Abstract
Overcoming the resistance of tumours to chemotherapy, often due to downregulation of Bax and Bak, represents a significant clinical challenge. It is therefore important to identify novel apoptosis inducers that bypass Bax and Bak. Potassium channels are emerging as oncological targets and a crucial role of mitochondrial Kv1.3 in apoptosis has been demonstrated. Here we report for the first time that Psora-4, PAP-1 and clofazimine, three distinct membrane-permeant inhibitors of Kv1.3, induce death by directly targeting the mitochondrial channel in multiple human and mouse cancer cell lines. Importantly, these drugs activated the intrinsic apoptotic pathway also in the absence of Bax and Bak, a result in agreement with the current mechanistic model for mitochondrial Kv1.3 action. Genetic deficiency or short interfering RNA (siRNA)-mediated downregulation of Kv1.3 abrogated the effects of the drugs. Intraperitoneal injection of clofazimine reduced tumour size by 90% in an orthotopic melanoma B16F10 mouse model in vivo, while no adverse effects were observed in several healthy tissues. The study indicates that inhibition of mitochondrial Kv1.3 might be a novel therapeutic option for the induction of cancer cell death independent of Bax and Bak.
Keywords: apoptosis, clofazimine, mitochondrial potassium channel, PAP-1, tumour model
INTRODUCTION
Chemoresistant tumour biology contributes to poor clinical prognosis and represents a fundamental limitation of current anti-tumour therapies. The pro-apoptotic proteins Bax and Bak are central players for the induction of apoptosis by many chemotherapeutic drugs including etoposide, cisplatin and adriamycin. Down-regulation of these proteins represents a common mechanism for tumour cells to develop resistance (e.g. Ionov et al, 2000; LeBlanc et al, 2002; McCurrach et al, 1997; Meijerink et al, 1998; Wang et al, 2001). Therefore, the identification of molecules that mediate the death of cancer cells independent of Bax and Bak is of great interest for the development of novel tumour therapies. Here, we tested the potential of mitochondrial Kv1.3 to serve as such a target for the induction of apoptosis.
Kv1.3, a potassium channel of the shaker family (Gutman et al, 2005), is functionally active in both the plasma membrane and the mitochondrial inner membrane (mitoKv1.3) in lymphocytes (Szabò et al, 2005), hippocampal neurons (Bednarczyk et al, 2010) and astrocytes (Cheng et al, 2010). Changes of Kv1.3-expression have been described in various cancers (Arcangeli et al, 2009), including human diffuse large B cell lymphoma (Alizadeh et al, 2000), glioma (Bielanska et al, 2009; Preussat et al, 2003), melanoma (Artym & Petty, 2002), breast (Abdul et al, 2003; Jang et al, 2009), prostate (Abdul & Hoosein, 2006), gastric (Lan et al, 2005), pancreas (Brevet et al, 2009) and colon cancers (Abdul & Hoosein, 2002).
Plasma membrane Kv1.3 has been shown to be critical for proliferation (for recent reviews see, e.g. Arcangeli et al, 2009; Cahalan & Chandy, 2009), while mitoKv1.3 has been demonstrated to be important for induction of apoptosis in different cell types (for a recent review see Szabò et al, 2010). Kv1.3 knock-down in human peripheral blood lymphocytes or deficiency in cytotoxic T lymphocytes (CTLL-2) impairs apoptosis triggered by various stimuli, while its expression in mitochondria is sufficient to restore apoptosis in CTLL-2 T lymphocytes (Szabò et al, 2008). Platelets from Kv1.3−/− mice are resistant to apoptosis (McCloskey et al, 2010). Furthermore, transfection of rat retinal ganglion cells, which express Kv1.1, Kv1.2, Kv1.5 and Kv1.3, with short interfering RNAs (siRNAs) directed against Kv1.1 or Kv1.3 channels greatly reduced apoptosis upon optic nerve transection, whereas Kv1.2- or Kv1.5-targeted siRNAs had only a small effect (Koeberle et al, 2009).
We previously reported that the presence of mitoKv1.3 is critical for mitochondrial apoptotic events (Szabò et al, 2008). In particular, we identified mitoKv1.3 as a novel target of the pro-apoptotic protein Bax and demonstrated a physical interaction between these two proteins in apoptotic cells (Szabò et al, 2008; Szabò et al, 2011). Incubating isolated Kv1.3-positive mitochondria with Bax or the known Kv1.3 inhibitors MgTx, ShK or Psora-4 triggered typical apoptotic events including membrane potential changes, reactive oxygen species (ROS) production and cytochrome c release (Szabò et al, 2008). These effects were not observed in Kv1.3-deficient mitochondria. Mutation of the highly conserved Bax lysine 128 (BaxK128E), which faces the intermembrane space after mitochondrial insertion of Bax (Annis et al, 2005), abrogated Kv1.3 inhibition and the pro-apoptotic effects of Bax both in isolated mitochondria and in intact cells expressing the mutant protein (Szabò et al, 2011). These data indicated that Bax binds to and inhibits Kv1.3 to trigger apoptosis.
However, to inhibit mito-Kv1.3 in intact cells, membrane permeable Kv1.3 inhibitors are required. Several membrane-permeant pharmacological inhibitors of Kv1.3 are available, in particular the non-peptidyl inhibitors Psora-4 (Kd 3 nM) (Vennekamp et al, 2004), PAP-1 (Kd 2 nM) (Schmitz et al, 2005) and clofazimine (IC50 300 nM) (Ren et al, 2008). Psora-4 and PAP-1 are non-phototoxic derivatives of 5-methoxy-psoralen that bind to a water-filled cavity below the selectivity filter of Kv1.3 (Schmitz et al, 2005; Vennekamp et al, 2004). Psora-4 inhibits Kv1.3 and Kv1.5 with comparable efficacy in the low nM range (Vennekamp et al, 2004), while PAP-1 is 23-fold selective for Kv1.3 (Kd 2 nM) over Kv1.5 (Schmitz et al, 2005). However, patch clamp experiments performed with micromolar concentrations showed that both inhibitors also act on other potassium channels of the Kv family (Schmitz et al, 2005). Clofazimine, a riminophenazine compound, rather selectively blocks Kv1.3 (Kd 300 nM) with tenfold higher potency than Kv1.1, Kv1.2, Kv1.5 and Kv3.1 (Ren et al, 2008). Clofazimine is already in use for the treatment of various pathologies, including psoriasis, chronic graft-versus-host disease and granulomatous cheilitis (e.g. Ren et al, 2008). Clofazimine has been shown to be safe for humans in over 70 years of clinical use. Importantly, administration of the most selective non-peptidyl Kv1.3 inhibitor, the Psora-4 derivative PAP-1, to monkeys did not result in toxicity and did not compromise the protective immune response to viral and bacterial infection (Pereira et al, 2007).
In the present work CTLL-2 lymphocytes either lacking Kv1.3 or stably transfected with Kv1.3 were employed, in order to provide genetic data for the observed effects of the membrane permeant inhibitors on the potassium channel. Further, we describe that these drugs efficiently kill a variety of Kv1.3-positive human and mouse tumour cells by inducing apoptosis, while they are inactive in Kv1.3-deficient cells. The cytotoxic effect, due to activation of the intrinsic apoptotic pathway, is independent of Bax and Bak expression. The potential value of Kv1.3 inhibitors as agents for tumour-treatment is supported by in vivo studies demonstrating that clofazimine greatly diminishes tumour growth in a mouse melanoma model. Our work thus identifies the specific targeting of mitoKv1.3 as a novel pharmacological tool to induce apoptosis in tumour cells independent of Bax and Bak. The safety of these Kv1.3 inhibitors emphasizes their potential for use in the treatment of malignant tumours.
RESULTS
Membrane-permeant Kv1.3 inhibitors induce apoptosis by targeting mitochondrial Kv1.3
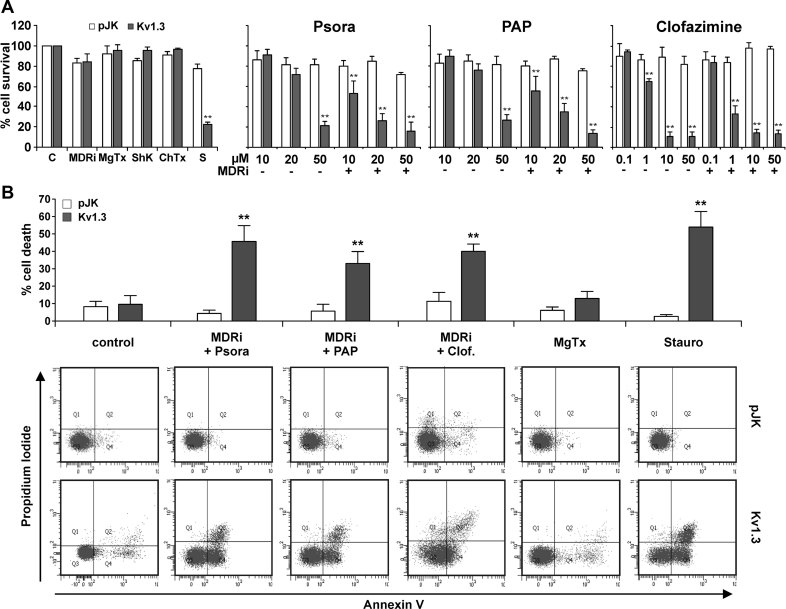
To test whether membrane-permeant Kv1.3 inhibitors are capable of inducing apoptosis by acting specifically via Kv1.3, we stably transfected murine CTLL-2, which lack Kv1.3 and other functional Kv potassium channels (e.g. Szabò et al, 2005) with an expression vector for Kv1.3 (CTLL-2/Kv1.3) or the empty vector control (CTLL-2/pJK). This model is suitable to study physiological processes linked to the activity of Kv1.3. Previous data showed that the transfection with Kv1.3 resulted in physiological expression levels of the channel protein (Szabò et al, 2005; Szabò et al, 2008). Psora-4, PAP-1 and clofazimine dose-dependently decreased cell viability, as determined by 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays, in Kv1.3-expressing CTLL-2/Kv1.3 cells (Fig 1A). Conversely, CTLL-2/pJK cells were resistant to these drugs (Fig 1A). CTLL-2/pJK cells were also resistant to staurosporine (1 µM for 12 h), a classical inducer of the intrinsic apoptotic pathway, emphasizing the importance of Kv1.3 for apoptosis (Fig 1A and Szabò et al, 2008). Since many tumours extrude drugs by multidrug resistance (MDR) pumps, we incubated the cells with the MDR pump inhibitors (MDRi) Cyclosporine H (CSH) and probenecid. These were used in order to prevent export of the applied drugs and thus allow the amplification of the effects of Kv1.3 inhibitors in the intracellular milieu. In fact, when combined with MDRi, Kv1.3 blockers were significantly more effective in reducing viability of CTLL-2/Kv1.3 cells. Clofazimine, a less potent Kv1.3 inhibitor (Kd 300 nM), was most effective (EC50 ∼1 µM). This is likely due to its ability to block both Kv1.3 (Ren et al, 2008) and MDR pumps (Van Rensburg et al, 1998). CSH and probenecid were not toxic at the concentrations we used (Fig 1A, left panel). Further, membrane-impermeant Kv1.3 inhibitors such as MgTx, ShK and ChTx did not affect cell survival of CTLL-2/Kv1.3 (Fig 1A, left panel) at a concentration >1000-fold higher than the channel-blocking dose (MgTx for example, fully blocks Kv1.3 at 1 nM) (Garcia-Calvo et al, 1993), indicating that inhibition of intracellular, but not plasma membrane Kv1.3, mediates cell death. Control studies also indicated that Kv1.3 expression did not alter protein levels of pro-apoptotic Bax, anti-apoptotic Bcl-xL or the effector caspase-3 in CTLL-2/Kv1.3 cells compared to CTLL-2/pJK cells (Supporting Information Fig S1A).
