Abstract
In the framework of biological control, the selection of effective natural enemies determines the final pest control. Thus, the genetic improvement of biocontrol agents could enhance the efficiency of biocontrol programs. Although promising, this approach has rarely been applied in this field. At the intraspecific level, hybridization between divergent populations of biocontrol agents is expected to promote hybrid vigor (heterosis), but it is not clear to what extent. An even more difficult task is the ability to predict the fitness of hybrids from the biological characteristics of their parents. We investigated these general questions by crossing seven populations of the parasitoid wasp Trichogramma chilonis (Hymenoptera: Trichogrammatidae). Our results show different levels of mating compatibilities among populations, including asymmetric or almost complete reproductive isolation. Hybrids' performance (fitness of the F1 generation) ranges from inbreeding depression to heterosis. It was possible, to some extent, to predict hybrid fitness from pairwise genetic and phenotypic distances among parents, in accordance with the ‘dominance’ hypothesis. This may provide general guidelines for the genetic improvement of biological control agents.
Keywords: biological control, genetic improvement, heterosis, intraspecific hybridization, reproductive compatibilities
Introduction
The identification of efficient biological control agents (organisms used to regulate the demography of specific pest species, hereafter referred to as BCA) is, in the most favorable cases, the result of a twofold screening process, which includes both inter- and intraspecific levels of investigation. At the intraspecific level, significant genetic variability has been repeatedly shown using molecular markers or investigating phenotypic traits (Hansen et al. 2012). Such variability can provide a basis for BCA's genetic improvement (Shapiro et al. 1997; Baker et al. 2003; Müller-Schärer and Schaffner 2008; Salame et al. 2010). Intraspecific hybridization – the genetic admixture of previously isolated populations – may be a relevant and applicable method to enhance BCA's fitness for at least two main reasons:
First, hybridization has been recently proposed as a mechanism capable to promote the demographic success of introduced species (Ellstrand and Schierenbeck 2000; Kolbe et al. 2004; Facon et al. 2005, 2008; Lavergne and Molofsky 2007; Rieseberg et al. 2007; Rosenthal et al. 2008; Schierenbeck and Ellstrand 2009; Keller and Taylor 2010). Because biological invasions and classical biological control (briefly defined as the intentional and perennial introduction of an exotic species for the durable control of a noxious species) are based on similar processes (Ehler 1998; Fagan et al. 2002; Fauvergue and Hopper 2009; Marsico et al. 2010), the latter provides a unique framework to test hypotheses derived from observations obtained in the field of invasion biology. The underlying rationale for the positive effect of hybridization relies on the fact that hybrids may exhibit, at the individual level, heterosis and/or, at the population level, high genetic variance for relevant phenotypic traits (e.g., Facon et al. 2008). These two features may help hybrids to adapt to novel and/or heterogeneous environments better than the parental lineages, as in the case, for instance, of exotic BCA used for classical biological control (Malausa et al. 2010; Benvenuto et al. in press).
Second, hybridization has been reported long ago in model organisms (Dobzhansky 1950) and it has also been proven effective for the genetic improvement of some agronomic resources (e.g., Falconer 1981; Crow 1998; Coors and Pandey 1999; Sadras and Calderini 2009). Thus, the added value provided by heterotic hybrids has stimulated numerous experimental and theoretical studies and has also generated commercial profit. By contrast, the genetic improvement of BCA arthropods has received less attention (Mackauer 1972, 1976; Hoy 1976; Hopper et al. 1993; Roderick and Navajas 2003), even though some experimental studies on insects (Legner 1989, 1993; Hoffmann et al. 2002; Mathenge et al. 2010; Szűcs et al. 2011), mites (Hoy and Knop 1981; Roush and Hoy 1981a,b; Hoy et al. 1983; Messing and Croft 1991), and nematodes (Hoy 1985; Shapiro et al. 1997; Segal and Glazer 2000; Strauch et al. 2004; Bai et al. 2005; Shapiro-Ilan et al. 2005; Mukuka et al. 2010a,b; Chaston et al. 2011) have been performed during the last three decades. Although genetic improvement approaches cannot be developed for all commercially available BCA, they could be applied to the ones broadly used in inoculative or inundative biological control programs (see Cock et al. 2010).
Second, hybridization has been reported long ago in model organisms (Dobzhansky 1950) and it has also been proven effective for the genetic improvement of some agronomic resources (e.g., Falconer 1981; Crow 1998; Coors and Pandey 1999; Sadras and Calderini 2009). Thus, the added value provided by heterotic hybrids has stimulated numerous experimental and theoretical studies and has also generated commercial profit. By contrast, the genetic improvement of BCA arthropods has received less attention (Mackauer 1972, 1976; Hoy 1976; Hopper et al. 1993; Roderick and Navajas 2003), even though some experimental studies on insects (Legner 1989, 1993; Hoffmann et al. 2002; Mathenge et al. 2010; Szűcs et al. 2011), mites (Hoy and Knop 1981; Roush and Hoy 1981a,b; Hoy et al. 1983; Messing and Croft 1991), and nematodes (Hoy 1985; Shapiro et al. 1997; Segal and Glazer 2000; Strauch et al. 2004; Bai et al. 2005; Shapiro-Ilan et al. 2005; Mukuka et al. 2010a,b; Chaston et al. 2011) have been performed during the last three decades. Although genetic improvement approaches cannot be developed for all commercially available BCA, they could be applied to the ones broadly used in inoculative or inundative biological control programs (see Cock et al. 2010).
Whatever the underlying biological control strategies (classical, inoculative, inundative, or even conservation – see Eilenberg et al. 2001 for the terminology), the production of heterotic hybrids is the cornerstone of genetic improvement projects. Such individuals can be obtained by chance or by labor-intensive crossing experiments, but a more ambitious and valuable goal would be to define predictors of hybrids' quality, such as genetic or phenotypic distance between parents or level of heterozygosity. These patterns have been particularly investigated in the framework of animal breeding programs but, taken as a whole, experiments have often produced inconsistent results (see for instance Riday et al. 2003; Geleta et al. 2004; Teklewold and Becker 2006; Li et al. 2008; Krystkowiak et al. 2009; Luan et al. 2010; Zhang et al. 2010 and references therein). Part of this discrepancy can be explained by different reasons, biological (including the taxa and the genetic scale investigated) or methodological (including the genetic markers and the statistical analyses applied) but ultimately we are still far from understanding the relative contributions of ‘dominance’, ‘overdominance’, and ‘epistasis’ to the heterotic genotypes (see Edmands 2002; Syed and Chen 2005 for definitions). However, contrary to the two other mechanisms, the ‘dominance’ hypothesis generates some predictions that can be experimentally tested.
According to the ‘dominance’ hypothesis, each parental population has fixed deleterious and recessive alleles at different loci, which reduce its fitness. Higher fitness can be restored in hybrids when advantageous and dominant alleles provided by one parent complement the deleterious alleles present in the other parents. As a consequence, two predictions can be made.
1) Hybrid fitness is assumed to be linked to the genetic distance between the parental lineages (Moll et al. 1965; Bateson 1983; Waser 1993). At least for small-to-moderate genetic divergences, we expect a positive correlation between hybrid fitness and the interparental genetic distance for neutral markers (Prediction 1). Indeed, genetically close populations have a greater probability to fix the same deleterious alleles because of their common genetic history. For larger genetic divergences, negative epistatic interactions may instead appear (Bateson 1983; Lynch 1991). The relationship is thus not linear: an ‘optimal’ genetic distance (to maximize fitness) should lie in an intermediate position in the spectrum of genetic distances among parental populations, which should ‘balance’ inbreeding and outbreeding costs (Bateson 1983) and should correspond to a peak of hybrid fitness (Moll et al. 1965; Waser 1993). This intermediate position is nevertheless difficult to quantify (Willi and Van Buskirk 2005).
2) Phenotypic distance among parents can be another candidate explanatory variable to predict hybrid fitness. Indeed, a negative linear correlation between the fitness of F1 hybrids and parental phenotypic distance is expected (Prediction 2). In fact, when there are very few fixed deleterious alleles in one of the two parents, there will be less chances of complementation capable to provide a significant increase of fitness in the hybrids.
The outcome of hybridization cannot be restricted to the sole fitness of viable offspring. Allopatric populations might have become reproductively incompatible and thus their ability to produce offspring might be completely impaired. This can be the consequence of premating isolation mechanisms (temporal, ecological, or behavioral differentiation can result in failure in mate recognition/preference; Schluter 2001; Turelli et al. 2001; Servedio and Noor 2003); the final result of postmating prezygotic mechanisms (reduced viability or fertility of mothers; Edmands 2002; Servedio and Noor 2003); or the effect of severe postmating postzygotic mechanisms (Orr and Presgraves 2000). Such reproductive incompatibilities are thought to increase with the genetic divergence among the parental populations (e.g., Coyne and Orr 1989, 1997). The relationship between parental divergence, reproductive barriers, and the fitness of viable hybrids is still not clear.
