Abstract
One hundred E. coli isolates from Norway (n = 37), Sweden (n = 24), UK (n = 20) and Spain (n = 19), producing CTX-M-type - (n = 84), or SHV-12 (n = 4) extended spectrum β-lactamases, or the plasmid mediated AmpC, CMY-2 (n = 12), were typed using multi-locus sequence typing (MLST) and multi-locus variable number of tandem repeat analysis (MLVA). Isolates clustered into 33 Sequence Types (STs) and 14 Sequence Type Complexes (STCs), and 58 MLVA-Types (MTs) and 25 different MLVA-Type Complexes (MTCs). A strong agreement between the MLST profile and MLVA typing results was observed, in which all ST131-isolates (n = 39) and most of the STC-648 (n = 10), STC-38 (n = 9), STC-10 (n = 9), STC-405 (n = 8) and STC-23 (n = 6) isolates were clustered distinctly into MTC-29, -36, -20, -14, -10 and -39, respectively. MLVA is a rapid and accurate tool for genotyping isolates of globally disseminated virulent multidrug resistant E. coli lineages, including ST131.
Introduction
Antibiotic resistance is a globally interrelated public health problem, the threat of which is ever increasing. Dispersion of large mobile genetic elements carrying multiple resistance determinants coupled with dissemination of successful clones have been chronicled in most bacterial species [1], [2]. Locally, antibiotic exposure selects and promotes the evolution of multidrug resistant (MDR) bacteria, [3], [4] and movement of people and transport of food facilitate their worldwide dispersion [5]. MDR bacteria both undermine empirical treatment and delay appropriate therapy, which in turn increases patient mortality [6]. Therefore preventing the spread of MDR bacteria is an infection control priority [2]. An integral part of infection control is the recognition of successful and emerging MDR strains or clones, and hence there is always a need for rapid and accurate typing tools.
β-lactams belong to our most important and widely used class of antibiotics. Bacteria belonging to the Enterobacteriaceae family have during the last decades evolved to enable carriage of multiple β-lactamases, including extended-spectrum β-lactamases (ESBLs), plasmid mediated AmpC enzymes as well as carbapenemases such as VIM, NDM, KPC and OXA-48, which has compromised the clinical utility of β-lactam drugs [7]. In particular, Enterobacteriaceae strains carrying CTX-M type ESBLs have become prevalent and dominant worldwide [8], [9], [10], and clonal spread of CTX-M producing E. coli co-resistant to trimethoprim-sulfamethoxazole and ciprofloxacin are increasingly reported [11]. The worldwide emergence of the clone O25:H4 - ST131 as a dominant host of ESBLs poses huge public health challenges, and never have there been a greater need for a rapid detection and an intercontinental monitoring system for these clones [12], [13].
ST131 was originally identified using the Achtman multi-locus sequence typing (MLST) scheme [14], but faster methods, specific for the detection of ST131 have also been reported, including commercial repetitive sequence-based PCR (rep-PCR) [15], single nucleotide polymorphism (SNP) analysis of mdh and gyrB combined with O25b rfb [16], PCR approach using O25-pabB [17], and a ST131 CTX-M-15 specific triplex PCR for operon afa FM95545 [18].
Several studies have explored the use of tandem repeated DNA as mean for identification of different bacterial strains. Multi-locus variable number of tandem repeat analysis (MLVA) has been successfully applied for rapid and interlaboratory typing of various bacteria such as Bacillus, Yersinia, Mycobacterium, Enterococcus, Staphylococcus, Neisseria, Salmonella and E. coli O157 [19]. In this study we explore the use of MLVA as a possible typing tool for different globally dispersed virulent multidrug resistant lineages of E. coli.
Materials and Methods
List of abbreviations and resistance genes given in Table 1.
