Abstract
Background
We investigated the effects of cardioplegic arrest and reperfusion (CP/Rep) on coronary arteriolar responses to endothelium-dependent and -independent vasodilators and associated signaling pathways in poorly controlled diabetic, well controlled diabetic and case-matched non-diabetic patients undergoing coronary artery bypass grafting (CABG).
Methods and Results
Coronary arterioles from harvested right-atrial tissues were dissected pre- and post-CP/Rep from poorly controlled diabetic (n = 10, hemoglobin A1c [HbA1c] = 9.3 ± 0.3), well controlled diabetic (n = 10, HbA1c = 6.2 ± 0.2) and non-diabetic patients (n = 10, HbA1c = 5.1 ± 0.1) undergoing CABG surgery. The baseline microvascular response to ADP, substance P and SNP of arterioles from poorly controlled diabetic patients were decreased as compared to the respective response from non-diabetic or well controlled diabetic patients (P < 0.05). The vasodilatory responses to ADP, and substance P after CP/Rep were significantly decreased in all three groups compared to pre-CP/Rep responses (P < 0.05). However, these decreases were more pronounced in the poorly controlled diabetic group (P < 0.05). The expression of protein kinase C-α (PKC-α), PKC-β, and protein oxidation in atrial tissues was significantly increased in the poorly controlled diabetic group as compared with those of non-diabetes or controlled diabetes.
Conclusion
Poorly controlled is associated with endothelium-dependent and -independent vascular dysfunction of coronary arterioles. Additionally, poorly controlled diabetes worsens the recovery of coronary arteriolar function after CP/Rep. These alterations are associated with the increased expression/activation of PKC-α and PKC-β, and enhanced oxidative stress.
Introduction
Cardioplegic arrest and reperfusion (CP/Rep) alters contractile responses of coronary arterioles to numerous vasoactive agents including phenylephrine, endothelin-1, and thromboxane-A2, 1–4 and impairs microvascular endothelial function.5,6 Diabetes is associated with increased risk of microvascular diseases and with increased morbidity and mortality after open heart operations involving cardioplegia and CPB. 7 Recent clinical trials have also shown that glycemic control significantly reduces microvascular complications, such as, retinopathy, nephropathy and peripheral arterial disease.8–10 In addition, peri-operative glucose control is associated with improved outcomes after coronary artery bypass graft (CABG) surgery.11 However, whether glycemic control improves the recovery of coronary microvascular function in patients with diabetes after CP/Rep is not established. The goal of this research is to investigate the effect of diabetes on CP/Rep-induced changed in coronary arteriolar function. Specifically, this study was designed to directly test the effect of CP/Rep on microvascular response of human coronary arterioles to endothelium-dependent and independent vasodilators and to examine study alterations in endothelium-related-protein expression/localization and gene expression in human atrial and vascular tissue from patients with or without diabetes.
Methods
Human Subjects and Tissue Harvesting
Samples of right atrial appendage were harvested from patients undergoing coronary artery bypass grafting (CABG) surgery before and after exposure of the heart to blood cardioplegia and short-term reperfusion under conditions of CPB. Samples were handled in a non-traumatic fashion. Double 3-0 polypropylene purse-string sutures (Ethicon, Somerville, NJ) were placed in the atrial appendage. The first sample of atrial appendage was harvested pre-CP/Rep. During placement of the venous cannula, the superior suture was tightened to secure the venous cannula. The inferior suture remained loose to allow this portion of the atrium to be exposed to blood cardioplegia, and reperfused (post-CP/Rep) after removal of the aortic cross-clamp. An initial 600--800mL of cold-blood (0–4°C) hyperkalemic (15 mmol/L K+) cardioplegic solution was delivered antegrade into the aortic root. This method was followed at 8–20 min intervals with 200–300mL of cold, cardioplegic solution (15 mmol/L K+). The composition of cardioplegic solutions have been previously described in detail.5,6
The second sample of atrial tissue (post-CP/Rep) was harvested between the purse-strings during removal of the venous cannula. Sections of atrial samples were immediately frozen in liquid nitrogen (immunoblotting), fixed in 10% formalin for 24 h followed by paraffinization and sectioning into 5- µm slices (immunoflourescent staining), or placed in cold (5–10°C) Krebs-Henseleit buffer (KHB, microvascular studies).
These patients were then divided into following three groups: 1) poorly controlled diabetic patients (n = 10, hemoglobin A1c [HbA1c] = 9.3 ± 0.3), 2) well controlled diabetic patients (n = 10, HbA1c = 6.2 ± 0.2) and 3) non-diabetic patients (n = 10, HbA1c = 5.1 ± 0.1). All procedures were approved by the Institutional Review Board of Rhode Island Hospital, Alpert Medical School of Brown University, and informed consent was obtained from all enrolled patients as required by the Institutional Review Board.
Microvessel Reactivity
Coronary arterioles (90–160 µm internal diameters) were dissected from pre- and post-CP/Rep right atrial-tissue samples. Microvessel studies were performed by in vitro organ bath video-microscopy as described previously.2–4 We have previously determined that human skeletal muscle microvascular responses to 5’-diphosphate (ADP) and Substance P are endothelium-dependent and the response to sodium nitroprusside (SNP) is endothelium-independent. 6
Immunofluorescence Microscopy
The detailed methods have been previously described.11 After PBS wash of skeletal-muscle tissue sections, sections were incubated overnight with anti-PKC-α (phospho- S657 + Y658), and anti-PKC-β1 (phospho- T642) (abcam, Cambridge, MA, each used at 1:200) at 4°C.
