Abstract
In order to search for new bioactive substances from marine organisms, we have investigated the acetone extracts of the soft coral Sarcophyton ehrenbergi collected at San-Hsian-Tai, Taitong County, Taiwan. Chromatographic fractionation of the extracts of the octocoral S. ehrenbergi led to the isolation of three new cembranoids, (+)-12-ethoxycarbonyl-11Z-sarcophine (1), ehrenbergol A and B (2 and 3). The structures of these isolated metabolites were elucidated through extensive spectroscopic analyses. Moreover, metabolites 1–3 were evaluated in vitro for their cytotoxicity towards selected cancer cell lines and antiviral activity against human cytomegalovirus (HCMV).
Keywords: Sarcophyton ehrenbergi, cembranoids, cytotoxicity, anti-HCMV
1. Introduction
Marine organisms, which have developed unique metabolic and physiological capabilities to ensure survival in extreme ocean habitats, offer the potential to produce new bioactive constituents that would not be observed from terrestrial organisms [1]. Soft corals belonging to the genus Sarcophyton (Alcyoniidae) have been well recognized as a rich source of terpenoids [1]. These constituents, mainly macrocyclic cembrane-type diterpenoids and their derivatives, represent important chemical defense substances for the animals against their natural predators [2]. Cembranoids have been previously reported to exhibit a range of biological activities including antitumor [3,4,5,6,7,8,9], ichthyotoxic [10], anti-inflammatory [11], neuroprotective [12], antibacterial [13], antiangiogenic [14], antimetastatic [14], anti-osteoporotic [15], and cytotoxic [16,17,18] properties. Among them, sarcophine (4) was reported to have antimetastatic activity [14].
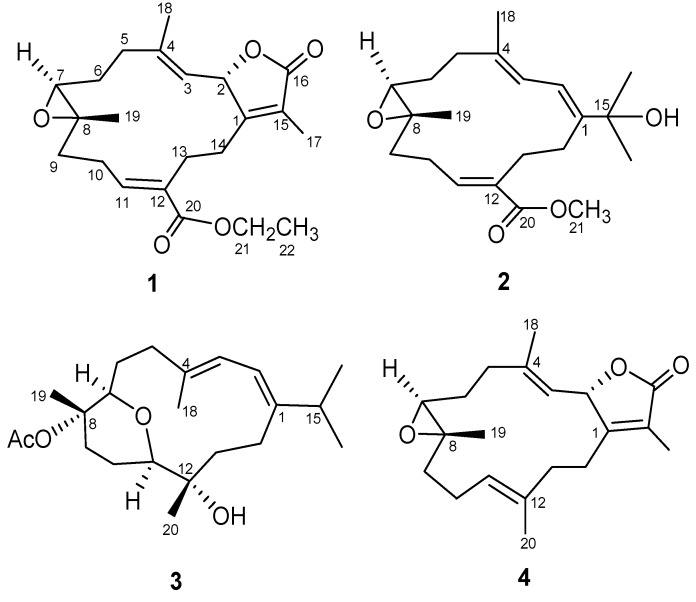
Twelve cembranoids were previously reported from the soft coral Sarcophyton ehrenbergi [1,19]. The samples for our previous studies on the secondary metabolites of the soft coral S. ehrenbergi were all collected at Dongsha Atoll [19,20]. Chemical investigation of the Taiwanese soft coral S. ehrenbergi (Figure 1) collected at San-Hsian-Tai (Taitong County) has afforded three new cembranoids, designated as (+)-12-ethoxycarbonyl-11Z-sarcophine (1), ehrenbergol A and B (2 and 3) (Figure 2). Herein, we describe the purification, structure elucidation, cytotoxicity and antiviral evaluation of these metabolites in detail.
Figure 1.
Soft coral Sarcophyton ehrenbergi.
Figure 2.
Structures of compounds 1–4.
2. Results and Discussion
The HRESIMS of 1 exhibited a pseudomolecular ion peak at m/z 397.1993 [M + Na]+, consistent with the molecular formula of C22H30O5, requiring eight degrees of unsaturation. IR absorption at 1754 cm−1 and NMR signals (Table 1) at δC 174.4 (qC, C-16), 160.7 (qC, C-1), 124.2 (qC, C-15), 78.2 (CH, C-2), and 8.6 (CH3, C-17); δH 5.57 (1H, dd, J = 10.0, 2.0 Hz, H-2) and 1.90 (3H, s, H3-17) were indicative of an α,β-unsaturated γ-lactone functionality by comparison with those of similar metabolites, such as the corresponding data of sarcophine (4) [6]. The IR absorption bands at 1705 cm−1 and NMR signals at δH 6.80 (1H, dd J = 10.4, 4.8 Hz, H-11), 4.23 (2H, m, H2-21), 1.32 (1H, t, J = 7.2 Hz, H-22); δC 166.9 (qC, C-20), 131.1 (qC, C-12), and 142.0 (CH, C-11) indicated the presence of α,β-unsaturated ethyl ester [20]. In addition, a trisubstituted epoxide was present in 1 from its 1H NMR signals at δH 2.56 (1H, br d, J = 6.4 Hz, H-7) and 13C NMR signals at δC 61.3 (qC, C-8) and 62.4 (CH, C-7). Moreover, the 13C NMR signals at δC 121.1 (CH, C-3), and 144.5 (qC, C-4) were assigned a trisubstituted double bond. The above functionalities account for seven of the eight degrees of unsaturation, suggesting a tricyclic structure in 1.
Table 1.
