Abstract
Objective
Cryopyrin-associated periodic syndromes (CAPS) represent a spectrum of CIAS1 gene-mediated autoinflammatory diseases characterized by recurrent systemic inflammation. The clinical spectrum of CAPS varies from mild to severe and includes the syndromes historically described as familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and neonatal-onset multisystem inflammatory disease (NOMID). This article presents the largest cohort of patients with CAPS. The objective is to describe the pathogenesis, otolaryngologic, and audiologic manifestations of CAPS.
Study Design
Prospective (2003–2009).
Setting
National Institutes of Health.
Subjects and Methods
Fifty-seven patients with a diagnosis of CAPS were identified (31 NOMID, 11 NOMID/MWS, 9 MWS, and 6 FCAS). Comprehensive data regarding clinical manifestations, audiologic phenotype, and fluid attenuation inversion recovery MRI (FLAIR-MRI) of the brain and inner ear were obtained.
Results
Complete audiologic data obtained on 70% of ears revealed conductive hearing loss in 4 (11%) NOMID ears and mixed hearing loss in 5 (13%) NOMID and 2 (14%) NOMID/MWS ears. Sensorineural hearing loss (SNHL), worse in higher frequencies, was the most common type of hearing loss and was present in 23 (61%) NOMID, 10 (71%) NOMID/MWS, and 4 (33%) MWS ears. All of the patients with FCAS had normal hearing except 2, who had SNHL from 4 to 8 kHz. On FLAIR-MRI sequence, cochlear enhancement was noted in 26 of 29 (90%) NOMID, 6 of 11 (55%) NOMID/MWS, 3 of 9 (33%) MWS, and 1 of 6 (17%) FCAS patients and was significantly associated with the presence of hearing loss. Maxillary sinus hypoplasia and mucosal thickening were found in 39% and 86% of the cohort, respectively.
Conclusion
CIAS1 pathway–mediated CAPS is associated with unregulated autoinflammation mediated by interleukin-1 in the cochlea and hearing loss. Timely diagnosis is crucial to initiate early treatment with interleukin-1 receptor antagonists.
Keywords: cryopyrin-associated periodic syndromes, familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystem inflammatory disease, chronic infantile neurological cutaneous and articular syndrome
Over the past decade, our understanding of the clinical and molecular characteristics of hereditary autoinflammatory diseases has expanded tremendously.1,2 The autoinflammatory diseases encompass a wide spectrum, characterized by recurrent episodes of fever and inflammation involving multiple organs and tissues. However, unlike autoimmune disorders, the episodes of inflammation are not associated with high titers of autoantibodies or antigen-specific T cells.1–4 Genetically defined disorders in this disease group include familial Mediterranean fever (FMF) and the tumor necrosis factor (TNF) receptor associated periodic syndrome (TRAPS), which present with episodic fevers and localized inflammation of the serosal membranes, joints, and skin.4 The focus of the current study is the cryopyrin-associated periodic syndromes (CAPS).
The disease spectrum of CAPS includes the familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and neonatal-onset multisystem inflammatory disease (NOMID), also known as CINCA (chronic infantile neurological cutaneous and articular syndrome). They are caused by missense mutations in the cold-induced autoinflammatory syndrome-1-gene (CIAS1), also referred to as NLRP3 or NALP3.4 This genetic defect has been linked to the overproduction of interleukin-1β (IL-1β) both in vitro and in patients, suggesting that IL-1 blocking therapy might be beneficial.5 Treatment with IL-1 blocking agents such as anakinra, a recombinant IL-1 receptor antagonist, has been very effective in these patients.6
The clinical manifestations of CAPS are a continuum of disease severity with overlapping phenotypic features, but can be separated on the basis of their organ manifestations and the disease triggers. FCAS, the mildest form of CAPS, is characterized by recurrent and short-lived episodes of rash, fever, arthralgias, and conjunctivitis that are triggered by exposure to cold.4,7 Symptoms occur early and in many patients within the first 6 months of life. Most patients have a normal life span, and the development of complications from chronic inflammation such as amyloidosis and hearing loss (HL) is rare. Patients described as having MWS present with recurrent attacks of rash, fever, arthralgias, and conjunctivitis. However, their disease is typically not triggered by exposure to cold. Patients with MWS develop more severe ocular and central nervous system (CNS) symptoms including uveitis, episcleritis, and headaches.4,7 Hearing loss is described in many patients in their second and third decades of life, and the development of amyloidosis has been reported in up to 30% of patients.4,7
NOMID is the most severe form of CAPS and has the worst prognosis. Because of the severe phenotype, patients do not reproduce and the mutations occur sporadically. Symptoms typically occur at birth or in early infancy and include rash, fever, and joint inflammation. Joint manifestations vary in severity, ranging from arthralgias with swelling and effusion to debilitating arthropathy resulting from excessive bony overgrowth occurring in one-third of the patients.3,4,7 Ocular manifestations of NOMID are also severe and include uveitis, optic atrophy, and blindness in 25% of patients.3 CNS involvement is the most devastating feature of NOMID and can present as chronic headaches, papilledema, aseptic meningitis, seizures, and cerebral atrophy.6,7 Interestingly, in NOMID patients, genetic mutations in CIAS1 can be found in only about 60% of patients; however, the clinical characteristics of “mutation-negative” patients with NOMID and their response to therapy do not differ.6 Sometimes, the phenotype between NOMID and MWS overlaps.
