Abstract
Histiocytic sarcoma (HS) and histiocyte–associated lymphoma (HAL) of mice are difficult to distinguish histologically. Studies of multiple cases initially diagnosed as HS or HAL allowed us to define HS as round, fusiform, or mixed cell types that were F4/80+, Mac–2+, and PAX5−; that lacked markers for other sarcomas; and that had immune receptor genes in germline configuration. Two other subsets had clonal populations of lymphocytes. The first, HAL, featured malignant lymphocytes admixed with large populations of normal–appearing histiocytes. The second appeared to be composites of lymphoma and HS. Several cases suggestive of B myeloid–lineage plasticity were also observed.
Keywords: histiocytic sarcoma, histiocyte–associated lymphoma, immunohistochemistry, lymphoma, Southern blotting, tissue microarray
Historically, lymphoid neoplasms have been mistaken for histiocytic proliferations. For example, one category in Rappaport’s 1966 classification of human hematopoietic neoplasms was “histiocytic lymphoma.”47 Now, virtually all cases from that category are recognized as diffuse large B cell lymphomas (DLBCL).28 Similarly for mice, many cases of “reticulum cell sarcoma–type B” from Dunn’s 1954 classification14 are now recognized as covering a spectrum of B cell–lineage neoplasia.41 Currently, histiocytic sarcoma (HS) is viewed as a rare neoplasm in both species.
HS in rodents has been described with a variety of terms, including reticulum cell sarcoma–type A, malignant histiocytosis, and Kupffer cell sarcoma, among others, all indicative of some early confusion about their cellular origins. More recent studies in mice showing expression for Mac–2 (LGALS3) and lysozyme are clearly consistent with HS originating from cells of the mononuclear phagocytic system.53 The incidence of these tumors varies significantly among mice, with strain, age, and sex being among the known variables.21,22,33
A recent report revealed a previously unappreciated heterogeneity among malignancies initially diagnosed as HS in AKXD recombinant inbred (RI) strains of mice.43 Through the combined use of histologic studies and molecular analyses for clonality of immunoglobulin heavy chain gene (IgH) and T cell receptor β chain gene (TCRβ) organization and ecotropic murine leukemia virus (MuLV) insertions, it was possible to define 3 categories of histiocyte–rich neoplasms: first, HS lacking clonal rearrangements of Ig or T cell receptor (TCR) loci; second, histiocyte–associated lymphoma (HAL), B cell lineage, exhibiting clonal rearrangements of Ig but not TCR genes; and, third, HAL associated with clonal rearrangements of Ig and TCR loci, indicting the coexistence of B and T cell lymphomas containing large numbers of histiocytes.43
In the present study, we have combined histologic, molecular, and immunohistochemical (IHC) analyses to study 55 histiocyte–rich neoplasms, 41 diagnosed as HS and 14 as HAL, derived primarily from NFS. V+ congenic mice6,24 with the goal of extending the range of features that differentiate HS from HAL.
Materials and Methods
Mice and Disease Classification
During an approximately 8–year period, 5,767 necropsies were performed in the Laboratory of Immunopathology, with more than half as part of studies of the mouse retrovirus–induced immunodeficiency syndrome, or MAIDS.42 Among other cases were NFS. V+ mice congenic for induction loci of ecotropic MuLVs of AKR or C58 mice,24 NZB/BlNJ mice (Jackson Laboratory, Bar Harbor, Maine), CFW mice,52 and a small series of genetically engineered mice—iMyc knock-in mice45,56 as well as κ-Myc and TCL1 transgenic (TG) mice25,26; 105 were diagnosed as HS and another 70 as HAL. The majority (n, 149) were NFS. V+ mice held for long–term observation for tumor development and necropsied at advanced stages, detected mainly by palpation of spleen and lymph nodes (LN). From the 175 mice diagnosed as HS or HAL, we selected 55 for further study that had material collected at necropsy for future molecular studies of DNA and RNA, with 41 diagnosed as HS and 14 as HAL.
The average age at diagnosis for HS and HAL was about 535 days. The male:female ratio was 1:1 for HS and 1.0:2.4 for HAL. Spleen weights of 900 mg or greater were recorded for 50% of cases diagnosed as HS and for 71% of cases diagnosed as HAL. HS involving only the uterine myometrium54 was diagnosed for 4 NZB and 3 iMyc TG mice. All initial histologic diagnoses were made on the basis of gross observations and histologic sections stained with hematoxylin and eosin (HE) by a pathologist experienced with the consensus nomenclatures for mouse hematopoietic neoplasms30,41 and the initial identification and dissection of histiocyte–rich neoplasms in mice.43 The same pathologist reviewed the diagnoses at the time the present study was undertaken and identified 6 cases originally diagnosed as HS as being splenic marginal zone lymphoma (MZL). All 6 had prominent clonal rearrangements of JH by Southern blot hybridization. Diagnoses of other cases were revised, as appropriate, with the addition of information gained from molecular studies of immune receptor gene organization and the results of IHC analyses.
Southern Blotting
High molecular weight DNA prepared from the tumors was digested with EcoRI for studies of IgH organization and ecotropic MuLV integrations and with HpaI for analyses of TCRβ organization, separated electrophoretically, and transferred to nitrocellulose. The membranes were hybridized with 32P–labeled probes for JH and TCRβ25,31 as previously described.20,24
Tissue Microarray Construction
Areas of tumor involvement seen on HE slides were marked by 2 pathologists to guide sample collection from the associated paraffin blocks for use in tissue microarrays (TMAs). Three types of tissues, including spleen, LN, and liver, were selected in each case, given that the tumors often involve more than one organ. The TMA was then manually constructed using an arrayer (Beecher Instruments Microarray Technology, Sun Prairie, Wisc) according to the TMA map. A 1–mm-diameter needle was used so that all samples could be arranged in a single block. The TMA was cut at 4 μm and mounted on Super-frost/Plus slides (Fisher Scientific, Pittsburgh, Pennia). One section was stained with HE, and the others were used for immunostaining. Histologic diagnoses based on evaluations of the TMA HE slide were validated against those made from the original HE slides.
Immunohistochemistry
An avidin–biotin–peroxidase method was employed for antigen detection23 with particular emphasis placed on antibodies recognizing PAX5, F4/80, and Mac-2. PAX5 was examined for its lineage-specific identification of the spectrum of B cells ranging from bone marrow B cells up to, but not including, plasma cells; F4/80, for increasing expression during monocyte to red pulp macrophage differentiation; and Mac-2, for identification of red pulp and white pulp macrophages.