NMR data for compound 1.
| Position | δH a (J in Hz) | δC b, Type | HMBC | COSY | NOESY |
|---|---|---|---|---|---|
| 1 | 160.7, C | ||||
| 2 | 5.57, dd (10, 2.0) | 78.2, CH | 3, 17 | 18 | |
| 3 | 5.08, d (10.4) | 121.1, CH | 5, 18 | 2, 18 | 5a |
| 4 | 144.5, C | ||||
| 5a | 2.39, m | 37.7, CH2 | 3, 4, 6, 18 | 5b, 6a | 3, 5b, 7 |
| 5b | 2.41, m | 3, 4, 6, 18 | 5a, 6a | 5a,18 | |
| 6a | 1.92, m | 22.9, CH2 | 6b, 5a, 5b | 6b | |
| 6b | 1.72, m | 6a, 7 | 6a,18, 19 | ||
| 7 | 2.56, br d (6.4) | 62.4, CH | 6 | 6b | 9a, 10a |
| 8 | 61.3, C | ||||
| 9a | 0.97, m | 38.2, CH2 | 10 | 9b | 9b, 11 |
| 9b | 2.22, m | 11 | 9a, 10a, 10b | 7, 9a, 19 | |
| 10a | 2.11, m | 25.9, CH2 | 12 | 9a, 10b | 7, 10b |
| 10b | 2.19, m | 9a, 10a, 11 | 10a | ||
| 11 | 6.80, dd (10.4, 4.8) | 142.0, CH | 10, 13, 20 | 10a | 9b |
| 12 | 131.1, C | ||||
| 13 | 2.36, m | 25.2, CH2 | 12, 20 | 14b | |
| 14a | 2.50, m | 27.0, CH2 | 1, 2, 15 | 14b | 14b |
| 14b | 2.11, m | 13a, 14a | 14a | ||
| 15 | 124.2, C | ||||
| 16 | 174.4, C | ||||
| 17 | 1.90, s | 8.6, CH3 | 1, 15, 16 | 2 | |
| 18 | 1.87, s | 15.3, CH3 | 3, 4, 5 | 3 | 2, 5b |
| 19 | 1.30, s | 16.8, CH3 | 7, 8, 9 | 6b, 9b | |
| 20 | 166.9. C | ||||
| 21 | 4.23, m | 60.8, CH2 | 20 | 22 | 22 |
| 22 | 1.33, t (7.2) | 14.3, CH3 | 21 | 21 | 21 |
a Spectra were measured in CDCl3 (400 MHz); b Spectra were measured in CDCl3 (100 MHz).
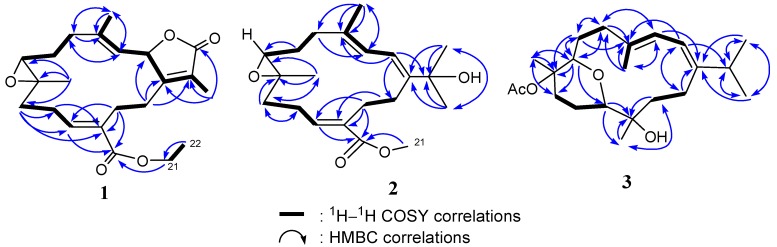
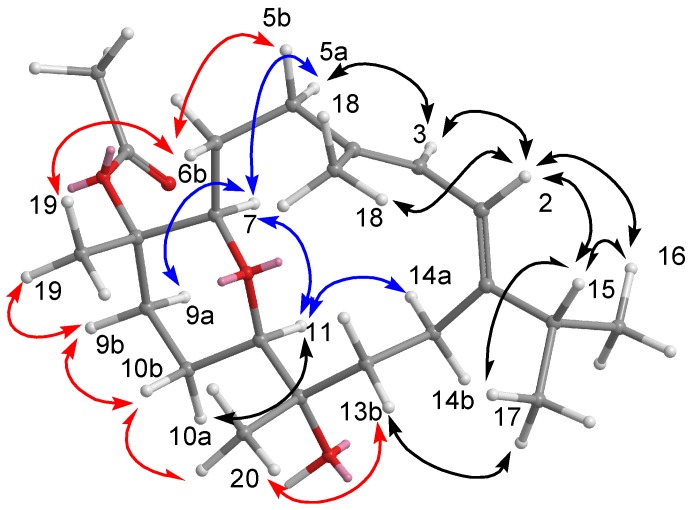
By interpretation of 1H-1H COSY correlations, it was possible to establish three partial structures of consecutive proton systems extending from H-2 to H-3, from H2-5 to H-7 through H2-6, from H2-9 to H-11 through H2-10, and from H2-13 to H2-14. Subsequently, the connectivities of these partial structures were further established by the HMBC correlations (Figure 3). HMBC correlations observed from H3-19 to C-7, C-8, and C-9 indicated the position of the epoxide at C-7 and C-8. Moreover, the HMBC correlations from H-11 to C-9, C-10, C-12, C-13, and C-20 and from H2-21 to C-20 as well as COSY correlation between H2-21 and H3-22, led the assignment of the ethoxycarbonyl at C-12. The locations of the double bond at C-3/C-4 was clarified by analysis of the HMBC correlations from Me-18 to C-3, C-4, and C-5. The molecular framework of 1 was further established by other HMBC correlations between H2-14 to C-1, C-2, C-15 and H3-17 to C-1, C-15, C-16.
Figure 3.
COSY and HMBC correlations of compounds 1–3.