Hearing loss and otolaryngologic manifestations of CAPS are not well described in the literature. The focus of our study is to present the otolaryngologic, audiologic, and imaging features from the largest cohort of patients with CAPS. We hope that familiarizing otolaryngologists and audiologists with the characteristics of CAPS will lead to timely diagnosis and early treatment with IL-1 receptor antagonists such as anakinra. As described later, such treatment has been shown to improve the inflammatory symptoms and hearing loss in some cases.6
Methods
Protocol and Data Collection
Prospective studies were conducted between 2003 and 2009 at the National Institutes of Health (NIH) Clinical Center, Bethesda, Maryland, to identify patients with CAPS for enrollment in 1 of the following treatment protocols: NOMID/anakinra (03-AR-0298), a natural history study (03-AR-0173), and FCAS/interleukin-1 Trap (05-AR-0014). These protocols were approved by the institutional review board at the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH. Written informed consent was obtained from adult subjects and parents of minor subjects. All participants were genetically tested as previously described.8 The diagnosis of CAPS was confirmed based on genetic testing or clinical criteria. Comprehensive data regarding genotype, clinical manifestations, audiologic phenotype, and fluid attenuation inversion recovery MRI (FLAIR-MRI) of the brain and inner ear were obtained. This article focuses on baseline data collected prior to enrollment in any NIH treatment protocols. A subset of our participants with NOMID were previously reported by Goldbach-Mansky et al.6
Classification of CAPS
The patients were placed in 4 categories based on clinical manifestations. Subjects with osseous dysplasia, bony overgrowth, or significant CNS manifestations and/or developmental delay were categorized as NOMID. If there was milder CNS disease without evidence of developmental delay, or brain atrophy and no bony overgrowth, patients were categorized as NOMID/MWS. Subjects with minimal CNS disease without significant developmental delay and CNS leukocytosis were categorized as MWS. Patients with cold-induced episodes of inflammation without CNS involvement were categorized as FCAS.
Clinical Examination
Baseline clinical characteristics including rash, headache, fever, aseptic meningitis, and joint involvement, including joint swelling, pain, or bony overgrowth, were obtained. Lumbar punctures were performed in patients with CNS symptoms. Aseptic meningitis was defined as cerebrospinal fluid (CSF) white blood cell count ≥6/mm3.
Otolaryngologic Examination
Comprehensive otolaryngologic examination included information regarding palpable cervical lymphadenopathy (size ≥1 cm), narrow or ogival palate resulting in the appearance of a high arched palate, and middle ear effusion (MEE).
Audiologic Examination
Age- and ability-appropriate audiologic evaluations including pure-tone air conduction (0.25–8 kHz) and bone conduction (0.25–4 kHz) thresholds were obtained in accordance with the American National Standards Institute standards.9,10 Distortion product otoacoustic emissions (DPOAEs) and acoustic reflex (AR) data were collected, when possible. Type and degree of HL are defined in Table 1.11
Table 1.
Definition of Type and Degree of Hearing Loss
| Definition | |
|---|---|
| Type | 3-Frequency (0.5/1/2 kHz) PTA for AC and BC |
| Normal | 3-Frequency AC PTA ≤20 dB HL and average A-B gap ≤10 dB HL |
| Conductive | 3-Frequency AC PTA >20 dB HL, BC PTA ≤20 dB HL, and average A-B gap >10 dB HL |
| Mixed | 3-frequency BC PTA >20 dB HL and average A-B gap >10 dB HL |
| Sensorineural | 3-Frequency BC PTA >20 dB HL and average A-B gap ≤10 dB HL |
| Degree | 4-Frequency (0.5/1/2/4 kHz) PTA for AC |
| Mild | >20 and ≤40 dB HL |
| Moderate | >40 and ≤70 dB HL |
| Severe | >70 and ≤95 dB HL |
| Profound | >95 dB HL |
Abbreviations: A-B, air–bone;AC, air conduction; BC, bone conduction; dB HL, decibel hearing level; PTA, pure-tone average.
Imaging Examination
Highly sensitive FLAIR-MRI of the inner ear and brain was performed in 55 of 57 subjects.12 FLAIR sequences after the administration of gadolinium were used to visualize potential inflammatory cochlear lesions. The imaging studies were reviewed by a neuroradiologist and an otologist. Cochlear enhancement (CE), sinonasal mucosal thickening, and maxillary hypoplasia were investigated. The criteria set by Scuderi et al13 were used to determine the presence or absence of maxillary hypoplasia.
Statistical Analysis
Descriptive statistics are used to summarize data stratified by disease severity. Fisher's exact test was used to evaluate differences among the groups for CE on FLAIR-MRI and HL.
