Abstract
The reliable isolation of primary oligodendrocyte progenitors cells (OPCs) holds promise as both a research tool and putative therapy for the study and treatment of central nervous system (CNS) disease and trauma. Stringently characterized primary mouse OPCs is of additional importance due to the power of transgenics to address mechanism(s) involving single genes. In this study, we developed and characterized a reproducible method for the primary culture of OPCs from postnatal day 5–7 mouse cerebral cortex. We enriched an O4+ OPC population using Magnetic Activated Cell Sorting (MACS) technology. This technique resulted in an average yield of 3.68 × 105 OPCs/brain. Following isolation, OPCs were glial fibrillary acidic protein− (GFAP−) and O4+. Following passage and with expansion, OPCs were O4+, A2B5+, and NG2+. Demonstrating their bi-potentiality, mouse OPCs differentiated into either more complex, highly arborized O4+ or O1+ oligodendrocytes (OLs) or GFAP+ astrocytes. This bi-potentiality is lost, however, in co-culture with rat embryonic day 15 derived dorsal root ganglia (DRG). Following 7–14 days of OPC/DRG co-culture, OPCs aligned with DRG neurites and differentiated into mature OLs as indicated by the presence of O1 and myelin basic protein (MBP) immunostaining. Addition of ciliary neurotrophic factor (CNTF) to conditioned media from OPC/DRG co-cultures improved OPC differentiation into mature O1+ and MBP+ OLs. This method allows for the study of primary mouse cortical OPC survival, maturation, and function without relying on oligosphere formation or the need for extensive passaging.
Keywords: Oligodendrocyte, Oligodendrocyte Precursor Cell, OPC Isolation, Cell Culture, O4, A2B5, Astrocyte, Oligodendrocyte Diferentiation, Oligodendrocyte Co-Culture
1. INTRODUCTION
The developing mammalian central nervous system (CNS) maintains an extensive population of progenitor cells which mature along the oligodendroglial lineage to yield myelinating oligodendrocytes (OLs) (Noble et al., 1992; Wolswijk and Noble, 1989). Of these progenitor populations, OPCs provide the largest source of maturing OLs, though a significant OPC subpopulation avoids differentiation maintaining their proliferative state (Nishiyama et al., 2009; Trotter et al., 2010). Isolation and subsequent in vitro study of OPCs from both the developing and adult CNS offers critical insights into putative disease and injury mechanisms involved in demyelinating disease states such as multiple sclerosis and CNS white matter infarctions as observed in ischemic stroke (Nishiyama, 1998). Furthermore, OPC engraftment into the injured CNS to replenish lost OLs continues to offer promise as a cell therapeutic method for the treatment of spinal cord injury (Cao et al., 2010).
A variety of cell surface markers indicate the state of OPC maturation along the oligodendroglial lineage. Though its antigen is found in multiple CNS cell types, A2B5, a monoclonal antibody that binds ganglioside, identifies a population of immature OPCs (Berg and Schachner, 1982; Eisenbarth et al., 1979; Raff et al., 1983; Schnitzer and Schachner, 1982). NG2, a chondroitin sulfate proteoglycan, expression is also indicative of the OPC population (Nishiyama et al., 2009). However, NG2+ cells are not specific to cell progenitors committed to an OL fate. In addition to having recently described synaptic associations to neurons, they also maintain the capacity to terminally differentiate into astrocytes as well as OLs (Emery, 2010; McTigue and Tripathi, 2008; Nishiyama et al., 2009). O4, a monoclonal antibody that binds cell surface pro-oligodendroblast antigen (POA) and galactocerebroside found on late-stage OPCs and post-mitotic immature OLs contrasts with A2B5 and NG2 expression in serving as a more reliable marker for OPCs committed to an oligodendroglial fate (Bansal and Pfeiffer, 1992; Bansal et al., 1992; Jackman et al., 2009; Nicolay et al., 2007; Nishiyama, 1998; Nishiyama et al., 2009; Trotter et al., 2010). O1, a monoclonal antibody that binds galactocerebroside on pre-myelinating OLs identifies OPCs that no longer maintain their proliferative potential but are transitioned to terminally differentiated OLs (Bansal and Pfeiffer, 1992; Cheng et al., 2007; Raff et al., 1978). As pre-myelinating OLs mature into myelinating OLs, the presence of O1 binding is accompanied by myelin basic protein (MBP) expression, one of the primary protein constituents of myelin and a marker for mature myelinating OLs (Nicolay et al., 2007; Trotter et al., 2010).
Numerous oligodendroglial cell lines have been developed for the study of OL function such as the CG-4 permanent cell line and Oli-neu immortalized cell lines (Jung et al., 1995; Lin et al., 2006; Louis et al., 1992; Mock et al., 2006; Pringproa et al., 2008). However, there is considerable variability between these cell lines largely depending on the source (i.e. chemically induced rodent tumors or spontaneous human tumors), isolation method, and growth conditions. Oftentimes, these lines maintain considerable proliferative potential, even once engrafted (a hallmark of their potential tumorigenicity), and may prove difficult for induction of differentiation (Lin et al., 2006; Pringproa et al., 2008). OPC cultures derived from the formation of neurospheres or oligospheres yield similar variability in genetic and phenotypic properties resulting in considerable departure from their in vivo counterparts (Chen et al., 2007; Zhao et al., 2010). Furthermore, with passage, subtle cellular changes can occur, often resulting in a molecularly different cell type than what was originally isolated (Lin et al., 2006). Lastly, nearly all existing primary OPC cultures from rodent models rely on more premature markers A2B5 and NG2 which may not have as extensive oligodendroglial lineage commitment (Behar et al., 1988; Hamanoue et al., 2009; Larsen and Yong, 2004; Shi et al., 1998). Primary OPC cultures may better maintain their endogenous phenotype using a later stage marker more limited to the oligodendroglial lineage but still maintain their proliferative capacity.
