Abstract
In atherosclerosis and diabetes mellitus, the concomitant presence of low-grade systemic inflammation and mild zinc deficiency highlights a role for zinc nutrition in the management of chronic disease. This review aims to evaluate the literature that reports on the interactions of zinc and cytokines. In humans, inflammatory cytokines have been shown both to up- and down-regulate the expression of specific cellular zinc transporters in response to an increased demand for zinc in inflammatory conditions. The acute phase response includes a rapid decline in the plasma zinc concentration as a result of the redistribution of zinc into cellular compartments. Zinc deficiency influences the generation of cytokines, including IL-1β, IL-2, IL-6, and TNF-α, and in response to zinc supplementation plasma cytokines exhibit a dose-dependent response. The mechanism of action may reflect the ability of zinc to either induce or inhibit the activation of NF-κB. Confounders in understanding the zinc-cytokine relationship on the basis of in vitro experimentation include methodological issues such as the cell type and the means of activating cells in culture. Impaired zinc homeostasis and chronic inflammation feature prominently in a number of cardiometabolic diseases. Given the high prevalence of zinc deficiency and chronic disease globally, the interplay of zinc and inflammation warrants further examination.
Keywords: zinc, inflammation, cytokines, atherosclerosis, diabetes mellitus
1. Introduction
Zinc was established as an essential trace element in 1961 following the discovery of zinc deficiency in humans [1]. It is one of the most abundant elements within cells and is necessary for a broad range of physiological processes. Zinc is an integral component of proteins involved in cell structures and the stabilisation of cell membranes. It functions to maintain the structural integrity of as many as 3000 transcription factors in the human genome and is essential for the biological activity of more than 300 zinc metalloenzymes [2]. In addition to its numerous structural and catalytic functions, zinc is involved in the regulation of an extensive variety of genes, impacting such diverse processes as protein-protein interactions, nucleic acid metabolism, cell replication, apoptosis, and signal transduction [3]. The small proportion of readily exchangeable or “free” zinc within cells [4] recently has been ascribed neurotransmitter functions [5], highlighting the diverse roles of zinc in biology.
Zinc deficiency is reported to contribute significantly to the global burden of disease [6]. Although severe zinc deficiency is relatively rare in developed countries, based on population estimates of dietary zinc intake less acute deficiency states are believed to be highly prevalent [7]. In addition to inadequate dietary zinc intake, deficiency may result from impaired absorption or resorption or increased excretion of zinc; conditions such as chronic diarrhea, extensive burns, or traumatic and surgical wounds increase endogenous zinc losses. An initial consequence of zinc deficiency is an impairment of immunological functions [8]. The wide involvement of zinc in the immune system [9] includes an ability to influence the production and signalling of numerous inflammatory cytokines in a variety of cell types [10,11] (Table 1).
Table 1.
Selected cytokines: cell sources and examples of their principal functions in inflammation.
| Cytokine | Primary Cell Sources | Key Functions in Inflammation |
|---|---|---|
| IL-1 | Macrophages Endothelial cells | Synthesis of acute phase proteins by hepatocytes; Local and systemic inflammatory effects |
| IL-2 | Activated T cells Th1 cells | Proliferation of T cells, B cells; Proliferation and activation of NK cells |
| IL-6 | Macrophages Th2 cells Endothelial cells Adipocytes Myocytes Osteoblasts | Synthesis of acute phase proteins by hepatocytes; Proliferation of B cells; Down-regulation of IL-1 and TNF production; Activation of immune cells, osteoclasts, endothelial cells; Hypothalamic Pituitary Axis—fever & hormone release |
| IL-10 | MacrophagesTh2 cells | Resolution of inflammation; Inhibition of Th1 inflammatory cytokine synthesis; Inhibition of activated macrophages and dendritic cells |
| IL-12 | Macrophages Dendritic cells | Promotion of Th1 differentiation; Stimulation of IFN-γ production by T cells, NK cells |
| TNF-α | Macrophages T cells NK cells Lymphoid cells Endothelial cells Adipocytes Cardiac myocytes Fibroblasts Neuronal cells | Synthesis of acute phase proteins by hepatocytes; Recruitment and activation of neutrophils and monocytes at sites of infection; Stimulation of CRP release from liver; Activation of NF-κB pathway; Induction of insulin resistance |
| TGF-β | Macrophages T cells | Resolution of inflammation; Limit production of IL-2, IFN-γ, and TNF; Inhibition of proliferation/activation of B cells, T cells, macrophages. |
| IFN-γ | Th1 cells NK cells | Activation of macrophages; Suppression of Th2 cell activity; Promotion of leukocyte migration |
Inflammation (INF) is an integral part of the innate immune system’s response to trauma or infection. The acute inflammatory response is initiated upon detection of inducers, such as microbial infections, oxygen radicals, and tissue damage, by sensors such as Toll-like receptors (TLR) and other pattern recognition receptors. The purpose of INF is to protect the host from the spread of infection or tissue damage, whereupon the inflammatory response is resolved and homeostasis is restored. In typical cases, the inflammatory response is localised to the site where the inflammatory inducer is present; however an increasing number of inflammatory conditions have been described where the initiating trigger is not well defined and INF appears to be chronic [12,13]. The redistribution of zinc in such conditions affects zinc homeostasis [14]. The aim of this review is to evaluate the literature on the interactions of zinc and cytokines in cardiometabolic disease. The presence of low-grade systemic INF in conjunction with perturbed zinc homeostasis in chronic disorders, such as atherosclerosis and diabetes mellitus (DM), highlights a role for zinc nutrition in the management of cardiometabolic symptoms [15].
2. Zinc Homeostasis and Inflammation
In humans, homeostatic mechanisms maintain plasma zinc within a concentration range of approximately 10 to 18 μmol/L. Cells are dependent on plasma to supply them with a constant supply of zinc to sustain normal function. In zinc deficiency, immune cells may be the first to respond to a change in zinc status even before plasma zinc concentrations fall below the normal range [16]. Cellular zinc concentrations are maintained by two classes of zinc transporters: the ZnT (SLC30) and Zip (SLC39) zinc transporter families. ZnT transporters promote cellular zinc efflux or its sequestration into intracellular organelles; conversely, Zip transporters facilitate extracellular or organellar zinc influx into the cytoplasm. Metallothionein (MT) also is believed to play a central role in the maintenance of zinc homeostasis by trafficking zinc through the cell and releasing it to zinc-requiring proteins. INF has been associated with modulated zinc transporter [17,18,19,20] and MT [21,22] expression in a variety of cell types. Inflammatory cytokines have been reported both to up- and down-regulate the expression of specific ZnT and Zip transcripts [19,23]; the net effect of the altered zinc transporter expression profile has been hypothesized to maintain or increase intracellular zinc in response to an increased demand for zinc in inflammatory conditions [19].
3. Zinc Status and Cytokines
The acute phase response to stress, trauma, and infection includes a transient and rapid decline in the plasma zinc concentration as a result of the redistribution of zinc into the cellular compartment. The increase in intracellular zinc is proposed to supply additional zinc for protein synthesis, neutralization of reactive nitrogen and oxygen species, and prevention of microbial invasion [24]. Zinc redistribution in inflammatory conditions appears to be mediated at least partially by cytokines; the exogenous administration of lipopolysaccharide (LPS) in healthy humans resulted in a rapid decrease in the serum zinc concentration that was preceded by prominent increases in TNF-α and IL-6 levels [25]. Cross-sectional studies support a relationship between cytokine and plasma zinc concentrations in trauma and infection. Patients with severe closed head injury exhibit hypozincemia along with a prominent cytokine and acute phase response [26]. In critically ill infected and noninfected adults assessed early after intensive care unit admission, lower plasma zinc concentrations were associated with higher illness scores and increased cytokine production [17].
