Abstract
In classical weed biological control, small collections of arthropods are made from one or a few sites in the native range of the target plant and are introduced to suppress the plant where it has become invasive, often across a wide geographic range. Ecological mismatches in the new range are likely, and success using the biocontrol agent may depend on postrelease evolution of beneficial life history traits. In this study, we measure the evolution of critical day length for diapause induction (day length at which 50% of the population enters dormancy), in a beetle (Diorhabda carinulata) introduced into North America from China to control an exotic shrub, Tamarix spp. Beetle populations were sampled from four sites in North America 7 years after introduction, and critical day length was shown to have declined, forming a cline over a latitudinal gradient At one field site, decreased critical day length was correlated with 16 additional days of reproductive activity, resulting in a closer match between beetle life history and the phenology of Tamarix. These findings indicate an enhanced efficacy and an increasingly wider range for D. carinulata in Tamarix control.
Keywords: adaptation, biocontrol, life history, phenology evolution, photoperiodism
Introduction
In classical biological control, specialized natural enemies (biological control agents) are collected from the native range of an invasive species (the target pest) and introduced into the new range, where management of the target is the goal. Ideally, the agent will experience a rapid population growth and become widespread in the new range of the target, decreasing pest densities to help achieve management goals. These circumstances fit those under which contemporary evolution has most often been documented, that is, in colonizing species undergoing rapid population growth (Reznick and Ghalambor 2001). Contemporary or rapid evolution occurs on ecological timescales, within tens of generations or fewer (Hairston et al. 2005; Carroll et al. 2007), and is no longer considered unusual, with numerous documented examples (Hendry and Kinnison 1999; Reznick and Ghalambor 2001). Because of this, an evolutionary framework is being incorporated into management of biological systems including conservation biology, natural resources management, and agriculture (Hendry et al. 2011; Lankau et al. 2011; Thrall et al. 2011), and scientists are garnering increased support for the incorporation of evolutionary principles into the practice of biological control (Roderick and Navajas 2003; Hufbauer and Roderick 2005).
The role of evolution in the practice of biological control is still being defined, with the safety and efficacy of biological control dependent on the nature and degree of postintroduction evolution (Hufbauer and Roderick 2005). The primary safety concern is that host range may evolve following the release of the agent, and the agent may have a negative impact on species not targeted for control. This has not been seen in weed biological control. There are instances of host use patterns shifting among plants within the fundamental host range (all plants that can be used by an agent) but evolution of fundamental host range has not been detected in weed biological control agents (van Klinken and Edwards 2002), and the stability of the fundamental host range is one reason for the excellent safety record of weed biocontrol (Pemberton 2000). Likewise, there is little evidence that evolution has been part of adaptation to new ranges that would increase postrelease efficacy of weed biological control agents.
The frequency and patterns of evolution in biological control thus remain largely unknown with few data with which to define a role for evolution in biocontrol (Roderick and Navajas 2003; Hufbauer and Roderick 2005). Evaluating biological control agents for the evidence of evolution will be an important part of applying evolutionary principles to biological control. In addition, the records available to researchers concerning release numbers, location, and population origin will provide a more complete information base for biological control organism than is usually available for introduced species (Marsico et al. 2010), making biological control agents potential model organisms for the study of evolution. The widespread occurrence of the invasive weed Tamarix spp. in North America (Friedman et al. 2005) and the recent introduction and success of an herbivore to control it (DeLoach et al. 2004) have provided ecological conditions under which the evolution of life history traits may occur, including those involved in phenological synchrony of the agent with the target plant.
Tamarisks (Tamarix spp.) are non-native shrubs that have become widely distributed in wetlands and riparian areas of the western United States (Friedman et al. 2005) where they are considered serious pests with many detrimental economic and ecological impacts (Shafroth et al. 2005). Tamarisks comprise at least five species of woody shrubs and their hybrids (Gaskin and Schaal 2003), which are difficult and expensive to control using conventional methods (O’Meara et al. 2010). The size and geographic scope of the infestation and cost of management make Tamarix an appropriate target for biological control (DeLoach et al. 2003). The first biocontrol agent introduced was the northern tamarisk beetle Diorhabda carinulata (Desbrochers) from central Asia (DeLoach et al. 2003). Beetles were initially released into the open in 2001 at seven test sites located in six western states (DeLoach et al. 2003) and subsequently redistributed to locations across the western United States, making biocontrol one of the primary options for tamarisk management (Tracy and Robbins 2009; O’Meara et al. 2010).
The collections of D. carinulata used for the biocontrol program were made in northwestern China near the city of Fukang (Fukang biotype) and in Kazakhstan near the town of Chilik (Chilik biotype) (DeLoach et al. 2003, 2004). Both collection sites are located at about 44°N latitude in a dry continental climate zone. In North America, initial releases of the northern tamarisk beetle established, and populations expanded in northern areas with similarly dry continental climates but introductions failed south of the 38th parallel (Lewis et al. 2003). One reason for the failure to establish at southern locations was the premature induction of reproductive diapause early in the summer at lower latitude sites, whereas at northern sites they entered diapause during mid- to late summer (Lewis et al. 2003; Bean et al. 2007a). During diapause in this species, reproductive development is arrested, and adults cease feeding and descend from the host plant into the leaf litter where they remain until the following season (Bean et al. 2007b).
The Fukang biotype of D. carinulata was shown to enter diapause primarily in response to day lengths shorter than 14 h 39 min in the field, with temperature having only a minor influence on diapause induction (Bean et al. 2007a). The day length of 14 h 39 min is the critical day length (CDL) for diapause induction defined as the genetically determined day length at which 50% of a population enters diapause and 50% remain in a state of continuous development (Beck 1980; Saunders 2002). Photoperiod is the most reliable cue for anticipating seasonal change and avoiding fatal temperatures or starvation (Beck 1980; Saunders 2002). It is common for insects to use photoperiod as a cue for the temporal organization of life history events (phenology) (Tauber et al. 1986; Danks 1987). One disadvantage of photoperiod as a biological cue is that the annual cycles in photoperiod vary across latitudes so that a photoperiod pattern that is a reliable indicator of seasonal change at one latitude becomes less reliable as a function of distance from that latitude. This is what happened with D. carinulata; the farther south insects were released from the latitude of origin in China (44.17°N) the less synchronized they were to local environmental conditions, including climate and host plant phenology. The mismatch could be traced to a CDL that was inappropriate for the latitude (Bean et al. 2007a; Dalin et al. 2010).
During the first few years of the tamarisk biocontrol program, from 2001 to 2004, newly introduced beetles continued to enter diapause when day lengths receded to <14 h 39 min. As a result, populations entered diapause in mid-August at the northernmost biocontrol release site located near Lovell, WY, 44.84°N, which corresponds closely to the latitude of origin for the Fukang biotype of D. carinulata. At a more southern site near Bishop, CA (37.08°N), a caged beetle population entered diapause in mid-July (Bean et al. 2007a). The timing of diapause induction enabled beetles to cease feeding and avoid the senescence of Tamarix foliage at the northern site, but at the southern site beetles entered diapause when green foliage remained available for at least 2 more months, long enough for the insects to have undergone an additional generation in the field (Lewis et al. 2003; Herrera et al. 2005; Bean et al. 2007a), which implied a fitness loss caused by premature diapause (Taylor and Spaulding 1988). In addition to curtailing population expansion, premature diapause may have caused the diapausing individuals to deplete metabolic stores and subjected them to high levels of predation in the leaf litter while temperatures remained warm (Bean et al. 2007a). Beetle phenology became increasingly mismatched with Tamarix phenology moving south from the latitude of beetle origin, and the phenology mismatch restricted the useful range of this species as a tamarisk biocontrol agent (Lewis et al. 2003; Bean et al. 2007a; Dalin et al. 2010).