To address these concerns in existing OPC isolation methods, we developed and characterized a reliable and reproducible primary culture of cortically-derived OPCs from mouse pups. We limited the scope of this characterization to no more than a single passage following each OPC isolation to limit the potential of genetic and phenotypic drift often observed with additional passages. Furthermore, we used the monoclonal antibody O4, meeting the criteria of isolating OPCs that are proliferative and have greater commitment to OL differentiation than the oligopotent A2B5 selected and NG2+ progenitor cells. Though differentiation of these isolated OPCs in vitro still yields both OLs and astrocytes, isolated mouse OPC/DRG co-cultures result in extensive OPC maturation with nearly complete absence of astrocytes. Overall this method will facilitate the continued study of OPC differentiation and OL function and provide in vitro findings that will be better recapitulated in vivo.
2. MATERIALS AND METHODS
2.1 Animals
E12–E15 time pregnant C57Bl/6 mice and E15 Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). All animal procedures were performed according to the guidelines of University of Louisville Institutional Animal Care and Use Committee protocols and the National Institutes of Health.
2.2 Preparation for mouse OPC isolation
Prior to OPC isolation, 13.5 μl of 50 mM β-mercaptoethanol (β-ME) was combined (Sigma, St. Louis, MO) to 10 ml of Solution 2 from the papain-based Neural Tissue Dissociation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Due to proprietary reasons, Miltenyi was unable to provide details on the specific composition of their reagents. Prior to the availability of the more efficient and cost-effective Miltenyi kit, attempts to gently dissociate tissue using 20 U/ml Papain, Trypsin, and DNAse I resulted in lower yet comparable OPC yields and viabilities. MACS Buffer was prepared by adding bovine serum albumin (0.5% BSA), EDTA (0.5 mM), and 5 μg/ml insulin in Dulbecco’s phosphate buffered saline supplemented with 1 g/L glucose (all from Sigma). OPC-A media was prepared by adding 2.1 g/L NaHCO3 (Sigma) to dissolved DMEM-F12 without HEPES powder (Invitrogen, Carlsbad, CA). N2 supplement (1%), B27 supplement (2%), Penicillin/Streptomycin (1%, all from Invitrogen), BSA (0.01%, Sigma), 40 ng/ml fibroblast growth factor 2 (FGF2, Millipore, Billerica, MA), and 20 ng/ml platelet-derived growth factor-AA (PDGF-AA, Sigma) were added to the previously prepared DMEM-F12. Differentiation media components included N2 supplement (1%), B27 supplement (2%), Penicillin/Streptomycin (1%), 50 μg/ml insulin, and 40 ng/ml triiodo-thyronine (T3, Sigma) in DMEM/F12. Differentiation media was supplemented with 1 ng/ml ciliary neurotrophic factor (CNTF, Sigma) where appropriate. Where described, “pre-equilibration” refers to cell media incubated for a minimum of 30 minutes in 37°C, 5% CO2 prior to use.
2.3 Dissection of mouse cortices
Whole mouse brains were harvested from ice-anesthetized postnatal day 5–7 mouse pups by decapitation following loss of reflexive movement (approximately 5–8 minutes in ice). The skin and skull were cut along the midline and reflected to expose the brain. The brain was then removed and placed in a pre-chilled Petri-dish with sufficient volume of HBSS without Ca+ and Mg+ (Lonza, Walkersville, MD) to submerge the brain. Afterwards, the cerebellum, brain stem, olfactory bulbs, and olfactory tracts were removed, which was followed by careful dissection of the meninges from the remaining cortex. This was repeated for as many brains as desired for a particular isolation. Finally, dissected cortices were transferred into a pre-weighed dish with pre-chilled HBSS without Ca+ and Mg+ and all cortices weighed.
2.4 Tissue dissociation of mouse cortices
Using the Neural Tissue Dissociation Kit (Miltenyi Biotec), an appropriate volume of Enzyme Mix 1 corresponding to the cortical tissue mass was prepared. For every 400 mg of tissue, 50 μl of Solution 1 was added to 1.9 ml of pre-made Solution 2 with β-ME. This was briefly vortexed and then preheated in a 37°C water bath for 10 minutes before use. As Enzyme Mix 1 was heating, 3 pasteur pipettes were fire polished with decreasing tip diameters and set aside for later use. Brains were transferred to a 0.5 ml drop of HBSS in the middle of a fresh 10 cm dish. Cortices were diced thoroughly (1 mm3 pieces) with a razor blade, then 5–6 ml of HBSS was added then tissue pippetted into a 15 ml conical tube. The dish was rinsed well with fresh HBSS to collect any remaining tissue and added into the 15 ml conical tube. Tissue was centrifuged at 300 × g for 2 minutes at room temperature (RT). Supernatant was then carefully aspirated, followed by addition of the pre-heated Enzyme Mix 1 to the pellet, and then gently mixed taking care to avoid air bubbles. Tissue was then incubated for 15 minutes in the 37 °C water bath, inverting the tube several times every 5 minutes to resuspend settled cells. During incubation, an appropriate volume of Enzyme Mix 2 was prepared. For every 400 mg of tissue, 20 μl of Solution 3 was added to 10 μl of Solution 4. Once the incubation was complete, Enzyme Mix 2 was added to the tube containing the tissue. This was inverted gently to mix and then slowly triturated approximately 15 times using the widest fire-polished pipette again taking care to avoid air bubbles. This was then incubated in a water bath for 10 minutes, inverting several times after 5 minutes of incubation. Tissue was slowly triturated approximately 15–20 times each using the remaining two fire-polished pipettes in decreasing diameter. Then tissue was incubated again in a water bath for 10 minutes, inverting several times after 5 minutes of incubation. During the incubation, a 40 μm cell strainer was pre-wetted with 5 ml DPBS into a 50 ml conical tube. DPBS was then aspirated from the tube and following the tissue incubation, the suspension was slowly decanted through the strainer. The 15 ml tube with the tissue was rinsed with 10 ml of HBSS with Ca+ and Mg+ (Invitrogen) and added to the strainer. The cell suspension was centrifuged at 300 × g for 10 minutes at RT. The supernatant was gently aspirated, leaving approximately 2–5 ml of supernatant in the tube, cells were washed with 10 ml of HBSS with Ca+ and Mg+ and centrifuged 300 × g for 10 minutes at RT. The supernatant was aspirated and the pellet gently resuspended in 5 ml MACS buffer. A small aliquot was then removed for a cell count and the cell suspension transferred to a 15 ml tube and centrifuged 300 × g for 5 minutes. During centrifugation, the total number of live cells was counted by Trypan Blue visualization. Approximately 107 total cells/brain were isolated.