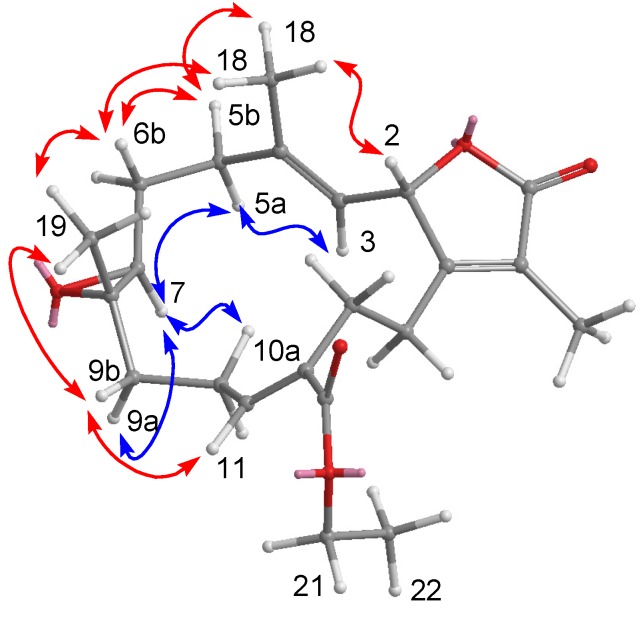
The relative configuration of 1 assigned by a NOESY spectrum was compatible with that suggested by computer modeling, in which the close contacts of atoms calculated in space were consistent with the NOESY correlations (Figure 4). The presence of a NOESY cross peak between the vinylic H-11 and H2-9 (δH 2.22) suggested the E geometry for the C-11/C-12 double bond, which was also identified by the chemical shift of H-11 at δH 6.80 [19,21]. The geometry of the trisubstituted olefin at C-3/C-4 was assigned as E based on the higher field chemical shift of the olefinic methyl signal for C-18 (δC 15.3). Furthermore, the crucial NOE correlations between H-2/Me-18, Me-18/H-6b (δH 1.72), Me-19/H-6b, Me-19/H-9b (δH 2.22), H-7/H-6a (δH 1.92), and H-7/H-9b, H-7/H-5a (δH 2.39), and H-3/H-5a demonstrated the 2S*, 7S*, and 8S* configurations as depicted in Figure 4. A careful analysis of all the NMR spectroscopic data (COSY, HSQC, HMBC, and NOESY) confirmed that 1 is actually the 12-ethoxycarbonyl derivative of (+)-11Z-sarcophine [19,22]. All of the NMR spectroscopic data of 1 were consistent with the structure shown as (+)-12-ethoxycarbonyl-11Z-sarcophine.
Ehrenbergol A (2) was assigned a molecular formula of C21H32O4, according to its HRESIMS and NMR spectroscopic data (Table 2). The IR absorptions of 2 at 1715 cm−1 revealed the presence of an α,β-unsaturated methyl ester functionality, which was confirmed by its NMR spectroscopic data [δH 6.89 (1H, dd, J = 10.4, 6.8 Hz, H-11) and 3.42 (3H, s, H3-21); δC 167.6 (qC, C-20), 133.9 (qC, C-12), 141.1 (CH, C-11), and 51.2 (CH3, COOMe)]. The NMR spectroscopic data also indicated that 2 possesses a trisubstituted epoxide [δH 2.67 (1H, dd, J = 10.8, 2.8 Hz, H-7); δC 60.6 (qC, C-8) and 62.1 (CH, C-7)], and two trisubstituted olefins [δH 6.53 (1H, d, J = 11.0 Hz, H-2) and 6.08 (1H, d, J = 11.0 Hz, H-3); δC 147.1 (qC, C-1), 118.4 (CH, C-2), 123.8 (CH, C-3), and 135.8 (qC, C-4)]. The above functionalities account for five of the six degrees of unsaturation, suggesting that 2 must consist of a 14-membered ring diterpenoid skeleton.
Figure 4.
NOESY correlations of compound 1.
Table 2.
NMR data for compound 2.
| Position | δH a (J in Hz) | δC b, Type | HMBC | COSY | NOESY |
|---|---|---|---|---|---|
| 1 | 147.1, C | ||||
| 2 | 6.53, d (11.0) | 118.4, CH | 4, 14, 15 | 3 | 16, 17, 18 |
| 3 | 6.08, d (11.0) | 123.8, CH | 5, 18 | 2, 18 | 5a, 7, 13a |
| 4 | 135.8, C | ||||
| 5a | 2.12, m | 37.8, CH2 | 3 | 5b, 18 | 3, 5b |
| 5b | 1.97, m | 3 | 5a, 6b, 18 | 5a, 6b, 18 | |
| 6a | 1.94, m | 25.5, CH2 | 7 | 6b, 7 | 6b |
| 6b | 1.17, m | 5b, 6a, 7 | 5b, 6a,19 | ||
| 7 | 2.67, dd (10.8, 2.8) | 62.1, CH | 6a, 6b | 3, 9a | |
| 8 | 60.6, C | ||||
| 9a | 0.85, td (12.4, 4.0) | 39.7, CH2 | 8, 19 | 9b, 10a, 10b | 9b, 7 |
| 9b | 1.95, m | 8, 10, 19 | 9a, 10a, 10b | 9a, 19 | |
| 10a | 2.16, m | 26.7, CH2 | 9, 10b, 11 | 10b | |
| 10b | 1.75,m | 9, 11, 12 | 9, 10a, 11 | 10a, 19 | |
| 11 | 6.89, dd (10.4, 6.8) | 141.1, CH | 20 | 10a, 10b | 9 |
| 12 | 133.9, C | ||||
| 13a | 2.26,m | 28.4, CH2 | 3, 13b, 14b | ||
| 13b | 2.38, m | 11, 12, 20 | 13a | ||
| 14a | 2.52, m | 28.6, CH2 | 12 | 14b, 17 | |
| 14b | 2.24, m | 12 | 14a | ||
| 15 | 73.6, C | ||||
| 16 | 1.33, s | 29.5, CH3 | 1, 15, 17 | 2 | |
| 17 | 1.44, s | 29.3, CH3 | 1, 16, 17 | 2 | |
| 18 | 1.58, s | 15.6, CH3 | 3, 4, 5 | 3, 5a, 5b | 2, 5b |
| 19 | 1.04, s | 15.6, CH3 | 7, 8, 9 | 6b, 9b, 10b | |
| 20 | 167.6, C | ||||
| 21 | 3.42, s | 51.2, CH3 | 20 |
a Spectra were measured in CDCl3 (400 MHz); b Spectra were measured in CDCl3 (100 MHz).
