Abstract
Objective
To determine whether there are identifiable factors associated with increased risk of aortic arch reintervention in patients who have undergone balloon aortoplasty (BD) for aortic arch obstruction (COA) after the Norwood procedure (NP).
Background
BD has been shown to be an effective acute therapy for COA after the NP. However, recurrent obstruction requiring repeat intervention is not uncommon.
Methods
All patients who underwent BD as the initial intervention for COA following the NP at our institution from to 01/93-05/09 were retrospectively analyzed (n=116).
Results
The median age at initial BD was 4.5 months. The median follow-up period was 3.4 years. The procedure was considered acutely successful in 92% of patients, with a median gradient reduction overall from 26 to 3 mmHg (p<0.0001) and COA diameter increase of 52% (p<0.0001). By Kaplan-Meier analysis, freedom from reintervention was 69% at 1 year, and 58% at 5 years; freedom from reoperation was 82% at 1 year, and 79% at 5 years. By Cox regression analysis, proximal arch obstruction, age <3 months at BD, ≥moderate ventricular dysfunction, ≥moderate atrioventricular valve regurgitation on pre-catheterization echocardiogram, and higher post-BD coarctation gradient were associated with shorter freedom from reoperation.
Conclusions
Despite a high acute success rate, a significant proportion of patients treated with BD for post-NP COA underwent reintervention during follow-up. The risk of arch reintervention is highest in patients with proximal arch obstruction, those who are younger than 3 months of age at the time of BD, and those with a less successful acute result.
Keywords: Coarctation, Balloon angioplasty, Norwood procedure, Reintervention, Hypoplastic left heart syndrome
Introduction
Obstruction of the reconstructed aortic arch (COA) remains an important problem in patients who have undergone the Norwood procedure (NP). It has been reported to occur in 9–37% of patients, and is a significant cause of morbidity1–7. COA has been associated with deterioration of ventricular function in this patient population, as well as atrioventricular valve regurgitation (AVVR), and imbalance of systemic and pulmonary blood flow8. It has also been associated with increased risk of death9, 10. Balloon aortoplasty (BD) has become the standard first line therapy for post-NP COA and is generally effective, with reported acute success rates ranging from 89 to 100%3–7. In fact, Zeltser et al. did not find a difference in survival between patients treated with BD for COA after the NP and those that did not develop arch obstruction7. Despite favorable acute results, little is known about the intermediate and long term outcomes following BD of the reconstructed aortic arch. Factors associated with reintervention after BD have not been characterized, and may have implications for decision-making around methods of aortic reconstruction, as well as timing and method of reintervention. We evaluated all patients who underwent BD as the primary modality of treatment for post-NP COA over the last 16 years with the objective of identifying factors associated with reintervention on the aortic arch in this patient population.
Materials and Methods
Patients
We reviewed all patients who underwent BD for post-NP COA at Children’s Hospital Boston from January 1993 to May of 2009. Patients were included if the NP had been performed for hypoplastic left heart syndrome (HLHS), or related single ventricle lesions and no prior arch interventions had been performed. Only patients who underwent their NP and BD at Children's Hospital Boston were included. Patients who underwent primary stenting were excluded. The diagnosis of HLHS was based on echocardiographic evidence of diminutive ascending aorta, aortic atresia or stenosis, mitral atresia or stenosis, and hypoplastic or dysfunctional left ventricle. NP was defined as a surgical procedure which included anastomosis of the native pulmonary trunk and aorta, excision of ductal tissue, reconstruction of the aortic arch, and creation of a systemic to pulmonary artery shunt or right ventricle-to-pulmonary artery conduit, with or without an open atrial septectomy. Patients were grouped by era, based on whether they were among the first or last 50% of patients. Cross-sectional follow-up was obtained at the time of most recent clinical evaluation, death, or transplant. The institutional review board of the Children’s Hospital Boston approved this study.
Data were obtained by review of medical records, pre-operative echocardiograms and angiograms from the initial BD procedure, which were reviewed by a single reviewer in blinded fashion. Preoperative patient factors included diagnosis, diameter of the ascending aorta, gender, age and weight at the time of the NP. Operative parameters included date of operation; type of pulmonary artery-to-aortic anastomosis (proximal side-to-side anastomosis vs. pulmonary trunk-to-underside of the aortic arch); whether a coarctectomy was performed; material used to augment the reconstructed aortic arch; and the source of pulmonary blood flow utilized (modified Blalock-Taussig shunt vs. right ventricle-to-pulmonary artery shunt). Coarctectomy was defined as resection of the aorta at the level of the coarctation, including wide resection of all ductal tissue and the posterior shelf and direct end-to-end anastomosis between the greater curvature of the native aortic arch and the posterior wall of the descending aorta. Precatheterization data included weight and age at the time of BD, as well as the degree of ventricular dysfunction and AVVR on the pre-catheterization echocardiogram.