Table 1. List of abbreviations and resistance genes.
| bla | β-lactamase | |
| CTX-M | Cefotaximase - Munich | |
| SHV | Sulfhydryl reagent variable | |
| CMY | Cephamycinase | |
| VIM | Verona imipenemase | |
| NDM | New Delhi metallo β-lactamase | |
| KPC | Klebsiella pneumoniae carbapenemase | |
| OXA | Oxacillinase | |
| MLST | Multi-locus sequence typing | |
| ST | Sequence type | |
| STC | Sequence type complex | |
| MLVA | Multi-locus variable number of tandem repeat analysis | |
| MT | MLVA type | |
| MTC | MLVA type complex | |
| SLV | Single locus variant | |
| DLV | Double locus variant | |
| TLV | Triple locus variant | |
| QLV | Quadruple locus variant |
Bacterial Strains
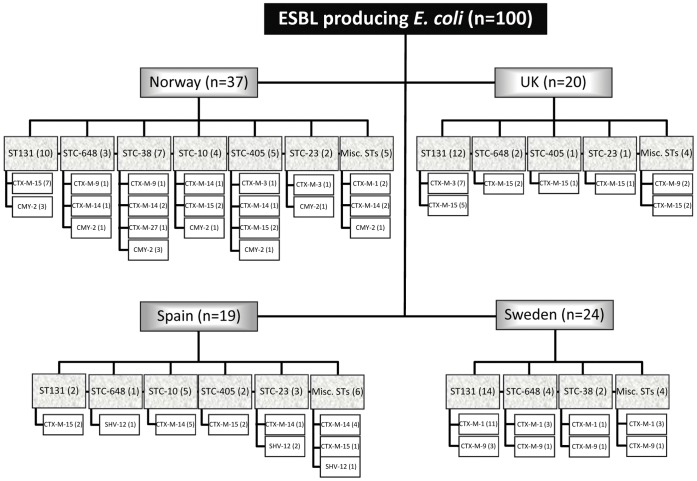
A total of 100 well-characterised clinical E. coli isolates producing ESBLs- or plasmid-mediated AmpC were tested. They had been collected between year 2000–2008, from Norway (n = 37), Sweden (n = 24), UK (n = 20) and Spain (n = 19), and of these, 84 isolates were CTX-M producers [CTX-M-1 (n = 20), CTX-M-3 (n = 9), CTX-M-15 (n = 27), CTX-M-27 (n = 1), CTX-M-9 (n = 10) and CTX-M-14 (n = 17)], 12 were CMY-2 producers and 4 were SHV-12 producers (Figure 1).
Figure 1. Overview of the 100 E. coli isolates included in this study from the four participating countries, their sequence types and genotypes.
Multi-locus Sequence Typing (MLST)
MLST was performed according to the scheme developed by Achtmans group targeting seven housekeeping genes; adk, fumC, gyrB, icd, mdh, purA, and recA, Target genes were amplified and sequenced using primers and conditions described on the web-site (http://mlst.ucc.ie/) [14]. Furthermore, allele numbers were assigned; Sequence Types (ST) were generated and assigned to the different Sequence Type Complexes (STCs) using the web-site from the respective laboratories. Collected MLST data were entered into BioNumerics (Applied Maths, Foster City, California) for comparative analysis.
Multi-locus Variable Number of Tandem Repeat Analysis (MLVA)
Total DNA of E. coli was prepared from overnight cultures using the RT Spin Bacteria DNA minikit (Invitek, Berlin, Germany). MLVA was performed in a single laboratory by targeting nine tandem repeats (CVN001, CVN002, CVN003, CVN004, CVN007, CVN014, CVN015, CCR001 and CVN016) identified and described by Lindstedt et al. [20], [21]. Repeats were amplified using PCR and multiple flour-labelled primers discernible by a genetic analyser. PCR products were subjected to capillary electrophoresis on an ABI-3130 Genetic Analyzer (Applied Biosystems, Oslo, Norway). Each peak was identified according to colour and size, and an allele number was assigned based on fragment sizes. Alleles for which amplicons were absent were designated an allele number of ‘0’. The allele numbers were entered into BioNumerics as character values and a dendrogram was constructed using categorical coefficients and the Ward algorithm. Numerical values were assigned for distinct MLVA-type profiles (MTs) and MLVA-Type Complexes (MTCs) were assigned for related isolates of up to four locus variations.
