Highlights
► In this study, we explored the function of IGF-1Ea propeptide in inducing cardiogenesis of stem cells. ► IGF-1Ea promoted cardiac mesodermal induction in uncommitted cells. ► Under differentiation condition, IGF-1Ea increased expression of cardiac differentiation markers. ► Furthermore, it promoted formation of finely organized sarcomeric structure. ► IGF-1Ea propeptide may be a good candidate to improve production of cardiomyocytes from pluripotent cells.
Keywords: Pluripotent stem cells, Cardiac differentiation, IGF-1Ea
Abstract
The mechanism implicated in differentiation of endogenous cardiac stem cells into cardiomyocytes to regenerate the heart tissue upon an insult remains elusive, limiting the therapeutical goals to exogenous cell injection and/or gene therapy. We have shown previously that cardiac specific overexpression of the insulin-like growth factor 1 propeptide IGF-1Ea induces beneficial myocardial repair after infarct. Although the mechanism is still under investigation, the possibility that this propeptide may be involved in promoting stem cell differentiation into the cardiac lineage has yet to be explored. To investigate whether IGF-1Ea promote cardiogenesis, we initially modified P19 embryonal carcinoma cells to express IGF-1Ea. Taking advantage of their cardiomyogenic nature, we analyzed whether overexpression of this propeptide affected cardiac differentiation program. The data herein presented showed for the first time that constitutively overexpressed IGF-1Ea increased cardiogenic differentiation program in both undifferentiated and DMSO-differentiated cells. In details, IGF-1Ea overexpression promoted localization of alpha-actinin in finely organized sarcomeric structure compared to control cells and upregulated the cardiac mesodermal marker NKX-2.5 and the ventricular structural protein MLC2v. Furthermore, activated IGF-1 signaling promoted cardiac mesodermal induction in undifferentiated cells independently of cell proliferation. This analysis suggests that IGF-1Ea may be a good candidate to improve both in vitro production of cardiomyocytes from pluripotent stem cells and in vivo activation of the differentiation program of cardiac progenitor cells.
1. Introduction
The design of experimental tools for repairing postmitotic organs with re-established functionality is a matter of intense research in regenerative studies. In particular, upon myocardial damage the mammalian heart activates a healing process that leads to a specific remodeling, consisting of fibrotic scar formation and cellular hypertrophy at the cost of functional muscle [1]. The replacement and regeneration of functional cardiac muscle and the surrounding vasculature is an important therapeutic goal. Several strategies in mammalian tissues have been explored to potentially support regeneration, which include supplementation of growth factors that function as cyto-protectants by inhibiting pro-death pathways, and improvement of candidate cells and their delivery into the injured myocardium to reconstitute the lost vasculature and musculature. The ideal donor cell should exhibit the structural and contractile properties of cardiomyocytes and should be able to integrate structurally and functionally into the host tissue. It must possess or acquire inherent properties to improve colonization of the scar tissue, survive an apoptotic and ischemic environment, and retain an initial high proliferative capacity [2]. Therefore, potential use of pluripotent or totipotent stem cells to replace tissue loss depends in part on efficient differentiation protocols to derive tissue-specific cells. It is known that Nodal and Wnt inhibition regulates formation of cardiomyocytes in mouse embryonic stem cells (mESCs) [3,4]. Furthermore, chemical mediators of cardiogenesis have been reported in mESCs, including cardiogenols, ascorbic acid, isoxazolyl-serines, sulfonyl hydrazones and DMSO [5,6]. Recently, it has been shown that pharmacological inhibition of Wnt signaling is sufficient to drive human mesoderm cells to form cardiomyocytes [7]. Nevertheless, although culture and differentiation techniques have improved [8,9], it remains challenging to obtain cardiomyocyte-enriched cultures, and the possibility to use exogenous cell therapy is therefore limited by this experimental gap. A promising tool would be to elicit endogenous cardiac stem cells to differentiate into novel cardiomyocytes to re-establish at least the lost musculature.
On this note, we recently demonstrated that the propeptide of the insulin-like growth factor 1 family IGF-1Ea, expressed in transgenic mouse muscle under cardiac-specific post-mitotic control, induces cardiac recovery by decreasing scar formation and increasing cell proliferation [10]. Although the mechanism is still under investigation, a possibility arising from our data is that IGF-1Ea may promote endogenous stem cell proliferation and differentiation into the cardiac lineage. Interestingly, previous analysis showed that inhibition of the PI3K in cultured ESCs induced decreased alpha-actinin staining and embryoid body (EB) beating areas, suggesting that the signaling mediated by PI3K leads to cardiogenesis [11]. Recently, IGF-1 has been identified as a mediator of Yap signaling to induce cardiomyocyte proliferation and survival during development [12]. Nevertheless, the direct involvement of the IGF-1 propeptides has not yet explored.