Briefly, slides were deparaffinized in xylene and rehydrated through a graded series of alcohol washes. Endogenous peroxidase activity was blocked by treatment with 3% H2O2 in methanol for 30 minutes. Slides to be stained for F4/80 (EMR1) and Mac–2 (LGALS3) were then digested in 0.1% trypsin in 0.1% calcium chloride solution (pH 7.8) at 37°C for 10 to 30 minutes while slides to be stained for PAX5 were heated in a pressure cooker in 0.01M citrate buffer for antigen retrieval (pH 6.0). After that, sections were incubated for 20 to 30 minutes in 10% normal goat serum for F4/80 and Mac–2 and in 10% horse serum for PAX5 and then incubated for 1 hour with antibody to F4/80 (Clone CI:A3–1; Serotec Inc, Raleigh, North Carolina) diluted 1:50 in 10% normal goat serum, Mac–2 (M3/38; Cedarlane Labs, Burlington, Ontario, Canada) diluted 1:200 in 10% normal goat serum, and mouse anti–human PAX5 (Clone 24; BD Bioscience, San José, Calif) diluted 1:25 in 10% normal horse serum. The slides were rinsed in phosphate buffered saline and incubated in biotinylated goat anti–rat immunoglobulin G (IgG) for F4/80 and Mac–2 (1:50 in 10% normal goat serum) and in biotinylated horse anti–mouse IgG (1:200 in 10% normal horse serum) for PAX5 for 30 minutes and subsequently incubated in VectorStain Elite ABC reagent for 30 minutes; 3,3′–diaminobenzidine (Vector Laboratories Inc, Burlingame, Conn) was used for chromogen. Slides were counterstained for 2 to 3 minutes with hematoxylin (Fisher Scientific) and mounted with Permount (Fisher Scientific). Using rat IgG2b in place of F4/80 and Mac–2 antibodies at a similar dilution generated a negative control. The method used for scoring immunoreactivity has been described.23 Positivity and intensity were combined for scoring F4/80 and Mac–2 expression, whereas only positivity was considered for PAX5 expression because PAX5 nuclear staining was uniform for B cell populations.
A similar procedure was used to stain TMA slides with antibodies for human smooth muscle actin, rat myogenin, human Von Willebrand factor, human lysozyme, and human desmin (purchased from Dako, Carpenteria, Calif).
Results
Characteristics of Mice With Histiocyte–Rich Neoplasms
In the present study, we selected 55 cases of histiocyte–rich neoplasms with material saved for molecular analyses: 41 diagnosed histologically as HS and 14 diagnosed as HAL. To simplify the process of understanding the data to be presented below, we can summarize the conclusions from these studies as follows: Only about 33% of cases initially diagnosed histologically as HS were confirmed as HS by molecular and IHC criteria. The remainders were divided almost equally among those previously defined as HAL—that is, a mixture of transformed B cells and invasive, seemingly nontransformed histiocytes43—and a hypothetical previously unrecognized diagnostic category, yet to be well defined, of composites composed of HS and lymphoma.
For the 55 histiocyte-rich cases, all cases but 8 were NFS. V+ (6, NFS. V− and 2, MuLV–infected NIH Swiss mice). The spleen was almost always involved, as it is for mouse hematopoietic neoplasms. Histiocytic proliferations were usually found to disseminate from spleen to various LN, including the cervical, axillary, parathymic, mediastinal, inguinal, splenic, and peritoneal, but a few appeared to originate in the LN. Liver was involved more frequently than kidney and lung.
Previous studies showed that many cases diagnosed histologically as HS among a large number of neoplasms of the AKXD RI and other strains harbored B and/or T cell lymphomas, as indicated by clonal rearrangements of Ig heavy and light chain or TCRβ genes.41 Rearrangements of Ig genes are characteristic features of normal B cells and B cell–lineage lymphomas, whereas almost all normal myeloid and T cells are negative. Some studies of normal cells and cell lines have shown that clonal JH rearrangements can be found in cells with features of macrophages17 and that a small proportion of human histiocytic/dendritic cell sarcomas can transdifferentiate from a follicular lymphoma clone.16 Thus, the lack of such rearrangements rules out the contribution of a clonal B cell population to a neoplasm, although their presence does not definitively exclude macrophages as possible contributors.
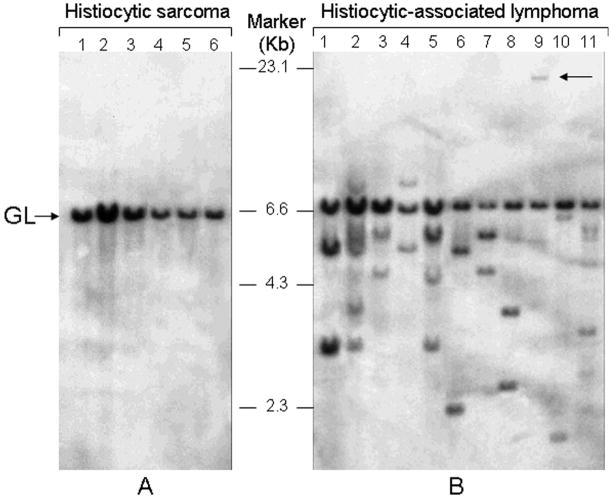
In the current study, we analyzed DNA from all 55 cases for the genomic organization of JH and all but 6 for the organization of TCRβ genes by Southern blot hybridization (Fig. 1; data not shown). Of these, 12 cases were found to have JH genes in germline configuration. One of the cases with germline organization for JH was rearranged for TCRβ (data not shown). At necropsy, this mouse was diagnosed with a histiocyte–rich nonthymic lymphoblastic lymphoma that now was clearly of T cell origin, as determined by positive staining for CD3 (not shown). DNA from 3 other cases had faint JH bands, possibly indicative of small populations of clonal B cells or, more likely, polyclonal B cell populations, with the faint bands representing multiple stochastic rearrangements of similar size. Further studies of DNA from 10 of these 14 cases with germline or faintly rearranged bands revealed germline configurations for IgH kappa light chain and TCRβ chain genes, consistent with an absence of B or T cell clones. We concluded that only 14 of the 55 cases could be diagnosed as HS, based on having immune receptor genes essentially in their germline configuration. Importantly, all 14 cases had initially been diagnosed histologically as HS.
Figure 1.
Southern blot analyses of mouse hematopoietic neoplasms originally diagnosed as histiocytic sarcoma. Genomic organization of immunoglobulin H genes was examined by hybridization of DNA digested with EcoRI and hybridized with a JH probe. Pathology number related to the 17 DNA samples studied are available from the senior author on request. A, histiocytic sarcomas exhibit only a germline JH band. B, for HA, clonal rearrangements of JH distinct from the germline band are seen in each lane. Cases showing 2 distinct bands of equal intensity are indicative of a single clone with both IgH alleles rearranged. Cases in lanes 2, 5, and 11 with 3 or more bands are judged to be oligoclonal. The arrow associated with lane 9 points to a rearrangement on a large EcoRI fragment.
Of the 41 other cases, 40 had one or more clear nongermline JH bands that hybridized at low to high intensity of varying strength, consistent with the presence of 1 or 2 B cell clones. Of the cases with B cell clones, 4 exhibited 1 or 2 clonal rearrangements of TCRβ, indicating the coexistence of T and B lymphomas (data not shown). Among the 40 cases with clonal JH rearrangements, 27 had initially been diagnosed histologically as HS and 13 as HAL. Among the 5 cases with TCRβ rearrangements, 3 had been diagnosed as HS and 2 as HAL. Because the diagnosis of HS for human cases requires the absence of clonal rearrangements of immune receptor genes,28 our results indicate that only a third of the mouse cases diagnosed histologically as HS fit the molecular criteria for their designation as true HS.
