Abstract
The present studies employed a novel microelectrode array recording technology to study glutamate release and uptake in the dentate gyrus, CA3 and CA1 hippocampal subregions in anesthetized young, late-middle aged and aged male Fischer 344 rats. The mossy fiber terminals in CA3 showed a significantly decreased amount of KCl-evoked glutamate release in aged rats compared to both young and late-middle-aged rats. Significantly more KCl-evoked glutamate release was seen from perforant path terminals in the DG of late-middle-aged rats compared young and aged rats. The DG of aged rats developed an increased glutamate uptake rate compared to the DG of young animals, indicating a possible age-related change in glutamate regulation to deal with increased glutamate release that occurred in late-middle age. No age-related changes in resting levels of glutamate were observed in the DG, CA3 and CA1. Taken together, these data support dynamic changes to glutamate regulation during aging in subregions of the mammalian hippocampus that are critical for learning and memory.
Keywords: l-Glutamate, Hippocampus, Voltammetry
1. Introduction
An understanding about how morphological and electrophysiological changes in brain regions critical for learning and memory translate into deficits in the cognitive functions of the hippocampus in aging is needed (for reviews see Rosenzweig and Barnes, 2003; Chawla and Barnes, 2007). In addition to preserved cell counts (West, 1993; West et al., 1993; Peters et al., 1996; Rapp and Gallagher, 1996; Rasmussen et al., 1996; Merrill et al., 2000; Merrill et al., 2001), most of the basic cellular characteristics of hippocampal principal cells do not change with advanced age including but not limited to resting membrane potential and amplitude and duration of Na+-mediated action potentials (for review see Rosenzweig and Barnes, 2003). Therefore, changes in hippocampal cognitive functions during aging do not appear to be attributable to changes in neuron number or basic cellular physiology. It is believed that structural changes in synaptic connections during aging affect functional connectivity in the hippocampus (reviewed by Rosenzweig and Barnes, 2003; Chawla and Barnes, 2007); however, age-associated alterations in neurotransmission and subsequent effects on function of the hippocampal circuitry are far less defined.
In the hippocampus, the chief excitatory neurotransmitter is l-glutamate. The NMDA and AMPA ionotropic glutamate receptor subtypes are critical for long-term potentiation and hippocampal-dependent learning and memory (Riedel et al., 2003). A reduction in ionotropic receptors and their constituent subunits with age has been correlated with a decline in memory function (Magnusson, 1998a,b; Adams et al., 2001; Tang et al., 2001; Clayton et al., 2002), which can be modulated with pharmacological agents that facilitate activation of the glutamate receptors (Müller et al., 1994; Wu et al., 2002; Rosenzweig and Barnes, 2003). Thus, age-related changes in presynaptic and glial regulation of glutamate release may be needed to help explain age-related changes in hippocampal neuron function during aging.
Glutamate regulation in the hippocampus has been extensively investigated using ex vivo methods like brain slices, tissue homogenates, and synaptosomes (for review see Segovia et al., 2001). Due to the limitations of these methods the literature has been filled with contradicting reports about increases, decreases or no changes in glutamate regulation with aging. The dentate gyrus (DG), cornu ammonis 3 (CA3) and cornu ammonis 1 (CA1) hippocampal subregions are distinct on a cellular, molecular, and functional basis, and are intricately connected via the trisynaptic circuit (for review Miller and O’Callaghan, 2005; Greene et al., 2008). Isolating the hippocampus from its extrinsic connections and disrupting the intrinsic connections could account for the inconsistency in previous aging studies. Only two prior microdialysis studies have reported on hippocampal glutamate regulation during aging using intact animals and the results are contradicting. Zhang et al. (1991) showed a decrease and Massieu and Tapia (1997) showed an increase in tonic glutamate levels in aged as compared to young rats. Though microdialysis does allow for in vivo measurements of tonic glutamate, controversy exists regarding the neuronal origin of the signals and its slowtemporal resolution limits the ability to investigate the rapid release and clearance dynamics associated with glutamate neurotransmission (Timmerman and Westerink, 1997; Segovia et al., 2001). This is important because alterations in release and/or clearance may not manifest with changes in tonic resting glutamate levels due to biological compensation, and therefore must be studied directly. Furthermore, due to the size of microdialysis probes (mm), the previous studies are limited because they do not address discrete subregional variation within the hippocampus.
We have recently demonstrated altered regulation of glutamate neurotransmission during aging in the striatum of rodents (Nickell et al., 2005, 2007) and the cortex of nonhuman primates (Quintero et al., 2007) using enzyme-based microelectrode arrays coupled to amperometry for in vivo glutamate recordings. In addition to a subsecond (2 Hz) temporal resolution that allows for rapid measurement of glutamate release and clearance in the extracellular space, our microelectrode technology measures from brain parenchyma with a spatial-resolution (µm) superior to that of microdialysis (Stephens et al., 2008) allowing accurate targeting of the DG, CA3 and CA1 subregions. These experiments investigated the capacity of glutamate release and uptake in subregions of the hippocampus (DG, CA1 and CA3) of young, late-middle aged and aged Fischer 344 (F344) rats. First, we studied the effects of aging on tonic (resting) levels of glutamate using a self-referencing enzyme-based microelectrode array recording technology (Day et al., 2006). Second, we used local application of a high potassium solution to evoke reproducible synaptic overflow of glutamate with a micropipette attached to the microelectrode arrays. This was carried out to simulate phasic bursts of glutamate release and determine the effects of aging on depolarization-induced release of glutamate. Finally, using the attached micropipette for evoked release we locally applied finite amounts of glutamate to study the effects of aging on glutamate clearance. The present studies are the first to provide insight into age-related changes in functional glutamatergic neurotransmission in subregions of the rat hippocampus.
2. Methods
2.1. Animals
Young adult (3–6 months, n = 18, mean weight = 337 ± 50 g), late-middle aged (18 months, n = 14, 452 ± 36 g) and aged (24 months, n = 19, 424 ± 32 g) male F344 rats were obtained from the National Institute on Aging colony (Harlan Sprague Dawley Inc., Indianapolis, IN) and used for all experiments. Protocols for animal use were approved by the Institutional Animal Care and Use Committee. In accordance with approved guidelines, animals were housed in a 12-h alternating light/dark cycle, with food and water available ad libitum.