RNA Isolation and Microarray Processing
Total RNA was isolated from ≈200 mg of skeletal muscle-tissue samples with a Trizol-based method, following the manufacturer’s protocol (Gibco BRL). The methods for RNA isolation and microarray processing have been previously described in detail.12,13,18
Microarray Analysis
Transcriptional profiling was performed on HG-U133 plus 2.0 Affymetrix chips. After quality control analysis, Chips were normalized using the Robust Multichip average (RMA) statistical method, and gene expression in post-CPB samples compared to pre-CPB samples or ND and UDM using one-way ANOVA. A post-hoc false detection rate (FDR) algorithm with alpha of 0.05 was applied to control for false positives. Fold Changes >2 or <-2 were considered real changes, and −log10(P value) > 3.48 were considered significant.
Chemicals
ADP, Substance P, SNP, were obtained from Sigma-Aldrich and dissolved in ultrapure distilled water and prepared on the day of the study.
Data Analysis
Data are presented as the mean and standard error of the mean (SEM). Microvascular responses are expressed as percent relaxation of the pre-constricted diameter. Microvascular reactivity was analyzed using 2 way repeated-measures ANOVA with a post hoc Bonferroni test. Clinical data were analyzed by one way ANOVA with Newman-Keuls Multiple Comparison post hoc test (GraphPad Software, Inc, San Diego, CA). P values < 0.05 were considered significant.
