Abstract
Chemical investigation of the ethanol extract of soft coral Sinularia sp. collected from the South China Sea led to the isolation of three new polyoxygenated sterols, (3S,23R,24S)-ergost-5-ene-3β,23α,25-triol (1), (24S)-ergostane-6-acetate-3β,5α,6β,25-tetraol (2), (24S)-ergostane-6-acetate-3β,6β,12β,25-tetraol (3) together with three known ones (4–6). The structures, including relative configurations of the new compounds (1–3), were elucidated by detailed analysis of spectroscopic data (IR, UV, NMR, MS) and by comparison with related reported compounds. The absolute configuration of 1 was further determined by modified Mosher’s method. Compound 5 exhibited moderate cytotoxicity against K562 cell line with an IC50 value of 3.18 μM, but also displayed strong lethality toward the brine shrimp Artemia salina with a LC50 value of 0.96 μM.
Keywords: soft coral, Sinularia sp., polyoxygenated sterols, cytotoxicity
1. Introduction
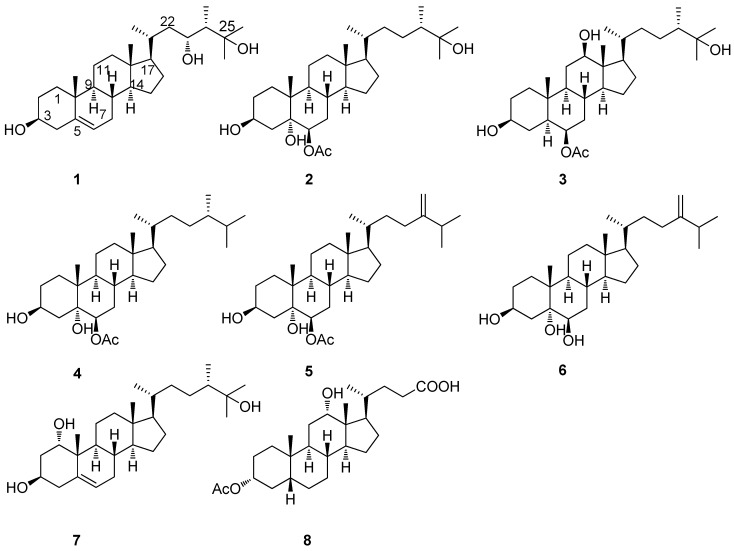
Soft coral of the genus Sinularia has been found to be a rich source of bioactive secondary metabolites [1,2], such as acylated spermidine [3,4], lipids and fatty acids [5], cyclic sesquiterpene peroxides [6], sterols [7,8], and norditerpenes [9,10]. A number of them showed an array of biological activities such as cytotoxic activities [3,4] and inhibitory effect on LPS-induced TNF-α production [9,10]. As part of our ongoing investigation of new natural bioactive compounds from marine invertebrates in the South China Sea [11,12,13,14,15,16], the soft coral Sinularia sp. attracted our attention because the crude extract of Sinularia sp. showed lethal activity toward brine shrimp Artemia salina. Bioassay-guided fractionation of the active extracts led to the isolation of three new polyoxygenated sterols (1–3) and three known ones (4–6) [17,18] (Figure 1).
Figure 1.
Structures of compounds 1–6 from Sinularia sp.
2. Results and Discussion
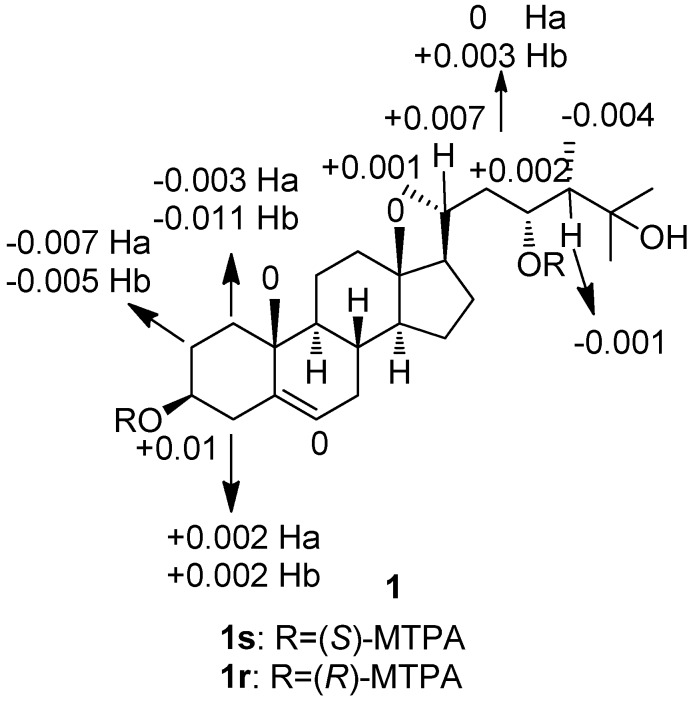
Compound 1 was obtained as a white, amorphous powder with [α]D25 −22.4 (c 0.05, CH3OH). IR spectrum showed its absorption at 3410–3584 cm−1 for hydroxy groups. The molecular formula was established as C28H48O3 based on HRESIMS ([M + Na]+ 455.3498 (calcd for C28H48O3Na 455.3496)) and NMR spectroscopic data. The 1H-NMR (Table 1) and 13C-NMR (Table 2) spectra implied that compound 1 was a polyhydroxylated sterol. The 1H-NMR spectrum of 1 revealed four methyl singlet signals at δH 0.70 (3H, s, H3-18), 1.00 (3H, s, H3-19), 1.23 (3H, s, H3-26) and 1.23 (3H, s, H3-27), and two methyl