Figure 1. Effect of membrane-permeant Kv1.3 inhibitors on lymphocytes lacking or expressing the Kv1.3 potassium channel.
CTLL-2/pJK and CTLL-2/Kv1.3 were incubated for 12 h with different compounds as indicated (MDRi: CSH 1 µM + Prob. 50 µM) (pJK: CTLL-2/pJK; Kv1.3: CTLL-2/Kv1.3).
- Cell viability was measured by MTT assay. Values are reported as the mean percentage of cell survival compared to untreated cells ± SD (n = 15–20; **p < 0.01). The concentration of ShK, MgTx and ChTx was 1 µM; staurosporine (S) was 1 µM; the concentrations of Psora-4, PAP-1 and clofazimine are given under the graph.
- Cell death was analyzed by FACS using double staining with Annexin V and propidium iodide. Values reported in the bar-graph indicate the mean percentage ± SD of dead cells calculated taking into account Q2 and Q4 (all annexin positive cells) (n = 5). Differences between CTLL-2/pJK and CTLL-2/Kv1.3 are statistically significant (t-test, **p < 0.01) in all cases, except for control, untreated cells. The lower panel illustrates a representative experiment.
In order to confirm that the observed decrease in cell viability was a result of increased apoptosis and not necrosis, we evaluated the effect of the PAP-1, Psora 4 and clofazimine in combination with MDRi by measuring phosphatidylserine exposure by Annexin V binding and propidium iodide staining. All three membrane-permeant Kv1.3 inhibitors induced phosphatidylserine exposure in CTLL-2/Kv1.3 cells, but not in Kv1.3 deficient CTLL-2/pJK cells. Annexin V binding was similar to that observed upon incubation of the cells with staurosporine (Fig 1B). Non-permeant inhibitors (MgTx and Shk) and MDRi alone did not induce significant apoptosis (Fig 1B and Supporting Information Fig S1B).
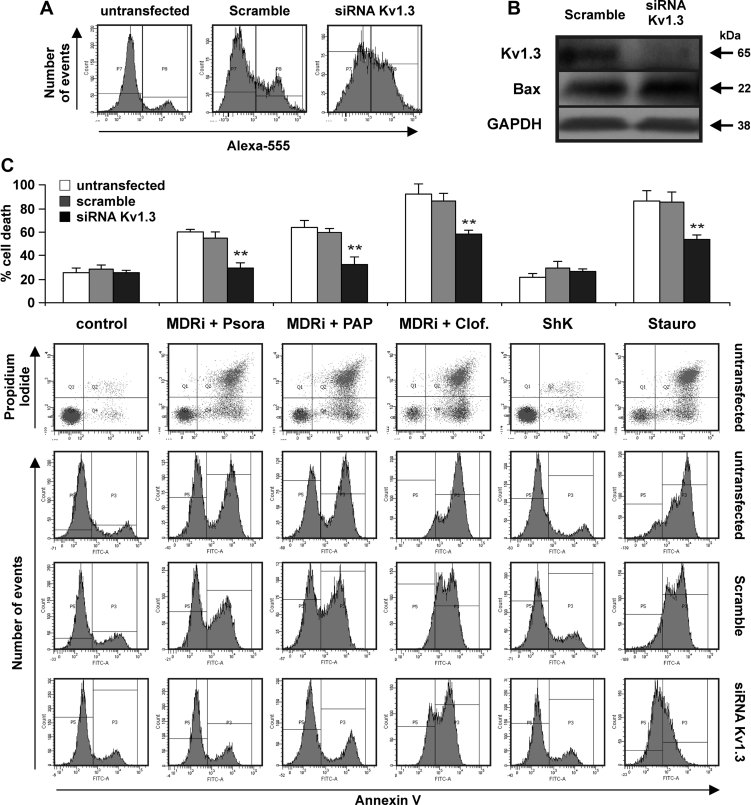
To further demonstrate that membrane-permeant Kv1.3 inhibitors induce apoptosis by blocking intracellular, most likely mitochondrial Kv1.3, we suppressed Kv1.3 expression in human Jurkat leukemic T cells by transient transfection with siRNA targeting Kv1.3. Kv1.3 is the only potassium channel of the Kv family expressed in human Jurkat leukemic T cells and is active in mitochondria (mitoKv1.3) (Szabò et al, 2005). Transfection efficiency was visualized by fluorescence activated cell sorter (FACS) analysis using an Alexa-555 labelled siRNA (Fig 2A) and suppression of Kv1.3 expression was confirmed by Western blotting (Fig 2B). The results show that Psora-4-, PAP-1- and clofazimine-induced apoptosis was significantly decreased in human Jurkat leukemic T cells upon reduction of channel expression by siRNA (Fig 2C), while cells transfected with control siRNA still underwent apoptosis to an extent similar to that observed in non-transfected cells.
Figure 2. Apoptosis-inducing effect of membrane permeant Kv1.3 inhibitors depend on Kv1.3 expression.
- A,B. Human Jurkat leukemic T cells were electroporated with either Alexa-555 labelled siRNA control (scramble) or siRNA against Kv1.3 as described in the experimental section. After 48 h the cells were treated as indicated for 24 h (20 µM Psora and PAP-1; 1 µM clofazimine, staurosporine (Stauro) and ShK) in the presence or absence of MDR-inhibitors (MDRi). Alexa-555 fluorescence (A) and Western blot (B) are shown to evaluate siRNA transfection and protein expression, respectively (100 µg/lane of total extract). Anti-Bax and anti-GAPDH were used as loading control. The results are representative of three independent studies.
- C. Upper panel: average percentages of cell death ± SD (n = 3). Asterisks indicate statistically significant differences (**p < 0.01). The lower panel displays a typical result using human Jurkat leukemic T cells from the same culture and processed together.
Although the above experiments suggest that the cytotoxic effect of PAP-1, Psora-4 and clofazimine is due to a specific action against mitochondrial Kv1.3, it is important to rule out a possible contribution of calcium-dependent potassium channels. CTLL-2 lymphocytes, like primary lymphocytes, express the KCa3.1 intermediate-conductance calcium-activated K+ channel (Supporting Information Fig S1A), while human Jurkat leukemic T cells express only the KCa2.2 small-conductance calcium-activated K+ channel (Fanger et al, 2001). These two channels are specifically blocked by TRAM-34 (IC50 20 nM) and UCL1684 (IC50 250 pM), respectively (Fanger et al, 2001). Notably, both drugs are membrane-permeant. Supporting Information Fig S2A illustrates that even 20 µM UCL1684 failed to induce apoptosis in human Jurkat leukemic T cells. Likewise, doses up to 5 µM TRAM-34 did not trigger death in CTLL-2/pJK cells. These data indicate that inhibition of calcium-activated K+ channels does not mediate cell death in these lymphocytes. In summary, these data demonstrate that PAP-1, Psora-4 and clofazimine induce cellular apoptosis by inhibition of intracellular Kv1.3.
Membrane-permeant Kv1.3 inhibitors induce the classical apoptosis pathway
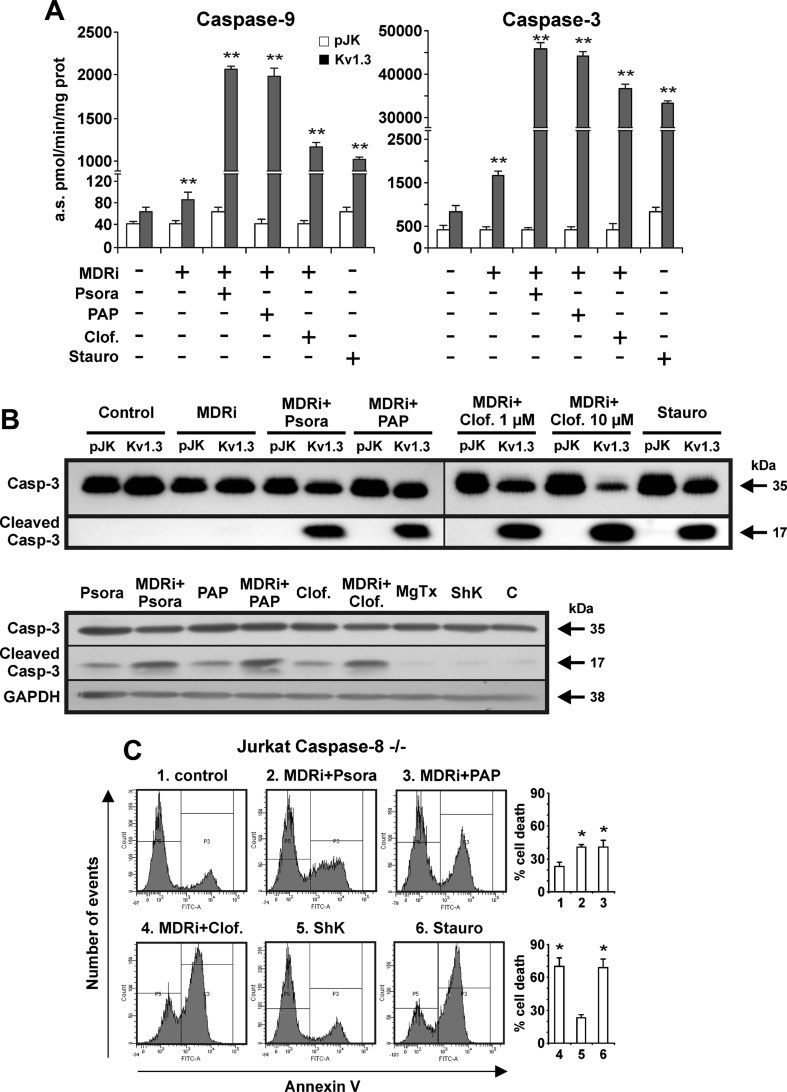
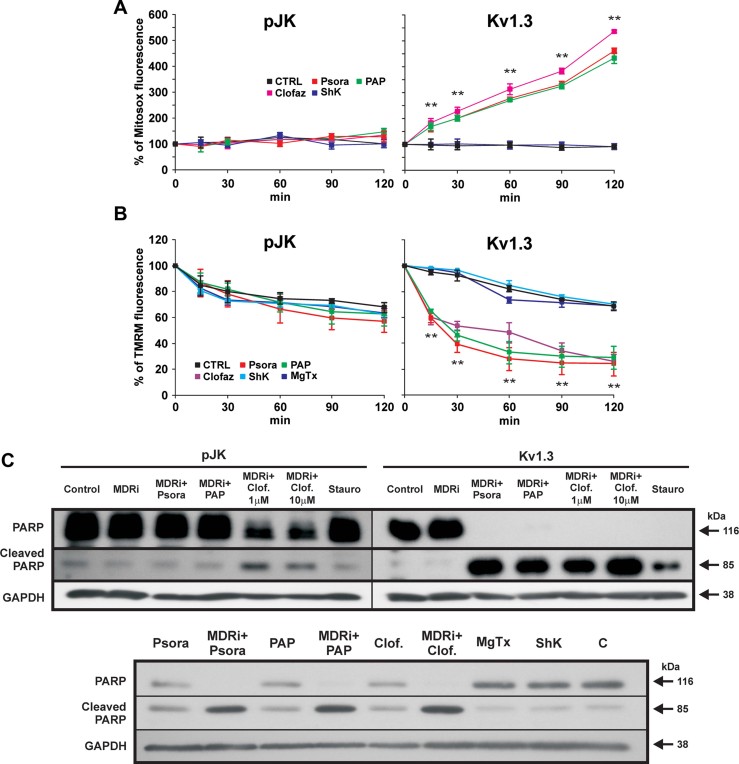
The apoptotic signalling cascade upon treatment with PAP-1, Psora-4 and clofazimine was next characterized by assaying hallmark apoptotic events in CTLL-2/Kv1.3 and CTLL-2/pJK cells. To this end, we evaluated the activity of caspase-9 and caspase-3, initiator and effector caspases, respectively, of the intrinsic pathway. We observed that caspase-9 and caspase-3 activity increased 25- and 30-fold, respectively, in the Kv1.3 expressing cells upon 12 h treatment with Psora-4, PAP-1 and clofazimine plus MDRi, while the drugs were without effect in CTLL-2/pJK cells (Fig 3A). Caspase-3 activation was confirmed by visualization of the cleaved form in Western blots (Fig 3B). Co-incubation of the cells with Kv1.3 blockers and MDRi significantly increased caspase-3 activity compared to the effects of the inhibitors alone (Fig 3B). A caspase-8 deficient lymphocyte cell line still underwent apoptosis, suggesting that caspase-8 is not involved in the apoptotic signalling induced by these drugs (Fig 3C). Mitochondrial cytochrome c release was observed only in Kv1.3-positive cells when treated with the membrane-permeant inhibitors (Fig 4A and B). To gain further insight into the mechanism of action of membrane-permeant Kv1.3 inhibitors, we monitored changes in mitochondrial ROS production in CTLL-2 cells (Fig 5A) and in human Jurkat leukemic T lymphocytes (Supporting Information Fig S4A) and mitochondrial membrane potential in CTLL-2 cells (Fig 5B). Membrane-permeant Kv1.3 inhibitors caused early ROS release and mitochondrial depolarization only in CTLL-2/Kv1.3 cells, but not in the cells lacking the channel (Fig 5A and B), indicating that membrane-permeable substances did not induce oxidative stress per se and that ROS production is downstream of Kv1.3. In accordance, cell death induced by oxidative stress via menadione occurred in both cell types independently of the presence of Kv1.3 (Supporting Information Fig S2B).