Results
Patient Characteristics
The patient characteristics are listed in table 1. All patients with preoperative hypertension were on anti-hypertensive medication (β-blocker, aspirin, calcium channel blocker, or angiotensin-converting enzyme inhibitor).
Table 1.
Patient Characteristics
| Patient Characteristics | Non-diabetes | Controlled Diabetes |
Uncontrolled Diabetes |
P values |
|---|---|---|---|---|
| Age (y) | 68 ± 4.5 | 66 ± 3.0 | 64 ± 4.0 | 0.5 |
| Male/Female | 10/3 | 10/2 | 10/3 | NS |
| Patient Blood Glucose (mg/dL) | 108 ± 10.0 | 164± 11.0 | 255 ± 15.0 | 0.05 |
| HbA1c (%) | 5.1 ± 0.1 | 6.2 ± 0.2 | 9.3 ± 0.3 | 0.05 |
| Obesity (BMI>30) | 3 | 4 | 4 | NS |
| Hypertension (n) | 9 | 8 | 9 | NS |
| Atrial fibrillation (n) | 2 | 2 | 1 | NS |
| Hypercholestesterolemia | 10 | 10 | 10 | NS |
| CABG only (n) | 10 | 10 | 10 | NS |
NS: no significance; BWI: body mass index;
Microvascular Reactivity
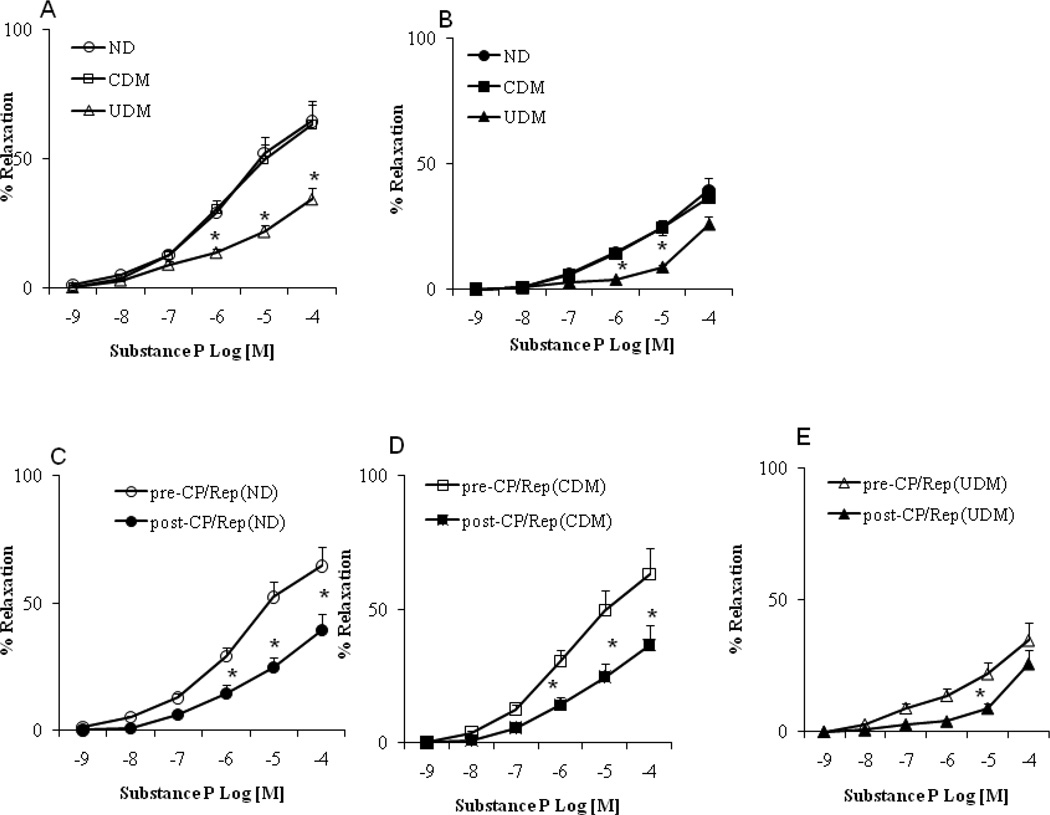
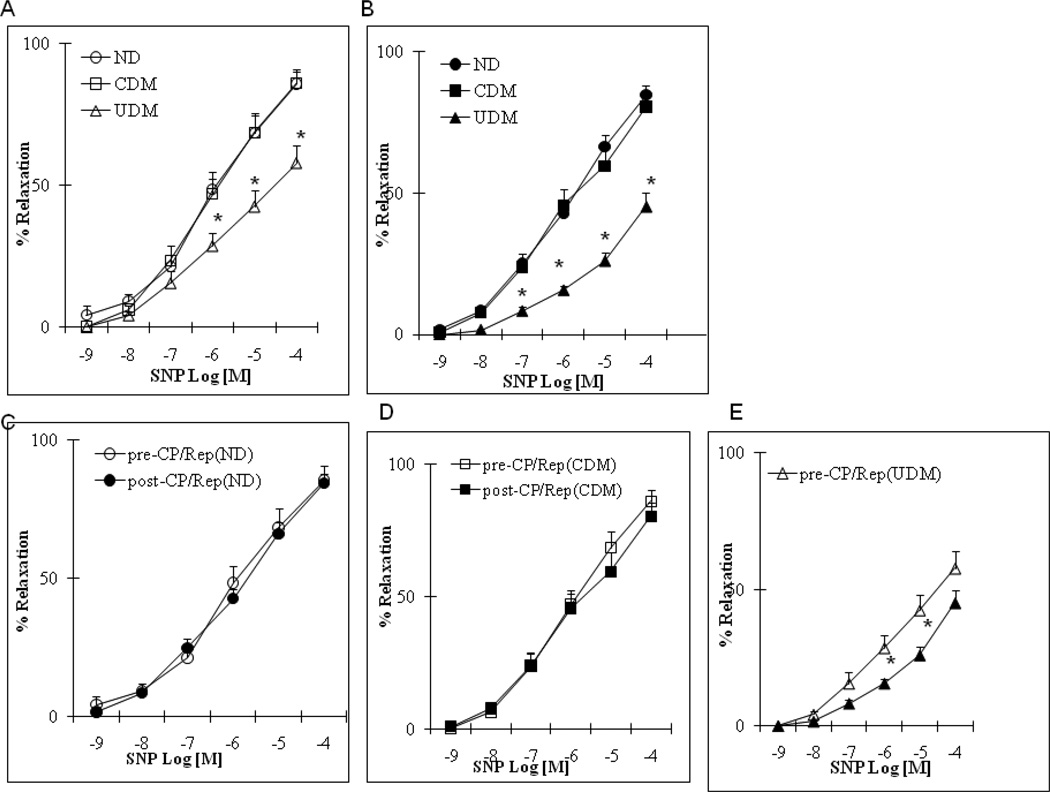
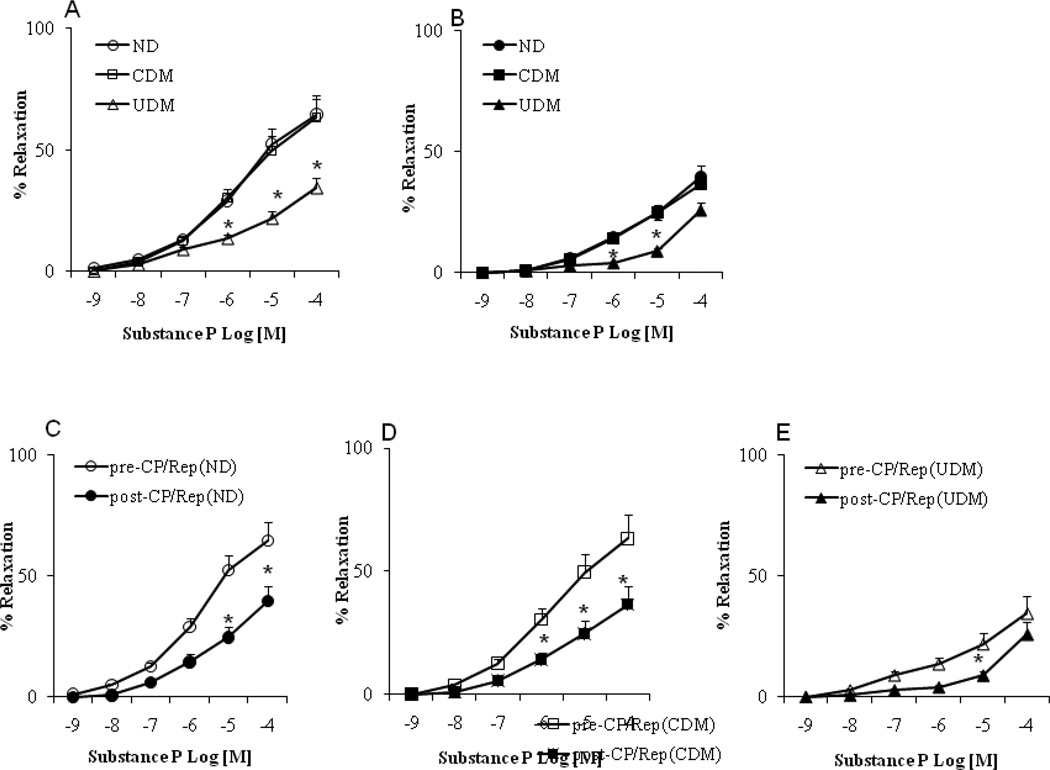
There were no significant differences in the arteriolar responses to ADP, substance P and SNP between the non-diabetic (ND) and well controlled diabetic (CDM) patients before CP/Rep (Figure 1–3A, respectively). There were significant decreases in the arteriolar responses to ADP, substance P and SNP of the poorly (un)controlled diabetic (UDM) patients