2.2. Amperometric recordings for rapid measurements of l-glutamate
Ceramic microelectrode arrays consisting of four platinum recording surfaces (15 µm × 333 µm each) arranged geometrically in two pairs (30 µm spacing between recording surfaces) stacked in a dorsal–ventral orientation on the microelectrode array tip (100 µm spacing between the pairs), were used in vivo for discrete targeting of the DG, CA3 and CA1 subregions of the rat hippocampus. Microelectrode arrays were manufactured and configured for selective glutamate detection as per our previously published methods yielding one pair of ‘glutamate-sensitive’ recording surfaces and one pair of background ‘sentinel’ recordings surfaces (Burmeister et al., 2002; Pomerleau et al., 2003; Day et al., 2006; Hascup et al., 2007; Stephens et al., 2008). All platinum recording surfaces were electroplated with meta-phenylenediamine (5 mM, Acros Organics, New Jersey, USA), which creates a molecular exclusion layer blocking interferents such as dopamine (DA), 3-4-dihydroxyphenylacetic acid (DOPAC), ascorbic acid and other neurochemicals based upon their size. Constant voltage amperometry (+0.7V vs. Ag/AgCl reference electrode) was performed using the Fast Analytical Sampling Technology high-speed electrochemistry instrument (FAST16, Quanteon, L.L.C., Nicholasville KY), and current from the four platinum recording sites was simultaneously recorded by the FAST software.
2.3. Microelectrode calibration
Individual recording specifications of each microelectrode, including the sensitivity to glutamate (current/concentration (pA/µM)) and the selectivity ratio of glutamate over an ascorbic acid interferent (glutamate:AA), were obtained by in vitro calibration prior to all experiments (Burmeister et al., 2002; Pomerleau et al., 2003; Day et al., 2006; Hascup et al., 2007; Stephens et al., 2008). Microelectrodes used for in vivo recordings had a selectivity ratio of at least 20:1 (95% ascorbic acid blockade), and a very linear response to the serial glutamate aliquots (r2 > 0.99). The slope of this line is referred to as the microelectrode sensitivity to glutamate (pA/µM). Microelectrodes with a sensitivity of at least 1 pA/µM and a limit of detection (signal-to-noise of 3) of at least 1 µM were used for in vivo glutamate recordings. Average microelectrode calibration parameters for these experiments were as follows (mean ± S.E.M.): sensitivity: 6 ± 0.3 pA/µM; limit of detection: 0.8 ± 0.2 µM; selectivity ratio: 150:1.
2.4. In vivo glutamate measurements in anesthetized rats
A pre-pulled single-barrel micropipette (1 mm o.d., 0.58 mm i.d. glass, A-M Systems Inc., Everett, WA) was attached to each ceramic microelectrode with Sticky Wax (Kerr Lab Corporation, Orange, CA). This allowed for intracranial application of a KCl solution (70 mM KCl, 79 mM NaCl, 2.5 mM CaCl2, pH 7.4) to study stimulus-evoked glutamate release or an exogenous glutamate solution (100 µM in 0.9% saline,pH7.4) to study glutamate clearance. The tips of the micropipettes were pulled to an inner diameter of 10 µm, and positioned 70 ± 20 µm away from the microelectrode surface, centered in the 100 µm space between the dorsal and ventral platinum recording pairs.
Male F344 rats were anesthetized with urethane (1.25 mg/kg, i.p.) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). A bilateral craniotomy was performed removing a window of bone between bregma and lambda to allow access to the hippocampal formation. A miniature Ag/AgCl reference electrode (200 µm) was implanted into the right frontal cortex. The micropipette/microelectrode assemblies were targeted to the dendritic trees of hippocampal principal cells in the DG, CA3 and CA1 subregions. Stereotactic coordinates were adapted from Paxinos and Watson (2006) to account for changes in animal size and skull thickness with age. Anterior–posterior and medial–lateral coordinates were taken from bregma, and dorsal–ventral coordinates were taken from the top of the skull. CA1 and CA3 recordings were performed at the same AP and ML coordinates with the microelectrode at two different depths. Placement coordinates were as follows: young, CA1{CA3} AP: −(3.9–4.3), ML: ± 3.4–3.6, DV: −3.5 {−4.1}; DGAP: −4.1, ML: ± 2.1, DV: −4.2; late-middle aged, CA1{CA3} AP: −(4.1–4.5), ML: ± 3.7–3.9, DV: −3.9 {−4.5}; DG AP: −4.4, ML: ± 2.2, DV: −4.5; aged, CA1{CA3} AP: −(4.4–4.8), ML: ± 3.7–4.0, DV: −4.0 {−4.6}; DG AP: −4.6, ML: ± 2.4, DV: −4.6.
Basal glutamate measurements were obtained from each region following a baseline period of 20 min, prior to the application of any solutions. The order in which the subregions were targeted and in which solutions were applied was randomized during experimentation, so as to not bias the study by always targeting one particular area first. A Picospritzer III (Parker Hannifin Corp. NJ, USA) was used to precisely control the volumes of KCl or glutamate delivered during intracranial applications.
For KCl-evoked glutamate release, a dose response was performed in the hippocampal subregions to determine the maximum glutamate release. Volumes delivered ranged from 12.5 to 75 nL and were quantified by using a microscope fitted with a reticule and calibrated to determine the volume of drug ejected from the micropipette (Day et al., 2006). Evoked glutamate release data were collected in young (n = 11), late-middle aged (n = 8) and aged (n = 10) F344 rats. For clearance studies, exogenous glutamate (100 µM) was locally applied in the hippocampal subregions (volumes ranging from 25 to 250 nL) to achieve a glutamate concentration in the microenvironment around the platinum recording sites within the range of concentrations most consistently obtained following KCl stimulation of glutamatergic terminals (10–20 µM, see Nickell et al., 2005). This allowed us to study clearance of glutamate at physiologically relevant concentrations, and offered the benefit of isolating glutamate clearance mechanisms, in contrast to studying glutamate clearance following KCl-evoked release, which causes a non-selective release of neurotransmitters such as GABA. This also allowed us to control for any effects of KCl on glutamate transporters. Glutamate clearance data were collected in young (n = 16), late-middle aged (n = 13) and aged (n = 14) F344 rats.
