Abstract
Study Objective
To determine the influence of Echinacea purpurea on the pharmacokinetics of lopinavir-ritonavir, and on CYP3A and P-glycoprotein (P-gp) activity using the probe substrates midazolam, and fexofenadine, respectively.
Design
Open label, single-sequence pharmacokinetic study.
Setting
Outpatient clinic in a Federal Government research hospital.
Subjects
Thirteen (8 males) healthy volunteers (median age: 31 yrs).
Measurements and main results
Healthy volunteers received lopinavir-ritonavir (400/100 mg) twice daily for 30 days. On study day 16, subjects began taking Echinacea purpurea 500 mg three times daily, which they continued for four weeks, the first two weeks in combination with lopinavir-ritonavir. On days 15 and 30 of lopinavir-ritonavir administration (pre and post-Echinacea, respectively), serial blood samples were collected over 12 hrs to determine lopinavir and ritonavir concentrations and subsequent pharmacokinetic parameters using non-compartmental methods. Study subjects also received single doses of midazolam (8 mg orally) and fexofenadine (120 mg orally) before- and after 28 days of Echinacea purpurea to assess CYP3A and P-glycoprotein (P-gp) activity, respectively. Neither lopinavir nor ritonavir pharmacokinetics were significantly altered by 2 weeks of Echinacea coadministration. The geometric mean ratios (GMR, 90% CI) for lopinavir area under the concentration vs. time curve from zero to 12 hrs (AUC0–12) and maximum concentration (post-Echinacea/pre-Echinacea) were 0.96 (0.83, 1.10) and 1.00 (0.88, 1.12), respectively (P > 0.05). Conversely, GMRs (90% CIs) for midazolam AUC from time zero to infinity (AUC0-∞) and oral clearance were 0.73 (0.61, 0.85) (P = 0.008) and 1.37 (1.10, 1.63) (P = 0.02), respectively. Fexofenadine pharmacokinetics did not significantly differ pre- and post-echinacea administration (P > 0.05).
Conclusion
Echinacea purpurea induced CYP3A activity but did not alter lopinavir concentrations, most likely due to the presence of the potent CYP3A inhibitor, ritonavir. Echinacea purpurea is unlikely to alter the pharmacokinetics of ritonavir-boosted protease inhibitors but may cause modest decreases in plasma concentrations of other CYP3A substrates.
Keywords: HIV, protease inhibitors, lopinavir, ritonavir, Echinacea purpurea, herb, cytochrome P450, P-glycoprotein, drug interaction
Despite the success of potent combination antiretroviral (ARV) therapy, complementary and alternative medications (CAM) remain widely used by patients with HIV infection. Indeed, more than half of HIV infected patients report using CAM at some point in time.1–4 Patients with HIV infection typically use CAM for symptomatic relief of side effects secondary to ARV therapy and/or general health benefits. Unfortunately, the co-administration of CAM and ARV medications can place patients at risk for clinically significant drug drug interactions. Because HIV protease inhibitors are primarily metabolized by cytochrome P450 (CYP) 3A4, herbal preparations that modulate this metabolic pathway have the potential to alter protease inhibitor pharmacokinetics potentially resulting in reduced ARV efficacy or increased toxicity.1 Piscitelli et al. found that St. John’s wort decreased the systemic exposure of the HIV protease inhibitor indinavir by 57% during coadministration.5 In a separate study by the same investigators, three weeks of garlic caplet supplementation decreased the area under the concentration versus time curve (AUC) of saquinavir, another HIV protease inhibitor, by 51%.6
In spite of the potential for clinically relevant interactions between CAM and ARVs, relatively few herbal products have been tested for their effects on ARV drug disposition in vivo; one such herbal supplement that has not been assessed for its influence on ARV pharmacokinetics is Echinacea purpurea. E purpurea is predominantly used to prevent and treat the common cold, influenza, and upper respiratory tract infections.7–9 In the setting of HIV infection, E. purpurea may be taken for its immunomodulatory and antiviral effects.1 Of note, Echinacea products ranked behind garlic as the second top-selling herbal dietary supplement in the food, drug, and mass market channel in the United States in 2005 with over 21 million U.S. dollars in sales.10
At least two studies have assessed the influence of E purpurea root on CYP3A activity in humans.11,12 Using single doses of both oral and iv midazolam as a probe for intestinal and hepatic CYP3A activity, respectively, Gorski et al. observed an 85% increase in the intestinal availability of midazolam (P=0.015) and a 15% reduction in the hepatic availability of the drug (P= 0.006) after 1600 mg (total daily dose) of E purpurea administration for 8 days.11 These data suggest that E purpurea selectively alters the catalytic activity of CYP3A in the liver vs. intestine. Conversely, Gurley et al. found that 28 days of E purpurea whole plant extract administration did not significantly alter CYP3A metabolic serum ratios of 1-hydroxymidazolam:midazolam collected one hr post-dose in 12 healthy volunteers.12
To this end, it is difficult to predict the influence of E purpurea on the pharmacokinetics of CYP3A substrates such as the HIV protease inhibitors. The presence or absence of such interactions may depend on the relative extraction of the coadministered drug by hepatic and intestinal CYP3A. Due to the potentially serious consequences of a drug-drug interaction between E purpurea and HIV protease inhibitors (i.e virologic and/or immunologic failure or drug toxicity) the current study was designed to assess the influence of E purpurea on the steady state pharmacokinetics of lopinavir plus ritonavir in healthy human volunteers.