doublet signals at δH 0.81 (3H, d, J = 6.6 Hz, H3-28) and 1.08 (3H, d, J = 6.6 Hz, H3-21), as well as two oxymethines (δH 3.71 and 3.56), and an olefinic proton signal at δH 5.34 (br d, J = 4.8 Hz). The 13C-NMR and DEPT spectra of 1 displayed 28 signals which were assigned to four quaternary carbons, nine methines, nine methylenes, and six methyls. The olefinic carbon signals appearing at δC 140.8 (C) and 121.6 (CH) corresponded to one trisubstituted double bond. The 13C-NMR chemical shifts at δC 75.8 (CH), 75.2 (C) and 71.8 (CH) confirmed the presence of three oxygenated carbons. Detailed analysis of the 1H-1H COSY spectrum in combination with HMQC and HMBC (Figure 2) experiments allowed the assignment of all of the chemical shifts in the 1H and 13C-NMR spectra and led to structure 1. Comparison of 1H-NMR and 13C-NMR of 1 with those of the known compound 7 (patusterol A) [19], a hydroxylated steroid from the Kenyan soft coral Lobophytum patulum, further confirmed the structure of 1. The obvious differences between the two compounds are the chemical shifts at δH3.71 (1H, ddd, J = 12.0, 8.4, 3.0 Hz, H-23) in 1 vs. 3.71 (1H, br t,J = 2.6 Hz, H-1) in 7, and δC 75.8 (CH) in 1 vs. 72.5 (CH) in 7, indicating that the hydroxyl group in 1 is at C-23 not as 7 at C-1. In addition, the 1H–1H COSY correlations of H-23 with H-24 and H-22, and the HMBC correlations from H3-28 to C-24, C-23 and C-25 enable the hydroxyl group to be placed at C-23. The relative stereochemistry of 1 was assigned on the basis of 2D NOESY experiment. In the NOE spectrum of 1, NOE correlations observed from H3-18 to H-20 indicated that H-20 was in β disposition. The α orientation of H-9 and H-14, and β orientation of H-8 were also determined by NOESY experiment. The absolute configuration at C-3 and C-23 of 1 was tried to determine using the modified Mosher’s method [20]. The (S)- and (R)-MTPA esters 1r and 1s were prepared using (R)- and (S)-MTPA chloride, respectively. The determination of Δδ values (δS − δR) (Figure 3) for protons neighboring C-3 and C-23 should lead to the assignment of the configuration at C-3 and C-23 in 1. The configuration at C-24 in this and congener sterols was suggested as 24S on biogenetic grounds, since almost all the 24-methylsterols isolated from corals have 24S stereochemistry according to the literature [21,22]. According to the relatively small difference of Δδ values (δS − δR), the absolute configuration of 1 was tentatively determined as (3S,23R,24S)-ergost-5-ene-3β,23α,25-triol.
Table 1.
1H-NMR data for compounds 1–3.