Results
Patient Demographics
Fifty-seven patients (31 NOMID, 11 NOMID/MWS, 9 MWS, and 6 FCAS) diagnosed with CAPS participated. The average age (range) in years was 14 (1–52), 18 (2–47), 15 (1–50), and 47 (15–69) for NOMID, NOMID/MWS, MWS, and FCAS, respectively.
Clinical Findings
Table 2 summarizes the clinical features of the 4 CAPS groups. Rash and conjunctivitis were the most common characteristics among the groups. Increased intracranial pressure, papilledema, and aseptic meningitis were common in NOMID and NOMID/MWS. Papilledema was not observed in any patients with FCAS.
Table 2.
Baseline Clinical Characteristics Among the CAPS Groups, n subjects (%)
| NOMID | NOMID/MWS | MWS | FCAS | |
|---|---|---|---|---|
| Rash | 30/31 (97) | 10/11 (91) | 9/9 (100) | 6/6 (100) |
| Fever | 29/31 (94) | 7/10 (70) | 8/9 (89) | 4/6 (67) |
| Conjunctivitis | 29/31 (94) | 10/11 (91) | 8/9 (89) | 6/6 (100) |
| Papilledemaa | 26/31 (84) | 9/11 (82) | 2/6 (33) | 0/6 (0) |
| Joint swelling/pain | 28/31 (90) | 8/11 (73) | 7/9 (78) | 6/6 (100) |
| Seizure | 8/30 (27) | 1/11 (9) | 0/9 (0) | 0/6 (0) |
| Increased ICP | 22/28 (79) | 9/11 (82) | 3/4 (75) | NR |
| Aseptic meningitis | 19/29 (66) | 11/11 (100) | 0/5 (0) | NR |
Abbreviations: FCAS, familial cold autoinflammatory syndrome; ICP,intra-cerebral pressure; MWS, Muckle-Wells syndrome; NOMID, neonatal onset multisystem inflammatory disease; NR, not recorded.
Papilledema present at the time of initial NIH examination.
Otolaryngologic Findings
A history of cervical lymphadenopathy or palpable lymphadenopathy was present in 14 (45%) NOMID, 6 (55%) NOMID/MWS, and 5 (56%) MWS patients. A high arched palate was noted in 16 (52%) NOMID, 4 (37%) NOMID/MWS, and 1 (11%) MWS patients. Lymphadenopathy and high arched palates were not present in patients with FCAS. MEE was present in 9 NOMID and 1 NOMID/MWS ears, and a tympanostomy tube was present in 3 NOMID ears.
Audiometric Findings
Mean air conduction (AC) and bone conduction (BC) thresholds are presented for each group in Figures 1 and 2, respectively. Hearing loss degree and type are presented for each group in Table 3. Because of patients' young age and cognitive delay, we were unable to obtain complete air- and bone-conduction data on every patient in order to assign type and degree of HL, as defined in Table 1. Sufficient data to calculate the type and degree of HL were obtained for 38 (61%) NOMID, 14 (64%) NOMID/MWS, 12 (67%) MWS, and 12 (100%) FCAS ears.
Figure 1.
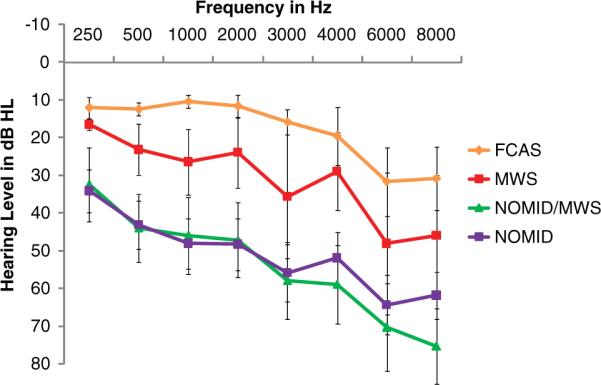
Mean (standard deviation) pure-tone air conduction thresholds for NOMID, NOMID/MWS, MWS, and FCAS groups.
Abbreviations: dB HL, decibel hearing loss; FCAS, familial cold autoinflammatory syndrome; MWS, Muckle-Wells syndrome; NOMID, neonatal-onset multisystem inflammatory disease.
Figure 2.
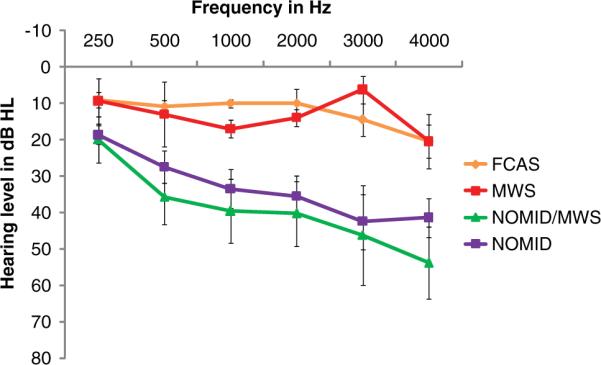
Mean (standard deviation) pure-tone bone conduction thresholds for NOMID, NOMID/MWS, MWS, and FCAS groups.