2.5. Histology procedures to confirm electrode locations
Because the ceramic microelectrode tips are cut by a diamond saw producing a smooth edge, they cause very little damage to the surrounding brain parenchyma during acute recordings, and leave virtually no electrode tract (see Rutherford et al., 2007). Therefore, upon completion of each experiment, Fast Green dye (Sigma–Aldrich) was applied through the micropipette (500 nL) to mark the locations of the microelectrode. Animals were euthanized with isoflurane, the brains removed, frozen at −20 °C and the positions of the microelectrode in the hippocampus verified by visual examination after slicing the brains (40 µm) on a cryostat (Fig. 1). Data were excluded if there was clear indication that the recording passes missed the CA1/CA3 or DG subregions of rat hippocampus (young, n = 2; late-middle aged, n=1; aged, n = 2).
Fig. 1.
Fast Green (Sigma–Aldrich) staining in the hippocampal formation confirming placement of the microelectrode/micropipette assembly in the dendritic tress of the CA1 and CA3 subregions (upper panel) and the DG subregion (lower panel).
2.6. Data analysis
For measurements of basal glutamate (resting levels), the current produced by the oxidation of background electroactive substances in the extracellular space and the charging current of the electrode surface was removed from the signal obtained with the glutamate-sensitive surfaces by subtraction. This is referred to as self-referencing (Burmeister and Gerhardt, 2001; Day et al., 2006). Current (pA) obtained by self-referenced in vivo measurements in each hippocampal subregion was divided by the microelectrode sensitivity (pA/µM) obtained during calibration, and reported as concentration of tonic glutamate (µM) (Quintero et al., 2007); young DG (n = 15), CA3 (n = 12), CA1 (n = 13); late-middle aged DG (n = 6), CA3 (n = 11), CA1 (n = 10); aged DG (n = 16), CA3 (n = 14), CA1 (n = 14).
Following local stimulation with 70 mM KCl (12.5–75 nL) in the DG, CA3, and CA1 hippocampal subregions, the maximum increase in the glutamate signal (pA) from baseline due to evoked glutamate release was recorded by the FAST software. Actual volumes delivered to obtain the maximum glutamate release were not significantly different across subregions or age groups (data not shown, two-way ANOVA, p = 0.95). Data were exported to a custom Excel™ spreadsheet for analysis. Maximum increase in glutamate signal from baseline (pA) was divided by the microelectrode sensitivity (pA/µM) and reported as the maximum amplitude of evoked-glutamate signal (µM). The first order rate of decay (k−1; s−1) was calculated for all glutamate signals to examine the rate of return from maximum amplitude to baseline. Based upon the known reproducibility of KCl-evoked glutamate signals in vivo (Nickell et al., 2005; Day et al., 2006), data from an individual subregion were only analyzed if at least three similar signals could be evoked, upon which the data were averaged and reported as a single number for that subregion: young DG (n = 8), CA3 (n = 6), CA1 (n = 6); late-middle aged DG (n = 5), CA3 (n = 5), CA1 (n = 7); aged DG (n = 6), CA3 (n = 5), CA1 (n = 5).
Following local application of 100 µM glutamate (25–250 nL) in the DG, CA3, and CA1 hippocampal subregions, maximum increase in the glutamate signal from baseline was recorded by the FAST software. Data were exported to a custom Excel™ spreadsheet for analysis where the maximum increase in glutamate signal from baseline (pA) was divided by the microelectrode sensitivity (pA/µM) and reported as the amplitude of glutamate signal following local application of exogenous glutamate (µM). Data from an individual subregion were only further analyzed if application of exogenous glutamate produced at least three signals in the range of 10–20 µM. For signals in the defined range, uptake rate (k−1 × maximum amplitude; µM/s) was calculated using an Excel™ spreadsheet and reported as the concentration of glutamate removed from the extracellular space per second. Data from individual subregions were averaged and reported as a single number for that subregion: young DG (n = 9), CA3 (n = 7), CA1 (n = 11); late-middle aged DG (n = 7), CA3 (n = 12), CA1 (n = 13); aged DG (n = 8), CA3 (n = 6), CA1 (n = 8).
A Grubb’s test for outliers removed two young animals and one aged animal from analysis. Mean and S.E.M. for all parameters of interest (tonic glutamate concentration, maximum amplitude of KCl-evoked glutamate signal and uptake rate following local exogenous glutamate application) were calculated for each subregion in each age group. Due to the defined guidelines for inclusion of data in the analysis, not every animal produced a complete set of tonic glutamate levels, KCl-evoked glutamate release and glutamate uptake rate data in all three subregions. Therefore, discrete comparisons were performed using a one-way analysis of variance (ANOVA, statistical significance defined as p < 0.05) in GraphPad Prism 5 along with a Bartlett’s comparison of data variance to look for differences in the trisynaptic circuit within each age group, and for differences within each subregion across age groups. A Tukey–Kramer post-hoc test for unequal sample sizes was performed where necessary.
3. Results
Histological evaluation confirmed MEA placement in the dendritic trees of the hippocampal principal cells in the DG, CA3 and CA1 subregions. The co-localized granule cell dendrites and entorhinal cortex terminals were targeted in the infrapyramidal blade of the DG. In the stratum lucidum of CA3, the MEA was placed among mossy fiber terminals and their target CA3 pyramidal cell dendrites. In CA1, MEA placement was confirmed in the suprapyramidal stratum radiatum were CA1 pyramidal cell dendrites and Schaffer collateral terminals are co-localized.