| H# | 1, δH (J in Hz) a | 2, δH (J in Hz) b | 3, δH (J in Hz) a |
|---|---|---|---|
| 1 | 1.82 (1H, d, J = 4.8 Hz, H-ax) | 1.75 (1H, br d, J = 12.0 Hz, H-ax) | 1.82 (1H, br d, J = 4.8 Hz, H-ax) |
| 1.12 (1H, m, H-eq) | 1.18 (1H, m, H-eq) | 1.27 (1H, m, H-eq) | |
| 2 | 2.30 (1H, ddd, J = 13.2, 4.8, 1.8 Hz, H-ax) | 2.01 (1H, dt, J = 12.0, 2.4 Hz, H-ax) | 1.99 (1H, 1H, dt, J = 12.6, 2.4 Hz, H-ax) |
| 1.49 (1H, m, H-eq) | 1.51 (1H, m, H-eq) | 1.49 (1H, m, H-eq) | |
| 3 | 3.56 (1H, m) | 3.99 (1H, m) | 4.09 (1H, m) |
| 4 | 2.22 (1H, td, J = 12.6, 2.4 Hz, H-ax) | 1.77 (1H, br d, J = 12.0 Hz, H-ax ) | 1.84 (1H, br d, J = 12.0 Hz, H-ax) |
| 1.48 (1H, d, J = 2.4 Hz, H-eq) | 1.53 (1H, m, H-eq) | 1.54 (1H, m, H-eq) | |
| 5 | – | – | 1.61 (1H, m) d |
| 6 | 5.34 (1H, br d, J = 4.8 Hz) | 4.68 (1H, t, J = 2.4 Hz) | 4.70 (1H, m) |
| 7 | 2.02 (1H, dt, J = 12.6, 4.8 Hz, H-ax) | 1.68 (1H, dd, J =13.8, 2.4 Hz, H-ax) | 1.64 (1H, m) |
| 1.96 (1H, m, H-eq) | 1.47 (1H, d, J = 2.4, H-eq) | 1.61 (1H, m) d | |
| 8 | 1.52 (1H, m) | 1.52 (1H, m) | 1.29 (1H, m) |
| 9 | 1.16 (1H, m) | 1.63 (1H, m) | 1.68 (1H, m) |
| 10 | – | – | – |
| 11 | 1.43 (1H, m) | 1.34 (1H, m) | 1.73 (1H, dd, J = 14.4, 7.2 Hz H-ax) |
| 1.32 (1H, m) | 1.32 (1H, m) | 0.77 (1H, m, H-eq) | |
| 12 | 1.28 (1H, m) | 1.58 (1H, m) | 4.31 (1H, td, J = 7.2, 0.6 Hz) |
| 0.97 (1H, m) | 1.42 (1H, m) | ||
| 13 | – | – | – |
| 14 | 1.33 (1H, m) | 1.25 (1H, m) | 1.30 (1H, m) |
| 15 | 1.44 (1H, m) | 1.55 (1H, m) | 1.51 (1H, m) |
| 0.94 (1H, m) | 1.03 (1H, m) | 1.02 (1H, m) | |
| 16 | 1.88 (1H, m) | 1.89 (1H, m) | 1.86 (1H, m) |
| 1.45 (1H, m) | 1.50 (1H, m) | 1.40 (1H, m) | |
| 17 | 1.15 (1H, m) | 1.05 (1H, m) | 1.10 (1H, m) |
| 18 | 0.70 (3H, s) | 0.78 (3H, s) | 0.68 (3H, s) |
| 19 | 1.00 (3H, s) | 1.14 (3H, s) | 1.16 (3H, s) |
| 20 | 1.50 (1H, m) | 1.36 (1H, m) | 1.39 (1H, m) |
| 21 | 1.08 (3H, d, J = 6.6 Hz) | 0.94 (3H, d, J = 6.6 Hz) | 0.93 (3H, d, J = 6.6 Hz) |
| 22 | 1.84 (1H, m) | 1.62 (1H, m) | 1.62 (1H, m) |
| 1.10 (1H, m) | 1.01 (1H, m) | 1.08 (1H, m) | |
| 23 | 3.71 (1H, ddd, J = 12.0, 8.4, 3.0 Hz) | 1.68 (1H, m) | 1.86 (1H, m) |
| 0.77 (1H, m) | 0.78 (1H, m) | ||
| 24 | 1.56 (1H, m) | 1.27 (1H, m) | 1.28 (1H, m) |
| 25 | – | – | – |
| 26 | 1.23 (3H, s) c | 1.09 (3H, s) | 1.15 (3H, s) e |
| 27 | 1.23 (3H, s) c | 1.10 (3H, s) | 1.15 (3H, s) e |
| 28 | 0.81 (3H, d, J = 6.6 Hz) | 0.87 (3H, d, J = 7.2 Hz) | 0.89 (3H, d, J = 7.2 Hz) |
| OAc | – | 2.02 (3H, s, CH3CO–) | 2.06 (3H, s, CH3CO–) |
a Spectra were measured in CDCl3 (600 MHz); b Spectra were measured in CD3OD (600 MHz). c,d,e Overlapping signals.
Table 2.
13C-NMR data for compounds 1–3.
