Abstract
Hexokinase-II (HKII) is highly expressed in the heart and can bind to the mitochondrial outer membrane. Since cardiac hypertrophy is associated with a substrate switch from fatty acid to glucose, we hypothesized that a reduction in HKII would decrease cardiac hypertrophy after pressure overload. Contrary to our hypothesis, heterozygous HKII-deficient (HKII+/−) mice displayed increased hypertrophy and fibrosis in response to pressure overload. The mechanism behind this phenomenon involves increased levels of reactive oxygen species (ROS), as HKII knockdown increased ROS accumulation, and treatment with the antioxidant N-acetylcysteine (NAC) abrogated the exaggerated response. HKII mitochondrial binding is also important for the hypertrophic effects, as HKII dissociation from the mitochondria resulted in de novo hypertrophy, which was also attenuated by NAC. Further studies showed that the increase in ROS levels in response to HKII knockdown or mitochondrial dissociation is mediated through increased mitochondrial permeability and not by a significant change in antioxidant defenses. Overall, these data suggest that HKII and its mitochondrial binding negatively regulate cardiac hypertrophy by decreasing ROS production via mitochondrial permeability.
Keywords: hexokinase, hypertrophy, mitochondria, mitochondrial permeability transition, reactive oxygen species
INTRODUCTION
Under basal conditions, the majority of cardiac energy derives from the oxidation of lipids; however, cardiac hypertrophy is associated with a shift in primary substrate utilization from fatty acids to glucose (Barger & Kelly, 1999; Sambandam et al, 2002; Taegtmeyer & Overturf, 1988). Glucose metabolism under conditions of pressure overload is mostly mediated through glycolysis, which starts with glucose uptake by glucose transporters (GLUTs) and phosphorylation by hexokinases (HK; Bell et al, 1993; Gould & Holman, 1993; Mueckler, 1994; Printz et al, 1997). Among the four mammalian HK isozymes, HKI and II are the primary isoforms expressed in the heart (Wilson, 2003), and both are shown to exert protective effects against cell death (Ahmad et al, 2002; Bryson et al, 2002; Sun et al, 2008). Hexokinase-II (HKII) contains two 50 kDa subunits, which are both active in the intact enzyme (Ardehali et al, 1999, 1996), and its transcription is regulated by insulin, glucocorticoids and hypoxia (Osawa et al, 1995). In addition to its catalytic subunits, HKII also contains a 21-amino-acid region in its N-terminal half that forms a hydrophobic α-helix and allows binding of the protein to the outer mitochondrial membrane (Aflalo & Azoulay, 1998; Azoulay-Zohar et al, 2004; Fiek et al, 1982; Linden et al, 1982). This binding of HKII to the mitochondria inhibits cell death, while dissociation of endogenous HKII from the mitochondria makes cells more susceptible to an injurious insult (Majewski et al, 2004; Pastorino et al, 2002). Furthermore, overexpression of full length HKII leads to greater protection than the form that lacks the mitochondrial binding domain (Sun et al, 2008). The binding to mitochondria may also allow preferential access of HKII to mitochondrially generated ATP (Pastorino & Hoek, 2003) and reduce cellular production of reactive oxygen species (ROS; da-Silva et al, 2004; Sun et al, 2008). This latter function may also contribute to the protective effects of HKII.
The role of HKII in cardiac glucose metabolism has been studied both in vitro and in vivo. Cardiomyocyte glucose metabolism in vitro is limited by glucose transport at normal insulin levels, but glucose phosphorylation becomes the rate-limiting step under hyperinsulinemic conditions (Henderson et al, 1961; Manchester et al, 1994). Animals with homozygous deficiency of HKII died at about 7.5 days after fertilization, suggesting that HKII is needed for proper embryogenesis. Mice with heterozygous HKII deficiency (HKII+/−) are viable, and there was no major effect on glucose uptake or global metabolism at baseline (Heikkinen et al, 1999). However, the impact of HKII deletion on glucose uptake becomes apparent under conditions of increased glucose demand such as exercise or insulin stimulation (Fueger et al, 2003, 2005). HKII+/− mice display no overt cardiac phenotype at baseline; however, we recently showed that the hearts of HKII+/− mice are more susceptible to ischemia/reperfusion injury after coronary ligation and HKII mitochondrial binding plays an important role in this process (Wu et al, 2011).
Since cardiac hypertrophy is associated with substrate switch from fatty acid to glucose, we hypothesized that a reduction of HKII in the heart leads to a less pronounced hypertrophic response after pressure overload. Contrary to our hypothesis, HKII+/− mice displayed an exaggerated response to transverse aortic constriction (TAC). To determine the mechanism for this unexpected observation, we studied the effects of HKII knockdown on ROS production in response to hypertrophic stimulation. Our results demonstrated that HKII knockdown increased ROS levels both in vitro and in vivo. Treatment with the antioxidant N-acetylcysteine (NAC) attenuated the hypertrophic response. We then studied the role of HKII mitochondrial binding in the hypertrophic response and showed that HKII dissociation from the mitochondria increased cellular hypertrophy, which is prevented by antioxidant treatment. Analysis of antioxidant expression and total antioxidant activity showed no significant changes with HKII knockdown or dissociation compared to control; however, HKII knockdown or mitochondrial dissociation increased mitochondrial permeability transition (MPT). Collectively, these results suggest that the role of HKII in cardiac hypertrophy goes beyond its effects on glucose metabolism, and the hypertrophy associated with a reduction in HKII levels and its mitochondrial binding is mediated through an increase in ROS production.
RESULTS
HKII+/− mice display exaggerated cardiac hypertrophy in response to pressure overload
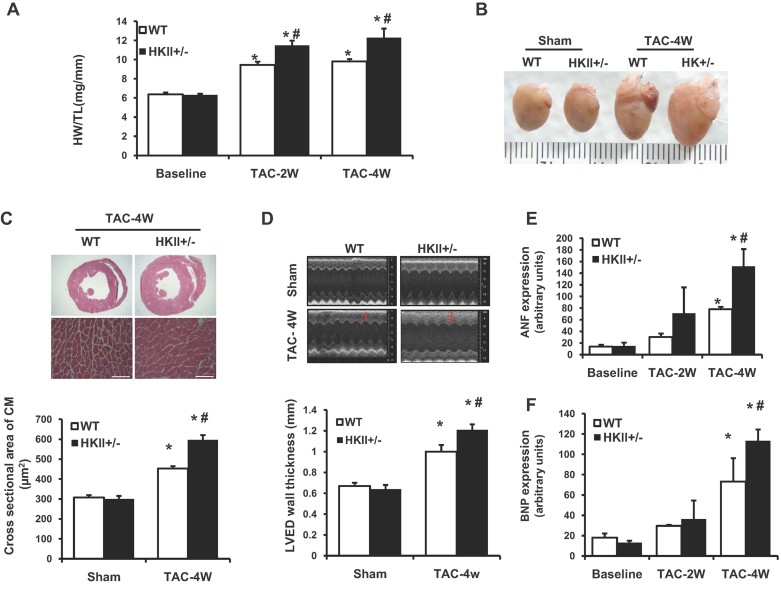
To test the effects of reduced HKII expression on hypertrophy, we subjected HKII+/− mice to pressure overload by TAC. Contrary to our initial hypothesis, HKII+/− mice demonstrated increased myocardial hypertrophy compared with wild-type (WT) mice. Heart weight to tibia length (HW/TL) and HW to body weight (HW/BW) were increased in HKII+/− mice 2 and 4 weeks after TAC (Fig 1A, Supporting Information Fig S1), however the systolic pressure gradients produced by TAC did not differ significantly (Supporting Information Fig S2). There was a gross increase in HKII+/− heart size compared with WT (Fig 1B), and histological analysis revealed an increase in individual cardiomyocyte cell size in HKII+/− mice compared to WT 4 weeks after TAC (Fig 1C, Supporting Information Fig S3). Echocardiography of the heart revealed a larger increase in left ventricular end diastolic (LVED) wall thickness after TAC in HKII+/− hearts (Fig 1D). Finally, the mRNA levels of atrial natriuretic factor (ANF) and B-type natriuretic peptide (BNP) were increased in HKII+/− mice compared with WT 4 weeks after TAC, indicating a stronger induction of foetal gene expression (Fig 1E and F). Overall, these data demonstrated that a reduction in HKII exacerbates the hypertrophy of the heart in response to pressure overload. As metabolic alterations in the hypertrophied heart have been considered as part of the phenotype, we investigated the changes in glycolysis and fatty acid oxidation in isolated cardiomyocyte from either WT or HKII+/− heart and did not observe any significant differences between the groups (Supporting Information Fig S4A and B).