Methods
Subjects
Healthy male and female volunteers between the ages of 18 and 50 were eligible for participation in this study. Each study candidate underwent an evaluation that included a medical history, physical examination and laboratory analysis (serum electrolytes, liver function tests, cholesterol and triglycerides) to rule-out any medical conditions that could place subjects at risk or potentially affect study results. Participants were also required to have a negative HIV ELISA test. Subjects were not allowed to have taken any medications (including prescription and non-prescription drugs, herbal supplements and oral contraceptives) within 30 days of study participation. Additional exclusion criteria included current or recent (within 6 weeks) tobacco use, drug or alcohol abuse, history of intolerance to any of the study medications, and persistent diarrhea. Acetaminophen, ibuprofen, and loperamide were allowed as needed to treat side effects associated with the study drugs; however, subjects were prohibited from taking these medications on pharmacokinetic sampling days. Volunteers were also instructed to refrain from ingesting fruit juices, including grapefruit juice, throughout the study period. Pregnant or breastfeeding females were excluded from study participation, and females of child-bearing potential were required to use a non-hormonal method of contraception throughout the study.
Informed consent was obtained from all study participants and clinical research was conducted in accordance with guidelines for human experimentation as specified by the U.S. Department of Health and Human Services. The study was approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board.
Study Design
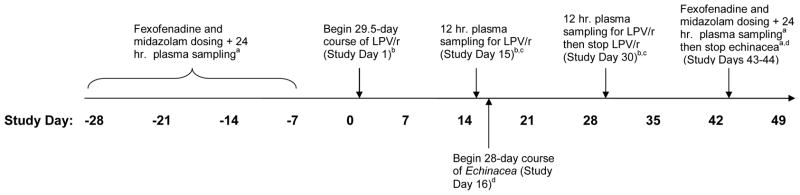
This study was a single-center, open-label investigation to evaluate the effect of two weeks of orally administered Echinacea purpurea on the steady-state pharmacokinetics of lopinavir and ritonavir in healthy volunteers (Figure 1). In addition, subjects underwent phenotyping for CYP3A and P-glycoprotein (P-gp) activity using oral midazolam and fexofenadine respectively, before and after 28 days of Echinacea purpurea administration. This study was conducted at the Clinical Research Center at the National Institutes of Health (Bethesda, MD, USA).
Figure 1. Study Design.
aFexofenadine 120 mg, single oral dose and midazolam 8 mg, single oral dose; plasma sample collected for determination of fexofenadine and midazolam concentrations used in pharmacokinetic analyses.
bLPV/r: lopinavir (100 mg) + ritonavir (400 mg), orally, twice daily.
cPlasma samples collected for the determination of steady state lopinavir and ritonavir concentrations used in pharmacokinetic analyses.
dEchinacea: Echinacea purpurea 500 mg three times daily
Treatment and Blood Sampling
Subjects were given a single 8 mg oral dose of midazolam syrup (Roche Laboratories, Nutley, NJ, USA) and 120 mg (2 × 60 mg tablets) of fexofenadine (Sanofi-Aventis, Bridgewater, NJ, USA) together on an empty stomach. Blood samples were collected for determination of midazolam and fexofenadine in plasma at 0 (pre-dose), 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, and 24 hr post-dose. After collection, samples were centrifuged immediately and plasma harvested and frozen at −80°C until analysis. Seven to 28 days after midazolam and fexofenadine administration, subjects began taking lopinavir/ritonavir 400 mg/100 mg (2 × 200 mg/50 mg Kaletra® tablets, Abbott, North Chicago, IL, USA) twice daily with meals for a total of 29.5 days. In clinic on day 15 of lopinavir/ritonavir administration, subjects received their morning lopinavir/ritonavir dose with food, followed by blood sample collection for the determination of steady state lopinavir and ritonavir plasma concentrations (phase 1). Blood samples were collected immediately before and 0.5, 1, 2, 3, 4, 6, 8 and 12 hours after the dose. The next morning, subjects began taking E purpurea 500 mg (2 × 250 mg tablets) three times daily (Echinamide® Natural Factors, WA USA), while continuing to take lopinavir/ritonavir twice daily. After two weeks of lopinavir/ritonavir and E purpurea coadministration, subjects returned to clinic for repeat lopinavir/ritonavir pharmacokinetic sampling (study day 30; phase 2) as performed in phase I. Following phase 2 pharmacokinetic sampling, subjects discontinued lopinavir/ritonavir and continued taking E purpurea alone for an additional 2 weeks. After a total of 4 weeks of E purpurea dosing, subjects returned to clinic for repeat fexofenadine and midazolam administration with post-dose blood sampling taking place as described earlier. Blood was also collected for end-of-study safety monitoring, including chemistry panel, complete blood count, pregnancy test, and non-fasting cholesterol and triglycerides.