Figure 3. Membrane-permeant Kv1.3 inhibitors trigger caspase activation.
CTLL-2/pJK and CTLL-2/Kv1.3 were incubated for 12 h with the different compounds (MDRi: CSH 1 µM + Prob. 50 µM; 20 µM Psora and PAP-1; 1 or 10 µM clofazimine; 1 µM staurosporine, ShK or MgTx) as indicated and total protein extracts were prepared.
- Caspase-3 and caspase-9 activities were measured as indicated in Materials and Methods Section. Four separate experiments were performed in triplicates. The values plotted are the average of 12 measurements ± SD (**p < 0.01).
- Upper part: Kv1.3 inhibitors trigger caspase-3 activation as indicated by proteolytic cleavage. Shown are Western blot studies with an anti-caspase 3 antibody using the same protein extracts (100 µg/lane) as in panel A. The lower panel displays caspase 3 cleavage in 106 CTLL-2/Kv1.3 cells treated as indicated or left untreated (‘C’). Caspase 3 cleavage by Kv1.3 inhibitors is enhanced by MDRi. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as loading control. The experiments were performed three times with similar results.
- Caspase-8 knock-out human Jurkat leukemic T cells undergo apoptosis when treated for 24 h with staurosporine (1 µM) and membrane-permeant Kv1.3 inhibitors (MDRi: CSH 4 µM + Prob. 100 µM; 20 µM Psora and PAP-1; 1 µM clofazimine, ShK). Mean of percentages of cell death ± SD is shown (n = 3, *p < 0.05).
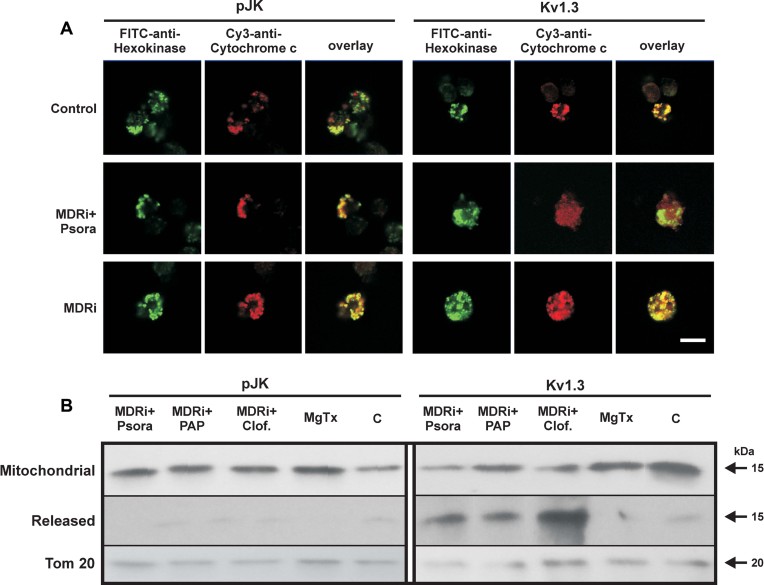
Figure 4. Kv1.3 inhibitors induce cytochrome c release only in Kv1.3-expressing lymphocytes.
- Cytochrome c release was detected in CTLL-2/pJK and CTLL-2/Kv1.3 cells by confocal microscopy following Psora-4 treatment (MDRi: CSH 1 µM + Prob. 50 µM; 20 µM Psora). Anti-hexokinase II was used to identify mitochondria. Shown is a representative result of 4 similar studies. Bar: 10 µm.
- CTLL-2/pJK and CTLL-2/Kv1.3 cells were treated with the different compounds (MDRi: CSH 1 µM + Prob. 50 µM; 20 µM Psora and PAP-1; 1 µM clofazimine, MgTx) as indicated. Release of cytochrome c was detected with a specific monoclonal antibody by Western blot. Tom20 was used as loading control. The experiment was performed 3 times with similar results.
Figure 5. Kv1.3 inhibitors induce ROS release, mitochondrial depolarization and PARP cleavage only in Kv1.3-expressing lymphocytes.
- A,B. Mitosox (A) and TMRM (B) fluorescence in CTLL-2/pJK and CTLL-2/Kv1.3 cells following incubation with each 20 µM Psora or PAP-1, each 1 µM clofazimine (all added in presence of MDRi: CSH 1 µM and Prob. 50 µM), MgTx or ShK or left untreated (CTRL). Mean values ± SD are shown (n = 4, **p < 0.01, ANOVA). Mitosox detects ROS produced by mitochondria, TMRM reflects mitochondrial membrane potential.
- C. PARP cleavage and activation was detected by Western blotting in the same extracts used in Fig 3B with an anti-PARP antibody (100 µg/lane). GADPH was used as loading control. Lower part: PARP activation in CTLL-2/Kv1.3 cells is more pronounced in the presence of MDRi.
Finally, PAP-1, Psora-4 and clofazimine induced poly (ADP-ribose) polymerase (PARP) cleavage, an event downstream of cytochrome c release and caspase-3 activation, only in cells expressing Kv1.3 (Fig 5C). PARP cleavage was more pronounced when these agents were used in combination with MDRi (Fig 5C). In contrast, neither MgTx nor ShK induced caspase-9 or caspase-3 activation, cytochrome c release, mitochondrial depolarization or PARP cleavage, further indicating that inhibition of plasma membrane Kv1.3 is not sufficient to trigger cell death.
Collectively, these data indicate that membrane-permeant Kv1.3 inhibitors activate typical intrinsic apoptotic signalling pathways, and suggest that this action is achieved by specifically targeting mitochondrial Kv1.3.
Kv1.3 inhibitors kill a variety of tumour cell lines by targeting mitochondrial Kv1.3
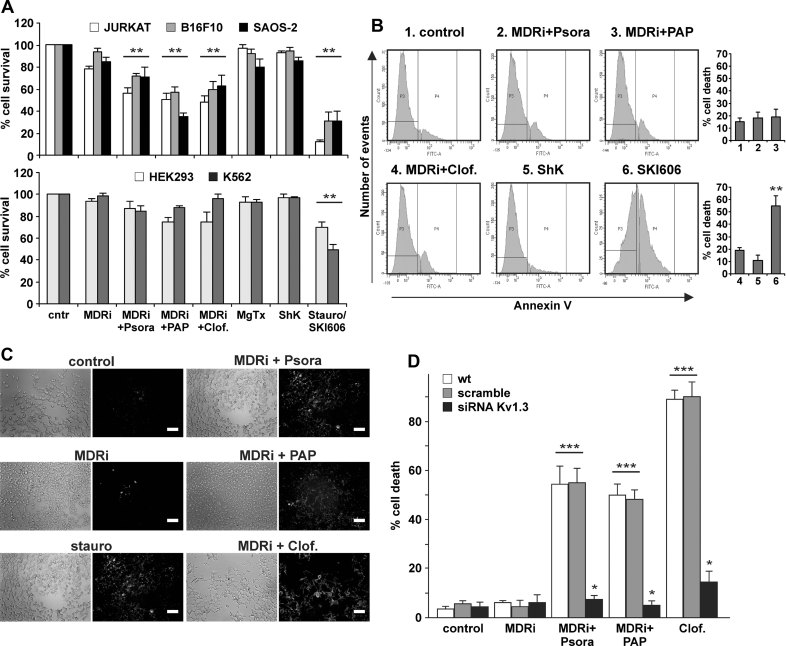
To evaluate the effects of Kv1.3 inhibitors on tumour cell lines, we tested cell survival by the MTT assay and cell death by fluorescein isothiocyanate (FITC)-annexin V binding in human Jurkat leukemic T lymphocytes, in human osteosarcoma SAOS-2 cells and in the mouse B16F10 melanoma cell line (Fig 6A–D). Western blot studies of lysates from Percoll-purified mitochondria employing two different anti-Kv1.3 antibodies confirmed expression of Kv1.3 in mitochondria of SAOS-2 and B16F10 cells (Supporting Information Fig S3A and B). Psora-4, PAP-1 and clofazimine induced apoptosis only in Kv1.3-expressing Jurkat, B16F10 and SAOS-2 cells, but not in HEK293 cells, known to express low levels of Kv potassium current (Yu & Kerchner, 1998), and not in the K562 human chronic myelogenous leukaemia cell line previously shown to lack Kv1.3 expression and current (Smith et al, 2002) (Fig 6A and B). Moreover, downregulation of Kv1.3 in B16F10 cells by transfection with siRNA prevented cell death upon treatment with Psora-4, PAP-1 and clofazimine (Fig 6D). Again, the drugs were more efficient in the presence of MDRi (Supporting Information Fig S3C).