Balloon Aortoplasty
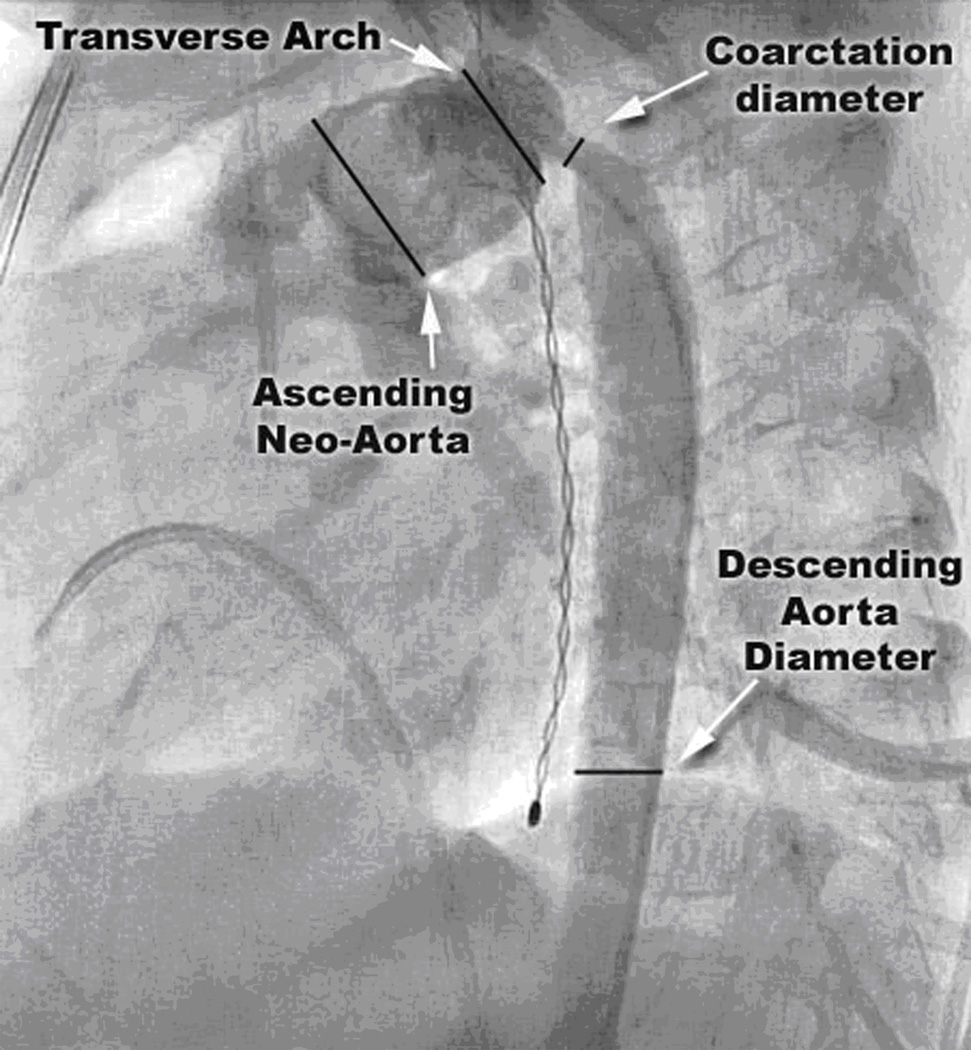
Patients presented for cardiac catheterization either based on clinical suspicion of arch obstruction or an unexplained deterioration in clinical status (unplanned catheterization group), or in anticipation of their next surgical procedure (planned catheterization group). The decision to perform BD was made on a case-by-case basis; no standardized criteria were in place. During the study period, BD was generally considered the first line of therapy for post-NP COA at our institution. Procedural factors obtained from the catheterization report included: peak gradient across the obstruction before and after balloon dilation (pre- and post-BD gradient), diameters of the first and largest balloons used for BD, and number of balloon inflations. The following measurements were made using the lateral or left anterior oblique projection of the aortic arch angiogram (Figure 1): neo-ascending aorta and transverse arch diameter, narrowest diameter at the site of obstruction (COA diameter), and diameter of the descending thoracic aorta at the level of the diaphragm (DAO). When the descending thoracic aorta at the level of the diaphragm was not visible in the angiograms, the DAO measurement was made as distal as possible in the descending thoracic aorta, beyond the area of post-stenotic dilation that is commonly present. The COA index was calculated by dividing the COA diameter by the DAO diameter. The arch geometry was also assessed, and classified as having Romanesque (semicircular rounded form), Gothic (triangular form with acute angulation between the ascending and descending aorta), or Crenel geometry (rectangular form)21. We also found that some patients had a tortuous or S-shaped arch.
Figure 1.
Lateral projection angiogram of the reconstructed neo-aorta. Measurements of the ascending aorta, transverse arch, aortic arch and descending thoracic aorta were performed at the indicated sites.
Strength of indication for BD was retrospectively assessed based on the gradient across the area of obstruction and the ventricular function. A strong indication was defined a priori as a COA index less than 50% and/or a peak gradient ≥15 mmHg in the presence of normal ventricular function, or a gradient ≥10 mmHg in the presence of significant ventricular dysfunction. Significant ventricular dysfunction was defined a priori as moderate or worse ventricular dysfunction on the pre-catheterization echocardiogram, or evidence of hemodynamic compromise (ventricular end-diastolic pressure ≥ 14 mmHg, and/or calculated cardiac index below 2.6 L/min/m2).
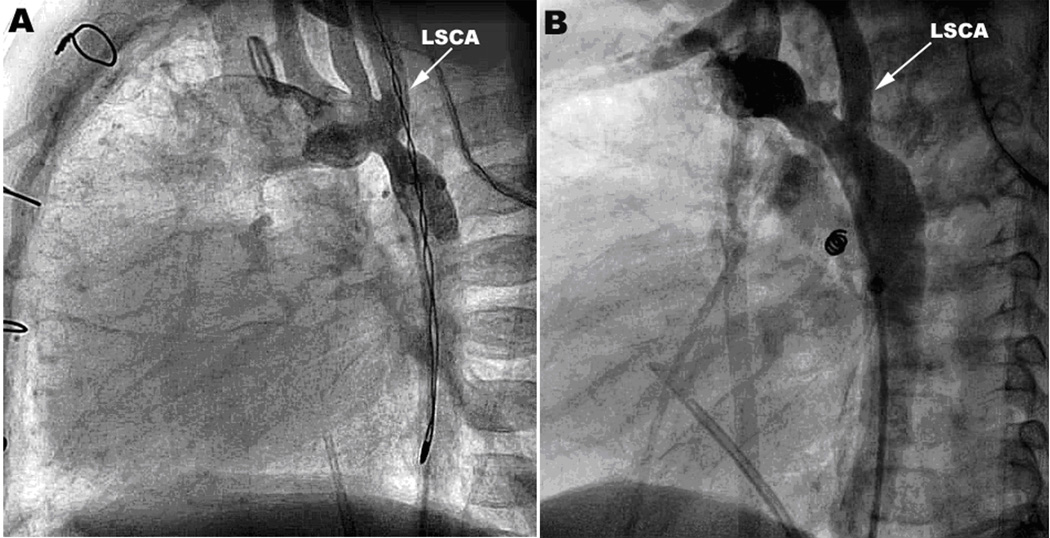
Patients were divided into two groups based on the anatomic region of the arch involved in the obstruction: distal COA was defined as obstruction immediately distal to the take-off of the left subclavian artery (LSCA) (Figure 2A); proximal COA,occurred at or proximal to the take-off of the LSCA (Figure 2B).