In order to investigate the possibility of using IGF-1Ea in a therapeutic-like approach to elicit endogenous differentiation of cardiac stem cells, we manipulated the growth and cardiomyocyte differentiation potential of P19 embryonal carcinoma cell lines expressing green fluorescent protein (GFP) under the control of the specific cardiac promoter myosin light chain 2v (MLC2v) [13]. We infected these cells with a lentivirus expressing Igf-1Ea gene and analyzed whether transgene overexpression could favor cardiomyocyte formation.
Our data show that overexpression of IGF-1Ea promoted increased cardiomyocyte markers’ regulation and mature sarcomeric structures’ formation in DMSO-treated cells. Furthermore, the pool of undifferentiated cells was stimulated to express mesodermal markers associated with cardiac lineage differentiation. Although cardiomyocyte number was not affected, the usage of this factor may be important to promote cardiogenesis for exogenous and/or endogenous therapies.
2. Materials and methods
An expanded Section 2 can be found in the online supplement.
2.1. Flow cytometry analysis and cell-sorting
Sorting and determination of the percentage of GFP positive cells was performed using a FACS Aria (BD, Bekton Dickinson). Confluent adherent differentiated (14 days post differentiation) and undifferentiated control and overexpressing IGF-1Ea cells were washed with 1× PBS and trypsinized. The cell suspension was filtered through a 30 μm sieve (Filcon, BD) and Sytox blue (Invitrogen) dye was added to discriminate alive from dead cells. Undifferentiated cells were used as negative control. For the cell sorting, a 100 μm nozzle (20 PSI) was used.
2.2. Transduction of P19-GFP cells
Transductions were performed following the protocol provided by the company (Invitrogen, ViraPower T-Rex Lentiviral Expression System User Manual, 2004). In summary, transduction was performed with 1 ml of pLenti4/TO/V5-DEST carrying IGF-1Ea cDNA in culture media containing Polybrene (Sigma, 6 μl). Cells were selected with zeocin (200 μg/ml), expanded and clones used for further experiments.
2.3. Statistical analysis
Statistical analyses were performed using GraphPad Prism 4. All measurements are reported as values ± standard error of the mean (SEM). Analysis of variance (ANOVA) or Student’s t-test was used to analyze significance among the samples as indicated. A significant difference was considered when p < 0.05 and was marked as (∗) for p < 0.05, (∗∗) for p < 0.01 and (∗∗∗) for p < 0.001.
3. Results
3.1. Generation of P19-GFP cells expressing IGF-1Ea
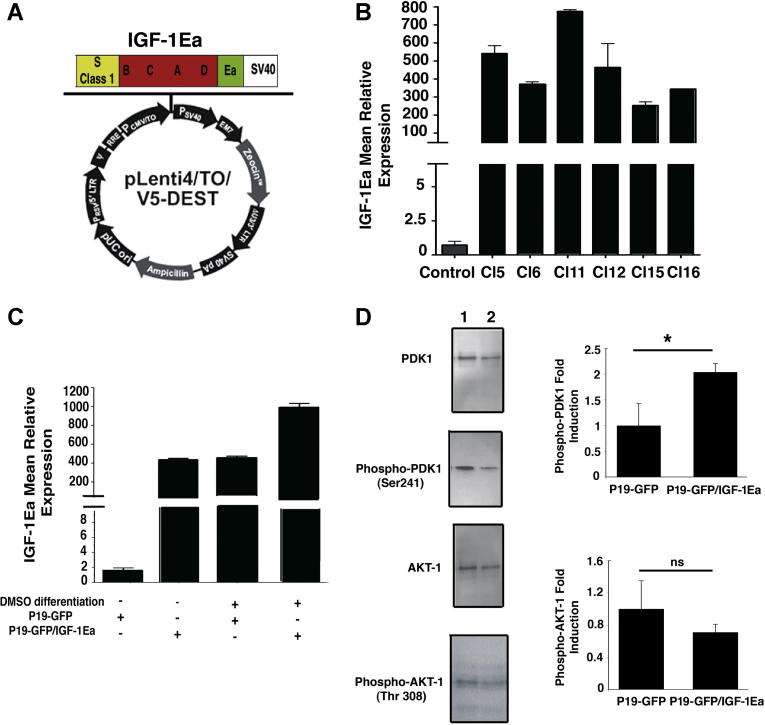
To analyze the function of IGF-1Ea propeptide in regulation of cardiac differentiation program, we made use of the pluripotent P19-GFP embryonal carcinoma cells [13]. These cells presented a clear advantage in this study, since they readily differentiate into cardiomyocytes by embryoid body techniques [13] and bona fide ventricular cardiomyocytes could be easily identified and sorted by FACS analysis through the cardiac specific expression of GFP. To induce the expression of the propeptide IGF-1Ea we generated a lentiviral vector carrying IGF-1Ea under the control of the CMV promoter (Fig. 1A). We selected by antibiotic resistance 16 stably transduced clones, of which 6 clones expressed high levels of IGF-1Ea (Fig. 1B). Clone 6 was selected for further experimentation, since it formed morphologically normal embryoid bodies and differentiate readily in ventricular cardiomyocytes [13], as shown by overexpression of GFP under the control of the MLC2v promoter (Supplementary Fig. 1A and B). P19-GFP cells transduced with an empty lentiviral vector served as control.