Studies of HE–stained slides from cases of HS, defined by the molecular criteria given above, revealed histiocytes of varying cytologies, ranging from large round cells with macrophage–like features to fusiform, spindle–shaped cells with a fibroblastoid or spindle–shaped appearance occurring in intertwining bands either as pure populations or in mixtures of varying proportions. In lung, liver, and kidney, they presented as single or multiple nodular masses, sometimes associated with histioblasts and mitotic figures. The Ki–67 index in all cases was less than 1%. In the spleen, they exhibited diffuse involvement of the red pulp and/or were localized to follicles, completely replacing the normal population of lymphocytes. LN frequently exhibited a profound intrasinusoidal histiocytosis in early cases but often contained solid masses replacing most or all the node in more advanced disease. Liver involvement was usually associated with perivascular infiltrates and often with Kupffer cell proliferation.
The round–cell type of HS is composed of sheets of large mononuclear cells with abundant eosinophilic cytoplasm that is often foamy or vacuolated (Fig. 2). The vesicular nuclei are usually round or ovoid; the nuclear membranes are thick; and the nucleoli are prominent. Mitotic figures are uncommon but present in large cells that clearly resembled histioblasts. Erythrophagocytosis and multinucleate giant cells are common features. Remnants of normal cell populations are usually present, but destruction of normal splenic architecture is common.
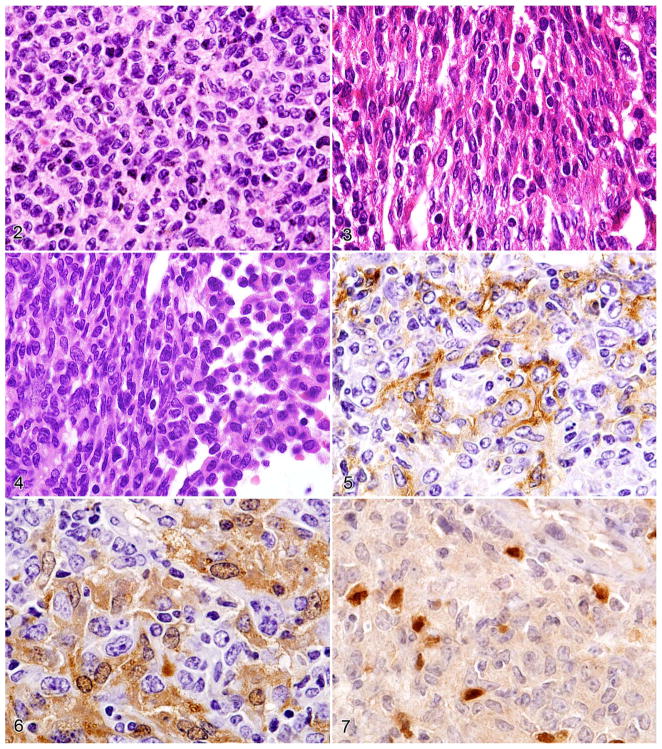
Figures 2–7.
Figure 2. HS, round–cell type. Note the round nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm. Figure 3. HS, spindle–cell type. Note the elongated and convoluted nuclei. Figure 4. HS, round- and spindle–cell type. Note round- (right side) and spindle-cell components (left side). Serial sections of HS stained with antibodies to the following: Figure 5. F4/80 (immunoreactive cell membrane). Figure 6. Mac–2 (immunoreactive cytoplasm and nucleus). Figure 7. PAX5 (note PAX5– HS tumor cells admixed with normal PAX5+ B cells).
The spindle–cell type of HS is characterized by solid sheets of elongated cells with much less cytoplasm than the round–cell type (Fig. 3). The nuclei are elongated, sometimes tear–shaped, and convoluted. Few lymphocytes or other cell populations are interspersed among the tumor cells. Mitotic figures have not been observed in spindle cells.
In the mixed–cell type of HS comprising round and spindle cells, the 2 components are either discrete and directly juxtaposed (Fig. 4) or intermingled. In spleen and liver, one can sometimes see transitions from round or ovoid cells to spindle cells. In round cell, spindle cell, or mixed cases, neoplastic histiocytes are F4/80+ and Mac-2-immunoreactive (Figs. 5, 6), and small round PAX5+ normal B cells can be seen in greater or lesser density to infiltrate the histiocyte accumulations (Fig. 7).
Histopathologic and immunohistochemical features of histiocytic sarcoma (HS):
Some earlier studies of mouse HS used cell type–specific markers to identify their origin from histiocytes54 but lacked a basis for distinguishing between antigen–positive subsets with or without clonal JH rearrangements. Other stromal tumors, such as leiomyomas, leiomyosarcomas, fibrosarcomas, hemangiosarcomas, and rhabdomyosarcomas, would be expected to have their Ig genes in germline configuration. To determine whether the diagnosed HS might arise from cells other than histiocytes, we studied them by IHC on constructed TMA slides for expression of antigens useful in establishing the diagnosis of soft tissue tumors, including smooth muscle actin, myogenin, desmin, and Von Willebrand factor.8 The neoplasms failed to stain with any of these reagents, even though vascular smooth muscle was positive for actin and desmin, and vascular endothelial cells and megakaryocytes were positive for Von Willebrand factor (data not shown). Although IHC for CD31, another endothelial marker, was not performed, probes for Cd31 transcripts present on the oligonucleotide microarrays used for transcriptional profiling of HS showed that levels of expression were not higher than those for normal spleen (data not shown). These findings exclude vascular smooth muscle cells, vascular endothelial cells, skeletal muscle cells, and fibroblasts as cellular origins for HS and support the suggestion that they are histiocyte derived. Together these data indicate that HS can be defined by the absence of immune receptor gene rearrangements; histologic presentations as histiocytes of round, fusiform, or mixed–cell types; and the lack of markers characteristic of sarcomas of other cellular origins.
Characteristics of Histiocyte–Rich Neoplasms Other Than True HS
Our molecular studies showed that 27 histiocyte–rich cases initially diagnosed as HS had clonal B or T cell populations, as did 14 others initially diagnosed as HAL. A histologic reevaluation of these 41 cases suggested, unexpectedly, that they fell into 2 distinct groups; specifically, 26 resembled the HAL previously described.43 These cases were dominated by large populations of histiocytes, not dissimilar to those seen in HS and most often of the round–cell type, but they also exhibited small and usually dispersed lymphoid accumulations with features of malignant B cells of different classes or malignant T cells. The B cell–lineage tumors included follicular B cell lymphomas (FBL), centroblastic (CBL) and immunoblastic (IBL) cases of DLBCL, splenic MZL, and one FBL with added splenic features of a Littoral cell angioma (data not shown).3,15
The second subset of histiocyte–rich neoplasms with clonal B cells appeared to be composites of HS and a B cell–lineage neoplasm (FBL, CBL, MZL, or plasmacytoma), a diagnostic category we had not recognized previously. The histiocytes disseminated to spleen, LN, and less often to liver, kidney, and lung were indistinguishable from those seen in HS. Nodular accumulation in splenic follicles often displaced lymphoma cells to the periphery, and normal LN architecture was destroyed. Perhaps most telling was the widespread distribution of large histiocytes and histioblasts, sometimes with mitotic figures. Lacking definitive phenotypic or genomic markers for true HS, we currently have no way of determining whether these cases represent the coexistence of lymphomas and HS or extremes of the spectrum of HAL (see below). Future studies using comparative genomic hybridization of DNA from micro-dissected HS cells should lend support to or disprove this hypothesis.