Abbreviations: dB HL, decibel hearing loss; FCAS, familial cold autoinflammatory syndrome; MWS, Muckle-Wells syndrome; NOMID, neonatal-onset multisystem inflammatory disease.
Table 3.
Distribution of the Type and Degree of Hearing Loss Among the CAPS Groups, n ears (%)
| NOMID (n = 38) | NOMID/MWS (n = 14) | MWS (n = 12) | FCAS (n = 12) | |
|---|---|---|---|---|
| Typea | ||||
| Normal | 6 (16) | 2 (14) | 8 (67) | 9 (75) |
| CHL | 4 (11) | 0 (0) | 0 (0) | 0 (0) |
| MHL | 5 (13) | 2 (14) | 0 (0) | 0 (0) |
| SNHL | 23 (61) | 10 (71) | 4 (33) | 3 (25) |
| Degreeb | ||||
| Normal | 9 (24) | 2 (14) | 8 (67) | 9 (75) |
| Mild | 7 (18) | 3 (21) | 0 (0) | 3 (25) |
| Moderate | 15 (39) | 8 (57) | 4 (33) | 0 (0) |
| Severe | 4 (11) | 0 (0) | 0 (0) | 0 (0) |
| Profound | 3 (8) | 1 (7) | 0 (0) | 0 (0) |
Abbreviations: CHL, conductive hearing loss; FCAS, familial cold autoinflammatory syndrome; MHL mixed hearing loss; MWS, Muckle-Wells syndrome; NOMID, neonatal-onset multisystem inflammatory disease; SNHL, sensorineural hearing loss.
Type of hearing loss was based on a 3-frequency (0.5/1/2 kHz) pure-tone average for air and bone conduction thresholds.
Degree of hearing loss was based on a 4-frequency (0.5/1/2/4 kHz) pure-tone average for air conduction thresholds.
All types of HL were observed in our total cohort (Table 3). SNHL was the most common type of HL in all groups; 23 of 38 (61%) NOMID, 10 of 14 (71%) NOMID/MWS, and 4 of 12 (33%) MWS ears. Two of 6 (33%) FCAS patients had high-frequency SNHL at 4 to 8 kHz. In 1 of these cases, the high-frequency SNHL may be multifactorial and influenced by presbycusis and military noise exposure. Conductive hearing loss (CHL) was present in 4 (11%) NOMID ears including 3 with subclinical CHL (normal AC threshold with air–bone gap). Mixed hearing loss was present in 5 (13%) NOMID and 2 (14%) NOMID/MWS ears.
The degree of HL (Table 3) ranged from normal to profound in patients with NOMID with normal hearing in 24% of the ears and was mild, moderate, severe, and profound in 18%, 39% 11%, and 8% of the ears, respectively. In the NOMID/MWS group, the degree of HL ranged from normal to profound, with normal, mild, moderate, and profound present in 14%, 21%, 57%, and 7% of the ears, respectively. Most of the ears with MWS had normal hearing (67%), whereas 4 (33%) ears had moderate HL. The cross-sectional data shown in Figure 3 may suggest that HL progressively worsens with age in those with NOMID, NOMID/MWS, and MWS. This pattern is not observed in our small cohort of FCAS.
Figure 3.
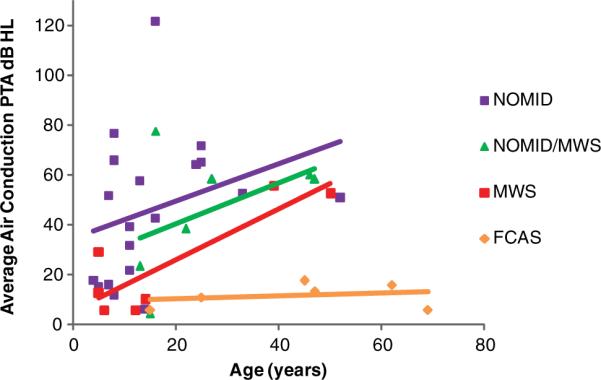
Air conduction thresholds for the 3-frequency pure-tone average (PTA) plotted against age at the time of the baseline test for NOMID, NOMID/MWS, MWS, and FCAS groups.
Abbreviations: FCAS, familial cold autoinflammatory syndrome; MWS, Muckle-Wells syndrome; NOMID, neonatal-onset multisystem inflammatory disease.
DPOAEs and ARs were present as expected based on peripheral hearing status and were appropriately absent in cases of conductive or SNHL. These data provided no evidence of neural or other retrocochlear dysfunction.