To document this relationship and to test predictions associated with the ‘dominance’ hypothesis of heterosis, we realized a factorial crossing experiment using seven populations of the egg parasitoid wasp Trichogramma chilonis Ischii (Hymenoptera: Trichogrammatidae), a worldwide biocontrol agent against lepidopteran pests (Smith 1996). These minute parasitoid wasps (<1 mm) are effective against a variety of agricultural pests, including the ones attacking sugarcane, rice, cotton, hot pepper, sorghum, pigeon pea, sweet potatoes, sweet corn, cabbage, cauliflower, and turnip (e.g., Smith 1996; Waterhouse 1998; Miura 2003). They are easy to mass rear under laboratory conditions and they can be stored in the cold (Pitcher et al. 2002). Most importantly, they are able to efficiently find their hosts in the field (Boo and Yang 2000; Fatouros et al. 2008).
Trichogramma females oviposit inside the host's eggs, whose embryos are quickly killed. As for other Trichogramma species, parasitized eggs turn black. This visible modification offers the opportunity to easily estimate female realized fecundity without waiting for offspring's emergence (Reay-Jones et al. 2006; Tabone et al. 2010). Most of the known T. chilonis populations are haplodiploid, with males and females developing, respectively, from unfertilized and fertilized eggs. As a consequence, only the production of daughters indicates mating success. We thus considered the production of daughters as a proxy for mating compatibilities and parasitism efficiency as a proxy for fitness. In both cases, we evaluated the relevance of different predictors (i.e., genetic and/or phenotypic distance) to obtain realistic predictions that can be used as general guidelines for the genetic improvement of BCA. Infections by both Wolbachia and Cardinium, the two endosymbionts possibly involved in the creation of reproductive barriers in Arthropods (Engelstädter and Hurst 2009), were previously checked.
Material and methods
Trichogramma chilonis strains
For this experiment, we used seven populations originating from geographically distinct locations: China (C1 and C2), Japan (J), Pakistan (P), Réunion Island (R), Taiwan (T), and Vietnam (V). These strains were collected between 1987 and 2003 (see Table 1) and then maintained in standardized conditions in the laboratory. All strains were routinely raised on the substitution host Ephestia kuehniella Zeller (Lepidoptera: Pyradidae) in plastic tubes at about 18–20°C, 75 ± 10% R.H., and L16:D8 (see also Tabone et al. 2006, 2010). No morphological characters (either on genitalia or antennae) allow to distinguish one strain from another (B. Pintureau, pers. comm.).
Table 1.
Origin and original hosts of the Trichogramma chilonis populations used in the crosses
| Code | Origin | Host | Collection date |
|---|---|---|---|
| C1 | China | Unknown | Before 1996 |
| C2 | China | Unknown | 1996 |
| J | Japan | Plutella xylostella (Linnaeus) | 1999 |
| P | Pakistan | Helicoverpa armigera (Hübner) | 2003 |
| R | Réunion | Chilo sacchariphagus Bojer | 1998 |
| T | Taiwan | Ephestia kuehniella Zeller | 1987 |
| V | Vietnam | Unknown | 2000 |
Molecular characterization of Trichogramma chilonis strains and possible endosymbionts
The seven allopatric T. chilonis populations used for the crossing experiment as well as two additional populations (a second population from Réunion Island and one population from Hawaii) were characterized for the cytochrome c oxidase subunit 1 (COI) using the primers LCO 1490 (50-GGTCAACAAATCATAAAGATATTGG-30) and HCO 2198 (50-TAAACTTCAGGGTGACCAAAAAATCA-30) (see Cheyppe-Buchmann et al. 2011 for technical details). For each population, the PCR products from about 9 individuals were directly sequenced on both strands by Genoscreen (Lille, France). These sequences were submitted to GenBank. Alignments were then manually edited with BioEdit 7.01 (Hall 1999). Pairwise genetic distances were calculated using the p-distance with the software MEGA 4 (Tamura et al. 2007).
The presence of both Wolbachia and Cardinium was tested using, respectively, FtsZ-F (5′-TTGCAGAGCTTGGACTTGAA-3′) – FtsZ-R (5′-CATATCTCCG CCACCAGTAA-3′) (Cheyppe-Buchmann et al. 2011) and 16S-CFB-F (5′-GCGGTGTAAAATGAGCGT G-3′) – 16S-CFB-R (5′-ACC TMT TCT TAA CTC AAG CCT-3′) (Weeks et al. 2003). PCR were performed in a final volume of 10 μL using 1 μL of DNA template. Concentrations of MgCl2 in buffer, dNTPs, primers, and Qiagen Taq polymerase (QIAGEN S.A.S., Courtaboeuf, France) were 1.5 mm, 0.2 mm, 0.5 μm, and 1 U, respectively. PCR conditions were as follows: 94°C for 5 min, 35 cycles at 94°C for 30 s, 58°C (ftsZ locus) or 56°C (Cardinium locus) for 60 s, 72°C for 1 min, with a final step at 72°C for 10 min. The DNA fragment presences/sizes were determined with QIAxcel DNA Fast Analysis Kit (QIAGEN S.A.S) on the Qiaxcel analyzer. Positive controls of infection by Wolbachia and Cardinium were, respectively, provided by individuals of Psyttalia lounsburyi (Cheyppe-Buchmann et al. 2011) and Bemisia tabaci (provided by L. Mouton, UMR 5558 ‘Biométrie et Biologie Evolutive’ – Lyon I, France).
Crossing experiments
During 2009, the seven populations were used in a complete diallel cross-mating design, including all reciprocals (for a total of 49 crosses). The whole design was replicated five times, each replicate being treated as a block in the statistical analysis. For each cross within each block, at least five different couples were created, each of which maintained in a vial with a supply of E. kuehniella eggs for oviposition. For each couple, the mating success was assessed checking for the presence of viable daughters in the offspring. When present, two of the firstly emerged daughters were individually isolated for 24 h and provided with food. Most of them (624/628) were still alive at the end of this period and were allowed to infest E. kuehniella eggs for 7 days. The four dead females out of the total (0.6%) were excluded from the statistical analysis. To maintain an adequate supply of hosts, E. kuehniella eggs were renewed once during the experiment. The total number of black eggs produced by each daughter was used as a proxy of their fitness.
Mating compatibilities among seven Trichogramma chilonis parental strains
Production of viable daughters
For each cross within each block, we calculated the proportion of couple giving birth to at least one viable daughter. These values were analyzed using a generalized linear mixed-effects model (GLMM) with binomial error distribution and logit link function using the lme4 package in R (R Development Core Team 2011). Our fixed explanatory variable was the type of mating (homotype: both parents belonging to the same strain; heterotype: the two parents belonging to different strains). The geographical origin of each parent as well as the block was considered as random effects. Akaike Information Criterion (AIC; Akaike 1974) scores indicated that the model with all the above-mentioned random effects (and no interactions among factors) was the best, implying a significant effect of the block on the production of viable daughters. This effect was small compared to the other random effects and was because of one specific block (block 3: once this block was removed, there was no block effect).
Reproductive isolation
Because we performed all possible crosses using a similar number of trials in each cross (Nosil et al. 2002; Coyne et al. 2005; Pérez-Figueroa et al. 2005; Carvajal-Rodriguez and Rolán-Alvarez 2006), we were able to use three classical estimators (IPSI, IAPSI, and W) to measure sexual isolation (see Rolán-Alvarez and Caballero 2000; Pérez-Figueroa et al. 2005). Briefly, the IPSI index (Rolán-Alvarez and Caballero 2000; Carvajal-Rodriguez and Rolán-Alvarez 2006) is a cross-specific estimator used to calculate sexual isolation between parental strains. IPSI varies between -1 (mating occurs only between parents of different origin) and + 1 (mating occurs only between parents of the same origin), with 0 indicating random mating.
The IAPSI index (‘index of asymmetry’) quantifies the asymmetry of the mating compatibilities (♀x mated with ♂y versus ♀y mated with ♂x) (Carvajal-Rodriguez and Rolán-Alvarez 2006). Values significantly different from 1 (symmetry) indicate asymmetric mating compatibilities.
The ‘cross-product estimator’ W is a sex- and strain-specific measure of the relative mating success (sexual fitness) of each population with respect to the most successful one (set to 1) (Rolán-Alvarez and Caballero 2000; Carvajal-Rodriguez and Rolán-Alvarez 2006).