IHC Analyses of F4/80, Mac–2, Lysozyme, and PAX5 Expression in Normal Tissue, HS, and HAL
To extend our characterizations of HS and HAL, we used IHC to study the expression of a series of proteins expressed by cells of the myeloid lineage (F4/80, Mac–2, and lysozyme) or the B cell lineage (PAX5).12,35,38,46,51 In normal spleen, F4/80 was exclusively expressed by macrophages in the red pulp but not in the white pulp; Mac–2 was expressed by macrophages in both compartments; and PAX5 was expressed in follicular, marginal zone, and red pulp B cells (data not shown). We examined the cytologic staining patterns for these antigens in cases diagnosed as HS, HAL, and HS plus lymphoma (Figs. 2, 5–7; data not shown). A case of HS with an intermixed population of round and spindle histiocytes stained with antibody to F4/80 exhibited membranous staining of the HS cells (Fig. 5). Mac–2 showed predominantly cytoplasmic but some nuclear staining in HS (Fig. 6). Staining with anti–PAX5 showed HS to be uniformly negative in round and fusiform cells, although small-and medium–sized B cells interspersed among the histiocytes showed intense nuclear staining (Fig. 7). Similar staining patterns were seen with the histiocytic component of the composite tumors (data not shown).
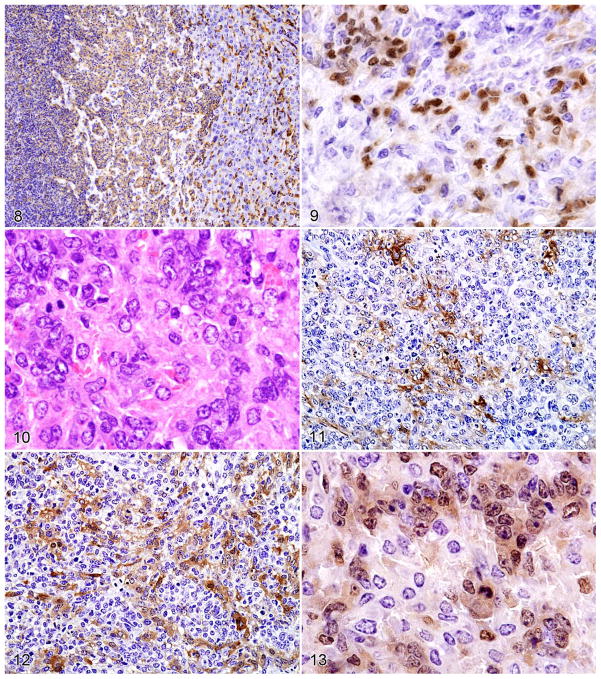
A case of HS in the liver, composed of populations of round and spindle cells (Fig. 4), was particularly informative for understanding the reactivity shown by antibody to F4/80. As shown in Fig. 4, an area of normal hepatocytes on the right was infiltrated by a population of large round histiocytes, seen best in the middle, that merged into a population of fusiform histiocytes on the left. Staining with antibody to F4/80 (Fig. 8) showed that reactivity was highest on the normal Kupffer cells in the normal hepatic portion. The population of round–to oval–shaped histiocytes stained less intensely, and the fusiform cells were the least reactive. This pattern of F4/80 reactivity on HS—intermediate to high on round cells and low to negative on fusiform cells—was characteristic of the staining seen throughout this survey of HS, HAL, and composite cases. Because expression of F4/80 increases during the normal maturation of monocytes to macrophages,38,51 these results suggest that the round histiocytes in HS represent a more mature population than that of the fusiform cells. PAX5 was not expressed by the HS cells but was positive on scattered normal B cells (Fig. 9)
Figures 8–13.
Figure 8. Histiocytic sarcoma in the liver stained with antibody to F4/80 showing high-level expression by normal Kupffer cells (right side), lower expression by the round–cell component of the histiocytic sarcoma (middle portion), and the lowest levels of expression by the spindle–cell component (left side). Figure 9. PAX5 staining of a spindle–type histiocytic sarcoma occupying the red pulp and surrounding a follicle with normal B cells. Note nuclear PAX5 staining of some spindle cells as well as normal B cells (upper left).
Histopathologic and immunohistochemical features of histiocyte-associated lymphoma. Serial sections of a histiocyte–associated lymphoma stained with HE showing the following: Figure 10. B-cell lymphoma and eosinophilic histiocytes. Figure 11. Antibodies to F4/80. Figure 12. Mac–2. Figure 13. PAX5. Note the more extensive staining of the histiocyte population by Mac–2 than F4/80 as they weave among the clusters of PAX5+ cells.
As reported previously,54 all round–cell histiocytes exhibited cytoplasmic localization of lysozyme with weak to strong immunoreactivity when compared with normal histiocytes (data not shown). In contrast to the patterns seen for F4/80 and Mac–2, expression of lysozyme in HS present in the liver was stronger than in normal Kupffer cells (data not shown).
The results of IHC analyses of HAL using antibodies to F4/80, Mac–2, lysozyme, and PAX5 were not remarkably different from those seen with HS. The round–cell histiocytes (Fig. 10) stained at varying levels with antibodies to F4/80 and more intensely with anti–Mac–2 (Figs. 11, 12, respectively). In addition, PAX5 was expressed only in B cells, either normal or neoplastic, but not in histiocytic elements (Fig. 13). Lysozyme was expressed in histiocytes/macrophages as well as monocytes in HAL (data not shown).
