See related article in EMBO Molecular Medicine http://dx.doi.org/10.1002/emmm.201200237
Atherosclerosis, the leading cause of death in Westernized countries, derives from a non-resolving inflammation of the large arteries. A critical step in the initiation of an atherosclerotic plaque is the expansion of intimal space (the area between the endothelial cell (EC) lining and the medial smooth muscle cell layer) in susceptible regions of arteries (Tabas et al, 2007). The disease-prone locations are largely determined by arterial blood flow characteristics, with branch points and the lesser curvatures of arteries, where the flow is turbulent, being particularly disposed to plaque formation. At these sites, the normally thin intimal space expands with the deposition of extracellular matrix (ECM), which then serves as a sticky surface to trap LDL particles that are constantly crossing the endothelial layer to enter the artery. One reason for this retention is that the apoB100 molecule on the LDL particle has a specific domain that binds tightly to the ECM, and genetic causation studies in a mouse model of atherosclerosis have shown that when this domain is deleted, plaque burden is significantly reduced despite equivalent hyperlipidemia (Skalen et al, 2002). As implied by the ‘Response to Retention’ hypothesis (Tabas et al, 2007), the retained lipoprotein particles set in motion the invasion of the arterial wall by monocytes that quickly become macrophages that engorge themselves on cholesterol and the other lipids to form foam cells. Foam cells not only physically expand the plaque, but by secreting a variety of chemokines and cytokines, they also create and amplify an inflammatory state (Fig 1).
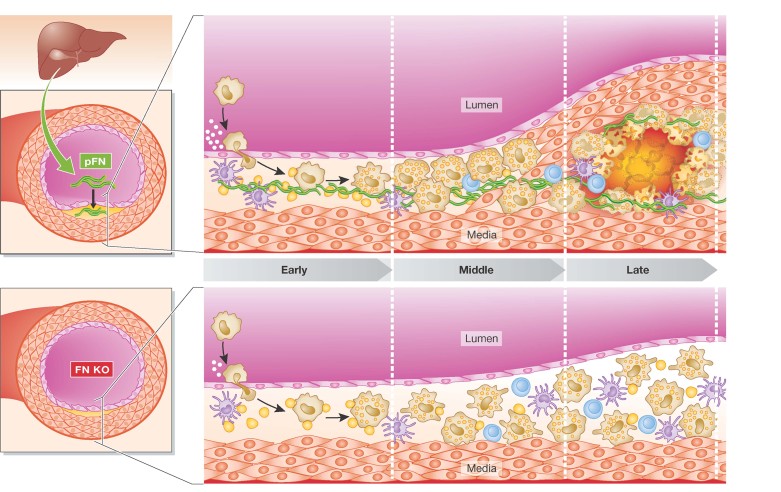
Figure 1. Plasma derived FN promotes atherogenesis in mouse models of atherosclerosis.
pFN, synthesized in the liver, is deposited in susceptible regions of the arteries and contributes to early atherosclerotic lesion formation and the recruitment of smooth muscle cells that shape the fibrous cap of advanced lesions (upper panel). Targeted deletion of FN in ApoE−/− mice results in smaller, less lipid-rich plaques, which lack a necrotic core and the overlying protective fibrous cap (lower panel).
As noted above, a key event in plaque initiation is the expansion of the ECM in susceptible arterial areas. The ECM includes proteoglycans, collagen, elastin, vitronectin, fibulin and importantly, fibronectin (FN), which is the subject of an intriguing report by Rohwedder et al in this issue of EMBO Molecular Medicine (Rohwedder et al, 2012). As the authors note, it has been known for over 20 years that the expression of FN is increased in atherosclerotic regions, particularly in the fibrillar form. Likely sources of this FN are haematopoietic cells in the plaques (e.g. macrophages), ECs activated by turbulent blood flow (Feaver et al, 2010), as well as plasma-derived FN (‘pFN’). Unlike the ‘local’ sources, which secrete an insoluble fibrillar matrix, pFN is derived from the liver, which secretes a soluble form. Depending on interactions with integrins, however, pFN can also be assembled into fibrils, raising the question of whether both local and plasma FN promote atherogenesis, or whether one of them predominates in this pathological process.
To address this question, the authors used a number of approaches in a standard model of human atherosclerosis, the apoE-deficient (ApoE−/−) mouse. First, they confirmed in ApoE−/− mice fed a high fat-high cholesterol diet to accelerate disease progression that lipid-rich plaques with FN deposits formed at aortic sites known to be susceptible to atherosclerosis. In the first variation of this experiment, they repeated the study in ApoE−/− mice genetically engineered to be deficient in FN expression in hepatic and haematopoietic cells (a ‘conditional knock out’ mouse), and observed the expected result that there were fewer, smaller, less lipid-rich plaques (Fig 1), presumably from the loss of FN from both distal and local sources.
To distinguish between the two sources, the authors next employed a different type of conditional knock out ApoE−/− mouse; that is one with specific deletion of FN in haematopoietic cells, but with normal levels of pFN. This time, the results resembled the findings from the initial studies in ApoE−/− mice: a large number of lipid-rich plaques were located in the susceptible aortic regions. These results point to liver-derived pFN as the relevant source of FN for atherogenesis. To directly support this suggestion, in a single experiment, the authors used control ApoE−/− mice as well as the hepatic/haematopoietic and the haematopoietic-only FN-deficient ApoE−/− mice. All three types of mice were fed the atherogenic diet, and the aortae were examined after 1 week of the diet to detect early changes in plaque-prone regions. Consistent with the longer-term studies, the control and haematopoietic FN-deficient mice already had continuous FN deposits in these regions, in contrast to the scant and spotty ones in the hepatic/haematopoietic-deficient mice. The logical inference was that pFN, derived from the liver, was the key source of the FN that is deposited early in the plaque initiation/progression processes.
