14;313(6003):552–557. doi: 10.1038/313552a0. [DOI] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Human fibronectin: molecular cloning evidence for two mRNA species differing by an internal segment coding for a structural domain. EMBO J. 1984 Jan;3(1):221–226. doi: 10.1002/j.1460-2075.1984.tb01787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautmann G., Matthes H. W., Gait M. J., Breathnach R. Synthetic donor and acceptor splice sites function in an RNA polymerase B (II) transcription unit. EMBO J. 1984 Sep;3(9):2021–2028. doi: 10.1002/j.1460-2075.1984.tb02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R., Maniatis T. A role for exon sequences and splice-site proximity in splice-site selection. Cell. 1986 Aug 29;46(5):681–690. doi: 10.1016/0092-8674(86)90343-0. [DOI] [PubMed] [Google Scholar]
- Reed R., Maniatis T. Intron sequences involved in lariat formation during pre-mRNA splicing. Cell. 1985 May;41(1):95–105. doi: 10.1016/0092-8674(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Green M. R. Role of the 3' splice site consensus sequence in mammalian pre-mRNA splicing. Nature. 1985 Oct 24;317(6039):732–734. doi: 10.1038/317732a0. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Greene J. M., Green M. R. Cryptic branch point activation allows accurate in vitro splicing of human beta-globin intron mutants. Cell. 1985 Jul;41(3):833–844. doi: 10.1016/s0092-8674(85)80064-7. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. On the origin of RNA splicing and introns. Cell. 1985 Sep;42(2):397–400. doi: 10.1016/0092-8674(85)90092-3. [DOI] [PubMed] [Google Scholar]
- Shelley C. S., Sharpe C. R., Baralle F. E., Shoulders C. C. Comparison of the human apolipoprotein genes. Apo AII presents a unique functional intron-exon junction. J Mol Biol. 1985 Nov 5;186(1):43–51. doi: 10.1016/0022-2836(85)90255-4. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., Jagadeeswaran P., Choudary P. V., Biro P. A., Elder J. T., deRiel J. K., Manley J. L., Gefter M. L., Forget B. G., Weissman S. M. Base substitution in an intervening sequence of a beta+-thalassemic human globin gene. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2455–2459. doi: 10.1073/pnas.78.4.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmappaya B., Shenk T. Nucleotide sequence analysis of viable deletion mutants lacking segments of the simian virus 40 genome coding for small t antigen. J Virol. 1979 Jun;30(3):668–673. doi: 10.1128/jvi.30.3.668-673.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert G., Feunteun J., Crawford L. V., Berg P., Fiers W. Nucleotide sequence deletions within the coding region for small-t antigen of simian virus 40. J Virol. 1979 Jun;30(3):674–682. doi: 10.1128/jvi.30.3.674-682.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann C. Molecular biology. Excision of introns in lariat form. Nature. 1984 Sep 13;311(5982):103–104. doi: 10.1038/311103a0. [DOI] [PubMed] [Google Scholar]
- Westaway D., Williamson R. An intron nucleotide sequence variant in a cloned beta +-thalassaemia globin gene. Nucleic Acids Res. 1981 Apr 24;9(8):1777–1788. doi: 10.1093/nar/9.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa B., Hofer E., Weissmann C. A minimal intron length but no specific internal sequence is required for splicing the large rabbit beta-globin intron. Cell. 1984 Jul;37(3):915–925. doi: 10.1016/0092-8674(84)90426-4. [DOI] [PubMed] [Google Scholar]
- Zannis V. I., Breslow J. L., SanGiacomo T. R., Aden D. P., Knowles B. B. Characterization of the major apolipoproteins secreted by two human hepatoma cell lines. Biochemistry. 1981 Dec 8;20(25):7089–7096. doi: 10.1021/bi00528a006. [DOI] [PubMed] [Google Scholar]
- van Santen V. L., Spritz R. A. mRNA precursor splicing in vivo: sequence requirements determined by deletion analysis of an intervening sequence. Proc Natl Acad Sci U S A. 1985 May;82(9):2885–2889. doi: 10.1073/pnas.82.9.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]