Figure 2.
Sites of Obstruction. A. Distal arch obstruction. Lateral angiogram showing obstruction distal to the left subclavian artery (LSCA). B. Proximal arch obstruction. Lateral angiogram showing obstruction proximal to the LSCA.
Statistical Analysis
The primary outcome variables were freedom from any arch reintervention and freedom from any arch reoperation after BD. Throughout the manuscript, “reintervention” and “reoperation” refer to any aortic arch reintervention (catheter-based or surgical) or reoperation after BD, respectively. Another outcome variable analyzed was transplant-free survival. Survival data are presented as percent survival (95% confidence interval) at defined intervals. Predictor variables included the demographic, surgical, pre-catheterization, and BD-related variables listed above.
Time-to-event outcomes were evaluated using Kaplan-Meier analysis with log-rank test or Cox regression analysis. The proportionality assumption of Cox regression analysis was checked by plotting cumulative hazards for the independent variables that were analyzed. Multivariable freedom from event analysis was not performed due to the relatively small number of outcome events. Hazard ratios are presented with 95% confidence intervals. Comparisons of paired pre-BD and post-BD pressure gradients and COA indexes were performed using the Wilcoxon Signed-Rank test. Comparisons between groups were performed using Fisher’s exact test, Student’s t-test, or the Wilcoxon rank-sum test, as appropriate. Logistic regression was used to adjust for strength of indication for BD when comparing patient-related factors (e.g., age, indication for catheterization) between patients who had a residual post-BD ≤10mmHg and those with a gradient >10mmHg. Data are presented as frequency, median (minimum - maximum), or mean ± standard deviation, as appropriate. SPSS (version 16.0, SPSS Inc. Chicago, Illinois) was used for statistical analysis.
Results
Patients
Between 1/93 and 5/09, 556 patients underwent a NP at Children's Hospital Boston; follow-up data at least 1 month after the NP was available to us for 462 of these patients (19 patients had no follow-up information after discharge from the hospital, and 75 died within 1 month of the NP). Of these 462 patients, 133 had an intervention for arch obstruction (29%). BD was the first intervention in 120 (90%) of these patients, but 4 were excluded from this study: 1 had BD previously performed at another institution, and 3 underwent primary stent placement to relieve COA (one 7 year-old with a tortuous arch and two infants in the early post-operative period). The other 116 patients were included in the study.
Arch Reconstruction
Table 1 summarizes the demographic and operative characteristics at the time of NP for the study cohort.
Table 1.
Demographic and Intraoperative Factors at the time of Stage I Norwood Operation in Patients that Subsequently Underwent Balloon Angioplasty for Aortic Arch Obstruction
| Intraoperative Factor | Number (%) or Median (Range) |
|---|---|
| Sex | |
| - Male | 68 (59%) |
| - Female | 48 (41%) |
| Diagnostic Category | |
| - HLHS | 84 (73%) |
| MS/AS | 36 (43%) |
| MS/AA | 22 (26%) |
| MA/AA | 26 (31%) |
| - Single LV | 11 (9%) |
| - Unbalanced AVC | 9 (8%) |
| - DORV | 12 (10%) |
| Age at time of NP (days) | 4 (0 – 83) |
| - ≤7 | − 91 (78%) |
| - 8–13 | − 20 (17%) |
| - 14 or older | − 5 (4%) |
| Weight at time of NP (Kg) | 3.2 (1.4 – 4.5) |
| - < 3 | − 32 (28%) |
| - ≥ 3 | − 84 (72%) |
| Type of systemic-to-pulmonary shunt | |
| - MBTS or central shunt | 85 (73%) |
| - RV-PA conduit | 31 (27%) |
| Type of Aorta-PA anastomosis1 | |
| - Proximal side-to-side anastomosis | 106 (92%) |
| - Pulmonary trunk to underside of arch | 9 (8%) |
| Coarctectomy1 | |
| - No | 81 (70%) |
| - Yes | 34 (30%) |
| Patch Material Used to Augment Arch1 | |
| - Aortic homograft | 66 (57%) |
| - Pulmonary homograft | 33 (29%) |
| - Autologous pericardium | 14 (12%) |
| - None | 2 (2%) |
AA, aortic atresia; AS, aortic stenosis; AVC, atrioventricular canal defect; DORV, double outlet right ventricle; HLHS, hypoplastic left heart syndrome; LV, left ventricle; MA, mitral atresia; MBTS, modified Blalock-Taussig shunt; MS, mitral stenosis; NP, Norwood Procedure; RV-PA, right ventricle to pulmonary artery.
Data not available for one patient.