Closer analysis of the aortae revealed a lower lipid content of the plaques in the mice with pFN deficiency that was associated with reduced macrophage accumulation. The authors investigated the basis for this with experiments in vitro and in vivo. An important step that determines the macrophage content of a plaque is the recruitment of circulating monocytes to cross the EC layer, which is facilitated in part by the adhesion molecule ICAM on the luminal surface of ECs. Notably, the in vitro studies showed that when ECs were cultured in the presence of pFN, ICAM-1 expression and the adhesion of macrophages increased. Using intravital microscopy, the authors went on to observe that the number of adherent leukocytes to the endothelium of carotid arteries was less in the mice with pFN deficiency.
Another striking finding from the histological examination of the plaques concerned the ‘fibrous cap’. This plaque structure is a collection of connective tissue elaborated by vascular smooth muscle cells (VSMCs) that migrate from the medial smooth layer to the sub-endothelium. Though mouse models with plaque rupture are not well established, in humans, ‘vulnerable’ plaques that cause heart attacks often have a thin or absent fibrous cap, which is thought to make them rupture-prone. In ApoE−/− mice deficient in pFN, the plaques lacked both VSMCs and, not surprisingly, then, fibrous caps. To begin to explore the mechanism for this, the authors noted in studies in vitro that pFN promoted the migration of VSMCs.
On balance, though the studies have undeniable strength, there are a number of caveats to note. For one, only one gender of mice (males) was studied. It is well known that a number of factors have different effects depending on the gender, and for the current studies to definitively speak to the other 50% of the animal kingdom, additional investigation in female mice are needed. Another qualification is the potential extrapolation of these mouse studies to human atherosclerosis. As noted above, mouse plaques do not generally rupture, no matter how advanced they become, so factors that are thought to affect plaque stability in people cannot be functionally characterized in mice. It is also not clear why it is the plasma, and not the local pool of FN that is regulatory. This is not to say that the data are not strong on this point, but the underlying molecular mechanisms will need to be further investigated to clarify the unique properties of pFN that lead to disease. Finally, as the authors themselves note, clinical studies linking pFN to atherosclerosis have borne mixed results.
…the report by Rohwedder et al. presents FN as a double-edged sword, which on the good edge, promotes the formation of a thick fibrous cap, but on the bad edge, expands the ECM, resulting in more atherogenic lipoprotein retention.
In summary, the report by Rohwedder et al. presents FN as a double-edged sword, which on the good edge, promotes the formation of a thick fibrous cap, but on the bad edge, expands the ECM, resulting in more atherogenic lipoprotein retention. This unfavorable consequence alone would result in the infiltration of inflammatory cells and plaque progression (Tabas et al, 2007), but at least in the mouse model, FN further contributes adversely by direct effects on ECs. As the authors rightly note, the interpretation of the effects of any factor on atherosclerosis should not be confined to what happens to the size of plaques-compositional changes, which can predominate [e.g. (Parathath et al, 2011)], must be also taken into account. In pre-clinical studies, a composite view of size/composition can be readily obtained by histological analyses of the plaques. Unfortunately, in clinical studies, we are left to imaging techniques (non-invasive and invasive) that are severely limited in what features of the plaque can be discerned besides size. Near-infrared catheter probes coupled with the use of injectable fluorescent dyes that detect different plaque components as well as other optical techniques have shown potential to discern compositional changes (Suter et al, 2011), but the need for catheterization clinically limits the applications to patients with acute coronary syndromes. Some promise from the non-invasive approach of fluorodeoxyglucose positron emission tomography (FDG-PET) to detect plaques rich in inflammatory cells has been thrown into question recently by the argument that this technique cannot distinguish inflammation from hypoxia (Folco et al, 2011). However, the ability to detect changes like those reported in Rohwedder et al in the typical patient awaits more sophisticated, non-invasive molecular imaging techniques.
Acknowledgments
The authors declare that they have no conflict of interest.
References
- Feaver RE, Gelfand BD, Wang C, Schwartz MA, Blackman BR. Atheroprone hemodynamics regulate fibronectin deposition to create positive feedback that sustains endothelial inflammation. Circ Res. 2010;106:1703–1711. doi: 10.1161/CIRCRESAHA.109.216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, Di Carli MF, Libby P. Hypoxia but not inflammation augments glucose uptake in human macrophages: implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-d-glucose positron emission tomography. J Am Coll Cardiol. 2011;58:603–614. doi: 10.1016/j.jacc.2011.03.044. [DOI] [PubMed] [Google Scholar]
- Parathath S, Grauer L, Huang LS, Sanson M, Distel E, Goldberg IJ, Fisher EA. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes. 2011;60:1759–1769. doi: 10.2337/db10-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwedder I, Montanez E, Beckmann K, Bengtsson E, Dunér P, Nilsson J, Soehnlein O, Fässler R. Plasma fibronectin deficiency impedes atherosclerosis progression and fibrous cap formation. EMBO Mol Med. 2012 doi: 10.1002/emmm.201200237. DOI: 10.1002/emmm.201200237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalen K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL, Boren J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–754. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- Suter MJ, Nadkarni SK, Weisz G, Tanaka A, Jaffer FA, Bouma BE, Tearney GJ. Intravascular optical imaging technology for investigating the coronary artery. JACC Cardiovasc Imaging. 2011;4:1022–1039. doi: 10.1016/j.jcmg.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]