In the summer and fall of 2005 (i.e., 4 years after the initial release), D. carinulata were observed to be reproductively active later in the season than they had been previously (T. Dudley and D. Eberts, US Bureau of Reclamation, personal observations), and this pattern was noted at two of the initial field release sites, one at 40.02°N and the other at 38.27°N. These observations led us to hypothesize that the CDL for diapause induction was evolving under the selection pressure brought about by the phenology mismatch of D. carinulata populations with host plant availability and local climate.
Introduced species are good subjects for the investigation of the evolution of life history traits, including CDL, in the new ecological settings they encounter (Tauber et al. 1986). The evolution of CDL for diapause induction has been observed or inferred for a number of insect species including species that have become agricultural pests after their unintentional introduction (Beck 1980; Tauber et al. 1986; Danks 1987) as well as species responding to environmental changes in their native range (Bradshaw and Holzapfel 2001). The evolution of CDL has not been documented in insects released for weed biological control although the practical importance of evolution in biocontrol systems has been well recognized, including the potential importance of adaptation to local environments (Hufbauer and Roderick 2005). In the case of D. carinulata, the evolution of a more appropriate CDL could greatly increase the usefulness of the beetle as a biocontrol agent by allowing beetles to be active later into the season and by enabling range expansion into more southern regions (Dalin et al. 2010). Adaptation could also create management concerns as beetles move into the areas previously considered outside the potential range of D. carinulata (Bateman et al. 2010; Dudley and Bean 2012).
In this study, we measure CDL for diapause induction in four populations of D. carinulata, all originating from the same source population, that have been collected from field sites across a latitudinal gradient from 44.84°N to 33.00°N. We measure CDL of populations under laboratory conditions, and we also measure beetle phenology at one site where phenology had been measured 5 years earlier (Bean et al. 2007a). The findings will help in the prediction of the potential range and efficacy of D. carinulata in North America and support the formulation of policies and recommendations regarding its use for tamarisk suppression.
Materials and methods
Study organisms and field sites
The taxonomy of the tamarisk-feeding Diorhabda has been recently revised with Diorhabda elongata (Brullé) deserticola (Chen) elevated to species Diorhabda carinulata, one of the five sibling Diorhabda species known to specialize on tamarisks in North Africa and Eurasia (Tracy and Robbins 2009). D. carinulata collected from two sites in Asia have provided the starting populations for all initial tamarisk biological control projects in North America (DeLoach et al. 2003). Beetles used in these studies were descendents of beetles that originated from the collections of fewer than 1000 beetles each made in northwestern China, near the city of Fukang (44.17°N; 87.98°E) in 1997 and 1998 (DeLoach et al. 2003, 2004).
To establish laboratory populations, 150–200 adult beetles were collected from field sites in Lovell, Wyoming (LWY, Fig. 1), in August 2007; Lovelock, Nevada (LNV, Fig. 1), in November 2007; Pueblo, Colorado (PCO, Fig. 1), in August, 2007, and Artesia New Mexico (ANM, Fig. 1), in July, 2007. Thus, the beetles had been exposed to local environmental conditions for at least 7 years except in the case of beetles collected at the ANM site. Beetles from that site had been in the field at the LNV site for 5 years and then introduced at the ANM site where they were exposed to local conditions for an additional 2 years. Temperature data were compiled for the four field sites with mean monthly and average high and low July, August, and September temperatures averaged from 1975 to 2011 and shown in Table 1. Temperature data were from weather stations in Lovell, WY, for the LWY site, Lovelock Derby Field, NV, for the LNV site, Pueblo Reservoir, CO, for the PCO site, and Artesia 6 s, NM, for the ANM site. Data were obtained from http://weather-warehouse.com.
Figure 1.
Map of the western United States showing D. carinulata collection locations in Lovell, Wyoming, Lovelock, Nevada, Pueblo, Colorado, and Artesia, New Mexico with latitudes shown next to each collection location.
Table 1.
Mean temperatures and mean high and low temperatures during July, August, and September at the four field sites used in this study. The difference between mean high and low is also given
| July | August | September | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Field Site | °N Lat. | Mean | High | Low | Diff. | Mean | High | Low | Diff. | Mean | High | Low | Diff |
| Lovell, Wyoming | 44.84 | 21.0 | 30.6 | 12.9 | 17.7 | 20.2 | 29.4 | 10.9 | 18.5 | 14.2 | 23.2 | 5.2 | 18.0 |
| Lovelock, Nevada | 40.02 | 24.3 | 35.4 | 13.3 | 22.1 | 22.6 | 33.8 | 11.4 | 22.4 | 17.9 | 28.9 | 6.9 | 22.0 |
| Pueblo, Colorado | 38.27 | 24.9 | 34.0 | 15.8 | 18.2 | 23.4 | 32.1 | 14.7 | 17.4 | 18.8 | 28.0 | 9.5 | 18.5 |
| Artesia New Mexico | 33.00 | 26.3 | 34.5 | 18.1 | 16.4 | 25.5 | 33.8 | 17.2 | 16.6 | 21.5 | 30.1 | 12.8 | 17.3 |
Beetles were held for at least one generation under laboratory conditions that allow continuous growth and reproduction (16:8 L:D, 25–27°C) before they were taken from stock cultures for use in experiments. This was carried out to minimize possible maternal effects on development, although no such effects had been noted in the previous studies (Bean et al. 2007a,b). Beetles were reared on fresh tamarisk foliage as previously described (Bean et al. 2007b) and used in experiments after one to five generations in the laboratory.
Diapause induction studies
Insects taken from stock cultures were transferred as eggs or newly hatched first instar larvae to constant temperature or thermoperiods with set photoperiods in programmable incubators (Model I30BLL; Percival, Perry, IA). Larvae were mass-reared in groups of at least 50 and transferred as prepupae to sand as a pupation substrate. Newly emerged adults were paired, and single pairs were moved to plastic cups and fed ad libitum with fresh Tamarix foliage. The number of pairs tested at each photoperiod and temperature ranged from 25 to 103 except in four instances where experimental day lengths were shorter than the CDL and no reproductive activity was measured, 7–19 pairs were tested. Paired adults were kept under experimental conditions for 20 days after emergence, and if no oviposition occurred, the insects were scored as ‘diapause.’ This protocol is based on the previous trials indicating that under a photoperiod of 16:8 (L:D) and 25°C, all females oviposit within the first 8 days after adult emergence (Bean et al. 2007b). In some experiments, diapausing insects were dissected after 20 days to verify developmental status (Bean et al. 2007b).
Photoperiod and temperature
Experimental photoperiods ranged from 13 h 30 min:10 h 30 min (L:D) to 15 h 10 min: 8 h 50 min (L:D) and were varied in 20-min increments. These day lengths were chosen because they bracketed the CDL for these populations (Bean et al. 2007a). In all treatments, photoperiod was held constant for the duration of the experiment.