Figure 6. Effects of membrane-permeant Kv1.3 inhibitors on various human cell lines.
- Jurkat, B16F10, SAOS-2 (upper panel) and HEK293 and K562 cells (lower panel) were incubated for 24 h as indicated (MDRi: 4 µM CSH + 100 µM Prob.; each 20 µM Psora-4 or PAP-1; each 1 µM clofazimine, MgTx, ShK or staurosporine). K562 cells are more sensitive to SKI606 (Puttini et al, 2006), an inhibitor of Src kinases, than to staurosporine, and SKI606 (5 µM) was therefore used as positive control. MTT assay was performed to determine cell survival. Data are shown as mean changes in percent compared to untreated cells ± SD (n = 12; **p < 0.01).
- Representative FACS analysis performed on K562 cells lacking Kv1.3 treated as in panel A. Right panel: average % of cell death ± SD is shown (n = 3; **p < 0.01).
- Annexin V binding in SAOS-2 cells after treatment with the different Kv1.3 inhibitors at the concentrations used in panel A shown by fluorescence microscopy. The result shown is representative of four other experiments giving similar results. Bar: 100 µm (except for MDRi + Clof, where bar is 50 µm).
- B16F10 melanoma cells die by apoptosis as assayed by FACS analysis after FITC-Annexin V staining. Furthermore, TUNEL staining after a 24 h treatment of B16F10 melanoma cells with Kv1.3 inhibitors confirmed apoptosis in B16F10 cells after Kv1.3 inhibition (data not shown). Suppression of Kv1.3 expression by transfection of siRNA targeting Kv1.3 prevents cell death by Kv1.3 inhibitors. B16F10 cells were treated as indicated for panel A. Given is the mean of the percentage of dead cells ± SD (n = 4; *p < 0.05, ***p < 0.001).
Kv1.3. inhibitors induce apoptosis independent of Bax and Bak
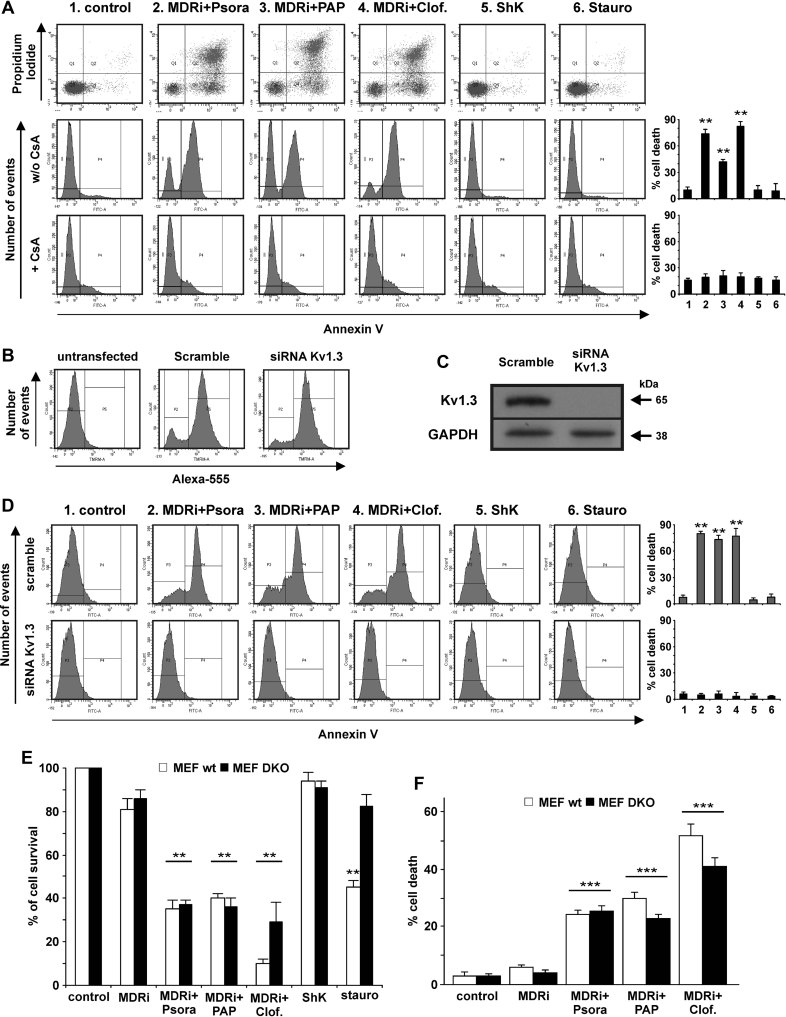
Tumour cells deficient in both Bax and Bak are protected against mitochondria-mediated apoptosis induced by many chemotherapeutic drugs, for instance etoposide, cisplatin or adriamycin (e.g. LeBlanc et al, 2002; McCurrach et al, 1997). Therefore, the often-observed deficiency of Bax and Bak in tumour cells (Ionov et al, 2000; LeBlanc et al, 2002; McCurrach et al, 1997; Meijerink et al, 1998; Wang et al, 2001) is a clinically relevant problem, and drugs inducing cell death downstream of Bax and Bak might offer novel treatment options for chemotherapeutic drug-resistant malignancies. We have previously shown that Kv1.3 is inhibited by Bax and contributes to a series of apoptotic events at the level of mitochondria (Szabò et al, 2008; Szabò et al, 2011). If the inhibition of mitochondrial Kv1.3 is a critical step for the induction of apoptosis, it should be possible to induce cell death in Bax/Bak-double knock-out cells by treatment with Psora-4, PAP-1 or clofazimine. In support of this idea, the three drugs efficiently induced apoptosis in Bax/Bak-double deficient human Jurkat leukemic T cells (Han et al, 2004) (Fig 7A) as well as in Bax/Bak-double knock-out murine embryonic fibroblasts (MEF DKO) (Scorrano et al, 2003) (Fig 7E and F). In contrast, Bax/Bak-deficient cells were resistant to staurosporine (Fig 7A and E), consistent with previous data. Control experiments confirmed that, as in human Jurkat leukemic T cells (Szabò et al, 2005), MEF DKO cells express Kv1.3 in their mitochondria (Supporting Information Fig S5A). Killing of Bax/Bak-deficient Jurkat cells by Kv1.3 inhibitors required expression of the Kv1.3 channel, since siRNA-mediated channel ablation (Fig 7B and C) prevented drug-induced apoptosis (Fig 7D). In Bax/Bak-double deficient human Jurkat leukemic T cells both drug-induced cell death (Fig 7A) and mitochondrial membrane potential changes (Supporting Information Fig S4B) were prevented by application of Cyclosporin A, an inhibitor of the permeability transition pore (PTP). Since UV-induced oxidative stress has been reported to induce autophagic death in MEF DKO cells (Buytaert et al, 2006a, b), we have investigated whether autophagy takes place in these cells treated with the Kv1.3 inhibitors used here. Conversion of the microtubule-associated protein light-chain C LC3 I (apparent mobility 18 kDa) into the autophagosome membrane-associated LC3 II form (apparent mobility 16 kDa) can be used to monitor autophagic activity (Mizushima et al, 2010). This conversion could be observed in DKO MEF cells treated with membrane-permeant Kv1.3 inhibitors, suggesting that activation of autophagy occurred to some extent (Supporting Information Fig S5B). However, DKO cells died to the same extent upon treatment with the drugs in the presence or absence of inhibitors of autophagy (Mizushima et al, 2010), indicating that this process did not contribute importantly to the death process observed (Supporting Information Fig S5C).
Figure 7. Membrane-permeant Kv1.3 inhibitors induce death in Bax/Bak-deficient human Jurkat leukemic T cells and Murine Embryonic Fibroblasts.
- A. Bax/Bak-deficient human Jurkat leukemic T cells are resistant to apoptosis induced by staurosporine, but are sensitive to Psora-4, PAP-1 and clofazimine (each 20 µM Psora-4 or PAP-1; each 1 µM clofazimine, ShK or staurosporine; MDRi: 4 µM CSH + 100 µM Prob.). These effects are abolished by preincubation with Cyclosporine A (CsA; 4 µM for 30 min) blocking the permeability transition pore (lower row). Average % of cell death ± SD is shown (n = 3; **p < 0.01).
- B–D. Bax/Bak-deficient human Jurkat leukemic T cells were electroporated with either Alexa-555 labelled siRNA control (scramble) or siRNA against Kv1.3 as described in the experimental section. After 48 h the cells were treated as indicated in panel A for 24 h. Alexa-555 fluorescence (B) and Western blot (C) are shown to evaluate siRNA transfection and protein expression, respectively (100 µg/lane of total extract). Anti-GAPDH was used as loading control. The results are representative for three independent studies. (D) Cell death was measured by FACS analysis after FITC-Annexin-V staining. In the plots are shown average percentages of cell death ± SD (n = 3; **p < 0.01).
- E,F. MTT assays (E) and TUNEL (F) on wildtype and Bax/Bak-deficient DKO MEFs indicate that permeant Kv1.3 inhibitors induce death independent of Bax and Bak expression (doses as in panel A). Apoptosis was confirmed also by Annexin-V staining, giving the same results (data not shown). Shown is the mean of the percentages of cell survival ± SD compared to untreated samples (n = 3; **p < 0.01, ***p < 0.001).