compared with those of ND or CDM before CP/Rep (P <0.05, Figure 1–3A). There were significant decreases in the vasodilatory response to ADP and substance P post-CP/Rep compared to pre-CP/Rep in all three groups (P <0.05, Figure 1–3C-E). However, the decreases were more pronounced in the UDM group (P <0.05 vs. ND or CDM, Figure 1–3B). There were no significant changes in the vasodilatory response to SNP post-CPB in ND and CDM groups compared to pre-CP/Rep (Figure 3A). In contrast, the post-CP/Rep responses to SNP in poorly controlled diabetic patients were significantly decreased (P<0.05, Figure 3C). There was no significant difference in the baseline diameter of the microvessels among the three groups (ND: 121 ± 4.5; CDM: 118 ± 4.6; UDM: 120 ± 5.0; P>0.05). The degrees of pre-contraction by the thromboxane A2 analog U46619 were 31 ±2 % in the ND group, the 30 ± 3 % in the CDM group and 28. ± 2 % in the UDM group, respectively.
Figure 1.
Coronary microvascular vasodilation in response to the endothelium-dependent vasodilator adenosine 5’-diphosphate (ADP): (A) pre-CP/Rep arterioles from non-diabetic (ND), controlled (CDM) and uncontrolled diabetic (UDM) groups; (B) post-CP/Rep arterioles of ND, CDM and UDM groups; (C) pre-CP/Rep vs. post-CPB (ND); (D) pre-CPB vs. post-CPB (CDM); (E) pre-CP/Rep vs. post-CP/Rep (UDM); *P<0.05 vs. pre-CP/Rep, or ND or CDM; n=10/group.
Figure 3.