Constant temperature experiments were performed at 25°C, which is near the mean July temperature at the four field sites, which ranged from 21.0 to 26.3°C (Table 1). Thermoperiods with amplitudes 10 and 20°C and an average of 25°C were superimposed on test photoperiods. Thermoperiods were structured to have the 9-h thermophase (either 30 or 35°C) occur during the photophase and the 9-h cryophase (either 20 or 15°C) occur during the scotophase. Transitions between cryophase and thermophase were programmed in steps, and the transitions took three hours. Steps were either a 1°C rise every 20 min (for the 10°C amplitude) or a 2°C rise every 20 min (for the 20°C amplitude) and were initiated when lights came on in the morning (lights on). Temperatures were programmed to decline by either 1°C every 20 min (for the 10°C amplitude) or 2°C every 20 min (for the 20°C amplitude) starting at 12 h after lights on, and reaching the scotophase temperature 15 h after lights on. Thermoperiods were structured so that the temperature did not begin to increase until lights on, peak temperature was reached during the middle of the photophase (day) and temperatures declined late in the photophase (equivalent to mid- to late afternoon), and temperatures were low (cryophase) during the night (scotophase). Thermoperiods with 20°C amplitude are typical of summer temperature patterns in the interior western United States (Bean et al. 2007a) as seen in differences between monthly mean high and low temperatures at the four field sites used in this study, which ranged between 16.6 and 22.4°C (Table 1).
The incidence of diapause in relation to photoperiod and temperature treatments was compared among populations using logistic regressions (PROC GENMOD, binominal, probit, type 3 analyses; SAS Institute 2008). The developmental pathway (diapause or reproductively active) of adults was the dependent binary response variable whereas Population, Photoperiod and Temperature were the independent nominal predictor variables. Two-way interaction between Population and Photoperiod was used to test for variation in photoperiodic responses among beetle populations. Similarly, we tested for two-way interactions between Population and Temperature, and between Temperature and Photoperiod. However, because of a lack of degrees of freedom, we were unable to test for potential three-way interaction of Population × Photoperiod × Temperature.
Linear regression models (PROC PROBIT INVERSECL; SAS Institute 2008) were used to estimate the critical photoperiod; i.e. the day length when there is a 50% probability of diapause, including 95% confidence intervals, by calculating the predicted 50% value for each population under each temperature treatments (Dalin et al. 2010). To conduct post hoc tests of variation among populations in photoperiod responses, we used similar logistic regressions described above (PROC GENMOD, binominal, probit, type 3 analyses) by comparing each population against the others under two of the three temperature treatments: the constant 25°C temperature treatment and the 10°C (30–20°C; average 25°C) amplitude thermoperiod treatment. Diapause incidence was the dependent binary response variable and Population and Photoperiod the independent variables. We did not conduct any post hoc tests for the higher amplitude thermoperiod treatment (35–15°C; average 25°C) because we were unable to rear all populations under the same photoperiod treatments. The purpose of the experiment was to characterize CDL under the different temperature treatments. Because we expected increased divergence of CDL with thermoperiod amplitude, we reared the southern populations under shorter day lengths than the northern populations and, thus, did not include the complete experimental design for the highest thermoperiod amplitude.
Determination of field CDL
Adult beetles were sampled weekly from the PCO field site (38.27°N) from mid-July to late August during the summer of 2008. They were collected from multiple tamarisk plants and at several locations within the field site for each sampling date. Beetles were held on ice and eventually frozen prior to analysis. Individuals were scored either as in diapause or as reproductive based on the condition of the reproductive systems and the fat body (Bean et al. 2007b). In some cases where an individual had neither fat body reserves nor reproductive development, it was not scored and did not enter into CDL calculations (Bean et al. 2007a). The date of diapause induction for the population was estimated using logistic regression analysis (JMP8, SAS Institute 2008). Reproductive status was the binary response, dependent variable (0 for diapause, 1 for reproductive) plotted against ordinal date. Inverse prediction was used to calculate the ordinal date at which there is a 0.50 probability of diapause along with 95% confidence limits.
Field critical day length was calculated by subtracting 13 days from the date of a 0.50 diapause probability, and determining day length on that calendar date using standard astronomical tables (Bean et al. 2007a). This formula was based on two observations. First was that insects required 10–20 days to switch from a reproductive state to a diapause state following photoperiod switches under laboratory conditions, from 16:8 (L:D) (under which beetles are reproductive) to 14:10 (L:D) or 14.5:9.5 (L:D) (shorter than the CDL for diapause induction) (Bean et al. 2007a). The second was direct; beetle populations reached 50% diapause at five widely separate field sites 13 days after day lengths had been 14 h 39 min. These observations allowed us to estimate the lag between perception of diapause-inducing photoperiods in the field, and the induction of diapause (Bean et al. 2007a).
Results
Logistic regression analyses revealed significant effects of Population, Photoperiod, and Temperature treatments on diapause incidence in D. carinulata (Table 2). There was a significant Population × Photoperiod interaction (Table 2), suggesting that beetles responded differently to photoperiod depending on population origin. The thermoperiod treatments resulted in reduced diapause incidence compared with the constant (25°C) temperature treatment. The nonsignificant Population × Temperature interaction (Table 2) suggests that all populations responded similarly to thermoperiod treatments. Below, we present CDL estimations for each of the four beetle populations under each of the temperature treatments and conduct separate post hoc regressions to reveal which populations differed from each other.
Table 2.
Effects of population origin (population), photoperiod and temperature on diapause incidence in Diorhabda carinulata released for the biological control of Tamarix spp. in western United States*
| Effect | χ2 | df | P |
|---|---|---|---|
| Population | 197.02 | 3 | <0.001 |
| Photoperiod | 44.57 | 5 | <0.001 |
| Temperature | 169.33 | 1 | <0.001 |
| Population × Photoperiod | 48.67 | 12 | <0.001 |
| Population × Temperature | 0.39 | 3 | 0.942 |
| Photoperiod × Temperature | 6.58 | 3 | 0.087 |
Results were obtained using logistic regression (binominal, logit, type 3).
CDL under constant temperature (25°C) in four populations of D. carinulata
The CDL measured at constant temperature (25°C) varied between 15.13 and 14.67 h for the four populations of D. carinulata (Table 3). The LWY population had the longest CDL (15.13); identical to that originally measured in D. carinulata (Fukang biotype) (Table 3). The other three populations had shorter CDLs which descended in order of the latitude of origin for the experimental source population (Table 3). Post hoc comparisons revealed that the two northernmost (LWY versus LNV) populations had similar photoperiod responses (Table 3; nonsignificant Population × Photoperiod interaction). The two southernmost (PCO versus ANM) populations also had similar photoperiod responses (Table 3), whereas all other population comparisons revealed significant Population and Population × Photoperiod interactions (df = 1; χ2 > 4.60; P < 0.04).
Table 3.
CDL of field-collected populations when reared under three different temperature treatments
| Temperature | Collection site | CDL (in h) | ±95% CI |
|---|---|---|---|
| 25°C | Original* | 15.13 | 15.06–15.23 |
| LWY | 15.13 | 15.07–15.21a | |
| LNV | 14.94 | 14.89–14.98a | |
| PCO | 14.88 | 14.80–14.95b | |
| ANM | 14.67 | 14.60–14.75b | |
| 30–20°C | Original | na | na |
| LWY | 15.00 | 14.96–15.04a | |
| LNV | 14.69 | 14.62–14.75b | |
| PCO | 14.85 | 14.75–15.03b | |
| ANM | 14.51 | 14.45–14.57c | |
| 35–15°C | Original† | 14.88 | 14.78–15.01 |
| LWY | 14.71 | 14.64–14.78 | |
| LNV | 14.16 | 14.08–14.24 | |
| PCO | 14.48 | 14.42–14.53 | |
| ANM | 13.96 | 13.90–14.03 |
ANM, Artesia New Mexico; LNV, Lovelock, Nevada; LWY, Lovell, Wyoming; PCO, Pueblo, Colorado.