Kv1.3 inhibitors prevent melanoma growth in vivo
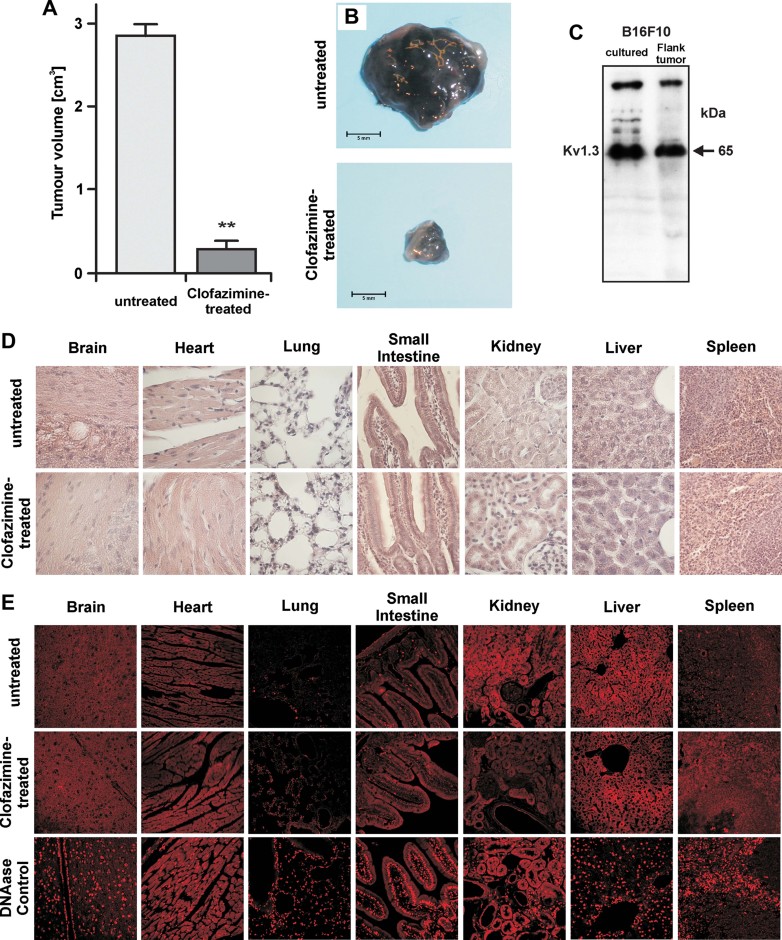
To test the significance of Kv1.3 inhibition-mediated apoptosis of tumour cells in vivo, we established a melanoma model by injecting 50,000 B16F10 melanoma cells subcutaneously into syngenic C57BL/6 mice. The tumour was allowed to establish and to grow for 5 days, after which, treatment was initiated with intraperitoneally injected clofazimine (5 µg/g mouse) at days 5, 7, 9 and 11 post-tumour injection. Clofazimine was chosen for these experiments due to its high efficacy in the in vitro experiments and its established clinical use (see Introduction Section). The tumours were removed at day 16. Analysis of the tumour size revealed a 90% reduction by clofazimine (Fig 8A and B). Control Western blots of the excised tumours displayed an expression of Kv1.3 comparable to that of cultured B16F10 cells (Fig 8C). Histological and TdT-mediated 2′-deoxyuridine, 5′-triphosphate (dUTP) nick end labelling (assay) (TUNEL) examination of the brain, heart, lungs, small intestine, kidney, liver and spleen from clofazimine treated animals revealed no gross abnormalities and cell death in these organs with respect to untreated ones (Fig 8D and E). In addition, we compared the effects of clofazimine with the membrane-impermeant Kv1.3 blocker MgTx. MgTx blocks proliferation of various cell types (Cahalan & Chandy, 2009) including lung adenocarcinoma (Jang et al, 2011). Administration of 50-times higher (taking into account the IC50 values) Kv1.3-inhibitory dose of MgTx with respect to clofazimine, reduced the tumour volume to 1.4 ± 0.3 cm3 (n = 6). In the same experimental setup, clofazimine reduced the tumour volume to 0.3 ± 0.16 cm3 (n = 8). Clofazimine may exert a larger tumour-reducing effect than MgTX because it blocks both plasma membrane and mitochondrial Kv1.3. However, differences in the pharmacokinetics of the two blockers might also contribute to their differences in efficacy.
Figure 8. Clofazimine reduces tumour size in an orthotopic melanoma model in vivo.
B16F10 melanoma cells were injected subcutaneously. On post-injection day five, mice were treated with intraperitoneal clofazimine at a dose of 5 µg/g. This was repeated on days 7, 9 and 11 post-tumour cell injection.
- A. Mean ± SD of the volume of the tumours after 16 days of growth in untreated (n = 8) and treated (n = 8) mice (**p < 0.01).
- B. Representative tumours.
- C. Cultured B16F10 cells or B16F10 melanoma were lysed, separated by 7.5% SDS–PAGE and Western blotted with anti-Kv1.3 antibodies. The results show expression of Kv1.3 in B16F10 cells in vitro and an in vivo.
- D,E. Haematoxylin/eosin (D) and TUNEL (E) staining from the brain, heart, lungs, small intestine, kidney, liver and spleen display no gross abnormalities in these organs after clofazimine treatment. Displayed are representative histologies from each three independent studies.
DISCUSSION
The results presented here indicate that three cell-permeant inhibitors of Kv1.3—PAP-1, Psora-4 and clofazimine—induce apoptosis in a panel of normal and tumour cells. We provide several lines of evidence that these inhibitors specifically target Kv1.3 to mediate apoptosis. Mouse CTLL-2 cells lacking Kv1.3 are resistant to PAP-1, Psora-4 and clofazimine and re-transfection of Kv1.3 to levels that are similar, or even lower, than in normal T lymphocytes restores sensitivity of these cells to the drugs. Moreover, siRNA-mediated down-regulation of Kv1.3 in human WT or Bax/Bak-deficient Jurkat leukemic T cells as well as in B16F10 melanoma cells prevents cell death induced by PAP-1, Psora-4 and clofazimine. In contrast, non-permeant Kv1.3 inhibitors such as MgTx, ShK or ChTx are unable to trigger death, in accordance with previous reports (Szabò et al, 1996; Jang et al, 2011). These data prove that intracellular (most likely mitochondrial) Kv1.3 is required for the induction of cell death by these inhibitors. We have previously shown in isolated mitochondria that inhibition of Kv1.3 results in the induction of mitochondrial changes that are typical for apoptosis including release of cytochrome c and mitochondrial depolarization. We have also shown that Bax binds to the pore of Kv1.3, subsequently resulting in the inhibition of Kv1.3 activity in isolated mitochondria. We proposed a model in which Lysine128 of Bax, like conserved Lysine-28 in MgTx, binds to the vestibule of the channel pore, inhibits the channel and triggers cell death (Szabò et al, 2008). Although acting at different regions of the channel than Bax and MgTx (Cahalan & Chandy, 2009), PAP-1, Psora-4 and clofazimine also inhibit Kv1.3 and induce cytochrome c release and mitochondrial membrane depolarization.
This model suggested that Kv1.3 inhibitors may be able to kill cells independently of Bax or Bak by promoting cytochrome c release downstream of the permeability transition or by other pathways such as VDAC oligomers. This prediction was confirmed in the present study using two genetically Bax/Bak-deficient cell lines, i.e. Bax/Bak-deficient human Jurkat leukemic T cells and MEF DKO. While these cells were resistant to staurosporine, they remained sensitive to PAP-1, Psora-4 and clofazimine and underwent apoptosis after treatment with these drugs.
The observation that PAP-1, Psora 4 and clofazimine trigger death independently of Bax and Bak underscores the potential of these drugs for use in tumour therapy. Bax and Bak are involved in the action of many chemotherapeutic agents including etoposide, cisplatin and adriamycin to name a few, and therefore, deficiency of Bax and Bak in tumour cells results in resistance to chemotherapy (e.g. LeBlanc et al, 2002; McCurrach et al, 1997). Thus, much effort is invested in identifying stimuli able to induce the intrinsic pathway of cell death in the absence of functional Bax/Bak. We demonstrate that PAP-1, Psora-4 and clofazimine induce apoptosis, in a Kv1.3-dependent and a Bax/Bak-independent way, in three cancer cell lines expressing mitoKv1.3. Moreover, we show that clofazimine effectively suppresses the in vivo growth of a transplanted mouse melanoma. Therefore, the present data provide a novel approach and specify a lead substance for the development of drugs that induce apoptosis independently of Bax and Bak.
Our in vivo data demonstrating that clofazimine prevents tumour growth raises the question as to why normal tissues that express mitoKv1.3, although generally at low levels (Bielanska et al, 2009), do not die after application of the membrane-permeant Kv1.3 inhibitor. PAP-1 has an excellent safety profile in rodents and monkeys (Pereira et al, 2007), and clofazimine has been shown to be safe in humans (Ren et al, 2008). Furthermore, in our study, clofazimine treatment did not induce histologically detectable changes in the brain, heart, lungs, small intestine, kidney, liver and spleen and, thus, appears to be non-toxic. Further, we did not detect a gross deletion of lymphocytes in clofazimine-treated mice, although the effect of the drug on lymphocyte sub-populations still requires definition. It may be relevant that channel protein expression does not necessarily correspond to the presence of functional channels in the mitochondrial or plasma membranes, as Kv1.3 may undergo several post-translational, activity-inhibiting (e.g. Cahalan & Chandy, 2009; Szabò et al, 1996) modifications. Also, channel activity is modulated by several factors including pH, temperature, calcium concentration and ROS (Cahalan & Chandy, 2009). The relatively high specificity of clofazimine for tumour cells might be explained by alterations of mitochondrial metabolism. For instance, some tumour cells may exhibit increased formation of ROS in mitochondria (e.g. Konstantinov et al, 1987; Szatrowski & Nathan, 1991; Zhou et al, 2003), or express decreased concentrations of ROS scavengers such as superoxide dismutase (Oberley et al, 1978; Van Driel et al, 1997). The increased ROS formation in tumour cell mitochondria seems to be caused by the action of oncogenes, mitochondrial gene mutations and the influence of cytokines in the microenvironment of the tumour (e.g. Pellicano et al, 2004). A constitutional increase in ROS concentration might sensitize tumour cells to inhibitors of Kv1.3, which trigger further ROS release (Figs 5A and S4A; Gulbins et al, 2010). Thus, a low concentration of ROS, or its efficient elimination in normal cells may protect these cells from the effects of Kv1.3 blockers. This would be in contrast to tumour cells, or to ex vivo cells prone to die such as cultured mouse lymphocytes, where treatment with Kv1.3 inhibitors may induce the release of additional ROS and result in apoptosis. This scenario would help to explain our findings and also provide a rationale to further develop Kv1.3 inhibitors as drugs that specifically target tumour cells independent of Bax and Bak.
The concentrations of Psora-4 and PAP-1 required for the induction of cell death (EC50 12 µM) are higher than those found to inhibit Kv1.3 in electrophysiological experiments: PAP-1 at 100 nM and Psora-4 at 50 nM are sufficient to completely block the Kv1.3 current in the plasmamembrane and can suppress human T cell proliferation (EC50 600 nM) (Schmitz et al, 2005; Vennekamp et al, 2004). Our previous patch clamp experiments revealed that 30 nM Psora-4 was sufficient to block mitoKv1.3 activity in mitoplasts as well as to induce ΔΨ changes and the release of cytochrome c from isolated mitochondria (Szabò et al, 2008). The requirement of higher concentrations to induce apoptotic changes when working with intact cells can be explained by a number of factors. The drugs are obviously exported by MDR proteins, and clofazimine may be more effective at lower concentrations due to its inhibitory action on both Kv1.3 and MDRs (Van Rensburg et al, 1998). Other reasons include the likely sequestration of these lipophilic drugs (log p values for PAP-1, Psora-4 and clofazimine are 4.03, 4.33 and 7.06, respectively) by cellular structures, in particular membranes. Therefore, the effective concentration of these drugs reaching the mitochondrial membrane may be significantly less than that added to the cell culture. Since the presence of 160 mM K+ on the external side of cloned Kv1.3 reduces the potency of PAP-1 tenfold (Schmitz et al, 2005), mitoKv1.3, whose vestibule is exposed to high cytoplasmic potassium, may be less sensitive to PAP-1 blockade. Regardless, our data unambiguously show an involvement of Kv1.3 in apoptosis mediated by these drugs because cells lacking or expressing reduced levels of Kv1.3 are significantly less sensitive to apoptosis induced by PAP-1, Psora-4 and clofazimine. The absence of death-inducing effects by membrane impermeant inhibitors of Kv1.3, and the observation that MDRis potentiated the effects of membrane-permeant Kv1.3 inhibitors, strongly indicate that PAP-1, Psora-4 and clofazimine act on intracellular Kv1.3. Moreover, the observed changes of the mitochondrial membrane potential upon incubation with these drugs compellingly suggest involvement of the mitoKv1.3. Therefore, we propose that inhibition of mitoKv1.3 is sufficient to induce intrinsic apoptosis.