2.5 Magnetic cell sorting (MACS) for OPC enrichment
Following the total cell count, the supernatant was gently aspirated and rat anti-mouse IgM magnetic beads (10% v/v in MACS Buffer, Miltenyi Biotec) were added to the cells to facilitate the removal of cell debris and dead cells. The pellet was gently resuspended and incubated for 15 minutes at 4°C with gentle shaking. As cells may settle, cells were mixed by tapping the tube every 5 minutes. Following incubation, cells were resuspended in 5 ml MACS buffer and centrifuged at 300 × g for 5 minutes at RT. During centrifugation, we set up a MACS MS column on a MACS magnetic stand (Miltenyi Biotec) and equilibrated the column with 0.5 ml of MACS buffer. A 15 ml conical tube was then positioned below the column to collect the flow-through. Following the spin, cells were resuspended in 0.5 ml MACS buffer, transferred to the column, and the column was washed four times with 0.5 ml MACS buffer. The initial flow through and all subsequent washes were collected in a single 15 ml tube and centrifuged at 300 × g for 5 minutes. Non-specifically bound cell debris and dead cells remained in the column and it was discarded. Following centrifugation, the cell pellet was resuspended in 400 μl of diluted O4 antibody (20% v/v in MACS buffer) (Cao et al., 2010). This was incubated 5–10 minutes at 4°C with gentle shaking and mixed once by tapping. The cell suspension was then washed two times by adding 5 ml MACS buffer followed by centrifugation at 300 × g for 5 minutes. During centrifugation, an additional MACS rat anti-mouse IgM beads mix was prepared based on the total cell count. For every 1 × 107 cells, 20 μl MACS beads were mixed with 180 μl MACS buffer. Following centrifugation, cells were resuspended in the second MACS beads mix and incubated 15 minutes at 4°C with gentle shaking. If cells settled, the suspension was mixed by tapping every 5 minutes. Afterwards, cells were resuspended in 5 ml MACS buffer and centrifuged at 300 × g for 5 minutes. During centrifugation, another MACS column was equilibrated with 0.5 ml MACS buffer as described above. The pellet was resuspended in 0.5 ml MACS buffer and added to the column, washed four times with 0.5ml MACS buffer. Afterwards, the column was removed from the magnet, positioned above a fresh 15ml tube, and bound cells eluted in 1 ml MACS buffer into the tube. Air bubbles were unavoidable during this step; however, we minimized their contact with the cell suspension at the bottom of the tube. A fresh column was quickly equilibrated, eluted cells added, the column washed four times with 0.5 ml MACS buffer, and cells eluted into a fresh 15 ml tube in 1 ml pre-equilibrated OPC-A medium. 1 ml of additional medium was added to this cell suspension and mixed thoroughly. Cells were counted, plated in a PDL/laminin-coated 10 cm tissue culture dish, and incubated in 37°C, 5% CO2. Counts averaged 3.68 × 105 OPCs/brain with a cell viability range of 85–95%. Approximately 9,000–15,000 cells/cm2 were plated for a successful OPC isolation.
2.6 OPC expansion, differentiation, and OPC/DRG co-culture
The day following OPC isolation, all medium was removed from the plate and replaced with fresh, pre-equilibrated OPC-A media. For subsequent daily medium changes, ½ medium was removed from the culture dish and replaced with pre-equilibrated OPC-A media. Cells were ready to passage within 6–9 days, depending on plating density and proliferative rate. Passaging was performed using Accutase® (Sigma), which contains a protease with collagenilytic activity to allow for gentle passaging of cells, according to the manufacturer’s instructions. Briefly, media was removed and plated OPCs were rinsed once with DPBS. Immediately afterwards, Accutase® was added (approximately 1 ml/10 cm2) and incubated at 37°C for 10 minutes. Following incubation, about half of the Accutase® containing cells was removed and the plate rinsed 2–3 times to remove remaining adherent cells. The cell suspension was transferred to a sterile tube, the plate washed with 3–4 ml of OPC-A media, and added to the cell suspension. Immediately, the cells were centrifuged at 300 × g for 5 minutes at RT. The supernatant was gently aspirated and cells resuspended in 2 ml of pre-equilibrated OPC-A media. Ideal plating densities of passage 1 OPCs typically ranged between 7,500–10,000 cells/cm2. Following passage, OPCs were fed with ½ media change with pre-equilibrated OPC-A every other day until used for experimentation.
For OPC differentiation, passage 1 OPCs were allowed to expand in OPC-A media for 3–5 days then replaced with pre-equilibrated Differentiation media. Depending on desired state of maturation, OPCs were allowed to differentiate in Differentiation media for 3–10 days with ½ media change with pre-equilibrated Differentiation media every other day. For OPC/DRG co-cultures, DRGs were isolated from E15 Sprague-Dawley rat embryos as previously described (Cao et al., 2010; Cheng et al., 2007; Plant et al., 2002). Following isolation, DRGs were plated 2,000 cells/cm2 and maintained on collagen coated coverslips in NB1 neurobasal medium to permit neurite outgrowth for at least three weeks and no more than four weeks prior to seeding with passage 1 OPCs. Once seeded, OPC/DRG co-cultures were maintained 7–14 days in Differentiation media without Penicillin/Streptomycin to permit OPC maturation with ½ media change every other day using pre-equilibrated media.