Figure 1. Cardiac hypertrophic response in HKII+/− hearts after TAC.
- HW/TL ratio in WT and HKII+/− mice subjected to TAC for 2 and 4 weeks as indicated, *p < 0.0001 versus WT sham at baseline, #p = 0.02 versus WT + TAC (2W) and #p < 0.0001 versus WT + TAC (4W) and n = 8–12.
- Representative gross images of hearts after sham or TAC operation for 4 weeks from WT and HKII+/− mice.
- Haematoxylin and eosin (H&E) staining of TAC operated WT and HKII+/− mice at 4 weeks. Quantification of myocyte size from cardiac histological sections of WT and HKII+/− heart 4 weeks after TAC is shown on the bottom, *p < 0.0001 versus WT sham, #p < 0.0001 versus WT + TAC and n > 400 cells from three animals in each group.
- Representative M-mode images of WT and HKII+/− hearts after 4 weeks of TAC. LVED septum wall thickness is shown on the bottom, red lines indicate interventricular septal wall thickness, *p < 0.0001 versus WT sham, #p = 0.008 versus WT + TAC and n = 8–10.
- Real-time PCR analysis of ANF levels from WT and HKII+/− hearts 2 and 4 weeks after TAC, *p < 0.001 versus WT sham at baseline, #p = 0.005 versus WT + TAC and n = 5.
- Real-time PCR analysis of BNP levels, *p = 0.010 versus WT sham at baseline, *p < 0.0001 HKII+/− versus WT sham at baseline, #p = 0.042 versus WT + TAC (4W) and n = 5. ANOVA was performed for all experimental conditions and data are presented as mean ± SEM.
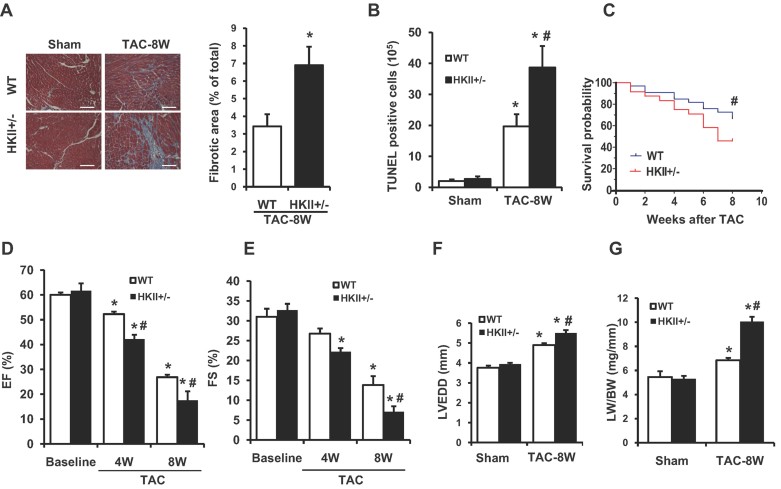
We then assessed the effects of HKII reduction on cardiomyocyte survival and cardiac function up to 8 weeks after TAC. Histological analysis revealed significantly higher levels of fibrosis in the HKII+/− mice compared with WT (Fig 2A). Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) staining showed an increase in cardiomyocyte cell death in HKII+/− mice after TAC (Fig 2B). Furthermore, prolonged exposure to pressure overload led to increased mortality in HKII+/− mice compared to WT, with 46% of these mice surviving up to 8 weeks after TAC compared with 67% in the WT group (Fig 2C). HKII+/− mice that did survive showed a significant decrease in cardiac ejection fraction and fractional shortening compared with WT after TAC (Fig 2D and E). HKII+/− mice also displayed a significant increase in LVED diameter (LVEDD; Fig 2F), and pulmonary oedema, as measured by lung weight over body weight 8 weeks after TAC, indicating the development of congestive heart failure (Fig 2G). Together, these results indicated that reduction of HKII expression exacerbated the maladaptive response to pressure overload and accelerated the transition from pathological hypertrophy to heart failure.
Figure 2. Transition from hypertrophy to heart failure after TAC.
- Histological view of cardiac sections stained with Masson's trichrome in WT and HKII+/− mice after 8 weeks of TAC. Quantification of fibrosis is shown next to the image, *p = 0.035 versus WT and n = 3. Scale bar = 50 µm.
- Quantification of TUNEL staining in the hearts of WT and HKII+/− mice after 8 weeks of TAC, *p < 0.0001 versus WT sham, #p = 0.01 versus WT + TAC and n = 3.
- Kaplan–Meier survival curve of WT and HKII+/− mice subjected to TAC for 8 weeks, #p = 0.032, n = 35 WT, n = 29 HKII+/− and log-rank test.
- EF% (ejection fraction) in WT and HKII+/− after 4 and 8 weeks of TAC as assessed by echocardiography, *p < 0.0001 versus WT sham at baseline, #p = 0.048 versus WT + TAC (4W), #p = 0.008 versus WT + TAC (8W) and n = 8–12.
- FS% (fractional shortening) in WT and HKII+/− after 4 and 8 weeks of TAC as assessed by echocardiography, *p = 0.0002 versus WT sham at baseline (HKII+/− + TAC 4W), *p < 0.0001 versus WT sham at baseline (TAC 8W), #p = 0.004 versus WT + TAC (8W) and n = 8–12.
- LVEDD in the hearts of WT and HKII+/− mice after 8 weeks of TAC, *p < 0.0001 versus WT sham; #p = 0.003 versus WT + TAC and n = 8–10.
- Lung weight to body weight ratio (LW/BW) in WT and HKII+/− subjected to TAC for 8 weeks, *p = 0.016 versus WT sham, *p < 0.0001 HKII+/− versus WT sham, #p < 0.0001 versus WT + TAC and n = 4–6. ANOVA was performed for all experimental conditions unless otherwise indicated, and data presented as mean ± SEM.
HKII knockdown in vitro results in exaggerated cardiomyocyte hypertrophy
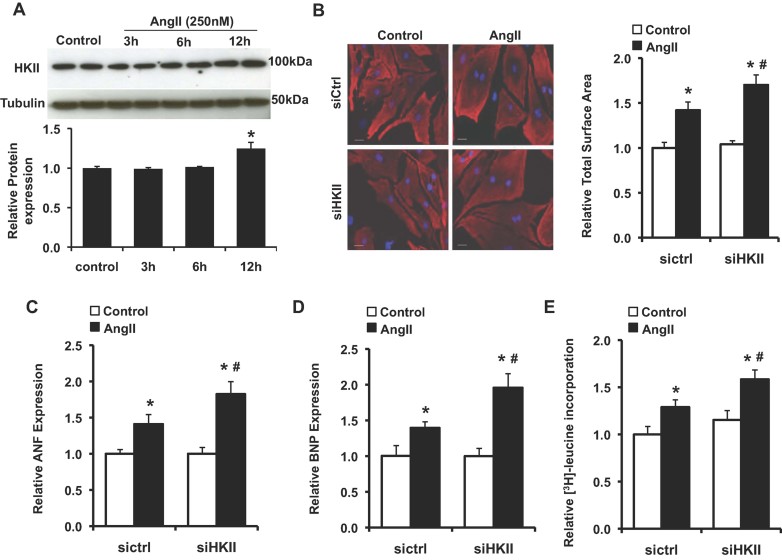
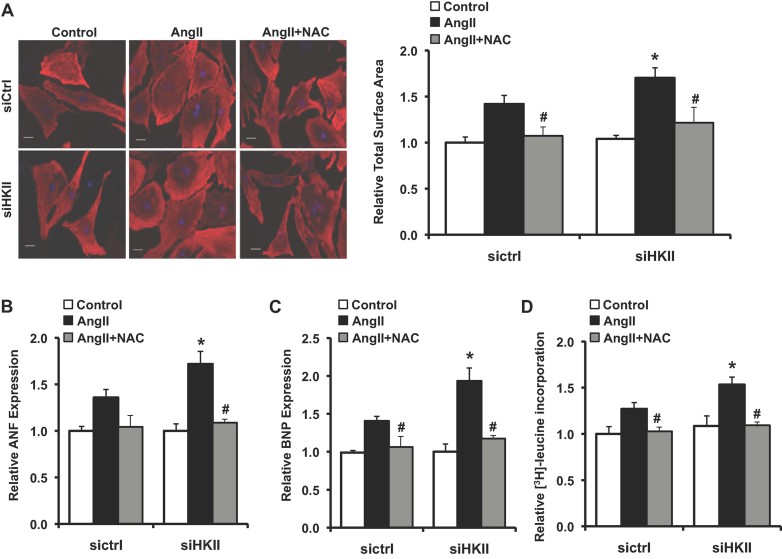
To confirm the results obtained in vivo and to provide a system to study the mechanism for the observed phenomenon, we next performed studies in neonatal rat cardiomyocytes (NRCM) treated with Angiotensin II (AngII) to induce hypertrophy. We observed a significant increase in total HKII expression in response to hypertrophic stimulation at the 12 h time point (Fig 3A). We then examined the effects of HKII reduction on the response to AngII in NRCM using small interfering RNA (siRNA)-mediated knockdown of HKII as previously described (Wu et al, 2011). Treatment with AngII resulted in a significant increase in cell size that was exaggerated with HKII knockdown (Fig 3B). Reduction of HKII also resulted in increased expression of ANF and BNP after AngII treatment (Fig 3C and D). Furthermore, knockdown of HKII increased AngII-stimulated [3H]-leucine incorporation, indicating a significant increase in protein synthesis (Fig 3E). Together, these results provide further evidence that knockdown of HKII exacerbated the hypertrophic response in cardiomyocytes.