All indexes were estimated using the program JMATING (Carvajal-Rodriguez and Rolán-Alvarez 2006). Bootstrapping (10 000 iterations) was used to calculate the standard deviation of these parameters as well as their departures from relevant thresholds (0, 1, and 1 for IPSI, IAPSI, and W, respectively); zero values in the dataset were replaced by 0.5 to allow for bootstrap analysis (Coyne et al. 2005; Carvajal-Rodriguez and Rolán-Alvarez 2006). We analyzed all five blocks together.
The correlation between IPSI and the COI genetic matrix was tested using the Mantel test (Mantel 1967), following Giokas et al. (2006) and Schug et al. (2008); 1000 iterations were thus performed utilizing the PopTools software (Hood 2010).
Fitness of Trichogramma chilonis hybrids and parental strains
Realized fecundity
Depending on the mating compatibilities between the two parents, not all crosses produced viable daughters. Among the ones that did, we randomly picked just one daughter from each cross. In two crosses (R♀ × V♂ and P♀ × C1♂), viable daughters were produced in only one block out of five and these females were unable to parasitize hosts. These two crosses were thus excluded from the analysis. Using this final dataset, we performed a generalized linear model (Poisson error distribution and log link function) to assess the influence of the ‘cross’ (considered here as a fixed factor), the variable ‘block’ being treated as a random factor.
Relative fitness of hybrids
From the raw data of fecundity, we then calculated, for each block, the relative daughter's fitness using two estimators: (i) the best parent heterosis (BPH) defined here as the difference between the hybrid's mean fitness and the mean fitness of the best parent (BP); (ii) the lowest parent heterosis (LPH), defined as the difference between the hybrid's mean fitness and the mean fitness of the lowest parent (LP). An average of BPH and LPH for the five blocks is reported in Table 4. According to its relative fitness, each hybrid was tested either against BP or LP using unilateral paired t-tests to detect either heterosis or hybrid breakdown.
Table 4.
Average values of lowest and best parent heterosis (LPH and BPH) with standard error and P-values (P) obtained from unilateral paired t-tests
| Cross | No. blocks | LPH | SE | P | BPH | SE | P |
|---|---|---|---|---|---|---|---|
| C1 × C2 | 5 | 15.83 | 49.81 | 0.26 | −22.73 | 44.11 | 0.16 |
| C1 × J | 5 | 44.10 | 41.91 | 0.09 | −0.73 | 52.19 | 0.49 |
| C1 × P | 5 | 4.54 | 28.87 | 0.37 | −15.21 | 33.61 | 0.19 |
| C1 × R | 5 | 44.92 | 46.27 | 0.05 | 21.76 | 56.45 | 0.22 |
| C1 × T | 5 | 28.89 | 18.43 | 0.01* | −2.18 | 22.49 | 0.42 |
| C2 × C1 | 5 | 35.44 | 47.38 | 0.09 | −3.13 | 46.21 | 0.45 |
| C2 × J | 5 | 54.53 | 12.46 | 0.00* | −0.29 | 53.26 | 0.50 |
| C2 × P | 5 | 6.61 | 20.13 | 0.25 | −26.65 | 38.76 | 0.10 |
| C2 × R | 5 | 36.08 | 43.02 | 0.07 | −8.08 | 75.15 | 0.41 |
| C2 × T | 5 | 26.33 | 4.17 | 0.00* | −19.72 | 31.93 | 0.12 |
| C2 × V | 2 | 11.63 | 42.96 | 0.39 | −52.27 | 1.32 | 0.01* |
| J × C1 | 5 | 42.44 | 20.55 | 0.01* | −2.39 | 22.57 | 0.41 |
| J × C2 | 3 | 45.11 | 30.70 | 0.20 | −29.79 | 65.45 | 0.26 |
| J × P | 3 | 43.56 | 24.52 | 0.05 | 24.56 | 28.66 | 0.14 |
| J × R | 5 | 26.85 | 41.92 | 0.08 | 2.69 | 54.73 | 0.37 |
| J × T | 5 | 14.00 | 24.08 | 0.27 | −5.04 | 38.24 | 0.39 |
| J × V | 3 | 9.00 | 19.35 | 0.25 | −23.08 | 29.65 | 0.16 |
| P × C2 | 2 | −24.33 | 51.38 | 0.31 | −52.60 | 80.04 | 0.26 |
| P × J | 4 | 43.38 | 46.48 | 0.08 | 6.53 | 49.76 | 0.41 |
| P × V | 5 | −0.82 | 22.61 | 0.47 | −22.46 | 11.60 | 0.01* |
| R × C1 | 5 | 46.58 | 31.76 | 0.02* | 23.41 | 45.77 | 0.16 |
| R × C2 | 5 | 46.58 | 17.25 | 0.00* | 2.42 | 43.21 | 0.46 |
| R × J | 5 | 47.21 | 19.07 | 0.01* | 23.05 | 20.24 | 0.03** |
| R × P | 5 | −1.50 | 32.83 | 0.46 | −23.80 | 29.19 | 0.07 |
| R × T | 5 | 45.12 | 20.90 | 0.01* | 32.53 | 19.74 | 0.01* |
| T × C1 | 5 | 35.55 | 21.99 | 0.01* | 4.48 | 30.49 | 0.38 |
| T × C2 | 5 | 1.51 | 15.88 | 0.04* | −44.54 | 42.97 | 0.04* |
| T × J | 5 | 54.58 | 16.63 | 0.00** | 35.54 | 9.96 | 0.00** |
| T × P | 5 | −11.76 | 8.59 | 0.02** | −40.66 | 21.51 | 0.01* |
| T × R | 5 | 23.65 | 22.74 | 0.04* | 11.05 | 22.85 | 0.17 |
| V × J | 1 | −3.00 | – | – | −31.70 | – | – |
Asterisks distinguish cases where the statistical test is significant but without particular relevance with regard to hybrid's fitness (*) from those where either hybrid depression or hybrid vigor are observed (**).
Prediction of hybrid fitness
To test our first prediction associated with the ‘dominance’ hypothesis (see introduction), we used a GLMM with Poisson error distribution and log link function taking the hybrid's fecundity as the response variable and the logarithm of the BP's fecundity as an offset. For the ‘fixed’ part of the GLMM, we took into account a quadratic relationship between the response variable and the pairwise genetic distance between the two parents. For the ‘random’ part of the GLMM, the block and the geographical origin of both the father and the mother (nested in each term of the quadratic regression) were initially used as random variables. The best model, according to AIC and Bayesian information criterion (BIC) scores, a posteriori evidenced the relevance of the mother's population (nested in the second-order term of the genetic distance), the father's population, and the block as random variables.
To test the second prediction of the ‘dominance model’ (see introduction), we saved the residuals from the previous analysis. These residuals, which represent the part of the hybrid fitness that could not be explained in the GLMM by genetic distance, were used as a response variable in a linear regression using the pairwise phenotypic difference between parents as an explanatory variable.
Results
Molecular characterization of Trichogramma chilonis and possible endosymbionts
Eighty-one sequences of the COI locus were obtained (Accessions: JQ598687-JQ598767). They were distributed between five different haplotypes. Only one haplotype was found in each population, providing some evidences for low genetic diversity in each strain. Three populations, the two Chinese ones (C1 and C2) and the population from Taiwan (T), shared the same haplotype and were thus not differentiated one with another. Nucleotide divergence varied from 0% to 4%, with the populations from Vietnam and Pakistan being more distant from the others (data not shown).
A total of 93 individuals were tested for the presence of Wolbachia, the mean number of individuals/population ranging from 11 to 21. No amplification was detected for these individual, contrary to the positive controls. Similarly, 88 individuals were tested for the presence of Cardinium, the mean number of individuals/population ranging from 10 to 21. Only one positive amplification was observed for one individual of the Réunion Island strain, positive control being successfully amplified. As 10 individuals were tested in the population, we can conclude that the prevalence of this endosymbiont is low in this population. These results strongly suggest a low probability of induction of cytoplasmic incompatibility by endosymbionts in or between our strains.
Mating compatibilities among seven Trichogramma chilonis parental strains
The number of crosses producing at least one viable daughter was affected by the mating type (χ2 = 105.57; df = 1; P < 0.0001). Overall, 79% of homotypic crosses produced viable daughters while the rate dropped to 46% in heterotypic crosses (Table 2). Variance associated with each random variable was, respectively, 1.21, 0.28, and 0.06 for mother's origin, father's origin, and block. Observing the influence of parents’ origin, we can draw two main conclusions. Within the homotypic crosses, the lowest percentage of successful mating was observed for Japan (48%). Within heterotypic crosses, most of those involving parents from Vietnam (83%) or females from Pakistan (81%) were unsuccessful, As shown in Table 2, these tendencies were confirmed by IPSI and IAPSI values (used to detect global sexual isolation and asymmetric compatibilities, respectively).