Balloon Aortoplasty
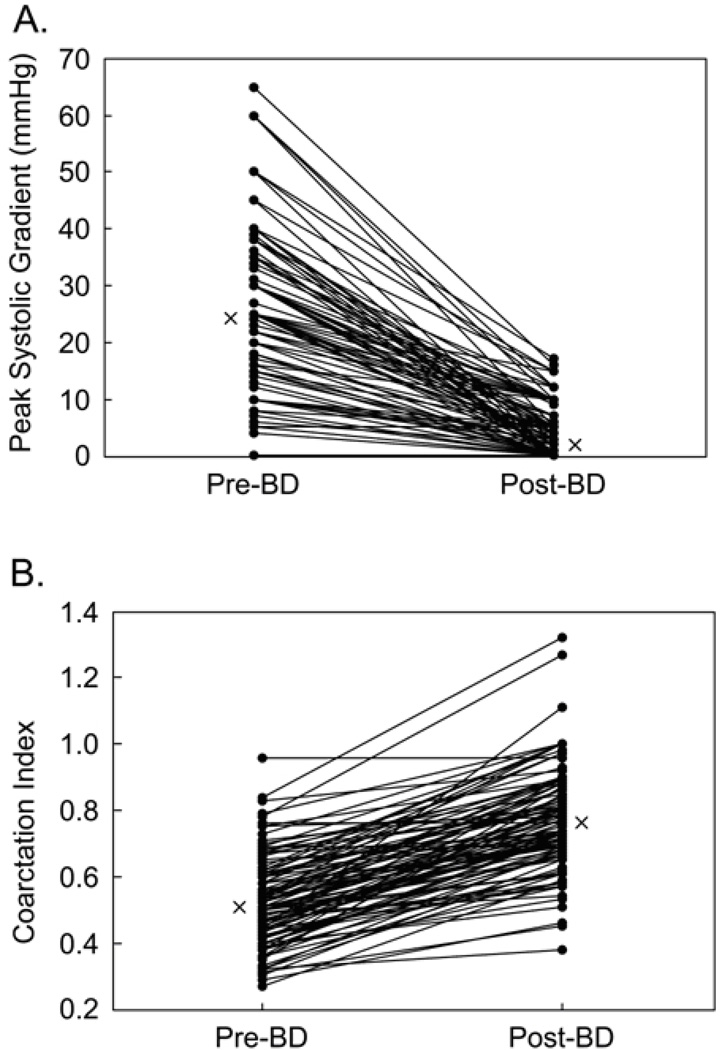
Table 2 summarizes the demographic, procedural and follow-up characteristics of patients undergoing BD. BD was acutely effective at reducing the peak gradient across the COA (Figure 3A), which fell from a median of 24 mmHg (0–65 mmHg) to 3 mmHg (0–17 mmHg; p<0.001). Of the 116 patients, 107 (92%) had a post-BD peak gradient ≤10 mmHg. There was no difference in the proportion of patients with a post-BD peak gradient ≤ 10 mmHg according to age at BD (p=0.21) or indication for the catheterization (p=0.5). This held true even after correcting for strength of indication for BD. The COA index after BD also increased significantly (Figure 3B; p<0.001).
Table 2.
Characteristics of Patients Undergoing Balloon Aortoplasty for Aortic Arch Obstruction Following the Norwood Procedure
| Variable | Number (%), Mean (+/− SD) or Median (Range) |
|---|---|
| Age at BD (months) | 4.5 (1.3–85.1) |
| - Infants (under 12 months of age) | − 100 (87%) |
| - ≤ 6 months of age | − 93 (80%) |
| - ≤ 3 months of age | − 45 (39%) |
| Weight (Kg) | 5.1 (2–24) |
| Circulation at time of BD | |
| - NP | 97 (83.6%) |
| - Bidirectional Glenn | 18 (15.5%) |
| - Fontan | 1 (0.9%) |
| Indication for Catheterization | |
| - Planned/Pre Surgical | 65 (56%) |
| - Unplanned | 51 (44%) |
| Peak Gradient across area of obstruction (mmHg) | |
| - Pre-BD | 24 (0–65) |
| - Post-BD | 3 (0–17) |
| Procedural Variables | |
| - First Balloon-to-COA diameter ratio | 1.8 (+/− 0.4) |
| - First Balloon-to-DAO diameter ratio | 0.9 (+/− 0.2) |
| - Maximum Balloon-to-COA diameter ratio | 2.0 (+/− 0.5) |
| - Maximum Balloon-to-DAO diameter ratio | 1.0 (+/− 0.2) |
| - Number of balloon inflations | 2 (1–7) |
| Follow-up Duration (years) | 3.4 (0.1 – 15.9) |
| Outcome | |
| - Reintervened | 47 (41%) |
| - Reoperated | 24 (21%) |
| - Repeat BD | 27 (23%) |
| - Death or transplant | 19 (16%) |
BD, balloon dilation of the aortic arch; COA, coarctation of the aorta; DAO, descending thoracic aorta at the level of the diaphragm; NP, Norwood procedure; SD, standard deviation.
Figure 3.
Acute results after balloon dilation (BD). A. Gradients across the site of obstruction before (Pre) and after (Post) BD. B. Coarctation index pre- and post-BD. Medians are indicated with an X.
Follow-up
All patients who did not undergo reintervention after BD (n=69) were followed for at least 6 months. During follow-up, 105 (91%) patients proceeded with staged single ventricle palliation: 27 patients (23%) underwent bidirectional Glenn only and 78 patients (67%) underwent a Fontan procedure. Six patients (5%) underwent conversion to a biventricular circulation, and 3 (3%) died before any subsequent surgeries.
Arch Reintervention
All reoperations (n=24) consisted of patch arch augmentation (homograft in 12 patients, autologous pericardium in 5, bovine pericardium in 3 and synthetic in 4). All but 3 of these patients underwent the reoperation at a subsequent surgical stage (not the sole indication for surgery). The indication given for reoperation was inadequate result of BD in 17 patients (71% of 24) and recurrence of obstruction in 7 (29%). All but 1 patient survived to discharge after reoperation.