Figure 3. Hypertrophic response to AngII in NRCM.
- Western blot of total HKII levels in NRCM treated with AngII (250 nM, 0–12 h), *p = 0.045, n = 3 and two-tailed t-test.
- In (B–E), NRCM were treated with scrambled or HKII siRNA for 36 h prior to media change and treatment with AngII (250 nM, 24 h). Representative images and cell size analysis of cardiomyocytes immunostained with sarcomeric α-actinin (red). Data represent the total cardiomyocyte area relative to untreated siRNA control from at least three-independent samples, a minimum of 75 total cells per group, *p = 0.001 siCtrl + AngII versus siCtrl, *p < 0.0001 siHKII + AngII versus siCtrl, #p = 0.037 versus siCtrl + AngII and two-tailed t-test. DAPI (blue) was used as a nuclear counterstain, and scale bar: 20 µm.
- Real-time PCR analysis of ANF expression ± AngII. Data represent average mRNA levels relative to the untreated control for each group, *p = 0.010 siCtrl + AngII versus siCtrl, *p = 0.001 siHKII + AngII versus siCtrl, #p = 0.049 versus siCtrl + AngII, n = 4–6 and two-tailed t-test.
- Real-time PCR analysis of BNP expression ± AngII. Data represent average mRNA levels relative to the untreated control for each group, *p = 0.016 siCtrl + AngII versus siCtrl, *p = 0.001 siHKII + AngII versus siCtrl, #p = 0.009 versus siCtrl + AngII, n = 4–6 and two-tailed t-test.
- Protein synthesis, evaluated by [3H]-leucine incorporation into total cellular protein normalized to cell number. Data are presented relative to untreated siRNA control, *p = 0.011 siCtrl + AngII versus siCtrl, *p = 0.0002 siHKII + AngII versus siCtrl, #p = 0.014 versus siCtrl + AngII, n = 4–6 and two-tailed t-test. All data are presented as mean ± SEM.
HKII knockdown increases ROS levels during hypertrophy
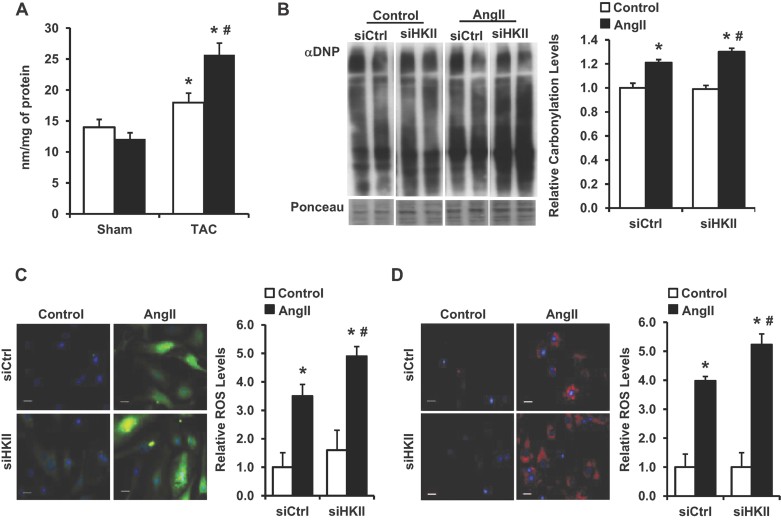
We next investigated the mechanism for the unexpected increase in the hypertrophic response observed after HKII reduction. Increased ROS activates the hypertrophic signalling cascade (Sabri et al, 2003; Seddon et al, 2007; Sugden & Clerk, 2006; Takimoto & Kass, 2007), and overexpression of HKII has been shown to reduce cellular ROS levels (Sun et al, 2008). Therefore, we focused our studies on ROS as a potential mechanism for the increased hypertrophy observed with HKII knockdown. Examination of left ventricular tissue from WT and HKII+/− mice 2 days after TAC revealed increased levels of lipid peroxidation (an indicator of ROS damage) in the HKII+/− hearts (Fig 4A). In NRCM, knockdown of HKII resulted in significantly higher levels of protein carbonylation after treatment with AngII (Fig 4B). Furthermore, 2′,7′-dichlorodihydrofluorescein (DCF) fluorescence increased significantly after treatment with AngII that was further increased by knockdown of HKII (Fig 4C). Finally, we used the MitoSox probe to assess mitochondrial superoxide production. Treatment of NRCM with HKII or scrambled siRNA resulted in no difference in MitoSox fluorescence at baseline, but there was increased generation of superoxide after AngII treatment in the setting of HKII knockdown (Fig 4D). These results suggest that HKII knockdown leads to increased ROS levels after hypertrophic stimulation.
Figure 4. ROS accumulation in response to HKII knockdown.
- Analysis of malondialdehyde (MDA) levels in left ventricular tissue from HKII+/− mice compared to WT 2 days after TAC, *p = 0.049 versus WT sham, *p = 0.001 HKII versus WT sham, #p = 0.009 versus WT + TAC and n = 3–5.
- ROS levels in NRCM, evaluated by Western blot of derivitized protein carbonyls (DNP) in lysates from NRCM treated with HKII or control siRNA ± AngII (250 nM, 24 h). All represented lanes were run non-contiguous on the same gel. Data are presented relative to untreated siRNA control, *p = 0.013 siCtrl + AngII versus siCtrl, *p = 0.002 siHKII + AngII versus siCtrl, #p = 0.046 versus siCtrl + AngII, n = 3 and two-tailed t-test.
- ROS levels in NRCM evaluated with DCF (green). Fluorescent intensity was measured in individual fields from 3 to 6 independent samples and presented relative to control, *p = 0.014 siCtrl + AngII versus siCtrl, *p = 0.0006 siHKII + AngII versus siCtrl, #p = 0.034 versus siCtrl + AngII and two-tailed t-test.
- Mitochondrial superoxide evaluated with MitoSox (red). Fluorescent intensity was averaged from 3 to 6 independent samples (three fields analysed per sample), *p = 0.0004 siCtrl + AngII versus siCtrl, *p = 0.0002 siHKII + AngII versus siCtrl, #p = 0.006 versus siCtrl + AngII and two-tailed t-test. In (C) and (D), nuclei are labelled with Hoechst 33342 (blue), scale bars: 20 µM. All data are presented as mean ± SEM.
We then studied whether the increase in ROS mediates the exaggerated hypertrophic response observed with HKII knockdown. Treatment with NAC significantly reduced ROS accumulation after treatment with AngII and attenuated the exaggerated increase observed with HKII knockdown (Supporting Information Fig S5A and B). Addition of NAC abrogated the AngII-induced cell size increase in NRCM and reduced the exaggerated response observed with knockdown of HKII (Fig 5A). NAC treatment also reduced the expression of ANF and BNP as well as the induction of protein synthesis in response to AngII and brought the levels with HKII knockdown to those of cells treated with control siRNA (Fig 5B–D). Together, these data suggest that increased ROS accumulation contributes to the exaggerated hypertrophic response observed with knockdown of HKII.