Analytical methods
Lopinavir and ritonavir plasma concentrations were determined by high performance liquid chromatography (HPLC) with liquid-liquid extraction using a method developed in our laboratory.13 Calibration curves for lopinavir and ritonavir were linear from 0.050 μg/mL to 15.0 μg/mL (R2 ≥ 0.0997). Percent errors, as a measure of accuracy, were < 15%, and the respective inter- and intra-assay coefficients of variation (CV) for ritonavir were 5.70–10.74% and 2.91–10.59%, while those of lopinavir were 4.07–9.08% and 3.16 – 9.36%, respectively, at four different concentrations. The limit of quantitation was 0.050 μg/mL and the limit of detection was 0.030 μg/mL.
Fexofenadine and midazolam were separated using Ultra Performance Liquid Chromatography (UPLC) with detection by tandem mass spectrometry (MS) using multiple reaction monitoring (MRM) as previously described.13 Calibration curves for midazolam and fexofenadine were linear from 1.0 to 100 ng/mL (R2 ≥ 0.998). Percent errors, as a measure of accuracy, were < 15%, and the inter- and intra-assay coefficients of variation (CV) were 1.31–8.48% and 3.53–6.03%, respectively, at three different drug concentrations. The limit of quantitation was 1.0 ng/mL and the limit of detection was 0.20 ng/mL.
Echinacea purpurea formulation
Echinacea purpurea fresh liquid extract 8:1 (250 mg) softgel capsules from a single lot (Echinamide® Natural Factors, lot no. 535285)) were used in this investigation. The extract formulation contained standardized amounts of alkylamides, polysaccharides, and cichoric acid via a patented extraction method. The product was manufactured in accordance with United States Pharmacopoeia (USP) guidelines and the Government of Canada’s Good Manufacturing Practices (GMP). The Echinamide® formulation used in this study did not undergo independent analysis by an outside laboratory.
Pharmacokinetic analysis
Plasma concentrations of lopinavir, ritonavir, fexofenadine, and midazolam were analyzed by non-compartmental methods using WinNonlin pharmacokinetic software, version 5.0 (Pharsight Corporation, Mountain View, CA, USA). The maximum plasma concentration (Cmax), and time to reach Cmax (Tmax), were obtained by direct inspection of the plasma concentration time profiles. The elimination rate constant (λZ) was determined by calculating the absolute value of the slope of the log linear regression using at least three points on the plasma concentration time plot. The AUC over 0–12 h (AUC0–12) at steady-state was determined for lopinavir and ritonavir using the log linear trapezoidal rule. Apparent oral clearance (CL/F) for lopinavir and ritonavir was obtained by dividing the dose by AUC0–12 at steady-state. For fexofenadine and midazolam, AUC from zero to the last quantifiable concentration (AUC0-last) was determined by the log-linear trapezoidal rule; AUC from zero to infinity (AUC0-∞) was calculated by dividing the last measured concentration by λZ and adding this value to AUClast. Apparent oral clearance (CL/F) was estimated for midazolam and fexofenadine as dose/AUC0–∞.
Statistical analysis
Results are reported as geometric means and geometric mean ratios (GMR) with 90% confidence intervals. Pharmacokinetic parameter values for lopinavir, ritonavir, midazolam and fexofenadine at baseline (phase I) and following E. purpurea administration (phase II) were compared using a two-tailed, paired, Student’s t-test, except for Tmax, which was analyzed using the Wilcoxon signed-rank test. P values < 0.05 were considered statistically significant for all analyses. SYSTAT Software, version 11 (Richmond, CA, USA) was used for statistical comparisons; Microsoft Excel 2003 (Microsoft Corp., Redmond, WA, USA) was used to generate descriptive data.
Sample Size
A difference in lopinavir AUC of at least 35% was considered to be clinically relevant for the purpose of estimating sample size. A standard deviation of 0.40 was assumed for lopinavir AUC based on previous data.14 With α set at 0.05, a sample size of 13 subjects was deemed necessary to provide 80% power to detect a 35% difference in lopinavir AUC before and after E. purpurea administration (SYSTAT Software, version 11 [Richmond, CA, USA]).
Results
Subjects
Fourteen subjects enrolled, and thirteen (8 males) completed study participation. One subject dropped out prior to study completion, citing personal reasons; there are no data to report for this individual. Demographic information for the study subjects is presented in Table 1.
Table 1.