The structure of 2 was established through COSY and HMBC experiments (Figure 3). Crucial HMBC correlations from Me-18 to C-3, C-4, and C-5, from Me-19 to C-7, C-8, and C-9, from H-11 to C-10, C-12, C-13, and C-20, and from Me-16/Me-17 to C-15 and C-1 confirmed the connectivity among these partial structures (Figure 3). The position of the methoxycarbonyl at C-12 was established by the HMBC correlations from H-11 and H-13 to C-20 and from H3-21 to C-20. A COSY experiment established a correlation between the two vinylic protons at δH 6.53 (H-2) and 6.08 (H-3). These results allowed the assignment of the planar structure of 2 as shown.
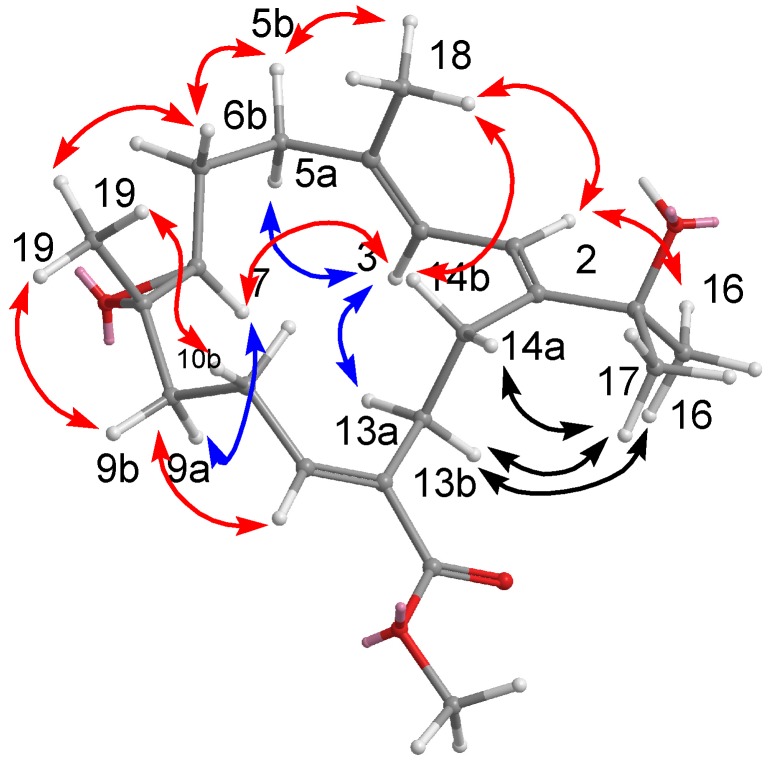
The configurations of all double bonds were determined from a NOESY experiment on 2. The crucial NOE correlations (Figure 5) between H-2 (δH 6.53)/Me-16 (δH 1.33), H-2/Me-18 (δH 1.44), and H-3 (δH 6.08)/H-5a (δH 2.12) indicated that the geometries of the conjugated diene at C-1/C-2 and C-3/C-4 were both E. The large coupling constant (J2,3 = 11.0 Hz) further suggested the s-trans geometry of the conjugated double bonds [20,21]. The presence of a NOESY cross peak between the vinylic H-11 and H2-9 made it possible to identify the configuration of the olefin at C-11/C-12 as the E geometry, which was also confirmed by the chemical shift of H-11 at δH 6.89 [20]. Moreover, the crucial NOESY correlations between Me-19/H-6b (δH 1.16), Me-19/H-9b (δH 1.94), H-7/H-6a (δH 1.97), H-7/H-9b (δH 1.95), and H-7/H-5a (δH 2.12) demonstrated the configurations of C-7 and C-8 as 7S∗ and 8S*, respectively. On the basis of the aforementioned observations and other detailed NOESY correlations (Figure 4), the structure of ehrenbergol A (2) was established.
Figure 5.
NOESY correlations of compound 2.
The positive HRESIMS spectrum of ehrenbergol B (3) exhibited a pseudo molecular ion peak at m/z 387.2509 [M + Na]+, consistent with the molecular formula of C22H36O4, implying five degrees of unsaturation. The presence of two oxygenated methine [δH 3.40 (d, 1H, J = 7.6 Hz) and δC 85.1 (C-7); δH 3.32 (dd, 1H, J = 11.2, 2.0 Hz) and δC 80.1 (C-11)] implied that an ether linkage is present between C-7 and C-11, which was confirmed by the HMBC correlations from H-7 to C-11, and from H-11 to C-7. The NMR spectroscopic data (Table 3) indicated that 3 possesses a conjugated diene [δH 5.89 (1H, d, J = 5.0 Hz) and 5.99 (1H, d, J = 5.0 Hz); δC 150.2 (qC, C-1), 119.6 (CH, C-2), 122.3 (CH, C-3), and 137.1 (qC, C-4)] and an acetoxy [δC 169.2 (qC), 21.9 (CH3) and δH 1.67 (3H, s)];. The above functionalities account for three of the five degrees of unsaturation, implying that 3 is a cembranoid characterized by the presence of an ether linkage between C-7 and C-11.