Results
Multi-locus Sequence Typing (MLST)
The 100 isolates were typed to 33 different Sequence Types (STs) and 14 different Sequence Type Complexes (STCs). A total of 81 of the 100 isolates were identified from 6 globally dispersed ST or STCs; i) ST131 (n = 39), ii) STC-648 (n = 10) spanning 3 different STs, iii) STC-38 (n = 9), spanning 2 different STs, iv) STC-10 (n = 9), spanning 4 different STs, v) STC-405 (n = 8), spanning 2 different STs, and vi) STC-23 (n = 6), spanning 4 different STs (Table 2). The remaining 19 isolates had 18 different STs which clustered into 9 different STCs (Table S1).
Table 2. Six major sequence type complexes (STCs) and sequence types (STs) in relation to assigned MLVA-types (MTs) and MLVA-type complexes (MTCs).
| STC | ST | No. (n) Isolates | bla | MT | MTC | Locus variation |
| None | 131 | 11 | CTX-M-15 | 6-0-0-10-3-4-1-6-0 | 29 | |
| 131 | 7 | CTX-M-3 | 6-0-0-10-3-4-1-6-0 | 29 | ||
| 131 | 4 | CTX-M-1 | 6-0-0-10-3-4-1-6-0 | 29 | ||
| 131 | 1 | CTX-M-9 | 6-0-0-10-3-4-1-6-0 | 29 | ||
| 131 | 2 | CTX-M-1 | 6-0-0-10-3-8-1-6-0 | 29 | SLV 29 (CVN014) | |
| 131 | 2 | CTX-M-15 | 6-0-0-10-3-3-1-6-0 | 29 | SLV 29 (CVN014) | |
| 131 | 1 | CTX-M-1 | 6-0-0-10-2-4-1-6-0 | 29 | SLV 29 (CVN014) | |
| 131 | 1 | CTX-M-1 | 6-0-0-10-3-11-1-6-0 | 29 | SLV 29 (CVN014) | |
| 131 | 1 | CTX-M-1 | 6-0-0-10-3-7-1-6-0 | 29 | SLV 29 (CVN014) | |
| 131 | 1 | CTX-M-9 | 6-0-0-10-3-10-1-6-0 | 29 | SLV 29 (CVN014) | |
| 131 | 1 | CTX-M-15 | 6-0-0-10-3-2-1-6-0 | 29 | SLV 29 (CVN014) | |
| 131 | 1 | CMY-2 | 6-0-0-10-3-5-1-6-0 | 29 | SLV 29 (CVN014) | |
| 131 | 1 | CTX-M-1 | 2-0-0-10-3-4-1-6-0 | 29 | SLV 29 (CVN001) | |
| 131 | 1 | CMY-2 | 6-3-0-10-3-4-1-6-0 | 29 | SLV 29 (CVN002) | |
| 131 | 1 | CTX-M-1 | 6-0-0-6-3-4-1-6-0 | 29 | SLV 29 (CVN004) | |
| 131 | 1 | CTX-M-9 | 6-0-0-10-3-6-1-39-0 | 29 | DLV 29 (CVN014, CCR001) | |
| 131 | 1 | CMY-2 | 5-0-0-10-3-5-1-0-41 | 29 | TLV 29 (CVN001, CVN014, CCR001) | |
| 131 | 1 | CMY-2 | 6-3-0-8-3-5-1-6-7 | 29 | QLV 29 (CVN002, CVN004, CVN014, CVN016) | |
| 648 | 648 | 2 | CTX-M-1 | 6-0-0-8-2-8-1-16-6 | 36 | |
| 648 | 1 | CTX-M-15 | 6-0-0-8-2-8-1-16-6 | 36 | ||
| 648 | 1 | CMY-2 | 6-3-0-8-2-8-1-16-6 | 36 | SLV 36 (CVN002) | |
| 648 | 1 | CTX-M-9 | 6-0-0-8-2-5-1-16-6 | 36 | SLV 36 (CVN014) | |