3.1. Cytokines in Chronic Inflammation
Chronic INF is characterised by elevated production of inflammatory cytokines [27]. A relationship between zinc status and cytokine production is reported in conditions associated with chronic INF. In overweight and obese adults, participants with low dietary zinc intakes (5.7 mg/day) were found to have lower plasma zinc concentration, intracellular zinc content, and intracellular free zinc levels and upregulated IL-1α, IL-1β, and IL-6 genes compared to overweight and obese participants with zinc intakes (12.2 mg/day) that met recommended dietary requirements [28].
3.2. Human Zinc Deficiency
The generation of a variety of cytokines, including IL-1β, IL-2, IL-6, and TNF-α, is reportedly influenced by mild to moderate zinc deficiency in humans. IL-1β production was found to be higher in LPS-stimulated peripheral blood mononuclear cells (PBMC) from zinc-deficient adults (as induced by experimental diet) compared to their zinc-sufficient counterparts [29,30]. Compared to zinc-sufficient individuals (as defined by the zinc concentration of lymphocytes, granulocytes, and platelets), phytohaemagglutinin (PHA)-induced production of IL-2 was lower in PBMC of zinc deficient patients with head and neck cancer and in zinc-deficient healthy volunteers [29]. Consistent results were observed also in an elderly population, with lower IL-2 and IL-2Rα mRNA observed in PBMC isolated from zinc-deficient (defined as plasma zinc <90 µg/dL (13.8 µmol/L)) compared to zinc-sufficient subjects [31]. After a 10 week zinc-restricted (4.6 mg/day) diet, the PHA-stimulated secretion of IL-2R was reduced in PBMC of healthy men [32].
Inconsistent results have been reported for IL-6. Zinc deficiency, defined as a plasma zinc concentration <9.95 mmol/L, in Indonesian infants was accompanied by lower production of IL-6 after ex vivo stimulation of whole blood with LPS and PHA [33], while no significant differences between zinc-sufficient and zinc-deficient adults were observed in the production of IL-6 in PBMC stimulated with PHA alone [29]. In a similar vein, divergent results have been reported for TNF-α, which was higher in LPS-stimulated [30] and lower in PHA-stimulated [29] PBMC from zinc-deficient subjects, suggesting that the source of cell-stimulation influences cytokine production.
3.3. Zinc Supplementation Studies in Humans
Human intervention studies measuring the effects of zinc on plasma cytokine concentrations or cytokine production in primary human blood cells are shown in Table 2 [34,35,36,37,38,39,40,41,42,43,44]. Supplementation with ≥45 mg zinc/day has been reported to decrease ex vivo generated levels of pro-inflammatory cytokine mRNA and proteins in stimulated mononuclear cells [34,35,37]. Conversely, increased cytokine concentrations have been shown in stimulated mononuclear cells isolated from populations supplemented with ≤20 mg zinc/day [39,40,41], suggesting a zinc dose-response. Measurements of plasma cytokine concentrations in response to zinc supplementation support a difference in effect depending on zinc dose; plasma concentrations of IL-6 have been shown to decrease with zinc supplementation of 45 mg/day [36] but to increase with 10 mg zinc/day [42,43]. The significance of these changes is unclear but the ability of zinc supplementation to influence cytokine concentrations in humans is consistently reported.
3.4. In Vitro Studies in Human Cells
A number of in vitro studies have measured inflammatory cytokine production in response to zinc depletion or supplementation in stimulated and unstimulated cells. In PHA- and phorbol myristate acetate (PMA)-stimulated HUT-78 cells, mRNA levels of IFN-γ were increased in zinc-sufficient (15 µM) compared to zinc-deficient (1 µM) cells [45]. In PHA- and PMA-activated human Jurkat T cells, supplementation with 50 or 100 µM zinc significantly reduced IFN-γ mRNA expression without affecting cell viability; in contrast, cells without stimulation did not express IFN-γ [46]. Together these results suggest that in inflammatory conditions IFN-γ gene expression is reduced both by zinc deficiency and zinc supplementation.
After PMA or LPS stimulation, human derived-promyelocytic leukemia (HL-60) and human vascular endothelial cells cultured in 1 µM zinc (zinc-deficiency) demonstrated significantly higher generation of TNF-α and IL-1β cytokines than cells cultured in 15 µM zinc (physiologic conditions) [47]. The effect of zinc deficiency on cytokine concentrations was not apparent in unstimulated cells; before PMA or LPS stimulation, both groups of cells produced only trace amounts of TNF-α and IL-1β regardless of the zinc concentration of the media and despite adverse changes in oxidative stress markers in the zinc deficient cells [47]. Similarly, in unstimulated THP-1 monocytic cells no significant effects of the addition of 120 µM zinc on TNF-α, IL-1β, and IL-6 concentrations were observed, however zinc activated the release of IL-8 [48]. In human promonocytic HL-CZ cells, 150 µM zinc increased IL-6 as well as IL-8 levels [49], which may reflect the higher zinc concentration or cell-specific differences in cytokine release.
Cell culture studies using primary human cells demonstrate effects of zinc on cytokines that differ according to the zinc concentration and activation state of the cell (Table 3) [50,51,52,53,54,55,56]. Zinc treatment enhanced IFN-γ & IL-10 concentrations in PHA-stimulated PBMC [50] and IL-1β & TNF-α in LPS-stimulated cells, while zinc down-regulated levels of IL-1β & TNF-α in PBMC stimulated with superantigens [55]. In PBMC isolated from healthy adults and treated for 24 hours with 3 or 30 µM zinc, no difference was observed in the concentration of TNF-α, IL-1β, IL-6, IL-12 and IFN-γ; however, an increase in all cytokines was seen with ≥100 µM zinc [52], which may indicate that high extracellular zinc concentrations act as inflammatory triggers in primary mononuclear cells.
Table 2.
Effects of zinc supplementation in humans on plasma cytokine concentrations or cytokine release in isolated blood cells.
| Author, Year | Description of Participants | Study Design/Cell Culture Conditions | Zn Dose (mg/day) | Outcome |
|---|---|---|---|---|
| Bao et al., 2008 [34] | 36 adults with sickle cell disease; M & F; 18–47 years | RCT, parallel, 13 weeks; isolated PBMCs stimulated with LPS or PHA-P for 24 h | 75 | Decrease in LPS-induced TNF-α & IL-1β mRNA & protein concentrations & increase in PHA-P induced IL-2 mRNA concentrations in Zn compared to placebo group |
| Prasad et al., 2007 [35] | 50 healthy older adults; M & F; 55–87 years/whole blood stimulated for 4 h & isolated PBMCs for 24 h with LPS | RCT, parallel, 52 weeks; isolated whole blood cells or PBMCs stimulated with LPS for 4 or 24 h | 45 | No change in % of whole blood cells positive for IL-1β or TNF-α in Zn supplement group; decrease in ex vivo generation of TNF-α in PBMCs from Zn supplement group |
| Bao et al., 2010 [36] | 40 healthy older adults; M & F; 56–83 years | RCT, parallel, 26 weeks | 45 | Decrease in plasma IL-6 concentrations in Zn supplement group |
| Prasad et al., 2004 [37] | 20 healthy adults; M & F; 19–50 years | RCT, parallel, 8 weeks; isolated PBMCs stimulated with LPS for 24 h | 45 | Zn reduced concentrations of LPS-induced TNF-α & IL-1β mRNAs |
| Raqib et al., 2004 [38] | 56 Shigella-infected children; M & F; 1–5 years | RCT, parallel, 2 weeks; isolated PBMCs stimulated with PHA for 72 h | 20 | No significant effects of Zn on PHA-induced release of IL-1β, IL-2, or IFN-γ |
| Sandstead et al., 2008 [39] | 54 children; M & F; 6–7 years | RCT, parallel, 10 weeks; isolated PBMC stimulated with PHA-P for 48 h | 20 a | Greater release of IL-2 & IFN-γ in stimulated cells derived from Zn supplemented compared to control subjects |
| Aydemir et al., 2006 [40] | adults; M; 19–31 years | controlled before & after trial, 10 days; isolated cells stimulated with LPS for 2 h or by antigen presentation for 2 days | 15 | Greater release of TNF-α, IL-1β, IFN-γ in stimulated cells (monocytes, granulocytes, & T lymphocytes, respectively) derived from Zn supplemented subjects compared to placebo |
| Kahmann et al., 2008 [41] | 19 healthy older adults; M & F; 65–82 years | uncontrolled before & after trial, 48 days; isolated PBMCs stimulated with LPS or SPEA for 72 h | 10 | Zn supplementation resulted in lower basal IL-6, higher LPS-induced IL-6, & higher SPEA-induced TNF-α & IFN-γ concentrations |
| Mariani et al., 2008 [42] | 39 healthy older adults; M & F; 60–83 years | uncontrolled before & after trial, 48 days | 10 | Increase in plasma IL-6 concentrations with Zn |
| Mocchegiani et al., 2008 [43] | 110 healthy older adults; M & F; 65–85 years | uncontrolled before & after trial, 48 days | 10 | No change in TNF-α but increase in plasma IL-6 concentrations with Zn |
| Kara et al., 2011 [44] | 40 athletes and sedentary young adults; M; 15–17 years | controlled before & after trial, 8 weeks | 5 b | Zn supplementation resulted in higher serum IL-2, TNF-α & IFN-γ concentrations compared to non-supplemented individuals, irrespective of exercise |
a Zinc supplement provided for 5 days per week; b Supplementation dose determined as mg/kg.