Fig. 1.
Characterization of P19-GFP embryonal carcinoma cells expressing IGF-1Ea. (A) The pLenti4/TO/V5-DEST contains the recombination sites attR1 and attR2 downstream the CMV-TO promoter that allowed recombination between this lentiviral vector and the one carrying IGF1Ea. (B) Transduced clones constitutively expressed IGF-1Ea transcripts. IGF-1 expression was analyzed by a rodent IGF-1 probe. 18S rRNA was used as internal reference gene. Values are Mean +/− SEM. (C) IGF-1Ea Clone 6 cells and control Clone 6 cells were differentiated in 1% DMSO and RNA extracts analyzed for quantitative Real Time PCR (qRT-PCR). 18S rRNA was used as internal reference gene. Results are expressed as mean ± SEM. (D) Undifferentiated P19-GFP cells expressing IGF-1Ea (lane 1) and control cells (lane 2) were analyzed for PDK1 and AKT1 activity. Normalization was performed with total AKT1 and PDK1. The figure shows the analysis of three independent experiments and their fold induction values in the right panel. Asterisk (∗) indicates significant values (p < 0.05); ns, no significant.
To verify whether differentiation into the cardiomyocytes lineage preserved IGF-1Ea expression, cells were treated with DMSO 1% and plated for 12 days on Petri dishes to allow formation of beating areas. Real time PCR (RT-PCR) data showed that expression of IGF-1Ea is increased significantly upon DMSO-induced cardiac differentiation (Fig. 1C). In parallel, IGF-1 mean relative expression of control cells after cardiomyocyte-induced differentiation showed upregulation of the IGF-1 transcript (Fig. 1C), indicating that differentiation into the cardiac lineage correlates with endogenous IGF-1 overexpression.
Taken together, these data indicate that constitutive expression of the gene of interest is ensured in undifferentiated and differentiated P19 teratocarcinoma cells using this lentiviral system.
3.2. IGF-1Ea activates PDK1 signaling and cell survival mechanisms but does not change cell cycle profile
To analyze whether IGF-1 signaling pathway is induced in cells overexpressing IGF-1Ea compared to control cells, cell extracts were analyzed for activation of the downstream mediators of IGF-1 signaling, PDK1 and AKT1. The results in Fig. 1D showed that overexpression of IGF-1Ea induced significant increase of PDK1 phosphorylation at residue Ser241 without affecting AKT1 phosphorylation levels compared to empty vector-transduced control cells (Fig. 1D). The data reported are in agreement with our previous analysis performed on IGF-1Ea overexpressing hearts [10], indicating that the downstream signaling activated by this specific propeptide is not mediated by AKT1 but employes a novel pathway in both mammalian heart and pluripotent teratocarcinoma cells.
Signaling pathways mediated by IGF-1 increase skeletal muscle and cardiac survival [14]. To verify whether overexpression of IGF-1Ea induced protective signaling, we mimicked infarct conditions in vitro exposing control and IGF-1Ea overexpressing cells to hypoxia (Fig. 2A). The effects of hypoxia on cell viability were quantified by measuring the activity of lactate dehydrogenase (LDH) released into the medium. LDH activity was significantly increased in both control and IGF-1Ea cells compared to normoxia-treated cells. Importantly, IGF-1Ea overexpression decreased LDH activity compared to control cells during hypoxia treatment, indicating a protective role of IGF-1Ea to low oxygen conditions.
Fig. 2.