Original refers to the laboratory colony derived from field-collected insects that came from three locations in western Nevada and eastern California as previously described (Bean et al. 2007a).
Original determination was made using a thermoperiod with 18°C amplitude, 34–16°C, with a 25°C average (Bean et al. 2007a).
Superscript alphabets indicate significant differences between CDLs within temperature treatments (P < 0.04).
CDL under thermoperiods in four populations of D. carinulata
CDLs measured under thermoperiods with amplitude of either 10°C (30–20°C) or 20°C (35–15°C) were consistently shorter than at constant 25°C (Table 3). Under the 10°C (30–20°C) amplitude thermoperiod treatment, the two northernmost populations (LWY versus LNV) responded differently to photoperiod for diapause induction with a shorter CDL in the LNV population (Table 3; Population × Photoperiod interaction: df = 1; χ2 = 25.99; P < 0.001). The two southernmost (PCO versus ANM) also showed significant differences (Table 3; Population × Photoperiod interaction: df = 1; χ2 > 6.0; P < 0.02), whereas the two central populations LNV and PCO were similar under these conditions (Table 3; nonsignificant Population × Photoperiod interaction).
CDLs measured at the higher amplitude thermoperiod (20°C) were shorter than those measured at the lower amplitude thermoperiod (10°C) in all four populations. The 95% confidence intervals did not overlap between populations within test amplitudes or within a population between the two amplitudes (Table 3). However, because the populations were reared under different photoperiods in the highest amplitude thermoperiod treatment, we were unable to analyze CDL responses statistically (see Materials and methods). The LWY strain had a slightly shorter CDL at the high amplitude thermoperiod than that measured in the original population although the original measurements were made with amplitude of 18°C instead of 20°C (Bean et al. 2007a). Because greater thermoperiod amplitudes induce the shorter CDLs (Table 3), the LWY CDL value is likely closer to the original CDL than indicated in Table 3, which would be consistent with the 25°C results where CDL was identical between LWY and the original laboratory culture.
Diapause induction and CDL in the field
The D. carinulata population at the PCO site entered diapause August 10, 2008 (ordinal date 223), which was 16 days later than they had entered diapause at the same site in 2003 (ordinal date 207) (Fig. 2). In the earlier study of D. carinulata, it was found that field CDL was best predicted by subtracting 13 days from the date when 50% of the population was in diapause, and using the day length on that date as the field critical day length (Bean et al. 2007a). The 13-day delay was thought to be the lag time between perception of the critical day length and detectable manifestation of the diapause developmental pathway (Bean et al. 2007a). Day length on August 10, 2008 was 13 h 48 min while day length 13 days before, on July 28, 2008, was 14 h 14 min. In the original studies, beetles entered diapause on July 26, 2003, when day length was 14 h 18 min, while 13 days before, on July 13, 2003, day length was 14 h 37 min. These data indicate that field critical day length for the Pueblo population (PCO) decreased by 23 min, from 14 h 37 min to 14 h 14 min, between 2003 and 2008. Data from laboratory studies show that the PCO population had a CDL of 14 h 29 min (14.48 h) under a thermoperiod with 20°C amplitude (Table 3), while the original measurements carried out in 2001–2003 using the source population showed a CDL of 14 h 53 min (14.88 h) under a thermoperiod with 18°C amplitude (Bean et al. 2007a). The 24-min decline in CDL, measured under laboratory conditions, is in agreement with the 23-min decline in CDL measured in the field.
Figure 2.
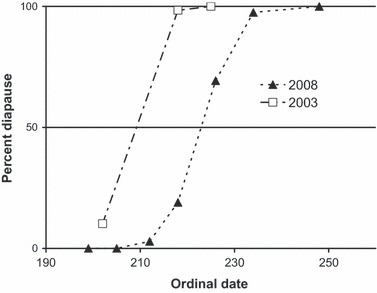
Diapause incidence in the field at the Pueblo, Colorado site during the summer of 2008. The 2003 values are from Bean et al. 2007a and are shown for comparison. The population reached 50% diapause on ordinal day 223 (August 10, 2008), while in 2003, the population reached 50% diapause on day 207 (July 26).
Postintroduction evolution of CDL shifts reproductive activity later into the season
At all four study sites, CDL measured under a thermoperiod with 20°C amplitude has decreased relative to the CDL originally measured in this population (Table 4). The shift is inversely proportional to the latitude of the field site (Fig. 3) and moved the predicted date of 50% diapause in the field back from July into August at two of the field sites and added an extra 5 days of reproductive activity in August at the northernmost field site (Table 5). At the southernmost field site, the original CDL would have constrained the population to a univoltine life history, but following the decrease in CDL, multiple generations are now possible (Fig. 5). Evolution has resulted in a latitudinal CDL cline in which CDL is inversely proportional to the latitude of origin (Fig. 3).
Table 4.
CDL changes since the open field introduction of D. carinulata into North America
| Population | CDL (35–15°C) | Difference (2008 minus original determination*) |
|---|---|---|
| Lovell, Wyoming | 14.71 | −0.16 (10 min shorter) |
| Lovelock, Nevada | 14.16 | −0.72 (43 min shorter) |
| Pueblo, Colorado | 14.48 | −0.41 (25 min shorter) |
| Artesia New Mexico | 13.96 | −0.90 (54 min shorter) |
| Pueblo, Colorado (field) | 14.23 | −0.38 (23 min shorter)† |
The original determination of CDL was made under a thermoperiod with 18°C amplitude and an average temperature of 25°C, while the CDL measured in 2008 was under a thermoperiod with amplitude 20°C. The CDL was originally measured at 14.88 h (14 h 53 min).
The original determination of field CDL was made from data taken during the summer of 2003 at the Pueblo site and was 14.62 h (14 h 37 min) (Bean et al. 2007a).
Figure 3.
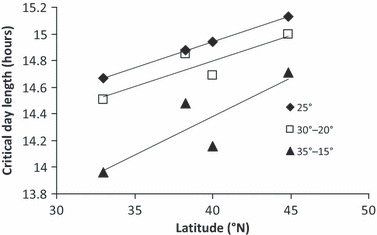
The relationship between CDL measured under three temperature regimes, and latitude at the four field sites in North America. For constant 25°C, y = 0.0388x + 13.392, R2 = 0.997; for a thermoperiod 30–20°C, y = 0.0386x + 13.257, R2 = 0.8011; for a thermoperiod 35–15°C, y = 0.058x + 12.064, R2 = 0.7246.
Table 5.
Predicted change in the date of field CDL based on CDL values measured under laboratory conditions*
| Predicted date for 50% diapause in the field† | ||||
|---|---|---|---|---|
| Field site | CDL | With original CDL | CDL from this study | Difference (days shifted) |
| LWY | 14.71 | August 14 | August 19 | 5 |
| LNV | 14.16 | July 31 | August 24 | 25 |
| PCO | 14.48 | July 24 | August 11 | 18 |
| ANM | 13.96 | Univoltine‡ | August 14 | – |
| PCO (field)§ | 14.23 | July 26 | August 10 | 15 |
ANM, Artesia New Mexico; LNV, Lovelock, Nevada; LWY, Lovell, Wyoming; PCO, Pueblo, Colorado.
CDL was originally measured at 14.88 h under controlled conditions, while field CDL was measured at 14.65 h based on the average at five field sites (Bean et al. 2007b). Field CDL is calculated by subtracting 0.23 h (14 min) from laboratory-measured CDL.