Normal and variant histiocytic sarcoma and histiocyte-associated lymphoma:
We also recognized anomalous presentations of 2 small subsets of histiocyte–rich neoplasms. The first type, exemplified by Fig. 9, featured a red pulp filled with sheets of fusiform histiocytes surrounding a normal B cell follicle with the intense PAX5 nuclear staining characteristic of normal B cells. Interestingly, some of the elongated or sharply angulated apparently histiocytic nuclei also exhibited high–level expression of PAX5. The intensity of the staining suggests that these cells might be normal centrocyte–like B cells within the matrix of histiocytes. Alternatively, they could represent instances of lineage infidelity with macrophages expressing B cell–lineage determinants. Studies of single cells obtained by laser capture microdissection would be required to determine whether this finding reflected B myeloid–lineage plasticity of clonally related neoplasms, as reported for normal cells7 or composites of HS and follicular lymphoma.16
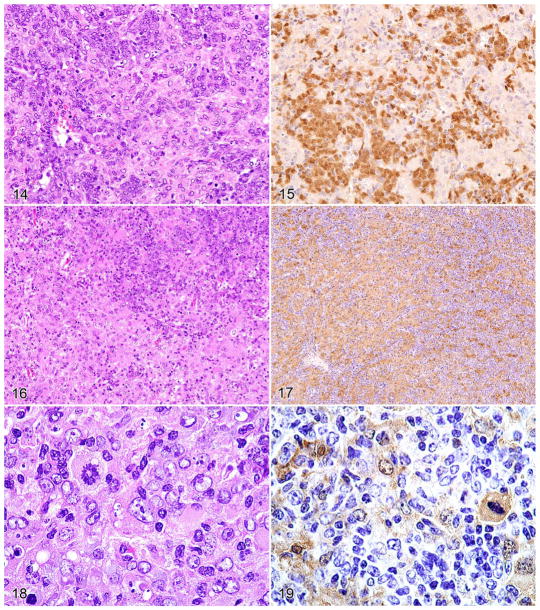
The second subset of atypical histiocyte–rich cases that are associated with clonal B cell lymphomas is illustrated in Figs. 14–19, with transformation of the associated histiocytes into HS. An area of the HAL shows an expanding population of neoplastic histiocytes (lower portion of Figs. 14, 16) that are Mac-2+ (Fig. 17). PAX5+ neoplastic B cells and nonreactive histiocytes are present (Fig. 15). Mac-2 staining of mitotic figures was also seen (Figs. 18, 19). These observations suggest that the cases may represent true composites of HS and lymphoma. Molecular criteria identifying transformed histiocytes will be required to substantiate this hypothesis.
Figures 14–19.
Histopathologic and immunohistochemical features of a HAL with foci of HS. Serial sections stained of a histiocytic sarcoma stained with HE (Figure 14), and antibodies to PAX5 (Figure 15). A focus of histiocytic sarcoma in the histiocyte-associated lymphoma, (Figure 16) stained for Mac-2 showing moderate histiocytic expression (Figure 17). Mitotic figures were seen in the focal case of histiocytic sarcoma (Figure 18) with a neoplastic mitotic histiocyte expressing Mac-2 (Figure 19).
Histopathologic and immunohistochemical features of a histiocyte-associated lymphoma with foci of histiocytic sarcoma. Serial sections stained with the following:
In summary, of the 41 cases initially diagnosed as HS, the final diagnoses included 14 HS, 15 HAL, and 12 HS plus lymphoma. Thus, 27 of 41 cases (65.8%) diagnosed as HS were associated with unapparent lymphomas. Over half the unsuspected lymphomas included MZL, alone or as a composite with FBL. The MZL may have been partially masked because of its characteristic spread into histiocyte–rich red pulp areas of HS or HAL. Almost all the rest were lymphomas of germinal center origin: FBL, CBL, and IBL. Of the 14 cases initially diagnosed as HAL, the final diagnoses included 11 HAL and 3 HS plus lymphoma. The lymphomas associated with the HAL were almost all of germinal center origin—FBL, CBL, and IBL—in addition to one composite of MZL plus anaplastic plasmacytoma.
Discussion
The combined histologic, molecular, and IHC studies of histiocyte–rich neoplasms described here provide important new insights into the origins and characteristics of spontaneous mouse HS and HAL while introducing a novel subset of these tumors: composites of HS and lymphoma. They also highlight the need for a phenotypic or molecular signature that defines transformed histiocytes. HS of humans is also of uncertain molecular pathogenesis.
In the absence of markers for transformed histiocytes, we suggest that HS is defined by germline configurations of immune receptor loci, the presence of myeloid markers, and the lack of expression of genes associated with other sarcoma types. The presence of abnormally large histioblasts is correlative but of uncertain significance. It is possible that IHC analyses for associated T and/or B cell lymphomas easily missed on examination of slides stained with HE could substitute for Southern blot hybridization analyses of immune receptor genes in diagnosing this malignancy.
By light microscopy, HS was shown to include cases with a predominance of round or spindle cells or mixed populations of the 2. Our phenotypic analyses provided strong evidence that the round cells represent a more mature population of histiocytes than that of the spindle cells, given the higher and lower levels of F4/80 expression, respectively. This perspective is based on the prior demonstration that expression of F4/80 increases with progressive maturation in the monocyte/macrophage lineage.35 Rare but highly informative cases from our series exhibited transitions from round to spindle cells in the same field in association with progressive reductions in expression of F4/80, a clear indication that the 2 histologic types were developmentally related in the same case. Analyses of other markers, including Mac–2 and lysozyme, confirmed a shared myeloid origin for the cytologically distinct populations of HS. Histopathology and immunophenotypic studies of a few cases in our series suggest that HS can originate from Kupffer cells in the liver as well as from macrophages in spleen or LN or from the uterus.
In addition to the categories of HS and HAL, we have tentatively identified a subset of histiocyte–rich tumors that are composites of HS and a lymphoid neoplasm. Support for this suggestion and distinction of the subset from HAL will require the identification and validation of molecular and/or IHC markers for transformed histiocytes. We also described another anomalous presentation, which included cases with histiocytes that appeared to express PAX5, suggesting lineage infidelity. This observation bears some similarities to rare human cases of clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas.16 In these patients, both cell types had identical IgH gene rearrangements, and they shared a t(14;18) translocation involving IgH and BCL2. Similar clonal relationships have been observed between T cell lymphoblastic leukemia/lymphoma and Langerhans cell histiocytosis and between acute lymphoblastic leukemia and HS.17 Studies of cultured cell lines have long suggested a poorly understood plasticity between the B cell and myeloid lineages,4,11,29,32 and this concept has gained increasing support from a variety of in vivo studies.*
The features of murine HS described in this report have many similarities to those of true HS in humans. HS in humans is a rare neoplasm that is sometimes associated with lymphomas and is difficult to distinguish from DLBCL or anaplastic large cell lymphoma without IHC studies. The cells are usually large, with abundant eosinophilic cytoplasm and large round nuclei with vesiculated chromatin. Spindle cells can be observed but are usually focal, a contrast with the diffuse expansions of spindle–shaped cells seen in some of our cases. In humans, the tumors are accompanied by variable numbers of eosinophils, lymphocytes, and plasma cells. The histiocytic cells variably express lysozyme and a number of other histiocytic markers, such as CD68, S–100, and CD11c. Absence of Ig and TCR gene rearrangements is required for definition of this disease by some,9 although they can clearly be found in an extremely low proportion of cases.16 Mouse and human cases are clearly distinguished by levels of mitotic activity, with frequencies of Ki67–expressing cells in the 80% range being common in human cases and with 1% representing the extreme end for cases in mice. The etiology of HS in humans is not known, although recent studies indicated that genetic or epigenetic inactivation of PTEN, p16INK4A, and p14ARF may play a role in some cases of both species.5
HAL in the mouse has a number of features in common with T cell–rich/histiocyte-rich lymphomas in humans as well as a number of distinct differences,1,36 although human and mouse cases can be readily distinguished on histologic grounds from other cases of stroma–rich B cell lymphomas. Most human cases are marked by large numbers of polyclonal T cells and lesser populations of histiocytes and clonal B cells, whereas the B cell component is more readily appreciated in mouse HA. In humans, there is a subset of cases with histiocytic predominance that more closely resembles the histologic features in mice.1,18 Although human T cell–rich/histiocyte–rich B cell lymphomas are recognized as a subset of DLBCL,27 splenic cases associated with MZL have been described.13 In the mouse, MZL is the most common lymphoma type associated with cases of HAL and compound cases of HS; lymphomas of germinal center origin (FBL, CBL, and IBL) are a close second.