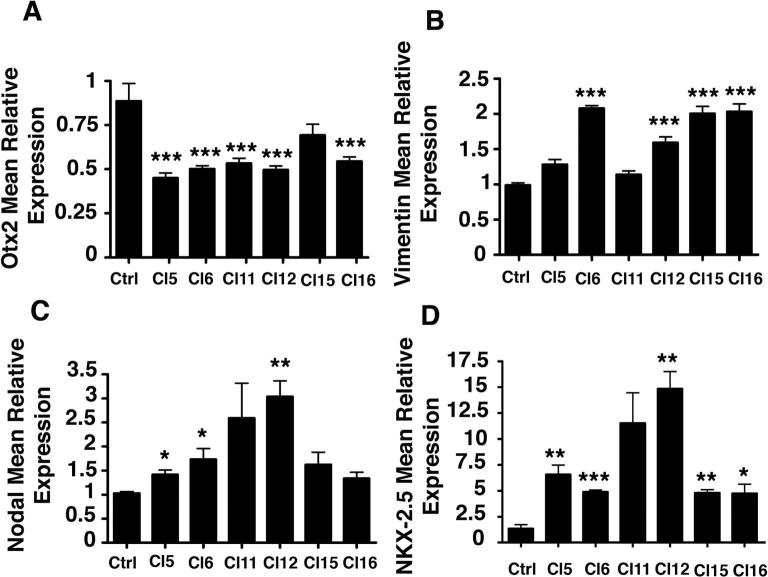
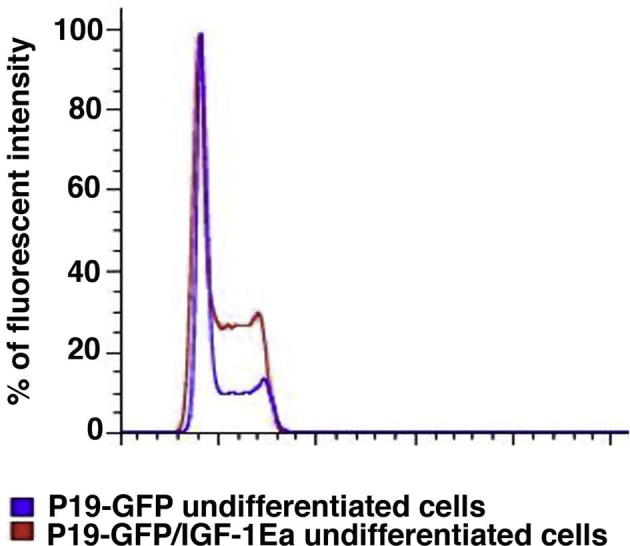
IGF-1Ea regulation of survival, proliferation and lineage commitment in undifferentiated P19-GFP cells. (A) P19-GFP/IGF-1Ea and control P19-GFP cells were exposed to 3% hypoxia for 24 h. Percentage of LDH activity was measured as described in Supplementary Materials and Methods by comparison of IGF-1Ea and control cells in normoxic (white bars) and hypoxic (black bars) conditions. Significance was measured by one-way ANOVA and ∗∗ indicates p < 0.01, whereas ∗∗∗ indicates p < 0.001. (B) Cell cycle analysis of undifferentiated P19Cl6 cells, transduced with empty (blue) or IGF-1Ea (red) viral vectors. Percentage of cells in S phase were similar in both empty and IGF-1Ea expressing cells. Results were obtained by PI staining using Flow Jo analyses. X-axis represents percentage of cells and y-axis fluorescent intensity. (C–G) qRT-PCR analysis for the mesodermal markers vimentin (C) and nodal (F), the ectodermal marker Otx2 (D), the endodermal GATA4 (E) and the cardiac marker NKX-2.5 (F) in undifferentiated IGF-1Ea and control cells. 18S rRNA was used as internal reference gene. Results are expressed as mean ± SEM. Asterisk (∗) indicates significant values (p < 0.05); ∗∗ indicates p < 0.01, whereas ∗∗∗ indicates p < 0.001. (For interpretation of the references in color in this figure legend, the reader is referred to the web version of this article.)
IGF-1 regulates proliferative response in different cell types in vitro [15,16] and during embryonic development [17]. Interestingly, G1, S and G2 cell cycle phases measured by flow cytometric analysis are comparable between control and IGF-1Ea cells, indicating that the signaling cascade mediated by this specific propeptide did not alter cell cycle in this clone (Fig. 2B). We report here that an altered proliferative profile was however observed in three of the 16 clones (e.g. clone 5, Supplementary Fig. 2), suggesting that the site of insertion rather than the expression of the growth factor causes cell cycle differences.
Taken together, these data showed that IGF-1Ea activates prosurvival signaling as assessed in the in vivo cardiac model, without altering cell cycle profile.