Figure 5. Hypertrophic response after treatment with the antioxidant NAC.
- In (A–D), NRCM were treated with scrambled or HKII siRNA, and exposed to AngII (250 nM, 24 h). NAC (1 mM) was added to the media 2 h after the addition of AngII where indicated. Representative images and cell size analysis of cardiomyocytes immunostained with sarcomeric α-actinin (red). Data represent the total cardiomyocyte area relative to untreated siRNA control from at least three-independent samples, a minimum of 75 total cells per group, *p = 0.037 versus siCtrl + AngII, #p = 0.010 versus siCtrl + AngII, #p = 0.014 versus siHKII + AngII and two-tailed t-test. DAPI (blue) was used as a nuclear counterstain and scale bar: 20 µm.
- Real-time PCR analysis of ANF levels. Data represent average mRNA levels for each marker relative to the untreated siRNA control for each group, *p = 0.015 versus siCtrl + AngII, #p = 0.004 versus siHKII + AngII, n = 3–6 and two-tailed t-test.
- Real-time PCR analysis of BNP levels. Data represent average mRNA levels for each marker relative to the untreated siRNA control for each group, *p = 0.016 versus siCtrl + AngII, #p = 0.035 versus siCtrl + AngII, #p = 0.020 versus siHKII + AngII, n = 3–6 and two-tailed t-test.
- Protein synthesis evaluated by [3H]-leucine incorporation into total cellular protein normalized to cell number. [3H]-leucine (1 µCi/ml) was added to cells to cells 1 h after treatment with NAC. Data are presented relative to untreated siRNA control, *p = 0.049 versus siCtrl + AngII, #p = 0.015 versus siCtrl + AngII, #p = 0.001 versus siHKII + AngII, n = 4–6 and two-tailed t-test. All data are presented as mean ± SEM.
Dissociation of HKII from the mitochondrial induces cardiomyocyte hypertrophy
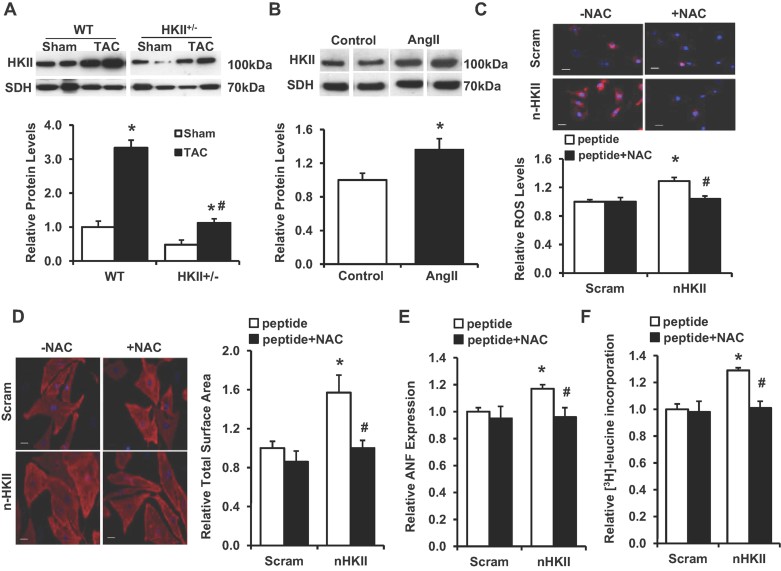
HKII binding to the mitochondrial outer membrane reduces cellular ROS production and protects against cell death in cardiomyocytes (Sun et al, 2008; Miyamoto et al, 2008; Wu et al, 2011). The levels of HKII bound to the mitochondria significantly increased after hypertrophic stimulation in both our in vivo and in vitro models (Fig 6A and B). In order to study the role of HKII mitochondrial binding in the development of hypertrophy, we used a synthetic cell permeable peptide (n-HKII) against the N-terminal hydrophobic domain of HKII to dissociate the enzyme from the outer membrane of the mitochondria (Majewski et al, 2004; Pastorino et al, 2002). Previous studies showed that treatment of NRCM with the n-HKII peptide resulted in a dose-dependent increase in cell death compared to a scrambled peptide control after 2 h; however, at low dose (5 µM) we observed mitochondrial dissociation without impacting cell viability (Wu et al, 2011). Treatment of NRCM with this dose resulted in increased ROS production compared to a scrambled peptide control after 3 h (Fig 6C). Also observed were significant increases in cell size, ANF levels and protein synthesis, all of which were attenuated by NAC administration (Fig 6C–F). Thus, dissociation of HKII from the mitochondria alone is sufficient to induce hypertrophy, and this effect is partially mediated by increased ROS levels.
Figure 6. Hypertrophic response to HKII dissociation from the mitochondria.
- Western blot demonstrating HKII levels in mitochondrial fractions from WT and HKII+/− hearts 4 weeks after TAC, *p < 0.0001 versus sham, #p < 0.0001 versus HKII+/− + TAC, n = 4–6 and determined by ANOVA. Mitochondrial inner membrane protein succinate dehydrogenase (SDH) was used as a loading control.
- Western blot of HKII levels in mitochondrial fractions from NRCM treated with AngII (200 nM, 6 h). Represented lanes for each Western blot were run non-contiguous on the same gel. Data are presented relative to untreated control, *p = 0.049, n = 3 and two-tailed t-test.
- Mitochondrial superoxide levels evaluated with MitoSox (red) after HKII dissociation from the mitochondria in NRCM using a competitive peptide (n-HKII) (5 µM, 3 h). A scrambled peptide (scram) was used as control. Cells were pretreated with NAC (500 µM) where indicated. Fluorescent intensity was measured from at least three-independent experiments, *p = 0.011 versus scram, #p = 0.019 versus n-HKII and two-tailed t-test.
- In (D–F), NRCM were treated with n-HKII or scrambled peptide (5 µM, 24 h), and pretreated with NAC (500 µM) where indicated. Representative images and cell size analysis of cardiomyocytes. Data represent the total cardiomyocyte area relative to scrambled peptide control from at least three-independent samples, a minimum of 75 total cells per group, *p = 0.001 versus scram and #p = 0.025 versus n-HKII. DAPI (blue) was used as a nuclear counterstain and scale bar: 20 µm.
- Real-time PCR analysis of ANF expression. Data are presented relative to scrambled control, *p = 0.004 versus scram, #p = 0.041 versus n-HKII, n = 4–6 and two-tailed t-test.
- Protein synthesis, evaluated by [3H]-leucine incorporation and normalized to cell number. Data are presented relative to scrambled control, *p = 0.005 versus scram, #p = 0.007 versus n-HKII, n = 3 and two-tailed t-test. All data are presented as mean ± SEM.