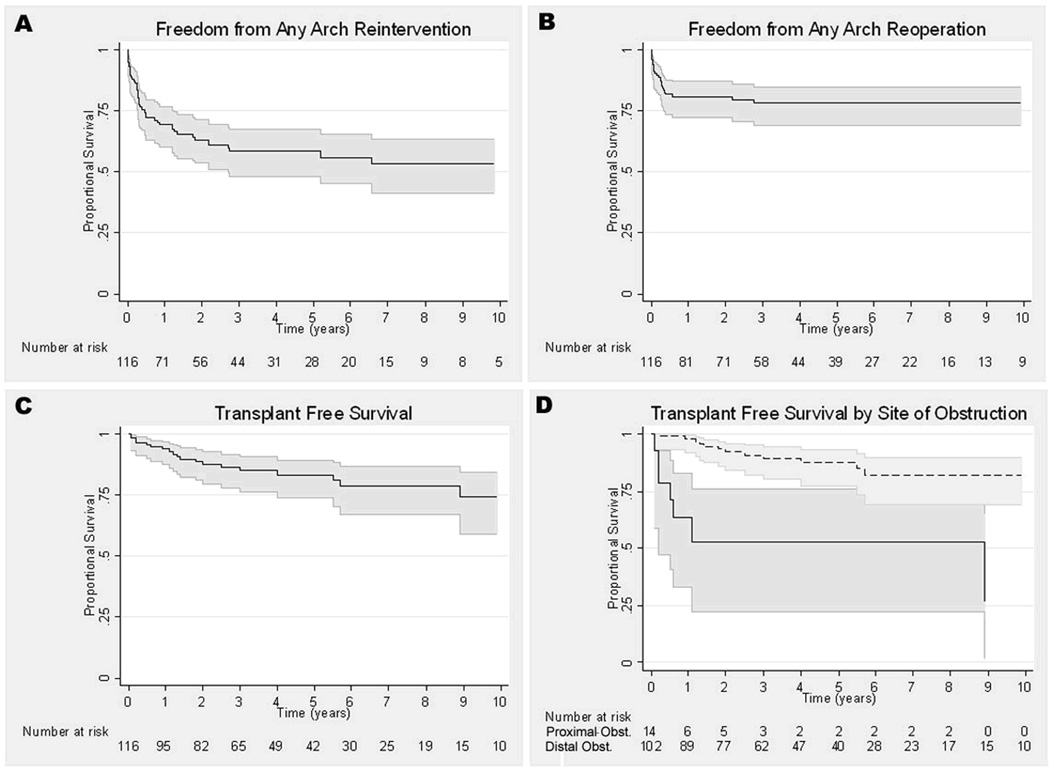
Survival analyses for each outcome are presented in Figure 4. Most of the reinterventions occurred in the first 6 months after BD (n=31; 66%). The primary indication given for reintervention in these patients was an inadequate result of the initial BD in 19 (61%), and recurrence of COA despite an acutely successful result in 12 patients (39%)(Figure 5). Freedom from reintervention was 69% at 1 year (60–76%) and 58% at 5 years (48–70%). Freedom from reoperation was 82% at 1 year (73–88%) and 79% at 5 years (71–85%). Transplant-free survival for the cohort was 94% at 1 year (88–97%), 83% at 5 years (67–87%), and 74% at 10 years (59–85%).
Figure 4.
Kaplan-Meier curves representing survival analysis for entire cohort. A. Freedom from any arch reintervention. B. Freedom from any arch reoperation. C. Transplant free survival. D. Transplant free survival by site of obstruction (obst.), proximal (solid line) or distal (dotted line). 95% confidence intervals are represented by the gray lines around the survival function (dark line).
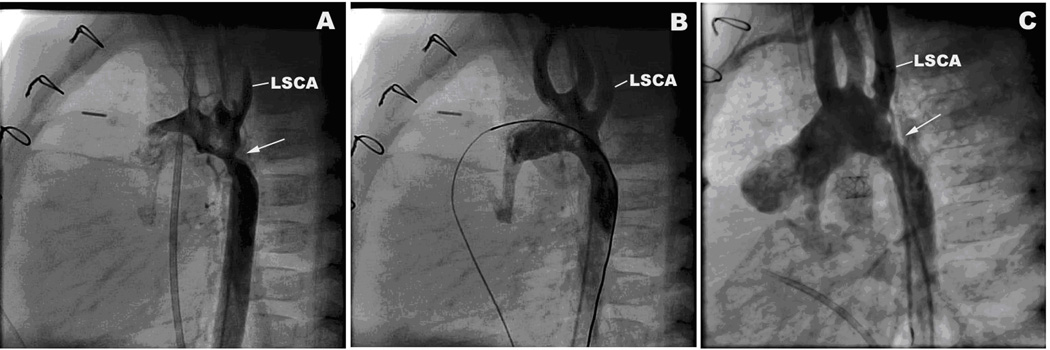
Figure 5.
Lateral projection aortic arch angiograms of infant with distal obstruction. A. Before balloon dilation there is a discrete obstruction (arrow) in the aortic arch immediately distal to the take-off of the left subclavian artery (LSCA). B. After balloon dilation there is resolution of the obstruction. C. On follow up catheterization 2 months later there is recurrence of obstruction (arrow) in the same location.
The results of univariable analysis for the primary outcomes are summarized in Table 3. Of note, there was no difference in the statistically significant variables when the analysis included only patients with a Stage I circulation at the time of BD (n=97), and no difference in freedom from reintervention or reoperation between patients who underwent BD in the first and second halves of the study period. Factors associated with shorter transplant-free survival were moderate or worse RV dysfunction at the time of pre-catheterization echocardiogram (HR: 5.3; 2.0–14.5; p=0.001) and proximal COA (HR: 7.4; 2.9–18.9; p<0.001).
Table 3.
Univariate Cox Proportional Hazard Ratio Model of Patient Factors with Relative Risk of Reintervention and Reoperation after Balloon Dilation for Arch Obstruction Following the Norwood Procedure
| Any Arch Reintervention | Any Arch Reoperation | |||
|---|---|---|---|---|
| Patient Factor | Hazard Ratio (95% CI) |
p Value |
Hazard Ratio (95% CI) |
p Value |
| Age at NP | 0.97 (0.91–1.03) | 0.33 | 0.84 (0.70–1.01) | 0.07 |
| BD era (compared to 1st half of series) | 1.16 (0.64–2.08) | 0.63 | 1.78 (0.78–4.09) | 0.17 |
| Weight < 3 Kg at NP | 0.77 (0.41–1.44) | 0.41 | 0.91 (0.38–2.19) | 0.83 |
| Ascending aorta diameter at time of NP (mm) | 0.91 (0.81–1.03) | 0.14 | 0.93 (0.79–1.10) | 0.42 |
| MBTS as source of pulmonary blood flow | 1.2 (0.62–2.27) | 0.60 | 1.70 (0.74–3.89) | 0.21 |
| Side-to-side anastomosis between PA and Ao | 0.63 (0.23–1.77) | 0.38 | 0.49 (0.14–1.63) | 0.24 |
| Coarctectomy performed | 1.44 (0.78–2.66) | 0.25 | 1.93 (0.85–4.35) | 0.11 |
| Homograft patch used for arch augmentation | 1.51 (0.67–3.8) | 0.32 | 1.90 (0.71–5.10) | 0.20 |
| Age <3 months at time of initial BD | 3.82 (2.12–6.91) | <0.001 | 3.78 (1.69–8.43) | 0.001 |
| ≥Moderate ventricular dysfunction on pre-BD Echo | 1.98 (1.07–3.65) | 0.03 | 2.33 (1.03–5.25) | 0.04 |
| ≥Moderate AVVR on pre-BD Echo | 1.62 (0.83–3.13) | 0.16 | 2.69 (1.17–6.10) | 0.02 |
| Proximal obstruction | 3.27 (1.56–6.84) | 0.002 | 5.74 (2.42–13.63) | <0.001 |
| Gothic arch geometry present | 1.57 (0.87–2.83) | 0.14 | 1.77 (0.78–4.05) | 0.18 |
| Unplanned catheterization | 3.87 (2.08–7.19) | <0.001 | 3.52 (1.46–8.49) | 0.005 |
| Gradient prior to BD (mmHg) | 1.01 (0.99–1.04) | 0.12 | 1.01 (0.98–1.03) | 0.66 |
| Gradient after BD (mmHg) | 1.06 (1.00–1.13) | 0.039 | 1.10 (1.02–1.19) | 0.02 |
| COA index prior to BD | 0.03 (0.002–0.34) | 0.006 | 0.06 (0.001–1.59) | 0.09 |
| COA index after BD | 0.02 (0.002–0.18) | <0.001 | 0.13 (0.01–2.22) | 0.16 |
Ao, aorta; AscAo, ascending aorta; AVVR, atrioventricular valve regurgitation; BD, balloon dilation of the aortic arch; COA: coarctation; echo, echocardiogram ; MBTS, modified Blalock-Taussig shunt; NP, Norwood Procedure; PA, pulmonary artery.