3.1. Effects of aging on tonic (resting) glutamate levels in the trisynaptic circuit
In these studies we utilized a novel microelectrode array recording technology to investigate the in vivo regulation of glutamate neurotransmission in the trisynaptic circuit of young, late-middle aged and aged F344 rat hippocampus with spatial (µm) and temporal (2 Hz) resolutions unique to the scientific literature. Self-referenced recordings in the DG, CA3 and CA1 hippocampal subregions were in the 3 µM range and showed no significant differences in tonic glutamate levels across the trisynaptic circuit within each age group (Fig. 2): young, DG: 3.4 ± 0.5 µM, CA3: 2.8 ± 0.6 µM, CA1: 2.5 ± 0.5 µM, F(2,35) = 2.72, p = 0.08; late-middle aged, DG: 2.9 ± 1.1 µM, CA3: 3.3 ± 0.7 µM, CA1: 2.1 ± 0.5 µM, F(2,24) = 0.80, p = 0.46; aged DG: 2.9 ± 0.5 µM, CA3: 3.0 ± 0.4 µM, CA1: 2.8 ± 0.4 µM, F(2,41) = 0.046, p = 0.96. Average tonic glutamate levels did not change for any individual subregion when compared across age groups (DG, F(2,34) = 0.19, p = 0.83; CA3,F(2,34) = 0.19, p = 0.83; CA1,F(2,34) = 0.65, p = 0.53). Thus, the resting or tonic levels of glutamate seem little affected with aging in the hippocampus, at least under these conditions and in anesthetized animals.
Fig. 2.
Mean tonic glutamate concentration in the trisynaptic circuit during aging. Aging did not significantly alter tonic glutamate levels in the DG (F(2,34) = 0.19, p = 0.83), CA3 (F(2,34) = 0.19, p = 0.83) or CA1 (F(2,34) = 0.65, p = 0.53).
3.2. Effects of aging on stimulus-evoked glutamate release in the trisynaptic circuit
Application of KCl (70 mM, isotonic, pH 7.4, 12.5–75 nL) allowed us to produce discrete and well-defined stimulation of the local environment around the microelectrode, resulting in robust (5–60 µM) and reproducible (every 60 s) release of glutamate from terminals in the DG, CA3 and CA1 hippocampal subregions (Fig. 3). The mean maximum amplitude of KCl-evoked glutamate signals was significantly increased in the DG of late-middle aged rats compared to young (p < 0.05) and aged (p < 0.05) rats (DG: young, 19.6 ± 3.9 µM; late-middle aged, 36.2 ± 7.7 µM; aged, 17.3 ± 1.8 µM, F(2,16) = 4.3, p = 0.032). The variances of these data were significantly different across age groups (Bartlett’s test, p = 0.038). Though no significant difference existed in the maximum amplitude of glutamate signals recorded by the microelectrode in the young and aged DG, the first order rate of decay of the signals was significantly faster in the aged rats as compared to the young rats (0.29 ± 0.03 s−1 vs. 0.21 ± 0.02 s−1, respectively; t-test p = 0.036), indicating an increased clearance capacity of the aged DG following phasic release of glutamate (Fig. 5). This was further investigated with exogenous glutamate clearance studies (see Section 3.2).
Fig. 3.
Representative glutamate release signals following local application of KCl (50 nL, 70 mM, isotonic, pH 7.4) in the trisynaptic circuit of F344 rats during aging. Each symbol in the traces is an individual glutamate measurement (2 Hz). Inset signals show reproducibility of evoked-glutamate release signals. (Right) Bar graphs represent the mean maximum amplitude of glutamate signals across the trisynaptic circuit. Terminals in CA3 and CA1 had significantly less glutamate release compared to terminals in the DG in the 18- and 24-month groups. (Bottom) Graphs represent the mean max. amplitude of glutamate signals across age groups. Each symbol is an individual animal. The perforant path terminals in the DG of late-middle aged rats released more glutamate compared to young (p < 0.05) and aged (p < 0.05) rats. The mossy fiber terminals in the CA3 of aged rats released less glutamate compared to young (p < 0.05) and late-middle aged (p < 0.05) animals.
Fig. 5.
Comparison of amplitude-matched signals in the DG showing faster clearance of glutamate in aged animals. Each symbol is an individual glutamate measurement (2 Hz). Arrow indicates local application of exogenous glutamate (100 µM in 0.9% saline, pH 7.4). (Inset) KCl-evoked glutamate signals from perforant path terminals in the DG of young and aged rats. Aged rats have a faster first order rate of glutamate clearance (0.29 ± 0.03 s−1 vs. 0.21 ± 0.02 s−1; t-test p = 0.036). Dashed line indicates predicted maximum amplitude of glutamate signal in an aged animal without increased glutamate clearance capacity.
Perhaps of greatest interest was that the CA3 subregion showed significantly less neurotransmitter release following KCl stimulation in aged rats when compared to young (p < 0.05) and late-middle aged (p < 0.05) animals (young, 14.6 ± 1.5 µM; late-middle aged, 16.7 ± 2.6 µM; aged, 8.4 ± 2.4 µM, F(2,13) = 3.82, p = 0.0495). Diminished release in CA3 also contributed to significantly altered glutamate release capacities throughout the trisynaptic circuit within the late-middle aged and aged groups. Compared to the DG in each respective group, the CA3 and CA1 subregions had significantly less glutamate release following KCl stimulation (late-middle aged: DG, 36.2 ± 7.7 µM; CA3, 16.7 ± 2.6 µM, p < 0.05; CA1, 16.5 ± 5.4 µM, p < 0.05; F(2,14) = 3.76, p = 0.0493; aged: DG, 17.3 ± 1.8 µM; CA3, 8.4 ± 2.4 µM, p < 0.05; CA1, 10.9 ± 2.3 µM, p < 0.05; F(2,13) = 4.89, p = 0.026). In contrast to the DG and CA3, no age-associated changes in maximum amplitude of evoked-glutamate signals were seen within the CA1 subregion across age groups (young, 12.7 ± 2.7 µM; late-middle aged, 16.5 ± 5.4 µM; aged, 10.9 ± 2.3 µM, F(2,15) = 0.47, p = 0.63). Thus, the major findings of this study were increased glutamate release from the entorhinal cortex terminals in the DG of late-middle aged rats, decreased glutamate release from mossy fiber terminals in the CA3 of aged rats, and diminished glutamate release capacity during aging from the mossy fibers and Schaffer collaterals comprising the trisynaptic circuit downstream of the DG.