3.3. Cardiac mesoderm formation is increased by IGF-1Ea overexpression in undifferentiated P19-GFP cells
It has been reported that PI3K signaling induces cardiac commitment in ES cells, suggesting that activators of this kinase such as IGF-1, TGFbeta, FGF, and insulin may promote cardiogenesis in vitro [18]. To test whether overexpression of the IGF-1Ea propeptide in undifferentiated P19-GFP cells was associated with a gene expression profile that correlates with ectodermal, endodermal or mesodermal lineage specification, we analyzed by RT-PCR the level of vimentin, GATA4, Otx2 and Nodal transcripts. Interestingly, we observed that vimentin, reported to favor cell migration into the primitive streak, is upregulated in the selected clone (Fig. 2C). The ectodermal marker Otx2 [19] is significantly downregulated, whereas the early endodermal marker GATA4 [20] (Fig. 2D and E) remained comparable to control cells. The mesodermal marker nodal was upregulated in IGF-1Ea expressing cells and this correlated with induction of the early cardiac marker NKX-2.5 (Fig. 2F and G). To distinguish whether this gene expression profile is a result of IGF-1Ea activity and it is not caused by differential site integration of the gene, we analyzed the above markers in the remaining clones (Supplementary Fig. 3). The data showed that Otx2 is significantly decreased in all clones (Supplementary Fig. 3A), whereas Vimentin (Supplementary Fig. 3B), nodal and NKX-2.5 (Supplementary Fig. 3C and D) significantly increased in the majority of the clones. Taken together, the data indicate that IGF-1Ea is a specific regulator of mesodermal cardiac induction in uncommitted cells.
3.4. IGF-1Ea overexpression induces increased cardiomyocyte differentiation in DMSO-treated cells without affecting their number
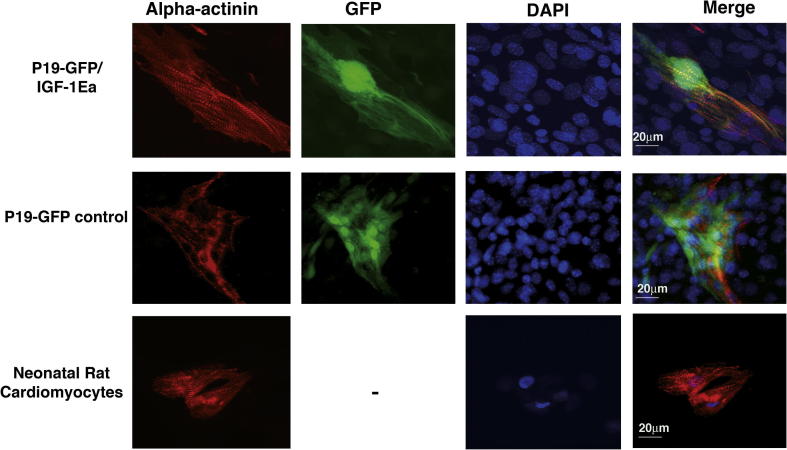
As shown previously, inhibition of the IGF-1-mediated signaling pathway abolishes cardiac commitment in ES cells without affecting endothelial cell formation [11,21]. As a result, induced IGF-1 overexpression could lead to increased amount of cardiac muscle cells or to a more differentiated state. Therefore, we initially analyzed whether the amount of ventricular cardiomyocytes, assessed by FACS analysis of GFP positive cells, is increased in IGF-1Ea transduced cells. As shown in Fig. 3A, the percentage of FACS-sorted GFP cells is comparable between IGF-1Ea transduced cells and control cells, indicating that IGF-1Ea overexpression did not alter the percentage of ventricular cardiomyocytes formed after DMSO-induced differentiation (Fig. 3A). To test whether IGF-1 overexpression correlates with increased cardiomyogenic differentiation, real time PCR of cardiac markers (Fig. 3B–D) and immunofluorescent staining with the structural sarcomeric protein alpha-actinin (Fig. 4) was performed in differentiated clone 6 and control cells. Interestingly, we observed that IGF-1Ea induced significant upregulation of NKX-2.5 and MLC2v in DMSO-treated cells compared to undifferentiated IGF-1Ea transduced cells, as well as differentiated control cells (Fig. 3B and C). When we measured the relative fold induction of the early endodermal and mesodermal marker GATA4 we noticed a significant increased in control cells upon DMSO-induced cardiac differentiation (Fig. 3D). Interestingly, IGF-1Ea overexpression decreased GATA4 upregulation (Fig. 3D) compared to differentiated control cells, suggesting that this specific IGF-1 propeptide may induce cardiomyogenic differentiation by regulating the expression of the early mesodermal and endodermal markers. To investigate whether the increased cardiac muscle markers correlated with mature cytoskeletal organization, IGF-1Ea clone 6 and control transduced cells were stained with alpha-actinin and the ventricular specific population was identified by GFP expression (Fig. 4). IGF-1Ea transduced cells showed localization of alpha-actinin in finely organized sarcomeric structure compared to control cells and to rat neonatal cardiomyocytes (Fig. 4). Taken together, the in vitro analysis showed that overexpression of IGF-1Ea correlates with increased cardiac differentiation upon DMSO-treatment. This response was not associated with increased GFP positive cell number.
Fig. 3.