2.7 Immunocytochemical analyses
Immunocytochemical analysis of OPC cultures in OPC-A and Differentiation media and OPC/DRG co-cultures were done as previously described (Cao et al., 2010). Briefly, cells were washed in DPBS and then incubated with the respective hybridoma supernatants for 45 minutes at 4°C. Afterwards, cells were fixed in 4% paraformaldehyde at RT for 10 minutes. Cells were blocked and incubated overnight with additional non-hybridoma primary antibodies in 4°C with 10% normal donkey serum (NDS, Jackson ImmunoResearch, West Grove, PA), 0.5% BSA (Sigma), and 0.1% Triton X-100 in DPBS. The following day, cells were incubated in secondary antibody at RT for one hour in 5% NDS, 0.5% BSA, and 0.1% Triton X-100 in DPBS. Antibodies used include mouse anti-A2B5 IgM, mouse anti-O4 IgM, and mouse anti-O1 IgM (hybridoma derived, 70% v/v in 20% DPBS, and 10% NDS), rabbit anti-NG2 (1:500, Millipore), rabbit anti-βIII-tubulin (1:2000, Covance, Princeton, NJ), rat anti-MBP (1:100, Millipore), rabbit anti-GFAP (1:500, Dako, Glostrup, Denmark), and rabbit anti-Map2 (1:100, Sigma). Cells were incubated with FITC, Texas Red, and Rhodamine Red conjugated F(ab′)2 fragment antibodies (Jackson Immunoresearch). Negative controls for antibody staining included the appropriate species-specific non-immune IgG or IgM antibodies substituted for the primary antibodies. 10X and 20X photomicrographs were captured with a Nikon TiE 300 inverted microscope equipped with a DXM-1200C coded digital camera and NIS Elements software (Nikon, Melville, NY). All quantitative data are presented as means +/− S.D.
3. RESULTS
3.1 OPC Enrichment
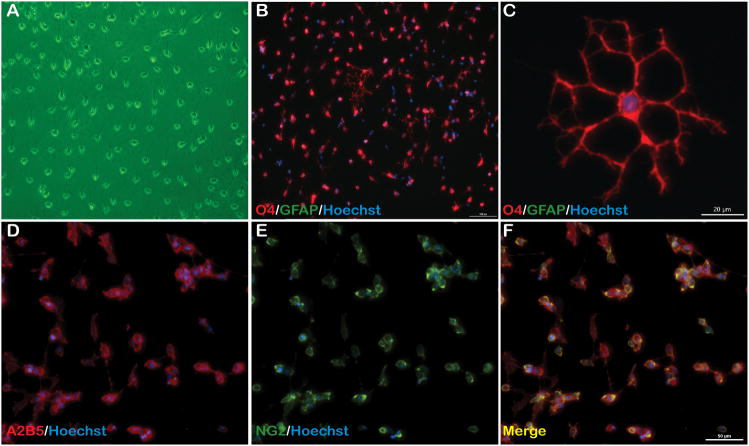
Using postnatal day 5–7 mouse cortices, we achieve a yield of 3.68×105 +/− 9.17×104 cortical OPCs/brain, which is approximately 3–4% of total dissociated cells. From 3 days post plating, enriched OPCs are phase bright with typical bipolar morphology (Fig. 1A). Furthermore, at passage 1 the cells remained almost entirely A2B5+ and NG2+ (Fig. 1D–F), demonstrating an expanded population of enriched OPCs positive for early OPC markers. All cells at passages 0 and1 were GFAP (Fig. 1B–C) and β-III tubulin (data not shown) negative indicating enrichment of a cell population devoid of astrocyte and neuronal contamination, respectively.
Figure 1. Isolated cells express multiple OPC specific surface markers.
(A) Phase contrast image of OPCs 3 days following isolation showing typical OPC morphology and (B) isolated cells are O4+ and GFAP−, 10X. (C) O4+ OPCs form multiple processes following plating; scale bar = 20 μm. (D–F) Following initial passage, OPCs are A2B5+/NG2+, indicative of early OPC surface markers; scale bar = 50 μm, 20X.
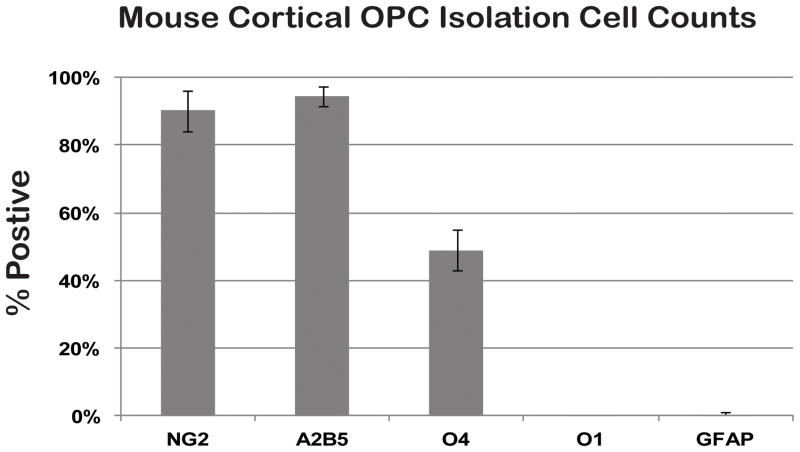
Following passage, despite being A2B5+ and NG2+, the percentage of O4+ cells declined from approximately 80% following OPC isolation (data not shown) to just under 50% of the total cell population (Fig. 2). Nonetheless, over 90% of all cells remained both A2B5+ and NG2+ with continued expansion (Fig. 2). Further quantification revealed that the isolated OPCs remained immature as they were not O1+ OLs and did not have any GFAP+ astrocytes (Fig. 2). These data suggest with continued expansion in vitro, O4 antibody binding declines but other markers remain positive indicating maintenance of the OPC phenotype.
Figure 2. P1 OPC cell counts show an enriched population of OPCs.
Approximately 50% of passage 1 OPCs are O4+ and over 90% of cells are both NG2+ and A2B5+. Cells were O1− and GFAP− indicating an enriched population of expanding OPCs. N=3, +/− SD.
