Abstract
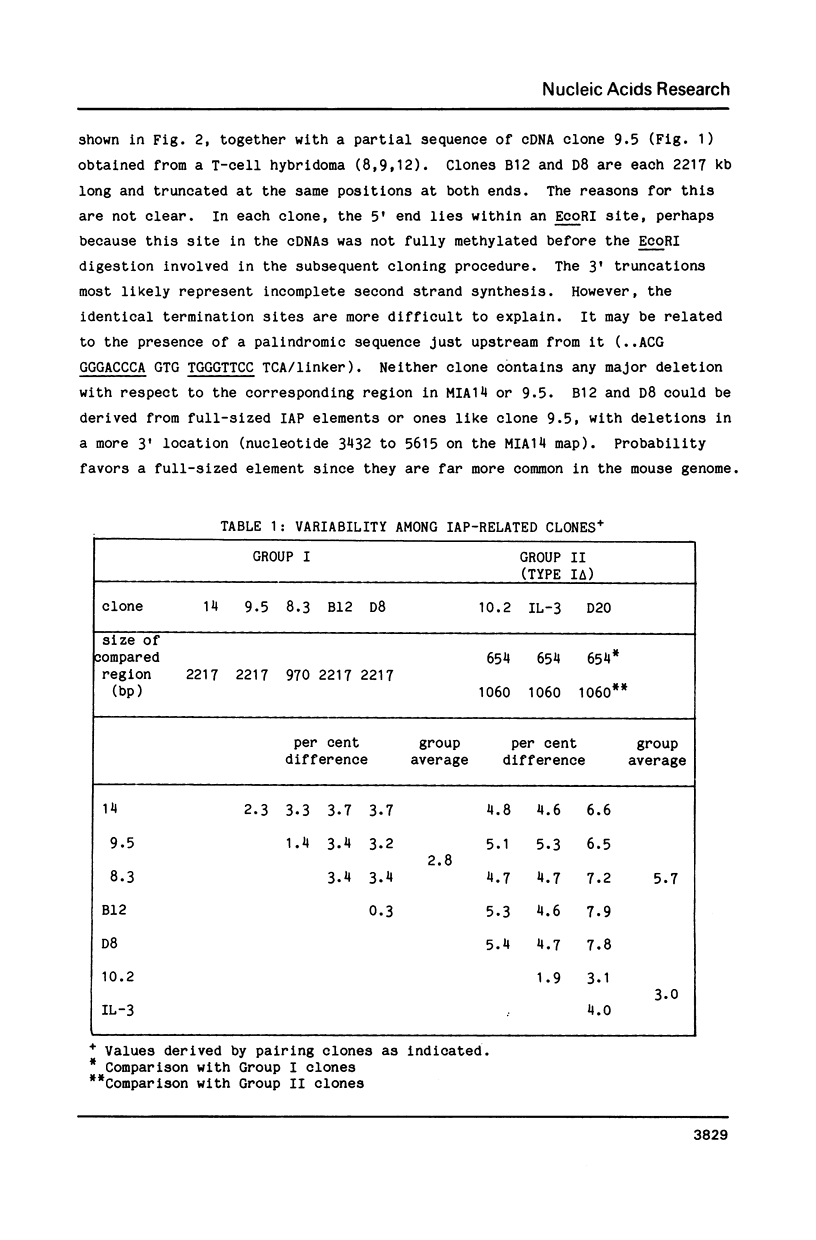
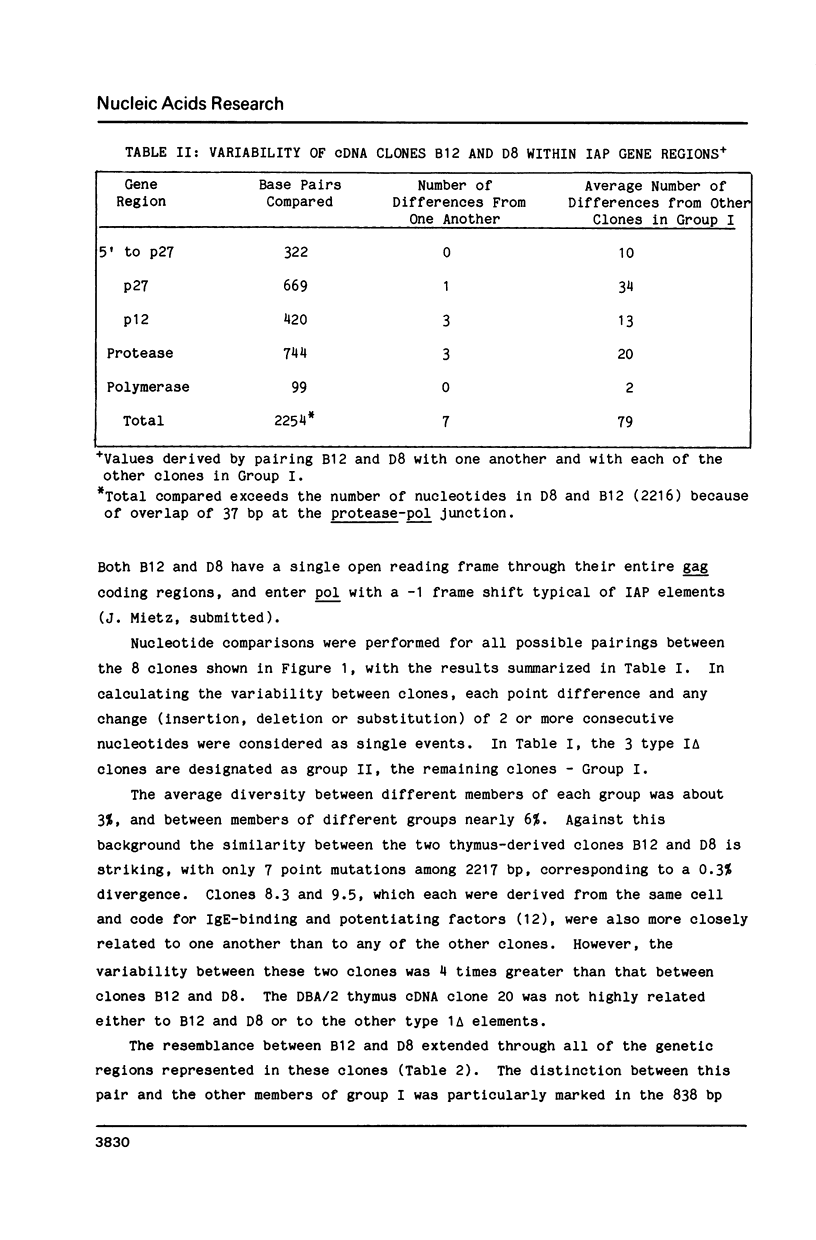
Poly(A)RNAs prepared from the thymuses of C57BL/6J and DBA/2J mice were used to construct cDNA libraries in the bacterial expression vector lambda gt11. The libraries were scanned first for protein production with polyvalent antiserum prepared against the 73kDa gag protein of mouse intracisternal A-particles (IAP). Reactive plaques were crossed-screened by hybridization with an IAP-specific DNA probe. Two IAP-specific protein-producing plaques were obtained from the C57BL/6 library and 4 from the DBA/2 library. One C57BL/6 cDNA clone (B12) and two DBA cDNA clones (D8 and D20) were sequenced in their entirety. Clones B12 and D8 were remarkably similar, particularly when compared to the 6 other IAP elements that have been sequenced thus far. We discuss the evidence which leads us to suggest that these clones may be derived from allelic IAP elements expressed in mouse thymus.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barklis E., Mulligan R. C., Jaenisch R. Chromosomal position or virus mutation permits retrovirus expression in embryonal carcinoma cells. Cell. 1986 Nov 7;47(3):391–399. doi: 10.1016/0092-8674(86)90596-9. [DOI] [PubMed] [Google Scholar]
- Burt D. W., Reith A. D., Brammar W. J. A retroviral provirus closely associated with the Ren-2 gene of DBA/2 mice. Nucleic Acids Res. 1984 Nov 26;12(22):8579–8593. doi: 10.1093/nar/12.22.8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin J. J., Weiss E. H., Paulson M., Flavell R. A. Duplicated gene pairs and alleles of class I genes in the Qa2 region of the murine major histocompatibility complex: a comparison. EMBO J. 1985 Dec 1;4(12):3203–3207. doi: 10.1002/j.1460-2075.1985.tb04066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feenstra A., Fewell J., Lueders K., Kuff E. In vitro methylation inhibits the promotor activity of a cloned intracisternal A-particle LTR. Nucleic Acids Res. 1986 May 27;14(10):4343–4352. doi: 10.1093/nar/14.10.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch W. M., Atchley W. R. Evolution in inbred strains of mice appears rapid. Science. 1985 Jun 7;228(4704):1169–1175. doi: 10.1126/science.4001935. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hojman-Montes de Oca F., Dianoux L., Peries J., Emanoil-Ravicovitch R. Intracisternal A particles: RNA expression and DNA methylation in murine teratocarcinoma cell lines. J Virol. 1983 Apr;46(1):307–310. doi: 10.1128/jvi.46.1.307-310.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff T. F., Uede T., Ishizaka K. Formation of rat IgE-binding factors by rat-mouse T cell hybridomas. J Immunol. 1982 Aug;129(2):509–514. [PubMed] [Google Scholar]