Table 3.
NMR data for compound 3.
| Position | δH a (J in Hz) | δC b, Type | HMBC | COSY | NOESY |
|---|---|---|---|---|---|
| 1 | 150.2, qC | ||||
| 2 | 5.89, br d (5.0) | 119.6, CH | 4, 14, 15 | 3 | 3, 15, 16, 18 |
| 3 | 5.99, br d (5.0) | 122.3, CH | 1, 5, 18 | 2, 18 | 2, 5a |
| 4 | 137.1, qC | ||||
| 5 | 2.16, m | 39.6, CH2 | 3, 4, 6, 7 | 6a, 6b | 3, 7, 6b, 18 |
| 6a | 1.42, m | 26.5, CH2 | 8 | 5a, 5b, 6b | 5b, 19 |
| 6b | 1.69, m | 4, 5, 7 | 5a, 5b, 6a | ||
| 7 | 3.40, d (7.6) | 85.1, CH | 5, 6, 8, 9, 11, 19 | 5a, 9a | |
| 8 | 80.7, qC | ||||
| 9a | 1.73, m | 35.4, CH2 | 9b, 10a, 10b | 7, 9b, 11 | |
| 9b | 2.83, dt (12.4, 4.0) | 19 | 9a, 10a, 10b | 9a, 10b, 19 | |
| 10a | 1.56, m | 23.0, CH2 | 10b | 10b, 11 | |
| 10b | 1.39, m | 10a | 10b, 20 | ||
| 11 | 3.32, dd (11.2, 2.0) | 80.1, CH | 7, 12, 20 | 10a, 10b | 9a, 10a, 14a |
| 12 | 73.0, qC | ||||
| 13a | 1.46, m | 41.0, CH2 | 20 | 13b, 14a | |
| 13b | 1.80, m | 12, 20 | 13a | 17, 20 | |
| 14a | 2.22, m | 24.0, CH2 | 1, 2 | 13a, 13b, 14b | 3, 11 |
| 14b | 1.77, m | 13 | 14a | ||
| 15 | 2.28, m | 35.2, CH | 16, 17 | 16, 17 | 2, 16, 17 |
| 16 | 1.08, d (7.2) | 22.0, CH3 | 1, 15, 17 | 15 | 2, 15 |
| 17 | 1.10, d (6.4) | 22.6, CH3 | 1, 15, 16 | 15 | 2, 13b, 15 |
| 18 | 1.64, s | 17.2, CH3 | 3, 4, 5 | 3 | 2, 5b |
| 19 | 1.50, s | 17.1, CH3 | 7, 8, 9 | 6b, 9b | |
| 20 | 1.01, s | 23.8, CH3 | 11, 12, 13 | 10b, 13b | |
| OAc | 1.67, s | 169.2, qC | |||
| 21.9, CH3 |
a Spectra were measured in C6D6 (400 MHz); b Spectra were measured in C6D6 (100 MHz).
The final assembly of 3 was determined by the information from COSY and HMBC experiments. The 1H-13C long-range correlations as determined from the HMBC spectrum allowed the connectivity of the structural fragments around each methyl group to be deduced (Figure 3). The crucial NOESY correlations (Figure 6) proved that the geometries of the conjugated diene at C-1/C-2 and C-3/C-4 were both E. The coupling constant (J2,3 = 5.0 Hz) further suggested the s-cis geometry of the above functionality [20,21]. The key NOESY correlations between H-3/H-14a (δH 2.22), H-14a/H-11, H-11/H-7, H-11/H-9a (δH 1.73), H-11/H-10a (δH 1.56), H-7/H-9b, H-7/H-3, H-7/H-5 (δH 2.16), Me-19/H-9b (δH 2.83), Me-20/H-10b (δH 1.39), and Me-20/H-13b (δH 1.80) suggested that H-7, and H-11 are on the same face (β), whereas Me-19 and Me-20 are oriented toward the other face (α), as shown in a computer generated 3D drawing. The above findings indicated the 7R*, 8S*, 11R*, and 12S* configurations as depicted in Figure 6. Therefore, the structure of 3 was elucidated as (7R*,8S*,11R*,12S*,1Z,3E)-8,12-dihydroxy-7,11-epoxycembra-1(2),3-diene 8-acetate.
Figure 6.
NOESY correlations of compound 3.
The cytotoxicities of metabolites 1–3 against P-388 (mouse lymphocytic leukemia), HT-29 (human colon adenocarcinoma) tumor cells, and human embryonic lung (HEL) cells are shown in Table 4. Metabolites 1–3 were also examined for antiviral activity against human cytomegalovirus (HCMV) using a human embryonic lung (HEL) and displayed antiviral activity against human cytomegalovirus, with IC50s of 60, 46, and 5.0 μg/mL, respectively.
Table 4.