HKII knockdown or mitochondrial dissociation increased ROS production
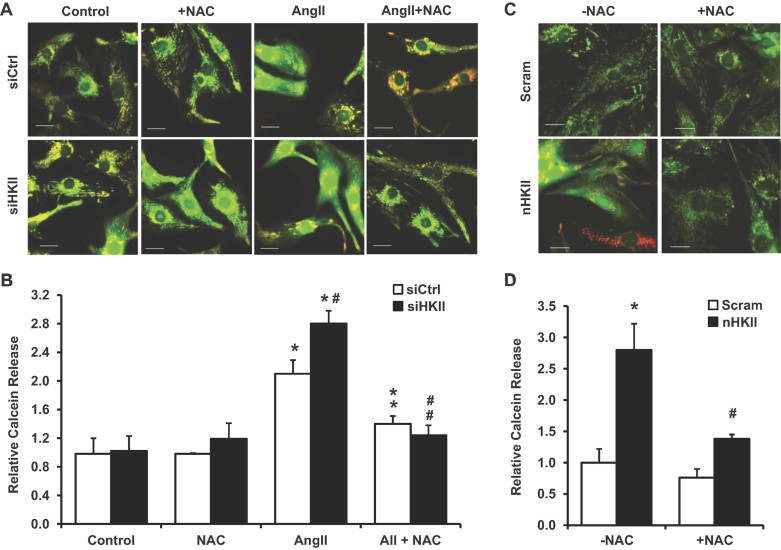
Our results thus far suggest that a reduction in HKII or its dissociation from the mitochondria induce cardiac hypertrophy through ROS accumulation, which results from an imbalance between ROS production and antioxidant activity (Giordano, 2005). We first examined a potential mechanism by which HKII regulates ROS production. It has been suggested that HKII plays a regulatory role in MPT, possibly through its binding to voltage-dependent anion channel (VDAC) on the mitochondrial outer membrane (Halestrap et al, 2002; Mathupala et al, 2006). Increased ROS, secondary to MPT, has been described in adult cardiomyocytes, and studies suggest that pharmacological inhibition of MPT can mitigate the hypertrophic response in NRCM (Halestrap, 2010; Javadov & Karmazyn, 2007; Zorov et al, 2000). We studied the effects of HKII knockdown and dissociation on MPT by measuring mitochondrial calcein release (Javadov et al, 2008; Petronilli et al, 1999). Treatment with AngII led to an increase in MPT that was exacerbated by HKII knockdown (Fig 7A and B). Peptide dissociation of HKII from the mitochondria also resulted in increased MPT (Fig 7C and D). NAC attenuated the MPT observed with AngII or peptide dissociation (Fig 7A–D), which was also observed after treatment with cyclosporine A (CsA, an inhibitor of cyclophilin D and MPT; Supporting Information Fig S6A and B). We did not observe a significant increase in cell death as a result of HKII knockdown under these conditions (Supporting Information Fig S7A); however, a modest increase in cell death was observed after HKII dissociation at the 24 h time point (Supporting Information Fig S7B). Overall, these studies suggest that the increased ROS levels leading to the exaggerated hypertrophic response with HKII knockdown and mitochondrial dissociation may be mediated by increased MPT.
Figure 7. Assessment of MPT after HKII knockdown or mitochondrial dissociation.
- Representative images of calcein distribution (green) after knockdown and treatment with AngII (250 nM, 2 h). NAC (500 µM) was added after 20 min where indicated. Mitochondrial are labeled with Mitotracker Red; scale bar: 20 µM.
- Quantification of cells demonstrating calcein release from the mitochondria after knockdown and treatment with AngII. A minimum of 150 total cells in each group were analysed from at least three-independent experiments. Data represent the percentage of cells with released calcein relative to untreated siRNA control, *p = 0.016 siCtrl + AngII versus siCtrl, *p = 0.002 siHKII + AII versus siCtrl, #p = 0.041 versus siCtrl + AngII, **p = 0.016 versus siCtrl + AngII and ##p =.001 versus siHKII + AngII.
- Representative images of calcein distribution after treatment with the n-HKII dissociation peptide or the scrambled (scram) control (5 µM, 2 h). Cells were pretreated with NAC (10 min, 500 µM) where indicated. Mitochondrial are labeled with Mitotracker Red; scale bar: 20 µM.
- Quantification of cells demonstrating calcein release from the mitochondria after peptide treatment. A minimum of 145 total cells in each group were analysed from at least three-independent experiments. Data represent the percentage of cells with released calcein relative to scrambled control, *p = 0.005 versus scram and #p = 0.008 versus n-HKII. All data are presented as mean ± SEM.
We then examined the possible effects of HKII reduction on antioxidant expression and activity. Analysis of total lysates from heart tissue demonstrated no significant difference between WT and HKII+/− mice after sham operation or TAC (Supporting Information Fig S8A). A decrease in antioxidant expression was observed in NRCM after AngII treatment; however, there were no significant differences observed with HKII knockdown (Supporting Information Fig S8B). Further in vitro analysis revealed that there was no significant difference in total antioxidant activity with HKII knockdown or dissociation (Supporting Information Fig S9A and B). Knockdown of HKII and treatment with AngII did result in a modest decrease in the relative ratio of NADPH to total NAD(P)H, as well as an increase in the relative ratio of oxidized glutathione (GSSG) to GSH (Supporting Information Fig 10A and B). Overall, the data implicate increased ROS production in the increased ROS levels observed after HKII knockdown or dissociation.
DISCUSSION
Cardiac hypertrophy is associated with a number of cellular changes, including a metabolic switch from fatty acid to glucose metabolism as the primary source of energy. In this paper, we hypothesized that a reduction in HKII would result in a decrease in cardiac hypertrophy and subjected HKII+/− mice to pressure overload, followed by assessment of cardiac size and function. Contrary to our hypothesis, HKII+/− mice displayed more hypertrophy in response to pressure overload, which was associated with more prominent cardiac dysfunction and mortality. Similar results were obtained in vitro, when HKII downregulation by siRNA was associated with an increase in cardiomyocyte hypertrophy in response to AngII treatment. We then studied the mechanism for this phenomenon and focused our studies on ROS accumulation. Previous studies had shown that HKII overexpression and mitochondrial binding reduces ROS levels (da-Silva et al, 2004; Sun et al, 2008), and ROS has been linked to cardiac hypertrophy (Sabri et al, 2003; Seddon et al, 2007; Sugden & Clerk, 2006; Takimoto & Kass, 2007). HKII knockdown both in vitro and in vivo was associated with increased ROS accumulation, and antioxidant treatment reduced hypertrophy associated with HKII knockdown. In addition, dissociation of HKII from the mitochondria led to cardiomyocyte hypertrophy that was also reversed with NAC. We then studied the mechanism for increased ROS levels. Our results demonstrated that HKII knockdown or mitochondrial dissociation resulted in an increase in MPT. Collectively, our results demonstrated that HKII knockdown or its dissociation from the mitochondria increases hypertrophy, at least in part, through increased ROS production and MPT.
Although our results were unexpected, similar results have been obtained with GLUT4 knockout mice. Abel et al. showed that cardiac specific knockout of GLUT4 results in cardiac hypertrophy with preserved cardiac function and no evidence of myocyte disarray and cardiac fibrosis (Abel et al, 1999). These changes occurred at baseline and in the absence of pressure overload, and homozygous knockout animals were studied, unlike the heterozygous HKII mice examined in our study. Nevertheless, these results along with those observed in this study suggest that the process of glucose transport and phosphorylation are needed for normal cardiac size, and a reduction in these steps leads to either cardiac hypertrophy at baseline (in case of GLUT4 knockout) or in response to pressure overload (in case of HKII knockout). Furthermore, the development of cardiac hypertrophy is not dependent on an intact metabolic process of glucose, and in fact, a reduction in this process leads to more exaggerated hypertrophy. We observed that fatty acid and glucose metabolism response to TAC did not significantly differ between WT and HKII+/− mice, suggesting that cardiac metabolic changes may not be the major mechanism that leads to exaggerated hypertrophy in HKII+/− in response to TAC.
Although our results do not exclude other mechanisms for the development of cardiac hypertrophy after HKII knockdown, ROS appears to play a major role in this process. ROS has been established as an inducer of cardiac hypertrophy in various models (Sabri et al, 2003; Seddon et al, 2007; Sugden & Clerk, 2006; Takimoto & Kass, 2007). In addition, a previous study suggested that administration of an antioxidant reverses cardiac hypertrophy in GLUT4-deficient mice (Ritchie et al, 2007). Thus, we propose that a reduction in HKII levels leads to cardiac hypertrophy not through changes in cardiac metabolism, but through oxidative stress on the heart. This increase in ROS by a reduction in HKII does not cause cell death, but induces an increase in cardiomyocyte size.
The mitochondrial binding of HKII has been shown to play an important role in cellular response to injurious agents (Majewski et al, 2004; Pastorino et al, 2002; Sun et al, 2008), production of ROS (da-Silva et al, 2004; Sun et al, 2008) and energy production (Pastorino & Hoek, 2003). Here, we showed that dissociation of HKII from the mitochondria also results in increased cardiomyocyte hypertrophy, and this effect is reversed by the addition of the antioxidant NAC. Thus, the binding of HKII to the mitochondria plays an important role in cardiac hypertrophy by mediating ROS levels. A great deal of work has been devoted to devising new methods to dislocate HKII from the mitochondria in oncology research in order to kill the rapidly dividing cancer cells (Pedersen, 2007). Since HKII mitochondrial binding is important in protection against I/R (Wu et al, 2011) and the development of hypertrophy, an approach that increases the binding of HKII to the mitochondria would be more applicable to these cardiovascular disorders.