3.3. Effects of aging on glutamate clearance in the trisynaptic circuit
To better determine potential age-related changes in glutamate uptake we locally applied an exogenous glutamate solution (100 µM in 0.9% saline, pH 7.4) into the DG, CA3, and CA1 hippocampal subregions of all three age groups to study clearance of glutamate from the extra-cellular space by glia and neuronal elements. Volumes ranging from 25 to 250 nL were delivered intracranially to obtain signals with maximum amplitudes in the range most frequently obtained during KCl-evoked glutamate release studies (10–20 µM). Because the high-affinity glutamate transporters operate under Michaelis–Menton kinetics, it is important to compare clearance rates where experimental conditions supply approximately the same amount of substrate to transporters as physiological conditions. Actual maximum amplitudes analyzed across subregions and age groups were not statistically different (data not shown, twoway ANOVA, p = 0.17); therefore, variability in the size of peaks analyzed did not contribute to differences in uptake rate.
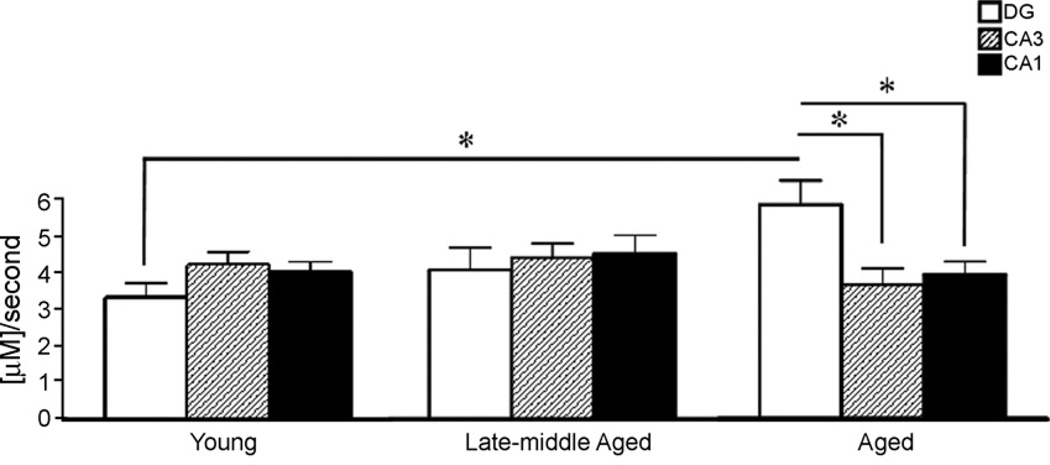
In the DG of aged rats, the glutamate uptake rate was significantly faster compared to the uptake rate in the DG of young rats (5.5 ± 0.7 µM/s vs. 3.3 ± 0.4 µM/s, p < 0.05), with the late-middle aged rats falling in between (4.1 ± 0.6 µM/s; F(2,22) = 3.88, p = 0.036) (Figs. 4 and 5). Also, within the aged trisynaptic circuit uptake rates were significantly different (F(2,19) = 5.38, p = 0.014), showing a faster clearance rate in the DG (5.5 ± 0.7 µM/s) when compared to the other hippocampal subregions (CA3: 3.7 ± 0.5 µM/s, p < 0.05; CA1: 3.9 ± 0.4 µM/s, p < 0.05) (Fig. 4). No significant differences in uptake rate across the trisynaptic circuit were seen in the young and late-middle aged groups (young, DG: 3.3 ± 0.4 µM/s, CA3: 4.2 ± 0.4 µM/s, CA1: 4.0 ± 0.3 µM/s, F(2,24) = 1.68, p = 0.21; late-middle aged, DG: 4.1 ± 0.6 µM/s, CA3: 4.4 ± 0.4 µM/s, CA1: 4.5 ± 0.5 µM/s, F(2,29) = 0.18, p = 0.84). The uptake rate within the CA3 and CA1 subregions was not different across age groups (F(2,22) = 0.86, p = 0.44 and F(2,29) = 0.61, p = 0.55, respectively) (Fig. 4).
Fig. 4.
Mean glutamate uptake rate (µM/s) following local application of exogenous glutamate (100 µM in 0.9% saline, pH 7.4) in the trisynaptic circuit F344 rats during aging. Uptake rate was significantly faster in the aged DG compared to young rats (5.5 ± 0.7 µM/s vs. 3.3 ± 0.4 µM/s, p < 0.05). Within the aged trisynaptic circuit, uptake rates were significantly different showing faster glutamate uptake rate in the DG when compared to the other hippocampal subregions (CA3: 3.7 ± 0.5 µM/s, p < 0.05; CA1: 3.9 ± 0.4 µM/s, p < 0.05).
4. Discussion
In this study, we have shown that tonic resting glutamate levels in discrete DG, CA3 and CA1 subregions of the hippocampus do not change significantly during aging in anesthetized F344 rats. However, the trisynaptic pathway did exhibit changes in glutamate release and uptake in specific subregions. Interestingly, evoked release of glutamate was greatest from perforant path terminals in the DG of late-middle aged rats as compared to young and aged animals. The most fascinating finding was that the CA3 subregion exhibited profound dysregulation during aging. Mossy fiber terminals in CA3 had diminished evoked glutamate release in aged rats compared to young and late-middle aged animals. Also, alterations within the trisynaptic circuit during aging showed a significant loss of glutamate release capacity in CA3 (mossy fibers) and CA1 (Schaffer collaterals) compared to the DG (perforant path) in both late-middle aged and aged rats. These findings suggest a diminished function or competence of the glutamate fibers in aging animals. Finally, the aged DG showed a significantly increased glutamate uptake capacity compared to the DG of young animals, suggesting that the increases in glutamate release in late-middle aged rats may result in increased glutamate uptake in aged rats.
4.1. Aging does not alter tonic glutamate levels in the trisynaptic circuit
Only two in vivo studies of resting glutamate in the hippocampus during aging have been reported. Their results are contradicting, showing an increase in aged compared to young Wistar rats (Massieu and Tapia, 1997) and a decrease in aged compared to young F344 rats (Zhang et al., 1991). A shortcoming of these studies is the lack of subregional resolution attained due to the large size of the microdialysis probes utilized.
In contrast to the previous in vivo studies, our data show that resting glutamate levels were approximately equal throughout the individual subregions of the trisynaptic circuit across age groups. We caution that these results too could be misleading. Our laboratory has recently begun to appreciate that urethane anesthesia can lower resting glutamate levels in the striatum and pre-frontal cortex (Rutherford et al., 2007). Tonic glutamate concentrations across the hippocampus could be affected by anesthesia. However, the microelectrode technology used in these studies is the first in vivo technology that allows for measures of resting glutamate in discrete subregions of the trisynaptic circuit. Additional studies are needed in awake rats to determine the effects of aging on resting levels of glutamate.