Table 2.
Estimates of coefficients for joint sexual isolation (IPSI) and index of asymmetry (IAPSI) ± standard deviation for each cross obtained from bootstrapping analysis with JMATING
| C1♂ | C2♂ | J♂ | P♂ | R♂ | T♂ | V♂ | ||
|---|---|---|---|---|---|---|---|---|
| C1♀ | n (tot) | 22 (25) | 22 (25) | 14 (25) | 17 (25) | 17 (25) | 23 (26) | 0 (25) |
| IPSI | −0.01 ± 0.11 | 0.11 ± 0.13 | 0.57 ± 0.08* | 0.11 ± 0.11 | −0.02 ± 0.11 | 0.96 ± 0.04* | ||
| IAPSI | 1.01 ± 0.04 | 0.98 ± 0.08 | 4.30 ± 1.52* | 0.99 ± 0.06 | 0.99 ± 0.03 | 0.43 ± 0.62 | ||
| C2♀ | n (tot) | 20 (25) | 19 (25) | 15 (25) | 24 (25) | 21 (24) | 23 (26) | 1 (25) |
| IPSI | 0.26 ± 0.14 | 0.42 ± 0.11* | 0.01 ± 0.11 | −0.07 ± 0.11 | 0.94 ± 0.05* | |||
| IAPSI | 1.30 ± 0.41 | 3.21 ± 1.49* | 1.00 ± 0.04 | 1.00 ± 0.04 | 0.84 ± 0.83 | |||
| J♀ | n (tot) | 16 (25) | 7 (25) | 16 (33) | 7 (25) | 10 (24) | 14 (27) | 4 (28) |
| IPSI | 0.37 ± 0.13* | 0.15 ± 0.12 | 0.12 ± 0.13 | 0.81 ± 0.08* | ||||
| IAPSI | 0.89 ± 0.22 | 0.89 ± 0.12 | 0.99 ± 0.08 | 2.26 ± 1.13 | ||||
| P♀ | n (tot) | 1 (24) | 2 (25) | 10 (25) | 20 (25) | 0 (25) | 0 (26) | 16 (25) |
| IPSI | 0.64 ± 0.07* | 0.57 ± 0.07* | 0.64 ± 0.07* | |||||
| IAPSI | 0.09 ± 0.12* | 0.09 ± 0.12* | 5.25 ± 1.39* | |||||
| R♀ | n (tot) | 19 (25) | 20 (25) | 20 (25) | 14 (25) | 23 (25) | 22 (27) | 1 (25) |
| IPSI | 0.01 ± 0.11 | 0.94 ± 0.04* | ||||||
| IAPSI | 1.00 ± 0.04 | 0.82 ± 0.82 | ||||||
| T♀ | n (tot) | 22 (25) | 23 (25) | 15 (25) | 20 (25) | 21 (25) | 21 (26) | 0 (25) |
| IPSI | 0.96 ± 0.04* | |||||||
| IAPSI | 0.439 ± 0.62 | |||||||
| V♀ | n (tot) | 0 (25) | 0 (25) | 1 (32) | 0 (25) | 0 (25) | 0 (28) | 28 (33) |
n, number of successful matings (production of daughters); tot, total number of realized crosses.
Indicate significant difference from random mating.
Values of IPSI not significantly different from zero (indicating random mating) were observed in all heterotypic crosses, with the exclusion of all the crosses with the Vietnamese and Pakistani populations. In particular, heterotypic crosses involving one parent from Vietnam mostly resulted in complete sexual isolation (IPSI scores ≍ 1). We found a positive correlation between the IPSI and the pairwise genetic divergence obtained with the COI genetic marker (IPSI vs. COI: r = 0.78847, P = 0.029, N = 21, Fig. 1). While the population from Vietnam is almost completely sexually isolated, the reproductive barrier between the Pakistani and most of the other populations is asymmetric, as evidenced by the deviation of the asymmetric incompatibility index (IAPSI) from 1. The production of viable daughters was in fact reduced in all heterotypic crosses with Pakistani females.
Figure 1.
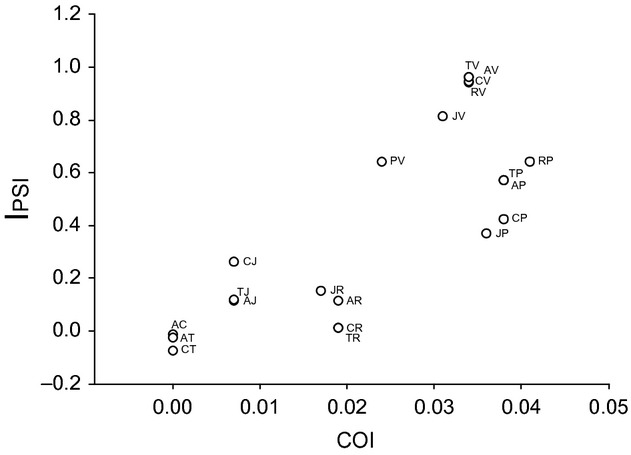
Relationship between mating incompatibility index (IPSI) and pairwise genetic distance obtained using the cytochrome c oxidase subunit 1 genetic marker (COI). Values of IPSI close to 1 indicate incompatible crosses.
The cross-product fitness estimators W (Table 3) confirmed low values of fitness for males and females from the Vietnamese lineage as well as for Pakistani females. It also evidenced the low fitness of Japanese females, which is not due to reproductive barriers but to an overall low performance. This trend was also observed assessing daughter's fecundity (see below).
Table 3.
Estimates of cross product estimator (W) ± standard deviation and corresponding P-values (P) for each sex and population obtained from bootstrapping analysis with JMATING
| Females | Males | |||
|---|---|---|---|---|
| Population | W | P | W | P |
| C1 | 0.97 ± 0.12 | 0.29 | 0.99 ± 0.14 | 0.46 |
| C2 | 1 | 0.92 ± 0.13 | 0.28 | |
| J | 0.57 ± 0.08* | 0.0001 | 0.83 ± 0.12 | 0.09 |
| P | 0.40 ± 0.07* | <0.0001 | 1 | |
| R | 0.96 ± 0.12 | 0.36 | 0.92 ± 0.13 | 0.27 |
| T | 0.99 ± 0.13 | 0.47 | 0.96 ± 0.13 | 0.36 |
| V | 0.21 ± 0.04* | <0.0001 | 0.47 ± 0.08* | <0.0001 |
Indicate statistical significance.
Fitness of Trichogramma chilonis hybrids and parental strains
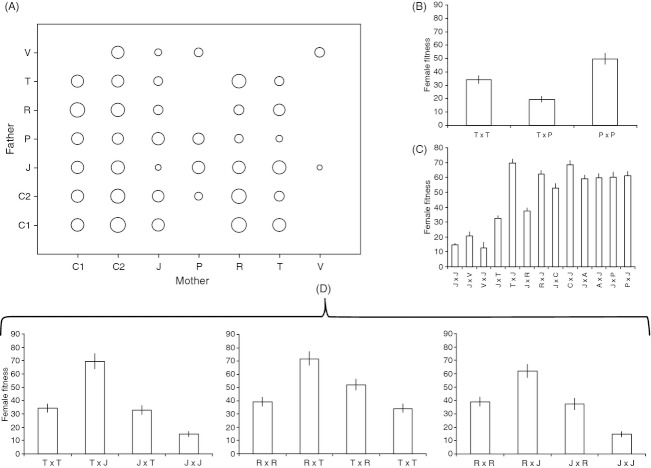
The fecundity of T. chilonis varied significantly among the seven parental populations with a magnitude of 3. The Chinese populations were indeed the most successful (average fecundity of about 65 parasitized hosts), while the Japanese population performed poorly, being able to parasitize an average of only 20 hosts in the same conditions (Fig. 2).
Figure 2.
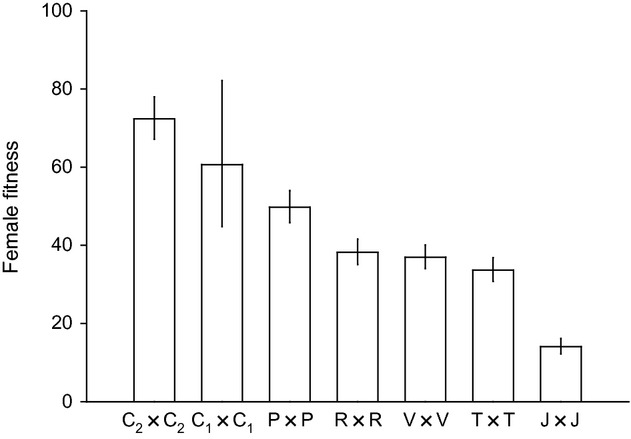
Genetic differentiation between the seven parental populations of Trichogramma chilonis. Estimates were obtained after fitting the most parsimonious generalized linear model to the observed data (see Material and methods). Error bars represent 95% confidence intervals.