Imaging Phenotype
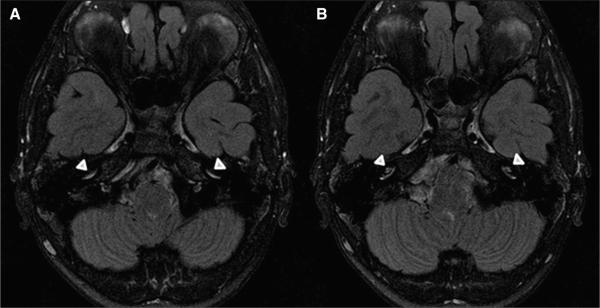
On FLAIR-MRI sequence (Figure 4), CE was noted in 26 of 29 (90%) NOMID, 6 of 11 (55%) NOMID/MWS, 3 of 9 (33%) MWS, and 1 of 6 (17%) FCAS patients. Maxillary sinus hypoplasia was observed in 20 of 30 (67%) NOMID and 2 of 11(18%) NOMID/MWS patients but not in MWS or FCAS patients. Mucosal thickening of the sinuses, especially the maxillary sinus, was present in 28 of 30 (93%) NOMID, 8 of 11 (73%) NOMID/MWS, 8 of 9 (89%) MWS, and 5 of 6 (83%) FCAS patients.
Figure 4.
Axial FLAIR-MRI images (A and B) after gadolinium administration demonstrating enhancement of the basal turn of bilateral cochleae (arrowheads).
Cochlear inflammation on FLAIR-MRI (Table 4) was present in those with HL more often than those without HL (P < .0001).
Table 4.
Distribution of Presence (+) and Absence (−) of SNHL or MHL and Cochlear Inflammation/Enhancement on FLAIR-MRI Among the CAPS Groups, n subjects
| HL(+)/CE(+) | HL(+)/CE(−) | HL(−)/CE(+) | HL(−)/CE(−) | |
|---|---|---|---|---|
| NOMID | 15 | – | 1 | 1 |
| NOMID/MWS | 3 | 2 | – | 1 |
| MWS | 1 | 1 | 1 | 3 |
| FCAS | 1 | – | – | 5 |
| Total | 20 | 3 | 2 | 10 |
Hearing loss defined as sensorineural or mixed hearing loss (Table 1). CE indicates cochlear enhancement; FCAS, familial cold autoinflammatory syndrome; HL, hearing loss; MWS, Muckle-Wells syndrome; NOMID, neonatal onset multisystem inflammatory disease.
Discussion
Cryopyrin-associated periodic syndromes are autoinflammatory disorders that form a clinical continuum ranging from a mild to a severe phenotype. These entities include NOMID, MWS, and FCAS. FCAS is mild with a good prognosis, whereas NOMID is the most severe phenotype with the worst prognosis. Although patients with FCAS have normal life spans, approximately 20% of NOMID patients die before reaching adulthood.14–16
The pathogenesis of CAPS involves either dominantly inherited or de novo gain of function mutations in the CIAS1 gene located on chromosome 1q44.3,4,7CIAS1 encodes cryopyrin, also known as NALP3 (NACHT, leucine-rich repeat and pyrin domain containing protein 3) or NLRP3. Most of the identified mutations occur in the region encoding the NACHT domain in exon 3. Cryopyrin is involved in the assembly of a macromolecular complex called the inflammasome that is involved in innate immunity. It leads to the activation of the caspase-1 enzyme, which in turn cleaves pro-IL-1beta and pro-IL-18 into their active proinflammatory forms, IL-1 and IL-18.1,3,6,7 The binding of IL-1beta to IL-1 receptor (IL-1R) initiates a cascade of signals resulting in early inflammatory responses. The balance between IL-1 and IL-1R is essential in distinguishing proinflammatory and anti-inflammatory responses in the body.17 The overproduction of activated ILbeta can result in excessive IL-1-mediated inflammation causing autoinflammatory disorders such as CAPS.
It is important to recognize the clinical features of patients with CAPS. Although multiple organ systems can be involved, a migratory maculopapular skin rash (Figure 5) resembling urticaria is usually the first notable manifestation in CAPS. The rash is nonpruritic, and when the skin is biopsied, it demonstrates perivascular infiltration with polymorphonuclear leukocytes and lymphocytes.4 As demonstrated in Table 2, rash was one of the most common presenting symptom in our subjects across groups. More severe presenting symptoms such as aseptic meningitis were common in the NOMID and NOMID/MWS groups. Findings suggestive of ongoing inflammatory processes in the head and neck region including cervical lymphadenopathy can also be present. In their literature review, Torbiak et al18 reported lymphadenopathy in all 32 patients with NOMID. Cervical lymphadenopathy was present in 45% to 56% of patients in our study groups. Additional phenotypic features observed in our cohort included high arched palate and MEE.
Figure 5.
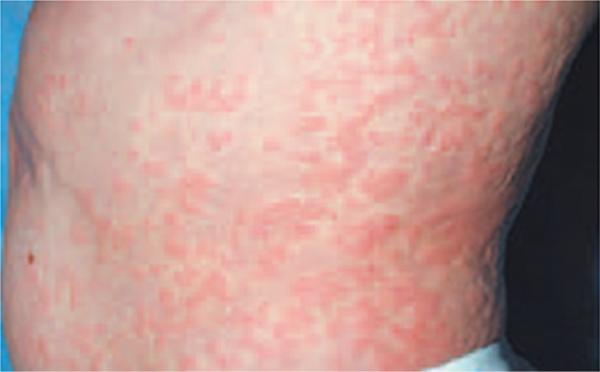
Typical maculopapular rash on the flank of a patient with CAPS. Printed with permission from Goldbach-Mansky R, Dailey NJ, Canna SW, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581-592.