3.2 OPC Differentiation
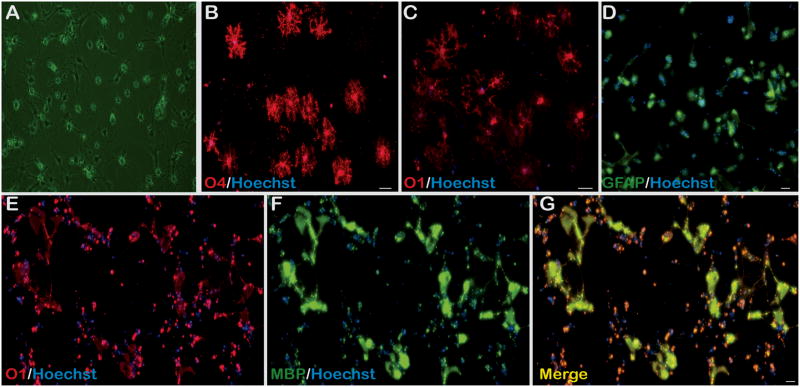
Mouse cortex-derived OPC differentiation may commit them into either GFAP+ astrocytes or OLs (Fig. 3). OPCs differentiate into astrocytes at 4–6 days in the presence of serum and the absence of PDGF-AA and FGF2 as evidenced by nearly 100% GFAP+ immunoreactivity with minimal O4 and O1 immunoreactivity (data not shown). However, when OPC growth media is replaced by low serum media without PDGF-AA and FGF2 but containing thyroid hormone (T3), differentiation into a mixed glial population occurs. There is reduced number of cells that are phase bright following 4 days of differentiation (Fig. 3A). Nearly 40% of all OPCs form a dramatically more complex arborization pattern of O4+ OLs (Fig. 3B, 4). This coincides with the approximately one-third O1+ population of differentiated OPCs with a comparatively complex arborization (Fig. 3C, 4). However, this is accompanied by differentiation of nearly one-third of OPCs to GFAP+ astrocytes (Fig. 3D, 4). OPCs differentiated into OLs also demonstrated myelin basic protein (MBP) co-immunostaining with O1+ OLs in the presence of our standard Differentiation media, though mature OLs were in the minority (Fig. 3E–G).
Figure 3. OPC differentiation into mature OLs.
(A) 4 days following initiation of OPC differentiation, cells are less phase bright and form more processes, 10X. (B–D) Following 7 days of incubation in differentiation media, OLs are highly arborized and are O4+ and O1+. However, there is astrocyte formation as well evidenced by GFAP+ cells, 10X. (E–G) O1+ OLs are also MBP+ indicating enhanced maturation and the beginning of myelin synthesis, 10X.
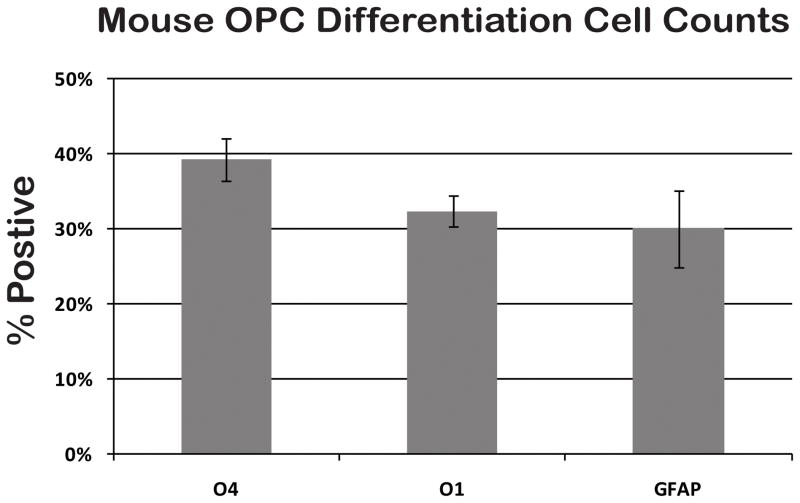
Figure 4. OPC differentiation counts.
Passage 1 OPCs differentiated for 4–6 days resulted in a relatively equal distribution of O1+ OLs and GFAP+ astrocytes with a slightly higher percentage of O4+ OLs. N=2, +/− SD.
3.3 OPC/DRG Co-Culture
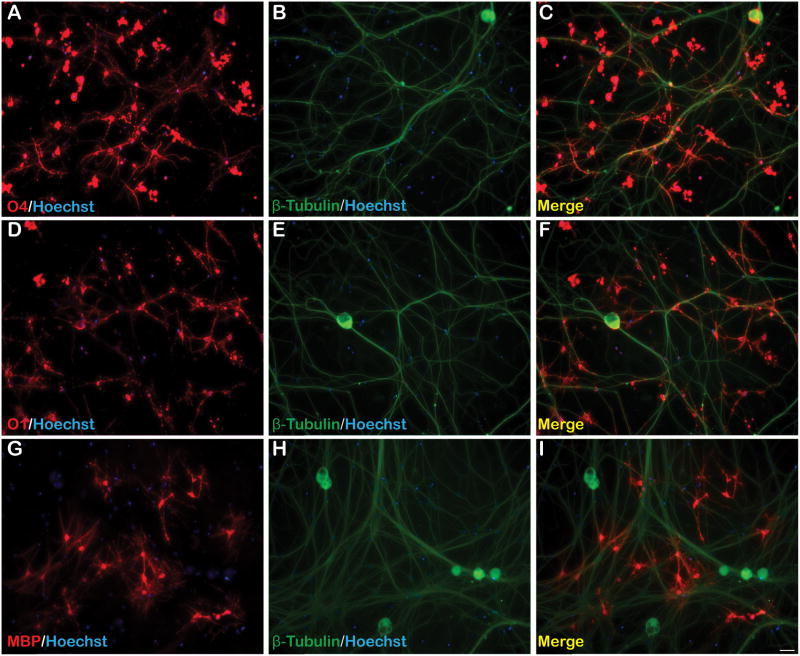
When co-cultured with rat embryonic day 15 DRGs, cortically isolated OPCs consistently aligned with DRG neurites and differentiated into mature OLs (Fig. 5). Following 7 days of incubation, co-cultures were A2B5− NG2− indicating maturation from their precursor phenotype (data not shown). Furthermore, OPCs differentiated into OLs as demonstrated by extensive O4+ and O1+ immunostaining of complex and highly arborized OLs (Fig. 5A–F). DRG neurite-aligned OLs were also MBP+ suggesting myelin synthesis along DRG axons (Fig. 5G–I). O4 and O1 immunoreactivities, and MBP expression were maintained in co-cultures following 14 days of incubation.