| C# | 1, a δC, type | 2, b δC, type | 3, a δC, type |
|---|---|---|---|
| 1 | 37.2, CH2 | 33.2, CH2 | 34.9, CH2 |
| 2 | 24.3, CH2 | 22.2, CH2 | 21.1, CH2 |
| 3 | 71.8, CH | 67.9, CH | 67.3, CH |
| 4 | 42.3, CH2 | 31.6, CH2 | 28.2, CH2 |
| 5 | 140.8, C | 75.5, C | 30.7, CH |
| 6 | 121.6, CH | 77.8, CH | 76.1, CH |
| 7 | 31.7, CH2 | 32.5, CH2 | 31.4, CH2 |
| 8 | 31.9, CH | 32.2, CH | 31.9, CH |
| 9 | 50.1, CH | 46.2, CH | 45.2, CH |
| 10 | 36.5, C | 39.6, C | 38.5, C |
| 11 | 21.1, CH2 | 29.1, CH2 | 40.5, CH2 |
| 12 | 39.7, CH2 | 41.0, CH2 | 73.7, CH |
| 13 | 42.5, C | 43.9, C | 42.7, C |
| 14 | 56.6, CH | 57.3, CH | 55.8, CH |
| 15 | 21.0, CH2 | 25.2, CH2 | 24.1, CH2 |
| 16 | 28.5, CH2 | 29.3, CH2 | 29.7, CH2 |
| 17 | 57.3, CH | 57.4, CH | 55.9, CH |
| 18 | 11.8, CH3 | 12.6, CH3 | 12.2, CH3 |
| 19 | 19.4, CH3 | 17.1, CH3 | 16.5, CH3 |
| 20 | 35.0, CH | 37.8, CH | 36.3, CH |
| 21 | 23.2, CH3 | 15.3, CH3 | 19.0, CH3 |
| 22 | 44.2, CH2 | 36.3, CH2 | 39.9, CH2 |
| 23 | 75.8, CH | 29.1, CH2 | 30.6, CH2 |
| 24 | 48.9, CH | 46.4, CH | 45.4, CH |
| 25 | 75.2, C | 74.2, C | 75.3, C |
| 26 | 30.7, CH3 | 26.0, CH3 | 26.2, CH3 |
| 27 | 30.7, CH3 | 27.2, CH3 | 27.2, CH3 |
| 28 | 14.1, CH3 | 19.6, CH3 | 14.8, CH3 |
| CH3CO | – | 21.4, CH3 | 21.4, CH3 |
| CH3 CO | – | 172.1, C | 164.5, C |
a Spectra were measured in CDCl3 (150 MHz); b Spectra were measured in CD3OD (150 MHz).
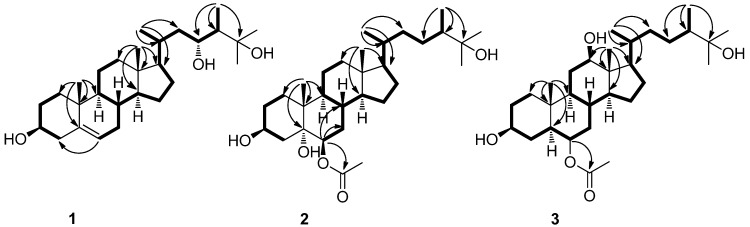
Figure 2.
1H-1H COSY(▬) and HMBC (→) correlations for compounds 1–3.
Figure 3.
Δδ values (δ(S) − δ(R)) for the MTPA esters of compound 1.
Compound 2 was obtained as a white, amorphous powder with [α]D25 −45.6 (c 0.90, CH3OH). Its positive ion HRESIMS revealed a pseudo molecular ion peak at m/z 515.3711 [M + Na]+ (calcd for C30H52O5Na 515.3712), corresponding to the molecular formula C30H52O5, possessing five degrees of unsaturation. IR spectrum showed its absorption at 3234–3587 cm−1 for hydroxyl groups, and 1652 cm−1 for carbonyl (acetate) group. Comparison of the 1H-NMR (Table 1) and 13C-NMR (Table 2) data of 2 with those of the known compound 4 [17], revealed that 2 shares the same structure nucleus as 4, differing from 4 only at the side chain where the C-25 was oxygenated, in agreement with the mass data. The oxygenation of the C-25 caused the 13C-NMR resonance of C-26 and C-27 to be shifted significantly downfield (from δC 21.5/21.6 to 26.0/27.2) and two singlet methyl signals appeared in 2 [δH 1.09 (3H, s), 1.10 (3H, s)] instead of two doublet methyls in 4 [δH 0.77 (3H, d, J = 6.6 Hz), 0.78 (3H, d, J = 6.6 Hz)] in the 1H-NMR spectrum. According to these data, compound 2 was assigned as the 25-OH derivative of 4. In addition, the HMBC (Figure 2) correlation from H-6 to ester carbonyl carbon at δC 172.1 (CH3CO), suggesting that the acetoxy group was positioned at C-6. The assigned relative configuration at C-6 was confirmed by that H-6 (δH 4.68 (1H, t, J = 2.4 Hz)) was coupled with H-7α (equatorial) (δH 1.47) with a small coupling constant of 2.4 Hz. Consequently, H-6 was in equatorial orientation, indicating the location of the acetoxy group was at axial position. Furthermore, the NOE cross-peaks observed between H3-19 (δH1.14) and both H-4β (δH 1.77) and H-2β (δH 2.01), and the absence of NOE correlations between H3-19 and both H-3 and H-6 implied that H-3 and H-6 are both α oriented. At the same time the NOE correlations observed between H-4α and both H-3 and H-6 further confirmed that H-3 and H-6 were α oriented. The configuration of the side chain of 2 was also confirmed by NOE correlations from H3-18 to H-20. So the structure of compound 2 was determined as (24S)-ergostane-6-acetate-3β,5α,6β,25-tetraol.