Coronary microvascular vasodilation in response to the endothelium-independent vasodilator sodium nitroprusside (SNP): (A) pre-CP/Rep arterioles from ND, CDM and UDM groups; (B) Post-CP/Rep arterioles of ND, CDM and UDM groups; (C) pre-CP/Rep vs. post-CP/Rep (ND); (D) pre-CP/Rep vs. post-CP/Rep (CDM); (E) pre-CP/Rep vs. post-CP/Rep (UDM); *P<0.05 vs. ND; #P<0.05 vs. CDM; n = 10/group.
Effect of CP/Rep on Microvessel Distribution of Phosphorylated PKC-α and PKC-β1
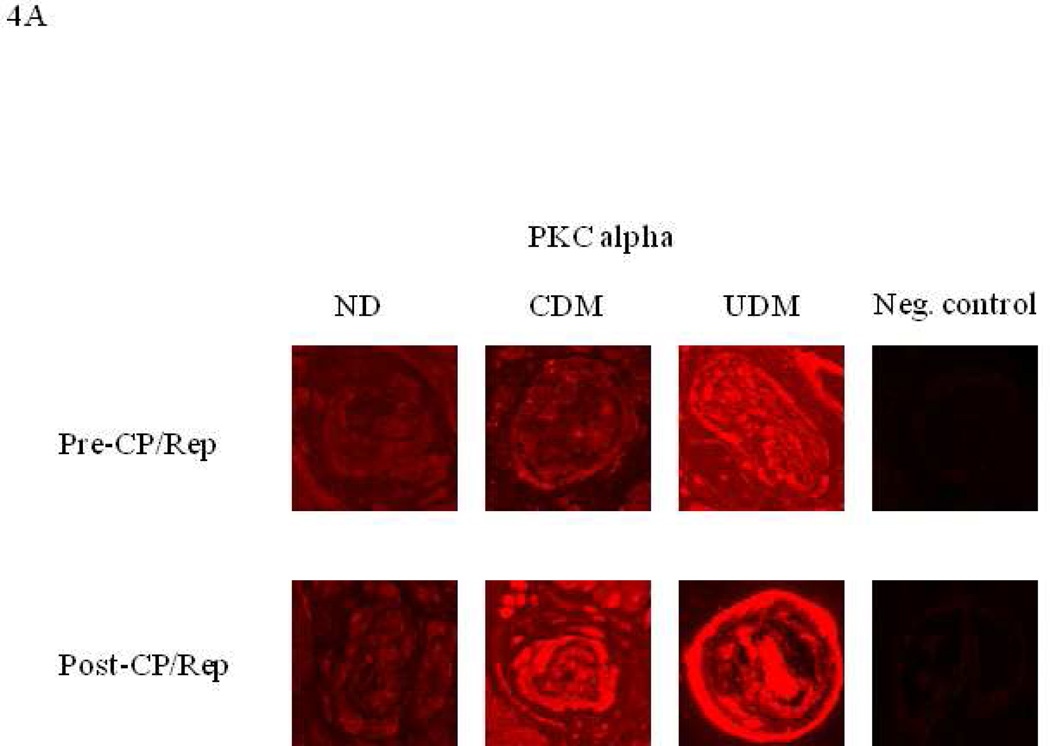
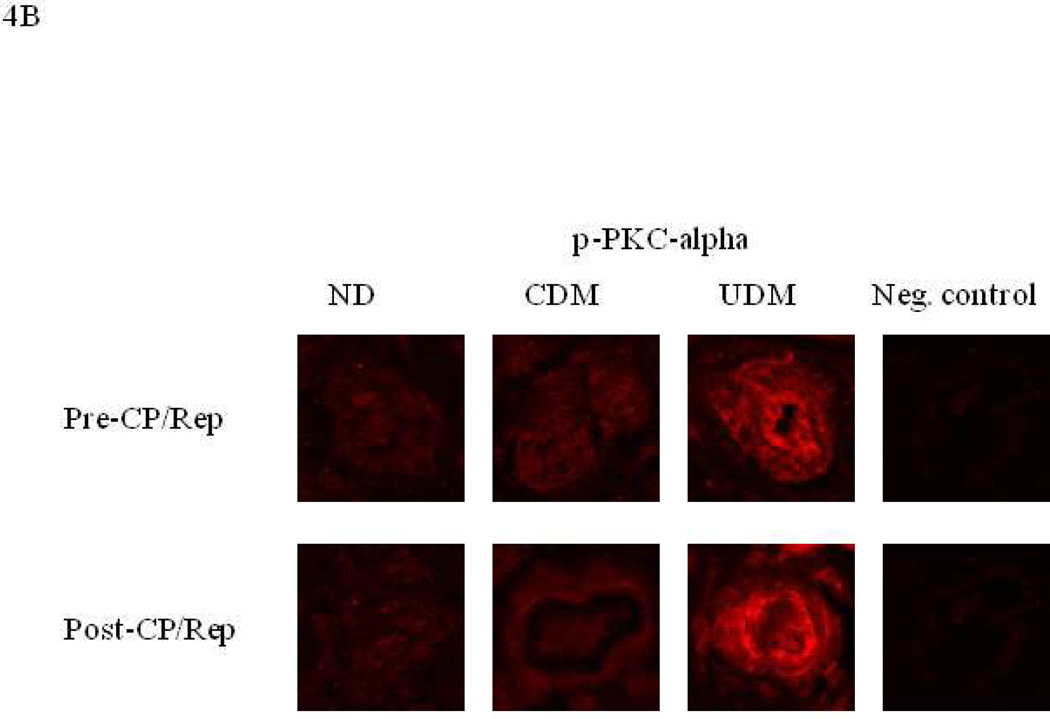
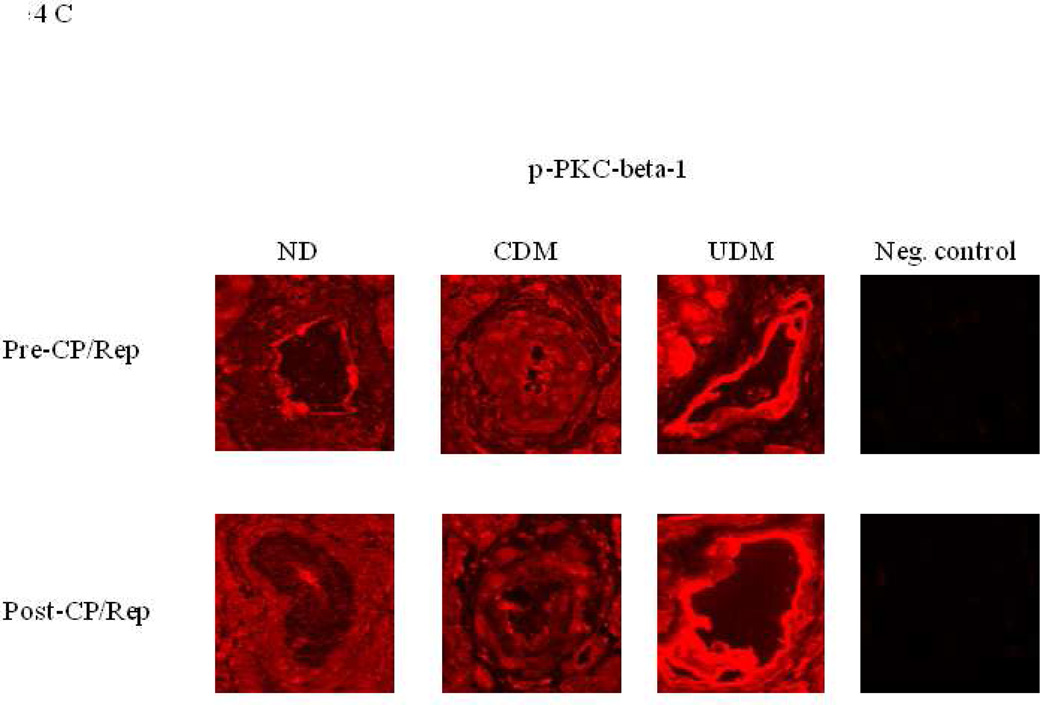
Immunofluorescent staining of coronary microvessels displayed a strong signal for PKC-α, phosphorylated PKC-α and phosphorylated PKC-β1 localized to the coronary microvasculature and stronger signals in the vessels of poorly controlled diabetics (UDM) compared with the vessels of non-diabetics (ND) or well-controlled diabetics (CDM) (Figure 4A-C). Post-CP/Rep microvascular immunofluorescent staining displayed an enhanced signal for PKC-α of poorly controlled diabetics and well controlled diabetics compared to that of non-diabetics (Figure 4A). Post-CP/Rep microvascular immunofluorescent staining displayed an enhanced signal for PKC-β1 and phosphorylated PKC-β1 of poorly controlled diabetics compared to that of non-diabetics or well controlled diabetics (Figure 4B, C). Negative controls documented a low level of background fluorescence.