The HS described here bears a number of similarities to HS identified in several strains of genetically engineered mice, including those deficient in the cytochrome C p450 gene, as well as Cyp1b153 or Cdkn2a knockout mice infected with MuLV,37 with incidences in the range of 55 to 65%. The tumors were F4/80+ and were found in the spleen, liver, and sometimes the bone marrow. A screening for common proviral integration sites in tumors of the Cdkn2a–deficient mice identified several genes associated with HS, including Ptpn6, Myst3, Dgke, and Kif13a.37 In addition, HS developed in most mice infected with an acutely transforming Ha–Ras–containing retrovirus.19 Whether any of these genes are involved in the pathogenesis of HS occurring in NFS. V+ mice will be the subject of future studies.
In summary, the present study demonstrated 3 morphologically distinct types of HS, with round, spindle, and mixed populations of histiocytes. All 3 are related on the basis of gene expression and differentiation patterns, with mature round cells expressing higher levels of F4/80 than those of spindle cells. The combination of histology with assessment of Ig clonality by Southern blotting and staining for F4/80, Mac–2, and PAX5 provides great value for distinguishing HS from HAL and other histiocyte–associated neoplasms while suggesting composites of HS with lymphoma as a new diagnostic category. The presence in some tumors of PAX5–expressing histiocytes may also provide opportunities for understanding B myeloid–lineage plasticity in neoplasia.
Acknowledgments
We thank Alfonso Macias for maintenance of the NFS. V+ mouse colony, for monitoring disease, and for necropsies; we also thank National Institute of Allergy and Infectious Diseases intramural editor Brenda Rae Marshall for assistance in preparation of the article. This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases, and National Cancer Institute and in part by a National Institute of Allergy and Infectious Diseases contract to SoBran Inc. Because X.H., T.N.F., W.H., J.M.W., C.–F.Q., J.W.H., and H.C.M. III are government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the National Institutes of Health reserves the right to provide the work to PubMedCentral for display and use by the public, and PubMedCentral may tag or modify the work consistent with its customary practices. You can establish rights outside of the United States subject to a government use license.
Financial Disclosure/Funding
The authors declared that they received no financial support for their research and/or authorship of this article.
Footnotes
Reprints and permission: sagepub.com/journalsPermissions.nav
It is not known if these lineage shifts are due to transdifferentiation or a process of dedifferentiation to a less mature cell before assumption of a second mature phenotype. Cases of composite HS and lymphoma with aberrant gene expression may provide new opportunities for dissecting this phenomenon.
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- 1.Achten R, Verhoef G, Vanuytsel L, De Wolf Peeters C. Histiocyte rich, T cell rich B cell lymphoma: a distinct diffuse large B cell lymphoma subtype showing characteristic morphologic and immunophenotypic features. Histopathology. 2002;40:31–45. doi: 10.1046/j.1365-2559.2002.01291.x. [DOI] [PubMed] [Google Scholar]
- 2.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 3.Arber DA, Strickler JG, Chen YY, Weiss LM. Splenic vascular tumors: a histologic, immunophenotypic, and virologic study. Am J Surg Pathol. 1997;21:827–835. doi: 10.1097/00000478-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Bauer SR, Holmes KL, Morse HC, III, Potter M. Clonal relationship of the lymphoblastic cell line P388 to the macrophage cell line P388D1 as evidenced by immunoglobulin gene rearrangements and expression of cell surface antigens. J Immunol. 1986;136:4695–4699. [PubMed] [Google Scholar]
- 5.Carrasco DR, Fenton T, Sukhdeo K, Protopopova M, Enos M, You MJ, Di Vizio D, Nogueira C, Stommel J, Pinkus GS, Fletcher C, Hornick JL, Cavenee WK, Furnari FB, Depinho RA. The PTEN and INK4A/ARF tumor suppressors maintain myelolymphoid homeostasis and cooperate to constrain histiocytic sarcoma development in humans. Cancer Cell. 2006;9:379–390. doi: 10.1016/j.ccr.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Chattopadhyay SK, Lowy DR, Teich NM, Levine AS, Rowe WP. Qualitative and quantitative studies of AKR type murine leukemia virus sequences in mouse DNA. Cold Spring Harbor Symp Quant Biol. 1974;39:1085–1101. doi: 10.1101/sqb.1974.039.01.124. [DOI] [PubMed] [Google Scholar]
- 7.Cobaleda C, Busslinger M. Developmental plasticity of lymphocytes. Curr Opin Immunol. 2008;20:139–148. doi: 10.1016/j.coi.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Coindre JM. Immunohistochemistry in the diagnosis of soft tissue tumours. Histopathology. 2003;43:1–16. doi: 10.1046/j.1365-2559.2003.01639.x. [DOI] [PubMed] [Google Scholar]
- 9.Copie Bergman C, Wotherspoon AC, Norton AJ, Diss TC, Isaacson PG. True histiocytic lymphoma: a morphologic, immunohistochemical, and molecular genetic study of 13 cases. Am J Surg Pathol. 1998;22:1386–1392. doi: 10.1097/00000478-199811000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Cotta CV, Zhang Z, Kim HG, Klug CA. Pax5 determines B versus T cell fate and does not block early myeloid lineage development. Blood. 2003;101:4342–4346. doi: 10.1182/blood-2002-10-3139. [DOI] [PubMed] [Google Scholar]
- 11.Davidson WF, Pierce JH, Rudikoff S, Morse HC., III Relationships between B cell and myeloid differentiation: studies with a B lymphocyte progenitor line, HAFTL 1. J Exp Med. 1988;168:389–407. doi: 10.1084/jem.168.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawe CJ, Potter M. Morphologic and biologic progression of a lymphoid neoplasm of the mouse in vivo and in vitro. Am J Pathol. 1957;33:603. [Google Scholar]