Compound 3 was obtained as a white, amorphous powder with [α]D25 −26.6 (c 0.50, CH3OH). It was found to have the same molecular formula (C30H52O5) as 2, as determined from high-resolution mass measurements which revealed a pseudo molecular ion peak at m/z 515.3710 [M + Na]+, (calcd for C30H52O5Na 515.3712). Both compounds (2 and 3) showed similarity in the 1H NMR and 13C NMR spectra (Table 1 and Table 2), with the most significant difference being the chemical shifts of C-5 (δC 75.5, C in 2 vs. δC 30.7, CH in 3) and C-12 (δC 41.0, CH2 in 2 vs. δC 73.7, CH in 3). This indicated that the location of a hydroxyl group in 3 was different from that of in 2. The established planar structure of 3 was further supported by the 2D NMR spectra. The diagnostic HMBC correlation from H3-18 to C-12 and 1H–1H COSY correlations between H-12 and H-11 (Figure 2) led the location of the hydroxyl group at C-12. The relative configuration of C-12 was established by comparison with the known compound 8 (3α-acetoxy-12α-hydroxy-5β-cholan-24-oic acid). The small coupling constant of H-12 (δH 3.99, 1H, t, J = 2.6 Hz) in 8 means that H-12 is at equatorial position according to the literature [23]. Whereas the large coupling constant of H-12 (δH 4.31, 1H, td, J = 7.2, 0.6 Hz) in 3 supported the axial position of H-12. Moreover, NOE correlations observed from both H-14 and H-9 to H-12 and the absence of NOE correlations between H3-18 and H-12 also implied H-12 was at α orientation. The chemical shift of H-3 (δH 4.09) suggested that 3-OH was β oriented and H-5 was α oriented by comparison with the 1H-NMR data of 3β-hydroxy-5α-oxygenated A/B trans sterols [24,25]. Based on the above analysis, the relative configuration of 3 was assigned, and the structure was elucidated as (24S)-ergostane-6-acetate-3β,6β,12β,25-tetraol.
The structures of compounds 4, 5 and 6 were identified as 24(S)-methylcholestane-3β,5α,6β-triol-6-monoacetate [17], 24-methylenecholestane-3β,5α,6β-triol-6-monoacetate [17], and ergost-24(28)-en-3β,5α,6β-triol [18], respectively, by comparison of their spectroscopic data with those in the literature.
All the isolated compounds (1–6) were evaluated for their cytotoxic activity against a panel of five human tumor cell lines (Hela, HL-60, K562, A-549 and SMMC-7721) and lethality toward brine shrimp A. salina. Only compound 5 exhibited moderate cytotoxicity against K562 cell line with an IC50 value of 3.18 μM. Moreover, compound 5 also displayed strong lethality toward brine shrimp A. salina with a LC50 value of 0.96 μM. For the other compounds, no cytotoxic activity at the concentration of 10 μM and no lethality toward brine shrimp at 25 μg/mL were found.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured in methanol using a JASCO P-1020 digital polarimeter. UV spectra were recorded on a Beckman DU 640 spectrophotometer. IR spectra were measured on a Bruker VECTOR 22 spectrophotometer. 1H- and 13C-NMR spectra were recorded on a JEOL Eclips-600 spectrometer. ESIMS and HRESIMS were measured on a Q-TOF Ultima Global GAA076 LC mass spectrometer. Silica gel (Qing Dao Hai Yang Chemical Group Co.; 200–300 and 300–400 mesh), octadecylsilyl silica gel (Unicorn; 45–60 μm) and Sephadex LH-20 (Amersham Biosciences) were used for column chromatography (CC). Precoated silica gel plates (Yan Tai Zi Fu Chemical Group Co.; G60, F-254) were used for thin layer chromatography (TLC). Semi-preparative HPLC was performed on a Waters 1525 system using a semi-preparative C18 (Kromasil 7 μm, 10 × 250 mm) column coupled with a Waters 2996 photodiode array detector.
3.2. Animal Materials
Soft coral Sinularia sp. was collected from the coral reef of Weizhou Island in the South China Sea in September 2008, and was identified by Prof. Hui Huang, South China Sea Institute of Oceanology, Chinese Academy of Sciences of China. The voucher specimen (No. GX-WZ-2008002-4) was deposited in the Key Laboratory of Marine Drugs, the Ministry of Education, Ocean University of China, Qingdao, China.
3.3. Extraction and Isolation
The frozen animals (dry weight 559.7 g) were cut into small pieces and exhaustively extracted with EtOH once (3000 mL) and CHCl3–CH3OH (1:1) for six times (3000 mL × 6) successively at room temperature. The organic extracts were evaporated to give a residue, which was suspended into H2O and partitioned with ethyl acetate. The ethyl acetate fraction was concentrated under reduced pressure to give a residue (28.0 g), which was subjected to gradient silica gel chromatography, eluting with 0%–100% ethyl acetate in light petroleum ether and 20%–100% CH3OH in CHCl3 to afford nine fractions (Fr. 1–Fr. 9). Fr. 6 was fractionated on silica gel column chromatography eluting with petroleum ether–ethyl acetate (5:1−1:2), and then chromatographed on Sephadex LH-20 eluted with petroleum ether–CHCl3–MeOH (2:1:1) to give Fr.61. Further purification of Fr. 61 by semi-preparative HPLC yielded compound 4 (8.4 mg). Fr. 7 and Fr. 8 were firstly isolated by repeated silica gel chromatography, further purified by Sephadex LH-20 (CHCl3–MeOH 1:1) and reversed-phase silica gel chromatography, and finally subjected to RP-HPLC to yield 5 (27.8 mg) and 6 (22.9 mg), respectively. Fr. 9 was chromatographed on silica gel eluting with petroleum ether–EtOAc (1:3), and further purified by RP-HPLC (MeOH/H2O 90:10, flow rate of 2.0 mL/min) to afford 1 (1.8 mg, tR = 26.5 min), 2 (11.3 mg, tR = 27.5 min), and 3 (1.3 mg, tR = 32.7 min) successively.