Figure 4.
Immuno-localization of PKC-α, phosphorylated-PKC-α and phospho-PKC-β1 in human right-atrial microvessels (n = 6/group). Vessels were stained for (A) PKC-α, (B) phosphorylated-PKC-α, (C) phosphorylated-PKC-β (red). Matched negative controls are displayed below each row of primary antibody.
Effect of Poorly Controlled Diabetes and CP/Rep on the Gene Expression of Endothelium Markers
There were no significant changes in the gene expression of endothelial markers in the atrial tissue samples between poorly controlled diabetic and non-diabetic groups, pre- or post-CP/Rep. The data obtained from microarray analysis were summarized in Table 2.
Table 2.
Data from Microarray Analysis (ND vs. UDM)
| ND vs. UDM(Pre−CP/Rep) | ND vs. UDM (Post−CP/Rep) | |||
|---|---|---|---|---|
| −Log (P) | Fold Change | −Log (P) | Fold Change | |
| CD34 | 1.4276 | −2.1159 | 1.353516 | −1.48858 |
| CD36 | 0.2626 | −0.25267 | 0.351938 | 0.081563 |
| CD47 | 0.5883 | −0.576 | 0.52975 | −0.224 |
| CD93 | 0.54005 | 0.0617 | 0.422063 | −0.12919 |
| CD151 | 1.704313 | −2.39938 | 1.303412 | −1.38512 |
| CD160 | 0.668 | −0.19964 | 0.447219 | 0.118125 |
| CDH1 | 0.643667 | −0.0658 | 0.526406 | −0.17144 |
| CDH2 | 1.0502 | −1.3318 | 0.365879 | −0.356 |
| CDH4 | 0.978333 | −0.5566 | 0.405063 | 0.22025 |
| CDH5 | 1.398571 | −1.25614 | 0.79 | −0.38873 |
| CDH12 | 0.843632 | −0.38221 | 0.489375 | −0.20944 |
| CDH13 | 0.682818 | −0.3597 | 0.658646 | 0.148854 |
| CEACAM1 (includes others) | 0.594692 | −0.00969 | 0.428625 | 0.149469 |
| CLDN3 | 0.701545 | −0.23564 | 0.39025 | −0.16294 |
| ENG | 1.033917 | −1.53908 | 0.802688 | −0.96944 |
| EPOR | 0.903237 | −0.67078 | 0.77463 | −0.66859 |
| ITGA1 | 1.599308 | 1.025769 | 1.029875 | 0.629094 |
| ITGA2 | 0.577 | −0.09233 | 0.440724 | −0.00634 |
| ITGA5 | 1.1321 | −1.1905 | 1.056857 | −0.11514 |
| ITGA6 | 0.595042 | 0.158042 | 0.742868 | 0.387755 |
| ITGA9 | 0.74672 | −0.3092 | 0.852154 | −0.27942 |
| ITGAV | 0.556743 | −0.32971 | 0.490837 | −0.31623 |
| ITGB1 | 0.759484 | −0.50865 | 0.753207 | −0.14697 |
| ITGB2 | 0.827176 | −0.73229 | 0.575333 | −0.60953 |
| ITGB3 | 0.645957 | 0.146085 | 0.552762 | 0.002405 |
| ITGB4 | 0.67125 | −0.59822 | 0.697864 | −0.39182 |
| ITGB5 | 0.696662 | −0.607 | 0.716844 | −0.20225 |
| ICAM1 | 0.696661538 | −0.607 | 0.624375 | 0.717375 |
| ICAM2 | 0.749285714 | −0.814816327 | 1.012067 | −0.12296 |
| ILK | 1.0824375 | −1.3874375 | 1.247563 | −0.50819 |
| JAM3 | 0.977529412 | −1.140588235 | 1.02568 | −0.52375 |
| LGALS1 | 0.776368421 | −1.588947368 | 0.912467 | 0.415067 |
| LGALS3 | 0.776368421 | −1.588947368 | 0.851767 | −0.61053 |
| MCAM | 0.590533333 | −0.137866667 | 0.969455 | −0.45009 |
| MFGE8 | 1.33275 | −1.7251875 | 1.997267 | −0.76967 |
| NRCAM | 0.7299375 | −0.134395833 | 0.600314 | −0.07986 |
| OLR1 | 0.619833333 | −0.004166667 | 0.383125 | 0.056875 |
| PECAM1 | 0.6404375 | −0.656604167 | 0.852804 | −0.49317 |
| PODXL | 1.029466667 | −1.4064 | 0.6714 | −0.6736 |
| PLXDC1 | 0.565571429 | −0.174142857 | 0.413563 | 0.222125 |
| PDPN (includes EG:10630) | 0.762770833 | −0.4220625 | 0.713868 | 0.120711 |
| POSTN | 0.6200625 | −0.8625 | 0.882 | −1.11827 |
| PVRL2 | 0.9055625 | −1.17725 | 1.012583 | −0.91138 |
| PROCR | 0.818625 | −0.70175 | 1.051455 | −0.55291 |
| SELE (includes EG:20339) | 0.675625 | −0.11075 | 0.413563 | 0.222125 |
| SELP | 1.0854375 | −0.852 | 0.6605 | −0.50806 |
| S1PR1 | 0.466375 | −0.293375 | 0.8175 | −0.3825 |
| S1PR2 | 1.003 | −0.51425 | 1.295111 | −0.49522 |
| STAB1 | 0.9026875 | −1.2669375 | 1.1958 | −0.72327 |
| THBD | 0.496214286 | −0.354428571 | 0.23375 | 0.429813 |