Table 3.
In vitro studies that report on the effects of zinc treatment on cytokine production in primary human blood cells.
| Author, Year | Treatment | Outcome |
|---|---|---|
| Metz et al., 2007 [50] | PBMC were supplemented with 30, 60 µM Zn or 1 µM TPEN for 7 days before stimulation with PHA for 24 h | PHA-stimulated IFN-γ & IL-10 concentrations were higher in cells pre-treated with 60 µM Zn |
| Poleganov et al., 2007 [51] | PBMC were supplemented with 7.5, 15, 30 µM Zn & stimulated with IL-1β, IL-12, or IL-18 for 39 h | Zn amplified the induction of IFN-γ by IL-1β, IL-12 & IL-18 |
| Chang et al., 2006 [52] | PBMC were treated with 3, 30, 100, 300, 1000 µM Zn for 24 h | TNF-α, IL-1β, IL-6, IL-12, and IFN-γ concentrations increased at zinc concentrations ≥100 µM in combination with decreased cell proliferation |
| von Bulow et al., 2005 [53] | PBMC were treated with 25 µM Zn plus 50 µM pyrithione & stimulated with LPS for 24 h; primary monocytes were incubated with 25, 125 µM Zn for 1 h before addition of LPS (250 ng/mL) for 24 h, or stimulated with 1 µM Zn plus pyrithione (50 µM) & LPS for 24 h | Application of zinc plus pyrithione blocked LPS-induced release of IL-1β & TNF-α in PBMC; in monocytes, 125 µM Zn significantly inhibited TNF-α release compared to controls; the application of zinc plus the pyrithione ionophore abrogated LPS-induced release of IL-1β & TNF-α |
| Wellinghausen et al., 1997 [54] | PBMC in serum-free medium were stimulated with 100 µM Zn for 48 h | Zn increased IL-1β concentrations; Zn-induced secretion of IFN-γ was not measurable |
| Driessen et al., 1995 [55] | PBMC were supplemented with 12.5, 25, 50, 100 µM Zn & stimulated with LPS, SEA, or SEE for 24 h (TNF-α), 48 h (IL-1β), & 72 h (IFN-γ) | IL-1β & TNF-α concentrations in LPS-stimulated cells were enhanced by the addition of Zn in a concentration-dependent manner; Zn down-regulated levels of IL-1β & TNF-α in cells stimulated with SEA & SEE superantigens |
| Scuderi, 1990 [ 56] | PBMC were incubated with 30, 60, 120, 250, 500, 1000, 2000 µM Zn for 18 h; in addition, cells were incubated with 63, 125, 250, 500, 1000 µM Zn plus a substimulatory concentration of LPS (0.01 pg/mL) | Addition of Zn resulted in a concentration-dependent stimulation of TNF (with a peak at 250 µM) & IL-1β (peak at 120 µM), IL-6 was unaffected by Zn; Zn & LPS in combination resulted in a synergistic stimulation of TNF but not IL-1β secretion |
4. Immune Cells
Zinc may affect the generation of cytokines by influencing the normal development and function of immune cells. Cytokines are produced by a variety of cells, although most commonly from T lymphocytes and macrophages [11]. Experimental zinc deficiency decreases the activity of serum thymulin, which is required for the maturation of T-helper cells [57], leads to an imbalance of T-helper 1 (Th1) and T-helper 2 (Th2) functions, decreases the recruitment of T-naive cells [58], and reduces natural-killer (NK) cell lytic activity [59].
A number of studies suggest that zinc may influence the function of polymorphonuclear neutrophils (PMNs). PMNs are an important component of the acute inflammatory response, providing the primary cellular defense against bacteria in humans [60]. Beyond their traditional role as professional phagocytes, neutrophils can be induced to express a variety of cytokines, including TNF-α, IL-1β, and IL-12, and chemokines, such as IL-8 [61]. In vitro zinc deficiency studies have found that zinc depletion disrupts cell membrane barrier integrity and induces increases in the secretion of IL-8 and neutrophil transmigration [62]. Zinc also may modulate the oxidative burst that is generated by PMNs as part of their microbicidal activity. Stimulated PMNs released lower levels of superoxide anion (O2−) in the presence of 20 μmol extracellular zinc compared to untreated cells; in contrast, PMNs exposed to 200 μmol zinc generated higher O2− levels [63]. Substantial evidence implicates a pathogenic role for PMN-derived oxygen metabolites in a range of disorders that are associated with perturbed zinc homeostasis and oxidative stress, including myocardial injury and arrhythmias during ischaemia and reperfusion [15,64].
Microarray analysis in the HUT-78 human T-lymphoma cell line provides evidence for a shift in global gene expression during zinc deficiency. Zinc deficient conditions affected gene expression of proteins that are associated with T-cell receptor subunits, antigen recognition molecules, adhesion molecules, and genes associated with lymphoid function. When zinc deficient HUT-78 cells were stimulated, gene expression of molecules associated with IL-1β responsiveness was increased and expression of IL-2R, IL-6R, and IL-4 was decreased compared to zinc adequate controls. An increase was observed also in the gene expression of molecules found in atherosclerosis, e.g., PTX3, which is rapidly induced by IL-1β [30].
5. Key Signaling Mechanisms: NF-κB and Nitric Oxide
The ability of zinc to regulate both negatively and positively the signalling pathways of TLR and the IL-1 and TNF receptors (IL-1R and TNF-R, respectively) may reflect its ability to induce or inhibit the activation of NF-κB, a ubiquitously expressed nuclear transcription factor that is critically involved in proliferation, immunity, INF, and apoptosis [15]. The TLR, IL-1R, and TNF-R signalling pathways converge on a common IκB kinase complex that phosphorylates the NF-κB inhibitory protein, IκBα, resulting in the release of NF-κB and its translocation to the nucleus [65].
Zinc has been depicted as both a negative and positive regulator of NF-κB. In vitro, incubation of HUT-78 (Th0) cells in media containing either low (1 μM) or high (50 μM or 100 μM) zinc concentrations decreased the activation of NF-κB and the expression levels of IL-2, IL-2R, and TNF-α compared to cells grown in 15 μM zinc medium. Cell growth (but not cell viability) was observed to be lower in the 1 μM, 50 μM and 100 μM zinc media, suggesting an altered cellular metabolism in cells exposed to non-physiological concentrations of extracellular zinc [66].