| 648 | 1 | CTX-M-9 | 6-1-0-8-2-7-1-16-6 | 36 | DLV 36 (CVN002, CVN014) | |
| 648 | 1 | CTX-M-1 | 6-18-0-8-2-7-1-16-6 | 36 | DLV 36 (CVN002, CVN014) | |
| 648 | 1 | SHV-12 | 6-3-0-8-2-7-1-16-6 | 36 | DLV 36 (CVN002, CVN014) | |
| 684 | 1 | CTX-M-15 | 5-0-7-0-3-1-1-6-0 | 6 | ||
| 62 | 1 | CTX-M-14 | 6-0-0-10-3-4-1-6-0 | 29 | ||
| 38 | 38 | 2 | CTX-M-14 | 5-3-0-10-3-8-1-64-20 | 20 | |
| 38 | 1 | CTX-M-9 | 5-3-0-10-3-8-1-64-20 | 20 | ||
| 38 | 1 | CMY-2 | 5-3-0-10-3-8-1-64-17 | 20 | SLV 20 (CVN016) | |
| 38 | 1 | CTX-M-1 | 5-3-0-10-3-7-1-64-15 | 20 | DLV 20 (CVN014, CVN016) | |
| 38 | 1 | CTX-M-9 | 5-3-0-10-3-4-1-64-10 | 20 | DLV 20 (CVN014, CVN016) | |
| 38 | 1 | CMY-2 | 5-3-0-10-3-7-1-64-17 | 20 | DLV 20 (CVN014, CVN016) | |
| 38 | 1 | CTX-M-27 | 5-3-0-8-3-8-1-55-17 | 20 | TLV 20 (CVN004, CCR001, CVN016) | |
| 778 | 1 | CMY-2 | 6-0-0-10-3-10-1-6-0 | 25 | ||
| 10 | 167 | 2 | CTX-M-15 | 5-10-7-8-3-1-1-6-7 | 14 | |
| 167 | 1 | CTX-M-14 | 5-10-7-8-3-1-1-6-7 | 14 | ||
| 167 | 1 | CTX-M-14 | 5-8-8-8-4-1-1-6-7 | 14 | TLV 14 (CVN002, CVN003, CVN007) | |
| 617 | 1 | CTX-M-14 | 5-10-7-8-3-1-1-6-7 | 14 | ||
| 10 | 1 | CTX-M-14 | 5-0-7-8-3-3-1-6-7 | 14 | DLV 14 (CVN002, CVN014) | |
| 10 | 1 | CMY-2 | 5-1-5-8-3-1-1-6-8 | 14 | TLV 14 (CVN002, CVN003, CVN016) | |
| 10 | 1 | CTX-M-14 | 5-0-0-8-3-5-1-35-0 | 5 | ||
| 48 | 1 | CTX-M-14 | 5-0-0-8-3-5-1-35-0 | 5 | ||
| 405 | 405 | 1 | CMY-2 | 5-1-0-10-3-4-1-0-47 | 10 | |
| 405 | 1 | CTX-M-15 | 5-1-0-10-3-4-1-0-47 | 10 | ||
| 405 | 1 | CTX-M-15 | 5-1-0-10-3-4-1-0-44 | 10 | SLV 10 (CVN016) | |
| 405 | 1 | CTX-M-3 | 5-1-0-10-3-4-1-64-0 | 10 | DLV 10 (CCR001, CVN016) | |
| 405 | 1 | CTX-M-14 | 5-1-0-10-3-5-1-64-21 | 10 | TLV 10 (CVN014, CCR001, CVN016) | |
| 964 | 2 | CTX-M-15 | 5-1-0-10-3-4-1-64-24 | 10 | DLV 10 (CCR001, CVN016) | |
| 405 | 1 | CTX-M-15 | 5-3-0-9-3-3-1-95-10 | 22 | ||
| 23 | 23 | 1 | SHV-12 | 6-0-0-8-3-5-1-6-0 | 39 | |
| 410 | 1 | CTX-M-15 | 6-0-0-8-3-5-1-6-0 | 39 | ||
| 88 | 1 | SHV-12 | 6-3-0-8-3-15-1-6-0 | 39 | DLV 39 (CVN002, CVN014) | |
| 90 | 1 | CTX-M-14 | 6-1-0-8-3-3-1-6-7 | 39 | TLV 39 (CVN002, CVN014, CVN016) | |
| 90 | 1 | CTX-M-3 | 6-1-0-8-3-4-1-6-7 | 39 | TLV 39 (CVN002, CVN014, CVN016) | |
| 88 | 1 | CMY-2 | 7-0-0-3-1-7-1-16-0 | 55 |
For complete data on all 100 isolates see Table S1.