Compound (1): White amorphous powder; [α]D25 −22.4 (c 0.05, CH3OH); IR (KBr) νmax 3410–3584 cm−1; UV (MeOH) λmax: 198 nm, 1H NMR (CDCl3, 600 MHz) and 13C NMR (CDCl3, 150 MHz) data in Table 1 and Table 2; HRESIMS m/z 455.3498 [M + Na]+ (calcd for C28H48O3Na, 455.3496).
Compound (2): White amorphous powder; [α]D25 −45.6 (c 0.90, CH3OH); IR (KBr) νmax at 3234–3587, 1652 cm−1; UV (MeOH) λmax: 195 nm, 1H NMR (CD3OD, 600 MHz) and 13C NMR (CD3OD, 150 MHz) data in Table 1 and Table 2; HRESIMS m/z 515.3711 [M + Na]+ (calcd for C30H52O5Na, 515.3712).
Compound (3): White amorphous powder; [α]D25 −26.6 (c 0.50, CH3OH); IR (KBr) νmax 3434, 3214, 1638 cm−1; UV (MeOH) λmax: 197 nm, 1H NMR (CDCl3, 600 MHz) and 13C NMR (CDCl3, 150 MHz) data in Table 1 and Table 2; HRESIMS m/z 515.3710 [M + Na]+ (calcd for C30H52O5Na, 515.3712).
3.4. Preparation of the (S)-and (R)-MTPA Esters of 1
Compound 1 (0.5 mg) was dissolved in 500 μL of pyridine, and dimethylaminopyridine (2.0 mg) and (R)-MTPACl (10 μL) were then added in sequence. The reaction mixture was stirred for 24 h at room temperature, and 1 mL of H2O was then added. The solution was extracted with 5 mL of CH2Cl2 and the organic phase was concentrated under reduced pressure. Then the residue was purified by semi-preparative HPLC (100% MeOH) to yield (S)-MTPA ester 1s (0.3 mg, tR = 23.40 min). By the same procedure, the (R)-MTPA ester 1r (0.3 mg, tR = 26.21 min) was obtained from the reaction of 1 (0.5 mg) with (S)-MTPACl (10 μL). Selected 1H NMR (CDCl3, 600 MHz) of (S)-MTPA ester (1s): δ 7.08–7.43 (5H, Ph), 5.348 (1H, m, H-23), 5.30 (1H, s, H-6), 5.17 (1H, m, H-3), 2.769 (1H, m, H-2a), 2.766 (1H, m, H-4a), 2.046 (1H, m, H-1a), 2.045 (1H, m, H-20), 2.01 (1H, m, H-22), 1.994 (1H, m, H-2b), 1.986 (1H, m, H-4b), 1.350 (1H, m, H-1b), 1.314 (1H, m, H-22), 1.269 (1H, m, H-24), 1.25 (6H, s, H3-26 and H3-27), 0.994 (3H, d, J = 6.6 Hz, H3-21), 0.99 (3H, s, H3-19), 0.879 (3H, d, J = 6.0 Hz, H3-28), 0.87 (3H, s, H3-18); selected 1H NMR (CDCl3, 600 MHz) of (R)-MTPA ester (1r): δ 7.08–7.43 (5H, Ph), 5.346 (1H, m, H-23), 5.30 (1H, s, H-6), 5.16 (1H, m, H-3), 2.776 (1H, m, H-2a), 2.764 (1H, m, H-4a), 2.049 (1H, m, H-1a), 2.038 (1H, m, H-20), 2.01 (1H, m, H-22), 1.999 (1H, m, H-2b), 1.984 (1H, m, H-4b), 1.361 (1H, m, H-1b), 1.311 (1H, m, H-22), 1.270 (1H, m, H-24), 1.25 (6H, s, H3-26 and H3-27), 0.993 (3H, d, J = 6.6 Hz, H3-21), 0.99 (3H, s, H3-19), 0.883 (3H, d, J = 6.0 Hz, H3-28), 0.87 (3H, s, H3-18).
3.5. Cytotoxicity Assay
The cytotoxicity against Hela (cervical cancer cells), HL-60 (Human promyelocytic leukemia cells), A-549 (human lung epithelial carcinoma), SMMC-7721 (human hepatocellular carcinoma cell line), and K562 (human immortalised myelogenous leukemia line) cell lines were evaluated by using SRB [26] and MTT [27] methods, respectively, according to the protocols described in previous literature. The test of brine shrimp toxicity on A. salina was performed according to standard protocols [28,29].