Figure 5. OPC maturation into MBP+ OLs in OPC/DRG co-culture.
Passage 1 OPCs co-cultured with rat embryonic DRGs resulted in OPC maturation into highly arborized (A–C) O4+ and (D–F) O1+ OLs 7 days following plating. (G–I) Numerous OLs formed MBP+ processes around β-tubulin+ DRG axons. All photomicrographs are 10X magnification.
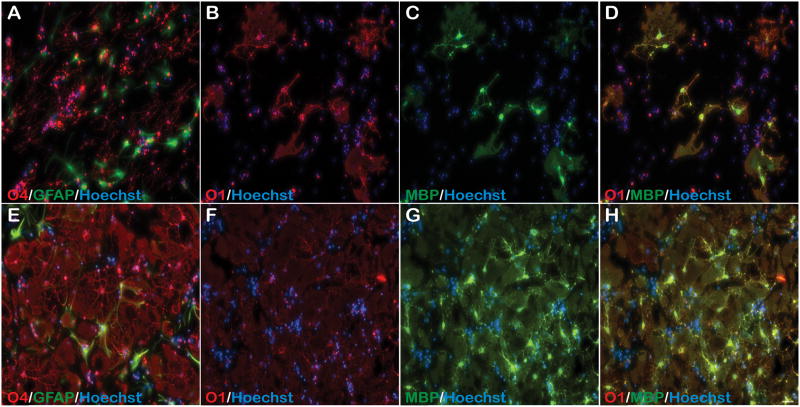
Due to reduced OPC- derived astrocyte differentiation in OPC/DRG co-cultures, we next differentiated OPCs in the presence of Differentiation media containing conditioned media (CM) collected from 7 day incubated co-cultures (Fig. 6). Following 7 days of incubation, OL maturation comparable to cultures without co-culture CM (Fig. 3) was observed as evident by increased O4+, O1+ and MBP+ OLs (Fig. 6A–D). However, GFAP+ astrocytes were still present as observed with standard Differentiation media (Fig. 6A).
Figure 6. CNTF enhances OPC differentiation in the presence of OPC/DRG co-culture CM.
(A–D) CM collected from OPC/DRG co-cultures were added to differentiating passage 1 OPCs for 7 days resulting in modest differentiation into more complex O4+, O1+ and MBP+ OLs. (E–H) However, when this was supplemented with CNTF, complex O4+, and highly mature O1+/MBP+ OLs formed. This too was accompanied by GFAP+ astrocyte differentiation. All photomicrographs are 10X magnification.
In vitro OPC differentiation leads to dramatic cell death (data not shown). We previously demonstrated ciliary neurotrophic factor (CNTF) improved adult rat spinal cord derived OPC survival both in vitro, upon transition to Differentiation media, and in vivo following engraftment into the injured spinal cord (Cao et al., 2010). Differentiation media supplemented with 1 ng/ml CNTF improved survival of mouse OPCs as well (data not shown). Therefore, 1ng/ml CNTF was also added to OPCs differentiating co-culture CM to determine if increased OPC survivability during the early phase of OPC maturation will preferentially increase the number of OPCs differentiating into OLs (Fig. 6). Following 7 days of incubation, highly complex and mature OLs that were O4+, O1+, and MBP+ had formed (Fig. 6E–H). This extent of OPC maturation was not previously seen and was only achieved with CNTF supplementation. However, OLs were also accompanied by OPCs differentiating into GFAP+ astrocytes revealing the continued bi-potentiality of cortically-derived postnatal OPC differentiation in vitro, with or without CNTF supplementation (Fig. 6E).
4. DISCUSSION
While numerous reports document the isolation of rat OPCs from embryonic, neonatal, and adult CNS, preparations of similarly-derived mouse OPCs has proved problematic (Chen et al., 2007; Lin et al., 2006). Previously described mouse OPC culture techniques involve mouse embryonic neurosphere formation followed by oligosphere induction for the production of large OPC cell numbers (Chen et al., 2007; Zhao et al., 2010). Additionally, immunopanning of postnatal mouse brains have previously been described. However, these OPCs were positively selected with an antibody identifying PDGFR-AA or using the A2B5 monoclonal antibody, the prior selecting for cells early in the OPC lineage and the latter may not select exclusively for cells that will differentiate within the oligodendroglial lineage (Cahoy et al., 2008; Dugas et al., 2010; Larsen and Yong, 2004; Nicolay et al., 2007). A2B5+ OPCs have been enriched from postnatal mouse brains by magnetic cell sorting (Hamanoue et al., 2009; Larsen and Yong, 2004). However, as the authors acknowledge, single-cell derived clones are selected for subsequent plating, raising concerns that the cells are in fact immortalized and may be genomically different from primary cells, a feature commonly observed with cell lines. To our knowledge, this is the first O4+ OPC primary culture method derived from mouse postnatal cortex that generates large numbers of OPCs without relying on cell immortalization, neurospehere/oligosphere formation, or clonal expansion.