- 13.Dogan A, Burke JS, Goteri G, Stitson RN, Wotherspoon AC, Isaacson PG. Micronodular T cell/histiocyte rich large B cell lymphoma of the spleen: histology, immunophenotype, and differential diagnosis. Am J Surg Pathol. 2003;27:903–911. doi: 10.1097/00000478-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Dunn TB. Normal and pathologic anatomy of the reticular tissue in laboratory mice, with a classification and discussion of neoplasms. J Natl Cancer Inst. 1954;14:1281–1433. [PubMed] [Google Scholar]
- 15.Falk S, Stutte HJ, Frizzera G. Littoral cell angioma: a novel splenic vascular lesion demonstrating histiocytic differentiation. Am J Surg Pathol. 1991;15:1023–1033. [PubMed] [Google Scholar]
- 16.Feldman AL, Arber DA, Pittaluga S, Martinez A, Burke JS, Raffeld M, Camos M, Warnke R, Jaffe ES. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: evidence for transdifferentiation of the follicular lymphoma clone. Blood. 2008;111:5433–5439. doi: 10.1182/blood-2007-11-124792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman AL, Minniti C, Santi M, Downing JR, Raffeld M, Jaffe ES. Histiocytic sarcoma after acute lymphoblastic leukaemia: a common clonal origin. Lancet Oncol. 2004;5:248–250. doi: 10.1016/S1470-2045(04)01428-7. [DOI] [PubMed] [Google Scholar]
- 18.Fraga M, Sánchez Verde L, Forteza J, García Rivero A, Piris MA. T cell/histiocyte rich large B cell lymphoma is a disseminated aggressive neoplasm: differential diagnosis from Hodgkin’s lymphoma. Histopathology. 2002;41:216–229. doi: 10.1046/j.1365-2559.2002.01466.x. [DOI] [PubMed] [Google Scholar]
- 19.Franz T, Löhler J, Fusco A, Pragnell I, Nobis P, Padua R, Ostertag W. Transformation of mononuclear phagocytes in vivo and malignant histiocytosis caused by a novel murine spleen focus forming virus. Nature. 1985;315:149–151. doi: 10.1038/315149a0. [DOI] [PubMed] [Google Scholar]
- 20.Fredrickson TN, Hartley JH, Morse HC, Chattopadhyay SK, Lennert K. Classification of mouse lymphomas. Curr Top Microbiol Immunol. 1995;194:109–116. doi: 10.1007/978-3-642-79275-5_14. [DOI] [PubMed] [Google Scholar]
- 21.Frith CH, Davis TM, Zolotor LA, Townsend JW. Histiocytic lymphoma in the mouse. Leuk Res. 1980;4:651–662. doi: 10.1016/0145-2126(80)90076-4. [DOI] [PubMed] [Google Scholar]
- 22.Frith CH, Ward JM, Chandra M. The morphology, immunohistochemistry, and incidence of hematopoietic neoplasms in mice and rats. Toxicol Pathol. 1993;21:206–218. doi: 10.1177/019262339302100213. [DOI] [PubMed] [Google Scholar]
- 23.Hao XP, Pretlow TG, Rao JS, Pretlow TP. Beta catenin expression is altered in human colonic aberrant crypt foci. Cancer Res. 2001;61:8085–8088. [PubMed] [Google Scholar]
- 24.Hartley JW, Chattopadhyay SK, Lander MR, Taddesse Heath L, Naghashfar Z, Morse HC, III, Fredrickson TN. Accelerated appearance of multiple B cell lymphoma types in NFS/N mice congenic for ecotropic murine leukemia viruses. Lab Invest. 2000;80:159–169. doi: 10.1038/labinvest.3780020. [DOI] [PubMed] [Google Scholar]
- 25.Hedrick SM, Germain RN, Bevan MJ, Dorf M, Engel I, Fink P, Gascoignec N, Heber Katz E, Kapp J, Kaufmann Y, Kaye J, Melchers F, Pierce C, Schwartz RH, Sorensen C, Taniguchi M, Davis MM. Rearrangement and transcription of a T cell receptor beta-chain gene in different T cell subsets. Proc Natl Acad Sci U S A. 1985;82:531–535. doi: 10.1073/pnas.82.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoyer KK, French SW, Turner DE, Nguyen MT, Renard M, Malone CS, Knoetig S, Qi CF, Su TT, Cheroutre H, Wall R, Rawlings DJ, Morse HC, III, Teitell MA. Dysregulated TCL1 promotes multiple classes of mature B cell lymphoma. Proc Natl Acad Sci U S A. 2002;99:14392–14397. doi: 10.1073/pnas.212410199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaffe ES, Banks PM, Nathwani B, Said J, Swerdlow SH. Recommendations for the reporting of lymphoid neoplasms: a report from the association of directors of anatomic and surgical pathology: the Ad Hoc Committee on Reporting of Lymphoid Neoplasms. Hum Pathol. 2002;33:1064–1068. doi: 10.1053/hupa.2002.129205. [DOI] [PubMed] [Google Scholar]
- 28.Jaffe ES, Harris NL, Stein H, Vardiman JW. Pathology and genetics of tumours of hematopoietic and lymphoid tissues. IARC Press; Washignton DC: 2001. [Google Scholar]
- 29.Klinken SP, Alexander WS, Adams JM. Hemopoietic lineage switch: v raf oncogene converts Emu myc transgenic B cells into macrophages. Cell. 1988;53:857–867. doi: 10.1016/s0092-8674(88)90309-1. [DOI] [PubMed] [Google Scholar]
- 30.Kogan SC, Ward JM, Anver MR, Berman JJ, Brayton C, Cardiff RD, Carter JS, de Coronado S, Downing JR, Fredrickson TN, Haines DC, Harris AW, Harris NL, Hiai H, Jaffe ES, MacLennan IC, Pandolfi PP, Pattengale PK, Perkins AS, Simpson RM, Tuttle MS, Wong JF, Morse HC., III Hematopathology Subcommittee of the Mouse Models of Human Cancers Consortium: Bethesda proposals for classification of nonlymphoid hematopoietic neoplasms in mice. Blood. 2002;100:238–245. doi: 10.1182/blood.v100.1.238. [DOI] [PubMed] [Google Scholar]
- 31.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 32.Lacaud G, Carlsson L, Keller G. Identification of a fetal hematopoietic precursor with B cell, T cell, and macrophage potential. Immunity. 1998;9:827–838. doi: 10.1016/s1074-7613(00)80648-2. [DOI] [PubMed] [Google Scholar]
- 33.Lacroix Triki M, Lacoste Collin L, Jozan S, Charlet JP, Caratero C, Courtade M. Histiocytic sarcoma in C57BL/6J female mice is associated with liver hematopoiesis: review of 41 cases. Toxicol Pathol. 2003;31:304–309. doi: 10.1080/01926230390204342. [DOI] [PubMed] [Google Scholar]