The relative performances of hybrids with regard to their parents (Fig. 3a) were estimated using the BPH and LPH (Table 4). Among the 31 available hybrids, five crosses gave negative values of LPH, suggesting hybrid breakdown. Nevertheless, this trend was statistically confirmed only for one cross involving females from Taiwan with males from Pakistan (Fig. 3b). On the other end, nine crosses gave positive values of BPH suggesting heterosis. Significant differences between the hybrid and the BP were nevertheless significant for only three of them (Fig. 3d): (i) the cross between females from Réunion Island and males from Taiwan (R × T), with the same tendency being also observed in the reciprocal cross (T × R); (ii) the cross between females from Taiwan and males from Japan (T × J); (iii) the cross between females from Réunion Island and males from Japan (R × J). In the last two instances, it is important to note that the fecundity values of the reciprocal crosses (J × T and J × R) are close to those of the BPs (Fig. 3d).
Figure 3.
Composite figure illustrating the variable outcomes of hybridization on female fecundity. (A) Bubble plot representing the mean fecundity of F1 generation. Estimates were obtained from the most parsimonious generalized linear mixed-effects model (GLMM) (see text for details). Bubbles along the diagonal represent the homotypic crosses (parents from the same population) that were detailed in Fig. 2. (B) Hybrid breakdown induced by the crossing between a female of Taiwan and a male of Pakistan. Statistical tests are given in Table 4. The reciprocal cross was not available. (C) Inbreeding depression in the Japanese population. (D) Evidences for heterosis. Statistical tests are given in Table 4.
As shown in Fig. 3c, another interesting point was the lower fitness of the Japanese population compared to 11 out of 12 of its associated hybrids. This pattern, combined with the low fitness observed for Japanese females (cf. previous paragraph), corresponds to what may be expected in case of inbreeding depression.
The GLMM evidenced a significant relationship between the hybrid fitness and both the second-order (z = −3.039, P = 0.00237) of and first-order (z = −2.013, P = 0.044117) terms of regression with the pairwise genetic distance (Fig. 4). This confirms the curvilinear relationship hypothesized by the ‘dominance’ model. Variances associated with the random variables (block, father's origin, and mother's origin) were, respectively, 0.09, 0.11, and 0.04. Finally, we did find a negative relationship between the residuals of hybrid fecundity and the phenotypic difference between parents (R2 = 0.1198; F1,134 = 19.38 P < 0.0001; Fig. 5), in accordance with the second hypothesis formulated in the introduction.
Figure 4.
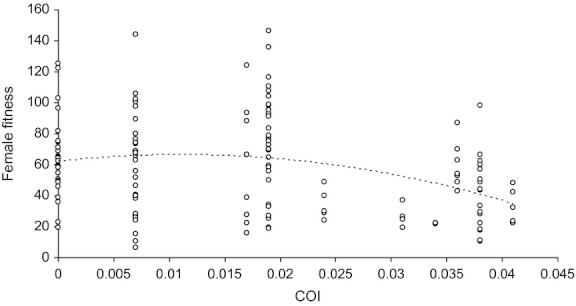
Relationship between F1 fitness and genetic distance between parents obtained using the cytochrome c oxidase subunit 1 genetic marker (COI). The line represents the quadratic effect of COI.
Figure 5.
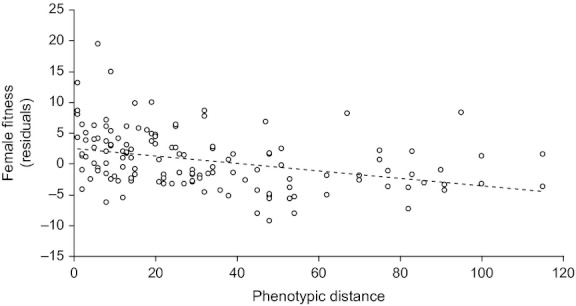
Relationship between F1 fitness [residual of previous generalized linear mixed-effects model (GLMM); see text for details] and phenotypic difference between parents.
Discussion
The phenotypic consequences of hybridization have always been subject of great debate in evolutionary biology (e.g., Arnold and Hodges 1995; Barton 2001; Burke and Arnold 2001). While there is no doubt that artificial hybridization has been successful for the genetic improvement of numerous agronomic resources, the benefits of hybridization in natural populations remain indeed controversial. Burke and Arnold (2001) reviewed many evidence of hybrid inferiority, describing numerous cases where hybrids are unviable, sterile, or less fit than their parents. These observations support the hypothesis that beneficial hybridization should be a rare phenomenon in the wild. Yet, successful cases of natural hybridization seem to be more widespread than initially thought and hybridization is now regarded as an important evolutionary process. This controversy may be partially explained by the diversity of taxa and genetic scales under investigation.
One particular field where the role of hybridization has been recently highlighted is invasion biology (Ellstrand and Schierenbeck 2000; Kolbe et al. 2004; Facon et al. 2005, 2008; Lavergne and Molofsky 2007; Rieseberg et al. 2007; Rosenthal et al. 2008; Schierenbeck and Ellstrand 2009; Keller and Taylor 2010). Hybrids with higher individual fitness and/or genetic variance than their parents are expected to adapt more successfully to novel conditions and/or heterogeneous environments (e.g., Facon et al. 2008). Although appealing, this hypothesis is difficult to be experimentally tested. Our approach utilizes crosses performed in the framework of a biological control program.
Experimental investigations are not only incredibly valuable to test hypothesis derived from invasion biology theory, but also beneficial to the overall practices of Research and Development in biological control (Cheyppe-Buchmann et al. 2011). In the particular case of hybridization, the ability to predict hybrid vigor could be pivotal to increase the success of classical biological control and also of other biological control strategies. Augmentative biological control (including here inoculative and inundative biocontrol sensu Eilenberg et al. 2001) is based on the regular releases of durable and massively produced BCA. To date, about 170 BCA species are commercialized worldwide (Cock et al. 2010), and although their total market value is far lower than other agronomic resources (e.g., cereal, cattle, and poultry), some of their biological features (short development time, intraspecific variability, etc.) offer opportunities for genetic improvement.
Unfortunately, lot of work still need to be carried out, particularly because not all parasitoids are easy to collect, rear, maintain under laboratory conditions, and manipulate in the field and laboratory. Moreover, it is also not always possible to sample populations covering the species’ distribution area (Santos and Quicke 2011), monitor their initial genetic features and successive modifications, evaluate their genetic background, and test for possibility of hybridization. Finally, we often lack ecological, life history, and behavioral data about the original populations in the wild (Hufbauer and Roderick 2005; Santos et al. 2011).
To our knowledge, the present study is the first experimental work specifically designed to test hypotheses on the outcome of hybridization in a BCA based on molecular and phenotypic distances. As demonstrated by the molecular characterization and the crossing experiment, the seven T. chilonis populations cover a broad range of intraspecific variability, with the population from Vietnam and to, a less extent, from Pakistan, being probably at the border between intra- and interspecific variability. At this genetic scale, we clearly evidenced the variability of the outcome of hybridization (Fig. 3). As a tendency, 16% of the available F1 had a lower mean fitness than their LP with a clear case of hybrid breakdown between the two populations of Pakistan and Taiwan. At the other extreme, 29% of the hybrids had a higher mean fitness than their parents with at least one remarkable case study of heterosis between Taiwan and Réunion Island populations. Another advantage of hybridization was the recovery of fitness observed in the hybrids obtained from the Japanese strain. This pattern strongly supports the hypothesis that the Japanese population suffers from inbreeding depression. However, it is not clear whether this situation represents the initial condition of the population in the field or the final condition after initiation or maintenance in the laboratory. More generally, each strain used in this study, as any captive population, may more or less rapidly diverge from its field source, limiting eco-evolutionary interpretations.