Multi-locus Variable Number of Tandem Repeat Analysis (MLVA)
The 100 isolates were typed to 58 different MLVA-types (MTs) and 25 different MLVA Complexes (MTCs) of related isolates. Isolates belonging to the six major STs or STCs were clustered as; i) ST131 (n = 39/39) and ST62 (n = 1/1) -isolates into MTC labelled 29, spanning 14 MTs, ii) STC-648 (n = 8/10) into MTC labelled 36, spanning 6 MTs, iii) STC-38 (n = 8/9) into MTC labelled 20, spanning 6 MTs, iv) STC-10 (n = 7/9) into MTC labelled 14, spanning 4 MTs v) STC-405 (n = 7/8) into MTC labelled 10, spanning 5 MTs, and vi) STC-23 (n = 5/6) into MTC labelled 39, spanning 4 MTs (Table 2). The remaining 26 isolates were distributed into 19 MTs (Table S1).
MLST and MLVA by Country and Genotypes
ST131 isolates (n = 39) were collected from Sweden (n = 14), UK (n = 12), Norway (n = 11), and Spain (n = 2), encoding β-lactamases CTX-M-1 (n = 11), CTX-M-3 (n = 7), CTX-M-15 (n = 14), CTX-M-9 (n = 3), and CMY-2 (n = 4). All 39 isolates were typed into a single MTC-29, with 24 identical isolates labelled MT29, 12 SLVs, 1 DLV, 1 TLV and 1 QLV (Table 2). Interestingly, 86% (n = 18/21) of the ST131 isolates with CTX-M-15 and its precursor CTX-M-3 producing isolates (all from the UK), had an indistinguishable MLVA genotype, MT29. Whereas greater MLVA diversity was seen among ST131 isolates with CTX-M-9 and CMY-2 enzymes.
STC-648 isolates (n = 10) were collected from Sweden (n = 4), Norway (n = 3), UK (n = 2) and Spain (n = 1), encoding β-lactamases SHV-12 (n = 1), CTX-M-1 (n = 3), CTX-M-15 (n = 2), CTX-M-9 (n = 2), CTX-M-14 (n = 1), and CMY-2 (n = 1). The majority of the strains (n = 8) were grouped into a single MTC-36, consisting of six different MTs, varying exclusively at loci CVN002 and/or CVN014.
The STC-38 isolates (n = 9) were collected only from Norway (n = 7) and Sweden (n = 2), encoding β-lactamases CTX-M-1 (n = 1), CTX-M-9 (n = 2), CTX-M-14 (n = 2), CTX-M-27 (n = 1) and CMY-2 (n = 3). Eight of these isolates were typed into MTC-20, consisting of six different MTs, varying at loci CVN014 and/or CVN016. All CTX-M-9 and CTX-M-14 isolates from Norway were grouped into a single genotype MT20.
Isolates belonging to the STC-405 (n = 8) were collected from Norway (n = 5), Spain (n = 2) and UK (n = 1), encoding β-lactamases CTX-M-3 (n = 1), CTX-M-15 (n = 5), CTX-M-14 (n = 1) and CMY-2 (n = 1). Seven of the isolates were grouped into MTC-10, consisting of five different MTs, mainly varying at loci CCR001 and CVN016.
The STC-10 isolates (n = 9) were collected from Spain (n = 5) and Norway (n = 4), encoding β-lactamases CTX-M-14 (n = 6), CTX-M-15 (n = 2) and CMY-2 (n = 1). Seven of these isolates were typed into MTC-14, and the remaining two CTX-M-14 producing isolates from Spain in MTC-5. The MTC-14 was consisting of four different MTs. Six isolates were collected from Spain (n = 3), Norway (n = 2) and UK (n = 1), encoding β-lactamases SHV-12 (n = 2), CTX-M-3 (n = 1), CTX-M-15 (n = 1), CTX-M-14 (n = 1), and CMY-2 (n = 1) of the STC-23. Five of the isolates were grouped into a single MTC-39, varying at loci CVN002, CVN014 and CVN016.