Study Subject Demographics
| Subject | Age (years) | Sex | Race/Ethnicity | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|---|---|
| 1 | 26 | Male | White | 86 | 24.4 |
| 2 | 40 | Male | White | 92 | 28.1 |
| 3 | 22 | Male | White | 89 | 26.5 |
| 4 | 48 | Female | White | 51 | 20.0 |
| 5 | 40 | Male | White | 88 | 27.9 |
| 6 | 33 | Male | Black | 87 | 28.1 |
| 7 | 30 | Female | White | 57 | 21.4 |
| 8 | 31 | Female | White/Hispanic | 51 | 22.4 |
| 9 | 23 | Female | White | 67 | 21.8 |
| 10 | 23 | Female | White/Hispanic | 94 | 38.4 |
| 11 | 36 | Male | White/Hispanic | 75 | 26.1 |
| 12 | 45 | Male | White | 78 | 24.4 |
| 13 | 31 | Male | White | 86 | 28.6 |
|
| |||||
| Median: | 31 | 86 | 26 | ||
Lopinavir and ritonavir
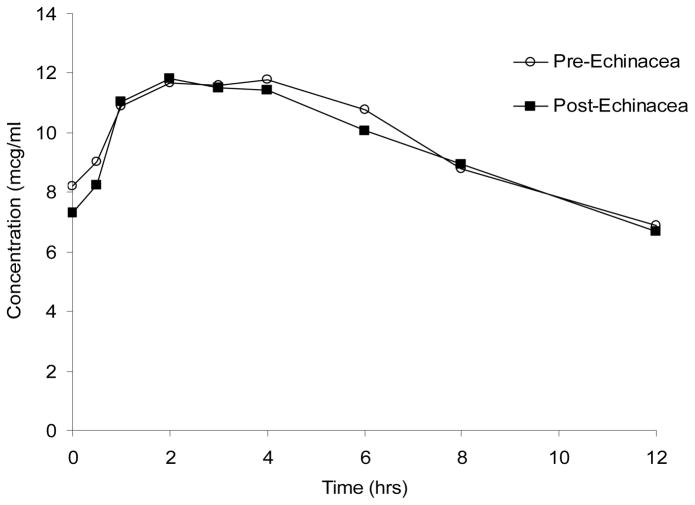
Neither lopinavir nor ritonavir pharmacokinetic parameter values were altered after two weeks of Echinacea administration (Table 1; Figure 2). The GMR (90% CI) for lopinavir AUC0–12 and Cmax (Post-echinacea/Pre-echinacea) were 0.96 (0.83–1.10; p = 0.82) and 1.00 (0.88–1.12; p = 0.72), respectively.
Figure 2.
Steady-state lopinavir concentration versus time curves before, and after two weeks of Echinacea purpurea administration
Midazolam and fexofenadine
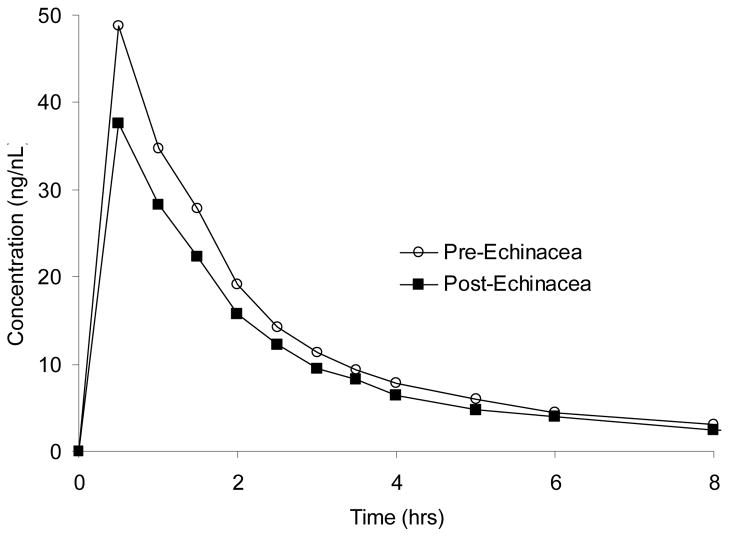
Midazolam AUC0-∞ and Cl/F were significantly decreased and increased, respectively, after echinacea administration (Table 2; Figure 3). The GMRs (90% CIs) for midazolam AUC0-∞ and Cl/F were 0.73 (0.61, 0.85) (P = 0.008) and 1.37 (1.10, 1.63) (P = 0.02), respectively. The GMR for midazolam T ½ was 0.55 (0.40, 0.70) (P=0.051), which bordered on statistical significance. Midazolam Cmax and Tmax were unchanged after echinacea administration (P > 0.05). In contrast to midazolam, fexofenadine pharmacokinetic parameter values showed no significant difference pre- and post-echinacea administration (P > 0.05 for all comparisons) (Table 2).
Table 2.