Hearing loss in CAPS has been reported in the literature. Prieur et al19 reported perceptive “deafness” of various degrees in 75% of patients with NOMID. Bilateral SNHL has been described in up to 85% of patients with MWS.20 However, given the rarity of CAPS, a comprehensive description of audiometric characteristics is lacking.
For those patients in our cohort for whom sufficient pure-tone threshold data were available, 62% of ears had hearing loss, and it was most prevalent in those with NOMID and NOMID/MWS. SNHL was the most common type, and the degree ranged from normal to profound (Table 3). Two FCAS patients had high-frequency SNHL; only 1 of these patients is suspected of having CAPS-related SNHL. Hearing loss is characteristically worse in the higher frequencies (Figures 1 and 2) and appears to progressively worsen with age in all groups except FCAS, the mildest form of CAPS (Figure 3).
Young age and cognitive delay were prohibitive factors for sufficient pure-tone data collection in 30% of ears in our CAPS patients. In these patients, comprehensive sedated auditory steady-state responses were used to estimate peripheral hearing sensitivity and determine appropriate clinical intervention. These data were not included in our analysis because of the variability associated with these predictions of behavioral thresholds.21 Clinicians should be aware that sedated evoked potential assessment of hearing may be required in this population.
Cochlear enhancement on FLAIR-MRI sequences was observed most often in those with NOMID and NOMID/MWS, the subgroups with a higher prevalence of HL. Cochlear enhancement appears to be an accurate predictor of cochlear HL (Table 4), and this relationship may provide insight into the mechanism of SNHL in CAPS.
The cochlear modiolus is highly porous and can potentially serve as a conduit between perilymph and cerebrospinal fluid.22 However, the cochlear–brain barrier maintains the composition of the inner ear fluids and protects the inner ear by limiting entry of nonnative substances such as IL-1. In an animal model, it has been shown that bacterial meningitis activates proinflammatory cytokines such as IL-1, IL-6, and TNF-α and initiates a cascade of events, resulting in alteration of the blood–brain barrier diffusion, polymorphonuclear leukocytes and serum protein infiltrations, meningeal inflammation, increased intracranial pressure, and decreased cerebral vascular perfusion.23 In CAPS, cryopyrin-mediated inflammasome and caspase-1 activation might cause inappropriate sustained secretion of inflammatory cytokines including IL-1, leading to chronic aseptic meningitis and subsequent increased permeability of cytokines between perilymph and CSF space via the modiolus. These cytokines can then stimulate the spiral ligament fibrocytes to produce downstream mediators that might induce an unchecked chronic inflammation responsible for cochlear dysfunction and HL.24 This mechanism of HL has been supported by several reports of successful treatment of CAPS patients with anakinra, a type of IL-1 inhibitor.6,25,26
Hearing loss was observed in 2 FCAS patients without detectable aseptic meningitis, yet 1 had CE on FLAIR-MRI. This suggests that CIAS1 mutation might result in uncontrolled local activation of IL-1 within the cochlea. Fujioka et al27 demonstrated the production of proinflammatory cytokines in the spiral ligament, stria vascularis, and spiral ganglion neuron after acoustic trauma to the cochlea. In CAPS, the CIAS1 mutation within the inner ear may lead to unregulated local production of cytokines, such as IL-1beta, and chronic inflammatory responses resulting in cochlear damage, SNHL, and MRI changes. Further pathologic study of temporal bone histology would be helpful to elucidate the mechanism of HL in CAPS.
At this time we do not know whether the mechanism of HL observed in CAPS might also be responsible for inner ear injury in a subset of autoimmune-mediated ear disease (AIED). The pathogenesis of AIED is often considered to be immune-mediated; however, no autoantibodies or antigen-specific T cells to the inner ear have been identified. Assessing patients with suspected AIED by studying the role of IL-1 inflammatory pathway and its blocking agents may help to identify responsive subsets within this group of AIEDs and elucidate their pathogenesis.
Mucosal thickening of the sinuses was detected in the majority of patients with CAPS, and MEE was more prevalent in NOMID patients than expected. Proinflammatory cytokines such as IL-1 and TNF-α are known to be stimulated after viral and bacterial infections and regulate the expression of mucin and other genes involved in the pathogenesis of sinusitis and otitis media.28 In NOMID, the most severe type of CAPS, excessive IL-1 secretion in respiratory mucosa of the middle ear and paranasal sinuses may lead to more frequent otitis media and chronic sinusitis.