Clofazimine, a riminophenazine compound, has been used in the treatment of cutaneous lupus, pustular psoriasis, chronic graft-versus-host disease and leprosy. Importantly, clofazimine has been reported to reduce hepatocellular carcinoma growth by oral administration (Ruff et al, 1998, but see Falkson & Falkson, 1999) or by intratumoural injection during in vivo studies (Pourgholami et al, 2004), but the mechanism was not investigated. It appears that the riminophenazines mediate their anti-proliferative effects at the level of the plasma membrane and/or the mitochondrial membrane. It has previously been shown that clofazimine activates cell membrane phospholipase A2, thus leading to the generation of lysophosphatidylcholine, which is a potent membrane-destabilizing agent capable of inducing cytotoxicity (Van Rensburg et al, 1993). A related action may be the ability of clofazimine to uncouple oxidative phosphorylation in the mitochondrial membrane, thus drastically affecting energy metabolism in tumour cells (Sri-Pathmanathan et al, 1994). Given that we observed a slight effect of clofazimine on hallmark apoptotic events in the Kv1.3-deficient CTLL-2 cells (see Fig 5A), our results do not exclude that clofazimine acts in part via the above-mentioned mechanisms in addition to initiating apoptosis via Kv1.3 inhibition. However, our observation that in five different cell lines of various origin clofazimine induces cell death, while at the same time three different cell lines lacking Kv1.3 are resistant to this drug, strongly argues that clofazimine mainly acts via inhibition of Kv1.3. The membrane-impermeant Kv1.3 inhibitor MgTx, at the dose used here, can partially reduce tumour volume, very likely by inhibition of cell proliferation, which is known to depend on plasma-membrane Kv1.3 (Cahalan & Chandy, 2009; Jang et al, 2011). Clofazimine, by acting on proliferation via the plasma-membrane-located Kv1.3 and in addition by inducing apoptosis via mitoKv1.3 is able to kill the cells, even in the absence of Bax and Bak. Active killing of tumour cells offers the perspective of a complete elimination of the cancer which could not be achieved by reducing only proliferation.
In summary, our studies demonstrate that Kv1.3 inhibitors are potent inducers of apoptosis in tumour cells expressing mitoKv1.3, and that their mode of action is independent of Bax and Bak. Our studies identify mitoKv1.3 as novel target for chemotherapy and suggest a strategy to circumvent a common resistance mechanism in tumour cells.
MATERIALS AND METHODS
Cells and drugs
Lymphocytes (Jurkat, CTLL-2, K562) were grown and stably transfected as described in Szabò et al, 2005. Adherent cells (HEK293, SAOS-2, MEF, B16F10) were grown in Dulbecco's modified Eagle medium (DMEM) under standard conditions (Sassi et al, 2010). Bax/Bak-deficient human Jurkat leukemic T cells were a kind gift of Prof. H. Rabinowich. These cells have the gene for Bax deleted and do not express Bak (Han et al, 2004). Bax/Bak-deficient MEF DKO were kindly provided by Prof. L. Scorrano. Lack of Bax and Bak expression in both cell lines was confirmed by Western blot (not shown) and is further indicated by their resistance to staurosporine. All membrane-permeant substances were strictly protected from UV sources, thus avoiding the formation of photo-oxidation by-products, which have previously been shown to induce apoptosis (Caffieri et al, 2007). All drugs were dissolved in dimethyl sulphoxide (DMSO) (except MgTx and ShK prepared in phosphate-buffer saline (PBS)) and diluted in DMEM. The final concentration of DMSO was ≤0.5% in all assays.
Purification of mitochondria
Mitochondria from SAOS-2, MEF and B16F10 cells were purified by differential centrifugation (Sassi et al, 2010). Briefly, approximately 80% confluent cells from four 150-cm2 flasks were washed once with PBS, detached by gentle scraping and spun down in a table centrifuge at room temperature. The pellet was resuspended in sucrose/N-[tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid (TES) buffer (300 mM sucrose, 10 mM TES, 0.5 mM EGTA, pH 7.4). After standing for 30 min on ice, cells were lysed in a Dounce homogenizer, and the lysate was centrifuged at 600×g for 10 min at 4°C. The pellet was again processed in the same way to maximize recovery. The combined supernatants were centrifuged once at 600×g, and the pellet was discarded. The mitochondria-containing supernatant from the last step was centrifuged at 6000×g for 10 min at 4°C. The pellet was gently homogenized and suspended in a small volume of TES buffer. A further purification was obtained by centrifugation (8500×g, 10 min, 4°C) on a discontinuous Percoll gradient (60, 30 and 18% Percoll in TES buffer). The floating material was discarded, and the fraction at the lower interface was collected and washed three times by centrifugation at 17,000×g for 5 min. The final pellet was resuspended in TES buffer.
Western blot
Samples were dissolved in sample buffer and subjected to 8, 12 or 15% SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to a polyvinyldifluoride (PVDF; Pall Corporation, Pensacola, FLA, USA) membrane. Primary antibodies used were: anti-Bak NT rabbit polyclonal (Upstate Biotechnology; cat. n. 06-536); anti-prohibitin mouse monoclonal (Lab Vision MS-261-P); anti-SERCA-2 ATPase mouse monoclonal (Affinity BioReagents MA3-910); anti-PMCA (plasma membrane calcium pump) ATPase mouse monoclonal (Affinity BioReagents MA3-914); anti-caspase-3 rabbit monoclonal; anti-PARP monoclonal; polyclonal anti-Kv1.3 (Alomone Labs APC-101), anti-Kv1.5 (Alamone Labs APC-004) and an anti-Kv1.3 antibody described in Szabò et al (2005); monoclonal mouse anti-cytochrome c antibody (clone 7H8.2C12, BD Biosciences Pharmingen, San Diego, CA, USA); anti-PARP (Sigma P248); anti-caspase-3 (Cell Signalling 8G10); anti-GAPDH (Millipore); anti-LC3 (Sigma L8918). Secondary antibodies (Calbiochem or Santa Cruz Inc.) were horseradish peroxidase- or alkaline phosphatase-conjugated and were used with chemiluminescence detection (Pierce) using film or digital imaging by a Bio-Rad ChemiDoc XRS apparatus.
MTT assay
To determine cell growth/viability in adherent cells, we employed the tetrazolium reduction (MTT) assay. To this end, adherent cells were seeded in standard 96-well plates and allowed to grow in DMEM (200 µl) for 24 h. The growth medium was then replaced with phenol red-free medium. Cells grown in suspension were seeded directly in a medium without phenol red and treated immediately for 12 (CTLL-2 cells) or 24 h (all other cell lines). Four wells were used for each condition. After 12 or 24 h incubation with the indicated drugs 10% CellTiter 96® AQUEOUS One solution (Promega) was added to each well. After 1 h of colour development at 37°C, absorbance at 490 nm was measured using a Packard Spectra Count 96-well plate reader.
The paper explained
PROBLEM:
Tumour cells deficient in both Bax and Bak are protected against mitochondria-mediated apoptosis induced by many chemotherapeutic drugs. Therefore, the often-observed deficiency of Bax and Bak in tumour cells is a clinically relevant problem, and drugs inducing cell death downstream of Bax and Bak might offer novel treatment options for chemotherapeutic drug-resistant malignancies.
RESULTS:
This study demonstrates that membrane-permeant Kv1.3 inhibitors are potent inducers of intrinsic apoptosis in tumour cells expressing mitochondrial Kv1.3, and that their mode of action is independent of Bax and Bak. Genetic deficiency or siRNA-mediated down-regulation of Kv1.3 abrogated the effects of the drugs. The potential value of Kv1.3 inhibitors as agents for tumour-treatment is supported by in vivo studies demonstrating that intraperitoneal injection of the membrane-permeant inhibitor clofazimine greatly reduced tumour volume in a mouse melanoma model, while healthy tissues were unaffected.
IMPACT:
Our work thus identifies the specific targeting of mitoKv1.3 as a novel pharmacological tool to induce apoptosis in tumour cells independent of Bax and Bak and suggests a strategy to circumvent a common resistance mechanism in tumour cells. The good safety profile of these Kv1.3 inhibitors emphasizes their potential for use in the treatment of malignant tumours.
FACS analysis
For the evaluation of apoptosis we plated cells (from 75,000 to 400,000 cells/well depending on cell line) in a 24- or 96-well plate. Cells were treated for 12 or 24 h with the indicated drugs. After treatment, CTLL-2 cells were harvested, washed with PBS, resuspended in FACS buffer consisting of 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 135 mM NaCl, 5 mM CaCl2, pH 7.4. The other cell lines were prepared in DMEM without serum and phenol red. In both cases, cells were incubated with propidium iodide and Annexin V-Fluos in the dark at 37°C for 15 min. Samples were then immediately analyzed by using a Beckton Dickinson FACSanto II flow cytometer (BD Biosciences) or a FACS-Calibur (λ = 488 nm). Data were processed using the BD VISTA software.
Release of cytochrome c
To detect the release of mitochondrial cytochrome c, CTLL-2 cells were either left untreated or treated with Kv1.3 inhibitors for 12 h, washed in cold HEPES/saline (H/S; 132 mM NaCl, 20 mM HEPES (pH 7.4), 5 mM KCl, 1 mM CaCl2, 0.7 mM MgCl2, 0.8 mM MgSO4), incubated for 30 min at 4°C in TES buffer and then Dounce-homogenized. Nuclei and unbroken cells were pelleted by centrifugation for 5 min at 600×g and 4°C. Supernatants were centrifuged at 6000×g for 10 min at 4°C. The supernatants were used to detect released cytochrome c and the pellets were used to detect mitochondrial cytochrome c. Proteins were separated on 15% SDS–PAGE, blotted onto a PVDF membrane, and developed with the indicated antibodies.
Cytochrome c release in intact cells
Cells were washed in PBS, fixed in PBS-buffered 2% paraformaldehyde (pH 7.3) for 10 min, washed again and permeabilized for 5 min with 0.1% Triton X-100. Cells were washed again and blocked with PBS/1% FCS for 10 min, washed and stained with Cy3-coupled anti-murine anti-cytochrome c antibodies (clone 7H8.2C12, BD-Biosciences) for 45 min at room temperature. Samples were washed 3-times in PBS and incubated with FITC-labelled anti-hexokinase antibodies. After intensive washing, confocal microscopy was performed.
PARP and caspase-3 cleavage; caspase activity assays
Cells were either left untreated or treated with Kv1.3 inhibitors for 12 h, washed in warm NaCl 0.9%, and lysed in a buffer consisting of 10 mM Tris–HCl pH 7.5, 0.2 M NaCl, 0.5% Triton X-100, 1 mM dithiothreitol (DTT), 2 mM ethylenediaminetetraacetic acid (EDTA) (Leanza et al, 2010). Particulate was removed by centrifugation for 10 min at 19,000×g at 4°C. The supernatant was used for Western blot and caspase activities were measured using a colorimetric assay system following the protocol described by the manufacturer (Promega for caspase-3 [G7220], Millipore for caspases 8 [APT171] and 9 [APT173]).