In contrast, the lipopolysaccaride (LPS)-induced activation of NF-κB was decreased in a concentration-dependent manner in human monocytes cultured in 10 μM, 20 μM, and 45 μM zinc/pyrithione (50 μM) [67]. In line with this result, HL-60 and human vascular endothelial cells cultured in 15 μM zinc demonstrated significantly increased concentrations of A20 protein after stimulation with PMA or LPS compared to stimulated cells cultured in 1 μM zinc [47]; A20 has been shown to inhibit TNF-α and IL-1β induced activation of NF-κB in endothelial cells [68], suggesting that it acts as a general inhibitor of NF-κB activation. Conversely, exposure of cultured human airway epithelial cells to 50 μM of zinc increased NF-κB-dependent transcriptional activity compared to control cells [69], indicating that the effects of zinc on NF-κB activation may be cell specific. Differences in cell type and study model, the zinc concentrations used, and the impact of various agents (such as chelating agents and zinc ionophores) on intracellular free zinc fluctuations make apparently contradictory in vitro observations of the effect of zinc on NF-κB activation difficult to reconcile.
In vivo, the effects of zinc on NF-κB activity appear to depend on the health and/or zinc status of the host. Diet-induced zinc deficiency in a murine model of polymicrobial sepsis enhanced NF-κB p65 DNA binding activity in vital organs and the expression of a range of NF-κB targeted genes known to increase systemic INF. Short term zinc repletion before the onset of sepsis significantly reduced these effects [70]. In humans, NF-κB activation and the mRNA levels of the NF-κB-regulated IL-2 cytokine and IL-2Rα receptor were found to be decreased in the peripheral blood mononuclear cells of elderly subjects with plasma zinc concentrations below the normal range (110 ± 10 µg/dL (16.8 ± 1.5 µmol/L)) compared to those with normal plasma zinc values. These effects were corrected with zinc supplementation of 45 mg/day Zn gluconate [31].
NO is an endogenous signalling molecule that is also involved in the inflammatory response of IL-1 and TLR ligands [71]. It is synthesized from L-arginine and O2 by members of the nitric oxide synthase (NOS) family of dimeric enzymes. The release of NO by the endothelium plays a key role in vascular homeostasis. Its anti-inflammatory actions include the inhibition of caspases, and thus IL-1β and IL-18 generation, and suppression of the clonal expansion of T-cells [72]. The zinc-dependent enzymes CuZnSOD and EC-SOD function to protect the cellular availability of NO, and therefore its anti-inflammatory functions, by controlling O2•− concentrations. Excess O2•− reacts with NO to form ONOO−. The ONOO− anion in turn oxidizes the zinc-thiolate cluster at the dimer interface of endothelial NOS (eNOS), leading to the release of zinc and consequent dimer disruption and uncoupling of the enzyme; uncoupled eNOS demonstrates increased O2•− production and decreased NO synthesis [73]. Numerous studies have reported that eNOS uncoupling is an important mechanism of pathologic O2•− production in the vascular endothelium [74]. NADPH oxidase also has been proposed to play a central role in eNOS uncoupling [75]. An increased expression of the p22phox subunit of NADPH oxidase has been demonstrated in the walls of human coronary atherosclerotic arteries [76], and NADPH oxidase appears able to be activated by zinc [77]. Localized zinc deficiency or the potential for zinc to be aberrantly redistributed among target proteins and intracellular compartments during the atherosclerotic process likely ameliorates this protective effect.
6. Cardiometabolic Disease
An altered distribution of zinc among its principal plasma proteins has been observed in atherosclerosis [15,78] and the ease with which labile zinc is transported into endothelial cells [79] suggests that the vascular endothelium may be particularly affected by perturbations in zinc homeostasis and metabolism [80]. There are conflicting reports of the relationship between atherosclerosis and zinc status, as assessed by dietary intake of zinc and/or the measurement of zinc concentrations in healthy and diseased tissues; the balance of epidemiological studies points to an association between zinc deficiency and atherosclerosis however the studies are hampered by the lack of a decisive biomarker of zinc status. Zinc has also been linked to several cardiovascular risk factors including plasma lipoprotein concentrations, haemostasis and antioxidant status [81]. Clinical trials are mainly of zinc supplementation, and these show a decrease in plasma high-density lipoprotein cholesterol concentrations leading to an increased risk of heart disease [82]. Impaired zinc homeostasis has been associated with increased levels of oxidative stress and the induction of widespread genomic and proteomic changes that relate to cardiovascular disease.
Zinc deficiency has been suggested to exacerbate the detrimental effects of specific fatty acids, such as linoleic acid, and inflammatory cytokines, such as TNF-α, on vascular endothelial functions. Endothelial cells rendered zinc deficient demonstrated considerably higher levels of apoptotic cell death and caspase-3 activity than control cells when stimulated with linoleic acid and TNF-α. This effect was blocked by concurrent administration of zinc to the culture medium [83]. Conversely, increases in intracellular free zinc concentrations resulted in a rise in oxidative stress-related apoptosis of endothelial cells [84], highlighting that a change in the cellular zinc concentration in either direction could promote cell death in the endothelium.
Potential mechanisms of the influence of zinc on atherogenesis studied in rodent models and in cell culture include its interaction with a wide range of cellular redox and inflammatory processes, such as NF-κB, NO, PPAR, and PKC signalling pathways. Cellular zinc deficiency has been shown to upregulate NF-κB activity in endothelial cells [85] and high concentrations of NF-κB have been found to be present in the smooth muscle cells of the atherosclerotic lesion [86]. NF-κB is a component of the adhesion molecule upregulation process; is involved in the promotion of smooth muscle cell proliferation [87]; and mediates signal transduction by TLR, which play an important role in the initiation of the innate immune response and are implicated in atherogenesis [88]. The release of NO by the endothelium plays a role in vascular homeostasis. One way in which the relationship between NO and zinc may promote atherogenesis relates to Nrf2 expression in vascular cells, which is a key factor in the cellular protection against oxidative stress and INF. A release of intracellular zinc from proteins containing zinc-sulfur complexes, stimulated by inducible NOS-derived NO, has been shown to be a critical component of an Nrf2-dependent signaling pathway that activates the glutathione redox cycle in endothelial cells, ultimately protecting against oxidative damage [89].
The anti-inflammatory and antioxidant potential of zinc is supported by a recent zinc supplementation study. In a trial in healthy elderly subjects, supplementation with 45 mg Zn/day for 6 months compared to placebo was associated with an increase in plasma antioxidant power (represented by ascorbate equivalent units, U/mL) and a decrease in plasma concentrations of CRP, IL-6, macrophage chemo-attractant protein 1 (MCP-1), vascular endothelial cell adhesion molecule 1 (VCAM-1), and oxidative stress markers [36]. Taken together with the potential high-density-lipoprotein-raising effect of zinc [82], these data indicate that zinc may exert an atheroprotective effect under some conditions [36].
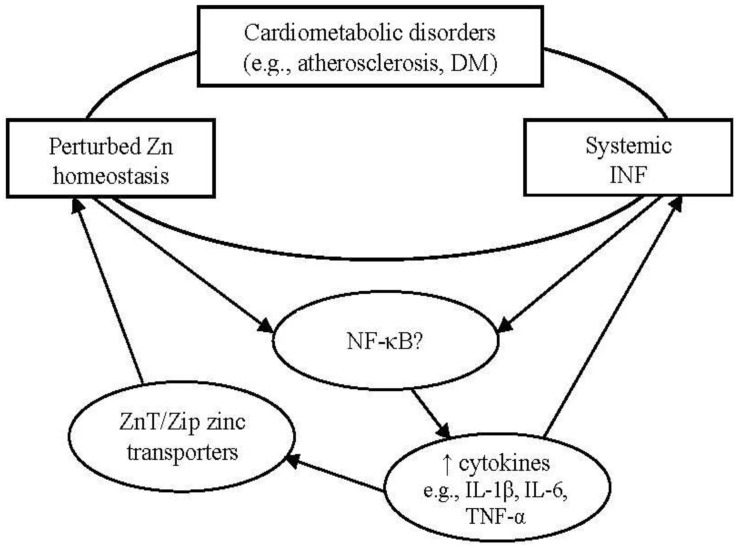
Perturbed zinc homeostasis has been observed also in DM. Both Type 1 and Type 2 DM exhibit an impaired immune function as part of their pathogenesis that ultimately results in a decreased functional β-cell mass; while Type 1 DM is primarily an autoimmune disorder that leads to rapid β-cell destruction, the failure of β-cells in Type 2 DM occurs over a prolonged period and involves the chronic activation of the innate immune system [90]. The sustained or aberrant expression in DM of a number of immune mediators, including NF-κB, IL-1β and IL-6, suggests a potential interaction between the impaired immunity and the perturbed cellular zinc homeostasis associated with the disease. An overview of the proposed interrelationship between impaired zinc homeostasis, systemic INF, and cardiometabolic disorders is depicted in Figure 1.