There was a high incidence of maxillary hypoplasia (67%) in patients with NOMID. Maxillary sinus hypoplasia is normally observed in up to 10% of computed tomography studies of the head and face.29 The pathophysiology of maxillary hypoplasia is controversial, but it is suggested that negative pressure within the sinus resulting from ostial obstruction can prevent normal pneumatization leading to hypoplasia. In NOMID, chronic sinusitis from uncontrolled inflammation at a very early age might result in ostial obstruction and consequently underdevelopment of maxillary sinuses. The presence of high arched palate and maxillary hypoplasia in patients with CAPS has been reported in other genetic disorders like Apert's syndrome.30
We present the baseline otolaryngologic and audiologic characteristics of patients with CAPS. Hearing loss, especially sensorineural, is one of the most common phenotypic features in CAPS, and it can be progressive. Because of the severe phenotype associated with NOMID, diagnosis is less likely to be delayed and complications such as hearing loss can be readily identified. However, cases involving less severe forms of CAPS may be misdiagnosed or underreported, especially if genetic testing for CIAS1 mutations is not available. CAPS should be included in the differential diagnosis for patients with what appears to be idiopathic or autoimmune HL and any other symptoms of periodic fever syndrome. Furthermore, routine audiological monitoring is recommended in all patients with NOMID, NOMID/MWS, or MWS. Hearing loss should be ruled out in patients with FCAS. Early diagnosis and management of hearing loss are important for appropriate speech–language, cognitive, and social development. Treatment of CAPS with IL-1 antagonists such as anakinra has been promising, and further studies are needed to determine the effectiveness of IL-1 antagonists in preventing, stabilizing, or even reversing cochlear damage and HL.6,25,26
Acknowledgments
We acknowledge Allyson Segar, AuD, for data entry and preliminary analysis of the initial NOMID patients; Deborah Stone, MD, and Dawn Chapelle, RN, for clinical evaluation of patients; Christopher Snyder and Bahar Afshar for data management; Scott Canna, MD, Josef Gregory Dolan, Innocent Njoku, Patrick Kicker, and Zachary Phillips for their help with the study logistics; and Carter Van Waes, MD, PhD, and Susan Rudy, RN, NP, for reviewing the manuscript.
Sponsorships: This work was sponsored by the intramural research programs of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Deafness and Other Communication Disorders at the NIH.
Funding source: This work was supported by the intramural research programs of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Deafness and Other Communication Disorders at the NIH.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Author Contributions Neda Ahmadi, data collection, data analysis, drafting the article, final approval for publication; Carmen C. Brewer, study design, data collection, data analysis, manuscript revision, final approval for publication; Christopher Zalewski, study design, data collection, data analysis, manuscript revision, final approval for publication; Kelly A. King, data collection, data analysis, manuscript revision, final approval for publication; John A. Butman, data collection, final approval for publication; Nicole Plass, data collection, manuscript revision, final approval for publication; Cailin Henderson, data collection, data analysis, manuscript revision, final approval for publication; Raphaela Goldbach-Mansky, study design, data analysis, manuscript revision, final approval for publication; H. Jeffrey Kim, study design, data analysis, manuscript revision, final approval for publication.
Disclosures
Competing interests: None.
References
- 1.Ryan JG, Goldbach-Mansky R. The spectrum of autoinflammatory diseases: recent bench to bedside observations. Curr Opin Rheumatol. 2008;20:66–75. doi: 10.1097/BOR.0b013e3282f1bf4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stojanov S, Kastner DL. Familial autoinflammatory diseases: genetics, pathogenesis and treatment. Curr Opin Rheumatol. 2005;17:586–599. doi: 10.1097/bor.0000174210.78449.6b. [DOI] [PubMed] [Google Scholar]
- 3.Neven B, Prieur AM, Quartier dit Maire P. Cryopyrinopathies: update on pathogenesis and treatment. Nat Clin Pract Rheumatol. 2008;4:481–489. doi: 10.1038/ncprheum0874. [DOI] [PubMed] [Google Scholar]
- 4.Hull KM, Shoham N, Chae JJ, et al. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol. 2003;15:61–69. doi: 10.1097/00002281-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldbach-Mansky R, Dailey NJ, Canna SW, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldfinger S. The inherited autoinflammatory syndrome: a decade of discovery. Trans Am Clin Climatol Assoc. 2009;120:413–418. [PMC free article] [PubMed] [Google Scholar]