Lopinavir and Ritonavir pharmacokinetic parameter values before- and after 14 days of Echinacea Purpurea Extract administration in 14 healthy volunteers.
| Lopinavir | Geometric Mean Values (90% CI)a | Geometric mean ratios (90% CI)a | Pb | |
|---|---|---|---|---|
|
| ||||
| Pre-Echinacea | Post-Echinacea | Post-Echinacea/Pre-Echinacea | ||
| AUC 0–12 (μg ·hr/mL) | 109 (88–131) | 105 (81–129) | 0.96 (0.83–1.10) | 0.82 |
| Cmax (μg/mL) | 11.9 (10.1–13.8) | 12.0 (9.6–14.3) | 1.00 (0.88–1.12) | 0.72 |
| Tmax (hr) | 2.0 (0–6)c | 2.0 (0.5–4.0)c | __ | 0.94 |
| T ½ (hr) | 8.7 (6.6–10.7) | 9.5 (7.4–11.6) | 1.09 (0.29–1.38) | 0.62 |
| Cl/Fss (L/hrç;) | 3.66 (3.84–4.28) | 3.80 (3.10–4.51) | 1.04 (0.90–1.18) | 0.59 |
|
| ||||
|
Ritonavir
| ||||
| AUC 0–12 (μg·.hr/mL) | 7.39 (4.43–10.35) | 6.79 (1.40–12.2) | 0.92 (0.66–1.18) | 0.76 |
| Cmax (μg/mL) | 1.03 (0.67–1.39) | 1.01 (0.11–1.91) | 0.98 (0.67–1.29) | 0.53 |
| Tmax (hr) | 2.9 (0–6.0)c | 1.8 (0–4.0)c | __ | 0.14 |
| T ½ (hr) | 3.97 (3.28–4.66) | 5.34 (4.46–6.22) | 1.35 (0.49–2.20) | 0.17 |
| Cl/Fss (L/hr·) | 13.53 (9.88–17.2) | 14.70 (9.87–19.50) | 1.09 (0.86–1.31) | 0.32 |
CI, confidence interval.
The Student’s paired, two-tailed T-test was used for statistical comparisons except for Tmax, for which the Wilcoxon Signed Rank test was used.
Due to several zero values in the data set, geometric means could not be calculated; therefore median values (and ranges) are reported instead; as a result, the post-Echinacea/pre-Echinacea ratio is calculated using median values, and ranges are reported in lieu of 90% CIs.
Figure 3.
Midazolam concentration versus time curves before, and after four weeks of Echinacea purpurea administration
Safety
Twelve of the 13 subjects experienced an adverse event consistent with those expected of the study medications. All of the adverse events were mild to moderate in severity and no serious adverse events were reported. Grades 1 and 2 diarrhea, abdominal pain, and nausea were the most reported adverse events; these events were comparable in incidence and severity in both phases of the study (i.e. lopinavir-ritonavir alone [phase 2] and with Echinacea coadministration [phase 2]). One subject reported conjunctivitis, sinus congestion, and acute sore throat (all grade 1), which was not felt to be related to the study medications. There were no significant laboratory abnormalities throughout the course of the investigation.
Discussion
The use of herbal supplements continues to be common among HIV-infected patients. Studies conducted previously have shown that concurrent use of certain herbal preparations, such as St. John’s wort and garlic, can significantly decrease plasma concentrations of unboosted protease inhibitors.5,6 However, ritonavir boosted protease inhibitor regimens are now preferred over regimens containing a single protease inhibitor; as a result, we chose to study the influence of E purpurea on the pharmacokinetics of the commonly used protease inhibitor combination, lopinavir/ritonavir.15 In addition to studying the influence of E purpurea on lopinavir/ritonavir, we also chose to study the isolated effects of E purpurea on CYP3A and P-gp activity (using the probe substrates midazolam and fexofenadine, respectively) since previous studies in healthy volunteers and in vitro demonstrate conflicting results.11,12,,16,17
In the current investigation, we did not observe significant changes in the pharmacokinetic profiles of lopinavir or ritonavir after 2 weeks of E purpurea exposure, nor did we see changes in fexofenadine pharmacokinetics after 4 weeks of E purpurea administration (Tables 1 and 2); however, we did see a modest, but statistically significant decrease in midazolam exposure (−27%; P = 0.008) and an increase in midazolam apparent oral clearance (37%; P = 0.02). The midazolam half-life was reduced by 45% after E purpurea administration, which trended toward statistical significance ( P = 0.051). Of note, Cmax and Tmax were unchanged by Echinacea administration. These results suggest induction of the CYP3A-mediated metabolism of midazolam by E purpurea.
Induction of CYP3A by E purpurea has been previously described in healthy volunteers.11 Gorski et al. observed contrasting modulatory effects of E purpurea at hepatic and intestinal sites (i.e. induction and inhibition, respectively). In their study, which used both intravenous and oral midazolam to differentiate intestinal versus hepatic effects of E purpurea on CYP3A activity, the investigators observed a significant increase in the oral availability of midazolam (≅ 43%; P = 0.028) and a significant decrease in hepatic availability (≅ 15%; P = 0.015). Of note, no significant changes were observed in midazolam pharmacokinetic parameter values after oral administration before- and after 8 days of echinacea dosing (400 mg 4 times daily).