These are predicted dates given the CDL measured in the laboratory for each population. The original CDL predictions had already been made for field sites LWY, LNV, and PCO and agreed closely with actual CDLs measured in the field (Bean et al. 2007a).
Day lengths never reach 14.65 h at the ANM site, so using the original CDL, there would be no reproduction in the first summer generation, making insects at this site univoltine.
These data are taken from field measurements carried out in 2003 and again in 2008 and represent actual developmental status in the field. Field measurements closely match laboratory based predictions in 2003 (July 24 versus July 26, Bean et al. 2007a) and again in 2008 (August 10 versus August 11, see Fig. 2).
Discussion
The critical day length for diapause induction has evolved over 5 years (about 10 generations) in the populations of D. carinulata introduced to control tamarisk. The preadapted CDL had restricted the range of D. carinulata in North America by affecting beetle phenology, putting it out of synchrony with tamarisk phenology and climate at lower latitudes (Lewis et al. 2003; Bean et al. 2007a; Dalin et al. 2010). The pattern of evolutionary change has resulted in the rapid formation of a cline in CDL that is proportional to the latitudinal gradient across which beetles have been introduced (Fig. 3). This is a pattern common to other insect species, reflecting adaptation to latitudinal gradients in the seasonal timing of developmentally permissive temperatures and host plant availability (Tauber et al. 1986), but the dynamics of cline formation have not been this closely monitored in other species.
Average temperatures at the four field sites increase with decreasing latitude, providing longer periods with permissive temperatures for beetle development at lower latitudes (Table 1). Tamarisk foliage remains green longer at more southern latitudes (Dalin et al. 2010), and it has been shown that a cline in the genetically programmed senescence date of tamarisk has developed along a latitudinal gradient, mostly likely through evolution following the introduction of tamarisks into North America (Friedman et al. 2011). At all four sites, the shift in CDL (Table 4) will extend the reproductive activity of beetles later into the season (Table 5) and enable them to better utilize locally available host plant foliage and temperatures permissive for continuous development. Shifting diapause later into the season will also decrease the amount of time beetles are exposed to warm summer conditions while dormant in the leaf litter.
At the site of origin near Fukang, D. carinulata is trivoltine and the adults of the third generation emerge in mid-August to enter diapause (Li et al. 2000; Lewis et al. 2003) a pattern consistent with a field CDL of 14 h 40 min. In North America, initial studies indicated a bivoltine life cycle at the successful release sites and a univoltine or partially bivoltine life cycle at sites where beetles did not establish, below the 38th parallel (Lewis et al. 2003; Bean et al. 2007a). In a study initiated in 2006, beetles were transferred from the LNV site to more southern latitudes including the PCO site, as well as sites below the 38th parallel (35.5°N and 35.4°N), and at all test locations, they were found to be bivoltine (Dalin et al. 2010), indicating sufficient CDL evolution to shift life history patterns. At the LVN site, beetles had been bivoltine in 2003–2004 with newly emerged second generation adults entering diapause after the beginning of August (Bean et al. 2007a). They have since shifted toward a trivoltine life history with second generation reproductively active beetles emerging in early to mid-August (T. Dudley and D. Bean personal observations). The recruitment of additional generations of D. carinulata at sites in North America is one consequence of shortened CDLs.
The original laboratory measurements of CDL in D. carinulata (Bean et al. 2007a) provided initial values for CDL in North America and divergence from those values, measured under controlled laboratory conditions, can be attributed to evolution. Initial measurements of CDL were made within 2–4 years after collection in China, and the laboratory cultures used for these measurements were derived from beetles collected from experimental cages at two field sites or from open field collections from the LNV site (Bean et al. 2007a). These collections were made in 2001–2002 before major population growth and dispersal. Given the short North American field history of the insects, we feel that evolution was minimal prior to adding beetles to stock cultures, and any incipient gene frequency changes would have been homogenized using beetles from three different sites. In addition, under field conditions, the beetles entered diapause when day lengths were shorter than 14 h 39 min at five widely separated field sites including three used in this study (PCO, LNV and LWY), which is an indication that CDLs were initially equivalent in all field populations. For these reasons, we believe the initial studies measured the state of the CDL in the introduced population of D. carinulata, and this study has measured evolution as divergence through time from the initial state, making this is an allochronic study (Hendry and Kinnison 1999). We now have two sets of CDL measurements, separated by at least five field seasons (about 10 generations) as reference points for future studies of CDL evolution in this population.
The contemporary evolution of CDL in D. carinulata occurred in spite of the fact that the starting population was collected from a single location in China and probably subjected to genetic bottlenecks in the prerelease phases of the program. This example fits the larger paradox that contemporary evolution is most frequently measured in colonizing populations (Reznick and Ghalambor 2001), which may have experienced genetic bottlenecks, reducing the genetic variation needed for evolution (Nei et al. 1975). On the other hand, colonizing populations may result from multiple introductions from geographically diverse sources, maintaining or even increasing genetic variation relative to populations in the native range (Frankham 2005; Dlugosch and Parker 2008a; Hufbauer 2008); yet, even within a single-source small introduction, there may be sufficient genetic variation to enable evolution (Dlugosch and Parker 2008b). Additive genetic variance, critical for quantitative trait heritability including life history traits, is resistant to loss of variation during genetic bottlenecks because additive variation is less dependent on rare alleles, and furthermore, there may be a conversion of epistasis into additive variance during bottlenecks (Cheverud and Routman 1996; Dlugosch and Parker 2008b) although the importance of the second point is still being evaluated (Turelli and Barton 2006).
The CDL for diapause induction is a quantitative life history trait shown to be under polygenic control (Tauber et al. 1986), and genetic and molecular genetic studies have indicated that CDL has a complex underlying mechanism (Emerson et al. 2009). CDL for diapause induction is then an example of a life history trait that may be less susceptible to the loss of variance following bottlenecking with laboratory and field examples to illustrate this point. Underlying genetic variation in photoperiodically induced diapause induction, reflected in response to artificial selection, is often retained after long periods in culture where loss of genetic variation would be expected (reviewed in Tauber et al. 1986). For instance, in laboratory populations of the fly Calliphora vicina, selection experiments have shown that photoperiodically induced diapause incidence remained a selectable trait even in a population that had been in culture for 10 years (Saunders 2001). In a number of species, CDL evolution has been measured or inferred in the field following colonization (Beck 1980; Tauber et al. 1986; Danks 1987) and while multiple introductions may have boosted genetic variance in some of these species other examples, including three discussed below, have occurred in genetically restricted populations.
The fall webworm Hyphantria cunea was first discovered in Japan in 1945 at a single location and has since expanded its range to 32°N in the south and 40°N in the north of Japan (Gomi 1997). In the process the moth, which was initially bivoltine, has become trivoltine in southern Japan, and the CDL for diapause induction has decreased (Gomi 1997, 2007). The CDL shift in the webworm has been rapid, with a 14-min decline occurring between 1995 and 2002 at one location (Gomi et al. 2007). Molecular genetic studies indicate that this species originated from a single location in North America (Gomi et al. 2004). Similarly, the Colorado potato beetle, Leptinotarsa decemlineata, was introduced from North America into Europe in the 1920s and has since expanded its range to cover an area from the Mediterranean to northern Russia (Grapputo et al. 2005; Piiroinen et al. 2011). Molecular genetic analysis has shown that this invasion likely resulted from an isolated introduction and that the invasive population shows a substantially reduced level of genetic variance (Grapputo et al. 2005). In spite of this, the Colorado potato beetle has become adapted to a wide array of climatic conditions, and this has included the evolution of CDL for diapause induction (de Wilde and Hsiao 1981). The third example involves the Asian tiger mosquito, Aedes albopictus, most likely introduced from Japan into the United States in 1985 (Kambhampati et al. 1991). This insect enters a photoperiodically induced embryonic diapause and has evolved a CDL cline, along a 15° latitudinal gradient, in the years between 1988 and 2008 (Urbanski et al. 2012). The introduction history of this mosquito is not precisely known, but mitochondrial haplotype analysis supports the hypothesis of a single introduction (Usmani-Brown et al. 2009), making this another possible example of a genetically restricted population undergoing rapid CDL evolution. These examples show that evolution of CDL in genetically restricted colonizing populations is not unique to D. carinulata, and they also underscore the value of D. carinulata as an example of ongoing evolution of CDL in a colonizing population in which the introduction history is well known.