The genetic, epigenetic, and molecular identity of OPCs derived from in vitro “sphere” formation are likely to be considerably different as their induction is biologically more distant from their in vivo environment than observed with primary cultures (D’Amour and Gage, 2003; Gage et al., 1995). Furthermore, selection of cell clones derived from a single cell following isolation is likely not representative of the primary isolated OPC population (D’Amour and Gage, 2003; Gage et al., 1995). The generation of single-cell derived clones necessitates that a single OPC will undergo putative proliferative changes in order to generate subsequent clones (Lin et al., 2006). Again, these changes are likely not present in primary cultures, particularly when limited in passage number.
Although this method enriches OPCs based on O4 antibody binding, once passaged, they are both A2B5+ and NG2+ as well, indicating overlapped expression of all three cell markers from cortical OPC isolates. With initial passage and expansion, the number of O4+ OPCs declines whereas A2B5+ NG2+ OPCs are maintained. Immunocytochemical studies of mouse OPCs 2–3 days following isolation show little to no A2B5+ staining suggesting O4+ later-stage OPCs de-differentiate into A2B5+ NG2+ O4− early-stage OPC phenotype (data not shown). Despite reduced O4 binding, the OPCs maintain the capacity to differentiate into OLs, though the potential to become astrocytes is still present.
Consistent with reported data for both rat and mouse OPCs, these OPCs maintain the capacity to become both astrocytes and OLs (Nishiyama, 1998; Noble et al., 1992; Trotter et al., 2010). Though CNTF did reduce the extent of cell death upon differentiation (as seen with adult rat spinal cord OPCs- data not shown), the number of GFAP+ astrocytes increased (data not shown) (Cao et al., 2010). Despite co-culturing in non-species matched proliferative precursors, OPC/DRG co-cultures resulted in OPC maturation into O1+ positive OLs with limited MBP immunoreactivity and minimal GFAP+ astrocyte formation. OPCs differentiated in the presence of co-culture conditioned media supplemented with CNTF resulted in extensive O1+ and MBP+ OL formation with highly complex arborization not seen without the presence of CNTF. However, a considerable number of differentiated astrocytes were observed as well supporting a previously described role for CNTF (Whittemore et al., 1999). This suggests that CNTF may be playing a synergistic role with soluble factors in the co-culture CM to dramatically enhance OPC differentiation, but further investigation is needed to determine whether greater differentiation along the oligodendroglial lineage vs. the astrocytic lineage in the presence of CNTF is possible and/or necessary.
There are a number of limitations of this cortical OPC isolation method. If desired, isolation of post-mitotic O4+ OPCs or OLs will be difficult using this protocol as these cells will be increasingly arborized and such cell processes will be damaged during tissue dissociation leading to their likely cell death. Though limiting passage number was preferred, attempts to passage primary OPCs beyond passage 2 resulted in cells morphologically resembling type 2 astrocytes (data not shown). Additionally, one freeze/thaw cycle is sufficient to cause OPCs to spontaneously differentiate into astrocytes (data not shown). Therefore, fresh OPC primary cultures are necessary and it is not recommended to take them further than passage 2.
Lastly, this protocol is yet to be fully optimized for OPC differentiation exclusively into mature myelinating OLs. This may be due to either mouse O4+ OPCs continuing to retain a capacity under these conditions to differentiate into astrocytes, O4+ OPC de-differentiating into A2B5+ NG2+ O4− early-stage OPCs resulting in cells that are permissive to an astrocytic lineage in addition to an oligodendroglial lineage once differentiation is induced, or a combination of the two. Nevertheless, this further demonstrates the need for identification of additional factors to accompany T3 that may be necessary for high percentage mouse OPC differentiation into OLs.
Despite these issues, this method permits isolation of mouse OPCs that obviate potential genomic instability and variability that occur with extended passage of other OPC culturing techniques. As a result, we contend that these mOPCs are as similar as possible to their counterparts in vivo and serve as an improved in vitro model for the study of mOPCs in development, disease, and trauma.
HIGHLIGHTS.
Characterization of a reproducible primary O4+ cortical mouse OPC isolation method.
Mouse OPCs remained O4+ and NG2+ with expansion and continued passaging.
OPC alignment with neurites and subsequent maturation occur in rat DRG co-culture.
Acknowledgments
We would like to thank Russell Howard for his continued assistance with cell culture and Kariena Andres for excellent animal care. This work was supported by the Kentucky Spinal Cord and Head Injury Research Trust, the Commonwealth of Kentucky Challenge for Excellence, NS054708, RR15576, and RR031159.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bansal R, Pfeiffer SE. Novel stage in the oligodendrocyte lineage defined by reactivity of progenitors with R-mAb prior to O1 anti-galactocerebroside. J Neurosci Res. 1992;32:309–16. doi: 10.1002/jnr.490320303. [DOI] [PubMed] [Google Scholar]
- Bansal R, Stefansson K, Pfeiffer SE. Proligodendroblast antigen (POA), a developmental antigen expressed by A007/O4-positive oligodendrocyte progenitors prior to the appearance of sulfatide and galactocerebroside. J Neurochem. 1992;58:2221–9. doi: 10.1111/j.1471-4159.1992.tb10967.x. [DOI] [PubMed] [Google Scholar]
- Behar T, McMorris FA, Novotny EA, Barker JL, Dubois-Dalcq M. Growth and differentiation properties of O-2A progenitors purified from rat cerebral hemispheres. J Neurosci Res. 1988;21:168–80. doi: 10.1002/jnr.490210209. [DOI] [PubMed] [Google Scholar]