4.2. Aging alters stimulus-evoked glutamate release in the trisynaptic circuit
In these studies we showed that significantly less glutamate release was evoked by local KCl application in the CA3 subregion of aged rats compared to the CA3 of young and late-middle aged rats. In the late-middle aged and aged groups, glutamate release was impaired from terminals in both CA3 and CA1 when compared to terminals in the DG. Changes in stimulus-evoked glutamate release in the hippocampus with aging has been previously demonstrated in brain slices (reviewed by Segovia et al., 2001). Diminished glutamate release indicates that less glutamate is available for receptor activation, potentially altering the relay of glutamatergic neurotransmission through the trisynaptic circuit, which is critical for hippocampal-dependent learning and memory.
The CA3 subregion has been repeatedly identified as having age-associated neurochemical alterations (Le Jeune et al., 1996; Kadar et al., 1998; Adams et al., 2001). Mossy fiber projections to CA3 have shown age-associated changes in dynorphin, an opioid-like peptide believed to be intimately involved with glutamatergic neurotransmission. Zhang et al. (1991) showed that an increase in dynorphin was localized to the DG granule cells and their MF projections in F344 rats, which correlated with an impaired memory performance and decreased glutamate release in vivo; however, their microdialysis study lacked anatomical resolution, localizing the diminished glutamate response only to the ventral hippocampus. Loss of glutamate release capacity in the CA3 across age groups, and within the trisynaptic circuitry of late-middle aged and aged rats is perhaps our most important finding. These experiments have potentially exposed the functional outcomes of the age-associated molecular changes in mossy fiber inputs previously described in the literature.
Our in vivo studies also showed that the DG subregion was significantly altered in late-middle aged rats as compared to younger and older animals because glutamatergic terminals in the late-middle aged DG released more neurotransmitter following local KCl stimulation. The augmented release of evoked-glutamate from perforant path fibers in the DG indicates that changes in input to the hippocampal formation occur by late-middle age. An age-related increase in glutamate release has been reported in brain slices (Meldrum et al., 1992; Saransaari and Oja, 1995). Prior work has shown that synapses from afferent projections to the DG decrease with age; however, the remaining synapses are electrophysiologically stronger (reviewed by Geinisman et al., 1995). Until now, the effects of these age-associated changes on glutamatergic neurotransmission in vivo have never been specifically investigated in the DG subregion.
A compensatory increase in synaptic strength is supported by our data because we showed an enhanced evoked glutamate release in late-middle aged rats. From this we can conclude that more glutamate is available from perforant path terminals in the late-middle aged DG to participate in glutamate-dependent processes. This is important because age-associated alterations in hippocampal connectivity due to loss of synapses may not immediately result in functional changes due to the ability of the late-middle aged glutamatergic afferents to the DG to compensate. The return in the aged DG to evoked glutamate release levels seen in young animals led us to two interesting, yet very different conclusions about the effects of aging on glutamate release in the DG. First, assuming the augmented release of glutamate in late-middle aged animals is a compensatory response to synaptic loss, the inability of the aged DG to maintain the increase may explain the loss of cognitive function in these animals. Alternatively, the aged DG may not be exhibiting a loss of compensation, but rather a different mechanism of compensation. The first order rate of glutamate clearance following evoked release was significantly faster in the aged DG compared to the young, even though the maximum release of glutamate measured by the microelectrode in both age groups was not significantly different. We believe the aged DG develops a compensatory increase in clearance capacity in response to the increased glutamate release in late-middle age. This is further supported by our exogenous glutamate clearance data (see Section 4.3). Increased clearance ability in the aged DG may be masking an increase in perforant path evoked-glutamate release similar to that seen in late-middle aged animals, such that higher release of glutamate in the aged DG is not measured by the electrode because a significant portion is rapidly cleared following release. If the increase in glutamate release during aging exceeds compensation and becomes pathologic, the faster glutamate clearance in aged animals may be a neuroprotective mechanism against excitotoxicity.
4.3. Aging alters glutamate clearance in the trisynaptic circuit
Our data indicate that the DG acquires significant glutamate clearance capacity with age, showing approximately a 66% increase in the uptake rate of aged rats as compared to young rats. A significant increase is seen when the DG is compared to other trisynaptic subregions within the aged hippocampus as well. These data are in contrast to prior work that has shown no consistent changes in glutamate clearance with aging (Najlerahim et al., 1990; Gilad et al., 1990; Palmer et al., 1994). Glutamate clearance from the synaptic cleft is controlled by uptake and diffusion (Syková, 2005). Approximately ninety percent of glutamate uptake is performed by high affinity transporters (GLAST, GLT-1) located predominantly on astroglia (reviewed by Danbolt, 2001). Syková et al. (2002) showed that a decrease in extracellular space (ECS) and loss of glial organization, especially in the DG, were evident in aged rats that were impaired learners. The aged F344 hippocampus maintains numbers of GFAP+ astrocytes at young levels (Hattiangady and Shetty, 2008), so it is possible that age-associated gliosis may be due to an increase in the fibrous character of astrocytes rather than an increase in astrocyte number (Wu et al., 2005). Syková et al. (2002) proposed that the compacted ECS could predispose the aging population to excitotoxicity due to synaptic ‘trapping’ of glutamate.
We believe the increased clearance capacity of the aged DG could be a very effective neuroprotective mechanism against over-activation of glutamate receptors resulting from age-associated increases in release capacity of perforant path terminals in the DG and excitotoxic trapping due to gliosis. The significant increase in uptake rate is likely related to increased surface expression of transporters on glia and neurons. We have recently reported that decreased surface expression of GLAST may contribute to slower regulation of glutamate uptake in the dorsal rat striatum (Nickell et al., 2007). How aging affects the function of astrocytic glutamate transporters in the hippocampus is a critical piece of information not yet fully understood. Additional studies are needed to investigate the mechanism (s) involved with enhanced clearance of glutamate in the aged DG observed in the present study.