Risk Factors for Arch Reintervention
Site of Obstruction
The most common site of COA was distal to the LSCA (n=102, 88%). Of the 14 patients with proximal COA, the obstruction was in the transverse arch (between the origins of the innominate and LSCA) in 10 patients and immediately proximal to the origin of the brachiocephalic vessels in 4.
Proximal COA was significantly associated with shorter freedom from reintervention, reoperation and transplant-free survival (Figure 4F). Patients with proximal COA underwent initial BD during an unplanned catheterization more often than patients with distal COA (86% vs. 38%; p=0.001). There were no other differences in demographic, surgical, or functional variables between the two groups. Although there was no difference between the pre-BD pressure gradients in the two groups (median of 25 vs. 23 mmHg; p=0.99), patients with proximal COA had a significantly higher post-BD gradient (median of 8 vs 2 mmHg; p=0.05).
Age at time of BD
Age less than 3 months at the time of BD was associated with shorter freedom from reintervention and reoperation. As would be expected based on our management of single ventricle patients, all patients that underwent BD at less than 3 months of age did so during an unplanned catheterization. Unplanned catheterization, regardless of age, was also associated with shorter freedom from reintervention and reoperation.
Pre-BD Catheterization Data
The pre-BD gradient was not associated with any of the outcomes. A lower pre-BD COA index was associated with shorter freedom from reintervention, but not with freedom from reoperation.
Acute response to BD
Post-BD COA index was associated with shorter freedom from reintervention, and the post-BD gradient was associated with shorter freedom from reoperation and reintervention.
Surgical Factors
We did not find any surgical factors that were associated with freedom from reintervention or reoperation after the initial BD. There was also no association between individual surgeons and patient outcomes. However, it should be mentioned that during the study period there were over 8 different surgeons performing this procedure. Analysis limited to the surgeons with the most cases showed no statistically significant difference.
Ventricular and Atrioventricular-valve Function
Both moderate or worse RV dysfunction and moderate or worse AVVR on the pre-catheterization echocardiogram were associated with shorter freedom from reoperation. Moderate or worse RV dysfunction was also associated with shorter freedom from reintervention.
Arch Geometry
The most common arch geometry was Gothic (50% or 56 patients out of the 111 patients with angiograms available for review). Romanesque arch was seen in 35 (32%), Crenel in 17 (15%), and S-shaped arch in 3 (3%). There was no statistically significant association between the presence of a Gothic arch and freedom from reintervention, freedom from reoperation, or transplant-free survival.
Sub-group Analysis
We performed separate analysis on the group of patients with distal arch obstruction (102 patients). The predictive variables of significance were the same. However, there were two additional variables that were significant in this sub-group by univariate analysis: (1) a higher pre-BD gradient was associated with shorter freedom from reintervention (p=0.03), and (2) a lower pre-BD coarctation index was associated with shorter freedom from reoperation (p=0.002).
Follow-up Echocardiography and Hemodynamic Evaluation
Follow-up echocardiograms were available in all but 1 patient. The median time from the BD to the last available echocardiogram was 37.4 months (0.3–192.8 months). Significant ventricular dysfunction (qualitatively moderate or worse) was present in 36 patients (31%) and did not differ in frequency between the patients who had reintervention and those who did not (32 vs. 31%; p=1.0). Significant AVVR (qualitatively moderate or worse) was present in 18 patients (16%), and did not differ in frequency between the patients who had reintervention and those who did not (22 vs. 12%; p=0.2).
Of the 69 patients who did not have reintervention, 52 (75%) had a follow-up catheterization. The median time from BD to the last available catheterization in these patients was 32.4 months (2.1–126.7 months). The median gradient across the aortic arch recorded at this catheterization was 0 mmHg (0–15 mmHg), and all but 1 patient had a gradient < 10 mmHg.
Discussion
Despite significant advances in the surgical management of patients with HLHS and related single ventricle defects, COA after the NP continues to be a significant problem1–8, 10, 11. When it occurs, BD has been established as the first line of therapy and is generally effective acutely3–7, as our study confirms. However, despite a high acute success rate, our data demonstrate that reintervention and reoperation on the aortic arch are common, especially in the 6 months following the initial BD. This finding is consistent with smaller series reported by other groups4, 6, 7.
As we expected, patients who underwent BD at a younger age and had symptomatic COA were at higher risk for reoperation and reintervention. Importantly, however, reduction in the COA gradient to levels ≤10 mmHg and increase in the COA index was achieved in the great majority of the patients, regardless of the indication for catheterization, the strength of the indication for BD, or the age of the patient.