When CDL was measured at constant temperature, the evolutionary divergence was small but significant, but when a thermoperiod was superimposed on the photoperiod regime, the differences between populations were larger and the divergence from the original CDL values became greater. This is an indication that the thermal sensitivity of the photoperiodic response is increasing in North America as beetles adapt to new environmental conditions. Temperature sensitivity of CDL is a case of phenotypic plasticity; that is, CDL may shift in response to environmental context, in this case temperature. The initial measurements of CDL in D. carinulata showed that increasing temperature resulted in slight decreases in CDL and that the superposition of a thermoperiod on a photoperiod regime also decreased CDL (Bean et al. 2007a). The effects were minor and could not compensate for the shorter day lengths found at southern latitudes as evidenced by beetles entering diapause during the warmest month of the year (July) at three field sites. So, while a small degree of phenotypic plasticity was evident in the photoperiodic response, it was not sufficient to compensate for the photoperiod mismatches initially experienced by the introduced beetle population at lower latitude sites. The evidence presented here suggests that not only is CDL decreasing in response to selection, but temperature sensitivity and thus phenotypic plasticity of CDL is increasing as well.
Increased plasticity of the photoperiodic response builds more flexibility into the phenology of D. carinulata, which may increase the likelihood of survival in novel environments. In spite of this, temperature sensitivity is not often considered as a critical component of insect CDL evolution. One exception is work on the fall webworm H. cunea in which the CDL has been shown to become more temperature sensitive as the introduced insect establishes in new ranges in Japan (Gomi 1997, 2007). Phenotypic plasticity allows species to better respond to novel environments (West-Eberhard 2003) and is itself subject to evolution (de Jong 2005). It appears that CDL plasticity is also evolving in D. carinulata allowing this species the developmental flexibility necessary to match host plant phenology and enable range expansion.
One of the outstanding questions in biological control is whether local adaptation occurs following the release of biocontrol agents, and if so, does adaptation enhance the efficacy of the agents (Roderick and Navajas 2003; Hufbauer and Roderick 2005). In the case of D. carinulata, evolution has resulted in a delayed diapause, which has allowed populations to achieve a greater level of phenological synchrony with host plants and climatic seasonality at sites across North America. This is important because multiple defoliations of Tamarix by the beetles cause incremental decline in stored carbohydrates, increasing stress on the host plant and eventually leading to plant mortality (Hudgeons et al. 2007). When beetles remain active later in the season, they are not only able to defoliate larger areas of Tamarix, but will also defoliate plants that have produced a second flush of foliage following an initial defoliation event (Dudley and DeLoach 2004). Furthermore, if defoliation occurs late in the season, the host plant is less likely to compensate for nutritional losses via re-foliation owing to its own phenological constraints, imposed by declining temperatures. Beetle populations will also have more time to increase in size in the mid- to late summer with decreasing CDL (Bean et al. 2007a). This is important because tamarisk defoliation requires locally dense populations, brought about by aggregation pheromones (Cossé et al. 2005), and such aggregations are rarer and more difficult to achieve when beetle numbers are low. So, it is almost certain that evolution of CDL has enhanced the efficacy of D. carinulata as a biocontrol agent.
Underlying the enhanced efficacy is a shift in the ecology of D. carinulata. A longer reproductive period, dispersal later into the season, and an enhanced overwinter survival are some of the potential ecological consequences of contemporary CDL evolution in D. carinulata. These factors will affect population growth of the beetles and ultimately change the ecological relationship between the insect and its host plant tamarisk. As noted above, this will result in a greater impact on tamarisk, which will influence the ecological dynamics in riparian ecosystems. This is an example of contemporary evolution affecting ecology, a pattern that has led to a recent reevaluation of the role of evolution in ecology (Hairston et al. 2005; Schoener 2011).
Clearly evolution of the seasonal timing of diapause induction has enabled D. carinulata to establish well beyond the geographic limits of the original introduced genotype, but less clear is how fast and how far it will continue to colonize new regions south of its current distribution. This is important because increased range will benefit Tamarix management efforts and alter decisions concerning the use of control techniques (O’Meara et al. 2010). There are also concerns that Tamarix biological control could impact a federally endangered passerine bird, the southwestern willow flycatcher, Empidonax traillii extimus, known to nest in Tamarix especially in the lower Colorado River basin and along the Rio Grande (Paxton et al. 2007; Bateman et al. 2010). In this case, it is essential to predict how quickly D. carinulata will move southward so that riparian restoration efforts, using native trees and woody shrubs that can serve as flycatcher nesting habitat, can be initiated in a timely manner prior to the decline of Tamarix (Dudley and Bean 2012). Continuing the measurement of evolving CDL values will be important in predicting further shifts in phenology, which will help determine the range of the insect (Chuine 2010). In addition, measurement of CDL and D. carinulata phenology at the moving front of southward population expansion will be an important component of watershed management in several western river basins (Bateman et al. 2010). Evolution at the edge of expanding populations is also of wider interest (Thomas et al. 2001; Phillips et al. 2010), and the linkage of the dispersal phenotype with reproductive development and photoperiodism (Bean et al. 2007b) makes this a particularly interesting case. These are some of the reasons why evolutionary principles are being considered in Tamarix biocontrol, much as they are now commonly incorporated into the formulation of natural resource management plans and policy in numerous other fields (Hendry et al. 2011; Lankau et al. 2011).
Acknowledgments
We are pleased to acknowledge Nina Louden, Jiana ten Brinke, Alison Blackwell, and Sonya Ortega for technical assistance on this project. We would also like to thank David Thompson, New Mexico State University, David Kazmer, US Department of Agriculture, and Debra Eberts, US Bureau of Reclamation for assistance at our field sites as well as for helpful discussions and invaluable contributions to the tamarisk biocontrol project. We also thank R. Hufbauer and two anonymous reviewers for helpful suggestions on an earlier version of this manuscript. This work was partially funded by the USDA Forest Service (Forest Health Protection) (STDP R4-2004-01) and the USDA-NRI (2006-35302-1664). We also thank The Carl Trygger Foundation for financial support (for PD).
Data archiving statement
Data deposited in the Dryad repository: doi: 10.5061/dryad.7qr3f154.