In contrast to the study by Gorski et al. we only studied the effects of E purpurea on oral midazolam pharmacokinetics; thus, our results are reflective of the net effect of E purpurea on both intestinal and hepatic CYP3A activity. It is interesting that, after oral midazolam administration, we observed results consistent with net CYP3A induction by E purpurea, whereas Gorski and coworkers observed no significant change in midazolam pharmacokinetics. This may be due to the fact that subjects undergoing CYP3A phenotyping in our study received E purpurea for 28 days compared to an 8-day course in the Gorski investigation. The longer duration of E purpurea administration in our study may have allowed for induction of hepatic CYP3A to predominate over intestinal CYP3A inhibition –resulting in a net reduction in overall CYP3A activity. However, since we did not administer intravenous midazolam in our study, it is not possible to definitively conclude that intestinal and hepatic CYP3A were differentially affected by E purpurea.
While our results are consistent with those of Gorski et al. in that we both observed CYP3A modulation with E purpurea administration, Gurley and coworkers found no effect of E purpurea (800 mg twice daily for 28 days) on CYP3A activity using a 1 hr. post-dose plasma concentration ratio of 1-hydroxymidazolam:midazolam to determine CYP3A phenotype (after an oral 8 mg midazolam dose).12 Of note, both studies administered a similar daily dose of E purpurea (1600 mg versus 1500 mg [our study]), and the same dose of oral midazolam (8 mg). Possible reasons for the disparity in results between our study and that conducted by Gurley et al. include different E purpurea manufacturers (potentially resulting in different amounts of phytochemicals [i.e. alkylamides] responsible for CYP3A modulation) and dissimilar CYP3A phenotyping methods.18
Despite observing enhanced CYP3A activity after 28 days of E purpurea administration, we did not observe reductions in the CYP3A substrates lopinavir and ritonavir, after 14 days of E purpurea dosing. The most likely explanation for our results is that ritonavir, a potent intestinal and hepatic CYP3A inhibitor, masked the CYP3A-inducing effects of E purpurea, resulting in the absence of a drug interaction.19 Indeed low-dose ritonavir (100 mg twice daily) is capable of attenuating CYP3A induction associated with other CYP3A inducers, such as rifabutin and efavirenz.20,21 Although it cannot be ruled out that E purpurea induced the metabolism of midazolam and not lopinavir-ritonavir due to the shorter course of E purpurea administration between lopinavir-ritonavir sampling periods compared to midazolam (14 vs. 28 days, respectively), this is unlikely, as 2 weeks of E purpurea administration should have been sufficient to produce CYP3A induction. Indeed, Gorski et al. observed CYP3A induction with E purpurea after only 8 days of administration to healthy volunteers.11
Despite in vitro reports that suggest that E purpurea may inhibit intestinal P-gp and alter the bioavailability of orally administered substrates, we did not observe any alteration in P-gp activity after 28 days of echinacea administration using fexofenadine as a P-gp probe substrate.17,22 This is consistent with the observations of Gurley et al. who did not observe a significant effect of Echinacea administration (267 mg three times daily for 14 days) on P-gp activity using digoxin as their P-gp probe medication.23 To this end, it is unlikely that E purpurea will produce clinically relevant interactions with coadministered medications via P-gp modulation.
Limitations to this study include the fact that we chose to administer oral midazolam in lieu of also administering intravenous midazolam. As such, it is not possible to compare and contrast the influence of E purpurea on intestinal versus hepatic CYP3A. In addition, we did not perform an independent phytochemical analysis for “marker compounds,” such as cichoric acid, echinacoside, or chlorogenic acid, in the echinacea product used in this study. 12 As such, it is possible that the E purpurea product we used differed in alkylamide content compared to other commercial preparations. Alkylamide content has previously been associated with the in vitro inhibitory potency of Echinacea.24 In addition, the product we used was produced using Echinacea purpurea fresh liquid extract, whereas other Echinacea products may also contain Echinacea angustifolia root, which has been shown to inhibit CYP3A4 in vitro.25 Nonetheless, the observation of a statistically significant interaction between E purpurea and midazolam in this study suggests that the product we used contained sufficient quantities of CYP3A-modulating constituent(s).