Differentiation program mediated by IGF-1Ea. (A) Percentage of GFP positive cells expressing IGF-1Ea and control cells quantified by FACS analysis. The analysis was performed in five independent experiments. Values are mean ± SEM. Significance was analyzed by Student’s t-test and p = 0.52. (B–D) qRT-PCR analysis for cardiac specific marker expression in IGF-1Ea and control cells in DMSO-differentiation conditions. (B) Nkx2.5, (C) MLC2v and (D) GATA4 expression were normalized by measuring levels of 18S rRNA.
Fig. 4.
IGF-1Ea mediated cardiomyocytes sarcomeric structure maturation. IGF-1Ea transduced cells (P19-GFP/IGF-1Ea), control cells transduced with lentivirus not expressing IGF-1Ea (P19-GFP) were cultured after embryoid bodies’ formation on 0.1% gelatin. Cardiomyocytes (GFP labeled panels) were stained with alpha actinin monoclonal antibody (Sigma). Nuclei were visualized by DAPI staining. Neonatal rat cardiomyocytes were used as positive control (lower panels). The measurement bar is 20 μm. The pictures are representative of two independent experiments.
4. Discussion
The limited potential of the mammalian myocardium for self-repair and renewal entails the necessity to find efficient molecular and cellular targeting repairing the scarred heart and improving cardiac function after myocardial ischemic disease.
One possibility is to mobilize endogenous cells able of regenerating the infarcted heart by differentiation into cardiomyocytes and endothelial cells. Alternatively, exogenous sources of cells should be used to replace damaged cardiac tissue.
Either ways are limited by the low number of cells capable of differentiating into contractile cardiomyocytes in order to repopulate the extensive loss of tissue occurring after myocardial infarct.
Our previous data showed that the insulin-like growth factor 1 propeptide IGF-1Ea induced cardiac repair after infarct by lowering scar formation and maintaining tissue integrity. To investigate the possibility that this propeptide can elicit cardiac lineage differentiation, in this manuscript we analyzed the effect of IGF-1Ea on pluripotent stem cells. As proof of principle, we used P19 embryonal carcinoma cells expressing GFP under the control of the cardiac specific promoter MLC2v. To ensure long-term constitutive expression of IGF-1Ea, we cloned Igf-1Ea cDNA into a lentiviral backbone. We selected 16 clones and clone 6 showed normal embryoid body formation and cardiomyocyte differentiation. Our initial in vitro analyses showed that IGF-1Ea was highly expressed in transduced P19-GFP cells and that overexpression was retained after DMSO-induced cardiac cell differentiation. Interestingly, expression of IGF-1 was upregulated also in differentiated control cells, indicating that DMSO-induced cardiac differentiation activates endogenous IGF-1 transcription.
Furthermore, IGF-1Ea induced increased cell survival upon hypoxic conditions and did not alter cell cycle. This was advantageous in our system, since proliferative signaling could change the expression of transcription factors, undermining the role of IGF-1Ea in regulating the differentiation program. In this manuscript, we aimed to understand whether the propeptide has a role in cardiogenesis for therapeutical use. We have observed that IGF-1Ea overexpression in undifferentiated cells correlated with increased vimentin and nodal expression. Vimentin is an intermediate filament found in mesenchymal cells [22]. Although vimentin knockout mice (vim−/−) have mild phenotype [23], it has been observed that migratory-deficient vim−/− fibroblasts failed to heal wounded epithelial tissue and that vim−/− lymphocytes have impaired transmigration and extravasation capacities [24,25]. The above data argue for a role of vimentin in cell migration. On this note, in bovine embryos the recruitment of cells from the epiblast to form the primitive streak as well as the endoderm and mesoderm is associated with expression of the intermediate filament vimentin [26], suggesting that vimentin can mark cells that specify the endodermal and the mesodermal field. Indeed, in our study we have found that IGF-1Ea-mediated vimentin regulation was associated with the presence of mesodermal but not ectodermal and endodermal lineage differentiation, as assessed by nodal overexpression. To our surprise upregulation of the early cardiac markers NKX-2.5 [27] was found in the undifferentiated cells, suggesting that IGF-1Ea regulates mesodermal lineage differentiation towards the myocardial fate in uncommitted cells.