- Berg GJ, Schachner M. Electron-microscopic localization of A2B5 cell surface antigen in monolayer cultures of murine cerebellum and retina. Cell Tissue Res. 1982;224:637–45. doi: 10.1007/BF00213758. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, He Q, Wang Y, Cheng X, Howard RM, Zhang Y, DeVries WH, Shields CB, Magnuson DS, Xu XM, Kim DH, Whittemore SR. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J Neurosci. 2010;30:2989–3001. doi: 10.1523/JNEUROSCI.3174-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Balasubramaniyan V, Peng J, Hurlock EC, Tallquist M, Li J, Lu QR. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc. 2007;2:1044–51. doi: 10.1038/nprot.2007.149. [DOI] [PubMed] [Google Scholar]
- Cheng X, Wang Y, He Q, Qiu M, Whittemore SR, Cao Q. Bone morphogenetic protein signaling and olig1/2 interact to regulate the differentiation and maturation of adult oligodendrocyte precursor cells. Stem Cells. 2007;25:3204–14. doi: 10.1634/stemcells.2007-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour KA, Gage FH. Genetic and functional differences between multipotent neural and pluripotent embryonic stem cells. Proc Natl Acad Sci U S A. 2003;100 (Suppl 1):11866–72. doi: 10.1073/pnas.1834200100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas JC, Cuellar TL, Scholze A, Ason B, Ibrahim A, Emery B, Zamanian JL, Foo LC, McManus MT, Barres BA. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron. 2010;65:597–611. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth GS, Walsh FS, Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979;76:4913–7. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–82. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–92. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- Hamanoue M, Matsuzaki Y, Sato K, Okano HJ, Shibata S, Sato I, Suzuki S, Ogawara M, Takamatsu K, Okano H. Cell surface N-glycans mediated isolation of mouse neural stem cells. J Neurochem. 2009;110:1575–84. doi: 10.1111/j.1471-4159.2009.06256.x. [DOI] [PubMed] [Google Scholar]
- Jackman N, Ishii A, Bansal R. Oligodendrocyte development and myelin biogenesis: parsing out the roles of glycosphingolipids. Physiology (Bethesda) 2009;24:290–7. doi: 10.1152/physiol.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Kramer E, Grzenkowski M, Tang K, Blakemore W, Aguzzi A, Khazaie K, Chlichlia K, von Blankenfeld G, Kettenmann H, et al. Lines of murine oligodendroglial precursor cells immortalized by an activated neu tyrosine kinase show distinct degrees of interaction with axons in vitro and in vivo. Eur J Neurosci. 1995;7:1245–65. doi: 10.1111/j.1460-9568.1995.tb01115.x. [DOI] [PubMed] [Google Scholar]
- Larsen PH, Yong VW. The expression of matrix metalloproteinase-12 by oligodendrocytes regulates their maturation and morphological differentiation. J Neurosci. 2004;24:7597–603. doi: 10.1523/JNEUROSCI.2092-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Xiang Z, Cui L, Stallcup W, Reeves SA. New mouse oligodendrocyte precursor (mOP) cells for studies on oligodendrocyte maturation and function. J Neurosci Methods. 2006;157:187–94. doi: 10.1016/j.jneumeth.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Louis JC, Magal E, Muir D, Manthorpe M, Varon S. CG-4, a new bipotential glial cell line from rat brain, is capable of differentiating in vitro into either mature oligodendrocytes or type-2 astrocytes. J Neurosci Res. 1992;31:193–204. doi: 10.1002/jnr.490310125. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Mock DJ, Strathmann F, Blumberg BM, Mayer-Proschel M. Infection of murine oligodendroglial precursor cells with Human Herpesvirus 6 (HHV-6)--establishment of a murine in vitro model. J Clin Virol. 2006;37 (Suppl 1):S17–23. doi: 10.1016/S1386-6532(06)70006-3. [DOI] [PubMed] [Google Scholar]
- Nicolay DJ, Doucette JR, Nazarali AJ. Transcriptional control of oligodendrogenesis. Glia. 2007;55:1287–99. doi: 10.1002/glia.20540. [DOI] [PubMed] [Google Scholar]
- Nishiyama A. Glial progenitor cells in normal and pathological states. Keio J Med. 1998;47:205–8. doi: 10.2302/kjm.47.205. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Noble M, Wren D, Wolswijk G. The O-2A(adult) progenitor cell: a glial stem cell of the adult central nervous system. Semin Cell Biol. 1992;3:413–22. doi: 10.1016/1043-4682(92)90012-k. [DOI] [PubMed] [Google Scholar]
- Plant GW, Currier PF, Cuervo EP, Bates ML, Pressman Y, Bunge MB, Wood PM. Purified adult ensheathing glia fail to myelinate axons under culture conditions that enable Schwann cells to form myelin. J Neurosci. 2002;22:6083–91. doi: 10.1523/JNEUROSCI.22-14-06083.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringproa K, Kumnok J, Ulrich R, Baumgartner W, Wewetzer K. In vitro characterization of a murine oligodendrocyte precursor cell line (BO-1) following spontaneous immortalization. Int J Dev Neurosci. 2008;26:283–91. doi: 10.1016/j.ijdevneu.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Raff MC, Abney ER, Cohen J, Lindsay R, Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983;3:1289–300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Mirsky R, Fields KL, Lisak RP, Dorfman SH, Silberberg DH, Gregson NA, Leibowitz S, Kennedy MC. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978;274:813–6. [PubMed] [Google Scholar]
- Schnitzer J, Schachner M. Cell type specificity of a neural cell surface antigen recognized by the monoclonal antibody A2B5. Cell Tissue Res. 1982;224:625–36. doi: 10.1007/BF00213757. [DOI] [PubMed] [Google Scholar]
- Shi J, Marinovich A, Barres BA. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J Neurosci. 1998;18:4627–36. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter J, Karram K, Nishiyama A. NG2 cells: Properties, progeny and origin. Brain Res Rev. 2010;63:72–82. doi: 10.1016/j.brainresrev.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore SR, Morassutti DJ, Walters WM, Liu RH, Magnuson DS. Mitogen and substrate differentially affect the lineage restriction of adult rat subventricular zone neural precursor cell populations. Exp Cell Res. 1999;252:75–95. doi: 10.1006/excr.1999.4621. [DOI] [PubMed] [Google Scholar]
- Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387–400. doi: 10.1242/dev.105.2.387. [DOI] [PubMed] [Google Scholar]
- Zhao X, He X, Han X, Yu Y, Ye F, Chen Y, Hoang T, Xu X, Mi QS, Xin M, Wang F, Appel B, Lu QR. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron. 2010;65:612–26. doi: 10.1016/j.neuron.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]