ND: nondiabetics; UDM: uncontrolled diabetics (poorly controlled); CDH1:cadherin 1; CDH2:cadherin 2; CDH4:cadherin 4; CDH5:cadherin 5, CDH12:cadherin 12, CDH13:cadherin 13, CEACAM1: carcinoembryonic antigen-related cell adhesion molecule 1 (biliary glycoprotein); CLDN3:claudin 3; ENG: endoglin; EPOR:erythropoietin receptor; ICAM1: intercellular adhesion molecule 1; ICAM2: intercellular adhesion molecule 2; ITGA1: integrin, alpha 1; ITGA2: integrin, alpha 2; ITGA5: integrin, alpha 5; ITGA6: integrin, alpha 6; ITGA9: integrin, alpha 9; ITGAV:integrin, alpha V; ITGB1: integrin, beta 1; ITGB2: integrin, beta 2; ITGB3: integrin, beta 3; ITGB4: integrin, beta 4; ITGB5: integrin, beta 5; PDPN: podoplanin; Jam3: junctional adhesion molecule 3; ILK: integrin-linked kinase; LGALS1:lectin, galactoside-binding, soluble, 1; LGALS3: lectin, galactoside-binding, soluble, 3; MCAM: melanoma cell adhesion molecule; MFGE8:milk fat globule-EGF factor 8 protein; NRCAM: neuronal cell adhesion molecule; OLR1: oxidized low density lipoprotein (lectin-like) receptor 1; POSTN: periostin, osteoblast specific factor; PECAM1:platelet/endothelial cell adhesion molecule; PODXL: podocalyxin-like; PVRL2: poliovirus receptor-related 2; PLXDC1: plexin domain containing 1; PROCR: protein C receptor, endothelial; SELP: selectin P; SELE: selectin E; S1PR1: sphingosine-1-phosphate receptor 1; S1PR2:sphingosine-1-phosphate receptor 2; STAB1: stabilin 1; THBD: thrombomodulin.
4. Discussion
We have previously found that poorly controlled diabetes alters contractile responses of human arterioles to numerous vasoactive substances.12 In the present study, using isolated coronary arterioles, we observed that poorly controlled diabetes, but, not well controlled diabetes significantly impairs endothelium-dependent and independent relaxation of human coronary microvasculature as compared to non-diabetes. These changes may contribute to the less favorable postoperative outcomes after cardiac surgery in poorly controlled diabetic patients.
We have previously reported that CP/Rep is associated with altered myogenic tone and endothelial function of coronary microvasculature.2–6 Recently, we have also found that permeability-modulating proteins such as vascular endothelial growth factor /vascular permeability factor (VEGF/VPF) are increased in expression in the poorly controlled diabetic patients and these changes are associated with increased edema formation and length of hospital stay in poorly controlled diabetic patients.13 In the present study, we further observed that poorly controlled diabetes worsens the recovery of endothelium-dependent and independent relaxation of coronary arterioles after CP/Rep.
PKC-α and PKC-β are two major isoforms of PKCs found in the smooth muscle and endothelial cells and are activated under conditions of hyperglycemia. 14,15 Treatment with a PKC-β inhibitor improves endothelial function in patients with diabetes mellitus.16 In the present study, we demonstrate that diabetes significantly up-regulates PKC-α and PKC-β protein expression and activation in the human atrial myocardium and coronary microvessels, suggesting that the increased PKC-α and β may contribute to diabetes-related endothelial dysfunction of coronary microvasculature. After CP/Rep, expression of PKC-α and β in poorly (un)controlled diabetes are still higher than those of non-diabetics or controlled diabetics, which may partially explain why CP/Rep worsens arteriolar endothelium function in the uncontrolled diabetic group.
Oxidative stress plays an important role in the pathogenesis of diabetic vascular complications. Reactive oxygen and nitrogen species trigger endothelial cell dysfunction through a multiple mechanisms.16,17 These increases in oxidative stress may also contribute to microvascular endothelial dysfunction in the diabetic arterioles after CP/Rep. Taking together, hyperglycemia, PKC activation, oxidative stress may cumulatively participated in the microvascular endothelial dysfunction of diabetic patients.
We have recently found that differential gene and protein expression of growth factors and their related genes between the patients with poorly controlled diabetes and patients without diabetes.13,18 These changes in gene and protein expression of growth factors could be associated with increased edema and weight gain in patients with uncontrolled diabetes after CP/Rep.18 In this study, the data from microarray analysis show no significant changes in the gene expression of tested endothelial markers such as, cadherin, integrin, and platelet/endothelial cell adhesion molecules between poorly controlled diabetic and non-diabetic groups, or between pre- and post-CP/Rep.