- Kuff E. L., Callahan R., Howk R. S. Immunological relationship between the structural proteins of intracisternal A-particles of Mus musculus and the M432 retrovirus of Mus cervicolor. J Virol. 1980 Mar;33(3):1211–1214. doi: 10.1128/jvi.33.3.1211-1214.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Fewell J. W. Intracisternal A-particle gene expression in normal mouse thymus tissue: gene products and strain-related variability. Mol Cell Biol. 1985 Mar;5(3):474–483. doi: 10.1128/mcb.5.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Mietz J. A., Trounstine M. L., Moore K. W., Martens C. L. cDNA clones encoding murine IgE-binding factors represent multiple structural variants of intracisternal A-particle genes. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6583–6587. doi: 10.1073/pnas.83.17.6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Smith L. A., Lueders K. K. Intracisternal A-particle genes in Mus musculus: a conserved family of retrovirus-like elements. Mol Cell Biol. 1981 Mar;1(3):216–227. doi: 10.1128/mcb.1.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Roberts L., Dobberstein B. Mouse histocompatibility genes: structure and organisation of a Kd gene. EMBO J. 1983;2(2):245–254. doi: 10.1002/j.1460-2075.1983.tb01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Intracisternal A-particle genes: identification in the genome of Mus musculus and comparison of multiple isolates from a mouse gene library. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3571–3575. doi: 10.1073/pnas.77.6.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Sequences associated with intracisternal A particles are reiterated in the mouse genome. Cell. 1977 Dec;12(4):963–972. doi: 10.1016/0092-8674(77)90161-1. [DOI] [PubMed] [Google Scholar]
- Martens C. L., Huff T. F., Jardieu P., Trounstine M. L., Coffman R. L., Ishizaka K., Moore K. W. cDNA clones encoding IgE-binding factors from a rat-mouse T-cell hybridoma. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2460–2464. doi: 10.1073/pnas.82.8.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor A. L., Weiss E. H., Kress M., Jay G., Flavell R. A. A nonpolymorphic class I gene in the murine major histocompatibility complex. Cell. 1984 Jan;36(1):139–144. doi: 10.1016/0092-8674(84)90082-5. [DOI] [PubMed] [Google Scholar]