- 8.Aksentijevich I, Putnam C, Remmers EF, et al. The clinical continuum of cryopyrinopathies: novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis Rheum. 2007;56:1273–1285. doi: 10.1002/art.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American National Standards Institute . S3.1-1999 American National Standard Maximum Permissible Ambient Noise Levels for Audiometric Test Rooms (Standard S3.1) American National Standards Institute; New York, NY: 2003. [Google Scholar]
- 10.American National Standards Institute . S3.1-1996 American National Standard Specification for Audiometers (Standard S3.6) American National Standards Institute; New York, NY: 2004. [Google Scholar]
- 11.Mazzoli M, Camp G, Newton V, et al. Recommendations for the description of genetic and audiological data for families with non-syndromic hereditary hearing impairment. Audiol Med. 2003;1:148–150. [Google Scholar]
- 12.Sone M, Mizuno T, Naganawa S, et al. Imaging analysis in cases with inflammation-induced sensorineural hearing loss. Acta Otolaryngol. 2009;129:239–243. doi: 10.1080/00016480802226163. [DOI] [PubMed] [Google Scholar]
- 13.Scuderi AJ, Harnsberger HR, Boyer RS. Pneumatization of the paranasal sinuses: normal features of importance to the accurate interpretation of CT scans and MR images. Am J Roentgenol. 1993;160:1101–1104. doi: 10.2214/ajr.160.5.8470585. [DOI] [PubMed] [Google Scholar]
- 14.Goldbach-Mansky R, Shroff SD, Wilson M, et al. A pilot study to evaluate the safety and efficacy of the long-acting interleukin-1 inhibitor rilonacept (interleukin-1 Trap) in patients with familial cold autoinflammatory syndrome. Arthritis Rheum. 2008;58:2432–2442. doi: 10.1002/art.23620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosengren S, Mueller JL, Anderson JP, et al. Monocytes from familial cold autoinflammatory syndrome patients are activated by mild hypothermia. J Allergy Clin Immunol. 2007;119:991–996. doi: 10.1016/j.jaci.2006.12.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldbach-Mansky R, Kastner DL. Autoinflammation: the prominent role of IL-1 in monogenic autoinflammatory diseases and implications for common illnesses. J Allergy Clin Immunol. 2009;124:1141–1149. doi: 10.1016/j.jaci.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torbiak RP, Dent PB, Cockshott WP. NOMID—a neonatal syndrome of multisystem inflammation. Skeletal Radiol. 1989;18:359–364. doi: 10.1007/BF00361425. [DOI] [PubMed] [Google Scholar]
- 19.Prieur AM, Griscelli C, Lampert F, et al. A chronic, infantile, neurological, cutaneous and articular (CINCA) syndrome: a specific entity analysed in 30 patients. Scand J Rheumatol Suppl. 1987;66:57–68. doi: 10.3109/03009748709102523. [DOI] [PubMed] [Google Scholar]
- 20.Gorlin R, Toriello H, Cohen M, editors. Hereditary Hearing Loss and Its Syndromes. Oxford University Press; New York, NY: 1995. p. 243. [Google Scholar]
- 21.Yeung KN, Wong LL. Prediction of hearing thresholds: comparison of cortical evoked response audiometry and auditory steady state response audiometry techniques. Int J Audiol. 2007;46:17–25. doi: 10.1080/14992020601102238. [DOI] [PubMed] [Google Scholar]
- 22.Rask-Andersen H, Schrott-Fischer A, Pfaller K, et al. Perilymph/modiolar communication routes in the human cochlea. Ear Hear. 2006;27:457–465. doi: 10.1097/01.aud.0000233864.32183.81. [DOI] [PubMed] [Google Scholar]
- 23.Yeung AH, Tinling SP, Brodie HA. Inhibition of post-meningitic cochlear injury with cerebrospinal fluid irrigation. Otolaryngol Head Neck Sur. 2006;134:214–224. doi: 10.1016/j.otohns.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Ichimiya I, Yoshida K, Hirano T, et al. Significance of spiral ligament fibrocytes with cochlear inflammation. Int J Pediatr Otorhinolaryngol. 2000;56:45–51. doi: 10.1016/s0165-5876(00)00408-0. [DOI] [PubMed] [Google Scholar]
- 25.Mirault T, Launay D, Cuisset L, et al. Recovery from deafness in a patient with Muckle-Wells syndrome treated with anakinra. Arthritis Rheum. 2006;54:1697–1700. doi: 10.1002/art.21807. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki T, Masumoto J, Agematsu K, et al. Anakinra improves sensory deafness in a Japanese patient with Muckle-Wells syndrome, possibly by inhibiting the cryopyrin inflammasome. Arthritis Rheum. 2008;58:864–868. doi: 10.1002/art.23261. [DOI] [PubMed] [Google Scholar]
- 27.Fujioka M, Kanzaki S, Okano HJ, et al. Proinflammatory cytokines expression in noise-induced damaged cochlea. J Neurosci Res. 2006;83:575–583. doi: 10.1002/jnr.20764. [DOI] [PubMed] [Google Scholar]
- 28.Kerschner JE, Khampang P, Erbe CB, et al. Mucin gene 19 (MUC19) expression and response to inflammatory cytokines in middle ear epithelium. Glycoconj J. 2009;26:1275–1284. doi: 10.1007/s10719-009-9245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolger WE, Woodruff WW, Jr, Morehead J, et al. Maxillary sinus hypoplasia: classification and description of associated uncinate process hypoplasia. Otolaryngol Head Neck Surg. 1990;103:759–765. doi: 10.1177/019459989010300516. [DOI] [PubMed] [Google Scholar]
- 30.Letra A, de Almeida AL, Kaizer R, et al. Intraoral features of Apert's syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e38–41. doi: 10.1016/j.tripleo.2006.04.006. [DOI] [PubMed] [Google Scholar]