4.4. Conclusions
To our knowledge, this is the first report in the literature describing age-associated subregional heterogeneity of hippocampal glutamatergic neurotransmission in vivo. Though resting levels of glutamate in the extracellular space were unchanged with aging, we were able to show subregion-specific alterations in stimulus-evoked glutamate release and clearance capacity in the trisynaptic circuit during aging. Taken with our previous aging studies in the rodent striatum and non-human primate cortex, we can begin to outline the vast variability of CNS susceptibility to changes in glutamate regulation during aging.
Acknowledgements
Support for these studies contributed from USPHS grants DA017186,AG00242, NS39787, AG013494, T32AG00242, and NSF DBI-0352848.
Footnotes
Disclosures
Dr. Gerhardt is the owner of Quanteon, LLC, which manufactures the FAST – 16 mk II system.
References
- Adams MM, Smith TD, Moga D, Gallagher M, Wang Y, Wolfe BB, Rapp PR, Morrison JH. Hippocampal dependent learning ability correlates with N-methyl-d-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J. Comp. Neurol. 2001 Apr 2;432(2):230–243. doi: 10.1002/cne.1099. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Gerhardt GA. Self-referencing ceramic-based multisite microelectrodes for the detection and elimination of interferences from the measurement ofl-glutamate and other analytes. Anal. Chem. 2001 Mar 5;73:1037–1042. doi: 10.1021/ac0010429. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Pomerleau F, Palmer M, Day BK, Huettl P, Gerhardt GA. Improved ceramic-based multisite microelectrode for rapid measurements of l-glutamate in the CNS. J. Neurosci. Meth. 2002 Sep 2;119:163–171. doi: 10.1016/s0165-0270(02)00172-3. [DOI] [PubMed] [Google Scholar]
- Chawla MK, Barnes CA. Hippocampal granule cells in normal aging: insights from electrophysiological and functional imaging experiments. Prog. Brain Res. 2007;163:661–678. doi: 10.1016/S0079-6123(07)63036-2. (review). [DOI] [PubMed] [Google Scholar]
- Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J. Neurosci. 2002 May 9;22:3628–3637. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001 Sep 1;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. (review). [DOI] [PubMed] [Google Scholar]
- Day BK, Pomerleau F, Burmeister JJ, Huettl P, Gerhardt GA. Microelectrode array studies of basal and potassium-evoked release of l-glutamate in the anesthetized rat brain. J. Neurochem. 2006 Mar 6;96:1626–1635. doi: 10.1111/j.1471-4159.2006.03673.x. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Detoledo-Morrell L, Morrell F, Heller RE. Hippocampal markers of age-related memory dysfunction: behavioral, electrophysiological and morphological perspectives. Prog. Neurobiol. 1995 Feb 3;45:223–252. doi: 10.1016/0301-0082(94)00047-l. (review). [DOI] [PubMed] [Google Scholar]
- Gilad GM, Gilad VH, Tizabi Y. Aging and stress-induced changes in choline and glutamate uptake in hippocampus and septum of two rat strains differing in longevity and reactivity to stressors. Int. J. Dev. Neurosci. 1990;8(6):709–713. doi: 10.1016/0736-5748(90)90064-9. [DOI] [PubMed] [Google Scholar]
- Greene JG, Borges K, Dingledine R. Quantitative transcriptional neuroanatomy of the rat hippocampus: evidence for wide-ranging, pathway-specific heterogeneity among three principal cell layers. Hippocampus. 2008 Oct; doi: 10.1002/hipo.20502. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup KN, Rutherford EC, Quintero JE, Day BK, Nickell JR, Pomerleau FP, Huettl P, Burmeister JJ, Gerhardt GA. Second-by-second measures of l-glutamate and other neurotransmitters using enzyme-based microelectrode arrays. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. Boca Raton, FL: CRC Press; 2007. pp. 407–450. [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol. Aging. 2008 Jan 1;29:129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadar T, Dachir S, Shukitt-Hale B, Levy A. Sub-regional hippocampal vulnerability in various animal models leading to cognitive dysfunction. J. Neural Transm. 1998;105(8–9):987–1004. doi: 10.1007/s007020050107. [DOI] [PubMed] [Google Scholar]
- Le Jeune H, Cécyre D, Rowe W, Meaney MJ, Quirion R. Ionotropic glutamate receptor subtypes in the aged memory-impaired and unimpaired Long-Evans rat. Neuroscience. 1996 Sep 2;74:349–363. doi: 10.1016/0306-4522(96)00213-8. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Aging of glutamate receptors: correlations between binding and spatial memory performance in mice. Mech. Ageing Dev. 1998a Sep 3;104:227–248. doi: 10.1016/s0047-6374(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. The aging of the NMDA receptor complex. Front. Biosci. 1998b May;3:e70–e80. doi: 10.2741/a368. (review). [DOI] [PubMed] [Google Scholar]
- Massieu L, Tapia R. Glutamate uptake impairment and neuronal damage in young and aged rats in vivo. J. Neurochem. 1997 Sep 3;69:1151–1160. doi: 10.1046/j.1471-4159.1997.69031151.x. [DOI] [PubMed] [Google Scholar]
- Meldrum MJ, Glenton P, Dawson R., Jr [3H]d-aspartic acid release in brain slices of adult and aged Fischer 344 rates. Neurochem. Res. 1992 Feb 2;17:151–156. doi: 10.1007/BF00966793. [DOI] [PubMed] [Google Scholar]
- Merrill DA, Roberts JA, Tuszynski MH. Conservation of neuron number and size in entorhinal cortex layers II, III, and V/VI of aged primates. J. Comp. Neurol. 2000 Jul 3;422:396–401. doi: 10.1002/1096-9861(20000703)422:3<396::aid-cne6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Merrill DA, Chiba AA, Tuszynski MH. Conservation of neuronal number and size in the entorhinal cortex of behaviorally characterized aged rats. J. Comp. Neurol. 2001 Oct 4;438:445–456. doi: 10.1002/cne.1327. [DOI] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Aging, stress and the hippocampus. Ageing Res. Rev. 2005 May 2;4:123–140. doi: 10.1016/j.arr.2005.03.002. (review). [DOI] [PubMed] [Google Scholar]