Independently from this relevant information that may be useful to improve the efficiency of T. chilonis as a BCA, this study offers the opportunity to test more in depth the role of ‘dominance’ as the main cause of heterosis. Globally, we confirmed the two main predictions associated with this model: (1) the curvilinear function between the hybrid performance and the molecular distance between the two parents, with an optimum of F1 fitness being observed for genetic distance of about 0.012 (Fig. 4); (2) the decreasing relationship between the hybrid performance and the phenotypic distance between the two parents (Fig. 5). These patterns rather confirm the pervasiveness of recessive and deleterious alleles in the seven T. chilonis populations under study. It is nevertheless not clear whether these alleles were already frequent in the wild or if their frequency has increased during the laboratory rearing. The extent to which such patterns are common for Hymenopteran parasitoids used as BCA remains to be tested but the ability to infer hybrid fitness without performing labor-intensive crosses could be of great practical value for further genetic improvement programs.
In addition to the information on the hybrid's phenotype, we were also able to investigate the formation of reproductive barriers in relation to genetic divergence. We observed various levels of mating compatibilities with the two extreme cases of Vietnam (almost complete isolation) and Pakistan (asymmetric isolation), which were not related to the presence of cytoplasmic incompatibility inducers. These reproductive barriers are thought to be the consequence of the divergence of either premating (Schluter 2001; Turelli et al. 2001; Servedio and Noor 2003) or postmating prezygotic mechanisms (Edmands 2002; Servedio and Noor 2003). As a consequence, the degree of reproductive incompatibilities is predicted to increase with increased genetic divergence among allopatric populations (e.g., Coyne and Orr 1989, 1997). This pattern was clearly confirmed here and sexual isolation became important at genetic distance of about 0.025 for our marker (Fig. 1). It is noteworthy that this threshold is higher than the optimum found for the hybrid's performance (Fig. 3), suggesting that the production of heterotic hybrids is likely to occur as soon as the two parental populations are (naturally or artificially) put into contacts.
Taken as a whole, this study clearly underlines the complex consequences of hybridization in a biological control agent. Selecting effective BCA is a difficult process per se (Hoelmer and Kirk 2005; van Lenteren et al. 2006; Barratt et al. 2010). Their genetic improvement can be even more challenging. In addition to experimental studies (or even better, in concomitance with these experimental studies), we recommend researcher to theorize and test relevant hypotheses associated with reproductive isolation and hybrid's performance. It would take only a little extra effort to test hypotheses while performing laboratory crosses. It seems to us that this approach, mixing theoretical and practical goals, should be considered as the best way to progressively build guidelines for the genetic improvement of BCA whatever the underlying strategies (classical, augmentative, or even conservation biological control).
Acknowledgments
This research was supported by the French Agency ANR (project BIOINV4I – ANR-06-BDIV-008 – Coordinator: Thomas Guillemaud) and the Scientific Department ‘Santé des Plantes et Environnement’ of INRA (grant 2008-1254-01). The authors want to thank Fadel Al Khatib (Institute Sophia-Agrobiotech) as well as Laurence Mouton (UMR 5558 ‘Biométrie et Biologie évolutive', Lyon I, France) for their contribution to the molecular work on T. chilonis and the analysis on endosymbionts.
Data archiving statement
Data for this manuscript available at Dryad: doi 10.5061/dryad.kc423.
References
- Akaike H. A new look at the statistical model identification. IEEE Transaction on Automatic Control. 1974;19:716–723. [Google Scholar]
- Arnold ML, Hodges SA. Are natural hybrids fit or unfit relative to their parents? Trends in Ecology & Evolution. 1995;10:67–71. doi: 10.1016/S0169-5347(00)88979-X. [DOI] [PubMed] [Google Scholar]
- Bai C, Shapiro-Ilan DI, Gaugler R, Hopper KR. Stabilization of beneficial traits in Heterorhabditis bacteriophora through creation of inbred lines. Biological Control. 2005;32:220–227. [Google Scholar]
- Baker DA, Loxdale HD, Edwards OR. Genetic variation and founder effects in the parasitoid wasp, Diaeretiella rapae (M'intosh) (Hymenoptera: Braconidae: Aphidiidae), affecting its potential as a biological control agent. Molecular Ecology. 2003;12:3303–3311. doi: 10.1046/j.1365-294x.2003.02010.x. [DOI] [PubMed] [Google Scholar]
- Barratt BIP, Howarth FG, Withers TM, Kean JM, Ridley GS. Progress in risk assessment for classical biological control. Biological Control. 2010;52:245–254. [Google Scholar]
- Barton NH. The role of hybridization in evolution. Molecular Ecology. 2001;10:551–568. doi: 10.1046/j.1365-294x.2001.01216.x. [DOI] [PubMed] [Google Scholar]
- Bateson P. Optimal outbreeding. In: Bateson P, editor. Mate Choice. Cambridge: Cambridge University Press; 1983. pp. 257–277. [Google Scholar]
- Benvenuto C, Cheyppe-Buchmann S, Bermond G, Ris N, Fauvergue X. Intraspecific hybridization, life history strategies and potential invasion success in a parasitoid wasp. Evolutionary Ecology. (In press) DOI 10.1007/s10682-011-9553-z. [Google Scholar]
- Boo KS, Yang JP. Kairomones used by Trichogramma chilonis to find Helicoverpa assulta eggs. Journal of Chemical Ecology. 2000;26:359–375. [Google Scholar]
- Burke JM, Arnold ML. Genetics and the fitness of hybrids. Annual Review of Genetics. 2001;35:31–52. doi: 10.1146/annurev.genet.35.102401.085719. [DOI] [PubMed] [Google Scholar]
- Carvajal-Rodriguez A, Rolán-Alvarez E. JMATING: a software for the analysis of sexual selection and sexual isolation effects from mating frequency data. Bmc Evolutionary Biology. 2006;6:40. doi: 10.1186/1471-2148-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaston JM, Dillman AR, Shapiro-Ilan DI, Bilgrami AL, Gaugler R, Hopper KR, Adams BJ. Outcrossing and crossbreeding recovers deteriorated traits in laboratory cultured Steinernema carpocapsae nematodes. International Journal for Parasitology. 2011;41:801–809. doi: 10.1016/j.ijpara.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyppe-Buchmann S, Bon MC, Warot S, Jones W, Malausa T, Fauvergue X, Ris N. Molecular characterization of Psyttalia lounsburyi, a candidate biocontrol agent of the olive fruit fly, and its Wolbachia symbionts as a pre-requisite for future intraspecific hybridization. BioControl. 2011;56:713–724. [Google Scholar]
- Cock MJW, Brodeur JC, van Lenteren J, Barratt BIP, Bigler F, Bolckmans K, Cônsoli FL, et al. Do new access and benefit sharing procedures under the convention on biological diversity threaten the future of biological control? BioControl. 2010;55:199–218. [Google Scholar]
- Coors JG, Pandey S. Genetics and Exploitation of Heterosis in Crops. Madison, WI: Crop Science Society of America; 1999. [Google Scholar]
- Coyne JA, Orr HA. Patterns of speciation in Drosophila. Evolution. 1989;43:362–381. doi: 10.1111/j.1558-5646.1989.tb04233.x. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. “Patterns of speciation in Drosophila” revisited. Evolution. 1997;51:295–303. doi: 10.1111/j.1558-5646.1997.tb02412.x. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Elwyn S, Rolan-Alvarez EL. Impact of experimental design on Drosophila sexual isolation studies: direct effects and comparison to field hybridization data. Evolution. 2005;59:2588–2601. [PubMed] [Google Scholar]
- Crow JF. 90 years ago: the beginning of hybrid maize. Genetics. 1998;148:923–928. doi: 10.1093/genetics/148.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. Genetics of natural populations. XIX. Origin of heterosis through natural selection in populations of Drosophila pseudoobscura. Genetics. 1950;35:288–302. doi: 10.1093/genetics/35.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmands S. Does parental divergence predict reproductive compatibility? Trends in Ecology & Evolution. 2002;17:520–527. [Google Scholar]
- Ehler LE. Invasion biology and biological control. Biological Control. 1998;13:127–133. [Google Scholar]
- Eilenberg J, Hajek A, Lomer C. Suggestions for unifying the terminology in biological control. BioControl. 2001;46:387–400. [Google Scholar]
- Ellstrand NC, Schierenbeck KA. Hybridization as a stimulus for the evolution of invasiveness in plants? Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7043–7050. doi: 10.1073/pnas.97.13.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelstädter J, Hurst GDD. The ecology and evolution of microbes that manipulate host reproduction. Annual Review of Ecology and Systematics. 2009;40:127–149. [Google Scholar]
- Facon B, Jarne P, Pointier JP, David P. Hybridization and invasiveness in the freshwater snail Melanoides tuberculata: hybrid vigour is more important than increase in genetic variance. Journal of Evolutionary Biology. 2005;18:524–535. doi: 10.1111/j.1420-9101.2005.00887.x. [DOI] [PubMed] [Google Scholar]
- Facon B, Pointier JP, Jarne P, Sarda V, David P. High genetic variance in life-history strategies within invasive populations by way of multiple introductions. Current Biology. 2008;18:363–367. doi: 10.1016/j.cub.2008.01.063. [DOI] [PubMed] [Google Scholar]
- Fagan WF, Lewis MA, Neubert MG, van den Driessche P. Invasion theory and biological control. Ecology Letters. 2002;5:148–157. [Google Scholar]
- Falconer DS. Introduction to Quantitative Genetics. London: Longman; 1981. [Google Scholar]
- Fatouros NE, Dicke M, Mumm R, Meiners T, Hilker M. Foraging behavior of egg parasitoids exploiting chemical information. Behavioral Ecology. 2008;19:677–689. [Google Scholar]
- Fauvergue X, Hopper KR. French wasps in the new world: experimental biological control introductions reveal a demographic Allee effect. Population Ecology. 2009;51:385–397. [Google Scholar]
- Geleta LF, Labuschagne MT, Viljoen CD. Relationship between heterosis and genetic distance based on morphological traits and AFLP markers in pepper. Plant Breeding. 2004;123:467–473. [Google Scholar]
- Giokas S, Mylonas M, Rolán-Alvarez E. Disassociation between weak sexual isolation and genetic divergence in a hermaphroditic land snail and implications about chirality. Journal of Evolutionary Biology. 2006;19:1631–1640. doi: 10.1111/j.1420-9101.2006.01115.x. [DOI] [PubMed] [Google Scholar]
- Hall T. Bioedit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/nt. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hansen MM, Olivieri I, Waller DM, Nielsen EE, The GeM Working Group Monitoring adaptive genetic responses to environmental change. Molecular Ecology. 2012;21:1311–1329. doi: 10.1111/j.1365-294X.2011.05463.x. [DOI] [PubMed] [Google Scholar]
- Hoelmer KA, Kirk AA. Selecting arthropod biological control agents against arthropod pests: can the science be improved to decrease the risk of releasing ineffective agents? Biological Control. 2005;34:255–264. [Google Scholar]
- Hoffmann JH, Impson FAC, Volchansky CR. Biological control of cactus weeds: implications of hybridization between control agent biotypes. Journal of Applied Ecology. 2002;39:900–908. [Google Scholar]
- Hood GM. PopTools version 3.2.3. 2010. Available on the internet. http://www.poptools.org.