Interestingly, IGF-1Ea potentiated the cardiogenic effects of DMSO treatment by increasing the expression of cardiac specific markers NKX-2.5 and MLC2v. In addition, we noted that GATA4 was downregulated by IGF-1Ea compared to control differentiated cells, suggesting a fine regulation of the endodermal/mesodermal cell lineage component by this specific propeptide. It has been reported that GATA4 is not necessary for cardiomyocyte differentiation [28], but it is capable of specifying endoderm fates to facilitate the generation of cardiomyocyte progenitors from associated mesoderm [20]. The extent of IGF-1Ea control of cardiac differentiation and endodermal/mesodermal specification is therefore a novelty and it will need to be further analyzed. Although IGF-1 is recognized as a potent mitogen in neoplasia [29] and during development [17], P19-GFP cells did not change their proliferative state during undifferentiated conditions. This may be caused by molecular and physiological differences between the in vivo and the in vitro system. Nevertheless, under DMSO-differentiation conditions the amount of GFP + cardiomyocytes remained invariant between IGF-1Ea overexpressing and control cells. On the contrary, IGF-1Ea overexpression correlated with mature sarcomeric structure, indicating that this specific isoform favors cardiac differentiation in both undifferentiated cells and cells stimulated to differentiate by DMSO treatment.
In addition to the above considerations, our analysis could be important to redirect gene therapy studies. Indeed, before gene therapy can become a permanent cure for any condition, the therapeutic DNA introduced into target cells must remain functional and the cells containing the therapeutic DNA must be long-lived and stable. Furthermore, while viruses are the carrier of choice in most gene therapy studies, they present a variety of potential problems to the patient such as toxicity, immune and inflammatory responses, as well as the ability to cause disease. These features preclude the usage of gene therapy in large scale clinical trial. Our study suggests that cell-mediated gene delivery could be used to reduce the risk of ectopic expression.
Taken together, our data propose to test cell-mediated IGF-1Ea delivery in therapeutical studies to induce cardiac stem cell differentiation into cardiomyocytes in vivo and suggest to further analyze the in vitro ability of the propeptide to elicit cardiac differentiation of pluripotent cells for transplantation experiments.
Acknowledgments
This work was supported by the British Heart Foundation Project Grant number PG/10/019. Dr. Bhawana Poudel was granted an EMBO fellowship (ASTF 108.00-2010) to perform FACS analysis at EMBL. P19-GFP cells have been kindly provided by Professor Christine Mummery at Leiden University Medical Centre, The Netherlands.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbrc.2011.11.028.
Appendix A. Supplementary data
Supplementary Figure 1.
Supplementary Figure 2.
Supplementary Figure 3.
References
- 1.Sutton M.G., Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 2.Capi O., Gepstein L. Myocardial regeneration strategies using human embryonic stem cell-derived cardiomyocytes. J. Control Release. 2006;116:211–218. doi: 10.1016/j.jconrel.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 3.Naito A.T., Shiojima I., Akazawa H., Hidaka K., Morisaki T., Kikuchi A., Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc. Natl. Acad. Sci. USA. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueno S., Weidinger G., Osugi T., Kohn A.D., Golob J.L., Pabon L., Reinecke H., Moon R.T., Murry C.E. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi T., Lord B., Schulze P.C., Fryer R.M., Sarang S.S., Gullans S.R., Lee R.T. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation. 2003;107:1912–1916. doi: 10.1161/01.CIR.0000064899.53876.A3. [DOI] [PubMed] [Google Scholar]
- 6.Wei Z.L., Petukhov P.A., Bizik F., Teixeira J.C., Mercola M., Volpe E.A., Glazer R.I., Willson T.M., Kozikowski A.P. Isoxazolyl-serine-based agonists of peroxisome proliferator-activated receptor: design, synthesis, and effects on cardiomyocyte differentiation. J. Am. Chem. Soc. 2004;126:16714–16715. doi: 10.1021/ja046386l. [DOI] [PubMed] [Google Scholar]
- 7.Willems E., Spiering S., Davidovics H., Lanier M., Xia Z., Dawson M., Cashman J., Mercola M. Small-molecule inhibitors of the wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circ. Res. 2011;109:360–364. doi: 10.1161/CIRCRESAHA.111.249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braam S.R., Denning C., van den Brink S., Kats P., Hochstenbach R., Passier R., Mummery C.L. Improved genetic manipulation of human embryonic stem cells. Nat. Methods. 2008;5:389–392. doi: 10.1038/nmeth.1200. [DOI] [PubMed] [Google Scholar]