The product of the enzymatic reaction catalysed by HKII, glucose-6-phosphate (G6P), is a substrate for the pentose phosphate pathway (PPP) in addition to its contribution to the glycolytic and glycogenesis pathways. G6P metabolism via the PPP generates reducing equivalents in the form of NADPH that are necessary for glutathione peroxidase (Gpx) activity and production of reduced glutathione. These results might suggest a reduction in G6P metabolism via the PPP that are necessary for the production of reduced glutathione. We did not thoroughly evaluate the PPP in ROS generation with HKII reduction, and cannot rule out a role for this pathway in this process.
During hypertrophy, cardiomyocytes encounter conditions that are known to illicit MPT, including oxidative stress and increased mitochondrial Ca2+ uptake (Matas et al, 2009). While MPT is typically associated with cell death due to irreversible opening of the MPT pore (mPTP), studies have shown that the pore can operate at a lower conductance level, which is transient and reversible (Di Lisa et al, 2003; Halestrap et al, 2007; Loor et al, 2011; Petronilli et al, 1999; Robin et al, 2007). Repeated and reversible MPT induction is known to trigger ROS induced ROS release; creating a positive feedback loop that can amplify ROS accumulation and cause mitochondrial dysfunction in the absence of immediate cell death (Zorov et al, 2000). MPT, associated with increased cell death, is readily observed in pathological hypertrophy during the transition to heart failure (Marcil et al, 2006; Ozcan et al, 2003; Sharov et al, 2007); however, recent studies have suggested that increased transient MPT may also play a causal role in the development of hypertrophy (Javadov et al, 2006; Matas et al, 2009; Nakayama et al, 2007).
In summary, our results show that a reduction in HKII leads to an exaggerated hypertrophic response to pressure overload. The increase in hypertrophy appears to be through an increase in ROS, possibly through the induction of MPT. Furthermore, dislocation of HKII from the mitochondria results in an increase in cardiomyocyte size due to elevated ROS production. Together our data indicate that methods that would increase HKII levels or its binding to the mitochondria may provide a novel approach for treatment of cardiac hypertrophy.
MATERIALS AND METHODS
Animal models
All animals were maintained and handled in accordance with the Northwestern Animal Care and Use Committee. Adult male HKII+/− mice (22–25 g) previously described (Fueger et al, 2003, 2007; Wu et al, 2011), were used in this study. HKII+/+ (WT) littermates were used as control. Chronic pressure overload was induced by TAC in mice at 10–12 weeks at the beginning of the study as described (Rockman et al, 1991). Details for screening of knockout mice, cardiac function analysis and histology can be found in the Supporting Information Materials and Methods Section.
Primary cell culture
NRCMs were isolated from 1- to 2-day-old Sprague–Dawley rats as previously described (Ardehali et al, 2005). Cardiomyocytes were cultured in DMEM/M199 (3:1), supplemented with 5% FBS, 1.5 mM vitamin B12 and 1 mM penicillin–streptomycin (Gibco). To prevent proliferation of non-myocytes, 100 µM bromodeoxyuridine (BrdU) was added to the culture media (Sigma). To induce hypertrophy, cells were cultured under serum free conditions in unsupplemented media for 12 h and then treated with AngII (250 nM; Sigma) for 24 h. For the antioxidant studies, 1 mM of N-acetyl-l-cysteine (NAC; Sigma) was added to the culture 2 h after the addition of AngII.
HKII peptide treatment
A peptide analogous to the N-terminal sequence of HKII (n-HKII: MIASHLLAYFFTELNHDQVQKVD), along with a scrambled peptide (Scram: VLIQKEVTDNLAFYMSHADHQLF) were synthesized by Genemed Synthesis, Inc., San Antonio, TX. To facilitate cell permeability, each peptide included a polyarginine sequence at the C-terminal. Mitochondrial dissociation was confirmed via Western blot of fractionated NRCM lysates (Wu et al, 2011). Peptides were added to NRCM cultured in serum free media, as indicated. For the antioxidant studies, 500 µM NAC was added to NRCM 20 min prior to the addition of peptide.
The paper explained
PROBLEM:
Glucose metabolism starts with glucose uptake and subsequent glucose phosphorylation by hexokinases (HK). Among the four mammalian HK isozymes, HKII is highly expressed in the heart and exerts protective effects against cell death. HKII also binds to the mitochondrial outer membrane through its N-terminal hydrophobic sequence, and this mitochondrial binding contributes to the cytoprotective effects of the protein. Since cardiac hypertrophy is associated with a substrate switch from fatty acid to glucose, we investigated how a reduction in HKII would alter cardiac hypertrophy after pressure overload. Our original hypothesis was that a reduction in HKII, as a major enzyme in glucose metabolism, would reduce cardiac hypertrophy in response to transverse aortic constriction (TAC).
RESULTS:
Here, we report that, contrary to our hypothesis, a reduction in HKII protein was associated with an increase in hypertrophy. Heterozygous HKII-deficient (HKII+/−) mice displayed increased hypertrophy and heart failure in response to TAC. The mechanism behind this phenomenon is increased production of ROS, as HKII knockdown increased ROS production, and the antioxidant N-acetylcysteine (NAC) attenuated this response. HKII mitochondrial binding is also important for the hypertrophic effect, as HKII dissociation from the mitochondria resulted in de novo hypertrophy, which was also attenuated by NAC. ROS production in response to HKII knockdown or mitochondrial dissociation was associated with increased mitochondrial permeability, which is attenuated by NAC.
IMPACT:
Overall, our data suggest that a reduction in HKII leads to an exaggerated hypertrophic response to pressure overload. The increase in hypertrophy is mediated through an increase in ROS, possibly through the induction of mitochondrial permeability transition (MPT). Furthermore, dissociation of HKII from the mitochondria results in an increase in cardiomyocyte size due to elevated ROS production. Thus, methods that would increase HKII levels or its binding to the mitochondria may provide a novel approach for treatment of cardiac hypertrophy.
RNA isolation and quantitative real-time PCR
Details for RNA isolation and amplification, including the primer sequences can be found in the Supporting Information Materials and Methods Section.
Isolation of mitochondria
Details on mitochondrial isolation and Western blot for the assessment of protein levels can be found in the Supporting Information Materials and Methods Section.
Cell size analysis
For cell size analysis, NRCM were cultured on glass coverslips treated with 0.1% gelatin. After the indicated treatment, cells were washed once with PBS and fixed in ice cold methanol for 10 min and then blocked with 1% goat serum for 30 min at room temperature. Cardiomyocytes were labelled with sarcomeric α-actinin (Sigma) followed by incubation with goat anti-mouse Cy3-conjugated secondary (Jackson Immunology Research). DAPI was used as a nuclear counterstain, and images were captured with the Zeiss AxioObserver.Z1 (510 nm-ex/580 nm-em). Total cell surface area was analysed using the ZeissAxio Imaging software. For each experimental conditions cell size was averaged from a minimum of five separate fields from at least three-independent samples. A minimum of 75 total cells were counted for each experimental condition examined.
Protein synthesis
To determine protein synthesis, [3H]-leucine (1 µCi/ml; Perkin-Elmer) was added to all treatment conditions 3 h after the addition of AngII. After 24 h, cardiomyocytes were washed with PBS, trypsinized and collected with ice cold PBS. Proteins were precipitated on ice with trichloroacetic acid (20%), washed with acetone and radioactivity was determined by liquid scintillation counting. Prior to protein isolation, an aliquot of cells was removed for counting, and radioactive levels were normalized to total cell number for each sample. For the peptide studies, [3H]-leucine was added to the media 3 h after treatment with the peptide began. After 24 h, lysates were isolated and analysed.
Evaluation of oxidative stress
Oxidative stress in mouse hearts was determined through measurement of lipid peroxides using the MDA kit from Oxford Biomedical Research according to manufacturers' instructions. Oxidative stress in neonatal cardiomyocytes was determined by levels of protein carbonylation using a modification of the OxyBlot Protein Oxidation Detection (Millipore). Briefly, protein carbonyls from lysates were derivitized to 2,4-dinitrophenylhydrazone (DNP) without the addition of reducing agents. After derivitization, samples were neutralized, run on an SDS–PAGE gel and transferred to nitrocellulose membrane. Membranes were exposed to Ponceau-Red (Sigma) to determine protein loading for each lane, washed and then analysed for DNP levels according to manufacturers' instructions. Band intensity for each lane was measured using Image J software (NIH) and normalized to the Ponceau loading control.