Results from this study suggest that E purpurea is unlikely to significantly alter the disposition of CYP3A substrates (i.e. protease inhibitors) when they are administered in combination with a potent CYP3A inhibitor (i.e. ritonavir). It is possible however, that E purpurea may cause mild reductions (≅ 25–30%) in the systemic exposure of CYP3A substrates that are not routinely coadministered with potent CYP3A inhibitors; the clinical relevance of such interactions will be greater in individuals taking CYP3A substrates whose plasma concentrations must be maintained above threshold values for optimal pharmacologic efficacy. Due to the variable effects of E purpurea on intestinal versus hepatic CYP3A activity as shown by Gorski et al, the influence of E purpurea on the net exposure of a coadministered CYP3A substrate will likely depend on the CYP3A extraction ratio of the concurrent medication.11 Drugs that are poorly absorbed due to significant intestinal metabolism via CYP3A, may undergo increased oral bioavailability secondary to intestinal CYP3A inhibition by E purpurea. Conversely, CYP3A substrates with adequate bioavailability and a low clearance may undergo increased oral clearance secondary to hepatic induction of CYP3A by E purpurea.11
In conclusion, echinacea purpurea is unlikely to alter the pharmacokinetics of ritonavir-boosted protease inhibitors such as the lopinavir-ritonavir combination assessed in this study. However, patients with HIV infection frequently take a variety of medications in addition to antiretrovirals, many of which are metabolized at least in part- by CYP3A; patients taking these medications in conjunction with E purpurea should be monitored closely for potential herb-drug interactions.
Table 3.
Midazolam and fexofenadine pharmacokinetic parameter values before- and after 28 days of Echinacea purpurea administration in 13 healthy volunteers
| Midazolam | Geometric Mean Values (90% CI)a | Geometric mean ratios (90% CI)a | Pb | |
|---|---|---|---|---|
|
| ||||
| Pre-Echinacea | Post-Echinacea | Post-Echinacea/Pre-Echinacea | ||
| AUC0-∞ (ng ·hr/mL) | 143 (119–166) | 104 (83–126) | 0.73 (0.61–0.85) | 0.008 |
| Cmax (ng/mL) | 50 (43–57) | 38 (30–47) | 0.77 (0.58–0.96) | 0.14 |
| Tmax (hr) | 0.5 (0.5–1.0)c | 0.5 (0.5–1.5)c | __ | 0.44 |
| T ½ (hr) | 5.6 (3.4–7.8) | 3.1 (2.3–3.9) | 0.55 (0.40–0.70) | 0.051 |
| Cl/F (mL/hr·) | 56 (46–66) | 77 (55–98) | 1.37 (1.10–1.63) | 0.02 |
|
| ||||
|
Fexofenadine
| ||||
| AUC0-∞ (ng ·hr/mL) | 1569 (1138–2000) | 1543 (1262–1824) | 0.98 (0.82–1.14) | 0.46 |
| Cmax (ng/mL) | 256 (181–332) | 232 (185–280) | 0.91 (0.77–1.04) | 0.18 |
| Tmax (hr) | 2.0 (1.5–3.5)c | 2.0 (1.0–8.0)c | __ | 0.311 |
| T ½ (hr) | 5.6 (5.2–6.1) | 5.5 (5.1–5.8) | 0.97 (0.90–1.04) | 0.46 |
| Cl/F (mL/hr) | 76 (57–96) | 78 (62–93) | 1.02 (0.88–1.16) | 0.75 |
CI, confidence interval.
The Student’s paired, two-tailed T-test was used for statistical comparisons except for Tmax, for which the Wilcoxon Signed Rank test was used.
Median values (ranges)
Acknowledgments
Sources of Support: The Intramural Research Programs of the National Institutes of Health (NIH) Clinical Center and the National Institute of Allergy and Infectious Diseases (NIAID).
Footnotes
Presentation: Data from this manuscript were presented in part at the Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA; September 12–15, 2009 (abstract A1-1307).