Fluorescence microscopy
Fluorescence imaging was performed using an Olympus IX71 microscope with an MT20 light source, and Cell-R software. Phosphatidylserine exposure was observed with the same apparatus after Annexin-V-Fluos (Roche) binding, following vendor-suggested procedures.
siRNA
The sequences for the siRNA targeting human or murine Kv1.3 were coupled to Alexa Fluo 555 (Quiagen). Transient transfection with 10 µg siRNA per 4 million cells was performed by electroporation as described in (Szabò et al, 2008).
Mitochondrial membrane potential measurements and ROS production
A count of 800,000 cells were incubated either with 20 nM tetramethyl rhodamine methyl ester (TMRM) or 1 µM Mitosox for 20 min in 0.5 ml DMEM without serum and phenol red. Subsequently, 0.3 ml of this mixture was added to a FACS tube containing 1.2 ml of DMEM without serum and phenol red. TMRM and Mitosox dilution avoided further uptake of the probe into the mitochondria. Samples were then analyzed by flow cytometry (FACSanto II, Becton Dickinson). Where indicated, cells were pre-treated for 30 min with 4 µM Cyclosporin A before addition of TMRM.
In vivo studies
In order to establish melanoma in vivo, B16F10 cells were grown to sub-confluency in minimum essential medium (MEM; Invitrogen, Karlsruhe, Germany) supplemented with 10 mM HEPES (pH 7.4), 2 mM l-glutamine, 1 mM sodium pyruvate, 100 µM non-essential amino acids, 100 units/ml penicillin and 100 µg/ml streptomycin (all from Invitrogen). The cells were detached with cell dissociation solution (Becton Dickinson, Heidelberg, Germany), washed twice in PBS and 50,000 B16F10 melanoma cells in a volume of 50 µl were subcutaneously injected into the right flank of C57BL/6 mice. Treatment with clofazimine was initiated at post-injection day 5 and repeated at days 7, 9 and 11. We intraperitoneally injected 5 µg/g clofazimine (IC50 for mouse Kv1.3: 470 nM), which had been freshly dissolved at 2 mg/ml in DMSO. Tumours were removed 16 days after initiation and the size of the tumours were measured with calipers. Volume was determined as the product of length, width and height. Margatoxin (Sigma) (IC50: 110 pM) was injected intravenously at a dose of 0.05 µg/g diluted in 100 µl PBS with identical post-injection day treatment regimen. Tumour volume was evaluated as outlined above. All procedures performed on mice were approved by the Animal Care and Use Committee of the Bezirksregierung Duesseldorf, Germany. All efforts were made to minimize the number of animals used and their suffering.
Western blotting of Kv1.3 in mouse flank tumours
At 16 days post-injection of B16F10 cells, untreated C57BL/6 mice were sacrificed, underwent laparotomy and sternotomy, and were immediately perfused via the right ventricle with 0.9% NaCl for 2 min. Tumours were removed and immediately shock-frozen in liquid nitrogen. Tissues were homogenized and lysed in a lysis buffer consisting of 0.1% SDS, 25 mM HEPES, 0.5% deoxycholate, 0.1% Triton X-100, 10 mM EDTA, 10 mM sodium pyrophosphate, 10 mM NaF, 125 mM NaCl and 10 µg/g aprotinin/leupeptin for 5 min at 4°C. Insoluble material was removed by 10 min centrifugation at 4°C and the supernatants were added to 5× SDS-sample buffer and boiled for 5 min at 95°C. Standardized aliquots of 10 µg protein were separated by 7.5% SDS–PAGE, blotted onto nitrocellulose and blocked with starting block buffer (Pierce Biotechnology, Rockford, IL, USA) for 45 min. Blots were incubated for 60 min at room temperature with anti-Kv1.3-antibodies, washed extensively in tris-buffered saline (TBS) supplemented with 0.05% Tween 20 and incubated for 60 min with alkaline phosphatase coupled anti-rabbit antibodies (dilution 1:20,000; Santa Cruz Inc.). The membrane was washed again and developed with the Tropix chemoluminescence system.
Histology and TUNEL assay
Untreated mice or those treated with clofazimine were sacrificed and immediately perfused at low pressure via the right heart with 0.9% NaCl for 2 min followed by 4% paraformaldehyde for 10 min. Organs, including the brain, heart, lungs, small intestine, liver, kidney and spleen, were then removed and further fixed in 4% paraformaldehyde for 36 h. Tissue was serially dehydrated and embedded in paraffin for sectioning at a thickness of 7 µm. The sections were then dewaxed, re-hydrated and incubated in 0.1 M citrate buffer (pH 6.0) at 350 W for 4 min in a microwave. The samples were immediately cooled in PBS and incubated with TMR- or FITC-coupled dUTP in the presence of terminal deoxynucleotidyl-transferase (Roche Diagnostics) for 30 min at 37°C. They were then placed in 70°C PBS for 10 min and subsequently cooled. These sections were stained for 2 min with hematoxylin and washed with water prior to being mounted in Mowiol and evaluated using a Leica TCS-SP2 microscope. Haematoxylin and eosin stainings were also performed with tissue prepared as described above.
Statistics
Statistical analysis was performed by using analysis of variance (ANOVA) or independent student t-test.
Acknowledgments
The authors are grateful to Profs. T. Pozzan, P. Bonaldo and Dr. P Grumati for helpful comments, to Prof. H. Rabinowich for the human Jurkat leukemic T Bax/Bak-less cell line, to Prof. L. Scorrano for MEF DKO cells and to Prof. A. Donella-Deana for K562 cells and SKI-606. Work carried out in the authors' laboratories was supported in part by Italian Association for Cancer Research grants (to I.S. n. 5118 and to M.Z.), an European Molecular Biology Organization Young Investigator Program and a Progetti di Rilevante Interesse Nazionale grant (to I.S.), by DFG-grant Gu 335/13–3 (to E.G.) and by a Fondazione Cassa di Risparmio di Padova e Rovigo (CARIPARO) grant (to M.Z.).
Supporting Information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author contributions
IS, EG, MZ and LL designed the study, LL, BH, NS, IS and EG performed experiments, all authors analyzed data and IS, EG, MZ and KGC wrote the paper. IS, EG and MZ provided funding.
For more information
http://www.iuphar-db.org/DATABASE/ObjectDisplayForward?familyId = 81&objectId = 540
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Abdul M, Hoosein N. Voltage-gated potassium ion channels in colon cancer. Oncol. Rep. 2002;9:961–964. [PubMed] [Google Scholar]
- Abdul M, Hoosein N. Reduced Kv 1.3 potassium channel expression in human prostate cancer. J. Membr. Biol. 2006;214:99–102. doi: 10.1007/s00232-006-0065-7. [DOI] [PubMed] [Google Scholar]
- Abdul M, Santo A, Hoosein N. Activity of potassium channel blockers in breast cancer. Anticancer Res. 2003;23:3347–3351. [PubMed] [Google Scholar]
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, Andrews DW. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO. J. 2005;24:2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti O. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr. Med. Chem. 2009;16:66–93. doi: 10.2174/092986709787002835. [DOI] [PubMed] [Google Scholar]
- Artym VV, Petty HR. Molecular proximity of Kv1.3 voltage-gated potassium channels and beta(1)-integrins on the plasma membrane of melanoma cells: effects of cell adherence and channel blockers. J. Gen. Physiol. 2002;120:29–37. doi: 10.1085/jgp.20028607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarczyk P, Kowalczyk JE, Beresewicz M, Dołowy K, Szewczyk A, Zabłocka B. Identification of a voltage-gated potassium channel in gerbil hippocampal mitochondria. Biochem. Biophys. Res. Commun. 2010;397:614–620. doi: 10.1016/j.bbrc.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Bielanska J, Hernández-Losa J, Pérez-Verdaguer M, Moline T, Somoza R, Ramón Y, Cajal S, Condom E, Ferreres JC, Felipe A. Voltage-dependent potassium channels Kv1.3 and Kv1.5 in human cancer. Curr. Cancer Drug Targets. 2009;9:904–914. doi: 10.2174/156800909790192400. [DOI] [PubMed] [Google Scholar]
- Brevet M, Fucks D, Chatelain D, Regimbeau JM, Delcenserie R, Sevestre H, Ouadid-Ahidouch H. Deregulation of 2 potassium channels in pancreas adenocarcinomas: implication of Kv1.3 gene promoter methylation. Pancreas. 2009;38:649–654. doi: 10.1097/MPA.0b013e3181a56ebf. [DOI] [PubMed] [Google Scholar]
- Buytaert E, Callewaert G, Vandenheede JR, Agostinis P. Deficiency in apoptotic effectors Bax and Bak reveals an autophagic cell death pathway initiated by photodamage to the endoplasmic reticulum. Autophagy. 2006a;2:238–240. doi: 10.4161/auto.2730. [DOI] [PubMed] [Google Scholar]
- Buytaert E, Callewaert G, Hendrickx N, Scorrano L, Hartmann D, Missiaen L, Vandenheede JR, Heirman I, Grooten J, Agostinis P. Role of endoplasmic reticulum depletion and multidomain proapoptotic BAX and BAK proteins in shaping cell death after hypericin-mediated photodynamic therapy. FASEB J. 2006b;20:756–758. doi: 10.1096/fj.05-4305fje. [DOI] [PubMed] [Google Scholar]
- Caffieri S, Di Lisa F, Bolesani F, Facco M, Semenzato G, Canton Dall'Acqua F. The mitochondrial effects of novel apoptogenic molecules generated by psoralen photolysis as a crucial mechanism in PUVA therapy. Blood. 2007;109:4988–4994. doi: 10.1182/blood-2006-08-037192. [DOI] [PubMed] [Google Scholar]
- Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol. Rev. 2009;231:59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Debska-Vielhaber G, Siemen D. Interaction of mitochondrial potassium channels with the permeability transition pore. FEBS Lett. 2010;584:2005–2012. doi: 10.1016/j.febslet.2009.12.038. [DOI] [PubMed] [Google Scholar]
- Falkson CI, Falkson G. A phase II evaluation of clofazimine plus doxorubicin in advanced, unresectable primary hepatocellular carcinoma. Oncology. 1999;57:232–235. doi: 10.1159/000012036. [DOI] [PubMed] [Google Scholar]
- Fanger CM, Rauer H, Neben AL, Miller MJ, Rauer H, Wulff H, Rosa JC, Ganellin CR, Chandy KG, Cahalan MD. Calcium-activated potassium channels sustain calcium signaling in T lymphocytes. Selective blockers and manipulated channel expression levels. J. Biol. Chem. 2001;276:12249–12256. doi: 10.1074/jbc.M011342200. [DOI] [PubMed] [Google Scholar]