Literature cited
- Bateman HL, Dudley TL, Bean DW, Ostoja SM, Hultine KR, Kuehn MJ. A river system to watch: documenting the effects of saltcedar (Tamarix spp.) biocontrol in the Virgin River valley. Ecological Restoration. 2010;28:405–410. [Google Scholar]
- Bean DW, Dudley TL, Keller JC. Seasonal timing of diapause induction limits the effective range of Diorhabda elongata deserticola (Coleoptera: Chrysomelidae) as a biological control agent for tamarisk (Tamarix spp.) Environmental Entomology. 2007a;36:15–25. doi: 10.1603/0046-225x(2007)36[15:stodil]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bean DW, Wang T, Bartelt RJ, Zilkowski BW. Diapause in the leaf beetle Diorhabda elongata (Coleoptera: Chrysomelidae), a biological control agent for tamarisk (Tamarix spp.) Environmental Entomology. 2007b;36:531–540. doi: 10.1603/0046-225x(2007)36[531:ditlbd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Beck SD. Insect Photoperiodism. 2nd edn. New York, NY: Academic Press; 1980. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Genetic shift in photoperiodic response correlated with global warming. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14509–14511. doi: 10.1073/pnas.241391498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SP, Hendry AP, Reznick DN, Fox CW. Evolution on ecological time scales. Functional Ecology. 2007;21:387–393. [Google Scholar]
- Cheverud JM, Routman EJ. Epistasis as a source of increased additive genetic variance at population bottlenecks. Evolution. 1996;50:1042–1051. doi: 10.1111/j.1558-5646.1996.tb02345.x. [DOI] [PubMed] [Google Scholar]
- Chuine I. Why does phenology drive species distribution? Philosophical Transactions of the Royal Society B. 2010;365:3149–3160. doi: 10.1098/rstb.2010.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossé AA, Bartelt RJ, Zilkowski BW, Bean DW, Petroski RJ. The aggregation pheromone of Diorhabda elongata, a biological control agent of saltcedar (Tamarix spp.): identification of two behaviorally active components. Journal of Chemical Ecology. 2005;31:657–670. doi: 10.1007/s10886-005-2053-2. [DOI] [PubMed] [Google Scholar]
- Dalin P, Bean DW, Dudley TL, Carney VA, Eberts D, Gardner KT, Hebertson E, et al. Seasonal adaptations to day length in ecotypes of Diorhabda spp. (Coleoptera: Chrysomelidae) inform selection of agents against saltcedars (Tamarix spp.) Environmental Entomology. 2010;39:1666–1675. doi: 10.1603/EN09270. [DOI] [PubMed] [Google Scholar]
- Danks HV. Insect Dormancy: An Ecological Perspective. Ottawa, Canada: Biological Survey of Canada; 1987. [Google Scholar]
- DeLoach CJ, Lewis PA, Herr JC, Carruthers RI, Tracy JL, Johnson J. Host specificity of the leaf beetle, Diorhabda elongata deserticola (Coleoptera: Chrysomelidae) from Asia, a biocontrol agent for saltcedars (Tamarix: Tamaricaceae) in the Western United States. Biological Control. 2003;27:117–147. [Google Scholar]
- DeLoach CJ, Carruthers R, Dudley T, Eberts D, Kazmer D, Knutson A, Bean D, et al. First results for control of saltcedar (Tamarix spp.) in the open field in the western United States. In: Cullen J, editor. Canberra, Australia: Eleventh International Symposium on Biological Control of Weeds; 2004. [Google Scholar]
- Dlugosch KM, Parker IM. Founding events in species invasions: genetic variation, adaptive evolution and the role of multiple introductions. Molecular Ecology. 2008a;17:431–449. doi: 10.1111/j.1365-294X.2007.03538.x. [DOI] [PubMed] [Google Scholar]
- Dlugosch KM, Parker IM. Invading populations of an ornamental shrub show rapid life history evolution despite genetic bottlenecks. Ecology Letters. 2008b;11:701–709. doi: 10.1111/j.1461-0248.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- Dudley TL, Bean DW. Tamarisk biocontrol, endangered species risk and resolution of conflict through riparian restoration. BioControl. 2012;57:331–347. [Google Scholar]
- Dudley T, DeLoach J. Saltcedar (Tamarix spp.), endangered species, and biological weed control – can they mix? Weed Technology. 2004;18:1542–1551. [Google Scholar]
- Emerson KJ, Bradshaw WE, Holzapfel CM. Complications of complexity: integrating environmental, genetic and hormonal control of insect diapause. Trends in Genetics. 2009;25:217–225. doi: 10.1016/j.tig.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Frankham R. Resolving the genetic paradox in invasive species. Heredity. 2005;94:385. doi: 10.1038/sj.hdy.6800634. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Auble GT, Shafroth PB, Scott ML, Merigliano MF, Preehling MD, Griffin EK. Dominance of non-native riparian trees in western USA. Biological Invasions. 2005;7:747–751. [Google Scholar]
- Friedman JM, Roelle JE, Cade BS. Genetic and environmental influences on leaf phenology and cold hardiness of native and introduced riparian trees. International Journal of Biometeorology. 2011;55:775–787. doi: 10.1007/s00484-011-0494-6. [DOI] [PubMed] [Google Scholar]
- Gaskin JF, Schaal BA. Molecular phylogenetic investigation of U.S. invasive Tamarix. Systematic Botany. 2003;28:86–95. [Google Scholar]
- Gomi T. Geographic variation in critical photoperiod for diapause induction and its temperature dependence in Hyphantria cunea Drury (Lepidoptera: Arctiidae) Oecologia. 1997;111:160–165. doi: 10.1007/s004420050220. [DOI] [PubMed] [Google Scholar]
- Gomi T. Seasonal adaptations of the fall webworm Hyphantria cunea (Drury) (Lepidoptera: Arctiidae) following its invasion of Japan. Ecological Research. 2007;22:855–861. [Google Scholar]
- Gomi T, Muraji M, Takeda M. Mitochondrial DNA analysis of the introduced fall webworm, showing its shift in life cycle in Japan. Entomological Science. 2004;7:183–188. [Google Scholar]
- Gomi T, Nagasaka M, Fukuda T, Hagihara H. Shifting of the life cycle and life-history traits of the fall webworm in relation to climate change. Entomologia Experimentalis et Applicata. 2007;125:179–184. [Google Scholar]
- Grapputo A, Boman S, Lindström L, Lyytinen A, Mappes J. The voyage of an invasive species across continents: genetic diversity of North American and European Colorado potato beetle populations. Molecular Ecology. 2005;14:4027–4219. doi: 10.1111/j.1365-294X.2005.02740.x. [DOI] [PubMed] [Google Scholar]
- Hairston NG, Ellner SP, Gerber MA, Yoshida T, Fox JA. Rapid evolution and the convergence of ecological and evolutionary time. Ecology Letters. 2005;8:1114–1127. [Google Scholar]
- Hendry AP, Kinnison MT. The pace of modern life: measuring rates of contemporary microevolution. Evolution. 1999;53:1637–1653. doi: 10.1111/j.1558-5646.1999.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Kinnison MT, Heino M, Day T, Smith TB, Fitt G, Bergstrom CT, et al. Evolutionary principles and their practical application. Evolutionary Applications. 2011;4:159–183. doi: 10.1111/j.1752-4571.2010.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera AM, Dahlsten DD, Tomic-Carruthers N, Carruthers RI. Estimating temperature-dependent developmental rates of Diorhabda elongata (Coleoptera: Chrysomelidae), a biological control agent of saltcedar (Tamarix spp.) Environmental Entomology. 2005;34:775–784. [Google Scholar]