References
- 1.Behm Dillon DM, Penzak SR, Bailey Klepser T. The use of herbals by patients with HIV. Advances in Pharmacy. 2004;2:41–60. [Google Scholar]
- 2.Fairfield KM, Eisenberg DM, Davis RB, et al. Patterns of use, expenditures, and perceived efficacy of complementary and alternative therapies in HIV-infected patients. Arch Intern Med. 1998;158:2257–64. doi: 10.1001/archinte.158.20.2257. [DOI] [PubMed] [Google Scholar]
- 3.Hsiao AF, Wong MD, Kanouse DE, et al. Complementary and alternative medicine use and substitution for conventional therapy by HIV-infected patients. J Acquir Immune Defic Syndr. 2003;33:157–65. doi: 10.1097/00126334-200306010-00007. [DOI] [PubMed] [Google Scholar]
- 4.Weber K, Schneider M, Sacks H, et al. Trends in complementary/alternative medicine (CAM) use in a large cohort of HIV infected women in the US from 1994–2001. XIV International AIDS Conference; Barcelona. July 7–12th, 2002; Abstract WePeB6004. [Google Scholar]
- 5.Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St. John’s wort Lancet. 2000;355:547–8. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- 6.Piscitelli SC, Burstein AH, Welden N, Gallicano KD, Falloon J. The effect of garlic supplements on the pharmacokinetics of saquinavir. Clin Infect Dis. 2000;34:234–8. doi: 10.1086/324351. [DOI] [PubMed] [Google Scholar]
- 7.Barnes J, Anderson LA, Gibbons S, Phillipson JD. Echinacea species (Echinacea angustifolia (DC) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2005;57:929–954. doi: 10.1211/0022357056127. [DOI] [PubMed] [Google Scholar]
- 8.Kligler B. Echinacea. Am Fam Physician. 2003;67:77–80. [PubMed] [Google Scholar]
- 9.Huntley AL, Coon JT, Ernst E. The safety of herbal medicinal products derived from Echinacea Species: A systematic review. Drug Safety. 2005;28(5):387–400. doi: 10.2165/00002018-200528050-00003. [DOI] [PubMed] [Google Scholar]
- 10.Blumenthal M, Ferrier GKL, Cavaliere C. Total sales of herbal supplements in the United States show steady growth. HerbalGram. 2006;71:64–66. [Google Scholar]
- 11.Gorski JC, Huang SM, Pinto AP, et al. The effect of echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clin Pharmacol Ther. 2004;75:89–100. doi: 10.1016/j.clpt.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Gurley BJ, Gardner SF, Hubbard MA, et al. In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, and saw palmetto. Clin Pharmacol Ther. 2004;76:428–40. doi: 10.1016/j.clpt.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Robertson SM, Davey RT, Voell J, Formentini E, Alfaro RM, Penzak SR. Curr Med Res Opin. 2008;24:591–99. doi: 10.1185/030079908x260871. [DOI] [PubMed] [Google Scholar]
- 14.Abbott Laboratories. Kaletra (lopinavir/ritonavir) package insert. North Chicago, Illinois: 2009. [Google Scholar]
- 15.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Nov 3, 2008. [Accessed (Oct. 10, 2008)]. pp. 1–139. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [page 38] [Google Scholar]
- 16.Yale SH, Glurich I. Analysis of the inhibitory potential of Ginkgo Biloba, Echinacea purpurea, and Serenoa repens on the metabolic activity of cytochrome P450 3A4, 2D6, and 2C9. J Alt Comp Med. 2005;11:433–39. doi: 10.1089/acm.2005.11.433. [DOI] [PubMed] [Google Scholar]
- 17.Hansen TS, Nilsen OG. Echinacea purpurea and P-glycoprotein drug transport in Caco-2 cells. Phytother Res. 2009;23:86–91. doi: 10.1002/ptr.2563. [DOI] [PubMed] [Google Scholar]
- 18.Toselli F, Matthias A, Gillam EMJ. Echinacea metabolism and drug interactions: the case for standardization of a complementary medicine. Life Sci. 2009;85:97–106. doi: 10.1016/j.lfs.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Yeh RF, Gaver VE, Patterson KB, et al. Lopinavir/ritonavir induces the hepatic activity of cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP1A2 but inhibits the hepatic and intestinal activity of CYP3A as measured by a phenotyping drug cocktail in healthy volunteers. J Acquir Immune Defic Syndr. 2006;42:52–60. doi: 10.1097/01.qai.0000219774.20174.64. [DOI] [PubMed] [Google Scholar]
- 20.Morse GD, Rosenkranz S, Para MF, et al. Amprenavir and efavirenz pharmacokinetics before and after the addition of nelfinavir, indinavir, ritonavir, or saquinavir in seronegative individuals. Antimicrob Agents Chemother. 2005;49:3373–81. doi: 10.1128/AAC.49.8.3373-3381.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallicano K, Khaliq Y, Carignan G, Tseng A, Walmsley S, Cameron DW. A pharmacokinetic study of intermittent rifabutin dosing with a combination of ritonavir and saquinavir in patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 2001;70:149–58. doi: 10.1067/mcp.2001.117612. [DOI] [PubMed] [Google Scholar]
- 22.Dresser GK, McDonald W, Kim RB, Bailey DG. Evaluation of herbal products as potential inhibitors of MDR1. Clin Pharmacol Ther. 2004;75:79. [Google Scholar]
- 23.Gurley BJ, Swain A, Williams DK, Barone G, Battu S. Gauging the clinical significance of P-glycoprotein-mediated herb-drug interactions: comparative effects of St. Jon’s wort, Echinacea, clarithromycin, and rifampin on digoxin pharmacokinetics. Mol Nutr Food res. 2008;52:772–79. doi: 10.1002/mnfr.200700081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shord SS, Shah K, Lukose A. Drug-botanical interactions: a review of the laboratory, animal, and human data for 8 common botanicals. Integr Cancer Ther. 2009;8:208–227. doi: 10.1177/1534735409340900. [DOI] [PubMed] [Google Scholar]
- 25.Budzinski JW, Foster BC, Vandenhoek S, Arnason JT. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine. 2000;7:273–282. doi: 10.1016/S0944-7113(00)80044-6. [DOI] [PubMed] [Google Scholar]