Discussion
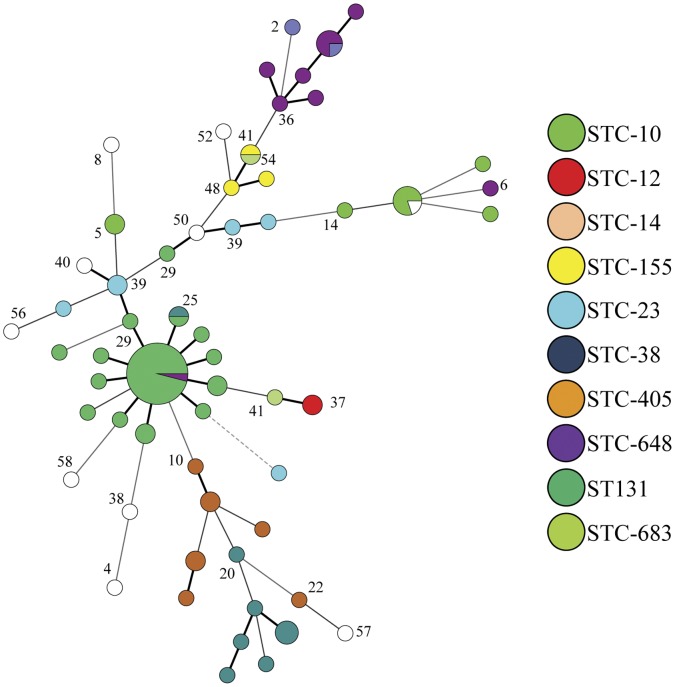
In this study we document MLVA as a tool for rapid and accurate typing of internationally-dispersed virulent ESBL producing E. coli lineages, including ST131. Independently collected isolates from four countries during 9 years displayed high concordance between MLST types and MLVA types with an increased resolution (Figure 2). Interestingly, the MLVA typing scheme was in particular able to genotype the six major STs and STCs responsible for the pandemic spread of ESBLs, into six distinctly different MTCs. No country-specific clustering of isolates was seen. The variability within the nine different loci under investigation suggested that the present MLVA scheme and its combination of highly variable and conservative loci can accurately serve the purpose of genotyping these strains. We recognized that in our collection, locus CVN015 had none, and loci CVN003 and CVN007 had little discriminatory power. CVN003 was only present in nine isolates with three different alleles, and CVN007 was identified with only a single allele 3 in 87% of the isolates. Nevertheless these loci have in the past proven to be important markers for genotyping enteropathogenic E. coli of diverse serotypes [21]. It is here also interesting to highlight that alleles 10 and 8 at locus CVN004 seem to be conserved (93%) across all isolates of the major STs and STCs, and in an MLVA database of E. coli isolates (n = 3417), allele 10 at locus CVN004 is only present in 4% of the isolates (n = 139) including the strains from this study (personal database of Lindstedt). Thus, the particular alleles 8 and 10, at locus CVN004 seem to be important indicators for the presence of the major STCs and ST131.
Figure 2. Minimum-spanning tree analysis of MLVA data from 100 E. coli isolates.
The circles represent unique MLVA types (numeric values); the diameter of the circles represents number of isolates. MLST data on the strains are color-coded: STC-10 (fluorescent green), STC-12 (red), STC-14 (dark blue), STC-155 (yellow), STC-23 (light blue) STC-38 (dark green), STC-405 (brown), STC-648 (purple), ST131 (green), STC-683 (light green).
Currently, phenotypic detection of the ST131 clone is not possible and molecular-based techniques are required. Accurate detection is an important first step towards monitoring and controlling ST131 dissemination. ST131 E. coli producing CTX-M-15 are usually co-resistant to quinolones and aminoglycosides [22], [23], which leaves few therapeutic options against infections in human or animal [24], [25]. In recent years, NDM-1 and KPC-2 carbapenemase producing ST131 has also been reported, substantiating the need for a rapid detection method of this clone [26], [27]. Indeed, newer, faster and more comprehensive typing technology is being developed and introduced. In wait of full genome sequencing as an affordable and manageable tool for typing, promising alternative typing techniques for E. coli in addition to MLVA include DNA microarray [28], [29] and Matrix-assisted laser desorption ionization-time of flight mass spectrometry, MALDI-TOF-MS [30]. Although these techniques are initially developed for accurate and rapid typing of specific pathogenic E. coli serotypes, they hold the potential for also typing MDR lineages of E. coli. Indeed, as we have successfully shown with the existing MLVA scheme; a potential for rapid, accurate and high resolution genotyping of ST131 and other internationally-dispersed, virulent multidrug resistant E. coli lineages.