Figure 1.
Potential interrelationship between cardiometabolic disorders, perturbed zinc homeostasis, and systemic inflammation. Cardiometabolic disorders, such as atherosclerosis and DM, often are associated with impaired zinc homeostasis and low-grade systemic INF. Depending on the health and/or zinc status of the host, zinc may enhance the expression of a range of NF-κB targeted genes known to increase systemic INF, including inflammatory cytokines. Cytokines have been shown to modulate the expression of zinc transporters, suggesting that non-resolving INF may contribute to perturbed zinc homeostasis.
Zinc has the ability to regulate both negatively and positively the signalling pathways of TLR and the IL-1 receptor, which may reflect its ability to induce or inhibit NF-κB activation. Increased concentrations of IL-1β have been observed in the pancreatic islet in humans with Type 2 DM [91], and human islets have been shown to respond to metabolic stress in vitro by increasing IL-6 release [92]. A prospective examination of the effects of IL-1β, IL-6, and TNF-α on the development of Type 2 DM found that participants with detectable levels of IL-1β and elevated concentrations of IL-6 in plasma had a threefold increased risk of developing DM compared to the reference group [93]. Both IL-1β and IL-6 are pleiotropic and are known to exert both beneficial and detrimental effects on a variety of cell types, including the pancreatic β-cells, depending on the cytokine concentration and the duration of exposure. Higher doses and longer exposure times impair glucose-stimulated insulin secretion and, at least in the case of IL-1β, increase β-cell apoptosis [94].
The characterizing feature of DM is the presence of chronic hyperglycemia, which is known to enhance oxidant production and impair antioxidant defense mechanisms [95,96]. Zinc supplementation has been reported to decrease cytokine levels [36] and glycemic marker concentrations [97,98], suggesting that hyperglycemia and chronic INF in type 2 DM are connected. In a preliminary evaluation of clinical trials investigating the effect of zinc supplementation on fasting blood glucose and serum insulin concentrations, a small but statistically significant reduction in fasting glucose concentrations was observed after zinc supplementation and in secondary analyses of participants with chronic metabolic disease, zinc supplementation produced a greater reduction in plasma glucose concentrations compared to the effect that was observed in healthy participants [99]. The significant albeit modest reduction in plasma glucose concentrations, suggest that zinc contributes to the management of hyperglycemia, and thereby reduces INF, in individuals with chronic metabolic disease.
β-cell destruction can be mediated by autoreactive T-lymphocytes such as CD4+ and CD8+ cells; cytokines have been shown to induce the expression of the Fas (CD95, APO-1) receptor in the β-cell, thereby sensitizing it to T-lymphocyte mediated destruction [90]. The involvement of T-cells in β-cell failure, and INF more broadly, may further implicate perturbations in zinc homeostasis in the pathogenesis of DM. Zinc deficiency is well-known to disrupt the development and function of T-cells by causing, for instance, a reduction in thymic involution [100] and a decrease in the CD4+ to CD8+ cell ratio [8], both effects that have been shown to be corrected by zinc supplementation in humans.
7. Conclusions
Recognition of the public health importance of zinc continues to expand, as does knowledge of the multitude of biological pathways affected by zinc and its interaction with INF. Impaired zinc homeostasis, chronic INF, and increased levels of oxidative stress feature prominently in a number of cardiometabolic diseases, including atherosclerosis and DM. Positive indications for zinc supplementation in ameliorating INF and oxidative stress in cardiometabolic disorders raise the possibility of specific therapeutic manipulation by zinc-based treatments [15,101], however caution is required in the administration of zinc in the clinical setting. Given the potential for high zinc doses to produce adverse effects, including the inhibition of T-cell functions and aberrant expression of cytokines [102] and a decrease in Cu-Zn superoxide dismutase activity [103], further well-designed randomized controlled trials are necessary to provide cogent insight into safe and desirable levels of zinc supplementation in varied populations. Additional investigations of the molecular mechanisms that underpin the sensing and distribution of zinc also are necessary to explain their effects in humans.
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Prasad A.S., Halsted J.A., Nadimi M. Syndrome of iron deficiency anaemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am. J. Med. 1961;31:532–546. doi: 10.1016/0002-9343(61)90137-1. [DOI] [PubMed] [Google Scholar]
- 2.Vallee B.L., Falchuk K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993;73:79–118. doi: 10.2466/pr0.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 3.Beyersmann D., Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14:331–341. doi: 10.1023/A:1012905406548. [DOI] [PubMed] [Google Scholar]
- 4.Krężel A., Maret W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 2006;11:1049–1062. doi: 10.1007/s00775-006-0150-5. [DOI] [PubMed] [Google Scholar]
- 5.Frederickson C.J., Koh J.Y., Bush A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 6.Ezzati M., Lopez A.D., Rodgers A., Vander Hoorn S., Murray C.J.L. Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 7.Wuehler S.E., Peerson J.M., Brown K.H. Use of national food balance data to estimate the adequacy of zinc in national food supplies: Methodology and regional estimates. Public Health Nutr. 2005;8:812–819. doi: 10.1079/phn2005724. [DOI] [PubMed] [Google Scholar]
- 8.Prasad A.S. Zinc in human health: Effect of zinc on immune cells. Mol. Med. 2008;14:353–357. doi: 10.1007/s00894-008-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kruse-Jarres J.D. The significance of zinc for humoral and cellular immunity. J. Trace Elem. Electrolytes Health Dis. 1989;3:1–8. [PubMed] [Google Scholar]
- 10.Abbas A.K., Lichtman A.H. Cellular and Molecular Immunology. 5th. Elsevier Saunders; Philadelphia, PA, USA: 2005. [Google Scholar]
- 11.Zhou X., Fragala M.S., McElhaney J.E., Kuchel G.A. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:541–547. doi: 10.1097/MCO.0b013e32833cf3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medzhitov R. Inflammation 2010: New adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Nathan C., Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Krebs N.E., Hambidge K.M. Zinc metabolism and homeostasis: The application of tracer techniques to human zinc physiology. Biometals. 2001;14:397–412. doi: 10.1023/A:1012942409274. [DOI] [PubMed] [Google Scholar]
- 15.Foster M., Samman S. Zinc and redox signaling: Perturbations associated with cardiovascular disease and diabetes mellitus. Antioxid. Redox Signal. 2010;13:1549–1573. doi: 10.1089/ars.2010.3111. [DOI] [PubMed] [Google Scholar]
- 16.Prasad A.S. Zinc and immunity. Mol. Cell. Biochem. 1998;188:63–69. doi: 10.1023/A:1006868305749. [DOI] [PubMed] [Google Scholar]