In our study, the site of obstruction relative to the take-off of the LSCA was strongly associated with outcome after BD for post-NP COA. The most common site of obstruction was distal to the LSCA, which accounted for 89% of the cases in this series. Patients with proximal COA were found to be less responsive to BD and to have worse outcomes. Although some reports have found an association between arch obstruction and mortality after the NP, more recent experience suggests that, in the balloon angioplasty era, mortality is similar for patients who do and do not undergo BD for arch obstruction7. Here, we identify a sub-group of patients in whom obstruction is less responsive to BD and is associated not only with increased reintervention rates, but also with decreased survival. Of the 14 patients with proximal arch obstruction, only 3 patients survived without reintervention. Symptomatic patients appear to be at particularly high risk: of the 12 patients with symptomatic proximal COA, 11 underwent reintervention, died or underwent transplant during the follow up period. Based on these findings, it would be reasonable to advocate for a more aggressive approach to the management of patients with proximal COA, especially those with symptomatic obstruction. It will also be important to determine risk factors for the development of proximal arch obstruction, although that is beyond the scope of this project.
Several studies have shown that surgical technique, patch materials and baseline anatomy influence the risk of arch obstruction after the NP1, 2, 11–14. We did not find any surgical factors that were associated with freedom from reoperation or reintervention after BD. Coarctectomy has been shown in some series to protect against late COA after the NP. In this study, patients with a history of a coarctectomy who had developed COA and underwent BD had no difference in freedom from subsequent reintervention compared with patients who did not have a coarctectomy. We take this to mean that coarctectomy does not provide an advantage in the response to BD, once COA develops. The presence of an aberrant right subclavian artery may alter the techinique of NP11; therefore this sub-population may respond differently to BD for arch obtrcution.. In our cohort, there were only 5 patients with this diagnosis, limiting our ability to analyze it as a risk factor for arch reintervention after BD.
The currently preferred surgical technique for arch reconstruction by most surgeons at our institution consists of posterior shelf resection (coarctectomy) and subsequent reconstruction of the greater curvature of the distal arch, followed by patch augmentation of the lesser curvature of the neo-aorta, preferably using glutaraldehyde-treated autologous pericardium. However, there continues to be some variation in practice among surgeons.
Pre-BD gradient was not associated with any of the outcomes in the group as a whole. However, among patients with distal COA, pre-BD gradient and pre-BD COA index were associated with a shorter freedom from reintervention and reoperation, respectively. These data suggest that, for patients with distal COA, more severe obstruction (as judged by the catheterization data) is a risk factor for reintervention on the aortic arch.
Several criteria have been applied to evaluate the acute results of balloon angioplasty for COA after the NP, mostly based on the post-BD gradient and/or the COA index3, 6, 7, 15. However, it is unclear whether these measures correlate with better long-term outcomes. We found that both higher post-BD gradient and a lower COA index after BD were associated with shorter freedom from reintervention, and that a higher post-BD gradient was also associated with shorter freedom from reoperation. However, these acute outcomes taken in isolation are unlikely to be reliable predictors of long-term success. Many factors, including the arch anatomy, the patient’s presentation, ventricular function, and AVVR likely contribute.
If untreated, COA can result in deleterious effects on ventricular function, which is a negative prognostic indicator16. In this study, moderate or worse ventricular dysfunction at the time of pre-catheterization echocardiogram was associated with significantly shorter transplant-free survival. Even though studies have shown that ventricular function does recover if the obstruction is relieved in a timely manner5, 8, it is still unclear whether there are long-term implications of the duration and magnitude of increased afterload to which these patients are exposed.
Patients with moderate or worse ventricular dysfunction or moderate or worse AVVR on the pre-catheterization echocardiogram had a shorter freedom from reoperation. These findings may reflect increased ventricular function and AVVR effects in patients with higher COA severity, or a lower threshold for arch reoperation in patients with higher grade ventricular dysfunction or AVVR.
In the bigger picture, the hydrodynamic efficiency of the reconstructed aortic arch of the NP, with geometric variability, compliance mismatch due to rapid tapering, heterogeneous aortic wall properties due to patch material and possibly scar, and other factors, is not well understood. Patients who have undergone a NP have decreased elasticity within the reconstructed aortic arch17, and elastic properties of the central arterial tree are known to have a profound influence on ventricular function18–20. In patients who have undergone anatomically successful repair of COA not associated with NP, abnormal arch geometry has been associated with abnormalities of central aortic fluid dynamics and biomechanics that may predispose to adverse cardiovascular outcomes21. We did not find an association between arch geometry and outcome after BD for COA following the NP. However, discrete COA is but one form of increased ventricular afterload, and further study is necessary to characterize ventricular-arterial interaction and to more fully understand the anatomic, hydrodynamic and mechanical load imposed by a reconstructed aorta after the NP.
Interpretation of our results is subject to certain limitations. Although we found that, of the patients who underwent NP and had follow-up for longer than 1 month, 29% had an intervention for COA, this may not accurately reflect the incidence of COA after the NP. The primary goal of this study was not to determine the incidence of, or risk factors for, arch reintervention after the NP. The study population represents a selected group, since patients that died with COA, those that underwent arch surgery without prior BD, and those with obstruction who did not undergo any arch reintervention were not included. Also, because of the retrospective nature of this study, there were no pre-established criteria for diagnosing recurrent COA; therefore, the indications for repeat intervention may not have been uniform across the study population.
In summary, BD was acutely successful in the large majority of patients with COA after the NP. However, both catheter-based and surgical reintervention were common in this patient population, especially in the first 6 months after BD. We did not find any association between surgical factors and acute or intermediate outcomes after BD. Obstruction that involved the neo-aortic arch proximal to the take-off of the LSCA was less responsive to BD and was associated with worse outcomes. Finally, younger patients and those that have a less successful acute result were more likely to undergo reintervention.
List of Abbreviations
- BD
balloon aortoplasty
- COA
coarctation of the aorta or aortic arch obstruction
- NP
Norwood Procedure
- HLHS
hypoplastic left heart syndrome
- AVVR
atrioventricular valve regurgitation
- DAO
descending thoracic aorta
- LSCA
left subclavian artery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This manuscript is not under consideration for publication elsewhere.
None of the contents of this manuscript have been previously published.
All authors have read and approved the manuscript.
The authors do not have any relationships with industry to disclose.