Cytotoxicit and anti-HCMV activity of 1–3.
| Compounds | ED50 (μg/mL) | ||||
|---|---|---|---|---|---|
| A549 | HT-29 | P-388 | HEL | Anti-HCMV | |
| 1 | 20.8 | >50 | 5.8 | >50 | 60 |
| 2 | >50 | >50 | 7.4 | >50 | 46 |
| 3 | 10.2 | >50 | 4.7 | >50 | 5.0 |
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were determined with a JASCO P1020 digital polarimeter. UV and IR spectra were obtained on JASCO V-650 and JASCO FT/IR-4100 spectrophotometers, respectively. NMR spectra were recorded on a Varian MR 400 NMR spectrometer at 400 MHz for 1H and 100 MHz for 13C. 1H NMR chemical shifts are expressed in δ (ppm) referring to the solvent peak δH 7.27 for CHCl3 or δH 7.15 for C6D6, and coupling constants are expressed in Hz. 13C NMR chemical shifts are expressed in δ (ppm) referring to the solvent peak δC 77.0 for CDCl3 or δC 128.0 for C6D6. MS were recorded by a Bruker APEX II mass spectrometer. Silica gel 60 (Merck, Germany, 230–400 mesh) and LiChroprep RP-18 (Merck, 40–63 μm) were used for column chromatography. Precoated silica gel plates (Merck, Kieselgel 60 F254, 0.25 mm) and precoated RP-18 F254s plates (Merck) were used for thin-layer chromatography (TLC) analysis. High-performance liquid chromatography (HPLC) was carried out using a Hitachi L-7100 pump equipped with a Hitachi L-7400 UV detector at 220 nm together with a semi-preparative reversed-phased column (Merck, Hibar LiChrospher RP-18e, 5 μm, 250 × 25 mm).
3.2. Biological Material
The soft coral S. ehrenbergi was collected by SCUBA at San-Hsian-Tai, Taitong County, Taiwan, in July 2008 at a depth of 8 m and stored in a freezer until extraction. The voucher specimen (SST-13) was identified by Professor Chang-Feng Dai, National Taiwan University and deposited at the Department of Marine Biotechnology and Resources, National Sun Yat-sen University, Taiwan.
3.3. Extraction and Isolation
A specimen of soft coral S. ehrenbergi (4.0 kg) was minced and extracted with acetone (4 × 3 L) at room temperature. The combined acetone extracts were then partitioned between H2O and EtOAc. The resulting EtOAc extract (46.9 g) was subjected to gravity silica gel 60 column chromatography (Si 60 CC) using n-hexane and n-hexane/EtOAc of increasing polarity, to give 20 fractions. Fraction 15 (2.26 g), eluted with n-hexane/EtOAc (1:1), was further subjected to Si 60 CC (n-hexane/EtOAc, 7:1) to give 8 subfractions. A subfraction 15-4 (250 mg), was purified by RP-18 HPLC (MeOH/H2O, 75:25) to afford 2 (2.0 mg, 0.0005%).The fraction 14 (3.56 g), eluted with n-hexane/EtOAc (2:1), was further subjected to Si 60 CC (n-hexane/EtOAc, 8:1) to give 5 subfractions. A subfraction 14-2 (299 mg), was separated by a RP-18 flash column (MeOH/H2O, 60:40 to 100% MeOH) to give 6 fractions. The subfraction 14-2-6, eluted with MeOH/H2O (90:10), was purified by RP-18 HPLC (MeOH/H2O, 85:15) to afford 3 (2.4 mg, 0.0006%). A subfraction 14-3 (248 mg), was separated by a RP-18 flash column (MeOH/H2O, 50:50 to 100% MeOH) to give 7 fractions. The subfraction 14-3-3, eluted with MeOH/H2O (70:30), was purified by RP-18 HPLC (MeOH/H2O, 65:35) to afford 1 (3.2 mg, 0.0008%).
(+)-12-Ethoxycarbonyl-11Z-sarcophine (1): White amorphous powder; 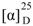 +77 (c 0.2, CHCl3); UV (MeOH) λmax (log ε) 228 (3.72) nm; IR (neat) νmax 3481, 2933, 1754, 1705, 1455, 1387, 1242, 1096, 991, 760 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) data in Table 1; HRESIMS m/z 397.1993 [M + Na]+ (calcd for C22H30O5Na, 397.1991).
+77 (c 0.2, CHCl3); UV (MeOH) λmax (log ε) 228 (3.72) nm; IR (neat) νmax 3481, 2933, 1754, 1705, 1455, 1387, 1242, 1096, 991, 760 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) data in Table 1; HRESIMS m/z 397.1993 [M + Na]+ (calcd for C22H30O5Na, 397.1991).
Ehrenbergol A (2): White amorphous powder; 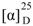 −184 (c 0.1, CHCl3); UV (MeOH) λmax (log ε) 221 (3.72), 242 (3.32) nm; IR (neat) νmax 3447, 2961, 2925, 2851, 1715, 1458, 1260, 1101, 1026, 799, 759 cm−1; 1H NMR (C6D6, 400 MHz) and 13C NMR (C6D6, 100 MHz) data in Table 1; HRESIMS m/z 371.2195 [M + Na]+ (calcd for C21H32O4Na, 371.2198).
−184 (c 0.1, CHCl3); UV (MeOH) λmax (log ε) 221 (3.72), 242 (3.32) nm; IR (neat) νmax 3447, 2961, 2925, 2851, 1715, 1458, 1260, 1101, 1026, 799, 759 cm−1; 1H NMR (C6D6, 400 MHz) and 13C NMR (C6D6, 100 MHz) data in Table 1; HRESIMS m/z 371.2195 [M + Na]+ (calcd for C21H32O4Na, 371.2198).
Ehrenbergol B (3): White amorphous powder; 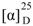 −84.0 (c 0.1, CHCl3); IR (neat) νmax 3461, 2959, 1737, 1634, 1456, 1378, 1259, 1089, 1026, 801 cm−1; 1H NMR (C6D6, 400 MHz) and 13C NMR (C6D6, 100 MHz) data in Table 2; HRESIMS m/z 387.2509 [M + Na]+ (calcd for C22H36O4Na, 387.2511).