- 17.Besecker B.Y., Exline M.C., Hollyfield J., Phillips G., Disilvestro R.A., Wewers M.D., Knoell D.L. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am. J. Clin. Nutr. 2011;93:1356–1364. doi: 10.3945/ajcn.110.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egefjord L., Jensen J.L., Bang-Berthelsen C.H., Petersen A.B., Smidt K., Schmitz O., Karlsen A.E., Pociot F., Chimienti F., Rungby J., Magnusson N.E. Zinc transporter gene expression is regulated by pro-inflammatory cytokines: A potential role for zinc transporters in beta-cell apoptosis? BMC Endocr. Disord. 2009;9:7. doi: 10.1186/1472-6823-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang C., Murgia C., Leong M., Tan L.W., Perozzi G., Knight D., Ruffin R., Zalewski P. Anti-inflammatory effects of zinc and alterations in zinc transporter mRNA in mouse models of allergic inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L577–L584. doi: 10.1152/ajplung.00280.2006. [DOI] [PubMed] [Google Scholar]
- 20.Liuzzi J.P., Lichten L.A., Rivera S., Blanchard R.K., Aydemir T.B., Knutson M.D., Ganz T., Cousins R.J. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA. 2005;102:6843–6848. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cousins R.J., Leinart A.S. Tissue-specific regulation of zinc metabolism and metallothionein genes by interleukin 1. FASEB J. 1988;2:2884–2890. doi: 10.1096/fasebj.2.13.2458983. [DOI] [PubMed] [Google Scholar]
- 22.De S.K., McMaster M.T., Andrews G.K. Endotoxin induction of murine metallothionein gene expression. J. Biol. Chem. 1990;265:15267–15274. [PubMed] [Google Scholar]
- 23.Lichten L.A., Cousins R.J. Mammalian zinc transporters: Nutritional and physiological regulation. Annu. Rev. Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 24.Kim S., Watanabe K., Shirahata T., Watarai M. Zinc uptake system (znuA locus) of Brucella abortus is essential for intracellular survival and virulence in mice. J. Vet. Med. Sci. 2004;66:1059–1063. doi: 10.1292/jvms.66.1059. [DOI] [PubMed] [Google Scholar]
- 25.Gaetke L.M., McClain C.J., Talwalkar R.T., Shedlofsky S.I. Effects of endotoxin on zinc metabolism in human volunteers. Am. J. Physiol. 1997;272:E952–E956. doi: 10.1152/ajpendo.1997.272.6.E952. [DOI] [PubMed] [Google Scholar]
- 26.Young B., Ott L., Kasarskis E., Rapp R., Moles K., Dempsey R.J., Tibbs P.A., Kryscio R., McClain C. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J. Neurotrauma. 1996;13:25–34. doi: 10.1089/neu.1996.13.25. [DOI] [PubMed] [Google Scholar]
- 27.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 28.Costarelli L., Muti E., Malavolta M., Cipriano C., Giacconi R., Tesei S., Piacenza F., Pierpaoli S., Gasparini N., Faloia E., et al. Distinctive modulation of inflammatory and metabolic parameters in relation to zinc nutritional status in adult overweight/obese subjects. J. Nutr. Biochem. 2010;21:432–437. doi: 10.1016/j.jnutbio.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Prasad A.S., Beck F.W., Grabowski S.M., Kaplan J., Mathog R.H. Zinc deficiency: Changes in cytokine production and T-cell subpopulations in patients with head and neck cancer and in noncancer subjects. Proc. Assoc. Am. Physicians. 1997;109:68–77. [PubMed] [Google Scholar]
- 30.Beck F.W., Li Y., Bao B., Prasad A.S., Sarkar F.H. Evidence for reprogramming global gene expression during zinc deficiency in the HUT-78 cell line. Nutrition. 2006;22:1045–1056. doi: 10.1016/j.nut.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Prasad A.S., Bao B., Beck F.W.J., Sarkar F.H. Correction of interleukin-2 gene expression by in vitro zinc addition to mononuclear cells from zinc-deficient human subjects: A specific test for zinc deficiency in humans. Transl. Res. 2006;148:325–333. doi: 10.1016/j.trsl.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Pinna K., Kelley D.S., Taylor P.C., King J.C. Immune functions are maintained in healthy men with low zinc intake. J. Nutr. 2002;132:2033–2036. doi: 10.1093/jn/132.7.2033. [DOI] [PubMed] [Google Scholar]
- 33.Wieringa F.T., Dijkhuizen M.A., West C.E., van der Ven-Jongekrijg J., van der Meer J.W., Muhilal Reduced production of immunoregulatory cytokines in vitamin A- and zinc-deficient Indonesian infants. Eur. J. Clin. Nutr. 2004;58:1498–1504. doi: 10.1038/sj.ejcn.1601998. [DOI] [PubMed] [Google Scholar]
- 34.Bao B., Prasad A.S., Beck F.W.J., Snell D., Suneja A., Sarkar F.H., Doshi N., Fitzgerald J.T., Swerdlow P. Zinc supplementation decreases oxidative stress, incidence of infection, and generation of inflammatory cytokines in sickle cell disease patients. Transl. Res. 2008;152:67–80. doi: 10.1016/j.trsl.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Prasad A.S., Beck F.W.J., Bao B., Fitzgerald J.T., Snell D.C., Steinberg J.D., Cardozo L.J. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007;85:837–844. doi: 10.1093/ajcn/85.3.837. [DOI] [PubMed] [Google Scholar]
- 36.Bao B., Prasad A.S., Beck F.W.J., Fitzgerald J.T., Snell D., Bao G.W., Singh T., Cardozo L.J. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: A potential implication of zinc as an atheroprotective agent. Am. J. Clin. Nutr. 2010;91:1634–1641. doi: 10.3945/ajcn.2009.28836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prasad A.S., Bao B., Beck F.W.J., Kucuk O., Sarkar F.H. Antioxidant effect of zinc in humans. Free Radic. Biol. Med. 2004;37:1182–1190. doi: 10.1016/j.freeradbiomed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Raqib R., Roy S.K., Rahman M.J., Azim T., Ameer S.S., Chisti J., Andersson J. Effect of zinc supplementation on immune and inflammatory responses in pediatric patients with shigellosis. Am. J. Clin. Nutr. 2004;79:444–450. doi: 10.1093/ajcn/79.3.444. [DOI] [PubMed] [Google Scholar]
- 39.Sandstead H.H., Prasad A.S., Penland J.G., Beck F.W.J., Kaplan J., Egger N.G., Alcock N.W., Carroll R.M., Ramanujam V.M.S., Dayal H.H., et al. Zinc deficiency in Mexican American children: Influence of zinc and other micronutrients on T cells, cytokines, and antiinflammatory plasma proteins. Am. J. Clin. Nutr. 2008;88:1067–1073. doi: 10.1093/ajcn/88.4.1067. [DOI] [PubMed] [Google Scholar]
- 40.Aydemir T.B., Blanchard R.K., Cousins R.J. Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations. Proc. Natl. Acad. Sci. USA. 2006;103:1699–1704. doi: 10.1073/pnas.0510407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahmann L., Uciechowski P., Warmuth S., Plümäkers B., Gressner A.M., Malavolta M., Mocchegiani E., Rink L. Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res. 2008;11:227–237. doi: 10.1089/rej.2007.0613. [DOI] [PubMed] [Google Scholar]
- 42.Mariani E., Cattini L., Neri S., Malavolta M., Mocchegiani E., Ravaglia G., Facchini A. Simultaneous evaluation of circulating chemokine and cytokine profiles in elderly subjects by multiplex technology: Relationship with zinc status. Biogerontology. 2008;7:449–459. doi: 10.1007/s10522-006-9060-8. [DOI] [PubMed] [Google Scholar]