- Moore K. W., Jardieu P., Mietz J. A., Trounstine M. L., Kuff E. L., Ishizaka K., Martens C. L. Rodent IgE-binding factor genes are members of an endogenous, retrovirus-like gene family. J Immunol. 1986 Jun 1;136(11):4283–4290. [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Cole M. D., White A. T., Huang R. C. Sequence organization of cloned intracisternal A particle genes. Cell. 1980 Sep;21(2):465–473. doi: 10.1016/0092-8674(80)90483-3. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Segal S., Lueders K. K., Kuff E. L. RNA associated with murine intracisternal type A particles codes for the main particle protein. J Virol. 1978 Jul;27(1):118–126. doi: 10.1128/jvi.27.1.118-126.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter R. M., Berson B., Griffin J. A., Hood L. Diversity in the germline antibody repertoire. Molecular evolution of the T15 VN gene family. J Exp Med. 1985 Dec 1;162(6):1998–2016. doi: 10.1084/jem.162.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikó L., Hammons M. D., Taylor K. D. Amounts, synthesis, and some properties of intracisternal A particle-related RNA in early mouse embryos. Proc Natl Acad Sci U S A. 1984 Jan;81(2):488–492. doi: 10.1073/pnas.81.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp R. A., Bailiff E. G. Sequence of amino acids in the major and minor chains of the diffuse hemoglobin from BALB-c mice. Biochim Biophys Acta. 1973 Mar 23;303(1):61–67. doi: 10.1016/0005-2795(73)90148-7. [DOI] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Cole M. D. Differing populations of intracisternal A-particle genes in myeloma tumors and mouse subspecies. J Virol. 1982 May;42(2):411–421. doi: 10.1128/jvi.42.2.411-421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wujcik K. M., Morgan R. A., Huang R. C. Transcription of intracisternal A-particle genes in mouse myeloma and Ltk- cells. J Virol. 1984 Oct;52(1):29–36. doi: 10.1128/jvi.52.1.29-36.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Campbell H. D., Young I. G. Nucleotide sequence of the intracisternal A-particle genome inserted 5' to the interleukin-3 gene of the leukemia cell line WEHI-3B. Nucleic Acids Res. 1986 Jul 25;14(14):5901–5918. doi: 10.1093/nar/14.14.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]