We have identified MLVA type complexes that correspond to the major MLST-defined complexes. Assuming extracted DNA and availability of all required equipment, the expected turnover time for MLVA of a single isolate is close to five hours, whereas it may require a couple of days for its complete MLST analysis. As such MLVA is a good alternative to MLST for epidemiological surveillance of virulent multidrug resistant E. coli in the future [2].
Supporting Information
MLST and MLVA data on all 100 isolates included in this study.
(XLSX)
Acknowledgments
We wish to thank all collaborators, technicians and personnel contributing to the collection and characterisation of these strains in Norway, Sweden, UK and Spain.
Funding Statement
This study was funded in part by the Northern Norway Regional Health Authority Medical Research Program. No additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Woodford N, Wareham DW (2009) Tackling antibiotic resistance: a dose of common antisense? J Antimicrob Chemother 63: 225–229. [DOI] [PubMed] [Google Scholar]
- 2. Woodford N, Turton JF, Livermore DM (2011) Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev 35: 736–755. [DOI] [PubMed] [Google Scholar]
- 3. Canton R, Morosini MI (2011) Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol Rev 35: 977–991. [DOI] [PubMed] [Google Scholar]
- 4. Barbosa TM, Levy SB (2000) The impact of antibiotic use on resistance development and persistence. Drug Resist Updat 3: 303–311. [DOI] [PubMed] [Google Scholar]
- 5. Pitout JD, Campbell L, Church DL, Gregson DB, Laupland KB (2009) Molecular characteristics of travel-related extended-spectrum-beta-lactamase-producing Escherichia coli isolates from the Calgary Health Region. Antimicrob Agents Chemother 53: 2539–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oteo J, Perez-Vazquez M, Campos J (2010) Extended-spectrum [beta]-lactamase producing Escherichia coli: changing epidemiology and clinical impact. Curr Opin Infect Dis 23: 320–326. [DOI] [PubMed] [Google Scholar]
- 7. Pitout JD (2010) Infections with extended-spectrum beta-lactamase-producing enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs 70: 313–333. [DOI] [PubMed] [Google Scholar]
- 8. Livermore DM, Canton R, Gniadkowski M, Nordmann P, Rossolini GM, et al. (2007) CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother 59: 165–174. [DOI] [PubMed] [Google Scholar]
- 9. Canton R, Coque TM (2006) The CTX-M beta-lactamase pandemic. Curr Opin Microbiol 9: 466–475. [DOI] [PubMed] [Google Scholar]
- 10. Castanheira M, Mendes RE, Rhomberg PR, Jones RN (2008) Rapid emergence of blaCTX-M among Enterobacteriaceae in U.S. Medical Centers: molecular evaluation from the MYSTIC Program (2007). Microb Drug Resist 14: 211–216. [DOI] [PubMed] [Google Scholar]
- 11. Canton R, Ruiz-Garbajosa P (2011) Co-resistance: an opportunity for the bacteria and resistance genes. Curr Opin Pharmacol 11: 477–485. [DOI] [PubMed] [Google Scholar]
- 12. Coque TM, Novais A, Carattoli A, Poirel L, Pitout J, et al. (2008) Dissemination of clonally related Escherichia coli strains expressing extended-spectrum beta-lactamase CTX-M-15. Emerg Infect Dis 14: 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Naseer U, Sundsfjord A (2011) The CTX-M Conundrum: Dissemination of Plasmids and Escherichia coli Clones. Microb Drug Resist. [DOI] [PubMed] [Google Scholar]
- 14. Wirth T, Falush D, Lan R, Colles F, Mensa P, et al. (2006) Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol 60: 1136–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lau SH, Cheesborough J, Kaufmann ME, Woodford N, Dodgson AR, et al. (2010) Rapid identification of uropathogenic Escherichia coli of the O25:H4-ST131 clonal lineage using the DiversiLab repetitive sequence-based PCR system. Clin Microbiol Infect 16: 232–237. [DOI] [PubMed] [Google Scholar]