- Hopper KR, Roush RT, Powell W. Management of genetics of biological-control introductions. Annual Review of Entomology. 1993;38:27–51. [Google Scholar]
- Hoy MA. Genetic improvement of insects: fact or fantasy. Environmental Entomology. 1976;5:833–836. [Google Scholar]
- Hoy MA. Recent advances in genetics and genetic improvement of the Phytoseiidae. Annual Review of Entomology. 1985;30:345–370. [Google Scholar]
- Hoy MA, Knop NF. Selection for and genetic analysis of permethrin resistance in Metaseiulus occidentalis: genetic improvement of a biological control agent. Entomologia Experimentalis et Applicata. 1981;30:10–18. [Google Scholar]
- Hoy MA, Westigard PH, Hoyt SC. Release and evaluation of a laboratory selected, pyrethroid resistant strain of the predaceous mite Metaseiulus occidentalis (Acari: Phytoseiidae) in Southern Oregon pear orchards and a Washington apple orchard. Journal of Economic Entomology. 1983;76:383–388. [Google Scholar]
- Hufbauer RA, Roderick GK. Microevolution in biological control: mechanisms, patterns, and processes. Biological Control. 2005;35:227–239. [Google Scholar]
- Keller SR, Taylor DR. Genomic admixture increases fitness during a biological invasion. Journal of Evolutionary Biology. 2010;23:1720–1731. doi: 10.1111/j.1420-9101.2010.02037.x. [DOI] [PubMed] [Google Scholar]
- Kolbe JJ, Glor RE, Rodríguez Schettino L, Larson CLAA, Losos JB. Genetic variation increases during biological invasion by a Cuban lizard. Nature. 2004;431:177–181. doi: 10.1038/nature02807. [DOI] [PubMed] [Google Scholar]
- Krystkowiak K, Adamski T, Surma M, Kaczmarek Z. Relationship between phenotypic and genetic diversity of parental genotypes and the specific combining ability and heterosis effects in wheat (Triticum aestivum L.) Euphytica. 2009;165:419–434. [Google Scholar]
- Lavergne S, Molofsky J. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3883–3888. doi: 10.1073/pnas.0607324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legner EF. Phenotypic expressions of polygenes in Muscidifurax raptorellus Hym, Pteromalidae, a synanthropic fly parasitoid. Entomophaga. 1989;34:523–530. [Google Scholar]
- Legner EF. Theory for quantitative inheritance of behavior in a protelean parasitoid, Muscidifurax raptorellus (Hymenoptera, Pteromalidae) European Journal of Entomology. 1993;90:11–21. [Google Scholar]
- van Lenteren JC, Bale J, Bigler E, Hokkanen HMT, Loomans AM. Assessing risks of releasing exotic biological control agents of arthropod pests. Annual Review of Entomology. 2006;51:609–634. doi: 10.1146/annurev.ento.51.110104.151129. [DOI] [PubMed] [Google Scholar]
- Li X, Yang G, Shi Y, Cong Y, Che S, Qu S, Li Z. Prediction of the heterosis of Laminaria hybrids with the genetic distance between their parental gametophyte clones. Journal of Applied Phycology. 2008;20:1097–1102. [Google Scholar]
- Luan F, Sheng Y, Wang Y, Staub JE. Performance of melon hybrids derived from parents of diverse geographic origins. Euphytica. 2010;173:1–16. [Google Scholar]
- Lynch M. The genetic interpretation of inbreeding depression and outbreeding depression. Evolution. 1991;45:622–629. doi: 10.1111/j.1558-5646.1991.tb04333.x. [DOI] [PubMed] [Google Scholar]
- Mackauer M. Genetic aspects of insect production. Entomophaga. 1972;17:27–48. [Google Scholar]
- Mackauer M. Genetic problems in the production of biological control agents. Annual review of Entomology. 1976;21:269–385. [Google Scholar]
- Malausa J-C, Blanchet A, Bon MC, Cheyppe-Buchmann S, Groussier-Bout G, Jones W, Pickett C, et al. Introduction of the African parasitoid Psyttalia lounsburyi in south of France for the classical biological control of Bactrocera oleae: will hybridization affect establishment and population growth? IOBC/WPRS Bulletin. 2010;53:49–55. [Google Scholar]
- Mantel N. The detection of disease clustering and a generalized regression approach. Cancer Research. 1967;27:209–220. [PubMed] [Google Scholar]
- Marsico TD, Burt JW, Espeland EK, Gilchrist GW, Jamieson MA, Lindström L, Roderick GK, et al. Underutilized resources for studying the evolution of invasive species during their introduction, establishment, and lag phases. Evolutionary Applications. 2010;3:203–219. doi: 10.1111/j.1752-4571.2009.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathenge CW, Holford P, Hoffmann JH, Zimmermann HG, Spooner-Hart RN, Beattie GAC. Hybridization between Dactylopius tomentosus (Hemiptera: Dactylopiidae) biotypes and its effects on host specificity. Bulletin of Entomological Research. 2010;100:331–338. doi: 10.1017/S0007485309990344. [DOI] [PubMed] [Google Scholar]
- Messing RH, Croft BA. Biosystematics of Amblyseius-Andersoni and a-Potentillae (Acarina, Phytoseiidae) – implications for biological-control. Experimental & Applied Acarology. 1991;10:267–278. [Google Scholar]
- Miura K. Suppressive effect of the egg parasitoid Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae) on the population density of the diamondback moth. Applied Entomology and Zoology. 2003;38:79–85. [Google Scholar]
- Moll RH, Lonnquist JH, Vélez Fortuno J, Johnson EC. The relationship of heterosis and genetic divergence in maize. Genetics. 1965;52:139–144. doi: 10.1093/genetics/52.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukuka J, Strauch O, Hoppe C, Ehlers R-U. Fitness of heat and desiccation tolerant hybrid strains of Heterorhabditis bacteriophora (Rhabditidomorpha: Heterorhabditidae) Journal of Pest Science. 2010a;83:281–287. [Google Scholar]
- Mukuka J, Strauch O, Hoppe C, Ehlers R-U. Improvement of heat and desiccation tolerance in Heterorhabditis bacteriophora through cross-breeding of tolerant strains and successive genetic selection. BioControl. 2010b;55:511–521. [Google Scholar]
- Müller-Schärer H, Schaffner U. Classical biological control: exploiting enemy escape to manage plant invasions. Biological Invasions. 2008;10:859–874. [Google Scholar]
- Nosil P, Crespi BJ, Sandoval CP. Host-plant adaptation drives the parallel evolution of reproductive isolation. Nature. 2002;417:440–443. doi: 10.1038/417440a. [DOI] [PubMed] [Google Scholar]
- Orr HA, Presgraves DC. Speciation by postzygotic isolation: forces, genes and molecules. BioEssays. 2000;22:1085–1094. doi: 10.1002/1521-1878(200012)22:12<1085::AID-BIES6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Pérez-Figueroa A, Caballero A, Rolan-Alvarez E. Comparing the estimation properties of different statistics for measuring sexual isolation from mating frequencies. Biological Journal of the Linnean Society. 2005;85:307–318. [Google Scholar]
- Pitcher SA, Hoffmann MP, Gardner J, Wright MG, Kuhar TP. Cold storage of Trichogramma ostriniae reared on Sitotroga cerealella eggs. BioControl. 2002;47:525–535. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. ISBN 3-900051-07-0. http://www.R-project.org/ [Google Scholar]
- Reay-Jones FPF, Rochat J, Goebel R, Tabone E. Functional response of Trichogramma chilonis to Galleria mellonella and Chilo sacchariphagus eggs. Entomologia Experimentalis Et Applicata. 2006;118:229–236. [Google Scholar]