4. Conclusions
In our continuing discovery for biological secondary metabolites from marine invertebrates in the South China Sea, this study provided a series of polyoxygenated sterols. The discovery of new compounds 1–3 has added to an extremely diverse and complex array of soft coral sterols. Further studies should be conducted to unambiguously establish their absolute configurations by total synthesis as well as to understand their biological/ecological roles in the life cycle of the animal.
Acknowledgements
This work was supported by the Key Program of National Natural Science Foundation of China (No. 41130858), the National Natural Science Foundation of China (Nos. 40976077; 30901879; 41176121; 81172977), Program for New Century Excellent Talents in University, Ministry of Education of China (No. NCET- 11-0472), the Research Fund for the Doctoral Program of Higher Education, Ministry of Education of China (No. 20090132110002), and the Program for Changjiang Scholars and Innovative Research Team in University, Ministry of Education of China (No. IRT0944).
Supplementary Files
PDF-Document (PDF, 510 KB)
Footnotes
Samples Availability: Available from the authors.
References
- 1.Blunt J.W., Copp B.R., Munro M.H.G., Northcote P.T., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2006;23:26–78. doi: 10.1039/b502792f. [DOI] [PubMed] [Google Scholar]
- 2.Chao C.H., Hsieh C.H., Chen S.P., Lu C.K., Dai C.F., Sheu J.H. Sinularianins A and B, novel sesquiterpenoids from the Formosan soft coral Sinularia sp. Tetrahedron Lett. 2006;47:5889–5891. [Google Scholar]
- 3.Choi Y.H., Schmitz F.J. Cytotoxic acylated spermidine from a soft coral, Sinularia sp. J. Nat. Prod. 1997;60:495–496. doi: 10.1021/np960662v. [DOI] [PubMed] [Google Scholar]
- 4.Ojika M., Islam M.K., Shintani T., Zhang Y., Okamoto T., Sakagami Y. Three new cytotoxic acylspermidines from the soft coral, Sinularia sp. Biosci. Biotechnol. Biochem. 2003;67:1410–1412. doi: 10.1271/bbb.67.1410. [DOI] [PubMed] [Google Scholar]
- 5.Imbs A.B., Yakovleva I.M., Pham L.Q. Distribution of lipids and fatty acids in the zooxanthellae and host of the soft coral Sinularia sp. Fish. Sci. 2010;76:375–380. doi: 10.1007/s12562-009-0213-y. [DOI] [Google Scholar]
- 6.Chao C.H., Hsieh C.H., Chen S.P., Lu C.K., Dai C.F., Wu Y.C., Sheu J.H. Novel cyclic sesquiterpene peroxides from the Formosan soft coral Sinularia sp. Tetrahedron Lett. 2006;47:2175–2178. [Google Scholar]
- 7.Sheu J.H., Chang K.C., Duh C.Y. A cytotoxic 5α,8α-epidioxysterol from a soft coral Sinularia species. J. Nat. Prod. 2000;63:149–151. doi: 10.1021/np9903954. [DOI] [PubMed] [Google Scholar]
- 8.Jia R., Guo Y.W., Mollo E., Gavagnin M., Cimino G. Two new polyhydroxylated steroids from the Hainan soft coral Sinularia sp. Helv. Chim. Acta. 2006;89:1330–1336. [Google Scholar]
- 9.Takaki H., Koganemaru R., Iwakawa Y., Higuchi R., Miyamoto T. Inhibitory effect of norditerpenes on LPS-induced TNF-α production from the Okinawan soft coral, Sinularia sp. Biol. Pharm. Bull. 2003;26:380–382. doi: 10.1248/bpb.26.380. [DOI] [PubMed] [Google Scholar]
- 10.Shoji N., Umeyama A., Arihara S. A novel norditerpenoid from the Okinawan soft coral Sinularia sp. J. Nat. Prod. 1993;56:1651–1653. doi: 10.1021/np50099a035. [DOI] [Google Scholar]
- 11.Wang C.Y., Chen A.N., Shao C.L., Li L., Xu Y., Qian P.Y. Chemical constituents of soft coral Sarcophyton infundibuliforme from the South China Sea. Biochem. Syst. Ecol. 2011;39:853–856. doi: 10.1016/j.bse.2011.04.005. [DOI] [Google Scholar]
- 12.Han L., Wang C.Y., Huang H., Shao C.L., Liu Q.A., Qi J., Sun X.P., Zhai P., Gu Y.C. A new pregnane analogue from Hainan soft coral Scleronephthya gracillimum Kuekenthal. Biochem. Syst. Ecol. 2010;38:243–246. doi: 10.1016/j.bse.2009.12.030. [DOI] [Google Scholar]