−84.0 (c 0.1, CHCl3); IR (neat) νmax 3461, 2959, 1737, 1634, 1456, 1378, 1259, 1089, 1026, 801 cm−1; 1H NMR (C6D6, 400 MHz) and 13C NMR (C6D6, 100 MHz) data in Table 2; HRESIMS m/z 387.2509 [M + Na]+ (calcd for C22H36O4Na, 387.2511).
3.4. Cytotoxicity Assay
Cytotoxicity was determined on P-388 (mouse lymphocytic leukemia), HT-29 (human colon adenocarcinoma), and A-549 (human lung epithelial carcinoma) tumor cells using a modification of the MTT colorimetric method according to a previously described procedure [23,24,25]. The provision of the P-388 cell line was supported by J.M. Pezzuto, formerly of the Department of Medicinal Chemistry and Pharmacognosy, University of Illinois at Chicago. HT-29 and A-549 cell lines were purchased from the American Type Culture Collection. To measure the cytotoxic activities of tested compounds, five concentrations with three replications were performed on each cell line. Mithramycin was used as a positive control.
3.5. Anti-HCMV Assay
To determine the effects of natural products upon HCMV cytopathic effect (CPE), confluent human embryonic lung (HEL) cells grown in 24-well plates were incubated for 1 h in the presence or absence of various concentrations of tested natural products with three replications. Ganciclovir was used as a positive control. Then, cells were infected with HCMV at an input of 1000 pfu (plaque forming units) per well of a 24-well dish. Antiviral activity was expressed as IC50 (50% inhibitory concentration), or compound concentration required to reduce virus induced CPE by 50% after 7 days as compared with the untreated control. To monitor the cell growth upon treating with natural products, an MTT-colorimetric assay was employed [26,27].
4. Conclusion
The first investigation of soft coral S. ehrenbergi collected at San-Hsian-Tai (Taitong County, Taiwan) has led to the isolation of three new cembranoids, (+)-12-ethoxycarbonyl-11Z-sarcophine (1) as well as ehrenbergol A and B (2 and 3). Metabolites 1–3 were not cytotoxic towards P-388 (mouse lymphocytic leukemia), HT-29 (human colon adenocarcinoma) tumor cells, and human embryonic lung (HEL) cells. However, metabolites 1–3 displayed antiviral activity towards human cytomegalovirus, with IC50s of 60, 46, and 5.0 μg/mL, respectively. Ehrenbergol B is the first cembranoid from Taiwanese soft corals to show potent anti-HCMV activity.
Acknowledgments
This research was financially supported by grants from the National Science Council (NSC99-2628-B-110-002-MY3) and Ministry of Education of Taiwan awarded to C.-Y.D.
Supplementary Files
PDF-Document (PDF, 245 KB)
Footnotes
Samples Availability: Not available.
References
- 1.Blunt J.W., Copp B.R., Hu W.-P., Munro M.H.G., Northcote P.T., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2009;26:170–244. doi: 10.1039/b805113p. [DOI] [PubMed] [Google Scholar]
- 2.Coll J.C. The chemistry and chemical ecology of octocorals (Coelenterata, Anthozoa, Octocorallia) Chem. Rev. 1992;92:613–631. [Google Scholar]
- 3.Gross H., Wright A.D., Beil W., König G.M. Two new bicyclic cembranolides from a new Sarcophyton species and determination of the absolute configuration of sarcoglaucol-16-one. Org. Biomol. Chem. 2004;2:1133–1138. doi: 10.1039/b314332e. [DOI] [PubMed] [Google Scholar]
- 4.Huang H.-C., Ahmed A.F., Su J.-H., Chao C.-H., Wu Y.-C., Chiang M.Y., Sheu J.-H. Crassocolides A–F, new cembranoids with a trans-fused lactone from the soft coral Sarcophyton crassocaule. J. Nat. Prod. 2006;69:1554–1559. doi: 10.1021/np060182w. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C., Li J., Su J., Liang Y., Yang X., Zheng K., Zeng L. Cytotoxic diterpenoids from the soft coral Sarcophyton crassocaule. J. Nat. Prod. 2006;69:1476–1480. doi: 10.1021/np050499g. [DOI] [PubMed] [Google Scholar]
- 6.Feller M., Rudi A., Berer N., Goldberg I., Stein Z., Benayahu Y., Schleyer M., Kashman Y. Isoprenoids of the soft coral Sarcophyton glaucum: Nyalolide, a new biscembranoid, and other terpenoids. J. Nat. Prod. 2004;67:1303–1308. doi: 10.1021/np040002n. [DOI] [PubMed] [Google Scholar]
- 7.El Sayed K.A., Hamann M.T., Waddling C.A., Jensen C., Lee S.K., Dunstan C.A., Pezzuto J.M. Structurally novel bioconversion products of the marine natural product sarcophine effectively inhibit JB6 cell transformation. J. Org. Chem. 1998;63:7449–7455. doi: 10.1021/jo9813134. [DOI] [PubMed] [Google Scholar]
- 8.Yan X.-H., Gavagnin M., Cimino G., Guo Y.-W. Two new biscembranes with unprecedented carbon skeleton and their probable biogenetic precursor from the Hainan soft coral Sarcophyton latum. Tetrahedron Lett. 2007;48:5313–5316. [Google Scholar]