- 9.Passier R., Van Laake L.W., Mummery C.L. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 10.Santini M.P., Tsao L., Monassier L., Theodoropoulos C., Carter J., Lara-Pezzi E., Slonimsky E., Salimova E., Delafontaine P., Song Y.H., Bergmann M., Freund C., Suzuki K., Rosenthal N. Enhancing repair of the mammalian heart. Circ. Res. 2007;100:1732–1740. doi: 10.1161/CIRCRESAHA.107.148791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klinz F., Bloch W., Addicks K., Hescheler J. Inhibition of phosphatidylinositol-3-kinase blocks development of functional embryonic cardiomyocytes. Exp. Cell Res. 1999;247:79–83. doi: 10.1006/excr.1998.4309. [DOI] [PubMed] [Google Scholar]
- 12.K.Y. Xin, L.B. Sutherland, X. Qi, J. McAnally, R.J. Schwartz, J.A. Richardson, R. Bassel-Duby, E.N. Olson, Regulation of insulin-like growth factor signaling by yap governs cardiomyocyte proliferation and embryonic heart size, Sci. Signal. 4 (2011) ra70. [DOI] [PMC free article] [PubMed]
- 13.Moore J.C., Spijker R., Martens A.C., de Boer T., Rook M.B., van der Heyden M.A., Tertoolen L.G., Mummery C.L. A P19Cl6 GFP reporter line to quantify cardiomyocyte differentiation of stem cells. Int. J. Dev. Biol. 2004;48:47–55. doi: 10.1387/ijdb.15005574. [DOI] [PubMed] [Google Scholar]
- 14.Chao W., Matsui T., Novikov M.S., Tao J., Li L., Liu H., Ahn Y., Rosenzweig A. Strategic advantages of insulin-like growth factor-I expression for cardioprotection. J. Gene Med. 2003;5:277–286. doi: 10.1002/jgm.347. [DOI] [PubMed] [Google Scholar]
- 15.Barres B.A., Hart I.K., Coles H.S., Burne J.F., Voyvodic J.T., Richardson W.D., Raff M.C. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- 16.Chakravarthy M.V., Fiorotto M.L., Schwartz R.J., Booth F.W. Long-term insulin-like growth factor-I expression in skeletal muscles attenuates the enhanced in vitro proliferation ability of the resident satellite cells in transgenic mice. Mech. Ageing Dev. 2001;122:1303–1320. doi: 10.1016/s0047-6374(01)00263-9. [DOI] [PubMed] [Google Scholar]
- 17.Liu J.P., Baker J., Perkins A.S., Robertson E.J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 18.Sachinidis A., Fleischmann B.K., Kolossov E., Wartenberg M., Sauer H., Hescheler J. Cardiac specific differentiation of mouse embryonic stem cells. Cardiovasc. Res. 2003;58:278–291. doi: 10.1016/s0008-6363(03)00248-7. [DOI] [PubMed] [Google Scholar]
- 19.Zakin L., Reversade B., Virlon B., Rusniok C., Glaser P., Elalouf J.M., Brulet P. Gene expression profiles in normal and Otx2−/− early gastrulating mouse embryos. Proc. Natl. Acad. Sci. USA. 2000;97:14388–14393. doi: 10.1073/pnas.011513398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holtzinger A., Rosenfeld G.E., Evans T. Gata4 directs development of cardiac-inducing endoderm from ES cells. Dev. Biol. 2010;337:63–73. doi: 10.1016/j.ydbio.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauer H., Rahimi G., Hescheler J., Wartenberg M. Role of reactive oxygen species and phosphatidylinositol 3-kinase in cardiomyocyte differentiation of embryonic stem cells. FEBS Lett. 2000;476:218–223. doi: 10.1016/s0014-5793(00)01747-6. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson J.E., Dechat T., Grin B., Helfand B., Mendez M., Pallari H.M., Goldman R.D. Introducing intermediate filaments: from discovery to disease. J. Clin. Invest. 2009;119:1763–1771. doi: 10.1172/JCI38339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colucci-Guyon E., Portier M.M., Dunia I., Paulin D., Pournin S., Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994;79:679–694. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 24.Eckes B., Colucci-Guyon E., Smola H., Nodder S., Babinet C., Krieg T., Martin P. Impaired wound healing in embryonic and adult mice lacking vimentin. J. Cell Sci. 2000;113(Pt 13):2455–2462. doi: 10.1242/jcs.113.13.2455. [DOI] [PubMed] [Google Scholar]
- 25.Nieminen M., Henttinen T., Merinen M., Marttila-Ichihara F., Eriksson J.E., Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat. Cell Biol. 2006;8:156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 26.Maddox-Hyttel P., Alexopoulos N.I., Vajta G., Lewis I., Rogers P., Cann L., Callesen H., Tveden-Nyborg P., Trounson A. Immunohistochemical and ultrastructural characterization of the initial post-hatching development of bovine embryos. Reproduction. 2003;125:607–623. [PubMed] [Google Scholar]
- 27.T.J. Lints, L.M. Parsons, L. Hartley, I. Lyons, R.P. Harvey, Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants, Development 119 (1993) 969. [DOI] [PubMed]
- 28.Narita N., Bielinska M., Wilson D.B. Cardiomyocyte differentiation by GATA-4-deficient embryonic stem cells. Development. 1997;124:3755–3764. doi: 10.1242/dev.124.19.3755. [DOI] [PubMed] [Google Scholar]
- 29.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.