- 13.Li L., Sheng L., Wang C.Y., Zhou Y.B., Huang H., Li X.B., Li J., Mollo E., Gavagnin M., Guo Y.W. Diterpenes from the Hainan soft coral Lobophytum cristatum Tixier-Durivault. J. Nat. Prod. 2011;74:2089–2094. doi: 10.1021/np2003325. [DOI] [PubMed] [Google Scholar]
- 14.Sun X.P., Wang C.Y., Shao C.L., Li L., Li X.B., Chen M., Qian P.Y. Chemical constituents of the soft coral Sarcophyton infundibuliforme from the South China Sea. Nat. Prod. Commun. 2010;5:1171–1174. [PubMed] [Google Scholar]
- 15.Li L., Wang C.Y., Shao C.L., Guo Y.W., Li G.Q., Sun X.P., Han L., Huang H., Guan H.S. Sarcoglycosides A-C, new O-glycosylglycerol derivatives from the South China Sea soft coral Sarcophyton infundibuliforme. Helv. Chim. Acta. 2009;92:1495–1502. doi: 10.1002/hlca.200900026. [DOI] [Google Scholar]
- 16.Li L., Wang C.Y., Shao C.L., Han L., Sun X.P., Zhao J., Guo Y.W., Huang H., Guan H.S. Two new metabolites from the Hainan soft coral Sarcophyton crassocaule. J. Asian Nat. Prod. Res. 2009;11:851–855. doi: 10.1080/10286020902867060. [DOI] [PubMed] [Google Scholar]
- 17.Bortolotto M., Braekman J.C., Daloze D., Tursch B. Chemical studies of marine invertebrates. XVIII. Four novel polyhydroxylated steroids from Sinularia dissecta (Coelenterata, Octocorallia, Alcyonacea) Bull. Soc. Chim. Belges. 1976;85:27–34. doi: 10.1016/0039-128x(76)90015-5. [DOI] [PubMed] [Google Scholar]
- 18.Lan W.J., Su J.Y., Zeng L.M. Studies on the secondary metabolites of the soft coral, Sinularia sp. collected from the South China Sea. Acta Sci. Nat. Univ. Sunyatseni. 2003;42:105–107. [Google Scholar]
- 19.Yeffet D., Rudi A., Ketzinel S., Benayahu Y., Kashman Y. Auroside, a xylosyl-sterol, and patusterol A and B, two hydroxylated sterols, from two soft corals Eleutherobia aurea and Lobophytum patulum. Nat. Prod. Commun. 2010;5:205–210. [PubMed] [Google Scholar]
- 20.Kusumi T., Fujita Y., Ohtani I., Kakisawa H. Anomaly in the modified Mosher’s method: Absolute configurations of some marine cembranolides. Tetrahedron Lett. 1991;32:2923–2926. [Google Scholar]
- 21.Kobayashi M., Hayashi T., Hayashi K., Tanabe M., Nakagawa T., Mitsuhashi H. Marine sterols. XI. Polyhydroxysterols of the soft coral Sarcophyton glaucum: Isolation and synthesis of 5-cholestane-1α,3β,5,6β-tetrol. Chem. Pharm. Bull. 1983;31:1848–1855. doi: 10.1248/cpb.31.1848. [DOI] [Google Scholar]
- 22.Al-Lihaibi S.S., Ayyad S.N., Shaher F., Alarif W.M. Antibacterial sphingolipid and steroids from the black coral Antipathes dichotoma. Chem. Pharm. Bull. 2010;58:1635–1638. doi: 10.1248/cpb.58.1635. [DOI] [PubMed] [Google Scholar]
- 23.Segura M., Alcfizar V., Prados P., Mendoza J.D. Synthetic receptors for uronic acid salts based on bicyclic guanidinium and deoxycholic acid subunits. Tetrahedron. 1999;53:13119–13128. [Google Scholar]
- 24.Yaoita Y., Amemiya K., Ohnuma H., Furumura K., Masaki A., Matsuki T., Kikuchi M. Sterol constituents from five edible mushrooms. Chem. Pharm. Bull. 1998;46:944–950. doi: 10.1248/cpb.46.944. [DOI] [Google Scholar]
- 25.Ishizuka T., Yaoita Y., Kikuchi M. Sterol constituents from the fruit bodies of Grifola frondosa (Fr.) S. F. Gray. Chem. Pharm. Bull. 1997;45:1756–1760. doi: 10.1248/cpb.45.1756. [DOI] [PubMed] [Google Scholar]
- 26.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J.T., Bokesch H., Kenney S., Boyd M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 28.Solis P.N., Wright C.W., Anderson M.M., Gupta M.P., Phillipson J.D. A microwell cytotoxicity assay using Artemia salina (brine shrimp) Planta Med. 1993;59:250–252. doi: 10.1055/s-2006-959661. [DOI] [PubMed] [Google Scholar]
- 29.Meyer B.N., Ferrigni N.R., Putnam J.E., Jacobson L.B., Nicols D.E., Mclaughlin J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–34. doi: 10.1055/s-2007-971236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDF-Document (PDF, 510 KB)