- Hudgeons JL, Knutson AE, Heinz KM, DeLoach CJ, Dudley TL, Pattison RR, Kiniry JR. Defoliation by introduced Diorhabda elongata leaf beetles (Coleoptera: Chrysomelidae) reduces carbohydrate reserves and regrowth of Tamarix (Tamaricaceae) Biological Control. 2007;43:213–221. [Google Scholar]
- Hufbauer RA. Biological invasions: paradox lost and paradise gained. Current Biology. 2008;18:R246–R247. doi: 10.1016/j.cub.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Hufbauer RA, Roderick GK. Microevolution in biological control: mechanisms, patterns and processes. Biological Control. 2005;35:227–239. [Google Scholar]
- de Jong G. Evolution of phenotypic plasticity: patterns of plasticity and the emergence of ecotypes. New Phytologist. 2005;166:101–118. doi: 10.1111/j.1469-8137.2005.01322.x. [DOI] [PubMed] [Google Scholar]
- Kambhampati S, Black IV WC, Rai KS. Geographic origin of the United States and Brazilian Aedes albopictus inferred from allozyme analysis. Heredity. 1991;67:85–94. doi: 10.1038/hdy.1991.67. [DOI] [PubMed] [Google Scholar]
- van Klinken RD, Edwards OR. Is host specificity of weed biological control agents likely to evolve rapidly following establishment? Ecology Letters. 2002;5:590–596. [Google Scholar]
- Lankau R, Jørgensen PS, Harris DJ, Sih A. Incorporating evolutionary principles into environmental management and policy. Evolutionary Applications. 2011;4:315–325. doi: 10.1111/j.1752-4571.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, DeLoach CJ, Knutson AE, Tracy JL. Biology of Diorhabda elongata deserticola (Coleoptera: Chrysomelidae), an Asian leaf beetle for biological control of saltcedars (Tamarix spp.) in the United States. Biological Control. 2003;27:101–116. [Google Scholar]
- Li B, Kong X, Meng L. An observation on the life cycle of Diorhabda elongata deserticola Chen: a potential biocontrol agent of saltcedar. Chinese Journal of Biological Control. 2000;16:48–49. [Google Scholar]
- Marsico TD, Burt JW, Espeland EK, Gilchrist GW, Jamieson MA, Lindström L, Roderick GK, et al. Underutilized resources for studying the evolution of invasive species during their introduction, establishment, and lag phases. Evolutionary Applications. 2010;3:203–219. doi: 10.1111/j.1752-4571.2009.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Maruyama T, Chakraborty R. The bottleneck effect and genetic variability in populations. Evolution. 1975;29:1–10. doi: 10.1111/j.1558-5646.1975.tb00807.x. [DOI] [PubMed] [Google Scholar]
- O’Meara S, Larsen D, Owens C. Shafroth PB, Brown CA, Merritt DM, editors. Methods to control saltcedar and Russian olive. 2010. pp. 65–102. Saltcedar and Russian olive control demonstration act science assessment: U.S. Geological Survey Scientific Investigations Report 2009–5247, 143 p.
- Paxton EH, Sogge MK, Durst SL, Theimer TC, Hatten JR. 2007. The ecology of the southwestern willow flycatcher in Central Arizona-a 10-year synthesis reportUSGS Open-File Report 2007-1381.
- Pemberton RW. Predictable risk to native plants in weed biological control. Oecologia. 2000;125:489–494. doi: 10.1007/s004420000477. [DOI] [PubMed] [Google Scholar]
- Phillips BL, Brown GP, Shine R. Life-history evolution in range-shifting populations. Ecology. 2010;96:1617–1627. doi: 10.1890/09-0910.1. [DOI] [PubMed] [Google Scholar]
- Piiroinen S, Ketola T, Lyytinen A, Lindström L. Energy use, diapause behavior and northern range expansion potential in the invasive Colorado potato beetle. Functional Ecology. 2011;25:527–536. [Google Scholar]
- Reznick DN, Ghalambor CK. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica. 2001;112–113:183–198. [PubMed] [Google Scholar]
- Roderick GK, Navajas M. Genes in new environments: genetics and evolution in biological control. Nature Review Genetics. 2003;4:889–899. doi: 10.1038/nrg1201. [DOI] [PubMed] [Google Scholar]
- Saunders DS. Geographical strains and selection for the diapause trait in Calliphora vicina. In: Denlinger DL, Giebultowicz JM, Saunders DS, editors. Insect Timing: Circadian Rhythmicity to Seasonality. New York, NY: Elsevier; 2001. pp. 113–122. [Google Scholar]
- Saunders DS. Insect Clocks. 3rd edn. Amsterdam, The Netherlands: Elsevier Science; 2002. [Google Scholar]
- SAS Institute. SAS/STAT User’s Guide, Version 9.2. Cary, NC, USA: SAS Institute; 2008. [Google Scholar]
- Schoener TW. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science. 2011;331:426–429. doi: 10.1126/science.1193954. [DOI] [PubMed] [Google Scholar]
- Shafroth PB, Cleverly JR, Dudley TL, Taylor JP, Van Riper CV, Weeks EP, Stuart JN. Control of Tamarix in the western United States: implications for water salvage, wildlife use, and riparian restoration. Environmental Management. 2005;35:231–246. doi: 10.1007/s00267-004-0099-5. [DOI] [PubMed] [Google Scholar]
- Tauber MJ, Tauber CA, Masaki S. Seasonal Adaptations of Insects. New York, NY: Oxford University Press; 1986. [Google Scholar]
- Taylor F, Spaulding JB. Fitness functions for alternative developmental pathways in the timing of diapause induction. American Naturalist. 1988;131:678–699. [Google Scholar]
- Thomas CD, Bodsworth EJ, Wilson JR, Simmons AD, Davies ZG, Musche M, Conradt L. Ecological and evolutionary processes at expanding range margins. Nature. 2001;411:577–581. doi: 10.1038/35079066. [DOI] [PubMed] [Google Scholar]
- Thrall PH, Oakeshott JG, Fitt G, Southerton S, Burdon JJ, Sheppard A, Russell RJ, et al. Evolution in agriculture: the application of evolutionary approaches to the management of biotic interactions in agro-ecosystems. Evolutionary Applications. 2011;4:200–215. doi: 10.1111/j.1752-4571.2010.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy JT, Robbins TO. Taxonomic revision and biogeography of the Tamarix-feeding Diorhabda elongata (Brullé, 1832) species group (Coleoptera: Chrysomelidae: Galerucinae: Galerucini) and analysis of their potential in biological control of Tamarisk. Zootaxa. 2009;2101:1–152. [Google Scholar]
- Turelli M, Barton NH. Will population bottlenecks and multilocus epistasis increase additive genetic variance? Evolution. 2006;60:1763–1776. [PubMed] [Google Scholar]
- Urbanski J, Mogi M, O’Donnell D, DeCotiis M, Toma T, Armbruster P. Rapid adaptive evolution of photoperiodic response during invasion and range expansion across climatic gradient. The American Naturalist. 2012;179:490–500. doi: 10.1086/664709. [DOI] [PubMed] [Google Scholar]
- Usmani-Brown S, Cohnstaedt L, Munstermann LE. Population genetics of Aedes albopictus (Diptera: Culicidae) invading populations, using mitochondrial nicotinamide adenine dinucleotide dehydrogenase subunit 5 sequences. Annals of the Entomological Society of America. 2009;102:144–150. doi: 10.1603/008.102.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental Plasticity and Evolution. Oxford: Oxford University Press; 2003. [Google Scholar]
- de Wilde J, Hsiao T. Geographic diversity of the Colorado potato beetle and its infestation in Eurasia. In: Lashcomb JH, Casagrande R, editors. Advances in Potato Pest Management. Stroudsburg, PA: Hutchinson Ross; 1981. pp. 47–68. [Google Scholar]