- Riday H, Brummer EC, Campbell TA, Luth D, Cazcarro PM. Comparisons of genetic and morphological distance with heterosis between Medicago sativa subsp sativa and subsp falcata. Euphytica. 2003;131:37–45. [Google Scholar]
- Rieseberg LH, Kim SC, Randell RA, Whitney KD, Gross BL, Lexer C, Clay K. Hybridization and the colonization of novel habitats by annual sunflowers. Genetica. 2007;129:149–165. doi: 10.1007/s10709-006-9011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick GK, Navajas M. Genes in new environments: genetics and evolution in biological control. Nature Reviews Genetics. 2003;4:889–899. doi: 10.1038/nrg1201. [DOI] [PubMed] [Google Scholar]
- Rolán-Alvarez E, Caballero M. Estimating sexual selection and sexual isolation effects from mating frequencies. Evolution. 2000;54:30–36. doi: 10.1111/j.0014-3820.2000.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal DM, Ramakrishnan AP, Cruzan MB. Evidence for multiple sources of invasion and intraspecific hybridization in Brachypodium sylvaticum (Hudson) Beauv. in North America. Molecular Ecology. 2008;17:4657–4669. doi: 10.1111/j.1365-294X.2008.03844.x. [DOI] [PubMed] [Google Scholar]
- Roush RT, Hoy MA. Genetic improvement of Metaseiulus occidentalis: selection with methomyl, dimethoate, and carbaryl and genetic analysis of carbaryl resistance. Journal of Economic Entomology. 1981a;74:138–141. [Google Scholar]
- Roush RT, Hoy MA. Laboratory, glasshouse, and field studies of artificially selected carbaryl resstance in Metaseiulus occidentalis. Journal of Economic Entomology. 1981b;74:142–147. [Google Scholar]
- Sadras V, Calderini D. Crop Physiology – Applications for Genetic Improvement and Agronomy. Elsevier Inc: Academic Press; 2009. [Google Scholar]
- Salame L, Glazer I, Chubinishvilli MT, Chkhubianishvili T. Genetic improvement of the desiccation tolerance and host-seeking ability of the entomopathogenic nematode Steinernema feltiae. Phytoparasitica. 2010;38:359–368. [Google Scholar]
- Santos AMC, Quicke DLJ. Large-scale diversity patterns of parasitoid insects. Entomological Science. 2011;14:371–382. [Google Scholar]
- Santos AMC, Besnard G, Quicke DLJ. Applying DNA barcoding for the study of geographical variation in host-parasitoid interactions. Molecular Ecology Resources. 2011;11:46–59. doi: 10.1111/j.1755-0998.2010.02889.x. [DOI] [PubMed] [Google Scholar]
- Schierenbeck KA, Ellstrand NC. Hybridization and the evolution of invasiveness in plants and other organisms. Biological Invasions. 2009;11:1093–1105. [Google Scholar]
- Schluter D. Ecology and the origin of species. Trends in Ecology & Evolution. 2001;16:372–380. doi: 10.1016/s0169-5347(01)02198-x. [DOI] [PubMed] [Google Scholar]
- Schug MD, Baines JF, Killon-Atwood A, Mohanty S, Das A, Grath S, Smith SG, et al. Evolution of mating isolation between populations of Drosophila ananassae. Molecular Ecology. 2008;17:2706–2721. doi: 10.1111/j.1365-294X.2008.03770.x. [DOI] [PubMed] [Google Scholar]
- Segal D, Glazer I. Genetics for improving biological control agents: the case of entomopathogenic nematodes. Crop Protection. 2000;19:685–689. [Google Scholar]
- Servedio MR, Noor MAF. The role of reinforcement in speciation: theory and data. Annual Review of Ecology Evolution and Systematics. 2003;34:339–364. [Google Scholar]
- Shapiro DI, Glazer I, Segal D. Genetic improvement of heat tolerance in Heterorhabditis bacteriophora through hybridization. Biological Control. 1997;8:153–159. [Google Scholar]
- Shapiro-Ilan DI, Stuart RJ, McCoy CW. Targeted improvement of Steinernema carpocapsae for control of the pecan weevil, Curculio caryae (Horn) (Coleoptera: Curculionidae) through hybridization and bacterial transfer. Biological Control. 2005;34:215–221. [Google Scholar]
- Smith SM. Biological control with Trichogramma: advances, successes, and potential of their use. Annual Review of Entomology. 1996;41:375–406. doi: 10.1146/annurev.en.41.010196.002111. [DOI] [PubMed] [Google Scholar]
- Strauch O, Oestergaard J, Hollmer S, Ehlers RU. Genetic improvement of the desiccation tolerance of the entomopathogenic nematode Heterorhabditis bacteriophora through selective breeding. Biological Control. 2004;31:218–226. [Google Scholar]
- Syed NH, Chen ZJ. Molecular marker genotypes, heterozygosity and genetic interactions explain heterosis in Arabidopsis thaliana. Heredity. 2005;94:295–304. doi: 10.1038/sj.hdy.6800558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szűcs M, Schwarzlander M, Gaskin JF. Reevaluating establishment and potential hybridization of different biotypes of the biological control agent Longitarsus jacobaeae using molecular tools. Biological Control. 2011;58:44–52. [Google Scholar]
- Tabone E, Bardon C, Pintureau B, Alauzet C. Importance of host oviposition pattern and plant size for the selection of Trichogramma strains to control the diamondback moth. Entomologia Experimentalis Et Applicata. 2006;119:47–51. [Google Scholar]
- Tabone E, Bardon C, Desneux N, Wajnberg E. Parasitism of different Trichogramma species and strains on Plutella xylostella L. on greenhouse cauliflower. Journal of Pest Science. 2010;83:251–256. [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Teklewold A, Becker HC. Comparison of phenotypic and molecular distances to predict heterosis and F1 performance in Ethiopian mustard (Brassica carinata A. Braun) Theoretical and Applied Genetics. 2006;112:752–759. doi: 10.1007/s00122-005-0180-3. [DOI] [PubMed] [Google Scholar]
- Turelli M, Barton NH, Coyne JA. Theory and speciation. Trends in Ecology & Evolution. 2001;16:330–343. doi: 10.1016/s0169-5347(01)02177-2. [DOI] [PubMed] [Google Scholar]
- Waser NM. Population structure, optimal outbreeding, and assortative mating in Angiosperms. In: Thornhill NW, editor. The Natural History of Inbreeding and Outbreeding. Chicago, IL: University of Chicago Press; 1993. pp. 173–199. [Google Scholar]
- Waterhouse DF. Biological Control of Insect Pests: Southeast Asian Prospects. Canberra, Australia: ACIAR Monograph, No 51, Australian Centre for International Agricultural Research; 1998. [Google Scholar]
- Weeks AR, Velten R, Stouthamer R. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proceedings. Biological Sciences/The Royal Society. 2003;270:1857–1865. doi: 10.1098/rspb.2003.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willi Y, Van Buskirk J. Genomic compatibility occurs over a wide range of parental genetic similarity in an outcrossing plant. Proceedings. Biological Sciences/The Royal Society. 2005;272:1333–1338. doi: 10.1098/rspb.2005.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Ni X, Jiang K, Deng H, He Q, Yang Q, Yang L, et al. Relationship between heterosis and parental genetic distance based on molecular markers for functional genes related to yield traits in rice. Rice Science. 2010;17:288–295. [Google Scholar]