- 16. Johnson JR, Menard M, Johnston B, Kuskowski MA, Nichol K, et al. (2009) Epidemic clonal groups of Escherichia coli as a cause of antimicrobial-resistant urinary tract infections in Canada, 2002 to 2004. Antimicrob Agents Chemother 53: 2733–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clermont O, Dhanji H, Upton M, Gibreel T, Fox A, et al. (2009) Rapid detection of the O25b-ST131 clone of Escherichia coli encompassing the CTX-M-15-producing strains. J Antimicrob Chemother 64: 274–277. [DOI] [PubMed] [Google Scholar]
- 18. Blanco M, Alonso MP, Nicolas-Chanoine MH, Dahbi G, Mora A, et al. (2009) Molecular epidemiology of Escherichia coli producing extended-spectrum β-lactamases in Lugo (Spain): dissemination of clone O25b:H4-ST131 producing CTX-M-15. J Antimicrob Chemother 63: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 19. Lindstedt BA (2005) Multiple-locus variable number tandem repeats analysis for genetic fingerprinting of pathogenic bacteria. Electrophoresis 26: 2567–2582. [DOI] [PubMed] [Google Scholar]
- 20. Lindstedt BA, Brandal LT, Aas L, Vardund T, Kapperud G (2007) Study of polymorphic variable-number of tandem repeats loci in the ECOR collection and in a set of pathogenic Escherichia coli and Shigella isolates for use in a genotyping assay. J Microbiol Methods 69: 197–205. [DOI] [PubMed] [Google Scholar]
- 21. Lobersli I, Haugum K, Lindstedt BA (2012) Rapid and high resolution genotyping of all Escherichia coli serotypes using 10 genomic repeat-containing loci. J Microbiol Methods 88: 134–139. [DOI] [PubMed] [Google Scholar]
- 22. Morosini MI, Garcia-Castillo M, Coque TM, Valverde A, Novais A, et al. (2006) Antibiotic coresistance in extended-spectrum-beta-lactamase-producing Enterobacteriaceae and in vitro activity of tigecycline. Antimicrob Agents Chemother 50: 2695–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rogers BA, Sidjabat HE, Paterson DL (2011) Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J Antimicrob Chemother 66: 1–14. [DOI] [PubMed] [Google Scholar]
- 24. Nicolas-Chanoine MH, Blanco J, Leflon-Guibout V, Demarty R, Alonso MP, et al. (2008) Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J Antimicrob Chemother 61: 273–281. [DOI] [PubMed] [Google Scholar]
- 25. Johnson JR, Miller S, Johnston B, Clabots C, Debroy C (2009) Sharing of Escherichia coli sequence type ST131 and other multidrug-resistant and Urovirulent E. coli strains among dogs and cats within a household. J Clin Microbiol 47: 3721–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morris D, Boyle F, Ludden C, Condon I, Hale J, et al. (2011) Production of KPC-2 carbapenemase by an Escherichia coli clinical isolate belonging to the international ST131 clone. Antimicrob Agents Chemother 55: 4935–4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peirano G, Schreckenberger PC, Pitout JD (2011) Characteristics of NDM-1-producing Escherichia coli isolates that belong to the successful and virulent clone ST131. Antimicrob Agents Chemother 55: 2986–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Geue L, Schares S, Mintel B, Conraths FJ, Muller E, et al. (2010) Rapid microarray-based genotyping of enterohemorrhagic Escherichia coli serotype O156:H25/H−/Hnt isolates from cattle and clonal relationship analysis. Appl Environ Microbiol 76: 5510–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Monecke S, Mariani-Kurkdjian P, Bingen E, Weill FX, Baliere C, et al. (2011) Presence of enterohemorrhagic Escherichia coli ST678/O104:H4 in France prior to 2011. Appl Environ Microbiol 77: 8784–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karger A, Ziller M, Bettin B, Mintel B, Schares S, et al. (2011) Determination of serotypes of Shiga toxin-producing Escherichia coli isolates by intact cell matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl Environ Microbiol 77: 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MLST and MLVA data on all 100 isolates included in this study.
(XLSX)