In conclusion, poorly controlled, but not well controlled diabetes is associated with arteriolar endothelium-dependent dysfunction. Additionally, poorly controlled diabetes worsens the recovery of arteriolar endothelial function after CP/Rep. Increased PKC-α and PKC-β expression/activation, and enhanced oxidative and nitrosative stress may cumulatively contribute to microvascular endothelial dysfunction of poorly controlled diabetes. In contrast, the well controlled diabetes is associated with improved peripheral arteriolar function after CP/Rep and cardiac surgery.
Figure 2.
Coronary microvascular vasodilation in response to the endothelium-dependent vasodilator substance P: (A) pre-CP/Rep arterioles from non-diabetics (ND), controlled diabetic (CDM) and uncontrolled diabetic (UDM) groups; (B) post-CP/Rep arterioles of ND, CDM and UDM groups; (C) pre-CP/Rep vs. post-CP/Rep (ND); (D) pre-CPB vs. post-CP/Rep (CDM); (E) pre-CP/Rep vs. post-CP/Rep of (UDM); *P<0.05 vs. pre-CP/Rep (ND) or CDM. n = 10/group.
Acknowledgment
We thank all physician assistants at Division of cardiothoracic surgery and operating room nurses, perfusionists at Rhode Island and Miriam Hospitals for their help to collect the consent forms and human tissue samples.
Financial Support: This research project was supported in part by the National Heart, Lung, and Blood Institute RO1 grants-HL-46716 and HL-69024 (F.W.S). L.M.C was supported by NIH training grants (T32-HL094300) and the Irving Bard Memorial Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at 7th American Surgical Congress, Nov. 12–16, 2012, Las Vegas, NV
References
- 1.Ruel M, Khan TA, Voisine P, Bianchi C, Sellke FW. Vasomotor dysfunction after cardiac surgery. Eur J Cardiothorac Surg. 2004;26:1002–1014. doi: 10.1016/j.ejcts.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 2.Sodha NR, Feng J, Clements RT, Bianchi C, Boodhwani M, Ramlawi R, et al. Protein Kinase C-α modulates microvascular reactivity in the human coronary and skeletal microcirculation. Surgery. 2007;142:243–252. doi: 10.1016/j.surg.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Feng J, Liu YH, Khabbaz KR, Hagberg R, Osipov RM, Sodha NR, et al. Endothelin-1 induced contractile responses of human coronary arterioles via endothelin-A receptors and PKC-α signaling pathways. Surgery. 2010;147:798–804. doi: 10.1016/j.surg.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng J, Liu YH, Chu LM, Clements RT, Khabbaz KR, Robich MP, et al. Thromboxane induced contractile response of human coronary arterioles is diminished post-cardioplegic arrest. Ann Thorac Surg. 2011;92:829–836. doi: 10.1016/j.athoracsur.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metias C, Li J, Simons M, Sellke FW. Serotonin-induced coronarycontraction increases after blood cardioplegia-reperfusion: role of COX-2 expression. Circulation. 1999;100:II328–II334. doi: 10.1161/01.cir.100.suppl_2.ii-328. [DOI] [PubMed] [Google Scholar]
- 6.Feng J, Liu YH, Clements RT, Sodha NR, Khabbaz KR, Senthilnathan V, et al. Calcium-activated potassium channels contribute to human coronary microvascular dysfunction after cardioplegia arrest. Circulation. 2008;118:S46–S51. doi: 10.1161/CIRCULATIONAHA.107.755827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thourani VH, Weintraub WS, Stein B, Gebhart SS, Craver JM, Jones EL, et al. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg. 1999;67:1045–1052. doi: 10.1016/s0003-4975(99)00143-5. [DOI] [PubMed] [Google Scholar]
- 8.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 9.Ismail-Beigi F, Craven T, Banerji MA, et al. ACCORD trial group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerstein HC, Miller ME, Genuth S, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furnary AP. Rationale for glycemic control in cardiac surgical patients: The portland diabetic project. Insulin. 2006;1:S24–S29. [Google Scholar]
- 12.Feng J, Liu YH, Khabbaz K, Hagberg R, Robich MP, Clements R, et al. Decreased contractile response to endothelin-1 of peripheral microvasculature from diabetic patients. Surgery. 2011;149:247–252. doi: 10.1016/j.surg.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emani S, Ramlawi B, Sodha NR, Li J, Bianchi C, Sellke FW. Increased vascular permeability after cardiopulmonary bypass in patients with diabetes is associated with increased expression of vascular growth factor and hepatocyte growth factor. J Thorac Cardiovasc Surg. 2009;138:185–191. doi: 10.1016/j.jtcvs.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King GL, Geraldes Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Creager MA. Inhibition of protein kinase C-β prevents impaired endothelium dependent vasodilation caused by hyperglycemia in humans. Circ Res. 2002;90:107–111. doi: 10.1161/hh0102.102359. [DOI] [PubMed] [Google Scholar]
- 17.Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voisine P, Ruel M, Khan TA, Bianchi C, Xu S, Kohane I, et al. Differences in gene expression profiles of diabetic and non-diabetic patients undergoing cardiopulmonary bypass and cardioplegic arrest. Circulation. 2004;110:II280–II286. doi: 10.1161/01.CIR.0000138974.18839.02. [DOI] [PubMed] [Google Scholar]