- 9.Cheng Y.-B., Shen Y.-C., Kuo Y.-H., Khalil A.T. Cembrane diterpenoids from the Taiwanese soft coral Sarcophyton stolidotum. J. Nat. Prod. 2008;71:1141–1145. doi: 10.1021/np0706668. [DOI] [PubMed] [Google Scholar]
- 10.Iwagawa T., Nakamura S., Masuda T., Okamura H., Nakatani M., Siro M. Irregular cembranoids containing a 13-membered carbocyclic skeleton isolated from a soft coral, Sarcophyton species. Tetrahedron. 1995;51:5291–5298. [Google Scholar]
- 11.Sawant S., Youssef D., Mayer A., Sylvester P., Wali V., Arant M., El Sayed K. Anticancer and anti-inflammatory sulfur-containing semisynthetic derivatives of sarcophine. Chem. Pharm. Bull. 2006;54:1119–1123. doi: 10.1248/cpb.54.1119. [DOI] [PubMed] [Google Scholar]
- 12.Badria F.A., Guirguis A.N., Perovic S., Steffen R., Müller W.E.G., Schröder H.C. Sarcophytolide: A new neuroprotective compound from the soft coral Sarcophyton glaucum. Toxicology. 1998;131:133–143. doi: 10.1016/S0300-483X(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 13.Bishara A., Rudi A., Benayahu Y., Kashman Y. Three biscembranoids and their monomeric counterpart cembranoid, a biogenetic Diels-Alder precursor, from the soft coral Sarcophyton elegans. J. Nat. Prod. 2007;70:1951–1954. doi: 10.1021/np070129n. [DOI] [PubMed] [Google Scholar]
- 14.Sawant S., Youssef D., Reiland J., Ferniz M., Marchtti D., El Sayed K.A. Biocatalytic and antimetastatic studies of the marine cembranoids sarcophine and 2-epi-16-deoxysarcophine. J. Nat. Prod. 2006;69:1010–1013. doi: 10.1021/np050527v. [DOI] [PubMed] [Google Scholar]
- 15.Cuong N.X., Tuan T.A., Kiem P.V., Minh C.V., Choi E.M., Kim Y.H. New cembranoid diterpenes from the Vietnamese soft coral Sarcophyton mililatensis stimulate osteoblastic differentiation in MC3T3-E1 cells. Chem. Pharm. Bull. 2008;56:988–992. doi: 10.1248/cpb.56.988. [DOI] [PubMed] [Google Scholar]
- 16.Wang S.-K., Duh C.-Y. New cytotoxic cembranolides from the soft coral Lobophytum michaelae. Mar. Drugs. 2012;10:306–318. doi: 10.3390/md10020306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin S.-T., Wang S.-K., Duh C.-Y. Cembranoids from the Dongsha atoll soft coral Lobophytum crassum. Mar. Drugs. 2011;9:2705–2716. doi: 10.3390/md9122705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng S.-Y., Chen P.-W., Chen H.P., Wang S.-K., Duh C.-Y. New cembranolides from the Dongsha atoll soft coral Lobophytum durum. Mar. Drugs. 2011;9:1307–1318. doi: 10.3390/md9081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng S.-Y., Wang S.-K., Chiou S.-F., Hsu C.-H., Dai C.-F., Chiang M.Y., Duh C.-Y. Cembranoids from the octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2010;73:197–203. doi: 10.1021/np900693r. [DOI] [PubMed] [Google Scholar]
- 20.Cheng S.-Y., Chiou S.-F., Tsai C.-W., Wang S.-K., Hsu C.-H., Dai C.-F., Chiang M.Y., Wang W.-H., Duh C.-Y. Ceramide and cerebrosides from the octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2009;72:465–468. doi: 10.1021/np800362g. [DOI] [PubMed] [Google Scholar]
- 21.Roengsumran S., Achayindee S., Petsom A., Pudhom K., Singtothong P., Surachetapan C., Vilaivan T. Two new cembranoids from Croton oblongifolius. J. Nat. Prod. 1998;61:652–654. doi: 10.1021/np9704765. [DOI] [PubMed] [Google Scholar]
- 22.Kashman Y., Zadock E., Néeman I. Some new cembrane derivatives of marine origin. Tetrahedron. 1974;30:3615–3620. doi: 10.1016/S0040-4020(01)97044-9. [DOI] [Google Scholar]
- 23.Geran R.I., Greenberg N.H., MacDonald M.M., Schumacher A.M., Abbott B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological syatems. Cancer Chemother. Rep. 1972;3:1–91. [Google Scholar]
- 24.Hou R.-S., Duh C.-Y., Chiang M.Y., Lin C.-N. Sinugibberol, a new cytotoxic cembranoid diterpene from the soft coral Sinularia gibberosa. J. Nat. Prod. 1995;58:1126–1130. doi: 10.1021/np50121a026. [DOI] [PubMed] [Google Scholar]
- 25.Chen W.-H., Wang S.-K., Duh C.-Y. Polyhydroxylated Steroids from the Bamboo Coral Isis hippuris. Mar. Drugs. 2011;9:1829–1839. doi: 10.3390/md9101829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens M., Balzarini J., Tabarrini O., Andrei G., Snoeck R., Cecchetti V., Fravolini A., de Clercq E., Pannecouque C. Cell-dependent interference of a series of new 6-aminoquinolone derivatives with viral (HIV/CMV) transactivation. J. Antimicrob. Chemother. 2005;56:847–855. doi: 10.1093/jac/dki328. [DOI] [PubMed] [Google Scholar]
- 27.Cheng S.-Y., Huang K.-J., Wang S.-K., Duh C.-Y. Capilloquinol: A novel farnesyl quinol from the Dongsha atoll soft coral Sinularia capillosa. Mar. Drugs. 2011;9:1469–1476. doi: 10.3390/md9091469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDF-Document (PDF, 245 KB)