- 43.Mocchegiani E., Giacconi R., Costarelli L., Muti E., Cipriano C., Tesei S., Pierpaoli S., Giuli C., Papa R., Marcellini F., et al. Zinc deficiency and IL-6–174G/C polymorphism in old people from different European countries: Effect of zinc supplementation. ZINCAGE study. Exp. Gerontol. 2008;43:433–444. doi: 10.1016/j.exger.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Kara E., Ozal M., Gunay M., Kilic M., Baltaci A.K., Mogulkoc R. Effects of exercise and zinc supplementation on cytokine release in young wrestlers. Biol. Trace Elem. Res. 2011;143:1435–1440. doi: 10.1007/s12011-011-9005-1. [DOI] [PubMed] [Google Scholar]
- 45.Bao B., Prasad A.S., Beck F.W., Bao G.W., Singh T., Ali S., Sarkar F.H. Intracellular free zinc up-regulates IFN-γ and T-bet essential for Th1 differentiation in Con-A stimulated HUT-78 cells. Biochem. Biophys. Res. Commun. 2011;407:703–707. doi: 10.1016/j.bbrc.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayashi K., Ishizuka S., Yokoyama C., Hatae T. Attenuation of interferon-gamma mRNA expression in activated Jurkat T cells by exogenous zinc via down-regulation of the calcium-independent PKC-AP-1 signaling pathway. Life Sci. 2008;83:6–11. doi: 10.1016/j.lfs.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 47.Prasad A.S., Bao B., Beck F.W.J., Sarkar F.H. Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-κB. Nutrition. 2011;27:816–823. doi: 10.1016/j.nut.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 48.Freitas M., Fernandes E. Zinc, cadmium and nickel increase the activation of NF-κB and the release of cytokines from THP-1 monocytic cells. Metallomics. 2011;3:1238–1243. doi: 10.1039/c1mt00050k. [DOI] [PubMed] [Google Scholar]
- 49.Tsou T.C., Chao H.R., Yeh S.C., Tsai F.Y., Lin H.J. Zinc induces chemokine and inflammatory cytokine release from human promonocytes. J. Hazard. Mater. 2011;196:335–341. doi: 10.1016/j.jhazmat.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 50.Metz C.H., Schröder A.K., Overbeck S., Kahmann L., Plümäkers B., Rink L. T-helper type 1 cytokine release is enhanced by in vitro zinc supplementation due to increased natural killer cells. Nutrition. 2007;23:157–163. doi: 10.1016/j.nut.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Poleganov M.A., Pfeilschifter J., Mühl H. Expanding extracellular zinc beyond levels reflecting the albumin-bound plasma zinc pool potentiates the capability of IL-1β, IL-18, and IL-12 to act as IFN-γ-inducing factors on PBMC. J. Interferon Cytokine Res. 2007;27:997–1001. doi: 10.1089/jir.2007.0037. [DOI] [PubMed] [Google Scholar]
- 52.Chang K.L., Hung T.C., Hsieh B.S., Chen Y.H., Chen T.F., Cheng H.L. Zinc at pharmacologic concentrations affects cytokine expression and induces apoptosis of human peripheral blood mononuclear cells. Nutrition. 2006;22:465–474. doi: 10.1016/j.nut.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 53.Von Bülow V., Rink L., Haase H. Zinc-mediated inhibition of cyclic nucleotide phosphodiesterase activity and expression suppresses TNF-alpha and IL-1 beta production in monocytes by elevation of guanosine 3′,5′-cyclic monophosphate. J. Immunol. 2005;175:4697–4705. doi: 10.4049/jimmunol.175.7.4697. [DOI] [PubMed] [Google Scholar]
- 54.Wellinghausen N., Martin M., Rink L. Zinc inhibits interleukin-1-dependent T cell stimulation. Eur. J. Immunol. 1997;27:2529–2535. doi: 10.1002/eji.1830271010. [DOI] [PubMed] [Google Scholar]
- 55.Driessen C., Hirv K., Wellinghausen N., Kirchner H., Rink L. Influence of serum on zinc, toxic shock syndrome toxin-1, and lipopolysaccharide-induced production of IFN-gamma and IL-1 beta by human mononuclear cells. J. Leukoc. Biol. 1995;57:904–908. doi: 10.1002/jlb.57.6.904. [DOI] [PubMed] [Google Scholar]
- 56.Scuderi P. Differential effects of copper and zinc on human peripheral blood monocyte cytokine secretion. Cell. Immunol. 1990;126:391–405. doi: 10.1016/0008-8749(90)90330-T. [DOI] [PubMed] [Google Scholar]
- 57.Prasad A.S. Zinc: Mechanisms of host defense. J. Nutr. 2007;137:1345–1349. doi: 10.1093/jn/137.5.1345. [DOI] [PubMed] [Google Scholar]
- 58.Beck F.W.J., Prasad A.S., Kaplan J., Fitzgerald J.T., Brewer G.J. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am. J. Physiol. 1997;272:E1002–E1007. doi: 10.1152/ajpendo.1997.272.6.E1002. [DOI] [PubMed] [Google Scholar]
- 59.Tapazoglou E., Prasad A.S., Hill G., Brewer G.J., Kaplan J. Decreased natural killer cell activity in zinc deficient subjects with sickle cell disease. J. Lab. Clin. Med. 1985;105:19–22. [PubMed] [Google Scholar]
- 60.Fantone J.C., Ward P.A. Polymorphonuclear leukocyte-mediated cell and tissue injury: Oxygen metabolites and their relations to human disease. Hum. Pathol. 1985;16:973–978. doi: 10.1016/S0046-8177(85)80273-2. [DOI] [PubMed] [Google Scholar]
- 61.Scapini P., Lapinet-Vera J.A., Gasperini S., Calzetti F., Bazzoni F., Cassatella M.A. The neutrophil as a cellular source of chemokines. Immunol. Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 62.Finamore A., Massimi M., Conti Devirgiliis L., Mengheri E. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J. Nutr. 2008;138:1664–1670. doi: 10.1093/jn/138.9.1664. [DOI] [PubMed] [Google Scholar]
- 63.Gavella M., Lipovac V., Car A. In vitro effect of zinc on superoxide anion production by polymorphonuclear leukocytes of diabetic patients. Acta Diabetol. 2000;37:135–137. doi: 10.1007/s005920070016. [DOI] [PubMed] [Google Scholar]
- 64.Siminiak T., Flores N.A., Sheridan D.J. Neutrophil interactions with endothelium and platelets: Possible role in the development of cardiovascular injury. Eur. Heart J. 1995;16:160–170. doi: 10.1093/oxfordjournals.eurheartj.a060880. [DOI] [PubMed] [Google Scholar]
- 65.Verstrepen L., Bekaert T., Chau T.L., Tavernier J., Chariot A., Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-κB: Variations on a common theme. Cell. Mol. Life Sci. 2008;65:2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bao B., Prasad A., Beck F.W., Suneja A., Sarkar F. Toxic effect of zinc on NF-kappaB, IL-2, IL-2 receptor alpha, and TNF-alpha in HUT-78 (Th(0)) cells. Toxicol. Lett. 2006;166:222–228. doi: 10.1016/j.toxlet.2006.07.306. [DOI] [PubMed] [Google Scholar]
- 67.von Bülow V., Dubben S., Engelhardt G., Hebel S., Plümäkers B., Heine H., Rink L., Haase H. Zinc-dependent suppression of TNF-α production is mediated by protein kinase A-induced inhibition of Raf-1, IκB Kinase β, and NF-κB. J. Immunol. 2007;179:4180–4186. doi: 10.4049/jimmunol.179.6.4180. [DOI] [PubMed] [Google Scholar]
- 68.Song H.Y., Rothe M., Goeddel D.V. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-κB activation. Proc. Natl. Acad. Sci. USA. 1996;93:6721–6725. doi: 10.1073/pnas.93.13.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim Y.M., Cao D., Reed W., Wu W., Jaspers I., Tal T., Bromberg P.A., Samet J.M. Zn2+-induced NF-κB-dependent transcriptional activity involves site-specific p65/RelA phosphorylation. Cell. Signal. 2007;19:538–546. doi: 10.1016/j.cellsig.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Bao S., Liu M.J., Lee B., Besecker B., Lai J.P., Guttridge D.C., Knoell D.L. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-κB. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;298:L744–L754. doi: 10.1152/ajplung.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dinarello C.A. Immunological and inflammatory functions of the Interleukin-1 family. Annu. Rev. Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 72.Bogdan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 73.Zou M.H., Shi C., Cohen R.A. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J. Clin. Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomas S.R., Witting P.K., Drummond G.R. Redox control of endothelial function and dysfunction: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 75.Xu J., Xie Z., Reece R., Pimental D., Zou M.H. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid. Role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler. Thromb. Vasc. Biol. 2006;26:2688–2695. doi: 10.1161/01.ATV.0000249394.94588.82. [DOI] [PubMed] [Google Scholar]