References
- 1.Ashcraft TM, Jones K, Border WL, et al. Factors affecting long-term risk of aortic arch recoarctation after the Norwood procedure. Ann Thorac Surg. 2008 Apr;85(4):1397–1401. doi: 10.1016/j.athoracsur.2007.11.054. discussion 1401-1392. [DOI] [PubMed] [Google Scholar]
- 2.Bautista-Hernandez V, Marx GR, Gauvreau K, et al. Coarctectomy reduces neoaortic arch obstruction in hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2007 Jun;133(6):1540–1546. doi: 10.1016/j.jtcvs.2006.12.067. [DOI] [PubMed] [Google Scholar]
- 3.Chessa M, Dindar A, Vettukattil JJ, et al. Balloon angioplasty in infants with aortic obstruction after the modified stage I Norwood procedure. Am Heart J. 2000 Aug;140(2):227–231. doi: 10.1067/mhj.2000.108238. [DOI] [PubMed] [Google Scholar]
- 4.Soongswang J, McCrindle BW, Jones TK, et al. Outcomes of transcatheter balloon angioplasty of obstruction in the neo-aortic arch after the Norwood operation. Cardiol Young. 2001 Jan;11(1):54–61. doi: 10.1017/s1047951100012427. [DOI] [PubMed] [Google Scholar]
- 5.Tworetzky W, McElhinney DB, Burch GH, et al. Balloon arterioplasty of recurrent coarctation after the modified Norwood procedure in infants. Catheter Cardiovasc Interv. 2000 May;50(1):54–58. doi: 10.1002/(sici)1522-726x(200005)50:1<54::aid-ccd11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Zellers TM. Balloon angioplasty for recurrent coarctation of the aorta in patients following staged palliation for hypoplastic left heart syndrome. Am J Cardiol. 1999 Jul 15;84(2):231–233. A239. doi: 10.1016/s0002-9149(99)00242-8. [DOI] [PubMed] [Google Scholar]
- 7.Zeltser I, Menteer J, Gaynor JW, et al. Impact of re-coarctation following the Norwood operation on survival in the balloon angioplasty era. J Am Coll Cardiol. 2005 Jun 7;45(11):1844–1848. doi: 10.1016/j.jacc.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 8.Larrazabal LA, Selamet Tierney ES, Brown DW, et al. Ventricular function deteriorates with recurrent coarctation in hypoplastic left heart syndrome. Ann Thorac Surg. 2008 Sep;86(3):869–874. doi: 10.1016/j.athoracsur.2008.04.074. discussion 869-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraisse A, Colan SD, Jonas RA, et al. Accuracy of echocardiography for detection of aortic arch obstruction after stage I Norwood procedure. Am Heart J. 1998 Feb;135(2 Pt 1):230–236. doi: 10.1016/s0002-8703(98)70086-9. [DOI] [PubMed] [Google Scholar]
- 10.Jonas RA. Intermediate procedures after first-stage Norwood operation facilitate subsequent repair. Ann Thorac Surg. 1991 Sep;52(3):696–700. doi: 10.1016/0003-4975(91)90980-5. [DOI] [PubMed] [Google Scholar]
- 11.Ishino K, Stumper O, De Giovanni JJ, et al. The modified Norwood procedure for hypoplastic left heart syndrome: early to intermediate results of 120 patients with particular reference to aortic arch repair. J Thorac Cardiovasc Surg. 1999 May;117(5):920–930. doi: 10.1016/s0022-5223(99)70373-9. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs ML. Aortic reconstruction in hypoplastic left heart syndrome-A reappraisal. J Thorac Cardiovasc Surg. 2000 Nov;120(5):872–874. doi: 10.1067/mtc.2000.110490. [DOI] [PubMed] [Google Scholar]
- 13.Larrazabal LA, del Nido PJ, Jenkins KJ, et al. Measurement of technical performance in congenital heart surgery: a pilot study. Ann Thorac Surg. 2007 Jan;83(1):179–184. doi: 10.1016/j.athoracsur.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Poirier NC, Drummond-Webb JJ, Hisamochi K, et al. Modified Norwood procedure with a high-flow cardiopulmonary bypass strategy results in low mortality without late arch obstruction. J Thorac Cardiovasc Surg. 2000 Nov;120(5):875–884. doi: 10.1067/mtc.2000.109540. [DOI] [PubMed] [Google Scholar]
- 15.Saul JP, Keane JF, Fellows KE, et al. Balloon dilation angioplasty of postoperative aortic obstructions. Am J Cardiol. 1987 Apr 15;59(9):943–948. doi: 10.1016/0002-9149(87)91130-1. [DOI] [PubMed] [Google Scholar]
- 16.Hosein RB, Clarke AJ, McGuirk SP, et al. Factors influencing early and late outcome following the Fontan procedure in the current era. The 'Two Commandments'? Eur J Cardiothorac Surg. 2007 Mar;31(3):344–352. doi: 10.1016/j.ejcts.2006.11.043. discussion 353. [DOI] [PubMed] [Google Scholar]
- 17.Cardis BM, Fyfe DA, Mahle WT. Elastic properties of the reconstructed aorta in hypoplastic left heart syndrome. Ann Thorac Surg. 2006 Mar;81(3):988–991. doi: 10.1016/j.athoracsur.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 18.Babalis D, Levy BI, Azancot I, et al. Ventricular function and arterial compliance in patients with congestive cardiomyopathy. Int J Cardiol. 1984 Mar;5(3):361–364. doi: 10.1016/0167-5273(84)90113-x. [DOI] [PubMed] [Google Scholar]
- 19.Eren M, Gorgulu S, Uslu N, et al. Relation between aortic stiffness and left ventricular diastolic function in patients with hypertension, diabetes, or both. Heart. 2004 Jan;90(1):37–43. doi: 10.1136/heart.90.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hundley WG, Kitzman DW, Morgan TM, et al. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001 Sep;38(3):796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 21.Ou P, Celermajer DS, Raisky O, et al. Angular (Gothic) aortic arch leads to enhanced systolic wave reflection, central aortic stiffness, and increased left ventricular mass late after aortic coarctation repair: evaluation with magnetic resonance flow mapping. J Thorac Cardiovasc Surg. 2008 Jan;135(1):62–68. doi: 10.1016/j.jtcvs.2007.03.059. [DOI] [PubMed] [Google Scholar]