- Garcia-Calvo M, Leonard RJ, Novick J, Stevens SP, Schmalhofer W, Kaczorowski GJ, Garcia ML. Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J. Biol. Chem. 1993;268:18866–18874. [PubMed] [Google Scholar]
- Gulbins E, Sassi N, Grassmè H, Zoratti M, Szabò I. Role of Kv1.3 mitochondrial potassium channel in apoptotic signalling in lymphocytes. Biochim. Biophys. Acta Bioenerg. 2010;1797:1251–1259. doi: 10.1016/j.bbabio.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stühmer W, et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- Han J, Goldstein LA, Gastman BR, Rabinovitz A, Wang GQ, Fang B, Rabinowich H. Differential involvement of Bax and Bak in TRAIL-mediated apoptosis of leukemic T cells. Leukemia. 2004;18:1671–1680. doi: 10.1038/sj.leu.2403496. [DOI] [PubMed] [Google Scholar]
- Ionov Y, Yamamoto H, Krajewski S, Reed JC, Perucho M. Mutational inactivation of the proapoptotic gene BAX confers selective advantage during tumor clonal evolution. Proc. Natl. Acad. Sci. USA. 2000;97:10872–10877. doi: 10.1073/pnas.190210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SH, Kang KS, Ryu PD, Lee SY. Kv1.3 voltage-gated K+ channel subunit as potential diagnostic marker and therapeutic target for breast cancer. BMB Rep. 2009;42:535–539. doi: 10.5483/bmbrep.2009.42.8.535. [DOI] [PubMed] [Google Scholar]
- Jang SH, Choi SY, Ryu PS, Lee SY. Anti-proliferative effect of Kv1.3 blockers in A549 human lung adenocarcinoma in vitro and in vivo. Eur. J. Pharmacol. 2011;651:26–32. doi: 10.1016/j.ejphar.2010.10.066. [DOI] [PubMed] [Google Scholar]
- Koeberle PD, Wang Y, Schlichter LC. Kv1.1 and Kv1.3 contribute to the degeneration of retinal ganglion cells after optic nerve transection in vivo. Cell Death Diff. 2009;17:134–144. doi: 10.1038/cdd.2009.113. [DOI] [PubMed] [Google Scholar]
- Konstantinov AA, Peskin AV, Popova EY, Khomutov GB, Ruuge EK. Superoxide generation by the respiratory chain of tumor mitochondria. Biochim. Biophys. Acta. 1987;894:1–10. doi: 10.1016/0005-2728(87)90206-4. [DOI] [PubMed] [Google Scholar]
- Lan M, Shi Y, Han Z, Hao Z, Pan Y, Liu N, Guo C, Hong L, Wang J, Qiao T, et al. Expression of delayed rectifier potassium channels and their possible roles in proliferation of human gastric cancer cells. Cancer Biol. Ther. 2005;4:1342–1347. doi: 10.4161/cbt.4.12.2175. [DOI] [PubMed] [Google Scholar]
- Leanza L, Miazzi C, Ferraro P, Reichard P, Bianchi V. Activation of guanine-β-d-arabinofuranoside and deoxyguanosine to triphosphates by a common pathway blocks T lymphoblasts at different checkpoints. Exp. Cell Res. 2010;316:3443–3453. doi: 10.1016/j.yexcr.2010.06.023. [DOI] [PubMed] [Google Scholar]
- LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D, Ashkenazi A. Tumor-cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat. Med. 2002;8:274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- McCloskey C, Jones S, Amisten S, Snowden RT, Kaczmarek LK, Erlinge D, Goodall AH, Forsythe ID, Mahaut-Smith MP. Kv1.3 is the exclusive voltage-gated K+ channel of platelets and megakaryocytes: roles in membrane potential, Ca2+ signalling and platelet count. J. Physiol. 2010;588:1399–1406. doi: 10.1113/jphysiol.2010.188136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurrach ME, Connor TM, Knudson CM, Korsmeyer SJ, Lowe SW. Bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA. 1997;94:2345–2349. doi: 10.1073/pnas.94.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijerink JP, Mensink EJ, Wang K, Sedlak TW, Slöetjes AW, de Witte T, Waksman G, Korsmeyer SJ. Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood. 1998;91:2991–2997. [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley LW, Bize IB, Sahu SK, Leuthauser SW, Gruber HE. Superoxide dismutase activity of normal murine liver, regenerating liver, and H6 hepatoma. J. Natl. Cancer Inst. 1978;61:375–379. [PubMed] [Google Scholar]
- Pellicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updates. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Pereira LE, Villinger F, Wulff H, Sankaranarayanan A, Raman G, Ansari AA. Pharmacokinetics, toxicity, and functional studies of the selective Kv1.3 channel blocker 5-(4-phenoxybutoxy)psoralen in Rhesus Macaques. Exp. Biol. Med. 2007;232:1338–1354. doi: 10.3181/0705-RM-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourgholami MH, Lu Y, Wang L, Stephens RW, Morris DL. Regression of Novikoff rat hepatocellular carcinoma following locoregional administration of a novel formulation of clofazimine in lipiodol. Cancer Lett. 2004;207:37–47. doi: 10.1016/j.canlet.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Preussat K, Beetz C, Schrey M, Kraft R, Wölfl S, Kalff R, Patt S. Expression of voltage-gated potassium channels Kv 1.3 and Kv 1.5 in human gliomas. Neurosci. Lett. 2003;346:33–36. doi: 10.1016/s0304-3940(03)00562-7. [DOI] [PubMed] [Google Scholar]
- Puttini M, Coluccia AM, Boschelli F, Cleris L, Marchesi E, Donella-Deana A, Ahmed S, Redaelli S, Piazza R, Magistroni V, et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res. 2006;66:11314–11322. doi: 10.1158/0008-5472.CAN-06-1199. [DOI] [PubMed] [Google Scholar]
- Ren YR, Pan F, Parvez S, Fleig A, Chong CR, Xu J, Dang Y, Zhang J, Jiang H, Penner R, et al. Clofazimine inhibits human Kv1.3 potassium channel by perturbing calcium oscillation in T lymphocytes. PLoS One. 2008;3:e4009. doi: 10.1371/journal.pone.0004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff P, Chasen MR, Long JEH, van Rensburg CEJ. A phase II study of oral clofazimine in unresectable and metastatic hepatocellular carcinoma. Ann. Oncol. 1998;9:217–219. doi: 10.1023/a:1008204911774. [DOI] [PubMed] [Google Scholar]
- Sassi N, De Marchi U, Fioretti B, Biasutto L, Gulbins E, Francolini F, Szabò I, Zoratti M. An investigation of the occurrence and properties of the mitochondrial intermediate-conductance Ca2+-activated K+ channel mtKCa3.1. Biochim. Biophys. Acta Bioenerg. 2010;1797:1260–1267. doi: 10.1016/j.bbabio.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Sankaranarayanan A, Azam P, Schmidt-Lassen K, Homerick D, Hansel W, Wulff H. Design of PAP-1, a selective small molecule Kv1.3 blocker, for the suppression of effector memory T cells in autoimmune diseases. Mol. Pharmacol. 2005;68:1254–1270. doi: 10.1124/mol.105.015669. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Smith GA, Tsui HW, Newell EW, Jiang X, Zhu XP, Tsui FW, Schlichter LC. Functional up-regulation of HERG K+ channels in neoplastic hematopoietic cells. J. Biol. Chem. 2002;277:18528–18534. doi: 10.1074/jbc.M200592200. [DOI] [PubMed] [Google Scholar]
- Sri-Pathmanathan RM, Plumb JA, Fearon KC. Clofazimine alters the energy metabolism and inhibits the growth rate of a human lung-cancer cell line in vitro and in vivo. Int. J. Cancer. 1994;56:900–905. doi: 10.1002/ijc.2910560624. [DOI] [PubMed] [Google Scholar]
- Szabò I, Gulbins E, Apfel H, Zhang X, Barth P, Busch AE, Schlottmann K, Pongs O, Lang F. Tyrosine phosphorylation-dependent suppression of a voltage-gated K+ channel in T lymphocytes upon Fas stimulation. J. Biol. Chem. 1996;271:20465–20469. doi: 10.1074/jbc.271.34.20465. [DOI] [PubMed] [Google Scholar]
- Szabò I, Bock J, Jekle A, Soddemann M, Adams C, Lang F, Zoratti M, Gulbins E. A novel potassium channel in lymphocyte mitochondria. J. Biol. Chem. 2005;280:12790–12798. doi: 10.1074/jbc.M413548200. [DOI] [PubMed] [Google Scholar]
- Szabò I, Bock J, Grassmé H, Soddemann M, Wilker B, Lang F, Zoratti M, Gulbins E. Mitochondrial potassium channel Kv1.3 mediates Bax induced apoptosis in lymphocytes. Proc. Natl. Acad. Sci. USA. 2008;105:14861–14866. doi: 10.1073/pnas.0804236105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabò I, Zoratti M, Gulbins E. Contribution of voltage-gated potassium channels to the regulation of apoptosis. FEBS Lett. 2010;584:2049–2056. doi: 10.1016/j.febslet.2010.01.038. [DOI] [PubMed] [Google Scholar]
- Szabò I, Soddemann M, Leanza L, Zoratti M, Gulbins E. Single point mutations of a lysine residue change function of Bax and Bcl-xL expressed in Bax- and Bak-less mouse embryonic fibroblasts—novel insights into the molecular mechanisms of Bax-induced apoptosis. Cell Death Diff. 2011;18:427–438. doi: 10.1038/cdd.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- Van Driel BE, Lyon H, Hoogenraad DC, Anten S, Hansen U, Van Noorden CJ. Expression of CuZn- and Mn-superoxide dismutase in human colorectal neoplasms. Free Radic. Biol. Med. 1997;23:435–444. doi: 10.1016/s0891-5849(97)00102-0. [DOI] [PubMed] [Google Scholar]
- Van Rensburg CEJ, Van Staden AM, Anderson R. The riminophenazine agents clofazimine and B669 inhibit the proliferation of cancer cell lines in vitro by phospholipase A2-mediated oxidative and nonoxidative mechanisms I. Cancer Res. 1993;53:318–323. [PubMed] [Google Scholar]
- Van Rensburg EJ, Joone G, Anderson R. α-Tocopherol antagonizes the multidrug resistance-reversal activity of cyclosporin A, verapamil, GF120918, clofazimine and B669. Cancer Lett. 1998;127:107–112. doi: 10.1016/s0304-3835(98)00020-2. [DOI] [PubMed] [Google Scholar]
- Vennekamp J, Wulff H, Beeton C, Calabresi PA, Grissmer S, Hänsel W, Chandy KG. Kv1.3 blocking 5-phenylalkoxypsoralens: a new class of immunomodulators. Mol. Pharmacol. 2004;65:1364–1374. doi: 10.1124/mol.65.6.1364. [DOI] [PubMed] [Google Scholar]
- Wang GQ, Gastman BR, Wieckowski E, Goldstein LA, Gambotto A, Kim TH, Fang B, Rabinovitz A, Yin XM, Rabinowich H. A role for mitochondrial Bak in apoptotic response to anticancer drugs. J. Biol. Chem. 2001;276:34307–34317. doi: 10.1074/jbc.M103526200. [DOI] [PubMed] [Google Scholar]
- Yu SP, Kerchner GA. Endogenous voltage-gated potassium channels in human embryonic kidney (HEK293) cells. J. Neurosi. Res. 1998;52:612–617. doi: 10.1002/(SICI)1097-4547(19980601)52:5<612::AID-JNR13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Hileman EO, Plunkett W, Keating MJ, Huang P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood. 2003;101:4098–4104. doi: 10.1182/blood-2002-08-2512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.