- 76.Azumi H., Inoue N., Takeshita S., Rikitake Y., Kawashima S., Hayashi Y., Itoh H., Yokoyama M. Expression of NADH/NADPH oxidase p22phox in human coronary arteries. Circulation. 1999;100:1494–1498. doi: 10.1161/01.CIR.100.14.1494. [DOI] [PubMed] [Google Scholar]
- 77.Matsunaga Y., Kawai Y., Kohda Y., Gemba M. Involvement of activation of NADPH oxidase and extracellular signal-regulated kinase (ERK) in renal cell injury induced by zinc. J. Toxicol. Sci. 2005;30:135–144. doi: 10.2131/jts.30.135. [DOI] [PubMed] [Google Scholar]
- 78.Foster M., Samman S. Zinc and Atherosclerosis: Clinical Observations and Potential Mechanisms. In: Rink L., editor. Zinc and Human Health: Biomedical and Health Research. Vol. 76. IOS Press; Amsterdam, The Netherlands: 2011. pp. 347–372. [Google Scholar]
- 79.Rowe D.J., Bobilya D.J. Albumin facilitates zinc acquisition by endothelial cells. Proc. Soc. Exp. Biol. Med. 2000;224:178–186. doi: 10.1046/j.1525-1373.2000.22418.x. [DOI] [PubMed] [Google Scholar]
- 80.Beattie J.H., Kwun I.S. Is zinc deficiency a risk factor for atherosclerosis? Br. J. Nutr. 2004;91:177–181. doi: 10.1079/BJN20031072. [DOI] [PubMed] [Google Scholar]
- 81.Hughes S., Samman S. The effect of zinc supplementation in humans on plasma lipids, antioxidant status, and thrombogenesis. J. Am. Coll. Nutr. 2006;25:285–291. doi: 10.1080/07315724.2006.10719537. [DOI] [PubMed] [Google Scholar]
- 82.Foster M., Petocz P., Samman S. Effects of zinc on plasma lipoprotein cholesterol concentrations in humans: A meta-analysis of randomised controlled trials. Atherosclerosis. 2010;210:344–352. doi: 10.1016/j.atherosclerosis.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 83.Meerarani P., Ramadass P., Toborek M., Bauer H.C., Bauer H., Hennig B. Zinc protects against apoptosis of endothelial cells induced by linoleic acid and tumor necrosis factor alpha. Am. J. Clin. Nutr. 2000;71:81–87. doi: 10.1093/ajcn/71.1.81. [DOI] [PubMed] [Google Scholar]
- 84.Wiseman D.A., Wells S.M., Hubbard M., Welker J.E., Black S.M. Alterations in zinc homeostasis underlie endothelial cell death induced by oxidative stress from acute exposure to hydrogen peroxide. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L165–L177. doi: 10.1152/ajplung.00459.2005. [DOI] [PubMed] [Google Scholar]
- 85.Hennig B., Meerarani P., Toborek M., McClain C.J. Antioxidant-like properties of zinc in activated endothelial cells. J. Am. Coll. Nutr. 1999;18:152–158. doi: 10.1080/07315724.1999.10718843. [DOI] [PubMed] [Google Scholar]
- 86.Bourcier T., Sukhova G., Libby P. The nuclear factor kB signaling pathway participates in dysregulation of vascular smooth muscle cells in vitro and in human atherosclerosis. J. Biol. Chem. 1997;272:15817–15824. doi: 10.1074/jbc.272.25.15817. [DOI] [PubMed] [Google Scholar]
- 87.De Martin R., Hoeth M., Hofer-Warbinek R., Schmid J.A. The transcription factor NF-κB and the regulation of vascular cell function. Arterioscler. Thromb. Vasc. Biol. 2000;20:e83–e88. doi: 10.1161/01.ATV.20.11.e83. [DOI] [PubMed] [Google Scholar]
- 88.Pasterkamp G., van Keulen J.K., de Kleijn D.P.V. Role of Toll-like receptor 4 in the initiation and progression of atherosclerotic disease. Eur. J. Clin. Invest. 2004;34:328–334. doi: 10.1111/j.1365-2362.2004.01338.x. [DOI] [PubMed] [Google Scholar]
- 89.Cortese-Krott M.M., Suschek C.V., Wetzel W., Kröncke K.D., Kolb-Bachofen V. Nitric oxide-mediated protection of endothelial cells from hydrogen peroxide is mediated by intracellular zinc and glutathione. Am. J. Physiol. Cell Physiol. 2009;296:811–820. doi: 10.1152/ajpcell.00643.2008. [DOI] [PubMed] [Google Scholar]
- 90.Donath M.Y., Størling J., Maedler K., Mandrup-Poulsen T. Inflammatory mediators and islet beta-cell failure: A link between type 1 and type 2 diabetes. J. Mol. Med. 2003;81:455–470. doi: 10.1007/s00109-003-0450-y. [DOI] [PubMed] [Google Scholar]
- 91.Maedler K., Sergeev P., Ris F., Oberholzer J., Joller-Jemelka H.I., Spinas G.A., Kaiser N., Halban P.A., Donath M.Y. Glucose-induced beta cell production of IL1-beta contributes to glucotoxicity in human pancreatic islets. J. Clin. Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ehses J.A., Perren A., Eppler E., Ribaux P., Pospisilik J.A., Maor-Cahn R., Gueripel X., Ellingsgaard H., Schneider M.K., Biollaz G., et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 93.Spranger J., Kroke A., Möhlig M., Hoffman K., Bergmann M.M., Ristow M., Boeing H., Pfeiffer A.F. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 94.Donath M.Y., Böni-Schnetzler M., Ellingsgaard H., Halban P.A., Ehses J.A. Cytokine production by islets in health and diabetes: Cellular origin, regulation and function. Trends Endocrinol. Metab. 2010;21:261–267. doi: 10.1016/j.tem.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 95.Arai K., Iizuka S., Tada Y., Oikawa K., Taniguchi N. Increase in the glucosylated form of erythrocyte Cu-Zn-superoxide dismutase in diabetes and close association of the nonenzymatic glucosylation with the enzyme activity. Biochim. Biophys. Acta. 1987;924:292–296. doi: 10.1016/0304-4165(87)90025-0. [DOI] [PubMed] [Google Scholar]
- 96.King G.L., Loeken M.R. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem. Cell Biol. 2004;122:333–338. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 97.Gupta R., Garg V.K., Mathur D.K., Goyal R.K. Oral zinc therapy in diabetic neuropathy. J. Assoc. Physicians India. 1998;46:939–942. [PubMed] [Google Scholar]
- 98.Kajanachumpol S., Srisurapanon S., Supanit I., Roongpisuthipong C., Apibal S. Effect of zinc supplementation on zinc status, copper status and cellular immunity in elderly patients with diabetes mellitus. J. Med. Assoc. Thail. 1995;78:344–349. [PubMed] [Google Scholar]
- 99.Samman S., Foster M., Capdor J., Stathakis E. Zinc, diabetes mellitus and cardiovascular disease in humans: A critical appraisal; Proceedings of International Society for Zinc Biology Conference; Melbourne, Australia. 15–19 January 2012; Abstract #71. [Google Scholar]
- 100.Wellinghausen N., Kirchner H., Rink L. The immunobiology of zinc. Immunol. Today. 1997;18:519–521. doi: 10.1016/S0167-5699(97)01146-8. [DOI] [PubMed] [Google Scholar]
- 101.Cave A.C., Brewer A.C., Narayanapanicker A., Ray R., Grieve D.J., Walker S., Shah A.M. NADPH oxidases in cardiovascular health and disease. Antioxid. Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 102.Ibs K.H., Rink L. Zinc-altered immune function. J. Nutr. 2003;133:1452–1456. doi: 10.1093/jn/133.5.1452S. [DOI] [PubMed] [Google Scholar]
- 103.Samman S. Dietary versus cellular zinc: The antioxidant paradox. Free Radic. Biol. Med. 1993;14:95–96. doi: 10.1016/0891-5849(93)90514-U. [DOI] [PubMed] [Google Scholar]