- Müller WE, Scheuer K, Stoll S. Glutamatergic treatment strategies for age-related memory disorders. Life Sci. 1994;55(25–26):2147–2153. doi: 10.1016/0024-3205(94)00395-5. (review). [DOI] [PubMed] [Google Scholar]
- Najlerahim A, Francis PT, Bowen DM. Age-related alteration in excitatory amino acid neurotransmission in rat brain. Neurobiol. Aging. 1990 Mar-Apr;11:155–158. doi: 10.1016/0197-4580(90)90049-6. [DOI] [PubMed] [Google Scholar]
- Nickell J, Pomerleau F, Allen J, Gerhardt GA. Age-related changes in the dynamics of potassium-evoked l-glutamate release in the striatum of Fischer 344 rats. J. Neural Transm. 2005 Jan 1;112:87–96. doi: 10.1007/s00702-004-0151-x. [DOI] [PubMed] [Google Scholar]
- Nickell J, Salvatore MF, Pomerleau F, Apparsundaram S, Gerhardt GA. Reduced plasma membrane surface expression of GLAST mediates decreased glutamate regulation in the aged striatum. Neurobiol. Aging. 2007 Nov 11;28:1737–1748. doi: 10.1016/j.neurobiolaging.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Palmer AM, Robichaud PJ, Reiter CT. The release and uptake of excitatory amino acids in rat brain: effect of aging and oxidative stress. Neurobiol. Aging. 1994;15(1):103–111. doi: 10.1016/0197-4580(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. San Diego: Academic Press; 2006. [Google Scholar]
- Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, Albert MS. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J. Neuropathol. Exp. Neurol. 1996 Aug 8;55:861–874. doi: 10.1097/00005072-199608000-00001. (review). [DOI] [PubMed] [Google Scholar]
- Pomerleau F, Day BK, Huettl P, Burmeister JJ, Gerhardt GA. Real time in vivo measures of l-glutamate in the rat central nervous system using ceramic-based multisite microelectrode arrays. Ann. NY Acad. Sci. 2003 Nov;1003:454–457. doi: 10.1196/annals.1300.051. [DOI] [PubMed] [Google Scholar]
- Quintero JE, Day BK, Zhang Z, Grondin R, Stephens ML, Huettl P, Pomerleau F, Gash DM, Gerhardt GA. Amperometric measures of age-related changes in glutamate regulation in the cortex of rhesus monkeys. Exp. Neurol. 2007 Dec 2;208:238–246. doi: 10.1016/j.expneurol.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Natl. Acad. Sci. U.S.A. 1996 Sep 18;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Schliemann T, Sørensen JC, Zimmer J, West MJ. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol. Aging. 1996 Jan-Feb;17:143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003 Mar 1–2;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. (review). [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog. Neurobiol. 2003 Feb 3;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rutherford EC, Pomerleau F, Huettl P, Strömberg I, Gerhardt GA. Chronic second-by-second measures of l-glutamate in the central nervous system of freely moving rats. J. Neurochem. 2007 Aug 3;102:712–722. doi: 10.1111/j.1471-4159.2007.04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Age-related changes in the uptake and release of glutamate and aspartate in the mouse brain. Mech. Ageing Dev. 1995 Jul 2–;81:61–71. doi: 10.1016/0047-6374(95)01583-l. [DOI] [PubMed] [Google Scholar]
- Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech. Ageing Dev. 2001 Jan 1;122:1–29. doi: 10.1016/s0047-6374(00)00225-6. (review). [DOI] [PubMed] [Google Scholar]
- Stephens ML, Spencer DD, Cavus I, Hsiao MC, Song D, Courellis SH, Deadwyler SA, Hampson RE, Putz D, Quintero JE, Bensalem-Owen MK, Hascup KN, Rutherford EC, Day BK, Nickell JR, Pomerleau F, Huettl P, Burmeister JJ, Talauliker PM, Marmarelis VZ, Granacki JJ, Berger T, Gerhardt GA. Microelectrode-based epilepsy therapy: a hybrid neural prosthesis incorporating seizure prediction and intervention with biomemetic maintenance of normal hippocampal function. In: Staley Soltesz., editor. Computational Neuroscience in Epilepsy. CH: Academic Press; 2008. p. 33. [Google Scholar]
- Syková E. Glia and volume transmission during physiological and pathological states. J. Neural Transm. 2005 Jan 1;112:137–147. doi: 10.1007/s00702-004-0120-4. (Epub Mar 19, 2004; review). [DOI] [PubMed] [Google Scholar]
- Syková E, Mazel T, Hasenöhrl RU, Harvey AR, Simonová Z, Mulders WH, Huston JP. Learning deficits in aged rats related to decrease in extracellular volume and loss of diffusion anisotropy in hippocampus. Hippocampus. 2002;12(2):269–279. doi: 10.1002/hipo.1101. [DOI] [PubMed] [Google Scholar]
- Tang YP, Wang H, Feng R, Kyin M, Tsien JZ. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology. 2001 Nov 6;41:779–790. doi: 10.1016/s0028-3908(01)00122-8. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink BH. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997 Nov 3;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. (review). [DOI] [PubMed] [Google Scholar]
- West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol. Aging. 1993 Jul-Aug;14:287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- West MJ, Amaral DJ, Rapp PR. Preserved hippocampal cell number in aged monkeys with recognition memory deficits. Soc. Neurosci. Abstr. 1993;19:599. [Google Scholar]
- Wu WW, Oh MM, Disterhoft JF. Age-related biophysical alterations of hippocampal pyramidal neurons: implications for learning and memory. Ageing Res. Rev. 2002 Apr 2;1:181–207. doi: 10.1016/s1568-1637(01)00009-5. (review). [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang AQ, Yew DT. Age related changes of various markers of astrocytes in senescence-accelerated mice hippocampus. Neurochem. Int. 2005 Jun 7;46:565–574. doi: 10.1016/j.neuint.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Zhang WQ, Mundy WR, Thai L, Hudson PM, Gallagher M, Tilson HA, Hong JS. Decreased glutamate release correlates with elevated dynorphin content in the hippocampus of aged rats with spatial learning deficits. Hippocampus. 1991 Oct 4;1:391–397. doi: 10.1002/hipo.450010407. [DOI] [PubMed] [Google Scholar]