- 34.Lang RB, Stanton LW, Marcu KB. On immunoglobulin heavy chain gene switching: two gamma 2b genes are rearranged via switch sequences in MPC 11 cells but only one is expressed. Nucleic Acids Res. 1982;10:611–630. doi: 10.1093/nar/10.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SH, Starkey PM, Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues: immunochemical studies with monoclonal antibody F4/80. J Exp Med. 1985;161:475–489. doi: 10.1084/jem.161.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim MS, Beaty M, Sorbara L, Cheng RZ, Pittaluga S, Raffeld M, Jaffe ES. T cell/histiocyte rich large B cell lymphoma: a heterogeneous entity with derivation from germinal center B cells. Am J Surg Pathol. 2002;26:1458–1466. doi: 10.1097/00000478-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Lund AH, Turner G, Trubetskoy A, Verhoeven E, Wientjens E, Hulsman D, Russell R, DePinho RA, Lenz J, van Lohuizen M. Genome wide retroviral insertional tagging of genes involved in cancer in Cdkn2a deficient mice. Nat Genet. 2002;32:160–165. doi: 10.1038/ng956. [DOI] [PubMed] [Google Scholar]
- 38.McKnight AJ, MacFarland AJ, Dri P, Turley L, Willis AC, Gordon S. Molecular cloning of F4/80, a murine macrophage restricted cell surface glycoprotein with homology to the G protein linked transmembrane 7 hormone receptor family. J Biol Chem. 1996;271:486–489. doi: 10.1074/jbc.271.1.486. [DOI] [PubMed] [Google Scholar]
- 39.Mikkola I, Heavey B, Horcher M, Busslinger M. Reversion of B cell commitment upon loss of Pax5 expression. Science. 2002;297:110–113. doi: 10.1126/science.1067518. [DOI] [PubMed] [Google Scholar]
- 40.Montecino Rodriguez E, Dorshkind K. Identification of B/macrophage progenitors in adult bone marrow. Semin Immunol. 2002;14:371–376. doi: 10.1016/s1044532302000714. [DOI] [PubMed] [Google Scholar]
- 41.Morse HC, III, Anver MR, Fredrickson TN, Haines DC, Harris AW, Harris NL, Jaffe ES, Kogan SC, MacLennan IC, Pattengale PK, Ward JM. Hematopathology Subcommittee of the Mouse Models of Human Cancers Consortium: Bethesda proposals for classification of lymphoid neoplasms in mice. Blood. 2002;100:246–258. doi: 10.1182/blood.v100.1.246. [DOI] [PubMed] [Google Scholar]
- 42.Morse HC, III, Chattopadhyay SK, Makino M, Fredrickson TN, Hügin AW, Hartley JW. Retrovirus induced immunodeficiency in the mouse: MAIDS as a model for AIDS. AIDS. 1992;6:607–621. doi: 10.1097/00002030-199207000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Morse HC, III, Qi CF, Chattopadhyay SK, Hori M, Taddesse Heath L, Ozato K, Hartley JW, Taylor BA, Ward JM, Jenkins NA, Copeland NG, Fredrickson TN. Combined histologic and molecular features reveal previously unappreciated subsets of lymphoma in AKXD recombinant inbred mice. Leuk Res. 2001;25:719–733. doi: 10.1016/s0145-2126(01)00022-4. [DOI] [PubMed] [Google Scholar]
- 44.Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401:556–562. doi: 10.1038/44076. [DOI] [PubMed] [Google Scholar]
- 45.Park SS, Kim JS, Tessarollo L, Owens JD, Peng L, Han SS, Chung ST, Torrey TA, Cheung WC, Polakiewicz RD, McNeil N, Ried T, Mushinski JF, Morse HC, III, Janz S. Insertion of c Myc into IgH induces B cell and plasma cell neoplasms in mice. Cancer Res. 2005;65:1306–1315. doi: 10.1158/0008-5472.CAN-04-0268. [DOI] [PubMed] [Google Scholar]
- 46.Principato M, Cleveland JL, Rapp UR, Holmes KL, Pierce JH, Morse HC, III, Klinken SP. Transformation of murine bone marrow cells with combined v raf v myc oncogenes yields clonally related mature B cells and macrophages. Mol Cell Biol. 1990;10:3562–3568. doi: 10.1128/mcb.10.7.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rappaport H. Atlas of Tumor Pathology. Armed Forces Institute of Pathology; Washington DC: 1966. Tumors of the hematopoietic system; pp. 97–161. [Google Scholar]
- 48.Rolink A, Nutt S, Busslinger M, ten Boekel E, Seidl T, Andersson J, Melchers F. Differentiation, dedifferentiation, and redifferentiation of B lineage lymphocytes: roles of the surrogate light chain and the Pax5 gene. Cold Spring Harb Symp Quant Biol. 1999;64:21–25. doi: 10.1101/sqb.1999.64.21. [DOI] [PubMed] [Google Scholar]
- 49.Rolink AG, Schaniel C, Bruno L, Melchers F. In vitro and in vivo plasticity of Pax5 deficient pre B I cells. Immunol Lett. 2002;82:35–40. doi: 10.1016/s0165-2478(02)00016-0. [DOI] [PubMed] [Google Scholar]
- 50.Rolink AG, Schaniel C, Melchers F. Stability and plasticity of wild type and Pax5 deficient precursor B cells. Immunol Rev. 2002;187:87–95. doi: 10.1034/j.1600-065x.2002.18708.x. [DOI] [PubMed] [Google Scholar]
- 51.Schaller E, MacFarlane AJ, Rupec RA, Gordon S, McKnight AJ, Pfeffer K. Inactivation of the F4/80 protein in the mouse germ line. Mol Cell Biol. 2002;22:8035–8043. doi: 10.1128/MCB.22.22.8035-8043.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taddesse Heath L, Chattopadhyay SK, Dillehay DL, Lander MR, Nagashfar Z, Morse HC, III, Hartley JW. Lymphomas and high level expression of murine leukemia viruses in CFW mice. J Virol. 2000;74:6832–6837. doi: 10.1128/jvi.74.15.6832-6837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward JM, Nikolov NP, Tschetter JR, Kopp JB, Gonzalez FJ, Kimura S, Siegel RM. Progressive glomerulonephritis and histiocytic sarcoma associated with macrophage functional defects in CYP1B1 deficient mice. Toxicol Pathol. 2004;32:710–718. doi: 10.1080/01926230490885706. [DOI] [PubMed] [Google Scholar]
- 54.Ward JM, Sheldon W. Expression of mononuclear phagocyte antigens in histiocytic sarcoma of mice. Vet Pathol. 1993;30:560–565. doi: 10.1177/030098589303000610. [DOI] [PubMed] [Google Scholar]
- 55.Yu D, Allman D, Goldschmidt MH, Atchison ML, Monroe JG, Thomas Tikhonenko A. Oscillation between B lymphoid and myeloid lineages in Myc induced hematopoietic tumors following spontaneous silencing/reactivation of the EBF/Pax5 pathway. Blood. 2003;101:1950–1955. doi: 10.1182/blood-2002-06-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu D, Qi CF, Morse HC, III, Janz S, Stevenson FK. Deregulated expression of the Myc cellular oncogene drives development of mouse “Burkitt like” lymphomas from naive B cells. Blood. 2005;105:2135–2137. doi: 10.1182/blood-2004-07-2573. [DOI] [PubMed] [Google Scholar]