Measurement of ROS
Intracellular ROS levels were analysed in NRCM plated on glass coverslips as described above. ROS levels were determined by image analysis using two-different fluorescent indicators in separate experiments. First, after the indicated treatment, cells were washed and loaded with 1 µM 2′,7′-dichlorofluorescein diacetate (CM-H2DCFDA; Molecular Probes) in Hank's Buffered Salt Solution (HBSS, Gibco). After 15 min, media was removed and replaced with HBSS containing the 0.5 µM Hoechst 33342 nuclear counterstain (Invitrogen). Cells were again washed and incubated in HBSS incubated for an additional 20 min before imaging. Fluorescence images were acquired with the Zeiss AxioObserver.Z1 (488 nm-ex/510 nm-em). To assess mitochondrial superoxide production, NRCM were loaded with 0.5 µM MitoSox (Molecular Probes) using the same protocol. Fluorescence images were acquired with the Zeiss AxioObserver.Z1 (510 nm-ex/580 nm-em). The average fluorescent intensity was analysed for each sample using Image J (NIH).
Evaluation of MPT
Induction of MPT in NRCM was determined using a calcein release assay previously described, with modifications (Javadov et al, 2006; Petronilli et al, 1999). For MPT analysis, NRCM were cultured on glass bottom culture dishes (MatTek) treated with 0.1% gelatin. Cardiomyocytes were incubated under ambient conditions for 5 min in with 10 nM of MitoTracker Red (Life Technologies) in HBSS (Sigma H1387) supplemented with HEPES (10 mM), pH 7.3. Cells were then washed twice, and incubated in HBSS for 20 min with 1 µM of calcein–acetoxymethylester (calcein–AM; Molecular Probes) along with 2 mM of cobalt chloride to quench cytosolic and nuclear calcein loading. After loading, cells were washed three times, and HBSS was replaced prior to the start of the assay. Eppifluorescent images of Mitotracker Red and calcein fluorescence were acquired with the ZeissAxioObserver.Z1 (40× magnification; 75 NA) using 575/599 and 488/515 excitation/emission, respectively. MPT was determined by the number of cells demonstrating calcein redistribution from the mitochondria to the cytosol at the end of the indicated treatment. In order to minimize phototoxicity, cells were imaged at a single time point. For the knockdown experiments calcein release was evaluated 2 h after the addition of AngII (250 nM). NAC (500 µM) was added after 20 min where indicated. For the HKII dissociation studies, calcein release was evaluated 2 h after the addition of the n-HKII or scrambled peptide control (5 µM). NAC (500 µM) was added 10 min prior to peptide treatment where indicated. CsA (0.2 µM) was added 10 min prior to treatment where indicated. Images for each experimental condition were acquired from a minimum of five separate fields from at least three-independent experiments. As calcein release and decreased calcein fluorescence were observed over time at baseline, the results were normalized to untreated siRNA or scrambled controls.
Statistical analysis
Data are expressed as mean ± SEM. Statistical significance was assessed with ANOVA, the unpaired Student's t-test and log-rank test.
Acknowledgments
HA was supported by NIH grant R01 HL087149. RW and EW are supported by the American Heart Association. We would like to thank Dr. Sami Heikkinen, University of Eastern Finland, for generating the HKII+/− mice. We also would like to thank Dr Gary Fang, Northwestern University, for assisting with adult cardiomyocyte isolation.
Supporting Information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author contributions
RW, EW and HA designed and performed the research; RW performed the animal surgeries and in vivo data acquisition and analysis; EW performed the in vitro data acquisition and analysis; KC assisted with tissue preparation, cell quantification and data analysis; MG performed the in vivo ROS analysis and assisted with cell quantification; MT and CLE performed the adult cardiomyocyte isolation; ML provided reagents and expertise; HA, EW and RW wrote the manuscript, which all authors commented on; HA supervised the project.
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest. 1999;104:1703–1714. doi: 10.1172/JCI7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aflalo C, Azoulay H. Binding of rat brain hexokinase to recombinant yeast mitochondria: effect of environmental factors and the source of porin. J Bioenerg Biomembr. 1998;30:245–255. doi: 10.1023/a:1020544803475. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Ahmad S, Schneider BK, Allen CB, Chang LY, White CW. Elevated expression of hexokinase II protects human lung epithelial-like A549 cells against oxidative injury. Am J Physiol Lung Cell Mol Physiol. 2002;283:L573–L584. doi: 10.1152/ajplung.00410.2001. [DOI] [PubMed] [Google Scholar]
- Ardehali H, Yano Y, Printz RL, Koch S, Whitesell RR, May JM, Granner DK. Functional organization of mammalian hexokinase II. Retention of catalytic and regulatory functions in both the NH2- and COOH-terminal halves. J Biol Chem. 1996;271:1849–1852. doi: 10.1074/jbc.271.4.1849. [DOI] [PubMed] [Google Scholar]
- Ardehali H, Printz RL, Whitesell RR, May JM, Granner DK. Functional interaction between the N- and C-terminal halves of human hexokinase II. J Biol Chem. 1999;274:15986–15989. doi: 10.1074/jbc.274.23.15986. [DOI] [PubMed] [Google Scholar]
- Ardehali H, O'Rourke B, Marban E. Cardioprotective role of the mitochondrial ATP-binding cassette protein 1. Circ Res. 2005;97:740–742. doi: 10.1161/01.RES.0000186277.12336.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. In self-defence: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem J. 2004;377:347–355. doi: 10.1042/BJ20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger PM, Kelly DP. Fatty acid utilization in the hypertrophied and failing heart: molecular regulatory mechanisms. Am J Med Sci. 1999;318:36–42. doi: 10.1097/00000441-199907000-00006. [DOI] [PubMed] [Google Scholar]
- Bell GI, Burant CF, Takeda J, Gould GW. Structure and function of mammalian facilitative sugar transporters. J Biol Chem. 1993;268:19161–19164. [PubMed] [Google Scholar]
- Bryson JM, Coy PE, Gottlob K, Hay N, Robey RB. Increased hexokinase activity, of either ectopic or endogenous origin, protects renal epithelial cells against acute oxidant-induced cell death. J Biol Chem. 2002;277:11392–11400. doi: 10.1074/jbc.M110927200. [DOI] [PubMed] [Google Scholar]
- da-Silva WS, Gomez-Puyou A, de Gomez-Puyou MT, Moreno-Sanchez R, De Felice FG, de Meis L, Oliveira MF, Galina A. Mitochondrial bound hexokinase activity as a preventive antioxidant defense: steady-state ADP formation as a regulatory mechanism of membrane potential and reactive oxygen species generation in mitochondria. J Biol Chem. 2004;279:39846–39855. doi: 10.1074/jbc.M403835200. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Canton M, Menabo R, Dodoni G, Bernardi P. Mitochondria and reperfusion injury. The role of permeability transition. Basic Res Cardiol. 2003;98:235–241. doi: 10.1007/s00395-003-0415-x. [DOI] [PubMed] [Google Scholar]
- Fiek C, Benz R, Roos N, Brdiczka D. Evidence for identity between the hexokinase-binding protein and the mitochondrial porin in the outer membrane of rat liver mitochondria. Biochim Biophys Acta. 1982;688:429–440. doi: 10.1016/0005-2736(82)90354-6. [DOI] [PubMed] [Google Scholar]
- Fueger PT, Heikkinen S, Bracy DP, Malabanan CM, Pencek RR, Laakso M, Wasserman DH. Hexokinase II partial knockout impairs exercise-stimulated glucose uptake in oxidative muscles of mice. Am J Physiol Endocrinol Metab. 2003;285:E958–963. doi: 10.1152/ajpendo.00190.2003. [DOI] [PubMed] [Google Scholar]
- Fueger PT, Shearer J, Krueger TM, Posey KA, Bracy DP, Heikkinen S, Laakso M, Rottman JN, Wasserman DH. Hexokinase II protein content is a determinant of exercise endurance capacity in the mouse. J Physiol. 2005;566:533–541. doi: 10.1113/jphysiol.2005.085043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fueger PT, Lee-Young RS, Shearer J, Bracy DP, Heikkinen S, Laakso M, Rottman JN, Wasserman DH. Phosphorylation barriers to skeletal and cardiac muscle glucose uptakes in high-fat fed mice: studies in mice with a 50% reduction of hexokinase II. Diabetes. 2007;56:2476–2484. doi: 10.2337/db07-0532. [DOI] [PubMed] [Google Scholar]
- Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GW, Holman GD. The glucose transporter family: structure, function and tissue-specific expression. Biochem J. 1993;295:329–341. doi: 10.1042/bj2950329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans. 2010;38:841–860. doi: 10.1042/BST0380841. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie. 2002;84:153–166. doi: 10.1016/s0300-9084(02)01375-5. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Clarke SJ, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta. 2007;1767:1007–1031. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen S, Pietila M, Halmekyto M, Suppola S, Pirinen E, Deeb SS, Janne J, Laakso M. Hexokinase II-deficient mice. Prenatal death of homozygotes without disturbances in glucose tolerance in heterozygotes. J Biol Chem. 1999;274:22517–22523. doi: 10.1074/jbc.274.32.22517. [DOI] [PubMed] [Google Scholar]
- Henderson MJ, Morgan HE, Park CR. Regulation of glucose uptake in muscle. IV. The effect of hypophysectomy on glucose transport, phosphorylation, and insulin sensitivity in the isolated, perfused heart. J Biol Chem. 1961;236:273–277. [PubMed] [Google Scholar]
- Javadov S, Karmazyn M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol Biochem. 2007;20:1–22. doi: 10.1159/000103747. [DOI] [PubMed] [Google Scholar]
- Javadov S, Baetz D, Rajapurohitam V, Zeidan A, Kirshenbaum LA, Karmazyn M. Antihypertrophic effect of Na+/H+ exchanger isoform 1 inhibition is mediated by reduced mitogen-activated protein kinase activation secondary to improved mitochondrial integrity and decreased generation of mitochondrial-derived reactive oxygen species. J Pharmacol Exp Ther. 2006;317:1036–1043. doi: 10.1124/jpet.105.100107. [DOI] [PubMed] [Google Scholar]
- Javadov S, Choi A, Rajapurohitam V, Zeidan A, Basnakian AG, Karmazyn M. NHE-1 inhibition-induced cardioprotection against ischaemia/reperfusion is associated with attenuation of the mitochondrial permeability transition. Cardiovasc Res. 2008;77:416–424. doi: 10.1093/cvr/cvm039. [DOI] [PubMed] [Google Scholar]
- Linden M, Gellerfors P, Nelson BD. Pore protein and the hexokinase-binding protein from the outer membrane of rat liver mitochondria are identical. FEBS Lett. 1982;141:189–192. doi: 10.1016/0014-5793(82)80044-6. [DOI] [PubMed] [Google Scholar]
- Loor G, Kondapalli J, Iwase H, Chandel NS, Waypa GB, Guzy RD, Vanden Hoek TL, Schumacker PT. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim Biophys Acta. 2011;1813:1382–1394. doi: 10.1016/j.bbamcr.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB, Hay N. Hexokinase–mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Manchester J, Kong X, Nerbonne J, Lowry OH, Lawrence JC., Jr Glucose transport and phosphorylation in single cardiac myocytes: rate-limiting steps in glucose metabolism. Am J Physiol. 1994;266:E326–E333. doi: 10.1152/ajpendo.1994.266.3.E326. [DOI] [PubMed] [Google Scholar]
- Marcil M, Ascah A, Matas J, Belanger S, Deschepper CF, Burelle Y. Compensated volume overload increases the vulnerability of heart mitochondria without affecting their functions in the absence of stress. J Mol Cell Cardiol. 2006;41:998–1009. doi: 10.1016/j.yjmcc.2006.08.117. [DOI] [PubMed] [Google Scholar]
- Matas J, Young NT, Bourcier-Lucas C, Ascah A, Marcil M, Deschepper CF, Burelle Y. Increased expression and intramitochondrial translocation of cyclophilin-D associates with increased vulnerability of the permeability transition pore to stress-induced opening during compensated ventricular hypertrophy. J Mol Cell Cardiol. 2009;46:420–430. doi: 10.1016/j.yjmcc.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Mathupala SP, Ko YH, Pedersen PL. Hexokinase II: cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008;15:521–529. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219:713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, et al. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa H, Printz RL, Whitesell RR, Granner DK. Regulation of hexokinase II gene transcription and glucose phosphorylation by catecholamines, cyclic AMP, and insulin. Diabetes. 1995;44:1426–1432. doi: 10.2337/diab.44.12.1426. [DOI] [PubMed] [Google Scholar]
- Ozcan C, Bienengraeber M, Hodgson DM, Mann DL, Terzic A. Mitochondrial tolerance to stress impaired in failing heart. J Mol Cell Cardiol. 2003;35:1161–1166. doi: 10.1016/s0022-2828(03)00204-9. [DOI] [PubMed] [Google Scholar]
- Pastorino JG, Hoek JB. Hexokinase II: the integration of energy metabolism and control of apoptosis. Curr Med Chem. 2003;10:1535–1551. doi: 10.2174/0929867033457269. [DOI] [PubMed] [Google Scholar]
- Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- Pedersen PL. Warburg, me and Hexokinase 2: multiple discoveries of key molecular events underlying one of cancers' most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr. 2007;39:211–222. doi: 10.1007/s10863-007-9094-x. [DOI] [PubMed] [Google Scholar]
- Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, Di Lisa F. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J. 1999;76:725–734. doi: 10.1016/S0006-3495(99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Printz RL, Osawa H, Ardehali H, Koch S, Granner DK. Hexokinase II gene: structure, regulation and promoter organization. Biochem Soc Trans. 1997;25:107–112. doi: 10.1042/bst0250107. [DOI] [PubMed] [Google Scholar]
- Ritchie RH, Quinn JM, Cao AH, Drummond GR, Kaye DM, Favaloro JM, Proietto J, Delbridge LM. The antioxidant tempol inhibits cardiac hypertrophy in the insulin-resistant GLUT4-deficient mouse in vivo. J Mol Cell Cardiol. 2007;42:1119–1128. doi: 10.1016/j.yjmcc.2007.03.900. [DOI] [PubMed] [Google Scholar]
- Robin E, Guzy RD, Loor G, Iwase H, Waypa GB, Marks JD, Hoek TL, Schumacker PT. Oxidant stress during simulated ischemia primes cardiomyocytes for cell death during reperfusion. J Biol Chem. 2007;282:19133–19143. doi: 10.1074/jbc.M701917200. [DOI] [PubMed] [Google Scholar]
- Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Jr, Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci USA. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri A, Hughie HH, Lucchesi PA. Regulation of hypertrophic and apoptotic signaling pathways by reactive oxygen species in cardiac myocytes. Antioxid Redox Signal. 2003;5:731–740. doi: 10.1089/152308603770380034. [DOI] [PubMed] [Google Scholar]
- Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev. 2002;7:161–173. doi: 10.1023/a:1015380609464. [DOI] [PubMed] [Google Scholar]
- Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–907. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov VG, Todor A, Khanal S, Imai M, Sabbah HN. Cyclosporine A attenuates mitochondrial permeability transition and improves mitochondrial respiratory function in cardiomyocytes isolated from dogs with heart failure. J Mol Cell Cardiol. 2007;42:150–158. doi: 10.1016/j.yjmcc.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden PH, Clerk A. Oxidative stress and growth-regulating intracellular signaling pathways in cardiac myocytes. Antioxid Redox Signal. 2006;8:2111–2124. doi: 10.1089/ars.2006.8.2111. [DOI] [PubMed] [Google Scholar]
- Sun L, Shukair S, Naik TJ, Moazed F, Ardehali H. Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Mol Cell Biol. 2008;28:1007–1017. doi: 10.1128/MCB.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taegtmeyer H, Overturf ML. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension. 1988;11:416–426. doi: 10.1161/01.hyp.11.5.416. [DOI] [PubMed] [Google Scholar]
- Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- Wu R, Smeele KM, Wyatt E, Ichikawa Y, Eerbeek O, Sun L, Chawla K, Hollmann MW, Nagpal V, Heikkinen S, et al. Reduction in hexokinase II levels results in decreased cardiac function and altered remodeling after ischemia/reperfusion injury. Circ Res. 2011;108:60–69. doi: 10.1161/CIRCRESAHA.110.223115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.